Abstract
Proton pump inhibitors (PPIs) are the most effective antisecretory drugs available for controlling gastric acid acidity and volume. They are the drugs of choice in the treatment of moderate-to-severe gastroesophageal reflux disease, hypersecretory disorders, and peptic ulcers. Currently in the United States, they are only available in an oral formulation. However, pantoprazole will soon be available in an intravenous formulation and will extend the power of PPIs to inpatient hospital settings. Intravenous pantoprazole has been shown to be effective and safe in clinical trials. Intravenous pantoprazole is indicated for the treatment of patients who require PPI therapy but who are unable to take oral medication. Intravenous pantoprazole has been shown to maintain acid suppression in patients switched from oral PPIs, so no change in dosage is required when switching from one formulation to the other. Potential hospital-based uses for intravenous PPI therapy include perioperative use as prophylaxis for acid aspiration syndrome during induction of anesthesia, prophylaxis for stress-related mucosal disease, and management of gastrointestinal bleeding from stress or acid peptic disease.
Keywords: Proton pump, Pantoprazole, Gastroesophageal reflux, Zollinger-Ellison syndrome, Peptic ulcer, Stress ulcer, Syndrome, Acid aspiration
For the ambulatory patient with gastroesophageal reflux disease (GERD), available therapies range from simple lifestyle changes to medical and surgical procedures.1,2 Patients who suffer from periodic or frequent heartburn often rely on antacids or other over-the-counter medications and/ or prescription-strength histamine H2-receptor antagonists (H2RAs) to relieve their discomfort.3,4 Patients with more severe acid-related disorders generally require medical attention and stronger acid-suppression therapy.5 Proton pump inhibitors (PPIs) are the most efficient antisecretory drugs available today and, therefore, the most effective oral therapy for acid-related diseases.6–9 However, for strong acid suppression in patients who are unable to take oral medication, switching to intravenous H2RAs at high doses is the only option currently available in the United States.10,11 Intravenous PPI formulations that are currently being developed will have several potentially important applications for use in the hospital setting (Table 1).10,12
TABLE 1.
Hospital-based uses for oral and intravenous PPI therapies
| Conditions requiring antisecretory therapy | Route of administration | References |
|---|---|---|
| Patients with established need for PPI therapy who cannot take oral medication | Oral to IV, IV to oral | Hartmann25 |
| GERD | Oral to IV, IV to oral | Bocheneck,18 Fumagalli,27 Metz,26 Wurzer,56 Plein63 |
| Hypersecretory disorders (e.g. ZES) | Oral to IV, IV to oral | Simon,58 Pisegna,11 Vinayek,59 Metz28 |
| Conditions affecting drug absorption* | Oral to IV, IV to oral | Gubbins41 |
| Management of peptic ulcers and gastrointestinal bleeding | Oral to IV, IV to oral, IV | Brunner,31 Labenz,30 Lind,29 Brunner52 |
| Stress ulcer prophylaxis | IV | Otani62 |
| Gastric outlet obstruction | IV | Brunner52 |
| Acid aspiration syndrome prophylaxis | IV | Cruickshank,32 Rocke33 |
| Diagnostic test for patients with NCCP† | IV | Fass55 |
Patients with chronic nausea and vomiting or severe diarrhea, such as those receiving chemotherapy.
Based on results of an oral PPI study.
IV indicates intravenous; and NCCP, noncardiac chest pain.
Oral formulations of PPIs (omeprazole, lansoprazole, rabeprazole, and pantoprazole) are currently available by prescription in the United States. All have been shown to be highly effective and superior to H2RAs in healing moderate-to-severe erosive esophagitis and in relieving GERD symptoms.7,13–20 Clinical studies have also shown all four PPIs to be superior to H2RAs in the initial management of duodenal and gastric ulcers.8,13,16,21–23 Because of their superior acid-suppressing activity, PPIs are preferred in the management of hypersecretory disorders such as Zollinger-Ellison syndrome (ZES).13,24
Although all four PPIs exist in oral formulations, only pantoprazole will be available in an intravenous formulation for acute use in hospital settings in the United States. Its availability will extend the power of PPI therapy to hospitalized patients unable to take oral medications.25,26 No change in efficacy has been observed upon switching between oral and intravenous PPI therapy in the treatment of patients with GERD or ZES.27,28 Studies also support the role of intravenous PPI therapy in the management of peptic ulcers and as a prophylaxis for acid aspiration syndrome.29–33 This review will address relevant hospital settings and guidelines for switching between oral and intravenous antisecretory therapy, with focus on the PPIs, particularly pantoprazole. Key studies supporting potential uses for intravenous PPI therapy and clinical efficacy and safety data on intravenous pantoprazole will also be reviewed.
SITUATIONS THAT REQUIRE SWITCHING ANTISECRETORY DRUG FORMULATIONS
Switching From Oral to Intravenous Therapy
Patients who are hospitalized for acute care are usually instructed to continue with their oral antisecretory therapies as long as these medications will not negatively impact their current condition or interfere with treatment. However, in cases in which a hospitalized patient may be unable to take a needed oral medication, a switch in route of administration may be warranted.
