Abstract
Background: This retrospective study investigated whether baseline serum lipoprotein(a) (Lp(a)) may predict subsequent stroke in patients under chronic peritoneal dialysis (PD).
Methods: Eight hundred and sixty incident PD patients, treated from 1 November 2005 to 28 February 2017, were enrolled, and followed until discontinuation of PD, death, or 31 May 2017. Hemorrhagic or ischemic stroke was the primary outcome. The population was stratified by baseline serum Lp(a) tertile. The risk of each stroke subtype was analyzed using the Cox proportional hazard models. Adjustments were made for: age; gender; history of stroke and hypertension; systolic blood pressure; lipid-lowering, antiplatelet and antihypertensive medications; laboratory profiles including hemoglobin, serum albumin, calcium, triglyceride, total and low-density lipoprotein cholesterol; and apolipoprotein A1.
Results: Among the 860 participants, 19.3% and 4.1% had diabetes mellitus and a history of stroke, respectively. The median baseline serum Lp(a) was 328 (172–585) mg/L. After 28 (14–41) months of follow-up, 33 (3.84%) and 12 (1.40%) patients developed hemorrhagic and ischemic stroke, respectively. Participants in the highest Lp(a) tertile had a significantly lower risk of hemorrhagic stroke compared with those in the lowest tertile (hazard ratio (HR) 0.3, 95% confidence interval (CI) 0.1–0.86; p = .026); the rates of ischemic stroke were comparable among the tertiles. Each 10 mg/L rise in serum Lp(a) was associated with a 2% (95% CI 0.96–1; p = .033) lower risk of hemorrhagic stroke.
Conclusions: Among patients with incident PD, a higher serum Lp(a) level may predict a lower risk of hemorrhagic stroke.
Keywords: End-stage renal disease, hemorrhagic stroke, lipoprotein(a), peritoneal dialysis, stroke
Background
Lipoprotein(a), or Lp(a), is a lipoprotein moiety with a lipid composition resembling that of low-density lipoprotein. Lp(a) consists of apolipoprotein(a), or apo(a), covalently bound to apolipoprotein B100 [1]. Prior studies have suggested that elevated serum Lp(a) levels were predictive of incident cardiovascular events, with mechanisms that involved the deposition of Lp(a) cholesterol in the intima, and thrombolysis and the clearance of fibrin were both inhibited, based on the competition between apo(a) and plasminogen for plasminogen receptors on vascular cells [2].
Stroke is the most common vascular complication among chronic dialysis patients [3]. The incidence of stroke in dialysis patients is 13.7–49.2/1000 patient-years [4–6]. The risk of stroke in these patients is more than threefold that of the general population [5,7]. Recent studies indicated a close correlation between serum Lp(a) level and risk of developing ischemic stroke [8]. Zhang and Zhang [9] reported that among patients with ischemic stroke, high serum Lp(a) levels were associated with increased severity and poor prognosis.
Most studies have addressed an association between Lp(a) and the risk of ischemic stroke, but rarely hemorrhagic stroke, the latter with conflicting results. A large meta-analysis of 36 prospective studies concluded that higher serum Lp(a) levels were associated with an increased risk of ischemic stroke, but there was no significant association with risk of hemorrhagic or total stroke [10]. In a prospective study, Ishikawa et al. [2] observed an inverse association between serum Lp(a) level and the risk of hemorrhagic stroke and overall stroke. Furthermore, high serum Lp(a) was associated with a decreased risk of cerebral and airway bleeding among a general population [11]. Few studies have investigated an association between Lp(a) and the risk of atherosclerotic cardiovascular disease in chronic dialysis patients [12,13].
We are not aware of any study of an association between serum Lp(a) and the risk of hemorrhagic stroke in patients under chronic dialysis. Considering that serum Lp(a) levels are usually higher in patients under peritoneal dialysis (PD) compared with either the general population or those under hemodialysis [14], we hypothesized that serum Lp(a) may be an important predictor of the risk of hemorrhagic stroke in patients undergoing PD.
The present retrospective study analyzed an association between serum Lp(a) and the risk of developing stroke (hemorrhagic or ischemic) in a longitudinal cohort of patients treated with PD, with a median follow-up of 28 months.
Methods
The Human Ethics Committee of Nanchang University approved this study (application ID: [2019]088), which complied with the ethical principles of the Declaration of Helsinki [15].
