Abstract
Hyaluronic acid (HA) is a linear polysaccharide of D-glucuronic acid and N-acetyl-D-glucosamine that is native to many tissues and interacts with cells via cell-surface receptors (e.g., CD44). HA has been extensively explored as a chemically-modified macromer for crosslinking into biomaterials, such as hydrogels and macroporous scaffolds. However, the influence of the extent and type of HA modification on its binding to CD44 is not well understood or quantified. To address this, we modified HA at either the carboxylic acid or the primary alcohol with various chemical groups (e.g., norbornenes, methacrylates) and magnitudes (~10, 20, or 40% of disaccharides) and then characterized binding in both soluble and hydrogel forms. HA binding to CD44 immobilized on plates or presented by cells was influenced by the extent and type of its modification, where increased modification (i.e., ~40%) generally decreased binding. The adhesion of CD44-modified beads to hydrogels as measured by atomic force microscopy revealed a similar trend, particularly with decreased adhesion with hydrophobic modifications to the carboxylic acid. Further, the chondrogenesis of mesenchymal stromal cells when encapsulated in hydrogels fabricated from modified HA macromers was reduced at high modification, behaving similarly to inert hydrogel controls. This work suggests that the types and extents of modification of polysaccharides are important factors that should be considered in preserving their biological function when processed as hydrogels.
Keywords: glycomaterials, hydrogels, tissue engineering, CD44, chondrogenesis
1. Introduction
Hyaluronic acid (HA) is a linear hydrophilic polysaccharide of alternating D-glucuronic acid and N-acetyl-D-glucosamine that is abundant in a variety of tissues and is relevant in both development and wound healing [1]. Cells interact with HA via surface receptors, including the primary receptor CD44 [2]. Interactions between CD44 and HA occur within the HA-binding domain (HABD, often 25-174aa), which is well-conserved across species, and reportedly requires a minimum of a 6-mer (hexasaccharide, or 3 HA repeat units) and optimally an 8-mer (octasaccharide, or 4 HA repeat units) for binding [3]. CD44 is understood to play a critical role in pericellular matrix assembly, retention, and organization, and its function is required for a range of cellular processes including morphogenesis, proliferation, and wound repair [1]. CD44 is widely expressed on a variety of cell types, including mesenchymal stromal cells (MSCs) [4].
HA lends itself to numerous biomedical applications due to these cellular interactions and its presence and role in the extracellular matrix of many tissues [5]. Applications include drug delivery and tissue bulking and some HA-based materials are already well established in the clinic (e.g., dermal fillers and viscosupplements) [6-11]. HA hydrogels have also been widely explored in tissue engineering, particularly as cell carriers where properties such as high water content, injectability into tissues, degradability, and the ability to mimic features of the native extracellular matrix are important [12-26].
To fabricate hydrogels using HA, HA macromers with chemical modifications that permit crosslinking are typically synthesized. There are numerous examples of HA modifications that have enabled covalent crosslinking of HA into hydrogels, including via Michael addition reactions or photoinitiated radical polymerizations (e.g., acrylates, methacrylates, maleimides), thiol-ene click reactions that may be performed with spatial control over crosslinking (e.g., norbornenes), and aqueous Diels-Alder reactions with furan- and maleimide-functionalized HA macromers [18, 27-30]. More recently, non-covalent assembly of HA hydrogels has been achieved via HA modifications that permit physical interactions, such as with the formation of guest-host complexes (e.g., cyclodextrin and adamantane, cucurbit[6]uril and diaminohexane) between modified HA macromers [31-33]. These modifications involve the addition of pendant groups of varying size, charge, and hydrophobicity to the HA backbone.
In addition to the types of modifications themselves, the extent of modification (i.e., the proportion of disaccharide repeats that are modified) can also be controlled through the synthesis reaction and can be used to alter the crosslink density of the hydrogel and final material properties. These reactions often target the carboxylic acids or primary alcohols within HA disaccharides [34, 35]. Carboxylic acid and primary alcohol groups are also involved in the interactions between HA and the HA binding domain (HABD) of CD44 - the carboxylic acid imbues much of the negative charge that is associated with HA and is reportedly involved in interactions with at least two residues (Ala102, Ala103) of the HABD, whereas the primary alcohol is involved with at least one residue (Tyr109) of the HABD [3, 36, 37]. Thus, the hydrophobicity and charge of the final HA macromer may be influenced not just by the modification introduced but also by where and how much of it is added to HA.
One notable target of tissue engineering approaches with HA hydrogels is the repair of cartilage tissue. Mature articular cartilage is avascular, alymphatic, and cell-sparse, and injury typically results in progressive degeneration and ultimately pain and loss of joint mobility [38]. Since HA is abundant in healthy cartilage (e.g., chondrocyte pericellular matrix) and is involved in cartilage homeostasis, it has been extensively studied as a component of hydrogels and scaffolds for cartilage repair [16, 17, 20, 23, 39-42]. Mesenchymal stromal cells (MSCs), which express CD44 as their primary receptor for HA, are often used together with HA hydrogels [2, 43]. Prior work has found that MSCs encapsulated within HA-based hydrogels exhibited greater expression of cartilage-specific markers both in vitro and in vivo when compared to those within inert polyethylene glycol (PEG) hydrogels [40]. Further, the blocking of CD44 with antibodies abrogated this increased chondrogenesis in HA hydrogels, further implicating CD44 in MSC-hydrogel interactions [41]. This work established that the choice of hydrogel affects cartilage matrix production and the chondrogenic differentiation of MSCs.
With all of this in mind, there is clear motivation to better understand the influence of the extent and type of HA modification on its binding to CD44, as well as the downstream consequences of these interactions on cell behavior (e.g., differentiation of encapsulated cells). To address this, we modified HA with either norbornenes (NorHA) or methacrylates (MeHA) to various extents, characterized their interactions with CD44 in a variety of contexts (e.g., soluble form, hydrogel form), and explored the downstream effects of HA modification on MSC chondrogenesis when encapsulated in HA hydrogels. PEG was used throughout for comparison as there is no direct binding between CD44 and PEG. Our better understanding of the interactions with CD44 and modified HA macromers will help in the design of hydrogels for biomedical applications.
