Abstract
Heart failure is a disease characterized by profound human suffering with limitations in survival despite treatment with guideline-directed medical therapies. Patients with heart failure frequently progress to advanced stages and often require cardiac transplantation or implantation of left ventricular assist devices (LVADs) to extend survival and improve quality of life. As the number of suitable heart donors, number of experienced medical centers and patient comorbidities place restrictions on the feasibility of cardiac transplantation, implantation of LVADs has emerged as a more frequently applied treatment as either a bridge to transplantation or as permanent therapy. Considerable data have documented improvements in survival, functional status and quality of life offered by LVADs, however, few studies have focused on identifying: (1) determinants of LVAD use across medical centers, (2) the relationship between the determinants of LVAD use and value (defined as quality divided by cost), and (3) how determinants of LVAD use are influenced or impacted by vulnerable populations. We propose a conceptual model that integrates the main determinants of LVAD utilization, which include technology, insurance coverage, market-, provider-and patient-level factors. We propose this paradigm as a necessary prerequisite for understanding LVAD usage and value. This conceptual framework provides a broader view for future studies, which are needed to inform emerging healthcare policies that influence dissemination of this expensive but life-prolonging medical therapy.
Advanced heart failure (HF) affects more than 250,000 Americans, and is characterized by severe limitations in survival, functional status and quality of life (1). Patients with advanced HF become refractory to guideline-directed medical therapy, and most will die within 2 years of diagnosis in the absence of advanced surgical therapies (2, 3). Cardiac transplantation (TXP) is the most successful surgical therapy to extend survival and improve symptoms for selected patients (4). However, most patients with advanced HF are not candidates for TXP given restrictive criteria imposed by a limited supply of donor hearts. Durable left ventricular assist devices (LVADs) offer patients with advanced HF an alternative therapeutic option as a bridge to transplantation (BTT) or as destination therapy (DT).
Use of durable LVAD therapy has grown dramatically in the past decade and now exceeds TXP as the dominant surgical treatment for advanced HF refractory to guideline-directed medical therapy (5). Rapid dissemination of durable LVAD therapy has occurred as a consequence of (1) a growing population of eligible recipients, (2) improvement in durable LVAD technology that has increased its acceptance by providers and patients, and (3) significantly fewer supply limitations compared with TXP.
The need to understand the determinants of LVAD use is urgent. The rapid and continuing growth of durable LVAD therapy for patients with advanced HF requires a timely understanding of potential determinants to inform targeted health care policies and ensure responsible and equitable dissemination of this life-prolonging technology. This review will focus on LVAD use in the adult population.
Potential determinants of LVAD usage
We introduce a conceptual model (FIGURE 1) as a paradigm for how to evaluate the value of durable LVAD therapy. Our model integrates multiple factors determining LVAD use, including technology innovation, insurer, and market-, provider-and patient-level factors. Each determinant is interdependent and likely contributes to overall clinical outcomes and health care spending. As value is defined as a clinical outcome achieved per dollar spent, each of these determining factors should be considered when evaluating the value of durable LVAD therapy.
Figure 1:
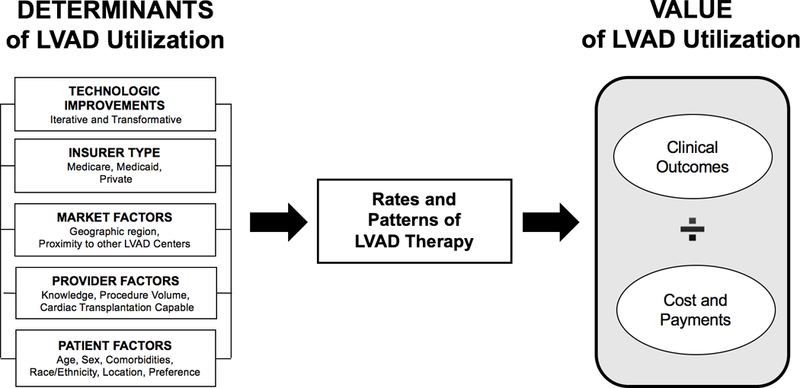
Our conceptual model integrates several factors determining LVAD use, including technology, insurer, and market-, provider-and patient-level factors. Technologic improvements can be iterative, such as the incremental improvements to reduce the rate of adverse events, or transformative, as evidenced by the shift from pulsatile to continuous flow LVADs. Insurer type has enormous implications for affecting trends in LVAD use (e.g., the inclusion of destination therapy as an indication in the Centers for Medicare and Medicaid Services National Coverage Determination led to a surge in VAD use beginning in 2008). Market-level factors account for proximity of alternate centers, which may influence thresholds for LVAD use depending on local or regional competition. Provider-level factors account for procedure volume and the offering of transplant services because these may affect clinical outcomes. Patient-level factors include characteristics such as age, sex, race, comorbidities, geography and patient preference.