Cohort and registration of clinical data
All patients with incident PD, defined as those for whom PD was their first modality of renal replacement therapy, were retrospectively enrolled in the present study. These patients received follow-up in the PD center at First Affiliated Hospital of Nanchang University, Jiangxi, China between 1 November 2005 and 28 February 2017, in accordance with the study protocol published previously [16]. For inclusion in this study, each patient was ≥18-years-old during PD initiation, and survived ≥90 days from the first day of PD treatment. Patients with any of the following were excluded: transferred from hemodialysis; had a failed renal allograft; received PD catheterization in other hospitals; or did not have available baseline serum Lp(a) data.
The patients were followed until discontinuation of PD, death, or 31 May 2017, whichever occurred first. Baseline demographic data included age, gender, diabetic status, prior history of stroke, and the cause of end-stage renal disease at baseline. Also recorded were body mass index, blood pressure, and medication regimens. Serum biochemical data included hemoglobin, albumin, blood urea nitrogen, creatinine, calcium, total cholesterol, high-density and low-density lipoprotein cholesterol (HDL-C and LDL-C, respectively), triglyceride, apolipoprotein A1 (Apo A1), apolipoprotein B (Apo B), and Lp(a). The patients’ residual renal function was assessed by the estimated glomerular filtration rate using the formula of the Chronic Kidney Disease Epidemiology Collaboration. The participants’ baseline data were collected during their first three months under PD.
The follow-up of study participants was done based on a comprehensive protocol, including a regular outpatient follow-up (every 2–3 months for patients who lived nearby and every 3–6 months for those who lived at distant areas), monthly phone call and home visits. The primary outcome of this study was the development of stroke events during the follow-up period. These were reported during the following: monthly phone calls; subsequent outpatient follow-up; electronic health records of hospitalization using the ICD-10 (tenth revision of the International Statistical Classification of Diseases and Related Health Problems) codes 163.9 and 161.9 for ischemic and hemorrhagic stroke, respectively; or the cause of death listed on death certificate. These events were adjudicated by a committee consisting of PD nurses and attending staff responsible for PD care.
Statistical analyses
Due to the limited number of stroke events, we divided the patients based on baseline serum Lp(a) tertiles: T1, ≤220 mg/L; T2, 220–468 mg/L; and T3, >468 mg/L. The patients’ characteristics in each tertile were expressed as percentage for categorical variables, means with standard deviations for continuous variables with normal distribution, or medians with interquartile ranges (IQRs) for continuous variables without normal distribution. The Kruskal–Wallis tests or analysis of variance was used for comparing continuous variables, and chi-squared tests for comparing categorical variables between participants within each Lp(a) tertile. The Kaplan–Meier technique was applied to estimate between-group survival, and compared the probability of survival between Lp(a) tertiles based on the log-rank test.
An association between serum Lp(a) level and the risk of stroke was examined using the Cox proportional hazards regression analyses, after the data of the following patients were censored: switch to hemodialysis; renal transplantation; transfer to another center; declined further treatments; or lost to follow-up. Univariate results were determined, and then multivariate adjustments were conducted to account for age, gender, prior history of stroke and hypertension, systolic blood pressure, lipid-lowering, antiplatelet and antihypertensive medication. Another regression analysis was conducted to evaluate whether baseline serum Lp(a) was independently associated with the risk of developing stroke. This analysis further incorporated hemoglobin, serum albumin, calcium, total cholesterol, LDL-C, triglyceride, and Apo A1. Covariates with p < .05 in the univariate Cox analyses were chosen, or those deemed of clinical importance for inclusion in the multivariate Cox regression analyses. The results are shown as hazard ratio (HR) and 95% confidence interval (CI).
All analyses were performed using SPSS version 22.0 (SPSS, Chicago, IL). A p Value <.05 was considered statistically significant.