2. Materials and Methods
2.1. Material synthesis
NorHA, MeHA, and PEG-diacrylate were synthesized as previously reported [27, 28, 44]. Briefly, to synthesize NorHA, sodium hyaluronate (75 kDa, Lifecore, Chaska, MN) was converted to HA tert-butyl ammonium salt (HA-TBA) using Dowex 50W proton exchange resin. Frozen and lyophilized HA-TBA was subsequently dissolved in DMSO and reacted with 5-norbornene-2-methylamine, coupling to either the carboxylic acid using benzotriazole-1-yl-oxytris-(dimethylamino)-phosphonium hexafluorophosphate (BOP) to make NorHA1 or to the primary hydroxyl using di-tertbutyl dicarbonate (Boc2O) to make NorHA2. To synthesize MeHA, methacrylic anhydride (Sigma, St. Louis, MO) was added to HA (75 kDa, Lifecore, Chaska, MN) in deionized water, and the pH was maintained between 8.0-8.5 with 5 N NaOH, and reacted on ice for 6 h. Each macromer solution was purified via dialysis (MW cut-off 6–8 kDa) against deionized water for a minimum of 96 h with 2 changes of water every 24 h. The final lyophilized HA macromers were characterized using 1H nuclear magnetic resonance (NMR) and then stored at −20° C (Bruker Advance 360 MHz, Bruker, Billerica, MA; Fig. S1). PEG diacrylate (PEG-DA) was synthesized from linear PEG (10 kDa), acrylated through reaction of PEG-OH (Fluka) with acryloyl chloride and trimethylamine in dichloromethane. This product was precipitated and then characterized by 1H NMR (Fig. S2).
2.2. Stochastic model to assess potential CD44 binding to modified HA
Using MATLAB R2016b software, a model was developed and run (n=1000) to determine the theoretical number of available binding sites for CD44 interactions with modified HA. In this model, the modification of any HA repeat unit is assumed to completely inhibit the binding of CD44 due to steric hindrance and/or functional group hydrophobicity/charge. Stochastic conversion of HA repeats was assumed for the conjugation of pendant groups to HA. Given a minimum of four (non-functionalized) HA repeat units in a row required per CD44 binding event, the distribution of binding site availabilities along a single 75 kDa HA chain was computed for low (10%), med (20%), and high (40%) extents of modification.
2.3. CD44 plate assay
Protein G coated plates (Thermo Fisher Scientific, Waltham, MA) were treated with recombinant human CD44 Fc chimera protein (1 μg/mL, R&D, Minneapolis, MN) and subsequently covalently crosslinked using bis(sulfosuccinimidyl) suberate (1 mM BS3, Thermo Fisher Scientific, Waltham, MA). Macromers modified with FITC-GCKK peptide (1:1 molar ratio, peptide:macromer) were added to wells at 200 μg/mL (HA backbone concentration, accounting for mass contribution of pendant groups to ensure constant HA molar amounts across groups) in PBS for 30 minutes. Wells were washed twice for 2 minutes each and then analyzed for FITC signal using a plate reader (Infinite M200, Tecan, Männedorf, Switzerland). Values were normalized to the signal for PEG macromers for reporting.
2.4. Flow cytometry
To determine the presence of CD44 receptors, human MSCs (Lonza Walkersville, Inc., Walkersville, MD; original passage number 2) were cultured on tissue culture plates to 80% confluency and then trypsinized. MSCs were labeled with Alexafluor 488 conjugated CD44 monoclonal antibody (clone IM7, BioLegend, San Diego, CA; clone 156-3C11, Cell Signaling, Danvers, MA) for 1 h on ice and analyzed using flow cytometry (BD FACSCanto II, Becton Dickinson, Franklin Lakes, NJ). To assess modified HA binding to cell-surface CD44, human MSCs were cultured and harvested as described above, incubated on ice with FITC-tagged macromers (1:1, peptide:macromer) at 200 μg/mL, washed twice for 2 minutes each, and then analyzed for FITC signal using flow cytometry. Values were normalized to the signal for PEG macromers for reporting.
2.5. Hydrogel fabrication
Macromers were sterilized using a germicidal lamp in a laminar flow hood for 30 min as needed. NorHA1 and NorHA2 were dissolved in sterile phosphate buffered saline (PBS) containing 0.05 wt% 2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure 2959, I2959, Ciba, Basel, Switzerland) and DL-dithiothreitol (DTT, Millipore Sigma) for polymerization, while MeHA and 4-arm PEG maleimide (purity >90%, Jenkem USA) were dissolved in a solution of PBS containing DTT (all concentrations of HA macromers calculated to account for mass contribution of pendant groups, thus ensuring constant HA molar amounts across groups). NorHA1 and NorHA2 were reacted with DTT via a light-mediated thiol-ene addition reaction between norbornene groups and thiols with ultraviolet light (Eiko, 1.9 mW/cm2, Topbulb, East Chicago, IN) for 10 min to produce crosslinked NorHA hydrogels. MeHA and PEG were reacted with DTT via Michael-type addition by the addition of triethanolamine (TEOA) buffer to pH 8 to yield MeHA and PEG hydrogels, respectively.
2.6. Hydrogel mechanical, swelling, and diffusivity characterization
Acellular hydrogels (2 wt%, ~5 mm in diameter and 1 mm thick) were tested in unconfined compression using a Dynamic Mechanical Analyzer Q800 (DMAQ800, TA Instruments, New Castle, DE). The weight percent was calculated to account for mass contribution of pendant groups added to ensure constant HA molar amounts across groups. Hydrogels were compressed at 0.5 N/min until they reached 70% of their initial thickness and the modulus was determined as the slope of the stress-versus-strain curve at low strains (10-20%). Diffusivity measurements were performed on a confocal microscope (TCS SP5, Leica, Wetzlar, Germany) using a fluorescence recovery after photobleaching (FRAP) technique. Hydrogels containing 100 μM soluble FITC-dextran (average molecular weight 150 kDa) were placed on glass slides and covered with a glass cover slip; the 488 nm line of an argon laser was set to 50% power and images were captured at 10x with the pinhole fully opened. Pre-bleach images were recorded over 6 seconds using 0.1% transmission. A 30 μm diameter circular region was bleached for 30 seconds at 100 % transmission, and post-bleach images were then captured at 0.1 % transmission for 120 seconds (Fig. S3, 60s recovery shown). Data was analyzed using a custom MATLAB script that fit recovery profiles using nonlinear least squares regression to the Soumpasis equation:
| (1) |
(1) where F(t) is the normalized fluorescence recovery profile, k is the mobile fraction, τD represents the characteristic diffusion time (s), t represents time (s), and I0 and I1 are zero and first order modified Bessel functions of the first kind. Effective diffusivities were then calculated according to:
| (2) |
(2) where Deff is the effective diffusivity (μm2/s) and w represents the bleach spot radius (μm). This protocol was adapted from prior studies that conducted similar analyses [45].
2.7. CD44 atomic force microscopy
Atomic force microscopy (AFM) was performed using custom CD44-functionalized beads on cantilevers. Briefly, protein G coated polystyrene beads (mean diameter 3.4±0.7 μm, Spherotech, Lake Forest, IL) were treated with recombinant human CD44 Fc chimera protein (1 μg/mL, R&D, Minneapolis, MN), which was covalently crosslinked using bis(sulfosuccinimidyl) suberate (1 mM BS3, Thermo Fisher Scientific, Waltham, MA). CD44-coated beads were washed in deionized water twice for 2 minutes, plated on glass, dried, and affixed to tipless silicon SPM-sensors (Arrow TL1, nominal spring constant 0.03 N/m, NanoAndMore). Hydrogels fabricated from macromers were probed with these custom CD44 bead tips in indentation testing at 10 μm/s with zero dwell time and 1.2±0.9 μm maximum indentation depth, and the maximum deflection from baseline in the output retraction curves were used to calculate retraction forces on the cantilever.
