Abstract
Membrane proteins on oligodendrocytes and CNS myelin (NI35/250) have been shown to block axon out-growth in culture, and this is thought to be one of the major reasons for severely limited regeneration of severed axons in the CNS of higher vertebrates. In a recent study, adult dorsal root ganglion (DRG) neurons, which are sensitive to these inhibitory proteins, regenerated successfully after transplantation into two white matter tracts of the rat brain without any intervention to suppress the inhibitory activity of CNS myelin. The results and implications of these two studies are considered.
Keywords: NI35/250, Axon regeneration, Proteoglycan, Gliosis, Oligodendrocytes, Astrocytes, Myelin, Spinal cord injury, Extracellular matrix
Spinal cord and traumatic brain injury primarily strike the young and healthy suddenly with effects that may alter their lives profoundly and permanently (Box 1). In contrast, injury to the peripheral nervous system is far less serious, because the motor, sensory, or autonomic dysfunction is localized to the affected nerve; more importantly, partial or complete recovery will ensue as the peripheral axons regenerate at a rate of roughly 1 mm/day. What differences between peripheral and central nervous system allow axon regeneration in the one case and prohibit it in the other?
Box 1. Spinal Cord and Traumatic Brain Injury: Statistical Overview.
A.Spinal Cord Injury
There are 200,000 cases of spinal cord injury (SCI) in the USA (not including deaths), with 10,000 new cases each year.
56% of the injured are young adults between the ages of 16–30 years.
82% of the injured are male.
A third of the cases are from motor vehicle injury, a third are from acts of violence, and a third are from falls, sports, or other.
31% of SCIs result in quadriplegia.
The annual medical expense for SCI is $6.2 billion.
B. Traumatic Brain Injury
There are 2 million cases of traumatic brain injury (TBI) each year in the USA, including 51,600 deaths.
TBI is the leading cause of death and disability among children and young adults.
Young men account for the largest number of cases.
Motor vehicle accidents account for 1/2 of all injuries.
Infants and elderly are the second highest risk group, usually through injury in a fall.
The annual medical expense for TBI is $25 billion.
For more information, contact the National Spinal Cord Injury Statistical Center, Birmingham, AL, and these sites on the World Wide Web:
One major factor influencing axon regeneration in the PNS and CNS derives from the different types of glial cells that myelinate PNS and CNS axons. The research of Martin Schwab and colleagues (Bandtlow et al. [1]) indicates that oligodendrocytes and central myelin contain cell surface molecules that inhibit axon outgrowth in culture and in vivo. Indeed, contact with a minute liposome containing the inhibitory molecule from CNS myelin will cause immediate collapse of growth cones from PNS or CNS neurons in vitro. Unless the growth inhibitory molecules in CNS myelin can be identified and neutralized, regeneration of CNS axons seems impossible. The efforts of the Schwab lab have been focused on this objective.
In a recent paper, Jerry Silver and colleagues (Davies et al. [2]) reported that adult sensory neurons transplanted into two white matter tracts in the adult brain were able to extend axons rapidly and vigorously over long distances without any intervention to block growth inhibitory factors in central myelin (2). The research of this laboratory concerns the injury response of glia to CNS trauma. There are two important aspects of this response that differ in the PNS and CNS. In the PNS, Schwann cells myelinate axons by forming contiguous chains of cells, which wrap short segments of myelin around the axon to form a series of myelinated segments. In the CNS, individual oligodendrocytes myelinate portions of several axons through multiple cytoplasmic extensions. When an axon in the PNS is severed, the chain of Schwann cells remains; this provides a pathway that guides axon regrowth to its proper destination. In the CNS, this physical pathway does not remain after axons are damaged. The tissue often becomes disorganized, and a physical barrier to axon regrowth can develop from astrocytes responding to CNS injury. In addition to the physical impediment the glial scar presents to regeneration, this research group has investigated the possibility that axon regeneration is inhibited by molecules secreted by reactive astrocytes into the extracellular matrix. A procedure was developed to introduce PNS neurons into the adult rodent brain without stimulating this injury response, and the relative influence of inhibitory molecules in myelin and gliosis in inhibiting axon regeneration could then be compared.