Results
Overview of the clinical features of PD participants
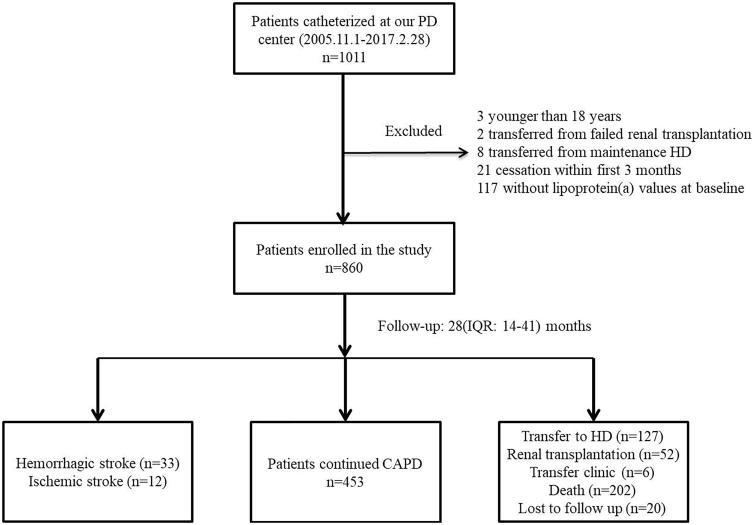
The records of 1011 patients treated with incident PD, monitored in our hospital, were reviewed. Among these, 151 were excluded for the following reasons: three were younger than 18 years; two had a failed renal allograft; eight were transferred from hemodialysis; 21 did not receive PD for more than 3 months; and 117 had no available baseline serum Lp(a) data. Thus, 860 patients were finally enrolled, and their data were recorded for subsequent analysis (Figure 1). In the PD center, all patients treated with PD received dialysates with 1.5% or 2.5% dextrose, and the connecting system was the twin-bag system type.
Figure 1.
A flowchart of study participant enrollment. CAPD: continuous ambulatory peritoneal dialysis; HD: hemodialysis.
The mean age of the study participants was 49.9 ± 14.5 years, and 57.6% were men (Table 1). Of the 860 participants, 4.1% and 19.3% had a prior history of stroke and diabetes, respectively. Only 9.9% received lipid-lowering medications to manage dyslipidemia. The most common causes of end-stage renal disease in these patients were chronic glomerulonephritis (64.3%), diabetic nephropathy (16.3%), and hypertension (12.8%).
Table 1.
Baseline characteristics of individuals stratified by tertiles of serum Lp(a) levels.
| Variables | Lp(a) tertiles (mg/L) |
Total (n = 860) | p Value | ||
|---|---|---|---|---|---|
| ≤220 (n = 288) | 220–468 (n = 286) | >468 (n = 286) | |||
| Age (years) | 50.6 ± 13.9 | 48.2 ± 15.0 | 50.8 ± 14.6 | 49.9 ± 14.5 | .064 |
| Men (%) | 166 (57.6) | 163 (57.0) | 166 (58.0) | 495 (57.6) | .968 |
| Body mass index (kg/m2) | 22.0 ± 3.7 | 22.1 ± 3.3 | 21.7 ± 3.3 | 22.0 ± 3.4 | .373 |
| Diabetes (%) | 48 (16.7) | 53 (18.5) | 65 (22.7) | 166 (19.3) | .170 |
| CVD (%) | 24 (8.3) | 34 (11.9) | 29 (10.1) | 87 (10.1) | .369 |
| Prior stroke (%) | 8 (2.8) | 14 (4.9) | 13 (4.5) | 35 (4.1) | .387 |
| Systolic pressure (mmHg) | 145 ± 25 | 149 ± 27 | 146 ± 27 | 147 ± 26 | .188 |
| Diastolic pressure (mmHg) | 87 ± 15 | 89 ± 16 | 87 ± 17 | 88 ± 16 | .170 |
| Total Kt/V | 2.2 (1.7, 2.8) | 2.2 (1.7, 2.7) | 2.2 (1.6, 2.7) | 2.2 (1.7, 2.7) | .445 |
| eGFR (ml/min per 1.73 m2) | 3.5 (1.8, 5.7) | 3.3 (1.9, 5.5) | 2.9 (1.6, 5.0) | 3.3 (1.8, 5.6) | .185 |
| Hemoglobin (g/L) | 79.5 ± 16.5 | 77.6 ± 16.7 | 80.0 ± 16.4 | 79.2 ± 16.7 | .171 |
| Albumin (g/L) | 36.3 ± 5.1 | 35.4 ± 4.7 | 34.2 ± 5.6 | 35.4 ± 5.2 | <.001 |
| Total cholesterol (mmol/L) | 3.83 ± 1.00 | 4.12 ± 1.09 | 4.64 ± 1.35 | 4.20 ± 1.20 | <.001 |
| Triglyceride (mmol/L) | 1.25 (0.83, 1.74) | 1.30 (0.94, 1.74) | 1.28 (0.89, 1.85) | 1.28 (0.89, 1.78) | .457 |
| Low density lipoprotein (mmol/L) | 2.05 (1.58, 2.64) | 2.28 (1.85, 2.96) | 2.57 (2.09, 3.30) | 2.30 (1.83, 2.94) | <.001 |
| High density lipoprotein (mmol/L) | 1.07 (0.84, 1.34) | 1.07 (0.88, 1.32) | 1.12 (0.92, 1.47) | 1.08 (0.89, 1.37) | .091 |
| Non-HDL (mmol/L) | 2.70 ± 0.89 | 2.98 ± 1.03 | 3.44 ± 1.24 | 3.04 ± 1.10 | <.001 |