Improvements in technology (transformative and iterative)
Improvements in durable LVAD technology represent the primary factor driving use, particularly after transformative changes in device design. This is evidenced by the rapid adoption of continuous-flow pump technology (over that of pulsatile technology) after approval by the United States Food and Drug Administration of the HeartMate II (Abbott Laboratories, Abbott Park, IL) for BTT indication in 2008 and for DT indication in 2010 (6, 7) (FIGURE 2). The Interagency Registry of Mechanically Assisted Circulatory Support (INTERMACS), a National Heart, Lung and Blood Institute-sponsored registry created to record the evolution of LVAD therapy and to assess and improve patient outcomes, now includes more than 20,000 patients, with nearly 100% of these patients receiving continuous-flow pumps in the most recent era (8). Use also increases with iterative improvements in LVAD technology that decrease the burden of device-related adverse events. Recent data from the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3) trial have demonstrated a significant reduction in pump thrombosis risk for the HeartMate 3 (Abbott Laboratories) compared to the HeartMate II (9). Because the risk of pump thrombosis has been a major barrier to greater durable LVAD adoption, particularly in those with less advanced stages of HF (10), the impending commercialization of the HeartMate 3 will likely lead to greater adoption, and perhaps broadening of clinical use among less ill patients.
Figure 2:
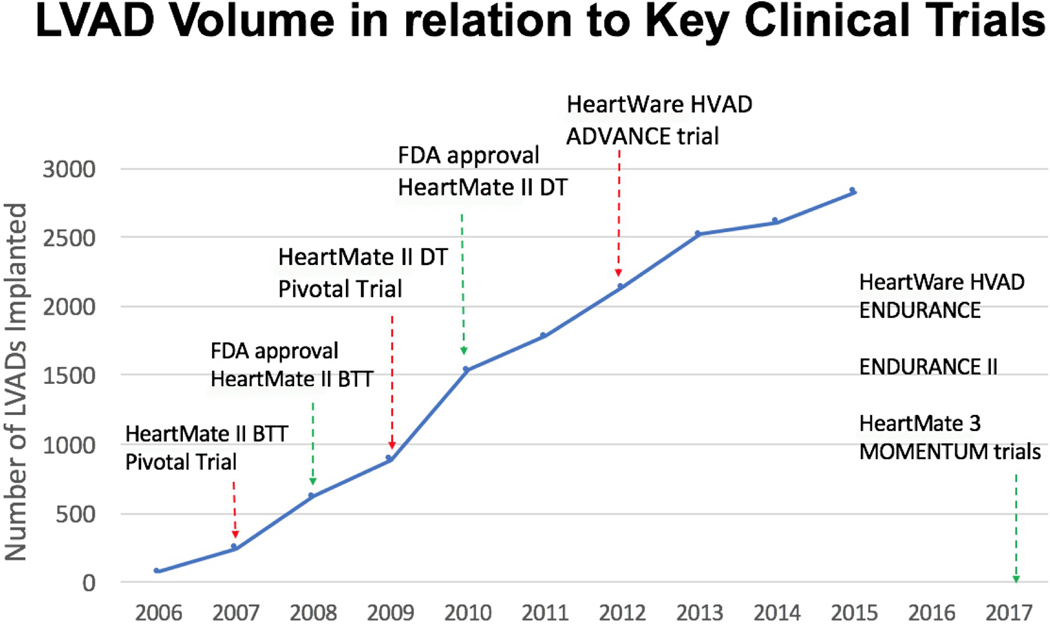
Key clinical trials for left ventricular assist devices (LVADs) are represented as a function of annual LVAD volume (source Interagency Registry for Mechanically Assisted Circulatory Support [INTERMACS] registry). Of note, the corresponding year of each clinical trial represents the date of publication of major findings from the trial. Although recent trials from 2017 (i.e., HeartWare HVAD [HeartWare Int.] A Clinical Trial to Evaluate the HeartWare® Ventricular Assist System [ENDURANCE], ENDURANCE II, HeartMate 3 [Abbott Laboratories] Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 [MOMENTUM 3]) are displayed, INTERMACS has yet to publish for the years 2016 and 2017. ADVANCE, HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure; BTT, bridge to transplant; DT, destination therapy; FDA, Food and Drug Administration.
