Capsule Summary:
This study demonstrates that the MAR-1 antibody to mouse FcepsilonRI unexpectedly binds to two Fcgamma receptors, raising concerns regarding numerous studies that used this antibody to assess the distribution and function of FcepsilonRI+ cells.
Keywords: Basophil, Dendritic Cell, Fc receptor, Fc epsilon RI, Fc gamma RI, Fc gamma RIV, IgE, Macrophage, Mast cell, Monocyte, Neutrophil
To the Editor:
IgE is a primary driver of allergic symptoms mediated by cells expressing the high affinity Fc receptor for IgE, FcεRI. The cell types expressing FcεRI and downstream pathways have been extensively studied in mouse models of allergic disease. The MAR-1 monoclonal antibody is thought to specifically recognize the alpha chain of mouse FcεRI. Although FcεRI was initially thought to be uniquely expressed by basophils and mast cells in mice,1 several recent studies using the MAR-1 antibody for flow cytometric analysis reported that FcεRI is expressed on dendritic cells, particularly monocyte-derived dendritic cells (moDCs), in various inflammatory conditions, such as exposure to house dust mite, the bacterial cell wall component lipopolysaccharide (LPS) or Sendai virus.2–4 The MAR-1 antibody has also been injected into mice for numerous functional studies, including depletion of basophils5 and rapid desensitization.6 Surprisingly, several results obtained with MAR-1-mediated basophil depletion have not been recapitulated in genetic models of basophil depletion5. These unexpected findings based on MAR-1 prompted us to reexamine the specificity of this antibody.
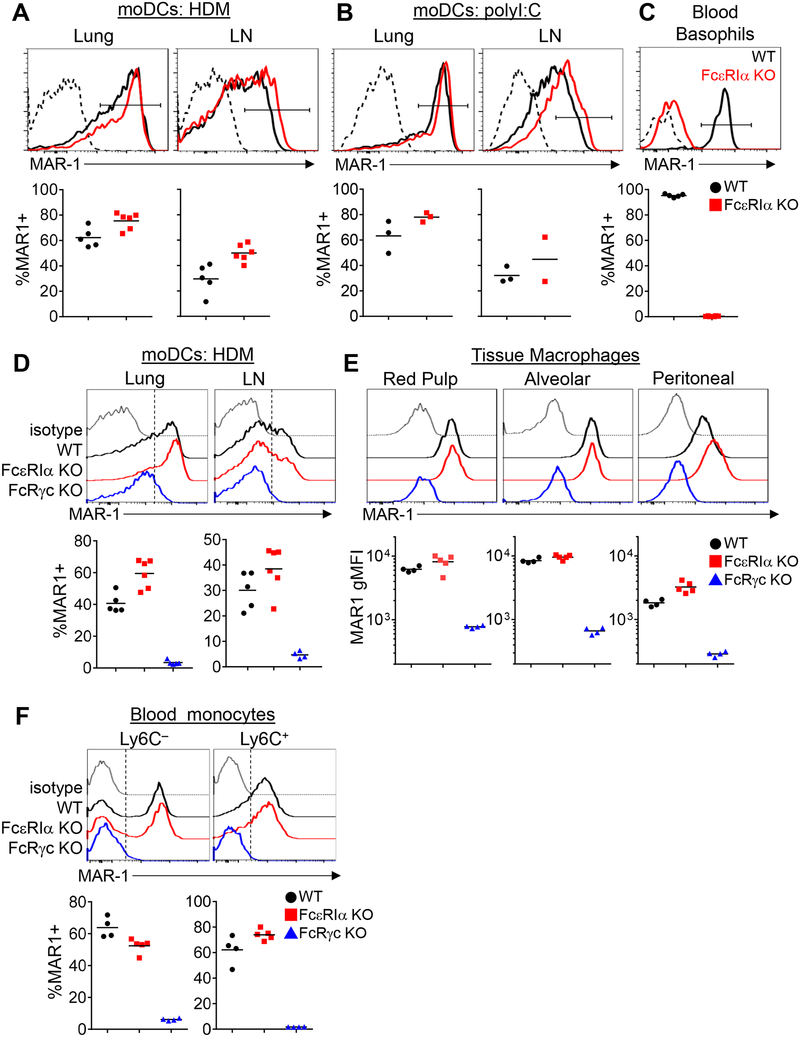
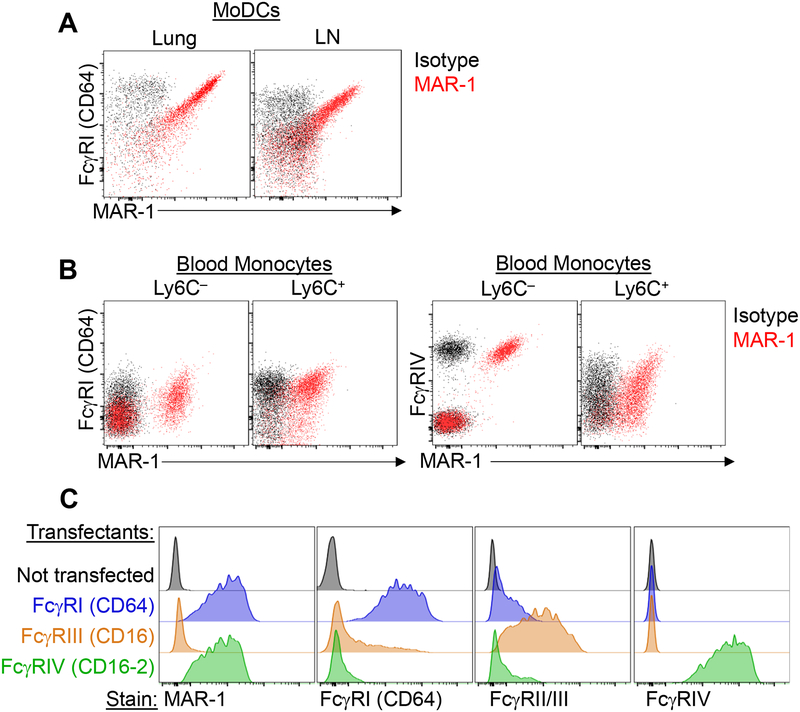
We first sought to clarify whether moDCs express FcεRI under inflammatory conditions. In mice treated intranasally with house dust mite extract (HDM) and examined 3 days later by flow cytometric analysis using the MAR-1 antibody, we were able to identify FcεRIα+ CD11c+ MHC-II+ CD11b+ inflammatory moDCs in the lungs and mediastinal lymph node (mLN), but we were surprised to find that these apparent FcεRIα+ moDCs were also present in FcεRIα-deficient mice (Fcer1a–/–, hereafter denoted as FcεRIα KO) (Fig 1A). Since the MAR-1 antibody labeled these cells in the absence of FcεRIα expression, we reasoned it would be more appropriate to call these MAR-1+ moDCs. As MAR-1+ moDCs have also been reported in viral infection3, we next tested MAR-1 staining of moDCs in this setting. To mimic viral infection, we administered intranasal poly I:C, a TLR3 ligand, and also detected MAR-1+ moDCs in the lungs. Similar to HDM exposure, we found that MAR-1+ moDCs could also be identified in poly I:C-treated FcεRIα KO mice similar to control mice (Fig 1B). In