| Apolipoprotein B (g/L) | 0.72 (0.58, 0.89) | 0.80 (0.64, 1.02) | 0.89 (0.70, 1.09) | 0.80 (0.63, 1.00) | <.001 |
| Apolipoprotein A1 (g/L) | 1.24 ± 0.27 | 1.25 ± 0.25 | 1.28 ± 0.32 | 1.26 ± 0.28 | .104 |
| C-reactive protein (mg/L) | 4.55 (2.29, 9.11) | 3.89 (2.01, 10.07) | 5.00 (2.52, 14.85) | 4.50 (2.30, 10.91) | .053 |
| Lipid-lowering agents use (%) | 26 (9.0) | 21 (7.3) | 38 (13.3) | 85 (9.9) | .049 |
| Antiplatelet medication (%) | 17 (5.9) | 17 (5.9) | 19 (6.6) | 53 (6.2) | .918 |
CVD: cardiovascular disease; Lp(a): Lipoprotein(a); Non-HDL: non-high density lipoprotein. P < 0.05 is considered statistically significant
Categorization of serum Lp(a) levels and associated factors
The overall median serum Lp(a) level was 328 (IQR, 172–585) mg/L. Participants were stratified into tertiles (T1, T2, and T3) based on serum Lp(a). The group with the highest Lp(a) (T3) had the greatest percentage of patients receiving lipid-lowering agents, high serum total cholesterol, LDL-C, non-HDL-C, and Apo B, but lowest serum albumin. Among the three tertile groups, there were no differences in age, gender ratio, or history of diabetes, cardiovascular disease, or stroke. The groups were also similar with regard to rates of patients receiving antiplatelet agents; blood pressure; hemoglobin; and serum C-reactive protein (Table 1).
Serum Lp(a) level significantly correlated with total cholesterol, LDL-C, HDL-C, non-HDL-C, Apo A1, and Apo B levels (r = 0.267, 0.282, 0.079, 0.267, 0.089, and 0.25, respectively; p < .001, <.001, = .020, <.001, = .009, and <.001; Table 2). Serum Lp(a) levels also positively correlated with C-reactive protein levels (r = 0.09; p = .018).
Table 2.
Correlation between serum Lp(a) levels, other parameters of lipid profile and inflammation.
| Lp(a) | CHOL | TG | LDL | HDL | Non-HDL | Apo A1 | Apo B | |
|---|---|---|---|---|---|---|---|---|
| CHOL | 0.267a | |||||||
| TG | 0.029 | 0.380a | ||||||
| LDL | 0.282a | 0.884a | 0.319a | |||||
| HDL | 0.079b | 0.426a | –0.302a | 0.281a | ||||
| Non-HDL | 0.267a | 0.920a | 0.538a | 0.871a | 0.009b | |||
| Apo A1 | 0.089b | 0.347a | –0.086a | 0.198a | 0.599a | 0.149a | ||
| Apo B | 0.250a | 0.722a | 0.460a | 0.739a | 0.048 | 0.782a | 0.033 | |
| CRP | 0.090b | –0.083b | 0.065 | –0.071 | –0.164a | –0.021 | –0.219a | 0.025 |
Apo A1: apolipoprotein A1; Apo B: apolipoprotein B; CHOL: cholesterol; CRP: C-reactive protein; HDL: high density lipoprotein; LDL: low density lipoprotein; Lp(a): lipoprotein(a); non-HDL: non-high density lipoprotein; TG: triglyceride.
Correlation is significant at the .01 level (two-tailed).
Correlation is significant at the .05 level (two-tailed).
Association between serum Lp(a) and the primary outcome
After a median follow-up of 28 months (IQR 14–41), 33 and 12 events of hemorrhagic and ischemic stroke occurred, respectively, while 202 patients died. Other causes of censoring included 52 receiving kidney transplantation, 127 transferred to hemodialysis, six transferred to other PD centers, and 20 lost to follow-up. Thus, 453 remained and were followed in the PD center.
