ABSTRACT
Objectives: To investigate effects of nicotine on biofilm formation of Streptococcus mutans isolates from oral washes of smoker and non-smoker human subjects.
Materials and methods: This study was conducted using 60 S. mutans isolates with three S. mutans isolates collected from oral washes of ten smoking subjects and ten from non-smoking subjects. Biofilm was formed by culturing each S. mutans strain (10 μl) in 190 μl of TSB supplemented with 1% sucrose (TSBS) containing 0, 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, and 32.0 mg/ml of nicotine for 24 h in 5% CO2 at 37°C in 96 well microtiter plates. The absorbance values of biofilm were measured at 490 nm in a microplate spectrophotometer.
Results: There was a significant effect (p-value < 0.05) of nicotine concentrations and smoking on the growth of biofilm, planktonic cells, and total absorbance, for all strains of S. mutans. Isolates from smokers had significantly more biofilm at 0–16 mg/ml of nicotine compared to those from non-smokers (p-value < 0.0001).
Conclusion: S. mutans smoker isolates are more affected by high nicotine concentrations than non-smoker isolates.
Clinical Relevance: The use of nicotine products increases the growth of S. mutans and may place tobacco users at risk for dental decay.
KEYWORDS: Biofilm, tooth decay, Streptococcus mutans, nicotine
Tooth decay is a complex dieto-bacterial disease with an association of social, behavioral and biological factors [1]. This complex disease is considered an infectious disease, which develops over time involving a complex interaction of oral microflora, specifically Streptococcus mutans, dietary carbohydrates, and a susceptible tooth surface [2]. It has been well defined that S. mutans and tooth decay are closely related, especially that S. mutans can adapt very well in a high carbohydrate environment under acidic conditions. S. mutans has the ability to metabolize sugars forming organic acids that bathe tooth surfaces causing its progressive mineral loss. It thrives in specific oral conditions with unique characteristics [3]. Adherence of S. mutans to hard tooth structures is considered one of the major characteristics that enable it to proliferate and microcolonize establishing a mature cariogenic biofilm. Numerous cariogenic factors of S. mutans are involved in its ability to adhere and aggregate to form cariogenic biofilms including initial sucrose-independent adherence in which antigen I/II is involved, and sucrose-dependent adherence based on the function of glucosyltransferases (Gtfs) and other glucan-binding proteins (Gbp) [3]. Oral microbial biofilm (dental plaque) formation involves four stages including salivary acquired pellicle formation, microorganism adherence, growth and maturation of the bacterial microcolonies, and lastly detachment to form a new biofilm [4]. In the first stage, if the tooth surface is clean, salivary molecules can adsorb to hydroxyapatite on enamel tooth surfaces by electrostatic interactions forming the acquired enamel pellicle. Initial microorganism adherence is the second stage that occurs when early colonizing bacteria attach to salivary acquired pellicle through a weak reversible attachment in the absence of sucrose utilizing specific receptors and ligands [4]. S. mutans has an important role in initial sucrose independent adherence involving a bacterial surface protein adhesin called antigen I/II that interacts specifically with a high molecular weight salivary agglutinin glycoprotein (SAG) found in the acquired enamel pellicle [5,6]. The third stage involves formation of an extracellular polysaccharide matrix and establishment of cariogenic biofilm attached to tooth surfaces which is contributed by an important cariogenic factor of S. mutans known as sucrose-dependent adherence involving Gtfs and Gbps [7,8]. S. mutans-associated Gtfs primarily produce both water soluble and insoluble glucans by metabolizing sucrose to glucose and fructose and subsequently polymerizing glucose to an extracellular adhesive insoluble glucan that binds bacterial cells together through Gbp and Gtf receptors and adhere the cells to the enamel tooth surface [8]. It was observed that deletion of the Gtfs genes remarkably decreases the cariogenic potential of S. mutans strains [9]. The synthesis of extracellular glucan enhances the adherence of S. mutans through a cell to cell interaction where streptococcal Gtfs bind to glucan that successively adheres cells to smooth tooth surfaces [10]. Additionally, S. mutans synthesizes Gbps that have a significant role in establishing a mature biofilm by adhering bacteria to the extracellular glucan. An in vitro study by Lynch et al. indicated that engineered S. mutans with deleted Gbps genes affected the adherence and aggregation of these organisms resulting in a decrease in the biofilm mass and change in its architecture [11].