Hospitalization itself does not appear to be a risk factor for GERD; however, the presence of cardiac or respiratory diseases, the use of certain drugs that reduce lower esophageal sphincter pressure, and the prone position frequently adopted by the convalescing patient may all increase the risk of developing GERD during hospitalization.34 Prolonged bed rest and use of nonsteroidal antiinflammatory drugs have been associated with increased risk of reflux symptoms.34 Drugs that inhibit smooth-muscle contraction, such as theophylline, which may be taken by patients with asthma or emphysema, may decrease lower esophageal sphincter pressure and affect the frequency and/or duration of reflux episodes.6,35,36
Hospitalized patients with GERD who are experiencing an increase in reflux activity, therefore, may be at increased risk for developing esophagitis. Furthermore, patients with GERD may have oral medication prohibited because of other conditions, making parenteral medication necessary. Hospitalized patients treated for esophageal strictures generally experience fewer relapses when undergoing PPI therapy than when undergoing H2RA therapy and may also benefit from an intravenous dosage form.14
In addition to its use in GERD, acid suppression therapy is also the mainstay in the management of patients with hypersecretory disorders (e.g., ZES, idiopathic hypersecretion, mastocytosis) or peptic ulcer disease (e.g., duodenal ulcer, gastric ulcer).13,37 For effective management of either of these conditions, many patients require PPI therapy.10 Changing their therapy to intravenous H2RAs because of an unexpected hospital admission is an option with a potentially poor outcome.10,11,38–40 Therefore, an intravenous formulation of a PPI is recommended for hospitalized patients with peptic ulcer disease or disorders involving acid hypersecretion who become temporarily unable to continue with their oral regimen.10
Complications of acid-related diseases are among many reasons why a patient may be unable to take medications orally. Some of the underlying health conditions may affect the efficacy of an orally administered medication, dictating a switch to intravenous drug administration. Conditions of nausea, vomiting, or severe diarrhea, which may occur perioperatively or be induced by chemotherapy, could affect the bioavailability of oral medications.11,26,41,42 The availability of an intravenous formulation circumvents this potential problem.
Switching from Intravenous to Oral Therapy
Patients who require continued antisecretory therapy after an acute need for acid suppression will generally be switched from intravenous to oral medication. Patients hospitalized for a previously untreated or ineffectively treated health condition, such as bleeding peptic ulcers or esophagitis, may start on intravenous PPI therapy and then switch to oral medication after their condition improves and they are able to eat solid foods. Continued oral acid-suppression therapy may be required upon hospital discharge. Perhaps the most likely drug-switching scenario involves the patient receiving long-term PPI therapy for an acid-related disease (e.g., GERD, ZES, peptic ulcer). This hypothetical patient is hospitalized and temporarily unable to take oral medications but will need to resume oral PPI therapy upon improving and leaving the hospital.
OTHER POTENTIAL INTRAVENOUS PROTON PUMP INHIBITOR USES
There are other uses for intravenous PPI therapy alone that may not require a switch in dosing form. During the induction of anesthesia, aspiration of gastric contents may lead to complications, including loss of life.33,43 The severity of the complications is influenced by both pH and volume of aspirated gastric content.43 Therapy with PPIs has been shown to increase gastric pH and reduce content volume.32,33,43 Perioperative use of PPI therapy may facilitate the emergency induction of anesthesia and help reduce the risk of acid aspiration syndrome.
Intravenous PPIs may be useful in treating acute gastrointestinal bleeding from ulcers or as stress ulcer prophylaxis in the management of patients in a medical or surgical intensive care unit.10 Known risk factors for stress-related mucosal damage include head trauma, surgery, respiratory failure requiring mechanical ventilation, and burns covering more than 25% of the body.44,45 Gastric acid-mediated mucosal damage frequently begins within 24 hours of major physiologic stress and can manifest as acute gastric and duodenal ulcers, hemorrhagic gastritis, or superficial gastric erosions.45
Controlling gastric acid output (AO) appears to be an effective prophylaxis against stress ulceration and related bleeding.44,46,47 Increasing and maintaining an intragastric pH above 3.5 may lower the incidence of stress ulceration and attenuate bleeding.44,47 For some patients with bleeding peptic ulcers who are treated and achieve hemostasis, increasing intragastric pH to above 6.0 may be required to reduce the risk of serious rebleeding.48 This is critical because ulcer rebleeding is associated with higher mortality.48 Intravenous H2RAs have been used as stress ulcer prophylaxis with mixed success. The principal problem is a lack of consistent intragastric pH control and tachyphylaxis.47 When administered once daily, intravenous H2RAs do not consistently maintain an intragastric pH above 3.5.47 Although continuous infusion can achieve this pharmacologic goal, higher doses are required and tolerance may develop after a few days of treatment.47
Only PPI therapy can reliably elevate intragastric pH to levels necessary to facilitate clot formation, halt gastrointestinal bleeding, and accelerate ulcer healing without inducing tolerance.31,47–51 However, because the intensive care patients at greatest risk for stress ulceration and related bleeding are also least likely to be permitted oral medications, PPI dosing adjustments may be required to achieve continuous acid suppression. Intravenous PPI regimens that involve higher-than-standard daily doses, supplied through continuous infusion or multiple daily bolus injections, appear most effective as an initial treatment step.48,49,51 Therefore, intravenous PPI therapy may be useful in providing rapid and definitive acid suppression in this patient population.