contrast, blood basophils only stained with MAR-1 from control mice but not from FcεRIα KO mice (Fig 1C), confirming the genotype of the FcεRIα KO mice. We next considered whether the MAR-1 antibody may bind to other Fc receptors and stained cells from mice lacking the Fc receptor common gamma chain (Fcer1g–/–, hereafter denoted as FcRγc KO), which is necessary for the normal surface expression of all activating Fc receptors. In FcRγc KO mice, MAR-1 staining was greatly diminished on moDCs from the lungs and mLN after HDM-treatment (Fig 1D). Macrophages and monocytes express various Fcγ receptors but are not known to express FcεRI in mice, yet one study had noted MAR-1 staining on monocytes.6 We tested whether MAR-1 would stain splenic red pulp macrophages, lung alveolar macrophages, peritoneal macrophages and blood monocytes. Indeed, MAR-1 stained all of these macrophages (Fig 1E) and a subset of blood Ly6C– and Ly6C+ monocytes in both wild-type and FcεRIα KO mice (Fig 1F). Similar to moDCs, MAR-1 staining was greatly reduced in these populations in FcRγc KO mice. These observations imply that MAR-1 may be cross-reacting with other Fc receptors, thereby resulting in the detection of MAR-1+ cells in FcεRIα KO mice. As our findings above suggested that MAR-1 binds one of the activating Fcγ receptors, we next attempted to identify which Fc receptor(s) MAR-1 may bind to. In mice, the activating Fc receptors that bind IgG are FcγRI (CD64), FcγRIII (CD16) and FcγRIV (CD16–2). We noticed that MAR-1 staining strongly correlated with FcγRI (CD64) staining on moDCs (Fig 2A), and that MAR-1 staining correlated with FcγRIV (CD16–2) staining on Ly6C– blood monocytes (Fig 2B). To definitively test whether MAR-1 was cross-reacting with FcγRI (CD64) and FcγRIV (CD16–2), we expressed specific Fc gamma receptors in a cell line and then stained with MAR-1 versus other Fc gamma receptor specific antibodies. Specifically, the Phoenix cell line, a modified 293T human embryonic kidney cell line, was individually transfected with the alpha chains of FcγRI, FcγRIII or FcγRIV together with the Fc receptor common gamma chain, and then stained with antibodies. Untransfected cells did not stain with MAR-1 or any of the activating Fc gamma receptor antibodies, as expected, confirming that this human cell line lacks endogenous reactivity with these mouse reagents (Fig 2C). In support of our observations in monocytes and macrophages, Phoenix cells transfected individually with FcγRI or FcγRIV stained with the MAR-1 antibody (Fig 2C). In contrast, MAR-1 did not stain FcγRIII-transfected cells (Fig 2C), demonstrating that MAR-1 binds to the alpha chains of FcγRI and FcγRIV, but not the shared Fc receptor common gamma chain.