Tobacco use is a behavioral risk factor that adversely affects oral health and is directly linked to common life threatening diseases such as cancer, and cardiovascular and respiratory diseases [12–14]. The oral cavity is the first place in the human body to get exposed to either chewing tobacco or tobacco smoke and its chemical components. Therefore, tobacco not only affects systemic organs but it also has a significant influence on periodontal and other oral tissues [15] as well as oral microorganisms. Nicotine is one of the major active ingredients of cigarette smoke [16]. This active chemical has a toxic effect on alveolar bone and clinical attachment loss [17]. The exact effects of nicotine associated with tooth decay has not been fully investigated. However, a study conducted on 824 male Mexican truck drivers found a remarkable association between tobacco use and dental caries experience. Drivers who smoked more than 10 cigarettes/day had twice as many carious lesions than non-smokers [18]. In an Italian military population it was determined that heavy smokers had double the number of decayed teeth than a general population [19]. Another study investigated the in vitro effect of cigarette smoke on the growth of S. mutans and Streptococcus sanguinis. They concluded that nicotine has a dose-dependent effect on the growth of S. mutans; since as the nicotine concentration in the cigarettes increased there was an increase in S. mutans growth [20]. In an in vivo study, it was reported that nicotine treated rats had a significant increase in S. mutans growth and developed more caries lesions than in nicotine untreated rats [21]. Recently, we determined that nicotine stimulates S. mutans planktonic cell Gtf and Gbp expression as a mechanism to increase planktonic cell attachment to biofilm matrix leading to an increased number of cells in the biofilm [22]. This may explain the development of more carious lesions in smokers. In another study from this laboratory, seven S. mutans strains were treated with different nicotine concentrations (ranging from 0–16 mg/ml) [23]. Biofilm formation, and metabolic activity of the strains were determined. Biofilm formation and metabolism of all seven S. mutans strains increased in a dose-dependent manner up to 16.0 mg/ml of nicotine. Planktonic cell growth exhibited the highest values between 2, 4 and 8 mg/ml of nicotine. Because of these significant effects of nicotine on S. mutans, it is possible that there may be a difference in the manner that S. mutans responds to nicotine in smokers. To date there is no information on the effect of nicotine on the biofilm formation of S. mutans isolates from smokers. Therefore, we proposed the use of an in vitro model to better understand the effects of nicotine on biofilm formation of S. mutans isolates from smokers and non- smoking subjects.
Materials and methods
Bacterial strains and media
Ten oral washes collected from smoking subjects and ten oral washes from non-smoking subjects were used in this study. Three S. mutans isolates were cultured from each oral wash. Therefore, a total of 30 presumptive S. mutans smoker isolates and 30 S. mutans non-smoker isolates were collected. The oral washes were collected as part of a large multicenter NIH-funded microbiome grant (HL098960) and were obtained under appropriate IRB approval (IRB number 1,401,371,742). Age, race, gender, smoking history and number of pack years history were obtained from each subject. The oral wash samples were stored at −80°C until used. Selective agar plates (MSSB; Mitis Salivarius Sucrose Bacitracin; Anaerobic Systems, Inc., Morgan Hill, CA) were used for culturing the oral wash samples in 5% CO2 at 37°C as an initial isolation step, and three different colonies representing S. mutans from each oral wash sample were selected and grown on different MSSB plates. The isolates were subcultured in tryptic soy broth (TSB, Acumedia, Baltimore, MA) for 24 h in 5% CO2 at 37°C. The isolates were stored in TSB with 20% glycerol at −80°C until used. Mannitol and raffinose carbohydrate fermentation assays were used to confirm the identity of the subcultured S. mutans isolates [24]. A total of 34 S. mutans isolates were confirmed (11 from smokers and 23 from non-smokers) from a total of 60 non-confirmed S. mutans isolates (30 from smokers and 30 from non-smokers). Nicotine from Sigma-Aldrich (St. Louis, MO) was used.
