TO THE EDITOR:
Prader–Willi syndrome (PWS) is a classical genomic imprinting disorder characterized by hypotonia, hypogonadism, hyperphagia, central obesity, short stature, small hands and feet and learning/behavioral problems due to a paternal deletion of chromosome 15q11–q13 region (about 70% of cases), maternal disomy 15 (UPD) (about 25%), or imprinting defects or translocations involving chromosome 15 (about 5%) [Butler, 1990; Bittel and Butler, 2005; Cassidy and Driscoll, 2009]. Several of these characteristics are consistent with endocrine and/or metabolic abnormalities involving the hypothalamic-pituitary axis. Similarly, findings of daytime drowsiness, obesity, sleep apnea and possibly scoliosis which are frequently seen in PWS may be linked to melatonin production and function [Bartness et al., 2002; Azeddine et al., 2007]. Two earlier studies reported normal melatonin levels in PWS subjects; however, the studies were limited by sample size, children only, and lack of genetic subtype information [Tamarkin et al., 1982; Willig et al., 1986].
Melatonin is secreted by the pineal gland in synchronization to light/dark cycles achieving maximum plasma concentrations of approximately 200 pg/ml at night and lower daytime levels of approximately 10 pg/ml [Cavallo, 1992; de Leersnyder et al., 2001]. Melatonin is produced from tryptophan with serotonin as an intermediate compound while high dietary tryptophan can elevate plasma levels. It can affect the autonomic regulation of the cardiovascular system, regulation of the hypothalamic–pituitary–gonadal axis, circadian rhythms and wake-sleep cycles [Cavallo, 1993; Commentz and Helmke, 1995; Nishiyama et al., 2001]. Melatonin levels are linked to energy expenditure and body mass regulation [Bartness et al., 2002] with obese pubertal males having an overall increase in melatonin production and release [Fideleff et al., 2006].
Because several PWS features could be influenced by melatonin, morning plasma melatonin levels were measured in 53 PWS subjects (25 males and 28 females) with a mean age of 23.6 years (range 10–50 years). All blood samples were drawn in the clinic setting under standard room lighting conditions. About 60% of the blood samples were collected between 7 and 8 AM and the remaining subjects between 8 and 11 AM with an average time of 8 AM. Ninety percent of the subjects were Caucasian. Age, obesity status, gender, genetic subtype, history of daytime drowsiness, sleep apnea, scoliosis, bone density measures, and diabetes status were recorded as well as any use of selective serotonin reuptake inhibitors (SSRIs), insulin and/or growth hormone. All subjects had genetic testing including DNA methylation, cytogenetic analysis with FISH and/or genotyping of informative markers from the 15q11–q13 region to identify the typical deletion type (type I or type II) or maternal disomy (UPD) as previously described [Bittel and Butler, 2005].
No PWS subjects were on thyroid or growth hormone, but four males were on testosterone and 12 were currently on SSRIs. Ten subjects were diabetic and four were on insulin. No PWS individuals were taking melatonin or dietary supplements with tryptophan. Body mass index (BMI) was calculated (kg/m2) for each subject and obesity defined as BMI >30 for adults (>18 years) and a BMI >95% using published standards for sex for subjects less than 18 years [Kuczmarski et al., 2000]. Demographic data and melatonin levels are summarized in Table I. Daytime drowsiness and sleep apnea were determined by the use of questionnaire forms and history at the time of enrollment and sleep apnea verified by reviewing medical records including sleep assessment studies. Bone density measurements were determined by dual X-ray absorptiometry (DEXA) scans at the time of blood collection and scoliosis determined by physical examination and inspection of DEXA scans as previously described [Butler et al., 2001].
TABLE I.
Demographic Data and Melatonin Levels in all Prader–Willi Syndrome (PWS) Subjects
| PWS total | PWS deletion type I | PWS deletion type II | PWS UPD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | P-value |
| Age (years) | 53 | 23.5 ± 9.3 | 16 | 26.8 ± 9.9 | 18 | 21.4 ± 9.1 | 19 | 22.6 ± 8.7 | 0.23* |
| BMI (kg/m2) | 53 | 32.2 ± 8.7 | 8 | 36.2 ± 10.6 | 18 | 34.9 ± 7.8 | 19 | 31.8 ± 7.9 | 0.29* |
| Melatonin (pg/ml) | 53 | 21.8 ± 29.7 | 8 | 17.9 ± 26.6 | 18 | 19.3 ± 17.2 | 19 | 27.3 ± 40.3 | 0.62** |
Daytime melatonin levels (mean±SD = 23.09±23.2 pg/ml) reported in the literature for the age range in our subjects [Laakso et al., 1990; Cavallo, 1992; de Leersynder et al., 2001].