Another potential use for the intravenous PPI is for the treatment of pyloric channel ulcers. Pyloric channel ulcers can lead to gastric outlet obstruction and, subsequently, can impair gastric emptying. Intravenous PPI therapy may promote ulcer healing and resolve pyloric stenosis in such cases.52
Noncardiac chest pain is one of the predominant atypical manifestations of acid reflux.53,54 Many costly diagnostic tests, some with only low sensitivity for GERD, are available to verify an esophageal source of noncardiac chest pain. Empiric PPI therapy is an effective, alternative, low-cost diagnostic test for reflux-induced noncardiac chest pain.53,55 Intravenous pantoprazole treatment similarly may be useful as a diagnostic tool in hospitalized patients with noncardiac chest pain.
CLINICAL STUDIES USING PARENTERAL PROTON PUMP INHIBITOR THERAPY
Equivalence of Pantoprazole Oral and Intravenous Dosing
Clinical studies of oral or intravenous PPIs support the potential uses discussed above. In particular, intravenous pantoprazole has been shown to be effective and safe in the treatment of patients with GERD or ZES. Furthermore, equivalent doses of pantoprazole tablets and intravenous pantoprazole effect equipotent inhibition of gastric acid secretion—a pharmacologic feature of pantoprazole that may facilitate switching between drug formulations by reducing concern for dosage adjustments.25,26 Recently, a randomized crossover study was undertaken to compare the effect of 40-mg pantoprazole tablets and intravenous pantoprazole on median 24-hour intragastric pH.25 Pantoprazole (oral or intravenous) was administered once daily to healthy subjects for 5 days in each phase of the study, and the median 24-hour intragastric pH was determined for each subject at baseline and at day 5 of each phase. The mean 24-hour pH was 3.3 and 3.1 for the intravenous and oral treatments, respectively. The mean percentages of time that intragastric pH was above 3 and above 4, likewise, were similar between the regimens.
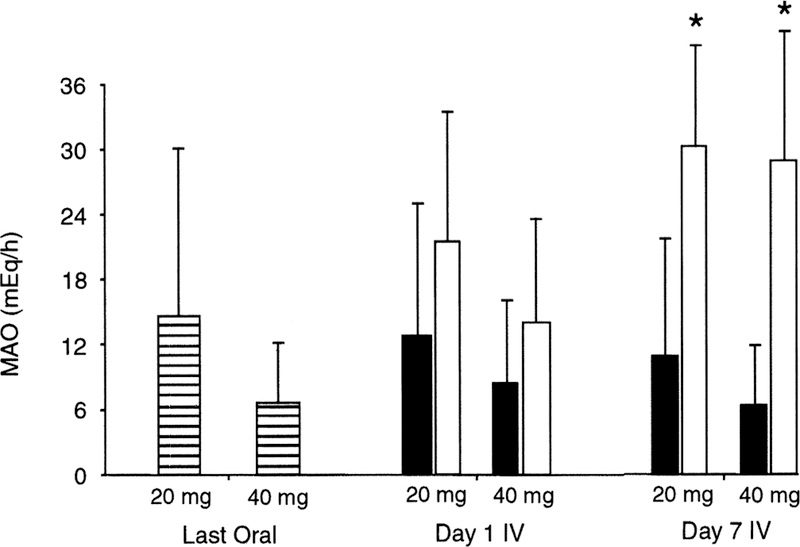
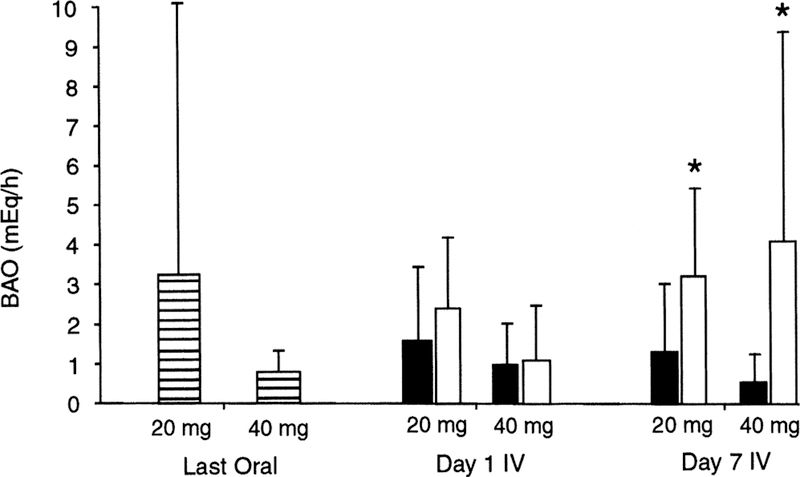
In another study involving patients with GERD, the ability of intravenous pantoprazole to maintain gastric acid suppression after a switch from oral medication was evaluated relative to placebo control.26 Patients were administered oral pantoprazole (20 or 40 mg) once daily for 10 days and then switched to either the same dose of intravenous pantoprazole or placebo for an additional 7 days. Basal and maximal (pentagastrin-stimulated) AO (BAO and MAO, respectively) measurements were made on the final day of the oral phase and compared with values determined on the last day of the intravenous phase. Gastric acid suppression (equivalent BAO and MAO values at end of each study phase) was maintained in patients switched from pantoprazole tablets to intravenous pantoprazole, but not in those switched to intravenous placebo (Fig. 1 and 2). These studies demonstrate the equipotency of oral and intravenous formulations of pantoprazole.