2.8. Cell encapsulation
Human MSCs were encapsulated at a density of 20 × 106 cells/mL in 2 wt% hydrogels (weight percent calculated to account for mass contribution of pendant groups added to ensure constant HA molar amounts across groups). MSC-laden hydrogels were cultured in chondrogenic media (DMEM, 1% v/v ITS+ Premix, 50 μg/ml L-proline, 0.1 μM dexamethasone, 0.9 mM sodium pyruvate, 50 μg/mL ascorbate, 1% v/v penicillin-streptomycin, supplemented with 10 ng/mL TGFβ3). For viability analysis, human MSCs encapsulated in hydrogels were stained using a Live/Dead® cell viability assay (Invitrogen) according to the manufacturer’s instructions and viability was quantified as the ratio of calcein-AM-stained cells to the total cell count.
2.9. Chondrogenic gene expression
After 3 days in culture, samples (cultured as described above or in the presence of a CD44 blocking antibody) were homogenized in Trizol (Invitrogen) using a handheld tissue homogenizer. RNA was extracted according to manufacturer protocol and measured using an ND-1000 spectrophotometer (Nanodrop Technologies). 1 μg RNA from each sample was used for cDNA synthesis using reverse transcriptase (Superscript II, Invitrogen) and random hexamers as the primers (Invitrogen). Polymerase chain reaction (PCR) was then performed using the Applied Biosystems 7300 system for Real-Time PCR with a 25 μL reaction volume for Taqman (5′-nuclease) and SYBR Green reactions (n = 4). Primers and probes for relevant targets glyceraldehyde 3-phosphate dehydrogenase (GAPDH, housekeeping gene), type I collagen (COL1A1), type II collagen (COL2A1), aggrecan (ACAN), and sox9 (SOX9) were selected (Table S1). Relative gene expression was assessed using the ΔΔCT method, where the fold difference is 2−ΔΔCt.
2.10. Biochemical and histological analysis
MSC-laden hydrogels were cultured for 8 weeks and subsequently digested using papain (1 mL/construct, 0.56 U/mL in 0.1 M sodium acetate, 10 M cysteine hydrochloric acid, and 0.05 M ethylenediaminetetraacetic acid) at pH 6.0 and 60 °C for 16 h. Samples were then analyzed for the presence of sulfated glycosaminoglycan (using dimethylmethylene blue), DNA (using PicoGreen), and collagen (orthohydroxyproline, using dimethylaminobenzaldehyde and chloramine T), as performed previously [42]. For histological analysis, samples were fixed in 10% formalin (24 h), embedded in paraffin and subsequently stabilized at 4 °C (24 h), and processed using standard histological protocols. Histological sections (8 μm) were stained using antibodies for type I collagen (Col I, mouse monoclonal anticollagen type 1, Millipore), type II collagen (Col II; mouse monoclonal anticollagen type II, Developmental Studies Hybridoma Bank), and sulfated glycosaminoglycan (alcian blue, pH 1.0). For quantification, images were first converted to 8-bit and then inverted; the mean staining intensity within randomly placed frames for each section was measured with Fiji (Fig. S4).
2.11. Statistical analysis
Values are reported as mean values ± the standard error of the mean (s.e.m.). Transformation and outlier removal were not performed unless otherwise specified. Normalization was performed for each assay using relevant control groups (e.g., gene expression was normalized to values for control cells prior to encapsulation). StatPlus:mac LE (AnalystSoft) was used for statistical analyses with one-way analysis of variance (ANOVA) (and Tukey’s honestly significant difference post hoc test of the means) to compare among groups (n ≥ 4), where culture duration and experimental group were independent factors.
3. Results and Discussion
Despite the widespread use of HA hydrogels in biomedical applications and the well-documented importance of HA on cell behavior, particularly via cell-surface receptors such as CD44, few studies have addressed how the modification of HA to form HA macromers for processing into hydrogels influences receptor binding. Here, we aimed to explore the effects of multiple important parameters in the design of HA macromers on HA-CD44 interactions in both soluble and hydrogel contexts. These included several types of chemical modifications that have been used in the biomaterials field, variations in the group on HA that is used for their conjugation, and changes in the level of modification on HA. We developed a number of novel methods to help quantify the interactions of CD44 with modified HA, such as atomic force microscopy, and also investigated how these modifications may influence cell behavior when encapsulated within HA hydrogels.
3.1. HA can be modified under diverse conditions to form HA macromers
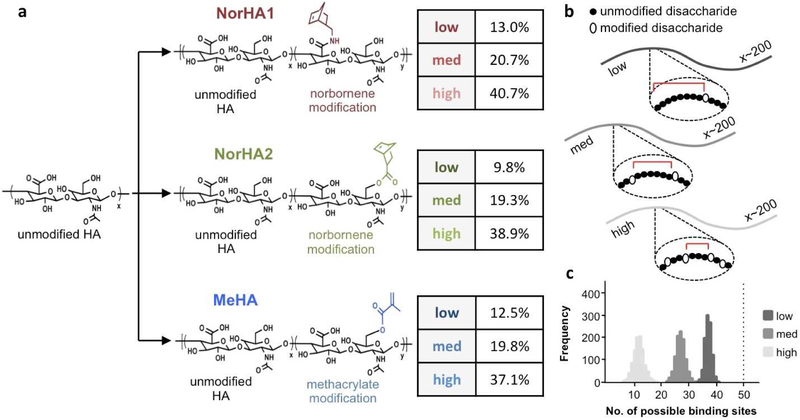
To examine the effect of modification of HA macromers on their binding to CD44, three modified HA macromers were synthesized with expected differences in charge and hydrophobicity and at three distinct levels of modification (targeting ~10, 20, and 40% of disaccharides on HA). These included: NorHA1, with HA modified at the carboxylic acid with norbornenes (low 13.0%, med 20.7%, and high 40.7% modification); NorHA2, with HA modified at the primary alcohol with norbornenes (9.8%, 19.3%, and 38.9% modification); and MeHA, with HA modified at the primary alcohol with methacrylates (12.5%, 19.8%, and 37.1% modification) (Fig. 1a). For all macromers, the difference between the expected extent of modification and the actual value was 3% or less. These macromers were identified for this study as they have all been used previously in cell encapsulation and tissue engineering. Additionally, they can all be covalently crosslinked into hydrogels using the same crosslinker (here, DTT) to eliminate any variables that may confound their comparisons.
Figure 1. Hyaluronic acid macromers used to investigate CD44 interactions.