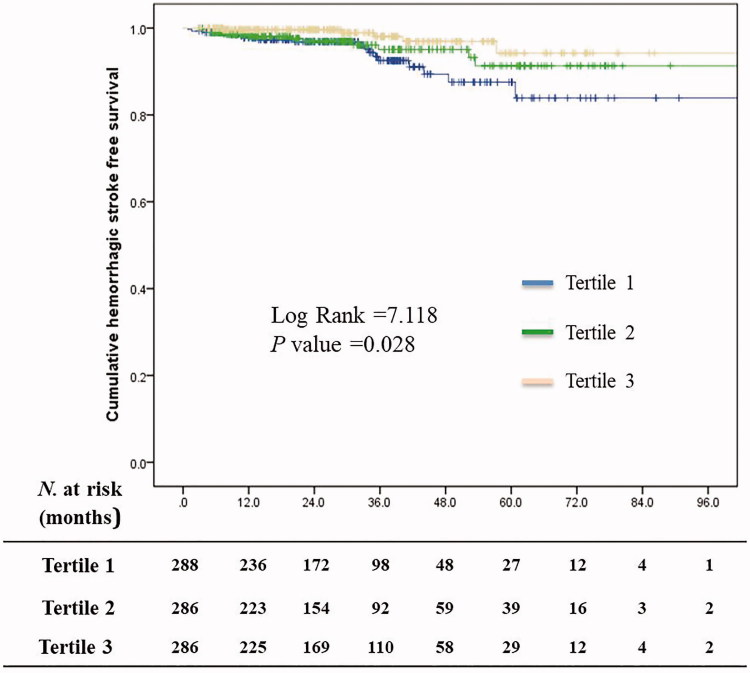
The percentages of patients with hemorrhagic stroke-free status at the first, third, and fifth year of follow-up were, respectively: in T1, 97.8%, 92.5%, and 87.5%; in T2, 98.1%, 95.1%, and 91.3%; and in T3, 99.6%, 98.1%, and 94.2% (Figure 2). The rate of hemorrhagic stroke-free status of the T3 group was the highest among all the tertiles (p = .028). For ischemic stroke-free survival, the 1, 3, and 5-year survival rate was 99.2%, 98.5%, and 98.5, respectively, in the T1 group; 99.6%, 98.5%, and 95.3%, respectively, in the T2 group; and 99.2%, 98.7%, and 98.7%, respectively, in the T3 group (p = .784).
Figure 2.
Hemorrhagic stroke-free survival curves for patients treated with peritoneal dialysis stratified by serum lipoprotein(a) tertile.
Associations between serum Lp(a) level and the risk of hemorrhagic or ischemic stroke were examined using the Cox proportional hazard regression (Table 3). After extensive adjustment for confounders, there was a significantly lower risk of hemorrhagic stroke in the T3 tertile (Lp(a) > 468 mg/L) compared with the T1 tertile (Lp(a) ≤ 220 mg/L) (HR, 0.30; 95% CI, 0.10–0.86; p = .026). The association between serum Lp(a) level and the risk of hemorrhagic stroke remained significant when serum Lp(a) was examined as a continuous variable. Furthermore, each 10-mg/L increase of serum Lp(a) level was associated with a 2% lower risk of hemorrhagic stroke (95% CI, 0.96–1.00; p = .033).
Table 3.
The associations between serum Lp(a) levels and the risk of stroke subtypes.
| Lp(a)a (mg/L) | Model 1b |
Model 2c |
Model 3d |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Hemorrhagic stroke | ||||||
| Continuous Lp(a)e | 0.98 (0.97–1.00) | .012 | 0.98 (0.96–1.00) | .009 | 0.98 (0.96–1.00) | .027 |
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | 0.64 (0.30–1.37) | .252 | 0.59 (0.27–1.28) | .181 | 0.64 (0.29–1.42) | .269 |
| Tertile 3 | 0.28 (0.10–0.76) | .012 | 0.25 (0.09–0.69) | .007 | 0.29 (0.10–0.83) | .022 |
| p for trend | .010 | .005 | .019 | |||
| Ischemic stroke | ||||||
| Continuous Lp(a)e | 1.00 (0.98–1.02) | .999 | 1.00 (0.98–1.01) | .723 | 1.00 (0.98–1.02) | .831 |
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | 1.22 (0.33–4.55) | .770 | 1.14 (0.30–4.31) | .843 | 1.41 (0.33–6.12) | .645 |
| Tertile 3 | 0.73 (0.16–3.28) | .686 | 0.57 (0.13–2.56) | .461 | 0.70(0.13–3.70) | .670 |
| p for trend | .872 | .461 | .683 | |||
Lp(a): lipoprotein(a).
Model 1: unadjusted.
Model 2: adjusted for age, gender, prior stroke, hypertension history, systolic blood pressure, lipid-lowering medication, antiplatelet medication, and antihypertensive medication.