FIG. 1.
Dose equivalency of pantoprazole oral and intravenous formulations as measured by MAO subsequent to the final dose of a 10-day oral treatment period, and on the first and seventh day of an intravenous treatment period. Patient enrollment was 20 mg oral (n = 32), 40 mg oral (n = 31), 20 mg intravenous (n = 24), 20 mg placebo (n = 8), 40 mg intravenous (n = 24), and 40 mg placebo (n = 7). Asterisk indicates p < 0.05 versus respective seventh day intravenous dose; MAO, maximal acid output; IV, intravenous; ▤, oral pantoprazole; ■, intravenous pantoprazole; ☐, intravenous placebo. Reprinted with permission from Am J Gastroenterol.26 Copyright 2000, The American College of Gastroenterology.
FIG. 2.
Dose equivalency of pantoprazole oral and intravenous formulations as measured by BAO subsequent to the final dose of a 10-day oral treatment period, and on the first and seventh day of an intravenous treatment period. Patient enrollment was 20 mg oral (n = 32), 40 mg oral (n = 31), 20 mg intravenous (n = 24), 20 mg placebo (n = 8), 40 mg intravenous (n = 24), and 40 mg placebo (n = 7). Asterisk indicates p < 0.05 versus respective seventh day intravenous dose; BAO, basal acid output; IV, intravenous; ▤, oral pantoprazole; ■, intravenous pantoprazole; ☐, intravenous placebo. Reprinted with permission from Am J Gastroenterol.26 Copyright 2000, The American College of Gastroenterology
Fumagalli et al.27 compared a therapeutic regimen of intravenous followed by oral pantoprazole with a regimen consisting of continuous oral pantoprazole in the treatment of grades II to III (Savary-Miller scale) GERD. Patients were randomized to receive pantoprazole (40 mg each morning) either orally or intravenously for 5 days followed by oral pantoprazole for a total duration of 8 weeks. Patients were assessed for symptom relief on each day of the initial intravenous phase and then at designated timepoints thereafter. Endoscopy was performed at baseline and at 8 weeks to assess GERD healing. Symptoms of GERD were markedly reduced after 3 days of either intravenous or oral treatment and were more than 90% improved by the first follow-up visit at 2 or 4 weeks. Esophageal grades II and III lesion healing rates were similar (greater than 80%) for both drug regimens by the end of the study. No serious adverse events occurred.
Similarly, Wurzer et al.56 concluded that a week of intravenous pantoprazole followed by oral administration of pantoprazole 40 mg was safe and effective in providing GERD symptom relief and healing grades II and III lesions. In their study of over 100 patients, complete healing rates were approximately 90% after 8 weeks of therapy. An initial report of another pantoprazole study comparing oral and oral-to-intravenous drug regimens in GERD patients indicated that both therapies significantly reduced the need and use of antacid medication to relieve symptom discomfort, relative to placebo control.57
Hypersecretory Conditions
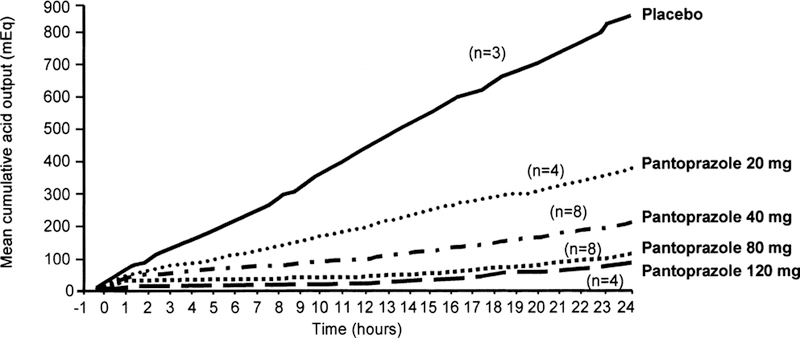
The utility of intravenous PPI therapy in the management of patients with artificially induced hypersecretory conditions has been examined in clinical trials. In a dose-response study, intravenous pantoprazole was compared with intravenous famotidine and placebo for gastric acid inhibitory ability in healthy subjects who were continuously infused with pentagastrin as a model of ZES.11 After 1 hour of pentagastrin infusion, the subjects were administered a single intravenous dose of pantoprazole (20, 40, 80, or 120 mg), famotidine (20 mg), or placebo. Efficacy outcome measurements were time to onset (when AO fell below 10 mEq/h), duration of response (total time AO was below 10 mEq/h), and cumulative AO over 24 hours. All doses of intravenous pantoprazole produced measurable acid suppression within 15 to 20 minutes after administration and reduced AO below the target value.11 Doses of intravenous pantoprazole 80 or 120 mg reduced AO below 10 mEq/h within the first hour after administration and achieved greater than 90% acid inhibition for more than 21 hours (Fig. 3). Although famotidine produced similar rates and inhibition of AO, it had a shorter duration of action—only 6 hours—compared with more than 20 hours for subjects treated with intravenous pantoprazole.11 These results support an earlier study that found that repeated daily 30-mg infusions of intravenous pantoprazole reduced short-term (4-hour) pentagastrin-stimulated AO by more than 50% after day 1 and by more than 95% by days 4 and 5.58
FIG. 3.
