Summary
Background
Fungal infections remain a major contributor to the opportunistic infections that affect people living with HIV. Among them, histoplasmosis is considered neglected, often being misdiagnosed as tuberculosis, and is responsible for numerous deaths in Latin America. The objective of this study was to estimate the burden of HIV-associated histoplasmosis compared with tuberculosis in Latin American countries.
Methods
For this modelling study, we estimated prevalence of previous exposure to Histoplasma capsulatum, HIV-associated histoplasmosis annual incidence, and number of deaths in 2012 in Latin American countries based on historical histoplasmin skin test studies in the general population, with an antigen dilution level of more than 1/10. Studies were identified in a literature search. Data on HIV-associated tuberculosis were extracted from the WHO notifications and outcomes tables and data on people living with HIV were extracted from the UNAIDS report for the year 2012. We systematically propagated uncertainty throughout all the steps of the estimation process.
Findings
Among 1310 articles identified as of June 1, 2015, 24 articles were included in the study, representing 129 histoplasmin skin test studies led in the general population of Latin American countries. For the year 2012, we estimated a range of 6710 (95% CI 5680–7867) to 15 657 (13 254–18 357) cases of symptomatic HIV-associated histoplasmosis in Latin America. Hotspot areas for histoplasmosis prevalence (>30%) and incidence (>1·5 cases per 100 people living with HIV) were Central America, the northernmost part of South America, and Argentina. According to realistic scenarios, we estimated a range of 671 (95% CI 568–787) to 9394 (7952–11 014) deaths related to histoplasmosis, compared with 5062 (3777–6405) deaths related to tuberculosis reported in Latin America.
Interpretation
Our estimates of histoplasmosis incidence and deaths are high and consistent with published data. For the first time, the burden of histoplasmosis is estimated to be equivalent in incidence and even higher in deaths when compared with tuberculosis among people living with HIV in Latin America.
Introduction
Fungal infections remain a major cause of opportunistic infections that affect people living with HIV, notably in low-income and middle-income countries.1 Histoplasma capsulatum is a fungus reported in five continents, including Latin America.2 It causes histoplasmosis, which can be asymptomatic or self-limited but also invasive and life-threatening in immunocompromised hosts.3
Prevalence of H capsulatum infection in the general population, based on histoplasmin skin test studies detecting previous exposure, varies widely worldwide by location, ranging from 0·8% to 89%.2,4,5 In people living with HIV, almost all untreated histoplasmosis cases will lead to death.3
With the spread of HIV, disseminated histoplasmosis became an increasing threat, with case-fatality rates ranging from 10% to 53% among culture-positive cases.6 Despite a sharp decline observed in a small number of reference centres, case-fatality rates remain high in almost all centres where the disease is recognised.6,7 Moreover, case-fatality rates are likely to be higher in the histoplasmosis-endemic areas of countries with high HIV prevalence and with limited clinician awareness, diagnostic capacity, and mycological expertise.3 Hence, histoplasmosis-related deaths might be greatly underestimated.8 Similarly, histoplasmosis is often mistaken for tuberculosis, and when patients fail to respond to tuberculosis treatment it might lead to a misdiagnosis of drug-resistant tuberculosis.9 Consequently, numerous cases and deaths from HIV-associated tuberculosis reported in national incidence and mortality statistics are in fact caused by histoplasmosis.8
According to regional data compiled by WHO,10 tuberculosis is endemic in Latin America, with a yearly incidence of 28 cases per 100 000 people living with HIV in Central America and 41–63 cases per 100 000 people living with HIV in South America in 2013. Additionally, an estimated 6100 (range 4600–8000) tuberculosis deaths among people living with HIV occurred across the Americas in 2013 (including the Caribbean, and North, Central, and South America). Supplementary material and references are available in the appendix.
In Latin America, the estimated number of people living with HIV is approximately 1·5 million.11 Histoplasmosis has been identified as a neglected disease, with a yearly number of deaths suspected as equivalent or greater than that from tuberculosis in people living with HIV.8 By contrast with tuberculosis, data on the burden of histoplasmosis are extremely scarce for Latin American countries and consist of academic publications reporting case series and incidence estimates at the country level. Intuitively, regions with high histoplasmin positivity rates and high HIV prevalence should have more deaths due to histoplasmosis than currently reported.8 A rigorous method is needed to estimate the burden of histoplasmosis in people living with HIV to improve knowledge and effectively plan future public health programme interventions.
We aimed to estimate the incidence of HIV-associated histoplasmosis and deaths at the regional and country levels for each Latin American country. As a secondary objective, we compared estimates of histoplasmosis incidence with tuberculosis cases reported among people living with HIV.
Methods
Literature review
From June 1, 2015, we searched for histoplasmosis prevalence estimates based on histoplasmin skin test studies done in Latin America using MEDLINE, Scielo, Lilacs, and Google Scholar. Latin America was defined as all South and Central American countries with the exception of the Caribbean region, according to the UN geoscheme definitions. The search terms were “histoplasmin” or “histoplasmosis prevalence” associated with “South America”, “Central America”, “Latin America”, or any Latin American country name. All types of published articles in any language were considered without time limitations since we assumed that, in endemic areas, the prevalence of this environmental fungal pathogen remained stable over time. We abstracted the following data from each article and included a study in our analysis when all data were present: name of the country and region (part of Central or South America), absolute number of people tested and number of people with a positive histoplasmin skin test, dilution level of histoplasmin antigen used for skin testing of more than 1/10 to avoid false positives due to cross-reactivity (range of dilution kept between 1/100 and 1/1000), and population tested. We included only studies that involved the general population and not a specific subgroup. However, when the only study from a given country involved a specific population subgroup, we included it to compute the country’s estimates (detailed search strategy, references, and results are available in appendix pp 2–3, 8, 11–30).
Our main aim was to estimate the annual incidence of HIV-associated histoplasmosis and number of deaths in 2012 in Latin American countries. We also compared histoplasmosis incidence estimates with tuberculosis cases and deaths reported among people living with HIV.
Statistical analysis
Histoplasmosis data and calculation of estimates in the general population
We extracted histoplasmin-positive rates from skin testing studies done in the general population. We calculated the mean prevalence for each country using the total number of positive patients identified divided by the total number of people tested. We estimated the mean prevalence at the continental level of Latin America in the same way for the whole Latin American region. We calculated a 95% CI using the relation:
where is the estimated prevalence, is the corresponding estimated variance, and n is the total number of people tested in all histoplasmin skin test studies done in one country of interest and at the regional level.
To further account for uncertainty around estimates, we assumed the normal approximation of a binomial distribution and then drew a random sample from the normal distribution built from 100 000 iterations of the prevalence values using Monte Carlo simulations. We then estimated for each country and region an expected prevalence with the associated 95% CI based on the median 2·5th and 97·5th percentiles of the probability distribution generated. Furthermore, these values allowed us to proceed with further computations to estimate histoplasmosis incidence and deaths.