Biofilm formation
Overnight cultures of each S. mutans strain (10 μl representing approximately 106 bacteria) grown in TSB were incubated with 0, 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, and 32.0 mg/ml of nicotine in TSB containing 1% sucrose (TSBS; 190 μl) for 24 h at 37°C in 5% CO2 in sterile 96 well microtiter plates (Fisher Scientific, Newark, DE). The total absorbance of each sterile 96 well microtiter plate was measured at 595 nm in a microplate spectrophotometer (SpectraMax 190; Molecular Devices, SunnyVale, CA) to assess the total bacterial growth (planktonic + biofilm cells). One hundred and twenty μl of planktonic cells of each sterile 96 well microtiter platewas transferred to other microtiterplates and the planktonic cell absorbance was determined at 595 nm. The biofilm remaining in each sterile 96 well microtiter platewas washed twice with deionized water, fixed with 200 μl of 10% formaldehyde (Sigma) for 30 min at room temperature, and washed three times with dionized water. Two hundred μl of 0.05% crystal violet was used to stain biofilm cells for 30 min. The wells were washed three times and 200 μl of isopropanol (Fisher, Pittsburg, PA) added for 60 min to extract the crystal violet from the biofilm cells. The absorbance values were measured at 490 nm.
Statistical methods
Each of the 34 confirmed S. mutans strains was tested three times in quadruplicate. Summary statistics (mean, standard deviation, standard error, range) of the absorbance values (total absorbance, planktonic and biofilm) were calculated for each of the strains. The effects of nicotine concentration, smoker vs. non-smoker S. mutans strain, and their interaction on biofilm formation were analyzed using ANOVA. The ANOVA included fixed effects for the two factors and their interaction and a random effect of absorbance values were examined. A transformation of the data (e.g. natural logarithm) was necessary to satisfy the ANOVA assumptions.
Results
Due to non-normality of the data, a rank transformation was used on the data prior to the analysis. A two-way ANOVA with a random effect for the multiple experiments was used for the analysis. There were significant effects of both nicotine concentrations and smoking on the growth of biofilm, planktonic cells, and total absorbance, for all strains of S. mutans (p < 0.0001; Figure 1–3). For biofilm, there was a significant interaction of nicotine concentrations and smoking for S. mutans smoker strains (Figure 3). For planktonic and total absorbance, there was a significant interaction of nicotine concentration and non-smoking S. mutans isolates (p < 0.0001; Figures 1 and 2, respectively). Biofilm formation of smoker isolates had dose-dependent effects up to 8.0 mg/ml. Isolates from smokers had significantly more biofilm at 0–16 mg/ml nicotine compared to those from non-smokers (p < 0.0001). Non-smoker isolates had significantly more total absorbance at all nicotine concentrations compared to smokers (p < 0.0001; Figure 1). There were significant differences of the planktonic cells between smoker and non–smoker isolates (Figure 2).
Figure 1.
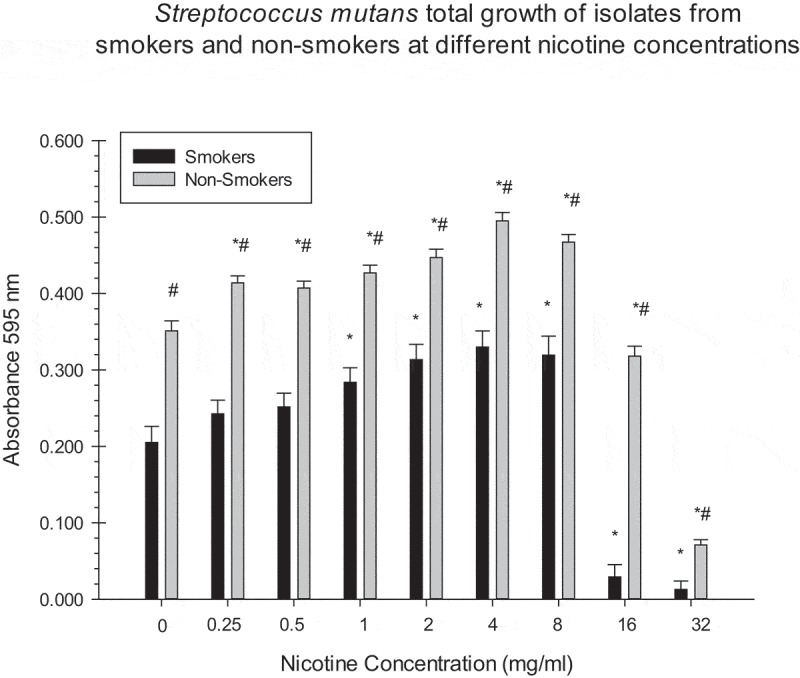
Asterisks indicate significant differences between total growth of S. mutans isolates (smokers/non-smokers) at different nicotine concentrations and the zero nicotine control. # indicate significant differences between total growth of S. mutans isolates of smokers/non-smokers at different nicotine concentrations.