Twenty-four hour mean cumulative acid output after 20-, 40-, 80-, and 120-mg intravenous doses of pantoprazole and placebo. Adapted from Am J Gastroenterol.11 Copyright 1999, The American College of Gastroenterology.
Clinical trials involving ZES patients also indicate that intravenous PPI therapy is safe and effective.28,59 An early study with intravenous omeprazole found that one 60-mg bolus injection every 12 hours adequately inhibited perioperative gastric hypersecretion (AO less than 5 mEq/h, 1 hour before the next dose) in 19 (95%) of 20 patients with ZES.59 More recently, the efficacy of intravenous pantoprazole therapy was investigated in patients with ZES receiving oral PPI therapy.28 Enrolled patients receiving either oral omeprazole (20 mg every day or 20, 40, 60, or 100 mg twice daily) or lansoprazole (30 mg every day or twice daily or 60 mg twice daily) were treated with intravenous pantoprazole (80 mg every 12 hours administered as 15-minute infusions) for 7 days. Of 14 patients, 13 (93%) maintained effective control of AO (less than 10 mEq/h), resulting in no significant change in mean or median AO values (p > 0.05). Only one patient required titration up to 120 mg twice daily to control AO. Although this study demonstrated that switching from omeprazole or lansoprazole to intravenous pantoprazole was safe and effective, switching between identical dosages of the equipotent pantoprazole formulations may be the least complicated therapeutic option.
OTHER POTENTIAL USES OF INTRAVENOUS PROTON PUMP INHIBITORS
Several clinical studies also support the recognized utility of intravenous PPIs in reducing the risk of perioperative acid aspiration, as well as peptic and stress-related mucosal bleeding. Parenteral administration of omeprazole 40 mg, delivered at least 30 minutes before surgery, was shown to reduce the risk of acid aspiration of gastric contents in women undergoing emergency cesarean section under general anesthesia.33 Two clinical studies testing a simplified suspension of omeprazole administered via nasogastric tube found that PPI therapy helped prevent clinically significant stress-related mucosal bleeding in mechanically ventilated patients.47,50 Another study investigating potential clinical roles for intravenous omeprazole found that parenteral PPI therapy effectively arrested stress-related mucosal bleeding in critically ill patients.52 In yet another study, intravenous omeprazole administered for up to 21 days to patients with peptic ulcer bleeding improved patient outcome and reduced the duration and maximum severity of gastrointestinal bleeding, the need for surgery, and the need for blood transfusions.29 In a recent study that included 240 patients, intravenous omeprazole administered as an 80-mg dose followed by an 8-mg/h infusion rate reduced the rebleeding rate from 23% to 7%.60 Investigators in an additional study involving healthy subjects, reported that intravenous PPI treatment (initial 80-mg bolus followed by continuous infusion at 8 mg/h) rapidly increased and maintained intragastric pH at levels sufficient to allow the successful provision of endoscopic hemostasis for upper gastrointestinal bleeding.31 Similar initial intravenous PPI treatment steps have been shown effective at increasing intragastric pH and reducing ulcer rebleeding in patients with duodenal or bleeding peptic ulcers.48,51
Numerous studies have also documented the clinical safety of intravenous pantoprazole.11,21,26,27,31,56,58 In clinical trials of GERD patients and healthy subjects, pantoprazole has been well tolerated with few serious adverse events reported. Furthermore, pantoprazole has been shown to have minimal potential for clinically relevant drug interactions, an important property in the hospital setting.61
SUMMARY
The imminent availability of intravenous pantoprazole in the United States will extend the power of PPI therapy to hospitalized patients who require definitive acid suppression but cannot take medications by mouth.62 A variety of needs for intravenous PPI therapy in hospital settings may involve switching PPI formulations. Studies involving GERD patients who could not take oral medications have shown no loss of efficacy upon switching from oral to intravenous PPI therapy. Intravenous pantoprazole will allow hospitalized patients undergoing oral PPI therapy to continue PPI therapy instead of switching to less effective H2RAs.