Histoplasmosis data and calculation of estimates in people living with HIV
Since incidence was estimated from the prevalence, we assumed that the proportion of people living with HIV previously exposed to H capuslatum was similar to the general population and that symptomatic histoplasmosis cases mostly occurred in people living with HIV with a CD4 count of less than 200 cells per μL (appendix pp 3–5, 8–9).
First, we estimated the annual incidence of histoplasmosis per 100 people living with HIV for each Latin American country separately and for the Latin American region using the relation:
and previously estimated histoplasmosis prevalence for each country and the Latin American region. Pp denotes the prevalence, Id incidence density, and Ei(D) expected value of duration of histoplasmosis obtained from an incidence (i) case series. Disease duration was set to 0·321 years. Hence, for each country and the Latin American region we calculated an estimated incidence with lower and upper bounds of 95% CI, corresponding to the median 2·5th and 97·5th percentile of the computed distribution of incidence values.
Then, to obtain the estimated number (and corresponding 95% CI) of all incident HIV-associated histoplasmosis cases (symptomatic and asymptomatic) for each country and the Latin American region, we multiplied the country-specific and region-specific HIV-associated histoplasmosis incidence estimates with the number of people living with HIV (all ages) estimated by the UNAIDS for the year 2012.11
Finally, to estimate the annual number of symptomatic incident HIV-associated histoplasmosis cases in each country and the entire region, we applied three scenarios to each value of the estimated annual number of all incident HIV-associated histoplasmosis cases (symptomatic and asymptomatic): the N(30) scenario corresponds to 30% of the estimated annual number of incident HIV-associated histoplasmosis cases with a CD4 count of less than 200 cells per μL, N(50) corresponds to 50% of cases, and N(70) corresponds to 70% cases. For each scenario, in each country and the entire region, we estimated the number of symptomatic incident HIV-associated histoplasmosis cases with lower and upper bounds of a 95% CI, corresponding to the median 2·5th and 97·5th percentiles of the computed incidence scenario.
Following incidence calculations we estimated the number of histoplasmosis-related deaths in 2012 and computed each value of the three scenario estimates of the number of symptomatic incident HIV-associated histoplasmosis cases using four case-fatality rate scenarios, for each country separately and the whole Latin American region: the F(10) scenario corresponds to 10% of histoplasmosis-related deaths in each incidence scenario, F(20) to 20% of deaths, F(40) to 40% of deaths, and F(60) to 60% of deaths. Four scenarios were chosen because case-fatality rates might vary according to country-specific conditions, such as access to antiretroviral therapy and effective antifungal therapy, availability of fungal diagnostic methods, and the level of clinician awareness.6,12,13 For each case-fatality scenario (F[10]N[30], F[10]N[50], F[10]N[70], F[20]N[30], F[20]N[50], F[20]N[70], F[40]N[30], F[40]N[50], F[40]N[70], F[60]N[30], F[60]N[50], and F[60]N[70]) in each country and Latin America, we estimated the number of deaths with lower and upper bounds of 95% CI, corresponding to the median 2·5th and 97·5th percentiles of the computed values of the number of deaths.
Tuberculosis data and calculation of estimates in people living with HIV
We extracted the number of incident tuberculosis cases and deaths in people living with HIV for each country and the Latin American region from the WHO notifications and outcomes tables for the year 2012.14 No data were available for French Guiana and calculation of estimates is detailed in the appendix (p 5).
HIV data and calculation of estimates
We extracted the 2012 estimates of the number of people living with HIV (all ages) by country and for Latin America from the 2013 UNAIDS Global Report.11 For each country and for the Latin American region, an estimated number of people living with HIV (all ages) was available together with a low and a high estimate.
Uncertainty analysis
To enhance the robustness of estimates, we systematically accounted for uncertainty at each step of the estimation process. We first describe the estimation process for histoplasmosis prevalence, incidence, and death, then for tuberculosis incidence; we then describe how we accounted for uncertainty around HIV data from UNAIDS. Finally, we describe the modelling strategy in more detail (conceptual overview on appendix p 10). We used the mean to describe input data estimates and the median to describe the model output estimates.
For histoplasmosis estimates we used the prevalence mean, with lower and upper bounds of the 95% CI, to generate 100 000 values of the prevalence in each country and the whole Latin American region. To propagate uncertainty across the estimation process, we set these values as starting data for the subsequent estimations of incidences (rate and numbers) and number of deaths.
To account for uncertainty of HIV data from UNAIDS, we generated 100 000 values of the number of people living with HIV in each country and the whole Latin American region. We combined these values with those generated throughout the estimation process of histoplasmosis incidence.
We did not compute HIV-associated tuberculosis incident case and death numbers. We only present these values as reported by WHO for each country and the Latin American region.
We used the beta–PERT (program evaluation and review technique) distribution as our basic descriptive distribution since it allowed us to specify a minimum, maximum, and modal value, and a fourth parameter that controls the spread (variance) of the distribution.15 This family of distributions is widely used and is an attractive choice for problems in which many estimates and sources of uncertainty need to be combined due to the intuitive nature of its parameters.15 We specified four parameters: the most likely value (value of the quantity of interest computed), the lowest and highest estimates (using the lower and upper values of the quantity of interest computed or the lower and upper bounds of the calculated 95% CI when available), and the shape or scale value (default values set at 4). We summarised the outputs of our models using posterior distributions calculated by Monte Carlo simulation, with 100 000 replicates. We expressed the final results with the median and 95% CI, corresponding to the 2·5th and 97·5th percentiles of the posterior distribution. We did the Monte Carlo simulation and uncertainty analysis using SAS software version 9.4 (Cary, NC, USA). The statistical source code and a conceptual overview of the data analysis, together with all input data and output estimates, are available in the appendix (pp 10–41).
Cartography
We developed maps to illustrate estimates of previous exposure to H capsulatum prevalence and HIV-associated histoplasmosis incidence. We used the mapping software MapInfo Pro version 11.0. Base maps were downloaded from ArcGIS Online.
Ethical approval was not required in this study since it used aggregated level published data and estimations based on open access secondary sources.