Figure 3.
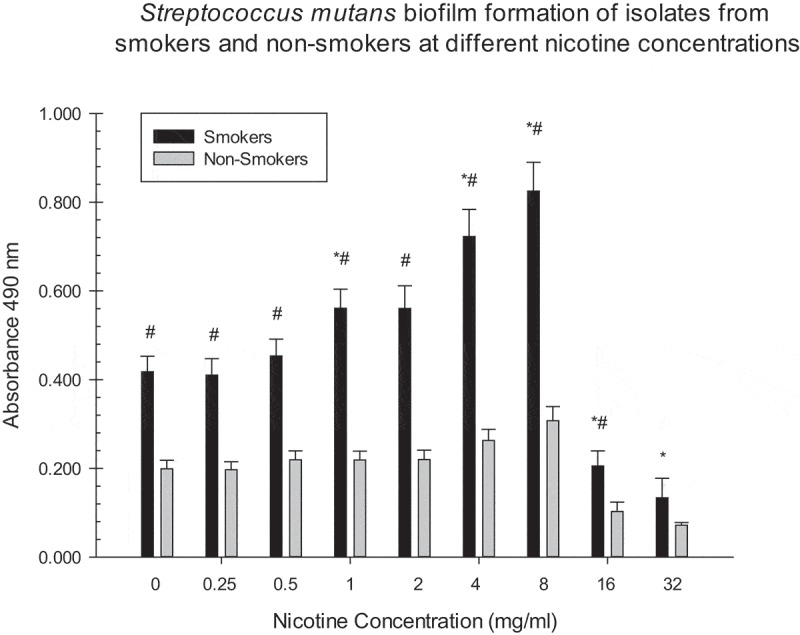
Asterisks indicate significant differences between S. mutans biofilm formation (smoker/non-smoker) at different nicotine concentrations and the zero nicotine control. # indicate significant differences between S. mutans biofilm formation of isolates from smokers and non-smokers at different nicotine concentrations.
Figure 2.
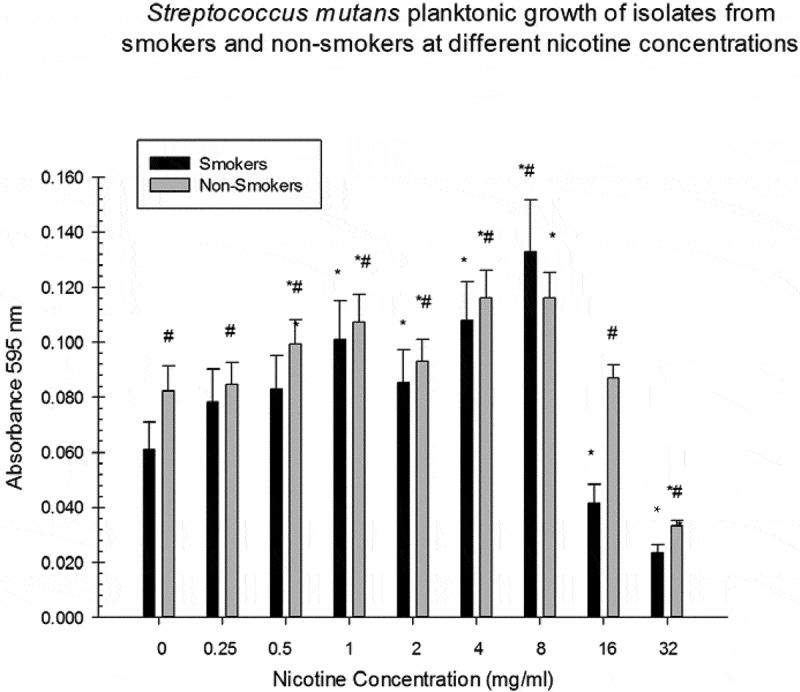
Asterisks indicate significant differences between S. mutans planktonic growth (smokers/non-smokers) at different nicotine concentrations and the zero nicotine control. # indicate significant differences between S. mutans planktonic growth of smokers/non-smokers at different nicotine concentrations.