Molecules Derived from Myelin that Inhibit Axon Outgrowth (Martin Schwab)
The capacity of many types of adult CNS neurons to regrow an axon after lesion has been demonstrated by transplantation experiments in which pieces of peripheral nerves were transplanted into various parts of the CNS, including cortex, thalamus, brainstem, spinal cord, and optic nerve (3). Retinal ganglion axons that reached their targets in the midbrain were shown to establish functional synapses and restore a pupillary light reflex (4, 5). Studies on the mechanism underlying this successful axonal elongation in peripheral nerves but not in CNS tissue led to the discovery of a very potent inhibitory activity for neurite growth present in oligodendrocytes and the myelin of the CNS (6, 7). A high-molecular-weight membrane protein (NI-35/250) has been found in rat, bovine, and human myelin as a main neurite growth inhibitory factor (8, 9). The protein has been purified recently to homogeneity and is a novel membrane protein (10).
Two types of in vivo experiments have shown the importance of these inhibitory environmental signals of axonal regeneration: blockade of oligodendrocyte differentiation or myelin formation on the one hand, and the application of neutralizing antibodies against the proteins NI-35/250 on the other hand. Oligodendrocytes were killed by X-irradiation of newborn rats or by antibodies and complement directed against myelin glycolipids (chick) (11–13). In both cases, successful regeneration of transected axons over long distances could be observed. These results are well in line with developmental studies in many parts of the CNS that showed a close temporal coincidence of the end of the plastic and growth permissive period of certain tracts or CNS regions and their myelination.
A monoclonal antibody that neutralizes the myelin-associated neurite growth inhibitory proteins NI-35/250 has been applied to rats with spinal cord or CNS lesion, and clear anatomical evidence for regrowth of lesioned corticospinal, optic nerve, and septo-hippocampal axons as well as for functional recovery was obtained (8, 14). Recently, similar results were obtained with a recombinant, partially humanized monovalent Fab fragment of this antibody (Fig. 1). Treatment of adult rats with IN-1 antibodies after a selective pyramidal tract lesion led also to a very significant enhancement of sprouting of intact fiber systems, in particular of the corticorubral, corticopontine, and intact corticospinal tract. These fiber systems established bilateral projections that may be linked to an almost complete recovery of fine motor control (15, 16).
Fig. 1.
Regenerated corticospinal axons in an adult rat spinal cord 2 weeks after lesion and infusion of a recombinant partially humanized IN-1 Fab’ antibody fragment. Sagittal section, rostral to the right, lesion is characterized by cavern.
These encouraging results confirm that adult neurons can sprout, grow, and regenerate under appropriate conditions, and that inhibitory environmental cues play a crucial role. However, the detailed neuroanatomical analysis showed (like in the grafts of peripheral nerve) that the percentage of successfully regenerating fibers from a given area or nucleus is relatively small. In addition to technical problems (e.g., antibody penetration), intrinsic neuronal determinants could play a crucial role in determining the degree of successful regeneration.
Neurons Can Modify their Response to Inhibitory Factors
Neuronal responses that have been correlated with a spontaneous regeneration response of CNS neurons include the upregulation of certain immediate early genes, of GAP-43, and also of specific tubulin subunits. Not much is known on the regulation of these responses, which may occur in different intensities in different types of neurons. It is probable that environmental signals, including trophic factors, cytokines, and inhibitory factors, influence these neuronal responses. Interestingly, the response of neurons to the above-mentioned myelin-associated neurite growth inhibitory proteins NI-35/250 is not uniform. Dilution experiments show a high affinity interaction of the purified inhibitory NI-250 protein with neurons, which leads to activation of an intracellular signal cascade that includes a rapid increase of intracellular calcium (17, 18). For a given neuron (e.g., dorsal root ganglion [DRG] cells or retinal ganglion cells), the response to these myelin proteins is subject to regulation: embryonic day 13 (El3) rat DRG neurons or E6 chicken retina neurons show a short, transitory, partial collapse of the growth cone followed by a normal continuation of axonal regeneration. In contrast, newborn rat DRG or E13 chick retina axons show long-lasting collapse and growth inhibition under the same conditions (19). These results suggest that neuronal populations could differ in their response to myelin proteins depending on the cell type, fiber type, age, and physiological or pathophysiological parameters (e.g., axotomy or grafting). Other factors could contribute to this variable regeneration, including additional inhibitory factors (e.g., those associated with myelin [myelin-associated glycoprotein][20], certain proteoglycans, and especially those associated with the lesion and scar area).