Model 3: adjusted for age, gender, prior stroke, hypertension history, systolic blood pressure, lipid-lowering medication, antiplatelet medication, antihypertensive medication, hemoglobin, albumin, calcium, total cholesterol, triglyceride, low-density lipoprotein cholesterol, and apolipoprotein A1.
Per 10 mg/L higher Lp(a).
No significant association was found between serum Lp(a) level and the risk of ischemic stroke in any regression model.
Discussion
In this retrospective cohort study, the clinical characteristics associated with serum Lp(a) level among patients treated with PD were examined. The main finding was that higher serum Lp(a) level was inversely associated with the risk of hemorrhagic stroke during follow-up. To our best knowledge, this study is the first to examine this association in patients receiving chronic dialysis.
Among patients with incident end-stage renal disease, cardiovascular disease is the most common morbidity and the predominant cause of mortality. Cardiovascular disease accounts for 33%, 37%, and 41% of these patients’ hospitalizations, rehospitalizations, and deaths, respectively [3]. Among all cardiovascular diseases, stroke is one of the most common causes of mortality and the leading etiology of disability, and about 2.5 million new stroke cases occur each year in China [17–19].
Patients with end-stage renal disease often suffer from defective coagulation and fibrinolysis, and uremic patients may be in a procoagulant state but simultaneously have an increased risk of bleeding [20,21]. The cause of this paradoxical phenomenon is debatable, but serum Lp(a) level may be an important but under-recognized contributing factor.
As a complex polymorphic lipoprotein, Lp(a) contains apo(a) a as a constituent, whose structure resembles plasminogen, a key component in the fibrinolysis cascade [22]. The presence of apo(a) confers unique anabolic and catabolic functions to Lp(a). Lp(a) can impair fibrinolysis through its competition with plasminogen due to its structural similarity [22]. Plasminogen circulates in a closed, activation-resistant conformation. Upon binding to fibrin clots, plasminogen adopts an open form and is converted into active plasmin by a variety of enzymes [23,24]. The apo(a) glycoprotein in Lp(a) has repeated copies of kringle-IV that are similar to the kringle-IV found in plasminogen [25]. As a result, plasminogen activation, plasmin generation, and fibrinolysis are impaired [26]. Another plausible explanation for the relationship between Lp(a) and the risk of hemorrhagic stroke may be related to the lysine-binding characteristics of Lp(a), allowing Lp(a) to bind lysine-rich components of the coagulation system. A potential candidate of such lysine-rich component is the tissue factor pathway inhibitor (TFPI), which has numerous lysine moieties in its carboxy-terminal portion, and has been found at high levels in patients with end-stage renal disease [20]. TFPI is a major endogenous regulator of tissue factor-mediated coagulation, and this pathway is prominently upregulated remarkably in patients with renal failure [27]. Inhibiting TFPI can also mitigate bleeding [28]. Caplice et al. [29] found that Lp(a) binds and inactivates TFPI in vitro, further lending support to the present study, in which an inverse association between Lp(a) and the risk of hemorrhagic stroke was found.
Among 109,169 individuals, researchers found that an increased Lp(a) level was associated with a low risk of major cerebral and airway bleeding [11]. Another study of 10,494 individuals similarly showed that, compared with those with the lowest Lp(a), participants with the highest Lp(a) had a significantly lower risk of cerebral hemorrhage, both in men (HR 0.34, 95% CI 0.15–0.76) and women (HR 0.44, 95% CI 0.21–0.96) [2]. The findings of the present study extend the above results from general populations to patients treated with PD, supporting an inverse association between Lp(a) and the risk of hemorrhagic stroke.
In the literature, high serum Lp(a) is considered a risk factor for ischemic stroke among the general population. Results from the Atherosclerosis Risk in Communities (ARIC) study indicated that higher serum Lp(a) levels were associated with an increased risk of ischemic stroke, primarily among those without atrial fibrillation [1]. Another clinical trial similarly revealed that increased serum Lp(a) levels were associated with a higher risk of ischemic stroke, with serum Lp(a)≥15 mg/dL predicting an 83% higher risk [30]. A recent meta-analysis of 90,904 subjects yielded the same conclusion [8], and Lp(a) levels could predict the severity of stroke and functional outcomes [31]. However, in the present study, no increase in the risk of ischemic stroke was shown among patients with elevated Lp(a) levels treated with PD, a finding that was more compatible with results from the EPIC-Norfolk [32] and PRIME study [33]. These studies did not identify a significant association between serum Lp(a) levels and the risk of ischemic stroke.