P-values calculated by using one-way ANOVA.
P-values calculated by using univariate analysis of variance among all three genetic subtype groups (deletion type I, deletion type II and UPD) adjusting for age and BMI.
Fasting plasma morning melatonin levels were determined by a double-antibody radioimmunoassay (RIA) using a melatonin direct RIA kit purchased from ALPCO Diagnostics (Salem, NH) following manufacturer’s instructions. Sample melatonin concentration in pg/ml was determined after developing a standardized curve based on known melatonin standards supplied in the kit. Two equal sample aliquots were counted and averaged. The intra- and inter-assay precision was 6.7% and 10.4%, respectively. The functional sensitivity of the assay was 1.3 pg/ml. Plasma melatonin levels collected between 7 and 11 AM with an average time of 8 AM were compared to our laboratory control subjects collected during the same time interval under the same standard room lighting conditions. Those reported in the literature at comparable ages and gender ratio also had blood collected in the morning during the same interval (e.g., 8 AM) [Laakso et al., 1990; Cavallo, 1992; de Leersnyder et al., 2001].
Experimental data (e.g., age and BMI) were analyzed by one-way ANOVA and parametric Pearsonian (r) or non-parametric Spear-man (rs) correlation coefficients calculated. The three genetic subgroups were analyzed by univariate analysis of variance with age correction. Male and female data were analyzed separately. Differences in melatonin concentration within genetic subtypes were examined by univariate analysis as a function of daytime drowsiness, sleep apnea, diabetic status, use of SSRIs or insulin, bone density data (e.g., total bone mineral density) as well as history of scoliosis. Statistical significance was set at 5% for all tests and determined using SPSS version 15 for Windows (SPSS, Inc. ChicagoIL).
For all PWS subjects (n =53), morning plasma melatonin levels ranged from 0.4 to 124 pg/ml with a mean value of 21.8 pg/ml, which was not significantly different compared with our available unaffected controls (four adult females and two adult males) and radioimmunoassay reference data (mean value of 23.9 pg/ml) reported in the literature on similarly aged controls [Laakso et al., 1990; Cavallo, 1992; de Leersnyder et al., 2001]. The highest level (124 pg/ml) was found in an 18-year-old PWS female with UPD who was taking an SSRI (fluvoxamine) for the past 3 years. The lowest level was reported from a 27-year-old PWS male with a type I deletion. The lowest overall mean melatonin concentration(17.9 pg/ml) occurred in PWS subjects with the type I deletion while the largest mean melatonin concentration (27.3 pg/ml) was found in PWS individuals with UPD. The majority of samples (e.g., 60%) were collected between 7 and 8 AM for both the PWS and comparison subjects and no difference in melatonin levels were observed in those samples collected later than 8 AM, but before 11 AM.
For all PWS females(n = 28), melatonin levels ranged from 1.0 to 124 pg/ml. For females with type I deletion (n = 8), melatonin values ranged from 1.3 to 88 pg/ml with one female above the reported morning reference range while seven females were within normal range (i.e., 0.7–47 pg/ml) reported in the literature [Laakso et al., 1990; Cavallo, 1992; de Leersnyder et al., 2001]. In PWS females with type II deletions, values ranged from 1.0 to 46 pg/ml. All of the 11 PWS females with type II deletions had melatonin levels in the reported reference range (mean ± SD = 23.9 ± 23.2 pg/ml). In the PWS female UPD group (n = 9), there were six females with expected levels and three with elevated levels (range 96–124 pg/ml).