If iterative device-related improvements can be similarly achieved in reducing the risk of stroke (to approximately 5% or less per year), further significant expansion of this therapy will likely follow (10). Further iterative or transformative improvements in technology (e.g., fully implantable systems) are needed to decrease the rates of device-related infections, because infection rates remain high in both the recent A Clinical Trial to Evaluate the HeartWare ® Ventricular Assist System (ENDURANCE) and MOMENTUM 3 clinical trials (9, 11).
Insurer factors (public vs private)
Health care spending for the care and management of HF is staggering and represents the second largest expenditure for the Centers for Medicare and Medicaid Services (CMS) (1). The CMS National Coverage Determination (NCD) continues to have a significant effect on LVAD use, as exemplified by the inclusion of DT as an approved indication in 2002. Consequently, LVAD usage for DT increased from 14.7% of total LVAD volume in 2006 to 41.6% in 2013, even as the total annual LVAD implantation volume increased approximately 10-fold during the same period (12). Changes in indications for durable LVAD therapy could arise as a result of the MOMENTUM 3 trial design (13). The MOMENTUM 3 trial eliminated BTT and DT designations as requirements for study participation and assessed eligibility for enrollment by symptoms, treatment modalities, and/or physiologic factors. Outcomes were then assessed based on short-term (i.e., 6 months) and long-term (i.e., 2 years) support.
Future decisions by CMS about whether BTT and DT designations will be required to meet requirements for reimbursement could potentially have a significant effect on LVAD use. Although the NCD provides criteria for reimbursement coverage for durable LVAD therapy, the NCD is not uniformly applied by private or public (Medicaid) insurance plans resulting in less or more restrictive criteria influencing availability of coverage (14, 15). This practice likely creates inequities in access and use across geography and different patient sub-groups.
Public insurance has been a means for a significant number of patients to gain access to advanced HF therapies. Compared with those with private insurance, patients requiring public insurance have been shown to have worse health care outcomes (16, 17). The role of insurance type on health outcomes has been studied in other areas of medicine but has not been extensively studied within the durable LVAD population (16, 17). Recent data have documented greater access to heart transplantation for states adopting the Affordable Care Act, in part, attributed to increased access to Medicaid services (16). This benefit was demonstrated for African Americans but not Hispanic or Caucasians populations (18). In reference to LVAD therapy, individual state-run Medicaid plans do not uniformly recognize the DT indication for LVAD therapy, creating a significant obstacle for economically disadvantaged patients relying on Medicaid services to receive DT (19). Whether access to durable LVAD therapy has been increased in states that have adopted the Affordable Care Act and expanded Medicaid access is unknown (20).