We think there may be three factors that are responsible for the absence of a significant association between serum Lp(a) levels and the risk of ischemic stroke, observed in the present study. First, the number of ischemic stroke events was low during the follow-up period, and therefore the statistical power was insufficient to determine the significance of this association. Second, the serum Lp(a) levels positively correlated with total cholesterol, LDL-C, and non-HDL-C. It is possible that an association between serum Lp(a) and the risk of ischemic stroke was neutralized by the effect of other lipid parameters. Third, the association between Lp(a) and ischemic stroke may differ between patients of different ethnicities. As reported in the REGARDS Study, the authors confirmed a positive association between Lp(a) and ischemic stroke in African Americans but not in Caucasians [34].
The strengths of the present study include the large sample size and the novelty of the hypothesis. It is the first to report that Lp(a) was protective against the risk of hemorrhagic stroke among patients treated with PD. However, this single-center study may be limited by its retrospective nature, which possibly lowered the reportage of hemorrhagic and ischemic stroke, which were the main outcomes. So too, only baseline serum Lp(a) levels were analyzed, which did not account for changes in Lp(a) during the follow-up, and the follow-up period might be too short to permit a concise evaluation of the incidence of stroke. Third, due to the single center nature of this study, the generalizability of our findings can be limited and needs to be improved by a multicenter study. Finally, the low outcome rates made it difficult to analyze all the known risk factors of stroke, and residual confounding factors could still be possible.
Conclusions
The present study showed that serum Lp(a) levels were independently and inversely associated with the risk of hemorrhagic stroke among patients treated with PD. These findings indicate that serum Lp(a) may serve as a clinical marker capable of estimating the risk of hemorrhagic stroke in patients treated with PD.
Acknowledgements
The authors thank Medjaden Bioscience Limited for scientific editing of this manuscript.
Disclosure statement
The authors declare that they have no competing interests.
References
- 1.Aronis KN, Zhao D, Hoogeveen RC, et al. Associations of lipoprotein(a) levels with incident atrial fibrillation and ischemic stroke: the ARIC (Atherosclerosis Risk in Communities) Study. J Am Heart Assoc. 2017;6:pii: e007372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishikawa S, Kotani K, Kario K, et al. Inverse association between serum lipoprotein(a) and cerebral hemorrhage in the Japanese population. Thromb Res. 2013;131:e54–e58. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Kim KH, Ahn SV, et al. Risk of major cardiovascular events among incident dialysis patients: a Korean national population-based study. Int J Cardiol. 2015;198:95–101. [DOI] [PubMed] [Google Scholar]
- 4.Fu J, Huang J, Lei M, et al. Prevalence and impact on stroke in patients receiving maintenance hemodialysis versus peritoneal dialysis: a prospective observational study. PLoS One. 2015;10:e0140887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Findlay M, MacIsaac R, MacLeod MJ, et al. Renal replacement modality and stroke risk in end-stage renal disease-a national registry study. Nephrol Dial Transplant. 2018;33:1564–1571. [DOI] [PubMed] [Google Scholar]
- 6.Weng SF, Shen YC, Wang JJ, et al. Reduced risk of new onset stroke after kidney transplantation in Asian dialysis patients: a propensity score-matched, competing risk study in Taiwan. QJM. 2019;112:489–495. [DOI] [PubMed] [Google Scholar]
- 7.Bansal VK, Herzog CA, Sarnak MJ, et al. Oral anticoagulants to prevent stroke in nonvalvular atrial fibrillation in patients with CKD stage 5D: an NKF-KDOQI Controversies Report. Am J Kidney Dis. 2017;70:859–868. [DOI] [PubMed] [Google Scholar]
- 8.Nave AH, Lange KS, Leonards CO, et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis. 2015;242:496–503. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Zhang XA. Prognostic value of serum lipoprotein(a) levels in patients with acute ischemic stroke. Neuroreport. 2014;25:262–266. [DOI] [PubMed] [Google Scholar]
- 10.Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and low risk of major bleeding in brain and airways in the general population: a Mendelian Randomization Study. Clin Chem. 2017;63:1714–1723. [DOI] [PubMed] [Google Scholar]