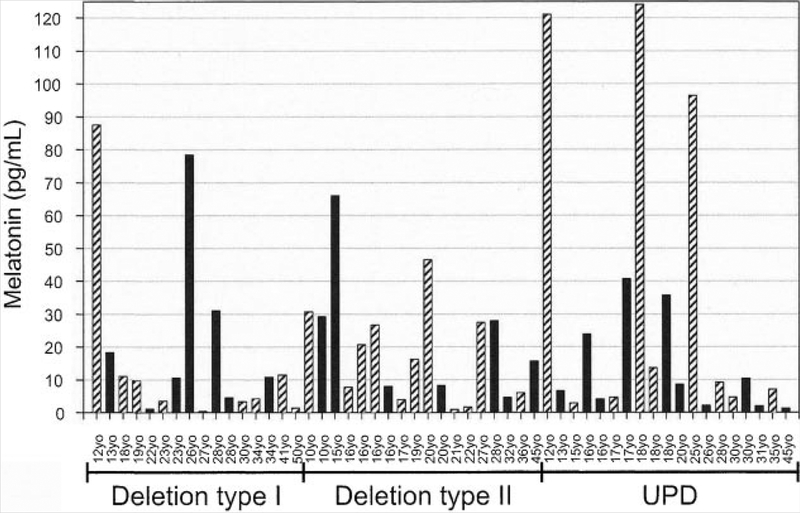
In all PWS males (n = 25), morning melatonin levels ranged from 0.4 to 78 pg/ml. In the 8 PWS males with type I deletions (range 0.4–78 pg/ml), one male had levels below the reference range, one above and six within the expected reference range. In seven PWS males with type II deletions, melatonin ranged from 4.5 to 29 pg/ml, all within the expected reference range. In the 10 PWS males with UPD, the range was 1.3–41 pg/ml which was within the expected reference range. These data are summarized in Figure 1.
FIG. 1.
Histogram showing daytime plasma melatonin levels (pg/ml) for subjects with Prader–Willi syndrome according to age, gender and genetic subtype. Solid bars represent males and diagonal bars represent females.
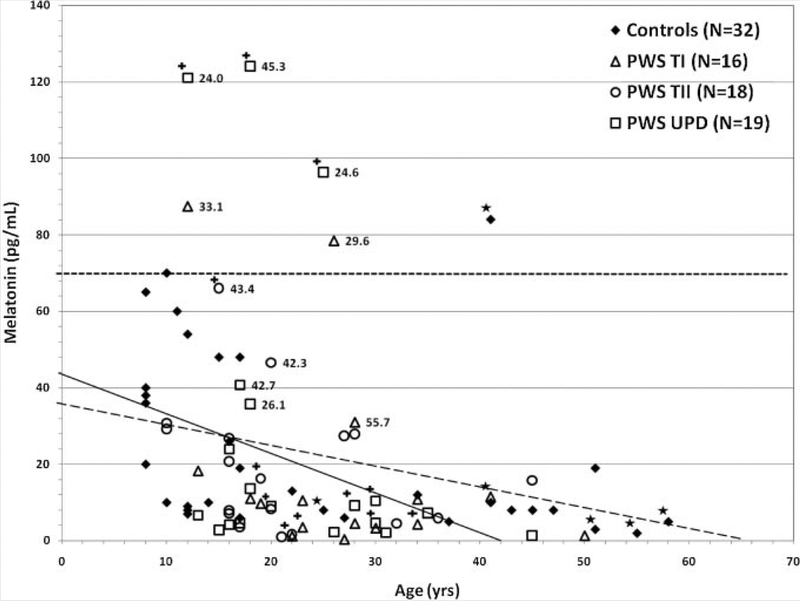
Plasma melatonin concentration was inversely correlated with age in both PWS (r = 0.32, P = 0.02) and control (r = 0.41, P = 0.02) subject groups (see Fig. 2). In addition, baseline total bone mineral content and density identified with DEXA scans were inversely correlated with melatonin levels (r = −0.62,P = 0.04 and r= − 0.002, P = 0.002 respectively) in all PWS males and females, with type I deletions. In all PWS males and females with UPD, melatonin levels were positively correlated (r = 0.59, P = 0.02) with a history of sleep apnea which was recorded in 28% of our PWS subjects; however, two with sleep apnea had the highest melatonin values (i.e., 121 and 124 pg/ml) and both were on SSRIs. However, there was no significant association between BMI and melatonin concentration but univariate analysis did show a significant increase (P = 0.03) in melatonin levels in PWS males and females with UPD who were taking SSRIs. No significant relationships were found with other variables including daytime drowsiness which was seen in 81% of our PWS subjects, scoliosis (25%), insulin use (8%) or diabetic status (19%) in our study.