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
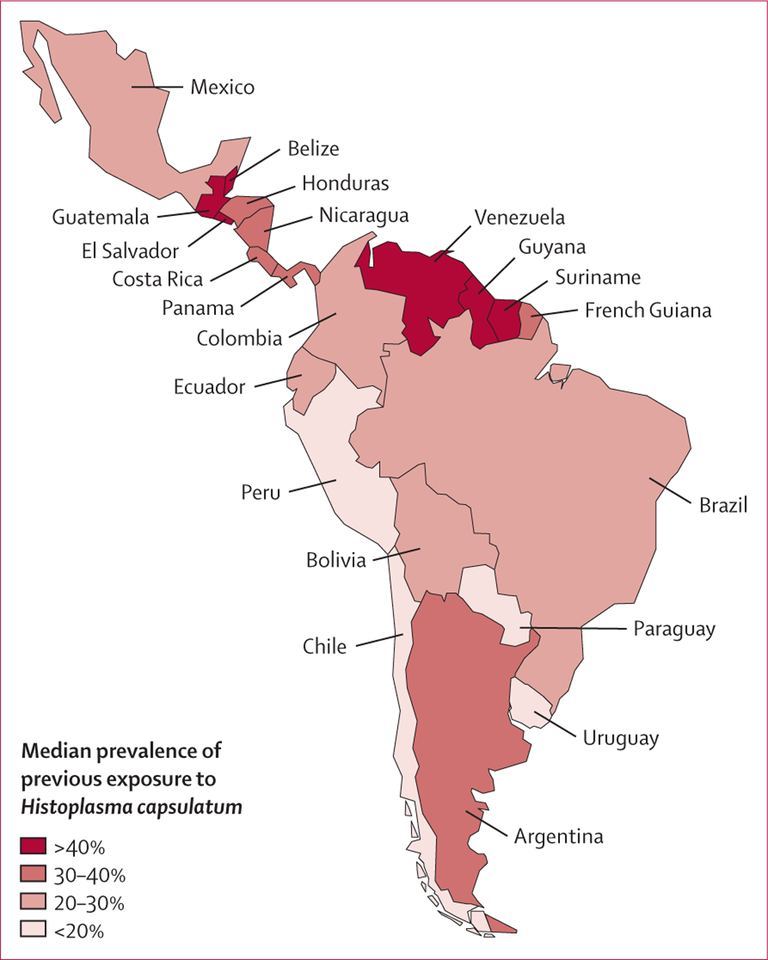
Among 1310 articles identified as of June 1, 2015, 24 articles were included in the study, representing 129 histoplasmin skin test studies done in the general population of Latin American countries (the description of all studies is detailed on appendix pp 11–30). The overall estimated prevalence of previous exposure to H capsulatum for Latin America was 32·2%, ranging from 0·1% in Chile to 57·2% in Guatemala (table 1; figure 1). Prevalence was over 20% in most countries except Chile, Paraguay, Peru, and Uruguay. Areas with the highest prevalence (>30%) were the Guiana Shield (French Guiana, Guyana, Suriname), Venezuela, Argentina, and Central America (except Mexico). These areas represented 57% (12 of 21) of the Latin American countries.
Table 1:
Estimated prevalence of previous exposure to Histoplasma capsulatum in the general population, annual incidence of histoplasmosis and tuberculosis in people living with HIV, Latin America, 2012
| Histoplasmosis prevalence* | People living with HIV(low-high)† | Histoplasmosis annual incidence in people living with HIV‡ | Symptomatic histoplasmosis annual incidence in people living with HIV‡ | Tuberculosis annual incidence in people living with HIV (low-high)§ | ||||
|---|---|---|---|---|---|---|---|---|
| Per 100 people living with HIV | N | N(30) | N(50) | N(70) | ||||
| Argentina | 37.8% (35.3–40.3) | 98000 (80000–120000) | 1.89 (1.70–2.10) | 1864 (1546–2232) | 559 (464–670) | 932 (773–1116) | 1305 (1082–1562) | 280 (270–630) |
| Belize | 49.4% (41.7–57.1) | 3100 (2800–3400) | 3.04 (2.22–4.15) | 94 (68–130) | 28 (21–39) | 47 (34–65) | 66 (48–91) | 29 (26–31) |
| Bolivia | 22.0% (20.9–23.1) | 16000 (8500–24000) | 0.88 (0.82–0.94) | 141 (93–191) | 42 (28–57) | 70 (47–96) | 99 (65–134) | 420 (390–560) |
| Brazil | 20.3% (19.7–20.8) | 595000 (530000–660000) | 0.79 (0.77–0.82) | 4714 (4321–5120) | 1414 (1296–1536) | 2357 (2161–2560) | 3300 (3025–3584) | 14000 (13990–14010) |
| Chile | 0.1% (0.03–0.17) | 39000 (25000–61000) | 0.003 (0.001–0.005) | 1.22 (0.35–2.36) | 0.37 (0.10–0.71) | 0.61 (0.17–1.18) | 0.85 (0.24–1.65) | 51 (48–120) |
| Colombia | 25.1% (24.4–25.7) | 150000 (110000–190000) | 1.04 (1.01–1.08) | 1565 (1264–1867) | 469 (379–560) | 782 (632–933) | 1095 (885–1307) | 1400 (1200–1700) |
| Costa Rica | 36.7% (34.5–38.5) | 9800 (8800–11000) | 1.81 (1.64–1.95) | 177 (156–199) | 53 (47–60) | 89 (78–100) | 124 (109–140) | 61 (55–68) |
| Ecuador | 22.6% (20.0–25.2) | 52 000 (36000–99000) | 0.91 (0.78–1.05) | 508 (346–762) | 153 (104–228) | 254 (173–381) | 356 (242–533) | 1200 (1100–1400) |
| El Salvador | 44.5% (35.9–55.0) | 25000 (16000–45000) | 2.50 (1.75–3.80) | 660 (383–1166) | 198 (115–350) | 330 (191–583) | 462 (268–816) | 220 (210–220) |
| French Guiana | 32.5% (29.3–35.7) | 2900 (1935–3390) | 1.50 (1.29–1.73) | 42 (32–53) | 13 (10–16) | 21 (16–26) | 30 (23–37) | 22 (14–30) |
| Guatemala | 57.2% (54.8–59.6) | 58000 (36 000–130 000) | 4.16 (3.78–4.60) | 2676 (1665–4322) | 803 (500–1297) | 1338 (833–2161) | 1873 (1166–3025) | 900 (810–1000) |
| Guyana | 47.0% (37.1–56.9) | 7200 (4300–12000) | 2.76 (1.84–4.12) | 204 (116–353) | 61 (35–106) | 102 (58–177) | 143 (81–247) | 200 (200–270) |
| Honduras | 38.8% (38.5–39.0) | 26000 (21000–33000) | 1.97 (1.95–1.99) | 518 (441–607) | 155 (132–182) | 259 (220–304) | 363 (308–425) | 460 (360–560) |
| Mexico | 22.8% (22.1–23.6) | 170000 (150000–210000) | 0.92 (0.88–0.96) | 1589 (1411–1818) | 477 (423–545) | 794 (706–909) | 1112 (988–1273) | 2500 (2500–2600) |
| Nicaragua | 36.7% (34.5–38.6) | 9600 (6600–15000) | 1.81 (1.64–1.95) | 179 (130–241) | 54 (39–72) | 89 (65–120) | 125 (91–168) | 110 (100–120) |
| Panama | 34.9% (33.8–36.0) | 17000 (12 000–22 000) | 1.67 (1.59–1.75) | 284 (224–346) | 85 (67–104) | 142 (112–173) | 198 (157–242) | 280 (250–310) |
| Paraguay | 17.5% (16.4–18.6) | 13000 (7400–24000) | 0.66 (0.61–0.71) | 90 (57–134) | 27 (17–40) | 45 (29–67) | 63 (40–94) | 280 (260–300) |
| Peru | 19.7% (18.4–21.0) | 76000 (36 000–230 000) | 0.76 (0.70–0.83) | 693 (334–1302) | 208 (100–391) | 346 (167–651) | 485 (234–912) | 2400 (2200–2700) |
| Suriname | 43.1% (40.7–45.5) | 4000 (3600–4400) | 2.36 (2.14–2.60) | 94 (83–107) | 28 (25–32) | 47 (42–53) | 66 (58–75) | 59 (51–67) |
| Uruguay | 10.2% (10.0–10.5) | 13000 (9800–19000) | 0.35 (0.35–0.36) | 47 (37–60) | 14 (11–18) | 24 (19–30) | 33 (26–42) | 130 (120–140) |
| Venezuela | 48.2% (47.8–48.6) | 110000 (74000–160000) | 2.90 (2.85–2.95) | 3243 (2422–4178) | 973 (727–1253) | 1622 (1211–2089) | 2270 (1695–2925) | 1200 (1000–1200) |
| Total for Latin America | 32.2% (32.0–32.4) | 1500000 (1200000–1900000) | 1.48 (1.47–1.49) | 22637 (18 934–26 225) | 6710 (5680–7867) | 11183 (9467–13112) | 15657 (13254–18357) | 26202 (25155–28032) |
Data are n (95% CI) or % (95% CI), unless otherwise specified.