Figure 1:
The MAR-1 antibody stains cells from FcεRIα KO mice but not from FcRγc KO mice. Flow cytometric analysis of MAR-1 staining; representative flow cytometric plots and summary graphs are shown. (A, B) MAR-1 staining on moDCs from the lung and mediastinal lymph node (LN) 3 d after intranasal HDM (A) or polyI:C (B) exposure in wild-type (WT, black) and FcεRIα KO (red) mice. (C) MAR-1 staining on blood basophils to confirm FcεRIα KO genotype. (D) MAR-1 staining on moDCs in the lung and mediastinal LN 3 d after HDM exposure in FcεRIα KO (red) and FcRγc KO mice (blue) compared to WT (black). (E) MAR-1 staining on splenic red pulp macrophages, alveolar macrophages and peritoneal macrophages from untreated mice of the indicated genotypes. (F) MAR-1 staining on blood monocytes from untreated mice. In histograms, dashed lines in A-C and gray lines in D-F denote isotype control staining (“isotype”). Data shown are representative of at least two independent experiments. In summary graphs, each data point represents one mouse, bars indicate the mean. The gating strategies to identify the represented populations of moDCs, basophils, macrophages and monocytes are shown in Fig E1.
Figure 2:
MAR-1 cross reacts with FcγRI and FcγRIV. (A) Representative plots showing flow cytometric analysis of MAR-1 and FcγRI (CD64) co-staining on moDCs in lung and mediastinal LN 3 d after HDM exposure. (B) Representative plots showing flow cytometric analysis of MAR-1 co-staining with FcγRI or FcγRIV on blood monocytes. For (A) and (B), black dots denote isotype control (“Isotype”) staining whereas red dots denote MAR-1 staining. The gating strategy to identify moDCs and monocytes is shown in Fig E1. (C) Phoenix cells were transfected individually with the indicated Fc receptors along with a YFP reporter gene. Transfected cells were gated by YFP expression and stained with MAR-1 or antibodies to FcγRI, FcγRII/III (CD16/32 clone 2.4G2) or FcγRIV (CD16–2), as indicated. Data shown are representative of at least 3 independent experiments.
We next further confirmed that the MAR-1 staining on myeloid cells in vivo, in the absence of FcγRI, was due to binding to FcγRI and FcγRIV. We analyzed splenocytes from mice deficient in the α chains of all four FcγR (FcγRα 4 KO) which showed an absence of MAR-1 staining on splenic red pulp macrophages and monocytes (Fig E2). Neutrophils, which are known to express FcγRIV7, also stained weakly with MAR-1 in control but not in FcγRα 4 KO mice (Fig E2). Some residual surface FcγRIV expression was also noted in the FcRexpression was also noted c KO mice compared with the FcγRα 4 KO mice (Fig E2), providing a potential explanation for the weak MAR-1 staining observed on some cell populations in the FcRγc KO mice. While our findings do not formally exclude the possibility that MAR-1 may bind additional receptors, taken together, our data indicate that MAR-1 binding to FcγRI and FcγRIV accounts for most of the MAR-1 staining on macrophages, monocytes, and neutrophils. Similar MAR-1 staining was observed on splenocytes from wild-type B6 and BALB/c mice, indicating our findings are broadly applicable (Fig E3). We conclude that MAR-1 antibody, which was thought to specifically recognize FcεRIα, in fact cross-reacts with FcγRI and FcγRIV. As a result, MAR-1 staining is specific for FcεRIα only on mast cells and basophils, whereas MAR-1 staining on dendritic cells, macrophages, monocytes, and neutrophils is due to FcγR expression. Contrary to prior reports,2–4 our results indicate that the MAR-1+ moDCs observed in inflammatory conditions do not express FcεRI. Therefore, normal mouse moDCs do not model the FcεRI expression by human DCs in allergic disease. However, some monocytes and macrophages that stain with MAR-1 express FcγRIV instead of FcεRI. Interestingly, FcγRIV is a low affinity receptor for IgE that binds to IgE-immune complexes.7 Engagement of FcγRIV by IgE-immune complexes on monocytes/macrophages may contribute to local inflammation.7