Inhibition of Axon Outgrowth by Gliosis (Jerry Silver)
It has been recognized for decades that the nonregenerative responses of CNS axons to injury are fundamentally different than the functionally reparative processes of the peripheral nervous system. Since the time of Cajal (21), this biological dichotomy has been explained, at least in part, by noting that the inherent growth-promoting tendency of the tubular, Schwann-cell environment to reform itself after PNS damage is unlike the disorganized and apparently impenetrable reactive astroglial scar that develops after injury to the CNS. However, the idea that reactive astrocytes form a major impediment to regeneration solely via mechanical means has been questioned in recent years. Thus, relatively normal tissue architecture is restored within days after a microlesion injury to the cingulum that transects basal forebrain axons without severely disrupting the astroglial frame-work. Nonetheless, despite the complete absence of a histologically obstructive glial scar, regenerating fibers, which, in this model, can be differentiated without error from uncut axons, never cross the lesion site. The vast majority become dystrophic on the spot, but a few can turn abruptly in the opposite direction, albeit for only short distances that would have carried them across the lesion had they been regrowing distally (22).
Inhibition of Regeneration by Extracellular Matrix Components
What then, in addition to the overall physical change in gliotic tissue, might contribute to immediate axon growth failure within the forming scar? Attention has turned to an understanding of one of the most important features of the response of the CNS to inflammation, the synthesis and modification of the extracellular matrix components made by reactive astroglia (23). One of the major constituents of the extracellular matrix (ECM), as in other tissues, is proteoglycans. Recent studies have identified a number of different types of proteoglycans in the CNS (24, 25) and many of these are now known to be potent inhibitors of neurite outgrowth (26). Of the many types of proteoglycans, chondroitin sulfate containing proteoglycans are among the most prevalent. Chondroitin sulfate proteoglycans are present in increased amounts within a variety of reactive tissues, including the CNS, after injury or disease (27–31). They are also temporarily expressed in boundary regions through which axons and dendrites fail to penetrate normally during development of the nervous system (32–33). Other studies from our lab have shown that the up-regulation of this family of molecules coincides developmentally with the age beyond which the CNS fails to support axon regeneration (34), notably at the end of two critical periods after which 1) axons can no longer regenerate back through the dorsal root entry zone into the spinal cord (35) and 2) when axons can no longer regenerate across the surface of glial scars harvested intact on nitrocellulose carriers from the forebrains of differently aged rats (30). Dystrophic axon tips in the cingulum appear to become entrapped within a thin lane of chondroitin sulfate proteoglycan, which appears within the first day after microlesion injury, precisely at the point where regeneration fails (2). Both reverse transcriptase-PCR analyses of gene expression as well as immunohistochemical surveys of protein production have led to the identification of at least three different proteoglycans (phosphacan, neurocan, and NG2) in adult CNS scar tissue (36). There are likely to be more.
The purified proteoglycans associated with reactive glia have been demonstrated by many different investigators to strongly inhibit cell as well as neurite out-growth in vitro (37). Their inhibitory action seems to occur either via their protein’s ability to bind to and mask the growth promoting potential of other molecules in their vicinity (e.g., LI) (29) or via a direct or indirect action of the highly sulfated glucosamino glycans (GAG) moieties attached to the core protein (32). Bound GAGs become increasingly potent local inhibitors of migrations of a large variety of cell types when their con-centrations are increased in relation to growth promoting molecules in their same environment. Indeed, their potency is so high that a gel formulation of a synthetic GAG has recently been approved for clinical use as an in vivo barrier for the successful prevention of epidural fibrosis and other types of painful or obstructive fibrotic adhesions in humans (38).