Histoplasmosis prevalence corresponded to estimates of the proportion (median [2·5th and 97·5th percentiles]) of the general population (including people living with HIV) previously exposed to Histoplasma capsulatum.
Low and high estimates of people living with HIV (all ages) reported by UNAIDS, 2012.11
Histoplasmosis incidence was estimated in people living with HIV from the prevalence estimates in the general population. The incident number of histoplasmosis cases in people living with HIV was estimated from the incidence combined with the number of people living with HIV. The three scenarios of symptomatic only histoplasmosis cases in people living with HIV corresponded to 30% (N[30]), 50% (N[50]), and 70% (N[70]) of all incident histoplasmosis cases in people living with HIV with a CD4 count <200 cells per μL. The median (2·5th and 97·5th percentiles) was presented for each estimate.
Tuberculosis incident cases, low and high estimates, in people living with HIV were extracted from the WHO notification table for the year 2012.14
Figure 1:
Estimated median prevalence of previous exposure to Histoplasma capsulatum in the general population of Latin American countries
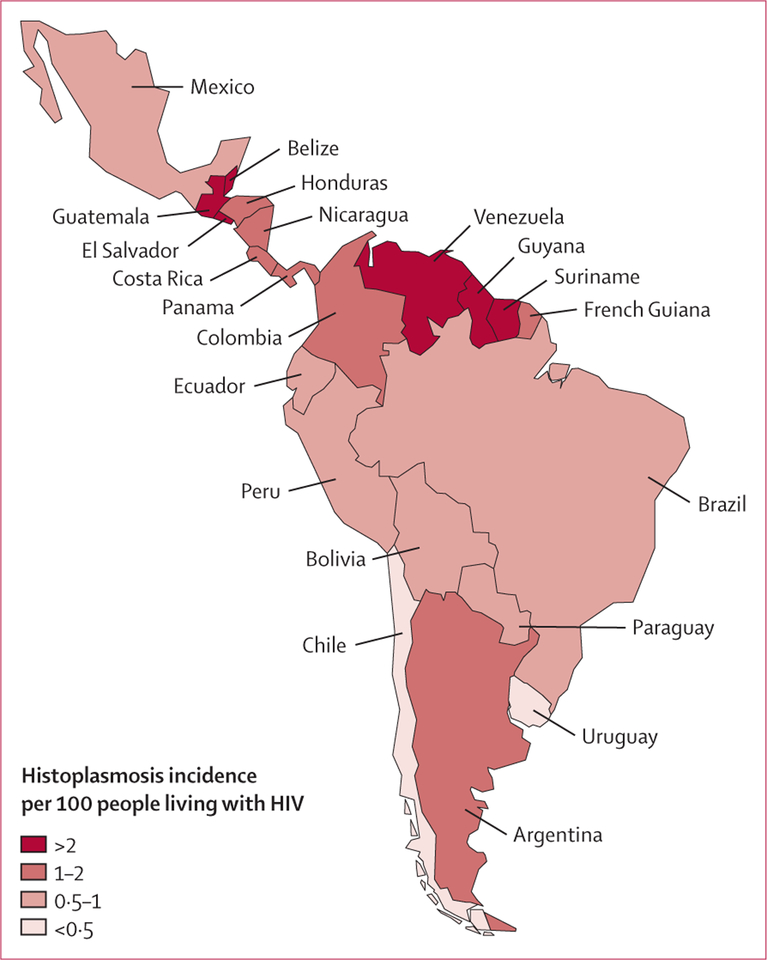
The estimated incidence of histoplasmosis (symptomatic and asymptomatic) in people living with HIV in 2012 ranged from 0·003 cases per 100 people living with HIV in Chile to 4·16 cases per 100 people living with HIV in Guatemala (table 1; figure 2). Areas with the highest incidence (≥1·5 cases per 100 people living with HIV) were the Guiana Shield, Central America (except Mexico), Argentina, and Venezuela. For the Latin America region, the incidence was estimated at 1·48 cases per 100 people living with HIV.
Figure 2:
Estimated annual incidence of histoplasmosis cases per 100 people living with HIV in Latin America, 2012
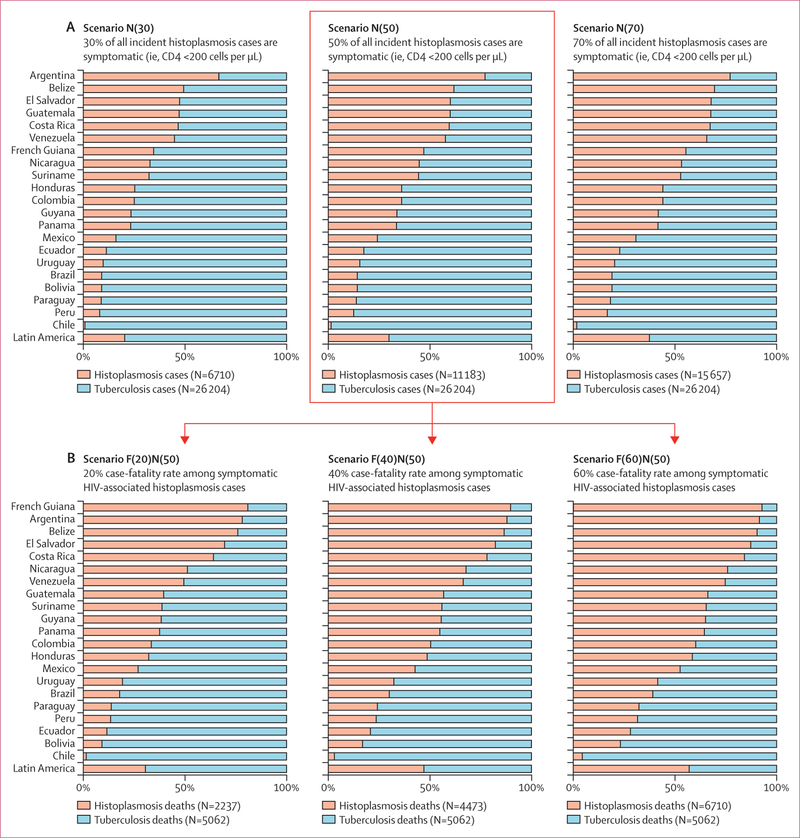
In Latin America, the estimated total number of HIV-associated histoplasmosis cases (symptomatic and asymptomatic) in people living with HIV in 2012 was 22 637. According to three scenarios where 30% (N[30]), 50% (N[50]), or 70% (N[70]) of these people living with HIV having a CD4 count of less than 200 cells per μL when they experienced histoplasmosis, the estimated total number of symptomatic histoplasmosis cases were 6710, 11 183, and 15 657, respectively (table 1; figure 3). In comparison, the total number of symptomatic HIV-associated tuberculosis cases in people living with HIV in 2012, reported by WHO, was 26 202.