(a) Hyaluronic acid (HA) modified with either norbornenes (NorHA, at either the carboxylic acid for NorHA1 or primary alcohol for NorHA2) or methacrylates (MeHA). The extent of modification of HA macromers was quantified using 1H NMR and categorized as either low (~10%), medium (~20%), or high (~40%). (b) Theoretical framework for stochastic modeling of HA modification, where a chain of ~200 disaccharides is modified stochastically at various rates and the distance between modifications is quantified for n=1000 simulations. (c) Histogram results from all simulations for the various modification levels, reported as the number of possible CD44 binding sites per HA chain based on the need for 4 unmodified disaccharides for binding (vertical dotted line indicates approximate maximum theoretical number of binding sites per HA chain).
The zeta potential of these synthesized HA macromers varied with modification types and extents - NorHA1 exhibited changes in effective surface charge of up to 45% with increasing extents of modification, while NorHA2 and MeHA did not show as pronounced of a trend (Fig. S5). Additionally, the zeta potentials of MeHA macromers were closest to that of unmodified HA (approximately 86% of unmodified). Given that the carboxylic acid within the HA disaccharide lends HA much of its negative charge, it is not surprising that the zeta potential is markedly altered when this moiety is modified during synthesis (i.e., NorHA1). The relatively more negative zeta potential of MeHA may be attributed to both the retention of the carboxylic acid as well as that methacrylates are less hydrophobic than norbornenes, which may influence the HA macromers in solution.
While it was apparent that modification of HA may alter its charge and hydrophobicity, we sought to model how this might influence its ability to bind to CD44. We expected that the number of possible CD44 binding sites along a macromer, defined here as needing 4 consecutive unmodified disaccharides, would decrease with increasing extents of modification (Fig. 1b). Based on this, we developed a theoretical stochastic model of CD44 binding to modified HA. With this model, assuming CD44 molecules would not sterically hinder the binding of other CD44 molecules along the length of one chain, an unmodified HA molecule that is 75 kDa would theoretically have 198 disaccharides or approximately 50 binding sites available.
Based on the stochastic model, 10% modification of HA reduces the mean number of binding sites available per chain to ~ 37 sites (Fig. 1c). Modifying to greater extents of 20% or 40% would further reduce the mean number of binding sites available per chain to ~27 and 11 sites, respectively. Compared to 10% modification, there would be a decrease of ~27% with 20% modification and a decrease of ~70% with 40% modification. Additionally, the average number of consecutive unmodified disaccharides is notably reduced with increasing extent of modification, where a high extent reduces this average value to below the threshold required for binding (Fig. S6). Although useful to represent potential changes in CD44 binding with HA modification, this model assumes that modification of a disaccharide unit completely abrogates binding by CD44, and this may not be true depending on the pendant group that is used and where on the disaccharide it is attached. For example, a bulky, hydrophobic pendant group coupled to a moiety that is critical for interactions with CD44 would result in the greatest disruption of binding and more closely approximate the outputs of this model.
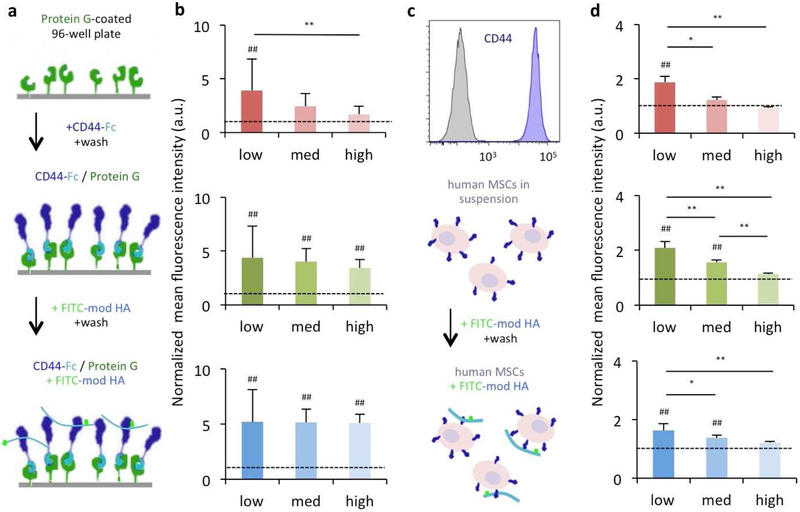
3.2. HA modification alters CD44 interactions with macromers in soluble form
We first characterized CD44 binding to HA macromers in solution to understand how HA modifications might disrupt interactions with CD44 in the absence of other factors such as crosslinking. Here, PEG was selected and added to the following studies as an inert control molecule that is readily fluorescently tagged and does not bind CD44, whereas unmodified HA cannot be readily labeled with fluorophores and was thus excluded from the study. In a first experiment, human CD44 chimera proteins were covalently linked to protein G-coated surfaces and allowed to interact with modified HA macromers (Fig. 2a). Quantification of these interactions between FITC-tagged modified HA macromers and CD44 indicated that the charge and hydrophobicity of modified HA may indeed affect binding to HA. For example, med- and high-modified NorHA1 macromers were similar to PEG in their binding ability, while all NorHA2 and MeHA macromers bound several-fold higher than PEG to CD44, with statistical differences across all modification levels (Fig. 2b). The greatest changes in binding were observed with NorHA1, with increased modification resulting in ~38% and ~57% decreases between modifications of 10–20% and 10–40%, respectively.
Figure 2. Modification of HA influences CD44 binding to HA macromers in soluble form.
(a) Protein G-coated plates were treated with a soluble CD44-Fc chimera for directional presentation of CD44. The plate surface was then exposed to FITC-modified HA macromers and the resulting fluorescent signal was quantified to assess (b) the influence of the extent and type of modification on CD44-HA interactions (red: NorHA1, green: NorHA2, blue: MeHA). (c) Human MSCs that express CD44 (shown through flow cytometry) were exposed to FITC-modified HA macromers and analyzed using flow cytometry to determine (d) the influence of the extent and type of modification on interactions between cell-surface CD44 and modified HA (red: NorHA1, green: NorHA2, blue: MeHA). n=8 for surface measurements and n=3 for flow measurements per group, dotted lines represent PEG controls, * P < 0.05; ** P < 0.01; ## P< 0.01 relative to PEG control.
To probe whether this might have implications in terms of actual receptor-ligand interactions in a cellular context, suspended human MSCs expressing CD44 were incubated with these same FITC-tagged modified HA macromers and subsequently analyzed via flow cytometry (Fig. 2c). Here, high-modified macromers across all modifications were bound by cell-surface CD44 similarly to PEG, as was the med-modified NorHA1 (Fig. 2d). In general, macromers with lower extents of modification bound more greatly to cells. As with the CD44-surface studies, the largest changes in binding (up to ~50%) with increased modification were observed with NorHA1 macromers. Importantly, the majority (~63%) of the antibody-binding epitope I/II (including the HA binding domain) of CD44 on these MSCs are retained after extended trypsinization to harvest cells from 2D culture, and epitopes that are lost are largely recovered within 25 minutes (~85%) so that these MSCs are capable of binding HA via CD44 even after extended trypsinization (Fig. S7) [46]. Epitope III (the variable stem) of CD44 was largely insensitive to extended trypsinization, which agrees with previous reports (Fig. S8) [47, 48].