In substrate choice assay situations, proteoglycans elicit their biological response on neurites not via growth cone collapse but via a strong turning response of the growth cone away from the proteoglycan rich terrain (39). In in vitro situations where the growth cone is surrounded by proteoglycan-containing matrix, the neurites come to resemble the so-called “sterile end balls” formed by cut axons within long term lesions of the CNS (21, 40–42). The GAG chains seem to be key players in the inhibitory environment created by glial scars after traumatic injury. Indeed, McKeon et al. (30) demonstrated that the growth of axons in vitro on glial scar tissue taken directly from the forebrain of mature rats is markedly enhanced by chondroitin lyase digestion of the wound tissue. It is important to stress that 1) astroglial cells by themselves do have the capacity, at least in vitro, to produce extracellular matrices that are inhibitory to axonal outgrowth and 2) that the inhibition is clearly caused by the proteoglycan content of the matrix (41). However, it is equally important to stress that 1) not all astroglial cells from the same region in the so-called reactive state (i.e., increased glial fibrillary acidic protein [GFAP] expression) are equivalent repulsors of neurite outgrowth, and that 2) different types of astroglia taken from different regions are unequally competent to become functionally reactive (37). One of the most dramatic and clear cut examples of this curious reactive heterogeneity occurs during the conversion of cortical astroglial cells when they interact with substrate bound β-amyloid peptide (38).
Regeneration in CNS White Matter
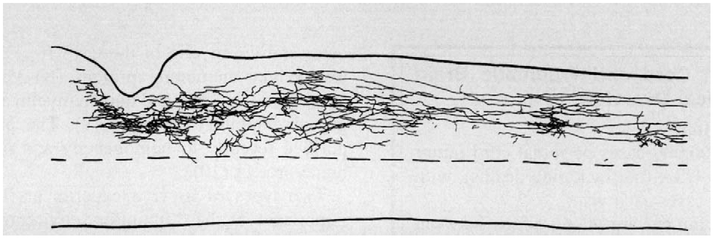
The critical question that has long remained unanswered concerning the role of gliosis in the CNS, however, is whether the reactive glial environment within the immediate vicinity of traumatic lesions in the brain and spinal cord is indeed a fundamental contributor to the problem of regeneration failure in the adult mammal. The approach we took to address this long-standing question was to quickly dissociate and reintroduce fully adult neurons back into adult CNS white matter without triggering the inflammatory cascade that leads to glial scarring and its associated inhibitory ECM component (2, 43–44). The goal was to use the microlesion pipette attached to a picospritzer (45) to nudge the axotomized neuronal cell bodies just beyond the developing line of reactive glial matrix and into the otherwise undisturbed, albeit purportedly inhibitory, white matter. The adult DRG was selected as the first cell for adult-to-adult transplantation for several reasons, but the most important was because it is clearly inhibited by myelin or by mature oligodendrocytes in vitro, at least when conditions are engineered such that the sole substratum for the regrowing axon is the oligodendrocyte. We saw no evidence of modulation of the inhibitory effect by the short in vitro exposure period. Despite the prediction from tissue culture studies and the expected strong negative influence from the resident myelin in which the growth cones were immersed, it was remarkable to find advancing, streamlined growth cones that had rapidly gained access to the host white matter glial terrain in the majority of the cases (34 of 41) (Fig. 2). The regenerating DRG axons grew with high efficiency and at surprisingly high speeds of 1–2 mm per day. In the callosum, they traveled the full distance from the trans-plant location, across the cerebral midline and into the contralateral cortex (a journey of about 8 mm) where the growing tips ramified into delicately branched terminal arbors. Confocal imaging of GFAP-positive astrocytes and calcitonin gene-related peptide- (CGRP) positive donor axons showed that many regenerating growth cones were closely associated and aligned with the beautifully organized array of longitudinal processes of the host astrocytes. The astroglia within successfully regenerating transplants showed no upregulation of proteoglycan; in addition, the cells themselves blended smoothly and continuously with those further away, which suggests that minimally reactive (i.e., normal resident intratract adult astroglia) may help to promote and guide adult axon regeneration even in the presence of a fully myelinated terrain.
Fig. 2.