REFERENCES
- 1.Long RG. Reflux oesophagitis and its treatment. Br J Clin Pract Symp Suppl 1994;75:36–40. [PubMed] [Google Scholar]
- 2.DeVault KR, Castell DO. Guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Arch Intern Med 1995;155:2165–73. [PubMed] [Google Scholar]
- 3.Graham DY, Smith JL, Patterson DJ. Why do apparently healthy people use antacid tablets? Am J Gastroenterol 1983;78:257–60. [PubMed] [Google Scholar]
- 4.Fass R, Hixson LJ, Ciccolo ML, et al. Contemporary medical therapy for gastroesophageal reflux disease. Am Fam Physician 1997;55:205–12. [PubMed] [Google Scholar]
- 5.Richter JE. Long-term management of gastroesophageal reflux disease and its complications. Am J Gastroenterol 1997;92:30S–5S. [PubMed] [Google Scholar]
- 6.Behrns KE, Koruda MJ, Herbst CA Jr. Gastroesophageal reflux disease. Pill, blade, or laparoscope? N C Med J 1997;58:436–8. [PubMed] [Google Scholar]
- 7.Chiba N, De Gara CJ, Wilkinson JM, et al. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology 1997;112:1798–810. [DOI] [PubMed] [Google Scholar]
- 8.Howden CW. Optimizing the pharmacology of acid control in acid-related disorders. Am J Gastroenterol 1997;92(suppl 4):17S–21S. [PubMed] [Google Scholar]
- 9.Franko TG, Richter JE. Proton-pump inhibitors for gastric acid-related disease. Cleve Clin J Med 1998;65:27–34. [DOI] [PubMed] [Google Scholar]
- 10.Berardi RR, Welage LS. Proton-pump inhibitors in acid-related diseases. Am J Health-Syst Pharm 1998;55:2289–98. [DOI] [PubMed] [Google Scholar]
- 11.Pisegna JR, Martin P, McKeand W, et al. Inhibition of pentagastrin-induced gastric acid secretion by intravenous pantoprazole: a dose-response study. Am J Gastroenterol 1999;94:2874–80. [DOI] [PubMed] [Google Scholar]
- 12.Langtry HD, Wilde MI. Lansoprazole. An update of its pharmacological properties and clinical efficacy in the management of acid-related disorders. Drugs 1997;54:473–500. [DOI] [PubMed] [Google Scholar]
- 13.Richardson P, Hawkey CJ, Stack WA. Proton pump inhibitors: pharmacology and rationale for use in gastrointestinal disorders. Drugs 1998;56:307–35. [DOI] [PubMed] [Google Scholar]
- 14.Schepp W Proton pump inhibitory therapy: then and now. Yale J Biol Med 1996;69:175–86. [PMC free article] [PubMed] [Google Scholar]
- 15.Boyce HW. Therapeutic approaches to healing esophagitis. Am J Gastroenterol 1997;92:22S–9S. [PubMed] [Google Scholar]
- 16.Castell DO, Richter JE, Robinson M, et al. Efficacy and safety of lansoprazole in the treatment of erosive reflux esophagitis. Am J Gastroenterol 1996;91:1749–57. [PubMed] [Google Scholar]
- 17.Dekkers CPM, Beker JA, Thjodleifsson B, et al. Double-blind, placebo-controlled comparison of rabeprazole 20 mg vs. omeprazole 20 mg in the treatment of erosive or ulcerative gastro-oesophageal reflux disease. Aliment Pharmacol Ther 1999;13:49–57. [DOI] [PubMed] [Google Scholar]
- 18.Bochenek WJ, the Pantoprazole GERD US Study Group. Pantoprazole heals erosive esophagitis (EE) more effectively and provides greater symptomatic relief than placebo or nizatidine in gastroesophageal reflux disease (GERD) patients [abstract]. Gastroenterology 1999;116:A125. [Google Scholar]
- 19.Mössner J, Hölscher AH, Herz R, et al. A double-blind study of pantoprazole and omeprazole in the treatment of reflux oesophagitis: a multicentre trial. Aliment Pharmacol Ther 1995;9:321–6. [DOI] [PubMed] [Google Scholar]
- 20.Corinaldesi R, Valentini M, Belaîche J, et al. Pantoprazole and omeprazole in the treatment of reflux oesophagitis: a European multicentre study. Aliment Pharmacol Ther 1995;9:667–71. [DOI] [PubMed] [Google Scholar]
- 21.Cremer M, Lambert R, Lamers CBHW, et al. A double-blind study of pantoprazole and ranitidine in treatment of acute duodenal ulcer. A multicenter trial. Dig Dis Sci 1995;40:1360–4. [DOI] [PubMed] [Google Scholar]
- 22.Brunner G, Luna P, et al. Pantoprazole IV—acid inhibitory treatment for upper GI-bleeding [abstract]. Gastroenterology 1996;110:A70. [Google Scholar]
- 23.Tanaka M, Maruoka A, Chijiiwa Y, et al. Endoscopic ultrasonographic evaluation of gastric ulcer healing on treatment with proton pump inhibitors versus H2-receptor antagonists. Scand J Gastroenterol 1994;29:1140–4. [DOI] [PubMed] [Google Scholar]