3.3. HA modification alters CD44 interactions with HA hydrogels
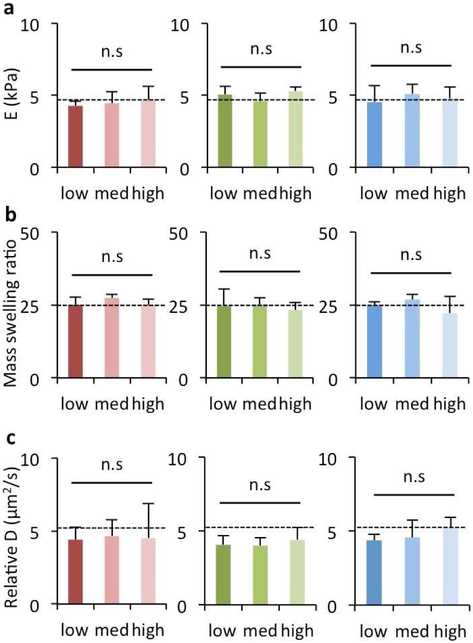
Following characterization of the interactions between CD44 and soluble HA macromers, we wanted to assess whether the changes in CD44 binding that were observed in a soluble context also translated to CD44 interactions with hydrogels formed from crosslinking of the same HA macromers. To investigate this, it was important that other features of the hydrogels did not change, so that the only variable being compared was the modification of HA. We controlled the crosslinking density by using the same dithiol crosslinker to form all hydrogels of interest via either a thiol-ene (i.e., NorHA1, NorHA2) or Michael-type addition (i.e., MeHA, PEG) reaction, and all hydrogels were formed with the same molar concentration of HA. This allowed us to maintain various hydrogel properties such as the elastic modulus (~5 kPa, Fig. 3a), mass swelling ratio (~25, Fig. 3b), and relative diffusivity (~5 μm2/s, Fig. 3c) across the hydrogels even with different extents and types of modification.
Figure 3. Matched properties of HA hydrogels with varying extents and types of modification.
Hydrogels were fabricated from modified HA macromers (red: NorHA1, green: NorHA2, blue: MeHA) using thiol-ene reactions for NorHA and Michael addition reactions for MeHA with di-thiol crosslinkers. The same crosslinker concentrations were used to match properties across modification levels. Hydrogels were analyzed for (a) elastic modulus (E), (b) mass swelling ratio, and (c) relative diffusivity (D). n=3 hydrogels per group, dotted lines represent PEG hydrogel controls, n.s. indicates no significant difference across groups and compared to the PEG hydrogel control.
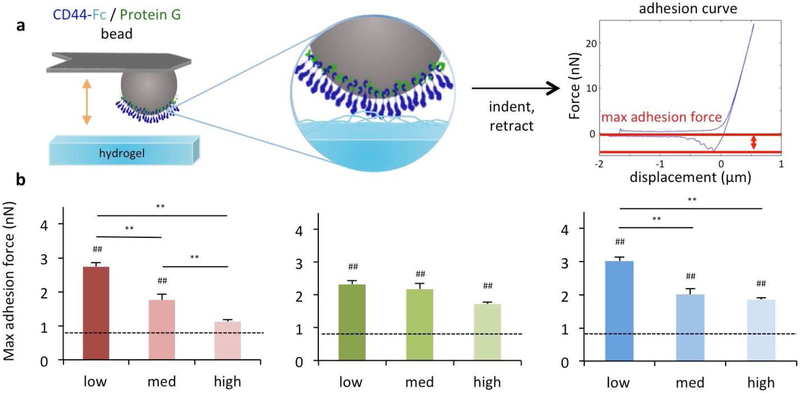
To investigate CD44 binding to HA in hydrogel form, a new method was devised using atomic force microscopy (AFM) to investigate the adhesivity of beads coated with CD44 to HA hydrogels. The beads were brought into contact with the hydrogels and the force measured upon retraction was used to assess adhesion, measured as the maximum adhesion force (Fig. 4a). Evaluation of this CD44 adhesion to the hydrogels fabricated from modified HA revealed that, in agreement with findings from the soluble macromer studies, NorHA1 hydrogels exhibited a downward trend in adhesion with increasing levels of modification such that a high modification yielded adhesion forces that were comparable to PEG hydrogels and were ~59% lower than adhesion to hydrogels from the low modified NorHA1 (Fig. 4b). In contrast, although hydrogels comprised of NorHA2 or MeHA macromers did demonstrate a modest downward trend in adhesion, adhesion to these hydrogels was still higher than adhesion to PEG hydrogels, regardless of their extent of modification.
Figure 4. Modification of HA influences CD44 adhesion to HA hydrogels.
Hydrogels were fabricated from modified HA macromers and characterized using (a) atomic force microscopy with CD44 modified beads for (b) quantification of adhesion forces (red: NorHA1, green: NorHA2, blue: MeHA). n ≥ 40 indentations across 3 hydrogels per group, dotted lines represent PEG hydrogel controls, * P < 0.05; ** P < 0.01; ## P< 0.01 relative to the PEG hydrogel control.
3.4. HA modification influences chondrogenesis in hydrogels
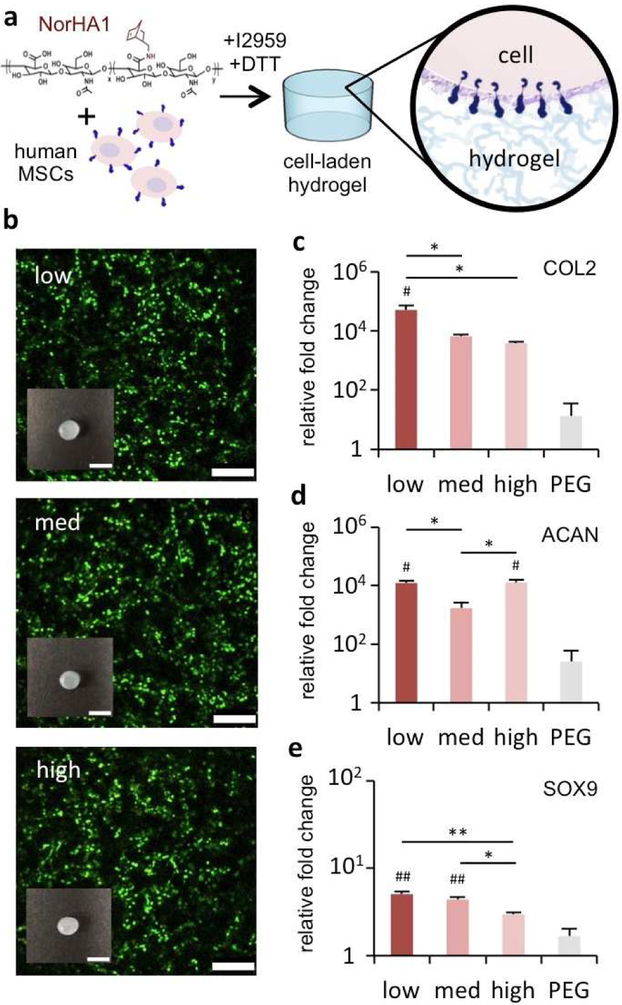
After exploring the effects of HA modification on CD44-HA interactions, we sought to probe the possible downstream consequences of these effects on outcomes relevant to tissue engineering. Because NorHA1 most consistently exhibited marked changes in CD44 binding in the various contexts, we selected this macromer for further investigation on cell behavior as we would anticipate the large effects at various modification levels. Human MSCs were encapsulated in 3D hydrogels from the NorHA1 macromers and PEG hydrogels were again used as an inert hydrogel control (Fig. 5a). Human MSCs demonstrated comparably high cell viabilities (>90%) when encapsulated in 3D NorHA1 hydrogels across the modification levels, as indicated by live/dead staining (Fig. 5b). The gross dimensions of the constructs were also comparable across these groups, which was expected since their levels of crosslinking and swelling were matched.