Figure 3: Comparison of the number of incident cases (A) and number of deaths (B) estimated for symptomatic histoplasmosis and reported for tuberculosis in people living with HIV in Latin America, 2012.
Tuberculosis data were extracted for all countries from the WHO notifications and outcomes tables.14 Data on symptomatic incident cases of HIV-associated histoplasmosis were estimated according to three scenarios of 30% (N[30]), 50% (N[50]), and 70% (N[70]) of the estimated annual number of all histoplasmosis cases (asymptomatic and symptomatic) occurring in people living with HIV and having a CD4 count <200 per μL. Data on the number of histoplasmosis-related deaths were estimated in each incident cases scenario using four case-fatality rates of HIV-associated histoplasmosis: 10% case-fatality rate F(10), 20% F(20), 40% F(40), and 60% F(60). The scenario F(10) N(50) is detailed in appendix (pp 6–7).
The N(30) scenario of symptomatic histoplasmosis cases represented 20% of the total combined number of symptomatic histoplasmosis and tuberculosis cases in people living with HIV across Latin America, N(50) represented 30% of cases, and N(70) represented 37% of cases (figure 3).
Areas where the estimated number of symptomatic HIV-associated histoplasmosis cases was higher or nearly equivalent to the reported number of HIV-associated tuberculosis cases were mainly Central America (excluding Mexico), the Guiana Shield, the northernmost part of South America, and Argentina. These areas represented 29% (six of 21) of the Latin American countries considering the N(30) scenario, 43% (nine of 21) of countries for the N(50) scenario, and 62% (13 of 21) of countries for the N(70) scenario (figure 3).
Estimates of the annual number of histoplasmosis-related deaths in people living with HIV in 2012 were expressed as proportions (10%, 20%, 40%, and 60% case-fatality rates) of the estimated number of symptomatic HIV-associated histoplasmosis cases (table 2; figure 3). The 10% case-fatality rate scenario is shown in the appendix (pp 6–7). Considering the median scenario (N[50]) for the number of symptomatic histoplasmosis cases, estimates for the total number of histoplasmosis-related deaths among Latin American people living with HIV was 1118 according to the 10% case-fatality rate scenario, 2237 for the 20% scenario, 4473 for the 40% scenario, and 6710 for the 60% scenario.
Table 2:
Annual number of deaths estimated for symptomatic histoplasmosis cases and reported for tuberculosis cases in people living with HIV, Latin America, 2012
| Histoplasmosis-related death annual incidence in people living with HIV* | Tuberculosis-related death annual incidence in people living with HIV (low-high)† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20% fatality, F[20] | 40% fatality, F[40] | 60% fatality, F[60] | ||||||||
| N[30] | N[50] | N[70] | N[30] | N[50] | N[70] | N[30] | N[50] | N[70] | ||
| Argentina | 112 (93–134) | 186 (155–223) | 261 (216–312) | 224 (186–268) | 373 (309–446) | 522 (433–625) | 335 (278–402) | 559 (464–670) | 783 (649–937) | 52 (16–110) |
| Belize | 6 (4–8) | 9 (7–13) | 13 (10–18) | 11 (8–16) | 19 (14–26) | 26 (19–36) | 17 (12–23) | 28 (21–39) | 40 (29–55) | 3 (2–4) |
| Bolivia | 8 (6–11) | 14 (9–19) | 20 (13–27) | 17 (11–23) | 28 (19–38) | 39 (26–54) | 25 (17–34) | 42 (28–57) | 59 (39–80) | 140 (100–170) |
| Brazil | 283 (259–307) | 471 (432–512) | 660 (605–717) | 566 (519–614) | 943 (864–1024) | 1320 (1210–1434) | 849 (778–922) | 1414 (1296–1536) | 1980 (1815–2151) | 2200 (1600–2800) |
| Chile | 0.07 (0.02–0.14) | 0.12 (0.03–0.24) | 0.17 (0.05–0.33) | 0.15 (0.04–0.28) | 0.24 (0.07–0.47) | 0.34 (0.10–0.66) | 0.22 (0.06–0.43) | 0.37 (0.10–0.71) | 0.51 (0.15–0.99) | 8 (4–14) |
| Colombia | 94 (76–112) | 156 (126–187) | 219 (177–261) | 188 (152–224) | 313 (253–373) | 438 (354–523) | 282 (228–336) | 469 (379–560) | 657 (531–784) | 310 (230–400) |
| Costa Rica | 11 (9–12) | 18 (16–20) | 25 (22–28) | 21 (19–24) | 35 (31–40) | 50 (44–56) | 32 (28–36) | 53 (47–60) | 74 (66–84) | 10 (8–13) |
| Ecuador | 31 (21–46) | 51 (35–76) | 71 (48–107) | 61 (41–91) | 102 (69–152) | 142 (97–213) | 92 (62–137) | 153 (104–228) | 214 (145–320) | 390 (320–460) |
| El Salvador | 40 (23–70) | 66 (38–117) | 92 (54–163) | 79 (46–140) | 132 (77–233) | 185 (107–327) | 119 (69–210) | 198 (115–350) | 277 (161–490) | 29 (20–39) |
| French Guiana | 3 (2–3) | 4 (3–5) | 6 (5–7) | 5 (4–6) | 8 (6–11) | 12 (9–15) | 8 (6–9) | 13 (10–16) | 18 (14–22) | 1 (NA–NA) |
| Guatemala | 161 (100–259) | 268 (167–432) | 375 (233–605) | 321 (200–519) | 535 (333–864) | 749 (466–1210) | 482 (300–778) | 803 (500–1297) | 1124 (699–1815) | 410 (340–480) |
| Guyana | 12 (7–21) | 20 (12–35) | 29 (16–49) | 25 (14–42) | 41 (23–71) | 57 (32–99) | 37 (21–64) | 61 (35–106) | 86 (49–148) | 33 (21–48) |
| Honduras | 31 (26–36) | 52 (44–61) | 73 (62–85) | 62 (53–73) | 104 (88–121) | 145 (123–170) | 93 (79–109) | 155 (132–182) | 218 (185–255) | 110 (76–150) |
| Mexico | 95 (85–109) | 159 (141–182) | 222 (198–255) | 191 (169–218) | 318 (282–364) | 445 (395–509) | 286 (254–327) | 477 (423–545) | 667 (593–764) | 430 (330–540) |
| Nicaragua | 11 (8–14) | 18 (13–24) | 25 (18–34) | 21 (16–29) | 36 (26–48) | 50 (36–67) | 32 (23–43) | 54 (39–72) | 75 (55–101) | 17 (13–22) |
| Panama | 17 (13–21) | 28 (22–35) | 40 (31–48) | 34 (27–41) | 57 (45–69) | 79 (63–97) | 51 (40–62) | 85 (67–104) | 119 (94–145) | 47 (35–61) |
| Paraguay | 5 (3–8) | 9 (6–13) | 13 (8–19) | 11 (7–16) | 18 (11–27) | 25 (16–38) | 16 (10–24) | 27 (17–40) | 38 (24–56) | 57 (46–69) |
| Peru | 42 (20–78) | 69 (33–130) | 97 (47–182) | 83 (40–156) | 139 (67–260) | 194 (94–365) | 125 (60–234) | 208 (100–391) | 291 (140–547) | 450 (340–570) |