There were significant differences between biofilm formation of smoker isolates at 1, 4, and 8 mg/ml of nicotine and the zero nicotine concentration (Figure 3). Significant differences between biofilm formation of non-smoker isolates and the zero nicotine concentration were observed at the 4, 8, 16, and 32 mg/ml concentrations. There were significant differences between biofilm formation of smoker and non-smoker isolates at 0.25, 0.5, 1, 2, 4, 8, 16 and 32 mg/ml nicotine concentrations (Figure 1). For total absorbance and planktonic measurements there were significant differences between smoker and non-smoker isolates at all nicotine concentrations (Figures 1 and 2). There was no significant relationship between the number of pack years smoked (Tables 1 and 2) and biofilm formation of S. mutans isolates at all nicotine concentrations. However, this correlation was statistically significant (negative) for the 0.25 mg/ml nicotine concentration for one of the three experiments. Several other correlations indicated some relationship although they did not reach statistical significance.
Table 1.
Average Demographic Factors of Smoking and Non-Smoking Human Subjects.
| Demographic Factors |
Smokers n = 10 |
Non-Smokers n = 10 |
| Gender | F = 1 M = 9 |
F = 4 M = 6 |
| Average Age | 40.5 years old | 42.0 years old |
| Race | White = 1 African American = 9 |
White = 5 African American = 5 |
| Average: Pack Years |
(8.5 cigarette per day/20) x 34.3 year smoking history = 14.5 pack years |
Table 2.
Individual Demographic Factors of Oral Washes from Smoking and Non-Smoking Human Subjects.
| IUPUI- ID # | Race | Sex | Age | Smoker | Smoking History | Pack Years |
|---|---|---|---|---|---|---|
| 891084OR01 | W | M | 52 | No | ||
| 891080OR02 | W | M | 32 | No | ||
| 891086OR01 | AA | F | 38 | No | ||
| 891090OR01 | AA | M | 37 | No | ||
| 891087OR01 | AA | M | 40 | No | ||
| 891085OR01 | W | M | 22 | Yes | ½ PPD~4 years | 3 years |
| 891091OR01 | W | M | 52 | No | ||
| 891088OR01 | AA | F | 42 | No | ||
| 891089OR01 | W | M | 35 | No | ||
| 891092OR01 | W | F | 56 | No | ||
| 005017OR01 | AA | M | 54 | Yes | 1 PPD~36 years | 36 years |
| 005022OR01 | AA | M | 43 | Yes | 3 cig PD~42 years | 6.3 years |
| 005016OR01 | AA | F | 46 | No | ||
| 005009OR01 | AA | M | 53 | Yes | 1 PPD~32 years | 32 years |
| 005010OR01 | AA | F | 48 | Yes | 8 cig PD~30 years | 12 years |
| 005011OR01 | AA | M | 51 | Yes | 1 PPD~37 years | 37 years |
| 005020OR01 | AA | M | 53 | Yes | 15 cig PD~34 years | 25.5 years |
| 005021OR01 | AA | M | 57 | Yes | ½ PPD~43 years | 22.5 years |
| 005024OR01 | AA | M | 58 | Yes | 1 PPD~41 years | 41 years |
| 005025OR01 | AA | M | 59 | Yes | ½ PPD~44 years | 22 years |
Discussion
To determine the effect of smoking history and the addition of nicotine on the formation of S. mutans biofilm, planktonic cells, and total growth in vitro, S. mutans isolates from smokers and non-smokers were compared in this study. To date, this is the first study that compares the effect of nicotine on both smoker and non-smoker isolates. In this study, nicotine enhanced biofilm growth in both S. mutans smoker and non-smoker isolates. Biofilm formation increased in a dose-dependent manner up to 8.0 mg/ml nicotine in both smoking and non-smoking oral strains. Furthermore, smoker isolates, when incubated with most of the nicotine concentrations, produced significantly more biofilm compared to the non-smoker isolates. However, the total growth of the non-smoking isolates was significantly more than smoker isolates at several nicotine concentrations. This is consistent with a previous in vitro study from this laboratory reporting that biofilm formation and metabolism of S. mutans increased in a dose-dependent manner up to 16.0 mg/ml of nicotine [23]. Planktonic cell growth was highest between 2, 4 and 8 mg/ml nicotine. The majority of isolates had MIC values of 16.0 mg/ml nicotine, MBC of 32.0 mg/ml nicotine, and MBIC of 16.0 mg/ml nicotine [23]. This previous study also indicated that nicotine had an antibacterial effect on both smoking and non-smoking isolates at high concentrations (16–32 mg/ml). Furthermore, a recent study indicated that