Market factors
Market-level factors may have a greater influence in the dissemination of LVAD therapy compared with TXP, given the significantly fewer barriers to initiating new LVAD programs. Given their expertise in delivery of other HF therapies (e.g., TXP), tertiary medical facilities have disproportionity been early adopters of LVAD therapy. A new era in durable LVAD therapy began after the Food and Drug Administration approved the HeartMate II for BTT in 2008, with increased proportion of durable LVADs subsequently implanted at non-academic, non-TXP facilities (late adopters) (21). A recent analysis of INTERMACS data has documented equivalent outcomes between TXP and non-TXP centers, suggesting that factors beyond traditional center designations (e.g., TXP, non-TXP) may contribute to patient outcomes (21). Further work is warranted to (1) characterize populations served by these emerging centers; 2) determine whether emerging centers are addressing unique patient populations or improving access to LVAD therapy; 3) analyze the role of market forces in influencing the threshold for durable LVAD use and value; 4) determine if current dissemination is providing access and increased value to patients receiving durable LVAD therapy, especially among our most vulnerable populations.
Provider factors
Provider factors at the hospital and physician level should be taken into account. Hospital-level factors to consider include the availability of board-certified HF specialists, annual procedure volume, and the offering of TXP services at that center. Moreover, there are considerable direct and indirect costs that are associated with establishing a successful LVAD program within a hospital that may serve as a barrier to the initiation or maintenance of a LVAD program.
Individual provider factors also play a key role in the use of LVAD therapy. Dissemination of medical knowledge is paramount, because durable LVAD therapy requires a high degree of provider specialization and expertise. Medical expertise is required because there are many unique aspects to LVAD therapy, including surgical implantation and device operation and management. Provider knowledge has been cited as a barrier to dissemination of other high technology medical therapies, such as implantable cardiac defibrillators (22, 23). Provider knowledge may thus play an important role in patient referral patterns for LVAD therapy.
An additional consideration is the regulatory requirements of individual providers. For example, individual LVAD providers are subject to less stringent requirements compared with TXP providers. For a center to perform LVAD therapy for DT, the NCD requires that a surgeon implant only 10 devices over a 3-year period (24) despite evidence suggesting a higher volume threshold to optimize outcomes (25, 26). By comparison, the United Network of Organ Sharing requires that a surgeon perform at least 20 heart transplants as primary surgeon or first assistant during an approved thoracic surgical residency, 12-month heart transplant fellowship, or within the first 2 years of clinical practice to qualify as surgical director of a heart transplant program (27). Additional training requirements for surgeons implanting LVADs are not specified in the NCD, despite more comprehensive recommendations from The Society of Thoracic Surgeons (28). In addition, as other markets in cardiac surgery (i.e., coronary artery bypass grafting) continue to decline, there may be an increase in the number of surgeons who pursue additional training to meet the NCD requirements to provide durable LVAD therapy.
Patient factors
The influence of patient factors is perhaps the most studied aspect of the determinants of LVAD use. Patient factors associated with limited access to LVAD therapy include age >65 years, female sex, black race, admission to a non-academic center and geographic region (11). Data from clinical trials and large patient registries (i.e., INTERMACS) have identified patient characteristics that influence outcomes after LVAD implantation that educate providers and patients about whether to pursue durable LVAD therapy (5). Individual patient factors are intimately associated with each of the other determinants of LVAD use including technology, insurance coverage, market and provider factors in ways that have yet to be studied.
Why a conceptual model? Disparities as a consequence of non-rational use
A conceptual model may serve as a useful lens for evaluating real-world problems, and affording frameworks for informing health policies. As an example, we describe how our model may help to evaluate existing disparities in LVAD use.
To date, large public and private payers have not instituted policies to ensure equity in LVAD use. Although not well studied to date, disproportionate access to LVAD therapy has likely resulted, especially among vulnerable populations (e.g., age, sex, race, ethnicity, geographic region, and socioeconomic status). For example, women represent one of the largest potential growth areas for LVAD therapy. Although the prevalence of HF is relatively equal among men and women, more women die of HF every year (29, 30). Advances in technology have produced smaller pumps that theoretically eliminate the size barrier as an impediment to smaller women receiving LVADs (31). Recent data have documented equivalent survival in patients with a small body size and supports LVAD use in this population (31). Despite these advances, women continue to receive fewer LVADs than men and remain under-represented in clinical trials (9, 32). Women are also more likely to receive LVAD therapy later in the course of their disease, thus reducing the potential benefit of this therapy (25, 26).