Early structural studies of rodent FcεRI demonstrated that surface expression could only be achieved when the alpha, beta and gamma chains were all transfected.1 Unlike rodents, in humans the beta chain is not necessary for FcεRI surface expression, and a trimeric form of FcεRI, consisting of one alpha chain and two gamma chains, is expressed on human dendritic cells and monocytes.1 Some recent studies have suggested that a trimeric form of mouse FcεRI lacking the beta chain may be naturally induced during inflammation, as a functional equivalent to human trimeric FcεRI.3, 8 However, in these studies, MAR-1 was used for co-immunoprecipitation of FcεRIα, which, based on our findings above, would likely have instead captured FcγRIα or FcγRIVα complexed with the shared FcRγ chain. Two mouse lines have been engineered to express human FcεRIα in DCs, which can form a trimeric receptor with mouse FcRγ chains, representing a potentially better model system for studies of IgE-mediated activation and antigen uptake.1, 9
Our findings also raise the possibility that the injection of the MAR-1 antibody into mice, as in the aforementioned basophil depletion studies, could have significant off-target effects. Hammad et al. reported that MAR-1 depleted certain populations of dendritic cells.4 A technical caveat, however, is that the authors used the same MAR-1 antibody to both deplete and detect cells, and thus the lack of MAR-1 staining may have been due to receptor occupancy. Another study noted that MAR-1 depleted a subpopulation of splenic dendritic cells that were CD11clo CD11b+ IgE+ and MAR-1+ 6; however, our flow cytometric analysis suggested that these cells were in fact splenic basophils, which express this set of markers (data not shown). In our own experiments, MAR-1 antibody administration did not significantly deplete known macrophage, monocyte, or moDC subsets (Fig E4). Nevertheless, it seems likely that injection of the MAR-1 antibody would cross-link activating Fcγ receptors on these and other myeloid cells, thereby potentially modifying immune responses. We therefore caution investigators in the use of the MAR-1 antibody, and we suggest that in future investigations, FcεRIα KO mice should be used as a critical control.
Supplementary Material
Acknowledgments
We thank F. Nimmerjahn for generously providing the Fc receptor constructs, J. Ravetch and P. Smith for generously providing tissues from FcγRα 4 KO mice, A. Greer and J.-S. Shin for contributions to a preliminary experiment and providing FcεRIα KO mice, A. Basbaum for providing FcRγc KO mice, C. Sokol for helpful advice, and C. Cho and Z. Yang for technical assistance.
This work was supported by the National Heart, Lung, And Blood Institute (Award Number DP2HL117752) and the National Institute of Allergy and Infectious Diseases (Award Numbers R01AI103146 and R21AI130495) of the National Institutes of Health, as well as the Cardiovascular Research Institute and the Sandler Asthma Basic Research Center at the University of California, San Francisco. Xin-Zi Tang was partially funded by the National Science Scholarship awarded by the Agency for Science, Technology and Research, Singapore. C.D.C.A. is a Pew Scholar in the Biomedical Sciences, supported by The Pew Charitable Trusts. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflicts of interest to report.
References
- 1.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol 1999; 17:931–72. [DOI] [PubMed] [Google Scholar]
- 2.Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, et al. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity 2016; 45:669–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grayson MH, Cheung D, Rohlfing MM, Kitchens R, Spiegel DE, Tucker J, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med 2007; 204:2759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med 2010; 207:2097–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voehringer D Basophils in allergic immune responses. Curr Opin Immunol 2011; 23:789–93. [DOI] [PubMed] [Google Scholar]
- 6.Khodoun MV, Kucuk ZY, Strait RT, Krishnamurthy D, Janek K, Lewkowich I, et al. Rapid polyclonal desensitization with antibodies to IgE and FcepsilonRIalpha. J Allergy Clin Immunol 2013; 131:1555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancardi DA, Iannascoli B, Hoos S, England P, Daeron M, Bruhns P. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J Clin Invest 2008; 118:3738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porcherie A, Mathieu C, Peronet R, Schneider E, Claver J, Commere PH, et al. Critical role of the neutrophil-associated high-affinity receptor for IgE in the pathogenesis of experimental cerebral malaria. J Exp Med 2011; 208:2225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platzer B, Baker K, Vera MP, Singer K, Panduro M, Lexmond WS, et al. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol 2015; 8:516–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.