- 24.Metz DC, Pisegna JR, Ringham GL, et al. Prospective study of efficacy and safety of lansoprazole in Zollinger-Ellison syndrome. Dig Dis Sci 1993;38: 245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann M, Ehrlich A, Fuder H, et al. Equipotent inhibition of gastric acid secretion by equal doses of oral or intravenous pantoprazole. Aliment Pharmacol Ther 1998;12:1027–32. [DOI] [PubMed] [Google Scholar]
- 26.Metz DC, Pratha V, Martin P, et al. Oral and intravenous dosage forms of pantoprazole are equivalent in their ability to suppress gastric acid secretion in patients with gastroesophageal reflux disease. Am J Gastroenterol 2000;95: 626–33. [DOI] [PubMed] [Google Scholar]
- 27.Fumagalli I, Klein M, Fischer R, et al. Comparison of intravenous with oral pantoprazole in symptom relief and healing of reflux esophagitis [abstract]. Gastroenterology 1998;114:A126. [Google Scholar]
- 28.Metz DC, Forsmark CE, Soffer E, et al. Zollinger-Ellison Syndrome (ZES) patients can replace oral proton pump inhibitors (PPIs) with intravenous (IV) pantoprazole (PANTO) without losing control of acid output (AO) [abstract]. Gastroenterology 2000;118:A1275. [Google Scholar]
- 29.Lind T, Aadland E, Eriksson S, et al. Beneficial effects of I.V. omeprazole (OME) in patients with peptic ulcer bleeding (PUB) [abstract]. Gastroenterology 1995;108:A150. [Google Scholar]
- 30.Labenz J, Peitz U, Leusing C, et al. Efficacy of primed infusions with high dose ranitidine and omeprazole to maintain high intragastric pH in patients with peptic ulcer bleeding: a prospective randomised controlled study. Gut 1997;40:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunner G, Luna P, Hartmann M, et al. Optimizing the intragastric pH as a supportive therapy in upper GI bleeding. Yale J Biol Med 1996;69:225–31. [PMC free article] [PubMed] [Google Scholar]
- 32.Cruickshank RH, Morrison DA, Bamber PA, et al. Effect of I.V. omeprazole on the pH and volume of gastric contents before surgery. Br J Anaesth 1989;63: 536–40. [DOI] [PubMed] [Google Scholar]
- 33.Rocke DA, Rout CC, Gouws E. Intravenous administration of the proton pump inhibitor omeprazole reduces the risk of acid aspiration at emergency cesarean section. Anesth Analg 1994;78:1093–8. [DOI] [PubMed] [Google Scholar]
- 34.Newton M, Kamm MA, Quigley T, et al. Symptomatic gastroesophageal reflux in acutely hospitalized patients. Dig Dis Sci 1999;44:140–8. [DOI] [PubMed] [Google Scholar]
- 35.McFadden ER Jr. Asthma. In: Fauci AS, Braunwald E, Isselbacher KJ, et al. , eds. Harrison’s principles of internal medicine, 14th ed. New York, NY: McGraw-Hill, 1998:1419–26. [Google Scholar]
- 36.Boushey HA. Bronchodilators and other agents used in asthma. In: Katzung BG, ed. Basic & clinical pharmacology, 7th ed. Stamford, CT: Appleton & Lange, 1998:325–42. [Google Scholar]
- 37.Schubert ML. Pharmacotherapy for acid/peptic disorders. Yale J Biol Med 1996; 69:197–201. [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy DM, Hyman PE. Effect of isopropamide on response to oral cimetidine in patients with Zollinger-Ellison syndrome. Dig Dis Sci 1982;27:353–9. [DOI] [PubMed] [Google Scholar]
- 39.Blum RA. Lansoprazole and omeprazole in the treatment of acid peptic disorders. Am J Health-Syst Pharm 1996;53:1401–15. [DOI] [PubMed] [Google Scholar]
- 40.Hirschowitz BI. Zollinger-Ellison syndrome: pathogenesis, diagnosis, and management. Am J Gastroenterol 1997;92(suppl):44S–8S. [PubMed] [Google Scholar]
- 41.Gubbins PO, Bertch KE. Drug absorption in gastrointestinal disease and surgery. Pharmacotherapy 1989;9:285–95. [DOI] [PubMed] [Google Scholar]
- 42.Altman DF. Drugs used in gastrointestinal diseases. In: Katzung BG, ed. Basic & clinical pharmacology, 7th ed. Stamford, CT: Appleton & Lange, 1998:1017–29. [Google Scholar]
- 43.Nishina K, Mikawa K, Maekawa N, et al. A comparison of lansoprazole, omeprazole, and ranitidine for reducing preoperative gastric secretion in adult patients undergoing elective surgery. Anesth Analg 1996;82:832–6. [DOI] [PubMed] [Google Scholar]
- 44.Wilcox CM, Spenney JG. Stress ulcer prophylaxis in medical patients: Who, what, and how much? Am J Gastroenterol 1988;83:1199–211. [PubMed] [Google Scholar]