Figure 5. Extent of HA modification influences early chondrogenesis in 3D hydrogels.
(a) Human MSCs were encapsulated in HA hydrogels from NorHA1 macromers (red) using a thiolene reaction, as well as PEG hydrogel controls (grey). After 3 days of culture, samples were analyzed for (b) live-dead staining (scale bar 250 μm) and gross appearance of individual specimens (inset scale bar 5 mm), and the expression of (c) collagen II (COL2), (d) aggrecan (ACAN), and (e) SOX9 genes (reported normalized to GAPDH and 2D control cells prior to encapsulation). n=4 hydrogels per group, * P < 0.05, ** P < 0.01; # P < 0.05 and ## P < 0.01 relative to the PEG hydrogel control.
MSCs expressed COL2A1, ACAN, and SOX9 at 3 days when encapsulated within hydrogels and incubated in chondrogenic media (Fig. 5c-e). COL2 and SOX9 gene expression appeared to decrease with increasing extent of modification, while ACAN expression did not show a clear trend. Although it represents a nearly 4-fold difference, SOX9 expression by MSCs in NorHA1 hydrogels with the highest extent of modification was not significantly greater than the expression levels in PEG; meanwhile, expression in the presence of low- and med- levels of modification was substantially higher than in PEG hydrogels (9- and 7-fold, respectively). All constructs showed relatively little expression of COL1, an undesired marker towards articular cartilage repair (Fig. S9). Overall, the expression of all chondrogenic genes in NorHA1 encapsulations tended to be higher than that in the PEG controls. However, when cultured with a CD44 blocking antibody, there were no differences across HA modification levels and PEG controls, further pointing to the involvement of CD44 in chondrogenesis (Fig. S10).
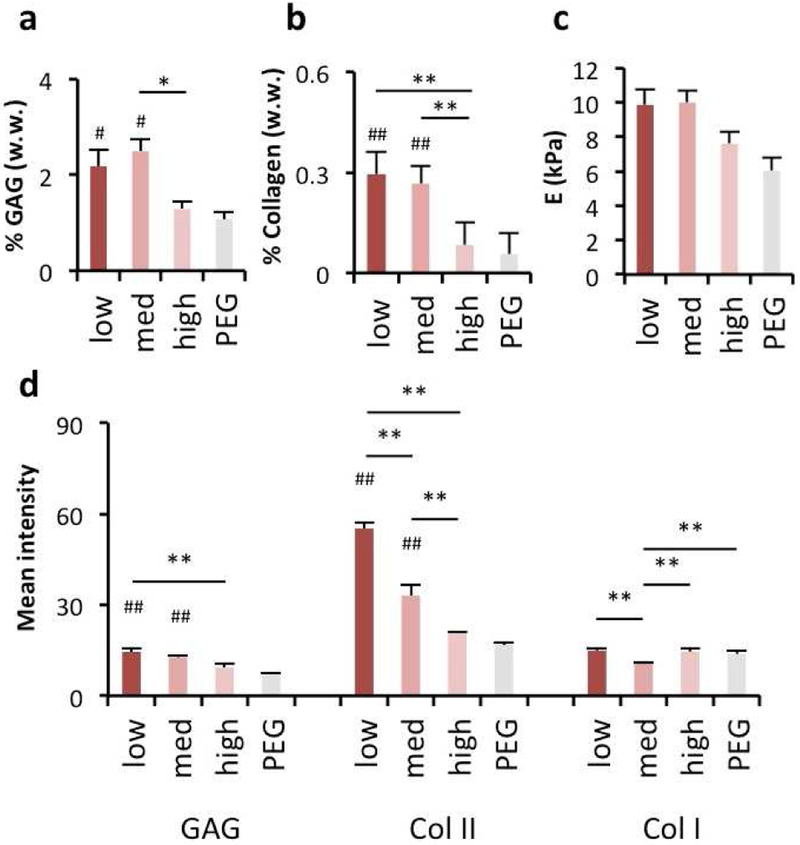
Since cartilage tissue engineering ultimately requires long-term tissue formation and repair, we also evaluated these constructs in long-term in vitro culture for up to 8 weeks. Here, the influence of HA modification on long-term MSC chondrogenesis agreed with short-term outcomes, as MSCs encapsulated in NorHA1 hydrogels exhibited smaller increases in sulfated GAG (Fig. 6a) and collagen (Fig. 6b) deposition when the NorHA possessed higher levels of modification, as determined by quantitative biochemical assays. Long-term mechanical properties (Fig. 6c) of NorHA1 encapsulations showed modest increases over PEG controls, likely due to variations in the amount of matrix being produced within the hydrogels (Fig. 6c). These biochemical levels matched histological findings for glycosaminoglycans and type II collagen, as shown through semi-quantitative image analysis of histological sections (Fig. 6d, Fig. S11). However, type I collagen was similar across all groups. Notably, the high level of modification led to similar results to that of the inert PEG hydrogel controls, implying that the macromer forms hydrogels that act more inertly rather than with the bioactivity typically considered by using HA in the hydrogel. The DNA content of these constructs did not vary with respect to extent of modification or PEG controls, suggesting that cell proliferation is not responsible for construct maturation in this context (Fig. S12). Importantly, the low- and med-modified groups contained comparable concentrations of glycosaminoglycans compared to reported values for healthy human articular cartilage, while the collagen content in all constructs remained lower than that of native tissue over the duration of the study [49, 50].
Figure 6. Extent of HA modification influences long-term chondrogenesis in 3D hydrogels.