Non-rational LVAD use in conjunction with spending on this therapy may worsen already existing health care disparities. Thus, careful attention to the various determinants of LVAD use is needed to ensure equitable access for vulnerable populations.
Conclusions
LVAD use has significantly increased during the past decade and is now on the precipice of dramatic dissemination to a broader population of patients with advanced HF. We provide a conceptual framework to serve as the foundation for (1) evaluating this effective, yet expensive therapy, and (2) informing health policy makers. Transformative device technology, along with the NCD, are likely the most important factors contributing to greater LVAD use. Although yet understudied, market-, provider-and patient-level factors likely influence LVAD use, but may lead to disproportionate use of this therapy, especially among our most vulnerable populations. Beyond LVAD use, we posit that our conceptual framework may also help guide the evaluation of similar costly but life-prolonging technologies (e.g., transcatheter aortic valve replacement), with the goal to develop policies that ensure their rationale use and dissemination.
Acknowledgments:
This project is supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant T32-HL007853.
Footnotes
The opinions expressed in this manuscript do not represent those of Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), National Heart, Lung and Blood Institute (NHLBI), Centers for Medicare and Medicaid Services (CMS) or United States Food and Drug Administration (FDA). The statistical analyses to support this study was performed at the University of Michigan.
Financial Disclosures:
Sarah T Ward MD - None.
Qixing Lian, BS - None
Francis D. Pagani, MD PhD - None.
Min Zhang, PhD - None.
Robert Kormos, MD - Advisory Board for Medtronic.
Keith D. Aaronson, MD MS - None.
Andrew Althouse, PhD - None
Brahmajee K. Nallamothu, MD MPH - None.
Donald S. Likosky, PhD - None.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. : Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers JG, Butler J, Lansman SL, et al. : Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. Journal of the American College of Cardiology 2007;50:741–7. [DOI] [PubMed] [Google Scholar]
- 3.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D: Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107. [DOI] [PubMed] [Google Scholar]
- 4.Agnetti G, Piepoli MF, Siniscalchi G, Nicolini F: New Insights in the Diagnosis and Treatment of Heart Failure. BioMed research international 2015;2015:265260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Pagani FD, et al. : Seventh INTERMACS annual report: 15,000 patients and counting. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2015;34:1495–504. [DOI] [PubMed] [Google Scholar]
- 6.Starling RC, Naka Y, Boyle AJ, et al. : Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). Journal of the American College of Cardiology 2011;57:1890–8. [DOI] [PubMed] [Google Scholar]
- 7.Jorde UP, Kushwaha SS, Tatooles AJ, et al. : Results of the destination therapy post-food and drug administration approval study with a continuous flow left ventricular assist device: a prospective study using the INTERMACS registry (Interagency Registry for Mechanically Assisted Circulatory Support). Journal of the American College of Cardiology 2014;63:1751–7. [DOI] [PubMed] [Google Scholar]
- 8.Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) website. Accessed at:https://www.uab.edu/medicine/intermacs/ Accessed on: March 17.
- 9.Mehra MR, Naka Y, Uriel N, et al. : A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. The New England journal of medicine 2017;376:440–50. [DOI] [PubMed] [Google Scholar]
- 10.Pagani FD, Aaronson KD, Kormos R, et al. : The NHLBI REVIVE-IT study: Understanding its discontinuation in the context of current left ventricular assist device therapy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2016;35:1277–83. [DOI] [PubMed] [Google Scholar]
- 11.Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC: Disparities in access to left ventricular assist device therapy. The Journal of surgical research 2009;152:111–7. [DOI] [PubMed] [Google Scholar]
- 12.Kirklin JK, Naftel DC, Pagani FD, et al. : Sixth INTERMACS annual report: a 10,000-patient database. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2014;33:555–64. [DOI] [PubMed] [Google Scholar]
- 13.Heatley G, Sood P, Goldstein D, et al. : Clinical trial design and rationale of the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2016;35:528–36. [DOI] [PubMed] [Google Scholar]
- 14.CIGNA coverage policy for VAD: https://cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/medical/mm_0054_coveragepositioncriteria_vad.pdf Accessed on: 3-20-2017 [Google Scholar]
- 15.Aetna: Ventricular Assist Devices Policy. Web: http://www.aetna.com/cpb/medical/data/600_699/0654.html Accessed on: 3-20-3017.