| Suriname | 6 (5–6) | 9 (8–11) | 13 (12–15) | 11 (10–13) | 19 (17–21) | 26 (23–30) | 17 (15–19) | 28 (25–32) | 40 (35–45) | 15 (11–19) |
| Uruguay | 3 (2–4) | 5 (4–6) | 7 (5–8) | 6 (5–7) | 9 (7–12) | 13 (10–17) | 9 (7–11) | 14 (11–18) | 20 (16–25) | 20 (15–26) |
| Venezuela | 195 (145–251) | 324 (242–418) | 454 (339–585) | 389 (291–501) | 649 (484–836) | 908 (678–1170) | 584 (436–752) | 973 (727–1253) | 1362 (1017–1755) | 330 (250–410) |
| Total for Latin America | 1342 (1136–1573) | 2237 (1893–2622) | 3131 (2651–3671) | 2684 (2272–3147) | 4473 (3787–5245) | 6263 (5302–7343) | 4026 (3408–4720) | 6710 (5680–7867) | 9394 (7952–11014) | 5062 (3777–6405) |
Data are n (95% CI). NA=not available.
Three case-fatality rate scenarios (F[20], F[40], and F[60] corresponding to 20%, 40%, and 60% case–fatality rate, respectively) were combined with three scenarios of the estimated number of symptomatic HIV-associated histoplasmosis cases (N[30], N[50], and N[70] corresponding to 30%, 50%, and 70% of the estimated annual number of HIV-associated histoplasmosis cases, symptomatic and asymptomatic, having a CD4 count <200 cells per μL). The median (2·5th and 97·5th percentiles) was presented for each estimate.
Tuberculosis-related deaths were reported as the number of deaths (low and high estimates).14
For the same year, 5062 tuberculosis-related deaths were reported by WHO among people living with HIV in Latin America. Considering the worst combined scenario of 60% case-fatality rate among a high number of symptomatic histoplasmosis cases (N[70]), the total number of histoplasmosis-related deaths in people living with HIV across Latin America was estimated at 9394. In the worst scenario, countries with more than 400 deaths per year resulting from symptomatic HIV-associated histoplasmosis cases were, in descending order, Brazil, Venezuela, Guatemala, Argentina, Colombia, and Mexico. For the same year, countries with more than 400 deaths reported among HIV-associated tuberculosis cases were, in descending order, Brazil, Peru, Mexico, and Guatemala.
Considering the median scenario (N[50]) for the number of symptomatic histoplasmosis cases, three case-fatality rate scenarios (20%, 40%, and 60%) of the estimated number of histoplasmosis-related deaths are graphically compared with the number of tuberculosis-related deaths observed in people living with HIV (figure 3). According to the 60% case-fatality rate scenario (F[60]N[50]), histoplasmosis and tuberculosis represented 11772 deaths in 2012, with 6710 (57%) deaths for histoplasmosis and 5062 (43%) deaths for tuberculosis. Areas with a higher or nearly equivalent number of deaths from histoplasmosis compared with tuberculosis deaths were Central America, the Guiana Shield, Colombia, Venezuela and Argentina. These countries represent 67% (14 of 21) of Latin American countries. Regardless of the case-fatality rate scenarios, the number of deaths from histoplasmosis was greater or similar to deaths from tuberculosis in five countries: Argentina, Belize, Costa Rica, El Salvador, and French Guiana.
Discussion
We found that histoplasmosis prevalence estimations in Latin America are high, with more than a third of the general population having been exposed to H capsulatum. With the exception of Chile, where the disease is rarely reported,16 histoplasmosis is widespread in Latin America and should be considered as a common disease by public health authorities. Specific guidance and requirements regarding histoplasmosis care and treatment in the general population will help reduce the burden of illness and death, notably for people with immunocompromising conditions at risk of severe histoplasmosis.17
Among Latin American people living with HIV, we found that the estimated histoplasmosis incidence is high at 1·48 cases per 100 people living with HIV, which amounts to over 22 000 cases per year. Countries with the highest histoplasmosis incidence in people living with HIV (≥1·5 cases per 100 people living with HIV) follow the same geographical distribution as histoplasmosis prevalence hotspots in the general population: Central America, Argentina, and the northernmost part of South America (Venezuela and the Guiana Shield). Nevertheless, despite lower incidence densities, countries with large populations (Brazil, Colombia, and Mexico) had a large number of estimated incident cases of histoplasmosis (>1500 cases per year).
Histoplasmosis incidence estimates computed in this study are concordant with incidence rates reported in retrospective cohorts of people living with HIV or in studies of the burden of histoplasmosis at the country level.6,18–23
With a number of symptomatic histoplasmosis cases in people living with HIV ranging from 6710 (N[30]) to 15 657 (N[70]) each year in Latin America, histoplasmosis is likely to be among the main AIDS-defining conditions. In hotspots described in this study, it might be the most common AIDS-defining condition, as shown in French Guiana18 and suspected in Guatemala and Argentina.24,25 The median scenario (N[50]) of symptomatic infections is realistic and probably even conservative. Indeed, apart from French Guiana, where 30% of people living with HIV are diagnosed with advanced disease, this proportion remains much higher (50–70%) across Latin America.26
Despite lower estimates at the Latin American level, the number of symptomatic histoplasmosis cases was similar or higher than tuberculosis cases in 43% (N[50]) to 62% (N[70]) of Latin American countries (figure 3). Regardless of the number of case scenarios, areas where the magnitudes for both diseases overlap were Central America and the northernmost part of South America (Venezuela).