Human MSCs were encapsulated in HA hydrogels from NorHA1 macromers (red) using a thiolene reaction, as well as PEG hydrogel controls (grey). After 8 weeks of culture, samples were analyzed for (a) glycosaminoglycan (GAG) content, (b) collagen content, (c) compressive modulus, and (d) histological quantification via staining intensity. n=4 hydrogels per group, * P < 0.05, ** P < 0.01; # P < 0.05 and ## P < 0.01 relative to the PEG hydrogel control.
The fact that HA is an important functional component of native cartilage tissue does indeed make HA-based hydrogels an attractive choice for cartilage tissue engineering. However, we established that since the carboxylic acid and primary hydroxyl groups are reportedly involved in interactions with residues in the CD44 HA binding domain, adding increasing numbers of bulky and hydrophobic pendant groups to those sites on a given HA molecule may be expected to perturb its bioactivity [37]. It is not surprising, then, that NorHA1, where the carboxylic acid is lost and replaced by a relatively bulky and hydrophobic norbornene pendant group, showed the greatest decreases in binding both in solution and in hydrogels. Other modifications showed decreased effects on CD44 binding; however, there was still typically a trend with increasing modification levels.
These findings suggest that early cell interactions with HA in these constructs, perhaps even starting from the point that cells are introduced into solutions of the macromers prior to crosslinking, may be altered by the extent and type of modifications made to the macromers used. Interactions between CD44 and HA appear to help drive expression of cartilage-specific markers, similar to that observed with increased CD44 levels during chondrogenesis, perhaps by enabling the “sensing” of local matrix, inducing CD44 clustering, and influencing SOX9 pathway activity by enhancing SOX9 expression, and such that expression of CD44 itself also increases during chondrogenesis [51, 52, 53]. With this in mind, it would be expected that high extents of modification that perturb CD44-HA interactions would have the greatest effects on downstream matrix deposition and tissue formation by inhibiting these interactions, which agrees well with our results. Interestingly, NorHA1 still enhanced both short- and long-term chondrogenesis relative to PEG even at 20% modification, and this may suggest that even for bulkier hydrophobic pendant groups added to the carboxylic acid moiety in synthesis, there is a permissive window of modification where some level of the bioactivity of HA is preserved.
There are a number of considerations that should be noted when interpreting these studies. For example, only one source (Lifecore) of HA was used in these studies, namely a pharmaceutical-grade HA that is synthesized by Streptococcus pyogenes. HA is commonly derived from Streptococcus subspecies for use in commercial products; however, it can also be derived from animal sources such as rooster comb and bovine vitreous, where preparations tend to be limited by high polydispersity and low yield [54, 55]. The HA used in this study is unmodified in its original state, and its molecular structure should be consistent with others from various sources and suppliers, but studies to confirm this were not performed. Further, the HA molecular weight may influence CD44 binding, clustering, and downstream signaling and we only utilized one molecular weight (74 kDa) that lends itself well to modification and the preparation of hydrogel precursors. We chose to investigate MSCs in this work based on their ability to undergo chondrogenesis and relevance in cartilage tissue engineering; however, the findings may vary based on the cell type of interest and the levels and importance of CD44 interactions in their function. Lastly, it should be noted that although we focused on CD44 interactions with modified HA, due to its importance in cell interactions and particularly chondrogenesis, other interactions or biological signaling from HA were not explored and may also drive interactions with HA that are important in biomedical applications. Overall, we believe that the insights gained from these studies will serve to inform the design of HA-based materials, particularly in cartilage tissue engineering, and future directions should also investigate the relevance of these findings in clinically meaningful in vivo models of cartilage injury.
5. Conclusions
In summary, CD44-HA interactions can be altered when HA is modified to synthesize HA macromers, with alterations dependent on the extent of modification, type of chemical group used for modification, and the site on HA used for modification. These effects are observable when the HA macromers are presented to CD44 both in soluble form or after crosslinking into hydrogels. Gene expression and long-term biochemical and histological analyses of MSCs encapsulated in HA hydrogels strongly suggest that modification levels of the HA macromer influences cell-hydrogel interactions and chondrogenic differentiation. Generally, a more hydrophobic pendant group attached to a more critical moiety for CD44 binding (e.g., charged acid) on the HA backbone can have marked effects on CD44-HA interactions. Importantly, low and moderately modified HA hydrogels still promoted significantly greater binding to CD44 when compared to inert molecules and upregulated chondrogenesis and cartilage formation were observed in HA hydrogels when compared to inert PEG hydrogel controls. We suggest that these considerations be incorporated into the design of HA hydrogels for tissue engineering with their significance dependent on the application and importance of CD44 binding.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Dr. Kwang Hoon Song and Dr. Leo Wang for assistance with macromer synthesis, Dr. Joshua E. Mealy for assistance with FRAP, Dr. Dror Seliktar for providing PEG-DA, and Minna H. Chen for helpful discussions. This work was supported by grants from the National Institutes of Health (R01 EB008722, T32 AR007132) and a National Science Foundation Graduate Research Fellowship to MYK.
Footnotes
Data Availability
The authors declare that all the relevant data supporting the findings of this study are available within the paper and its supplementary information, and from the corresponding authors upon reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mi Y. Kwon, Department of Bioengineering, University of Pennsylvania, 210 S. 33rd St, Philadelphia PA, 19104, USA
Chao Wang, Department of Biomedical Engineering, Drexel University, Philadelphia, PA 19104.
Jonathan H. Galarraga, Department of Bioengineering, University of Pennsylvania, 210 S. 33rd St, Philadelphia PA, 19104, USA
Ellen Puré, Department of Biomedical Sciences, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA, 19104, USA.
Lin Han, Department of Biomedical Engineering, Drexel University, Philadelphia, PA 19104.
Jason A. Burdick, Department of Bioengineering, University of Pennsylvania, 210 S. 33rd St, Philadelphia PA, 19104, USA.