adding 1.0 mg/ml nicotine to S. mutans biofilm cultures increases the production of lactate by two folds compared to S. mutans biofilm cultures with zero nicotine [25,26]. The results of the present study demonstrated that there was a significant difference in biofilm formation between smoker and non-smoker isolates at almost all nicotine concentrations. There was a more significant increase in biofilm formation of smoker isolates at 1, 4, 8, 16 and 32 mg/ml compared to biofilm at the zero nicotine concentration. In this study, it was clear that S. mutans isolates from smokers are more influenced by high nicotine concentrations (up to 16 mg/ml) than non-smokers. In addition, this study indicated that planktonic cell growth was greater in non-smoking isolates at all nicotine concentrations compared to the planktonic cell growth of smoker isolates at the same nicotine concentrations. The possible mechanism of nicotine on enhancement of biofilm growth of S. mutans strains tested in the present study can be explained by a recent study that demonstrated the effect of nicotine on the expression of Gbps and Gtfs genes [22]. Interestingly enough, it was found that nicotine up-regulates the expression of Gbps and Gtfs genes of S. mutans planktonic cells and down-regulates Gbps and Gtfs of S. mutans biofilm cells [22]. Thus, an increase of planktonic cell attachment to biofilm results in increased growth of biofilm. In this study, there was not a significant relationship between the number of pack years smoked and biofilm formation of S. mutans isolates at all nicotine concentrations. The present study hypothesized that nicotine produces significant differences in biofilm formation between smoker and non-smoker S. mutans isolates. According to the study results, this hypothesis was confirmed. The rationale for this hypothesis was derived from preliminary data indicating that S. mutans can become tolerant to increased nicotine concentrations and this tolerance appears to be stable (unpublished data). This may allow smoker isolates to be able to respond more vigorously to higher nicotine concentrations than non-smoker isolates. This preliminary study suggested that S. mutans becomes adapted with stable resistance at high nicotine concentrations by some type of mutation and possible stable upregulation of antigen I/II after it had been passed at least three times on 0 mg/ml nicotine. The use of nicotine products increases the growth of S. mutans and may place tobacco users at risk for dental decay [27].
Results of this study suggest that there is more increased dental caries in smokers than non-smokers because of the significant increase of biofilm formation in the S. mutans smoker isolates compared to non-smoker S. mutans isolates. Further investigations in the effects of nicotine on different stages of biofilm formation of smoker S. mutans isolates can lead to understanding the complete picture and future development of more effective strategies and methods that prevent the development of dental biofilm and tooth decay in smokers. Also, to further learn the types of mechanisms and regulations that these strains use to tolerate high nicotine concentrations. The investigation of the effects of nicotine on smoker and non-smoker S. mutans isolates provides information that high nicotine concentrations can enhance more biofilm formation in smoker isolates than non-smoker isolates and this suggests a strong relationship between smoking and risk of developing dental decay.
Funding Statement
The work was supported by Indiana University School of Dentistry in Indiana, U.S.A.
Disclosure statement
Author Nasreen Farouk El-ezmerli declares that she has no conflict of interest. Author Richard L Gregory has no conflict of interest. This manuscript was derived from Dr. El-ezmerli’s MSD thesis and has not been published elsewhere.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. However, the oral washes were collected as part of a large multicenter NIH-funded microbiome grant (HL098960) and were obtained under appropriate IRB approval (IRB number 1,401,371,742).
Informed consent
For this type of study, formal consent is not required.