Regenerating adult dorsal root ganglion (DRG) axons in adult rat corpus callosum. A, Confocal montage of a portion of an intracallosal adult DRG microtransplant scanned through two adjacent 60-mm horizontal sections. CGRP positive donor adult axons (red) exited the graft and extended within host white matter to cross the midline (M, arrowhead) to enter the contralateral hemisphere. Satellite cells (p75 positive) (green) were associated with donor neuron cell bodies (e.g., arrow), but not with axons. Asterisk indicates a calcitonin gene-related peptide- (CGRP) positive donor neuron near the transplant interface with host white matter. Survival at 4 days; scale bar 100 mM. B, High power confocal image through 17 mm of tangentially sectioned tissue showing a CGRP positive (red) adult donor axon with a streamlined growth cone extending through the myelin rich (CNS myelin specific Mab 328; green) adult host callosum, 2.5 mm from the graft. Inset, low power image of Mab 328 immunohistochemistry shows typical myelin rings when white matter is cut in cross section. Survival, 4 days; scale bar 10 mm. C, Confocal image of donor adult CGRP positive axon terminal within host cortical grey matter. Survival 6 days, scale bar 50 mm. Reprinted with permission from Nature (Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 1997;390:680–683) Copyright 1997 Macmillan Magazines Limited.
Another important aspect of the study by Davies et al. (2) was the association of regenerative failure (in 7 of 34 transplants) with the dramatic upregulation of proteoglycan rich ECM but, interestingly, without further changes in the geometry of the astroglial substructure. Thus, in each of the transplants that failed to regenerate, there was a surround of dense matrix that contained chondroitin sulfate proteoglycans, but without other morphological changes in the astroglial terrain. Triple staining for axons, matrix, and astrocytes showed clearly that the reactive matrix predicted precisely where fibers had become blocked or had actively turned away from the boundary and looped back into the transplant interior. There was no obvious relationship between the point of growth arrest and differences in myelin. Taken together, our results strongly suggest a potent role for reactive extracellular matrix in causing regeneration failure in the adult CNS.
Discussion
The possibility that a single factor would account for the difference between axon regeneration in the CNS and PNS must be dismissed upon consideration of the multiple processes involved in the regenerative response. From an intellectual and clinical standpoint, the elegant studies from both groups are impressive because they isolate, on a molecular level, potent inhibitory factors that limit regeneration in the CNS. Does myelin inhibit regeneration in the CNS? Clearly the in vitro evidence and improved regeneration in vivo when NI 35/250 is blocked by antibodies proves that it does. It is also clear that secretion of other inhibitory molecules into the ECM by reactive astrocytes is a powerful contributing factor in regenerative failure in the CNS. The work of these two groups provides a more comprehensive understanding of the regenerative response of axons to CNS trauma, and provides molecular insight into how the microenvironment in the CNS can be manipulated to promote axon regeneration.
The microtransplantation experiments show that when other impediments to CNS regeneration can be suppressed, such as physical disorganization and reactive gliosis, PNS neurons can overcome inhibitory effects of CNS myelin or seek out permissive substrates, such as nonreactive astrocytes, to extend regenerating axons. The facility of neurons (and glia) to adapt to extrinsic influences provides multiple avenues for overcoming impediments to axon regrowth. Axon outgrowth within the CNS varies with age, lesion type, and location, type of neuron, and the trophic and adhesive properties of the microenvironment. Neurons can alter their responses to many types of factors inhibiting axon outgrowth, through changes in second messenger responses (46–47), by altering the cell surface expression of receptors for the inhibitory molecules (19), or by masking or modifying the inhibitory molecule (through proteolysis, for example) (48). Given the right opportunity, axons can regrow in the inhospitable CNS environment: an absolutely exciting and hopeful prospect.
The insights from these studies also stimulate a number of questions for further investigation. Thus far only one type of neuron (DRG) has been characterized in the microtransplant model. Intrinsic limitations of different neurons to regenerate in the CNS may become apparent when other types of neurons, especially those taken from the CNS, are studied in the microtransplant model. Secondly, practical and effective methods for suppressing the secretion of inhibitory molecules into the ECM must be developed. However, we still do not know at present the specific components of the matrix that are crucial for axon repulsion. Also, the molecules that trigger the up-regulation of proteoglycan matrix, which seem to be passing through the compromised blood-brain barrier, are unknown and their characterization may help in developing therapies for intervention. In addition, the normal functions of NI35/250 in CNS myelin (apart from axon repulsion) are not well understood, and other myelin components (such as MAG) (49) may also inhibit neurite regeneration, which would require further interventions to improve regeneration in the clinical setting.