- 45.Lu WY, Rhoney DH, Boling WB, et al. A review of stress ulcer prophylaxis in the neurosurgical intensive care unit. Neurosurgery 1997;41:416–25. [DOI] [PubMed] [Google Scholar]
- 46.Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA 1996;275:308–14. [PubMed] [Google Scholar]
- 47.Lasky MR, Metzler MH, Phillips JO. A prospective study of omeprazole suspension to prevent clinically significant gastrointestinal bleeding from stress ulcers in mechanically ventilated trauma patients. J Trauma 1998;44:527–33. [DOI] [PubMed] [Google Scholar]
- 48.Lin HJ, Lo WC, Lee FY, et al. A prospective randomized comparative trial showing that omeprazole prevents rebleeding in patients with bleeding peptic ulcer after successful endoscopic therapy. Arch Intern Med 1998;158:54–8. [DOI] [PubMed] [Google Scholar]
- 49.Aoki T Intravenous administration of lansoprazole: a preliminary study of dose ranging and efficacy in upper gastrointestinal bleeding. Aliment Pharmacol Ther 1995;9(suppl 1):51–7. [DOI] [PubMed] [Google Scholar]
- 50.Phillips JO, Metzler MH, Palmieri TL, et al. A prospective study of simplified omeprazole suspension for the prophylaxis of stress-related mucosal damage. Crit Care Med 1996;24:1793–800. [DOI] [PubMed] [Google Scholar]
- 51.Hasselgren G, Keelan M, Kirdeikis P, et al. Optimization of acid suppression for patients with peptic ulcer bleeding: an intragastric pH-metry study with omeprazole. Eur J Gastroenterol Hepatol 1998;10:601–6. [DOI] [PubMed] [Google Scholar]
- 52.Brunner GH, Thiesemann C. The potential clinical role of intravenous omeprazole. Digestion 1992;51(suppl 1):17–20. [DOI] [PubMed] [Google Scholar]
- 53.Fass R, Fennerty MB, Johnson C, et al. Correlation ambulatory 24 hour esophageal pH monitoring results with symptom improvement in patients with non-cardiac chest pain due to gastroesophageal reflux disease. J Clin Gastroenterol 1999;28:36–9. [DOI] [PubMed] [Google Scholar]
- 54.Mujica VR, Rao SSC. Recognizing atypical manifestations of GERD. Asthma, chest pain, and otolaryngologic disorders may be due to reflux. Postgrad Med 1999;105:53–66. [DOI] [PubMed] [Google Scholar]
- 55.Fass R, Fennerty MB, Ofman JJ, et al. The clinical and economic value of a short course of omeprazole in patients with noncardiac chest pain. Gastroenterology 1998;115:42–9. [DOI] [PubMed] [Google Scholar]
- 56.Wurzer H, Schütze K, Bethke T, et al. Efficacy and safety of pantoprazole in patients with gastroesophageal reflux disease using an intravenous-oral regimen. Hepatogastroenterology 1999;46:1809–15. [PubMed] [Google Scholar]
- 57.Metz DC, Pratha V, Martin P, et al. Oral and intravenous dosage forms of pantoprazole are equivalent in their ability to suppress gastric acid secretion in patients with gastroesophageal reflux disease. Am J Gastroenterol 2000;95: 626–33. [DOI] [PubMed] [Google Scholar]
- 58.Simon B, Müller P, Hartmann M, et al. Pentagastrin-stimulated gastric acid secretion and pharmacokinetics following single and repeated intravenous administration of the gastric H+, K+-ATPase-inhibitor pantoprazole (BY1023/ SK&F96022) in healthy volunteers. Z Gastroenterol 1990;28:443–7. [PubMed] [Google Scholar]
- 59.Vinayek R, Frucht R, London JF, et al. Intravenous omeprazole in patients with Zollinger-Ellison syndrome undergoing surgery. Gastroenterology 1990;99:10–6. [DOI] [PubMed] [Google Scholar]
- 60.Lau JYW, Sung JJY, Lee KKC, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med 2000;343:310–6. [DOI] [PubMed] [Google Scholar]
- 61.Steinijans VW, Huber R, Hartmann M, et al. Lack of pantoprazole drug interactions in man: an updated review. Int J Clin Pharmacol Ther 1996;34:243–62. [PubMed] [Google Scholar]
- 62.Otani Y, Kitajima M, Sugiyama M, et al. Inhibitory effects of intravenous lansoprazole on gastric acid hypersecretion in patients with postoperative stress. J Clin Gastroenterol 1995;20(suppl 2):S22–6. [DOI] [PubMed] [Google Scholar]
- 63.Plein K, Holtz J. Clinical efficacy and safety of an intravenous (IV) pantoprazole formulation in erosive reflux esophagitis [abstract]. Gastroenterology 1997;112: A226. [Google Scholar]