- 12.Longenecker JC, Klag MJ, Marcovina SM, et al. High lipoprotein(a) levels and small apolipoprotein(a) size prospectively predict cardiovascular events in dialysis patients. J Am Soc Nephrol. 2005;16:1794–1802. [DOI] [PubMed] [Google Scholar]
- 13.Longenecker JC, Coresh J, Marcovina SM, et al. Lipoprotein(a) and prevalent cardiovascular disease in a dialysis population: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study. Am J Kidney Dis. 2003;42:108–116. [DOI] [PubMed] [Google Scholar]
- 14.Mikolasevic I, Zutelija M, Mavrinac V, et al. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renovasc Dis. 2017;10:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dentists. 2014;81:14–18. [PubMed] [Google Scholar]
- 16.Zhan X, Chen Y, Yan C, et al. Apolipoprotein B/apolipoprotein A1 ratio and mortality among incident peritoneal dialysis patients. Lipids Health Dis. 2018;17:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HH, Hung SY, Sung JM, et al. Risk of stroke in long-term dialysis patients compared with the general population. Am J Kidney Dis. 2014;63:604–611. [DOI] [PubMed] [Google Scholar]
- 18.Drew DA, Sarnak MJ. Ischemic and hemorrhagic stroke: high incidence in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2014;63:547–548. [DOI] [PubMed] [Google Scholar]
- 19.Tu WJ, Dong X, Zhao SJ, et al. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J Neuroendocrinol. 2013;25:771–778. [DOI] [PubMed] [Google Scholar]
- 20.Contaifer D Jr., Carl DE, Warncke UO, et al. Unsupervised analysis of combined lipid and coagulation data reveals coagulopathy subtypes among dialysis patients. J Lipid Res. 2017;58:586–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brophy DF, Carl DE, Mohammed BM, et al. Differences in coagulation between hemodialysis and peritoneal dialysis. Perit Dial Int. 2014;34:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saeed A, Virani SS. Lipoprotein(a) and cardiovascular disease: current state and future directions for an enigmatic lipoprotein. Front Biosci (Landmark Ed). 2018;23:1099–1112. [DOI] [PubMed] [Google Scholar]
- 23.Law RH, Caradoc-Davies T, Cowieson N, et al. The X-ray crystal structure of full-length human plasminogen. Cell Rep. 2012;1:185–190. [DOI] [PubMed] [Google Scholar]
- 24.Miles LA, Hawley SB, Baik N, et al. Plasminogen receptors: the sine qua non of cell surface plasminogen activation. Front Biosci. 2005;10:1754–1762. [DOI] [PubMed] [Google Scholar]
- 25.Romagnuolo R, DeMarco K, Scipione CA, et al. Apolipoprotein(a) inhibits the conversion of Glu-plasminogen to Lys-plasminogen on the surface of vascular endothelial and smooth muscle cells. Thromb Res. 2018;169:1–7. [DOI] [PubMed] [Google Scholar]
- 26.Anglés-Cano E, de la Peña Díaz A, Loyau S. Inhibition of fibrinolysis by lipoprotein(a). Ann N Y Acad Sci. 2001;936:261–275. [DOI] [PubMed] [Google Scholar]
- 27.Mercier E, Branger B, Vecina F, et al. Tissue factor coagulation pathway and blood cells activation state in renal insufficiency. Hematol J. 2001;2:18–25. [DOI] [PubMed] [Google Scholar]
- 28.Maroney SA, Cooley BC, Ferrel JP, et al. Absence of hematopoietic tissue factor pathway inhibitor mitigates bleeding in mice with hemophilia. Proc Natl Acad Sci USA. 2012;109:3927–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caplice NM, Panetta C, Peterson TE, et al. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood. 2001;98:2980–2987. [DOI] [PubMed] [Google Scholar]
- 30.Uchiyama S, Nakaya N, Mizuno K, et al. Risk factors for stroke and lipid-lowering effect of pravastatin on the risk of stroke in Japanese patients with hypercholesterolemia: analysis of data from the MEGA Study, a large randomized controlled trial. J Neurol Sci. 2009;284:72–76. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Zhao J, Gui Y, et al. Elevated lipoprotein (a) and risk of poor functional outcome in Chinese patients with ischemic stroke and type 2 diabetes. Neurotox Res. 2018;33:868–875. [DOI] [PubMed] [Google Scholar]
- 32.Gurdasani D, Sjouke B, Tsimikas S, et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2012;32:3058–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canoui-Poitrine F, Luc G, Bard JM, et al. Relative contribution of lipids and apolipoproteins to incident coronary heart disease and ischemic stroke: the PRIME Study. Cerebrovasc Dis. 2010;30:252–259. [DOI] [PubMed] [Google Scholar]
- 34.Arora P, Kalra R, Callas PW, et al. Lipoprotein(a) and risk of ischemic stroke in the REGARDS study. Arterioscler Thromb Vasc Biol. 2019;39:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]