FIG. 2.
Scatterplot of morning plasma melatonin levels versus age for subjects with Prader–Willi syndrome and controls. Open symbols represent subjects with Prader–Willi syndrome classified by genetic subtype. The closed symbol represents comparison subjects taken from the literature [Laakso et al., 1990; Cavallo, 1992; de Leersynder et al., 2001]and our six control subjects represented by (*) analyzed in our laboratory at the same time as the subjects with Prader–Willi syndrome. All measurements were made by radioimmunoassay on morning fasting plasma samples. The mean ± SD 21.8 ± 29.7 pg/ml (range = 0.4–124 pg/ml; r = 0.316, P = 0.021) for PWS subjects (N = 53) and mean ± SD = 23.9 ± 23.2 pg/ml (range = 1.4–84 pg/ml; r = 0.414,P = 0.018) for control subjects (N 32). The PWS subjects designated with (+) represent those individuals on SSRI medication. The numbers next to the PWS symbol represent the BMI measurements for those PWS subjects with the top 10 melatonin levels. The dash line represents the correlation for the control subjects and the solid line represents the correlation for the PWS subjects. The upper broken line represents the upper limits (+ 2 SD) of the melatonin range (70 pg/ml).
Melatonin is a ubiquitous molecule linked to numerous physiological processes including regulation of time keeping functions, immune regulation, energy expenditure, sexual maturation and bone metabolism [Pandi-Perumal et al., 2006]. The physiological actions of melatonin suggest a possible role in the observed phenotype of Prader–Willi syndrome characterized by hypogonadism, central obesity and behavioral problems. However, melatonin levels were found to be inversely correlated with bone mineral content and total bone mineral density as measured by DEXA scans in 11 PWS subjects with type I deletion. Butler et al. [2001] and others [Vestergaard et al., 2004] have shown that bone mineral density in PWS subjects is low compared to control subjects matched for age, sex and BMI. The decreased bone mineral density and lack of development of bone mass may be attributed to decreased muscle mass, hypotonia and sex steroid deficiency. Koyama et al. [2002] also reported that melatonin increases bone mineral density through inhibition of bone resorption. However, data from our PWS subjects with type I deletions implies an opposite association in that higher melatonin levels were associated with lower bone mineral density. This finding may suggest that in PWS the signaling pathway for melatonin to increase bone density is altered, perhaps through the melatonin receptor complex.
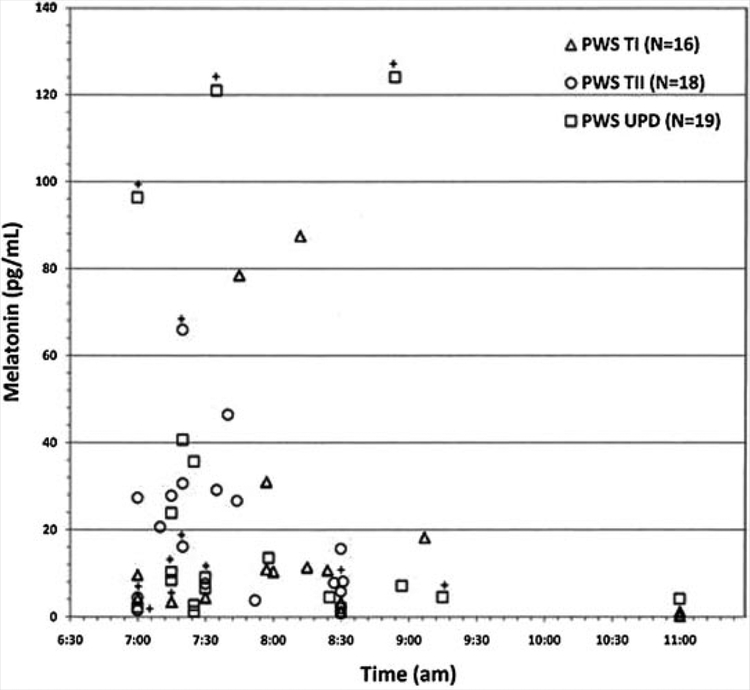
The finding of significant elevations of morning melatonin levels (P = 0.03) in PWS subjects with UPD taking SSRIs medications at the time of study compared to those not on SSRIs is not surprising. Using morning melatonin levels (mean ± SD =23.9 ± 23.2 pg/ml) calculated in the literature from control subjects using the same radioimmunoassay methodology and collection time (average time of 8 AM) [Laakso et al., 1990; Cavallo, 1992; de Leersnyder et al., 2001] with an upper cut-off level (± 2 SD) of 70 pg/ml, then five of the 53 PWS subjects and two of the 32 control subjects showed levels above the upper cut-off level (see Figs. 2 and 3). The control subject with the highest level (84 pg/ml) was a healthy 41-year-old white female with a BMI of 24. She was taking no medications and blood collection occurred at 11 AM. Interestingly, three of the five PWS subjects with the highest melatonin levels were on SSRIs at the time of blood collection which is in agreement with the literature, that is fluvoxamine (an SSRI) significantly increases melatonin levels in healthy individuals [von Bahr et al., 2000]. Six (32%) of the 19 PWS subjects with UPD and six (18%) of the 34 PWS subjects with deletions were on SSRIs. PWS individuals with type I or II deletions taking SSRIs showed higher melatonin levels, as well, but not statistically significantly different.