References
- 1.Knudson CB. Hyaluronan and CD44: strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res C Embryo Today. 2003; 69:174–196. [DOI] [PubMed] [Google Scholar]
- 2.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990; 61:1303–1313. [DOI] [PubMed] [Google Scholar]
- 3.Teriete P, Banerji S, Noble M, Blundell C, Wright A, Pickford A, et al. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Molec Cell. 2003; 13:483–496. [DOI] [PubMed] [Google Scholar]
- 4.Maleki M, Ghanbarvand F, Behvarz M, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult cells. Intern J Stem Cells. 2014; 7:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent TC, Fraser JRE. Hyaluronan. The FASEB Journal. 1992; 6:2397–2404. [PubMed] [Google Scholar]
- 6.Fakhari A, Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler, and in osteoarthritis treatment. Acta Biomater. 2013; 9:7081–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schanté CE, Zuber G, Herlin C, Vandamme TF. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr Polym. 2011; 3:469–489. [Google Scholar]
- 8.Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003; 5:54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kogan G,. Šoltés L, Stern R, Gemeiner P Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007; 29:17–25. [DOI] [PubMed] [Google Scholar]
- 10.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman III JH, Gorman RC, Burdick JA. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci USA. 2010; 107:11507–11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tous E, Ifkovits J, Koomalsingh KJ, Shuto T, Soeda T, Kondo N, Gorman JH, Gorman RC, Burdick JA. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules. 2011; 12:4127–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balakrishnan B, Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem Rev. 2011; 111:4453–4474. [DOI] [PubMed] [Google Scholar]
- 13.Shu XZ, Ghosh K, Liu Y, Palumbo FS, Luo Y, Clark RA, Prestwich GD. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J Biomed Mater Res A. 2003; 68A:365–375. [DOI] [PubMed] [Google Scholar]
- 14.Wade RJ, Bassin EJ, Gramlich WM, Burdick JA. Nanofibrous hydrogels with spatially patterned biochemical signals to control cell behavior. Adv Mater. 2015; 27:1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Kim IS, Cho Th, Lee KB, Hwang SJ, Tae G, Noh I, Lee SH, Kim HC, Park Y, Sun K. Bone regeneration using MMP sensitive-hyaluronic acid based hydrogels. Biomaterials. 2007; 28:1830–1837. [DOI] [PubMed] [Google Scholar]
- 16.Kim IL, Khetan S, Baker B, Chen CS, Burdick JA. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials. 2013; 34:5571–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009; 30:4287–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khetan S, Katz JS, Burdick JA. Sequential crosslinking to control cellular spreading in 3-dimensional hydrogels. Soft Matter. 2009; 5:1601–1606. [Google Scholar]
- 19.Khetan S, Burdick JA. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials. 2010; 31:8228–8234. [DOI] [PubMed] [Google Scholar]
- 20.Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials. 2013; 34:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002; 3:1304–1311. [DOI] [PubMed] [Google Scholar]
- 22.Shu XZ, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004; 25:1339–1348. [DOI] [PubMed] [Google Scholar]
- 23.Erickson IE, Kestle SR, Zellars KH, Farrell MJ, Kim M, Burdick JA, Mauck RL. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 2012; 8:3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HJ, Yu C, Chansakul T, Hwang NS, Varghese S, Yu SM, Elisseeff JH. Enhanced chondrogenesis of mesenchymal stem cells in collagen mimetic peptide-mediated microenvironment. Tissue Eng Part A. 2008; 14:1843–1851. [DOI] [PubMed] [Google Scholar]
- 25.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009; 103:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryant SJ, Durand KL, Anseth KS. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res A. 2003; 67A:1430–1436. [DOI] [PubMed] [Google Scholar]
- 27.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005; 6:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gramlich WM, Kim IL, Burdick JA. Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry. Biomaterials. 2013; 34:9803–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013; 12:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan H, Rubin JP, Marra KG. Direct synthesis of biodegradable polysaccharide derivative hydrogels through aqueous Diels-Alder chemistry. Macromol Rapid Comm. 2011; 32:905–911. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Zhao X, Lin Y, Su Z, Wang Q. Dual stimuli-responsive supramolecular hydrogel of bionanoparticles and hyaluronan. Polym Chem. 2014; 5:6754–6760. [Google Scholar]
- 32.Rodell CB, Kaminski AL, Burdick JA. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules. 2013; 14:4125–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park KM, Yang JA, Jung H, Yeom J, Park JS, Park KH, Hoffman AS, Hahn SK, Kim K. In situ supramolecular assembly and modular modification of hyaluronic acid hydrogels for 3D cellular engineering. ACS Nano. 2012; 6:2960–2968. [DOI] [PubMed] [Google Scholar]
- 34.Huang G, Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Delivery. 2018; 25:766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdick JA, Prestwich GD. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv Mater. 2011; 23:H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Highley CP, Prestwich GD, Burdick JA. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol. 2016; 40:35–40. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharya DS, Souchek DJ, Hill T, Taylor M, Natarajan A, Mohs A. Impact of structurally modifying hyaluronic acid on CD44 interaction. J Mater Chem B. 2017; 5:8183–8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2007; 60:243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 2011; 32:8771–8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009; 15:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci USA. 2013; 110:10117–10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A. 2011; 18:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caplan AI. Mesenchymal stem cells. J Ortho Res. 1991; 9:641–650. [DOI] [PubMed] [Google Scholar]
- 44.Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005; 26:2467–2477. [DOI] [PubMed] [Google Scholar]
- 45.Mealy JE, Rodell CB, Burdick JA. Sustained small molecule delivery from injectable hyaluronic acid hydrogels through host-guest mediated retention. J Mater Chem B Mater Biol Med. 2015; 3:8010–8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masson D, Vusio P, Loirat M, Spring F, Anstee D, Denis M. et al. Epitope mapping of four novel CD44 monoclonal antibodies using surface plasmon resonance and soluble CD44. Transfus Med. 2001; 11:447–454. [DOI] [PubMed] [Google Scholar]
- 47.Tsuji K, Ojima M, Otabe K, Horie M, Koga H, Sekiya I, et al. Effects of different cell-detaching methods on the viability and cell surface antigen expression of synovial mesenchymal stem cells. Cell Transplant. 2017; 26:1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picker L, De los Toyos J, Telen MJ, Haynes B, Butcher E. Monoclonal antibodies against the CD44 [In(Lu) related p80] and Pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors. J Immunol. 1989; 142:2046–2051. [PubMed] [Google Scholar]
- 49.Rogers BA, Murphy CL, Cannon SR, Briggs TWR. Topographical variation in glycosaminoglycan content in human articular cartilage. J Bone Joint Surg. 2006; 88-B:1670–1674. [DOI] [PubMed] [Google Scholar]
- 50.Hoemann CD. Molecular and biochemical assays of cartilage components. Methods Mol Med. 2004; 101:127–156. [DOI] [PubMed] [Google Scholar]
- 51.Nicoll SB, Barak O, Csoka A, Bhatnagar RS, Stern R. Hyaluronidases and CD44 undergo differential modulation during chondrogenesis. Biochem Biophys Res Commun. 2002; 292:819–825. [DOI] [PubMed] [Google Scholar]
- 52.Wu SC, Chen CH, Wang JY, Lin YS, Chang JK, Ho ML. Hyaluronan size alters chondrogenesis of adipose-derived stem cells via the CD44/ERK/SOX-9 pathway. Acta Biomater. 2018; 66:224–237. [DOI] [PubMed] [Google Scholar]
- 53.Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. CMLS, Cell Molec. Life Sci 2002; 59:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varga L Studies on hyaluronic acid prepared from the vitreous body. J Biol. Chem. 1955; 217:651–658. [PubMed] [Google Scholar]
- 55.Swann DA. Studies on hyaluronic acid. I. The preparation and properties of rooster comb hyaluronic acid. 1968; 156:17–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.