References
- [1].Zero DT. Dental caries process. Dent Clin North Am. 1999;43:635–7. [PubMed] [Google Scholar]
- [2].Seow WK. Biological mechanisms of early childhood caries. Community Dent Oral Epidemiol. 1998;26:8–27. [DOI] [PubMed] [Google Scholar]
- [3].Lee SF, Progulske-Fox A, Bleiweis AS. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988;56:2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204–211. [DOI] [PubMed] [Google Scholar]
- [5].Pecharki D, Petersen FC, Assev S, et al. Involvement of antigen I/II surface proteins in Streptococcus mutans and Streptococcus intermedius biofilm formation. Oral Microbiol Immunol. 2005;20:366–371. [DOI] [PubMed] [Google Scholar]
- [6].Jakubovics NS, Stromberg N, van Dolleweerd CJ, et al. Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands. Mol Microbiol. 2005;55:1591–1605. [DOI] [PubMed] [Google Scholar]
- [7].Mattos-Graner RO, Smith DJ, King WF, et al. Water-insoluble glucan synthesis by mutans streptococcal strains correlates with caries incidence in 12- to 30-month-old children. J Dent Res. 2000;79:1371–1377. [DOI] [PubMed] [Google Scholar]
- [8].Matsumura M, Izumi T, Matsumoto M, et al. The role of glucanbinding proteins in the cariogenicity of Streptococcus mutans. Microbiol Immunol. 2003;47:213–215. [DOI] [PubMed] [Google Scholar]
- [9].Tsumori H, Kuramitsu H. The role of the Streptococcus mutans glucosyltransferases in the sucrose-dependent attachment to smooth surfaces: essential role of the GtfC enzyme. Oral Microbiol Immunol. 1997;12:274–280. [DOI] [PubMed] [Google Scholar]
- [10].Mooser G, Wong C. Isolation of a glucan-binding domain of glucosyltransferase (1,6-alphaglucan synthase) from Streptococcus sobrinus. Infect Immun. 1988;56:880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lynch DJ, Fountain TL, Mazurkiewicz JE, et al. Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol Lett. 2007;268:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Akaji EA, Folaranmi N. Tobacco use and oral health of inmates in a Nigerian prison. Niger J Clin Pract. 2013;16:473–477. [DOI] [PubMed] [Google Scholar]
- [13].Chockalingam K, Vedhachalam C, Rangasamy S, et al. Prevalence of tobacco use in urban, semi urban and rural areas in and around Chennai city, India. PLoS One. 2013;8:e76005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Johnson NB, Hayes LD, Brown K, et al. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors–USA, 2005-2013. MMWR Surveill Summ. 2014;63(Suppl 4):3–27. [PubMed] [Google Scholar]
- [15].Reibel J. Tobacco and oral diseases. Update on the evidence, with recommendations. Med Princ Pract. 2003;12(Suppl 1):22–32. [DOI] [PubMed] [Google Scholar]
- [16].Jacob P 3rd, Yu L, Shulgin AT, et al. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am J Public Health. 1999;89:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Al-Habashneh R, Al-Omari MA, Taani DQ. Smoking and caries experience in subjects with various form of periodontal diseases from a teaching hospital clinic. Int J Dent Hyg. 2009;7:55–61. [DOI] [PubMed] [Google Scholar]
- [18].Aguilar-Zinser V, Irigoyen ME, Rivera G, et al. Cigarette smoking and dental caries among professional truck drivers in Mexico. Caries Res. 2008;42:255262. [DOI] [PubMed] [Google Scholar]
- [19].Campus G, Cagetti MG, Senna A, et al. Does smoking increase risk for caries? a cross-sectional study in an Italian military academy. Caries Res. 2011;45:40–46. [DOI] [PubMed] [Google Scholar]
- [20].Zonuz AT, Rahmati A, Mortazavi H, et al. Effect of cigarette smoke exposure on the growth of Streptococcus mutans and Streptococcus sanguis: an in vitro study. Nicotine Tob Res. 2008;10:63–67. [DOI] [PubMed] [Google Scholar]
- [21].Liu S, Wu T, Zhou X, et al. Nicotine is a risk factor for dental caries: an in vivo study. J Dent Sci. 2018;13:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huang R, Li M, Gregory RL. Nicotine promotes Streptococcus mutans extracellular polysaccharide synthesis, cell aggregation and overall lactate dehydrogenase activity. Arch Oral Biol. 2015;60:1083–1090. [DOI] [PubMed] [Google Scholar]
- [23].Huang R, Li M, Gregory RL. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur J Oral Sci. 2012;120:319–325. [DOI] [PubMed] [Google Scholar]
- [24].Setterstrom JA, Gross A, Stanko RS. Comparison of Minitek and conventional methods for the biochemical characterization of oral streptococci. J Clin Microbiol. 1979;10:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li M, Huang R, Zouhl X, et al. Effect of nicotine on cariogenic virulence of Streptococcus mutans. Folia Microbiol. 2016;61:505–512. [DOI] [PubMed] [Google Scholar]
- [26].Wagenknecht DR, BalHaddad AA, Gregory RL. Effects of nicotine on oral microorganisms, human tissues and the interactions between them. Curr Oral Health Reports. 2018. doi: 10.1007/s40496018-0173-3 [DOI] [Google Scholar]
- [27].Tomar SL, Hecht SS, Jaspers I, et al. Oral health effects of combusted and smokeless tobacco products. Adv Dent Res. in press;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