The order of magnitude of HIV-associated histoplasmosis deaths (9600 cases) across Latin America was crudely estimated as similar to HIV-associated tuberculosis deaths (6000 cases) for the year 2013.8 Our country-by-country approach based on historical data and a conservative median scenario (F[40]N[50]) also suggests that estimates of HIV-associated histoplasmosis deaths (4473 cases) and reported HIV-associated tuberculosis deaths (5062 cases) were comparable. Hence, together histoplasmosis and tuberculosis might account for a fifth of the 52 000 AIDS-related deaths estimated by UNAIDS in Latin America for the year 2012,11 with histoplasmosis ranking among the top AIDS-related cause of deaths.25
The estimates of histoplasmosis-related deaths are conservative because the disease is widely undiagnosed and untreated across Latin America.8,27 The true burden of deaths might be closer to the worst scenario (F[60]N[70]), with 9394 deaths per year. Under this scenario, 67% (14 of 21) of Latin American countries had more histoplasmosis-related than tuberculosis-related deaths, and histoplasmosis-related deaths alone would account for a fifth of AIDS-related deaths in Latin America.
The burden of histoplasmosis-related deaths can be reduced with increased awareness, early diagnosis, and appropriate therapy, which has been shown to lead to a marked reduction of mortality.6 Enzyme immunoassay urinary antigen tests and training of health-care providers have resulted in an increase in the diagnosis of histoplasmosis12 and a reduction of mortality.13 New rapid and simple diagnostic tools in development and improvements in antifungals available on the WHO essential medicines list could speed up the goal of reducing histoplasmosis mortality and AIDS-related deaths by 2020.28–30
The present study has limitations. The estimated prevalence (95% CI) of histoplasmin skin test positivity from available studies is a crude approximation of a country’s overall rate, since prevalence might vary widely within a country, which could have led to underestimations or overestimations of incidence and deaths. Costa Rica, Nicaragua, and El Salvador used the mean of bordering counties for histoplasmosis skin test prevalence. Guatemala, Guyana, and Suriname only had histoplasmosis skin test prevalence studies of patients in hospitals available. Moreover, the different scenarios might not always accurately reflect the reality in all countries. However, in the absence of relevant and reliable data in most countries, rigorous computations were done using realistic estimations from the available literature.
Tuberculosis data reported by WHO and HIV data estimated by UNAIDS might not always reflect the exact burden of disease because of permanent evolutions regarding quality and methods of reporting.
Despite antiretroviral therapy scale-up, histoplasmosis remains an important cause of morbidity and mortality in people living with HIV. These crude estimations emphasise the urgency of diagnosis and treatment of histoplasmosis in Latin America, where far too many people living with HIV die from a treatable infection, in the absence of empirical antifungal therapy and rapid diagnostic tools.30,31 To our knowledge, this is the first study to quantify and map the burden of this neglected public health problem. This study is aimed to help Latin American countries and international institutions prioritise future interventions and research. Prospective studies are now needed to map precisely the risk of exposure to H capsulatum and refine these estimates with direct measures of the burden of histoplasmosis among people living with HIV and other populations at risk of life-threatening disease development in endemic countries (eg, transplant, haematological malignancies, treatment with corticosteroids, and other immunosuppressive medications).32
Supplementary Material
Research in context.
Evidence before this study
Progress in care and treatment of HIV infection, notably antiretroviral therapy, has greatly reduced the incidence of AIDS and AIDS-related deaths in high-income countries. In low-income and middle-income countries, AIDS and AIDS-related deaths remain challenging, with fungal opportunistic infections being important and often unsuspected contributors. Histoplasmosis is suspected to be the main AIDS-defining condition and AIDS-related cause of death in Latin America. The 2017 WHO guidelines for managing advanced HIV disease recommended for the first time to screen and treat for histoplasmosis in Latin America. Among the research gaps listed, the lack of simple diagnostic tests limits knowledge of the disease burden, which is often misdiagnosed as tuberculosis. We searched the MEDLINE, Scielo, Lilacs, and Google Scholar databases with no limitation of time until Feb 1, 2018. The search terms were “histoplasma” or “histoplasmosis” associated with “South America”, “Central America”, “Latin America”, or any Latin American country name. All types of articles in any language were considered. We selected studies describing the burden of histoplasmosis with prevalence or incidence data. Thus, epidemiological data published on histoplasmosis were mainly from histoplasmin skin test studies, case reports, or case series, with a few seroprevalence studies done in different populations. The burden of this disease has been mapped as points or areas of endemicity on maps but is not known at the country level. Data on histoplasmosis in people living with HIV were mainly found in case reports or series, with few recent studies of burden at the country level. This paucity of knowledge contributes to high mortality of HIV-associated histoplasmosis: a major neglected, unsuspected, undiagnosed, and untreated disease across Latin America.
Added value of this study
To the best of our knowledge, this is the first study to estimate with an accurate method (following the Guidelines for Accurate and Transparent Health Estimates Reporting statement) the burden of HIV-associated histoplasmosis at the country and region levels for Latin America. Similarly, for the first time we present burden estimates at the country level, instead of the commonly employed mapping using points and areas at risk. HIV-associated histoplasmosis incidence estimates per country were compared with HIV-associated tuberculosis country data reported by WHO for the year 2012 to provide a comparison of the magnitude of this suspected public health issue. The study findings showed that in Latin America, incidence of and deaths from histoplasmosis and tuberculosis in people living with HIV were similar, with numerous overlapping hotspots for the two diseases. Histoplasmosis, which is underdiagnosed in most countries, is suspected to be among the top AIDS-defining conditions and AIDS-related causes of death in Latin America.
Implications of all the available evidence
Estimates of the burden of histoplasmosis compared with tuberculosis are now available for the first time to policy makers. The country-level estimates can help inform public health officials who want to revise strategic plans, upgrade fungal diagnosis and treatment capacity, directly measure the burden of histoplasmosis in their country, and otherwise work to reduce histoplasmosis incidence and mortality. These data show that HIV-associated histoplasmosis is a major problem for the Latin American region, which requires consideration by international organisations. Finally, histoplasmosis is not only a problem for people living with HIV but it is also likely to be a threat to people with other immunocompromising conditions in Latin America (eg, transplant, haematological malignancies, treatment with corticosteroids, and other immunosuppressive medications).
Acknowledgments
AAA acknowledges an Investissement d’Avenir grant of the Agence Nationale de la Recherche (CEBA: ANR-10-LABX-25–01). AAA is supported by the Agence Nationale de la Recherche sur le SIDA et les hépatites virales—Agence autonome de l’Institut National de la Santé et de la Recherche Médicale (ANRS-Inserm no. 12 260) and by the European Union (PO FEDER 2007–2013, No. Présage: 31 362, Guyane Française). We thank Larissa Valmy who contributed to the statistics and Valentin Dufit who contributed to the cartography for the study.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of interests
We declare no competing interests.
