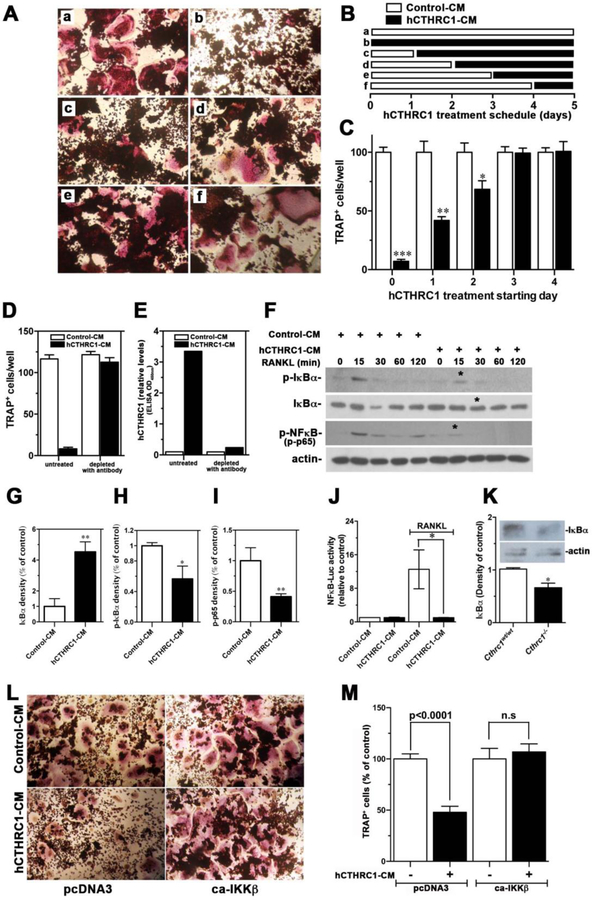
Figure 6. Effects of CTHRC1 on RANKL-induced osteoclastogenic differentiation and intracellular signaling transduction in RAW264.7 cells.
(A-C) RAW264.7 cells were differentiated with RANKL for 5 days and the time-dependent presence of hCTHRC1 to inhibit osteoclast differentiation was assessed. TRAP-positive multinucleated cells were quantified. Data represent means±SEM of ≥3 experiments with hCTHRC1-CM versus control-CM treated cells. (D) Osteoclast differentiation of RAW264.7 cells in the presence of hCTHRC1 (hCTHRC1-CM) is inhibited. Depleting hCTHRC1-CM with anti-CTHRC1 monoclonal antibodies abolishes this effect, demonstrating specificity. (E) Effectiveness of hCTHRC1-CM depletion was verified by measuring hCTHRC1 levels before and after depletion using an established ELISA. (F) RAW264.7 cells were stimulated with RANKL for the indicated length of time in the presence of hCTHRC1 or control medium. Western blotting of cell lysates shows reduced activation of NFκB (p-NFκB), reduced degradation of IκBα and reduced phosphorylation of IκBα (p-IκBα in the presence of hCTHRC1 (asterisks). (G-H) Quantification of immunoblot data marked with * in (F) are shown. (J) hCTHRC1 inhibits RANKL-induced NFκB-dependent luciferase reporter activity. (K) Western blot analysis of IκBα levels in femur lysates from wildtype and Cthrc1 null mice shows significantly reduced levels in the mutants. (L, M) RAW264.7 cells treated with control-CM or hCTHRC1-CM were transfected with control vector (pcDNA3) or a vector expressing a constitutively active form of IKKβ (ca-IKKβ), which reversed inhibition of osteoclast differentiation by hCTHRC1. All data represent means±SEM of replicates ≥3 with * = p<0.05, ** = p<0.01, and *** = p<0.005.