These two lines of research show important similarities. Both studies underscore the importance of glia in limiting regeneration in the CNS, and in both investigations, it is the effect of glia on the biochemical features of the microenvironment that renders the CNS inhospitable for neurite outgrowth. At the same time, both studies suggest that differences intrinsic to neurons may be important in overcoming obstacles to regeneration in the CNS. DRG neurons of 13 days of development show weak sensitivity to NI35/250 (19), and it is not yet known if other types of neurons will be as successful in the microtransplant experiments as DRG neurons. Anatomical considerations, such as the extent and placement of the lesion, and the degree of physical dis-organization in the scar region, influence regeneration, and these effects differ markedly between the PNS and CNS. Studies by Schwab and colleagues (Thallmair et al. [15], Z’Graggen et al. [16])show that smaller lesions allow structural plasticity of spared axons to restore a high degree of function even when regeneration of axons across the lesion does not occur to an appreciable extent. Antibodies against NI35/250 promote this structural plasticity by allowing axonal sprouting to ipsilateral and contralateral targets to restore function. Inhibitory molecules secreted into the ECM in response to injury could, presumably, also impede outgrowth of sprouts in types of lesions that stimulate reactive gliosis.
Glia can have both positive and negative affects on regeneration. Astrocytes, by secreting CSPG into the scar, restrict axon outgrowth, but the nonreactive astrocytes that are insinuated among the oligodendrocytes of white matter tracts can provide a permissive pathway for axon outgrowth in the CNS. Other myelin associated proteins, such as MAG, which are expressed by oligodendrocytes, may have important influences in myelination that would be beneficial in later stages of recovery from injury to myelinated fibers. Clearly, injury results in secretion of molecules that are inhibitory to regeneration, but it also induces secretion of factors that pro-mote axon survival, sprouting, and reinnervation of appropriate structures. Developing methods to control glial responses that promote and inhibit regeneration should lead to better therapies for treating traumatic injuries of the central nervous system.
A combined approach using results from both lines of research offers real promise for improving regeneration after CNS trauma. However, the percentage of axons that regenerate in both studies is quite small. This shows quantitatively how much there is yet to be understood of the multiple processes that limit CNS regeneration in higher vertebrates. Fortunately, even a small amount of anatomical recovery can produce an impressive degree of functional recovery in experimental animals (15, 50). In patients with sensory loss, for example, even a modest gain of sensory function would improve the quality of life and reduce the increased risk of further injury.
In conclusion, neutralization of growth inhibitory environmental signals, addition of appropriate growth enhancers, as well as switching the lesioned neurons into a growth mode (e.g., by axotomy of peripheral neurons) are viable ways to induce and enhance axonal regeneration, and possibly functional restoration, in the adult CNS. The nature of all these signals and their interrelationships represent a large area of future research in this exciting field at the cross-road of developmental neurobiology and clinical neuroscience.
Contributor Information
R. DOUGLAS FIELDS, Laboratory of Developmental Neurobiology, NICHD, National Institutes of Health, Bethesda, Maryland.
MARTIN E. SCHWAB, Brain Research Institute, University of Zurich, Swiss Federal Institute of Technology, Zurich, Switzerland
JERRY SILVER, Department of Neurosciences, Case Western Reserve University, Cleveland, Ohio.
References
- 1.Bandtlow C, Zachleder T, Schwab ME. Oligodendrocytes arrest neurite growth by contact inhibition. J Neurosci 1990;10:3837–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 1997;390:680–683. [DOI] [PubMed] [Google Scholar]
- 3.Bray GM, Villegas-Perez MP, Vidal-Sanz M, Carter DA, Aguayo AJ. Neuronal and nonneuronal influences on retinal ganglion cell survival, axonal regrowth, and connectivity after axotomy. Ann N Y Acad Sci 1991;633:214–228. [DOI] [PubMed] [Google Scholar]