- 16.Oliveira GH, Al-Kindi SG, Simon DI: Implementation of the Affordable Care Act and Solid-Organ Transplantation Listings in the United States. JAMA cardiology 2016;1:737–8. [DOI] [PubMed] [Google Scholar]
- 17.DuBay DA, MacLennan PA, Reed RD, et al. : Insurance Type and Solid Organ Transplantation Outcomes: A Historical Perspective on How Medicaid Expansion Might Impact Transplantation Outcomes. Journal of the American College of Surgeons 2016;223:611–20.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breathett K, Allen LA, Helmkamp L, et al. : The Affordable Care Act Medicaid Expansion Correlated With Increased Heart Transplant Listings in African-Americans But Not Hispanics or Caucasians. JACC Heart failure 2017;5:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Waivers/1115/downloads/in/Healthy-Indiana-Plan-2/in-healthy-indiana-plan-support-20-Health-Benefit-Booklet.pdf.
- 20.Schultz WYM, Ko Y-A, Vega J, Morris A. : Medicaid Insurance Is Associated with Shorter Time to Transplant Listing After LVAD Implantation. . Am J Transplant 2016;16 (suppl 3). [Google Scholar]
- 21.Katz MR, Dickinson MG, Raval NY, et al. : Outcomes of patients implanted with a left ventricular assist device at nontransplant mechanical circulatory support centers. The American journal of cardiology 2015;115:1254–9. [DOI] [PubMed] [Google Scholar]
- 22.Sherazi S, Zareba W, Daubert JP, et al. : Physicians’ knowledge and attitudes regarding implantable cardioverter-defibrillators. Cardiology journal 2010;17:267–73. [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Khatib SM, Sanders GD, Carlson M, et al. : Preventing tomorrow’s sudden cardiac death today: dissemination of effective therapies for sudden cardiac death prevention. American heart journal 2008;156:613–22. [DOI] [PubMed] [Google Scholar]
- 24.Pagani FD, Acker MA, Camacho MT, et al. : Clinical statement on the requirements for surgeon certification for implantation of durable Ventricular Assist Devices (VADs). Ann Thorac Surg 2013;95:1834–9. [DOI] [PubMed] [Google Scholar]
- 25.Hsich EM, Naftel DC, Myers SL, et al. : Should women receive left ventricular assist device support?: findings from INTERMACS. Circulation Heart failure 2012;5:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook JL, Grady KL, Colvin M, Joseph SM, Brisco MA, Walsh MN: Sex differences in the care of patients with advanced heart failure. Circulation Cardiovascular quality and outcomes 2015;8:S56–9. [DOI] [PubMed] [Google Scholar]
- 27.OPTN UNOS Public Comment Proposal Available at: https://optn.transplant.hrsa.gov/media/1927/mpsc_primary_surgeon_txcases_20160815.pdf Date accessed: March 17.
- 28.Pagani FD, Kormos RL, Calhoon JH, Higgins RS, Rich JB: Certification for implantation of durable, implantable ventricular assist devices in the United States: the need for clarification of the process. Ann Thorac Surg 2013;95:1520–2. [DOI] [PubMed] [Google Scholar]
- 29.Adams KF Jr., Fonarow GC, Emerman CL, et al. : Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). American heart journal 2005;149:209–16. [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Abraham WT, Albert NM, et al. : Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. American heart journal 2004;148:43–51. [DOI] [PubMed] [Google Scholar]
- 31.Zafar F, Villa CR, Morales DL, et al. : Does Small Size Matter With Continuous Flow Devices?: Analysis of the INTERMACS Database of Adults With BSA </=1.5 m2. JACC Heart failure 2017;5:123–31. [DOI] [PubMed] [Google Scholar]
- 32.Rogers JG, Pagani FD, Tatooles AJ, et al. : Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. The New England journal of medicine 2017;376:451–60. [DOI] [PubMed] [Google Scholar]