References
- 1.Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis 2017; 17: e334–43. [DOI] [PubMed] [Google Scholar]
- 2.Colombo AL, Tobon A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49: 785–98. [DOI] [PubMed] [Google Scholar]
- 3.Adenis AA, Aznar C, Couppie P. Histoplasmosis in HIV-infected patients: a review of new developments and remaining gaps. Curr Trop Med Rep 2014; 1: 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comstock GW, Vicens CN, Goodman NL, Collins S. Differences in the distribution of sensitivity to histoplasmin and isolations of Histoplasma capsulatum. Am J Epidemiol 1968; 88: 195–209. [DOI] [PubMed] [Google Scholar]
- 5.Principe A, Convit J, Pifano F. Results of the epidemiological investigations on histoplasmosis, coccidioidomycosis and tuberculosis carried out in various regions of Venezuela (in Spanish: ). Mycopathol Mycol Appl 1961; 15: 11–52. [DOI] [PubMed] [Google Scholar]
- 6.Adenis A, Nacher M, Hanf M, et al. HIV-associated histoplasmosis early mortality and incidence trends: from neglect to priority. PLoS Negl Trop Dis 2014; 8: e3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peigne V, Dromer F, Elie C, Lidove O, Lortholary O. Imported acquired immunodeficiency syndrome-related histoplasmosis in metropolitan France: a comparison of pre-highly active anti-retroviral therapy and highly active anti-retroviral therapy eras. Am J Trop Med Hyg 2011; 85: 934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nacher M, Adenis A, Arathoon E, et al. Disseminated histoplasmosis in Central and South America, the invisible elephant: the lethal blind spot of international health organizations AIDS 2016; 30: 167–70. [DOI] [PubMed] [Google Scholar]
- 9.Adenis A, Nacher M, Hanf M, et al. Tuberculosis and histoplasmosis among human immunodeficiency virus–infected patients: a comparative study. Am J Trop Med Hyg 2014; 90: 216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Granado M, Lopez R, Volz A, et al. Tuberculosis in the Americas—regional report 2014. Epidemiology, control, and financing Geneva: Pan American Health Organization and World Health Organization, 2015. [Google Scholar]
- 11.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Contract No.: UNAIDS / JC2502/1/E Geneva: UNAIDS, 2013. [Google Scholar]
- 12.Caceres DH, Zuluaga A, Arango-Bustamante K, et al. Implementation of a training course increased the diagnosis of histoplasmosis in Colombia. Am J Trop Med Hyg 2015; 93: 662–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samayoa B, Mercado D, Argueta E. Disseminated histoplasmosis (DH) before and after the implementation of urine antigen detection ELISA (UADE) in an HIV clinic in Guatemala Interscience Conference on Antimicrobial Agents and Chemotherapy. Poster M-1694; San Francisco, CA, USA; Sept 9–12, 2012. [Google Scholar]
- 14.WHO. Global Health Observatory data repository. Tuberculosis and HIV notifications and outcomes tables. 2013. http://apps.who.int/gho/data/view.main.TBHIVWHOREG (accessed Jan 6, 2015).
- 15.Vose D Risk analysis: a quantitative guide, 2nd edn Chichester: John Wiley & Sons, 2000. [Google Scholar]
- 16.Cruz R, Opazo H, Barthel E, Campos S, Piontelli E. Disseminated allocthonous histoplasmosis in a acquired immunodeficience syndrome patient. Boletín Micológico 2006; 21: 77–84. [Google Scholar]
- 17.Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30: 207–27. [DOI] [PubMed] [Google Scholar]
- 18.Nacher M, Adenis A, Adriouch L, et al. What is AIDS in the Amazon and the Guianas? Establishing the burden of disseminated histoplasmosis. Am J Trop Med Hyg 2011; 84: 239–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bava AJ. Histoplasmosis in the Muniz Hospital of Buenos Aires. Rev Inst Med Trop Sao Paulo 1995; 37: 531–35. [DOI] [PubMed] [Google Scholar]
- 20.Unis G, Oliveira Fde M, Severo LC. Disseminated histoplasmosis in Rio Grande do Sul (in Portugese). Rev Soc Bras Med Trop 2004; 37: 463–68. [DOI] [PubMed] [Google Scholar]
- 21.Velásquez Uribe G, Rueda ZV, Vélez LA, Aguirre DC, Gómez-Arias RD. Histoplasmosis in AIDS patients: a cohort study in Medellín, Colombia: Infectio 2010; 14: s99–106. [Google Scholar]
- 22.Corzo-León D, Armstrong-James D, Denning D. Burden of serious fungal infections in Mexico Mycoses 2015; 58: 34–44. [DOI] [PubMed] [Google Scholar]
- 23.Medina N, Samayoa B, Lau-Bonilla D, et al. Burden of serious fungal infections in Guatemala. Eur J Clin Microbiol Infect Dis 2017;36: 965–69. [DOI] [PubMed] [Google Scholar]
- 24.López Daneri AG, Arechavala A, Iovannitti CA, Mujica MT. Histoplasmosis diseminada en pacientes HIV/Sida (in Spanish: ). Medicina (B Aires) 2016; 76: 332–37. [PubMed] [Google Scholar]
- 25.Debroy P, Medina N, Herrera R, et al. Opportunistic infections as a mayor cause of mortality during the first year of HIV diagnosis in a national reference clinic in Guatemala—PUB007 20th International AIDS Conference. Melbourne, VIC, Australia; July 20–25, 2014. [Google Scholar]
- 26.Piñeirúa A, Sierra-Madero J, Cahn P, et al. The HIV care continuum in Latin America: challenges and opportunities. Lancet Infect Dis 2015; 15: 833–39. [DOI] [PubMed] [Google Scholar]
- 27.Nacher M, Adenis A, Mc Donald S, et al. Disseminated histoplasmosis in HIV-infected patients in South America: a neglected killer continues on its rampage. PLoS Negl Trop Dis 2013; 7: e2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathieu N, Antoine A. Proceedings of first histoplasmosis in the Americas and the Caribbean meeting, Paramaribo, Suriname, Dec 4–6, 2015. Emerging Infect Dis 2016; 22. [Google Scholar]
- 29.Denning DW. Minimizing fungal disease deaths will allow the UNAIDS target of reducing annual AIDS deaths below 500 000 by 2020 to be realized. Philos Trans R Soc Lond B Biol Sci 2016;371: 20150468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samayoa B, Roy M, Cleveland AA, et al. High mortality and coinfection in a prospective cohort of human immunodeficiency virus/acquired immune deficiency syndrome patients with histoplasmosis in Guatemala. Am J Trop Med Hyg 2017; 97: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nacher M, Adenis A, Sambourg E, et al. Histoplasmosis or tuberculosis in HIV-infected patients in the amazon: what should be treated first? PLoS Negl Trop Dis 2014; 8: e3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. Lancet Infect Dis 2017; 17: e367–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.