- 4.Thanos S Adult retinofugal axons regenerating through peripheral nerve grafts can restore the light-induced pupilloconstriction reflex. Eur J Neurosci 1992;4:691–699. [DOI] [PubMed] [Google Scholar]
- 5.Sauvé Y, Sawai H, Rasminsky M. Functional synaptic connections made by regenerated retinal ganglion cell axons in the superior colliculus of adult hamsters. J Neurosci 1995;15:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neuro-trophic factors. J Neurosci 1985;5:2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci 1988;8:2381–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 1996;76:319–370. [DOI] [PubMed] [Google Scholar]
- 9.Spillmann AA, Amberger VR, Schwab ME. High molecular weight protein of human central nervous system inhibits neurite outgrowth: an effect which can be neutralized by the monoclonal antibody IN-1. Eur J Neurosci 1997;9:549–555. [DOI] [PubMed] [Google Scholar]
- 10.Spillmann AA, Bandtlow CE, Lottspeich F, Keller F, Schwab ME. Identification and characterization of a bovine neurite growth inhibitor (bNI-220). J Biol Chem 1998;273:19283–19294. [DOI] [PubMed] [Google Scholar]
- 11.Savio T, Schwab M. Lesioned corticospinal tract axons regenerate in myelin-free rat spinal cord. Proc Natl Acad Sci U S A 1990; 87:4130–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keirstead HS, Hasan SJ, Muir GD, Steeves JD. Suppression of the onset of myelination extends the permissive period for the functional repair of embryonic spinal cord. Proc Natl Acad Sci USA 1992;89:11664–11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keirstead HS, Dyer JK, Sholomenko GN, McGraw J, Kelaney KR, Steeves JD. Axonal regeneration and physiological activity following transection and immunological disruption of myelin within the hatching chick spinal cord. J Neurosci 1995;15:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 1990;343:269–272. [DOI] [PubMed] [Google Scholar]
- 15.Thallmair M, Metz GAS, Z’Graggen WJ, Raineteau O, Kartje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nature Neurosci 1998;1:124–131. [DOI] [PubMed] [Google Scholar]
- 16.Z’Graggen WJ, Metz GAS, Kartje GL, Thallmair M, Schwab ME. Functional recovery and enhanced cortico-fugal plasticity after unilateral pyramidal tract lesion blockade of myelin-associated neurite growth inhibitor in adult rats. J Neurosci 1998;15:4744–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandtlow CE, Schmidt MF, Hassinger TD, Schwab ME, Kater SB. Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science 1993;259:80–83. [DOI] [PubMed] [Google Scholar]
- 18.Loschinger J, Bandtlow CE, Jung J, Klostermann S, Schwab ME, Bonhoeffer F, et al. Retinal axon growth cone responses to different environmental cues are mediated by different second-messenger systems. J Neurobiol 1997;33:825–834. [DOI] [PubMed] [Google Scholar]
- 19.Bandtlow CE, Loschinger J. Developmental changes in neuronal responsiveness to the CNS myelin-associated neurite growth inhibitor NI 35/250. Eur J Neurosci 1997;2743–2752. [DOI] [PubMed] [Google Scholar]
- 20.Filbin MT. The muddle with MAG. Mol Cell Neurosci 1996;8: 84–92. [DOI] [PubMed] [Google Scholar]
- 21.Ramon y Cajal S Degeneration and regeneration of the nervous system (May RM, trans.) London: Oxford University Press; 1928. [Google Scholar]
- 22.Davies SJA, Field PM, Raisman G. Regeneration of cut adult axons fails even in the presence of continuous aligned glial path-ways. Exp Neurol 1996;142:203–216. [DOI] [PubMed] [Google Scholar]
- 23.Fitch MT, Silver J. Activated macrophages and the blood brain barrier: Inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol 1997;148:587–603. [DOI] [PubMed] [Google Scholar]
- 24.Margolis RK, Margolis RU. Nervous tissue proteoglycans. Experientia 1993;49:429–446. [DOI] [PubMed] [Google Scholar]
- 25.Lander AD. Proteoglycans in the nervous system. Curr Opin Neurobiol 1993;3:716–723. [DOI] [PubMed] [Google Scholar]
- 26.Hoke A, Silver J. Proteoglycans and other repulsive molecules in glial boundaries during development and regeneration of the nervous system. Prog Brain Res 1996;108:149–163. [DOI] [PubMed] [Google Scholar]
- 27.Oohira A, Matsui F, Katoh SR. Inhibitory aspects of brain chon-droitin sulfate proteoglycans on neurite outgrowth from PC 12 cells. J Neurosci 1991;11:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine JM. Increased expression of the NG-2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci 1994; 14:4716–4–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grumet M, Flaccus A, Margolis RU. Functional characterization of chondroitin sulfate proteoglycans of brain: Interactions with neurons and neural cell adhesion molecules. J Cell Biol 1993;120: 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans in-hibit the potential for laminin mediated axon growth on astrocytic scars. Exp Neurol 1995;136:32–43. [DOI] [PubMed] [Google Scholar]
- 31.Maeda N, Noda M. 6B4 proteoglycan/phosphacan is repulsive substratum but promotes morphological differentiation of cortical neurons. Development 1996;122:647–658. [DOI] [PubMed] [Google Scholar]
- 32.Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol 1990;109:110–130. [DOI] [PubMed] [Google Scholar]
- 33.Brittis PA, Silver J. Multiple factors govern intraretinal axon guidance: A time-lapse study. Mol Cell Neurosci 1995;6:413–132. [DOI] [PubMed] [Google Scholar]
- 34.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in amodel of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci 1991;11:3398–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pindzola RR, Doller C, Silver J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev Biol 1993;156:34–48. [DOI] [PubMed] [Google Scholar]
- 36.McKeon RJ, Buck CR. Gene expression in glial scars following CNS injury. Soc Neurosci Abstr 1997;23:777. [Google Scholar]
- 37.Hoke A, Silver J. Heterogeneity among astrocytes in reactive gliosis. Perspect Dev Neurobiol 1994;2:269–274. [PubMed] [Google Scholar]
- 38.Hoke A, Canning DR, Malemud CJ, Silver J. Regional differences in reactive gliosis induced by substrate bound beta-amyloid. Exp Neurol 1994;130:56–66. [DOI] [PubMed] [Google Scholar]
- 39.Wujek JR, Ahmad S, Harel A, Maier KH, Roufa D, Silver J. A carbohydrate polymer that effectively prevents epidural fibrosis at laminectomy sites in the rat. Exp Neurol 1991;114:237–245. [DOI] [PubMed] [Google Scholar]
- 40.Snow DM, Letoumeau PC. Neurite outgrowth on a step gradient of chondroitin sulfate proteoglycan. J Neurobiol 1992;23:322–336. [DOI] [PubMed] [Google Scholar]
- 41.Canning DR, McKeon RJ, DeWitt DA, Perry G, Wujek JR, Frederickson RC, et al. Beta-amyloid of Alzheimer’s disease in-duces reactive gliosis that inhibits axonal outgrowth. Exp Neurol 1993;124:289–298. [DOI] [PubMed] [Google Scholar]
- 42.Canning DR, Hoke A, Malemud CJ, Silver J. A potent inhibitor of neurite outgrowth that predominates in the extracellular matrix of reactive astrocytes. J Dev Neurosci 1996;14:153–175. [DOI] [PubMed] [Google Scholar]
- 43.Fitch MT, Silver J. Glial cell extracellular matrix: Boundaries for axon growth in development and regeneration. Cell Tissue Res 1997:290:379–384. [DOI] [PubMed] [Google Scholar]
- 44.Fitch MT, Silver J. Beyond the glial scar: Cellular and molecular mechanisms by which glial cells contribute to CNS regenerative failure In: Tuszynski MH, Kordower H. Bankiewicz K, editors. CNS regeneration: Basic science and clinical applications. New York: Academic Press; 1998. In press. [Google Scholar]
- 45.Davies SJA, Field PM, Raisman G. Long fibre growth by axons of embryonic mouse hippocampal neurons microtransplanted into the adult rat fimbria. Eur J Neurosci 1993;5:95–106. [DOI] [PubMed] [Google Scholar]
- 46.Fields RD, Guthrie PG, Russell JT, Kater SB, Malhotra BS, Nelson PG. Accommodation of mouse DRG growth cones to electrically induced collapse: Kinetic analysis of calicum transients and set-point theory. J Neurobiol 1993;24:1080–1098. [DOI] [PubMed] [Google Scholar]
- 47.Song HJ, Ming GL, Poo MM. CAMP-induced switching in turning direction of nerve growth cones. Nature 1997;388:275–279. [DOI] [PubMed] [Google Scholar]
- 48.Amberger VR, Hensel T, Ogata N, Schwab ME. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res 1998;58:149–158. [PubMed] [Google Scholar]
- 49.Li M, Shibata A, Li C, Braun PE, McKerracher L, Roder J, et al. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J Neurosci Res 1996;46:404–414. [DOI] [PubMed] [Google Scholar]
- 50.Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 1995;378:498–501. [DOI] [PubMed] [Google Scholar]