FIG. 3.
Scatterplot of morning melatonin levels versus time of blood collection. Open symbols represent subjects with Prader–Willi syndrome classified by genetic subtype. The PWS subjects designated with (+) represent those individuals on SSRI medications. The upper limits of normal for the melatonin range is 70 pg/ml.
Given that melatonin levels are circadian and peak during the night in normal individuals, a single sample in the morning may not be adequate for those who may have an unusual rhythm, especially, if subjects with PWS have abnormal 24 hr patterns compared with control subjects. However, as illustrated in the scatterplot of melatonin levels with time of blood collection, there was no trend for increased melatonin levels in those PWS subjects with blood samples collected later in the morning.
In conclusion, there was no evidence that single measures of fasting morning melatonin levels in PWS were significantly different from morning measures seen in individuals within the general population reported in the literature or measured in our laboratory setting. Higher levels were noted in PWS subjects with UPD particularly those taking SSRI medications. There was no increase in melatonin levels seen in PWS subjects with blood collections later in the morning further indicating an apparent normal circadian rhythm in this syndrome using methods described in this study.
REFERENCES
- Azeddine B, Letellier K, Wang da S, Moldovan F, Moreau A. 2007. Molecular determinants of melatonin signaling dysfunction in adolescent idiopathic scoliosis. Clin Orthop Relat Res 462:42–52. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Demas GE, Song CK. 2002. Seasonal changes in adiposity: The roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med (Maywood) 227:363–376. [DOI] [PubMed] [Google Scholar]
- Bittel DC, Butler MG. 2005. Prader–Willi syndrome: Clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med 7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. 1990. Prader–Willi syndrome: Current understanding of cause and diagnosis. Am J Med Genet 35:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Haber L, Mernaugh R, Carlson MG, Price R, Feurer ID. 2001. Decreased bone mineral density in Prader–Willi syndrome: Comparison with obese subjects. Am J Med Genet 103:216–222. [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, Driscoll DJ. 2009. Prader–Willi syndrome. Eur J Hum Genet 17:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo A 1992. Plasma melatonin rhythm in normal puberty: Interactions of age and pubertal stages. Neuroendocrinology 55:372–379. [DOI] [PubMed] [Google Scholar]
- Cavallo A 1993. Melatonin and human puberty: Current perspectives.J Pineal Res 15:115–121. [DOI] [PubMed] [Google Scholar]
- Commentz JC, Helmke K. 1995. Precocious puberty and decreased melatonin secretion due to a hypothalamic hamartoma. Horm Res 44:271–275. [DOI] [PubMed] [Google Scholar]
- de Leersnyder H, De Blois MC, Claustrat B, Romana S, Albrecht U, von Kleist-Retzow JC, Delobel B, Lyonnet S, Vekemans M, Munnich A. 2001. Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J Pediatr 139:111–116. [DOI] [PubMed] [Google Scholar]
- Fideleff HL, Boquete H, Fideleff G, Albornoz L, Lloret SP, Suarex M, Esquifino AI, Honfi M, Cardinali DP. 2006. Gender-related differences in urinary 6-sulfatoxymelatonin levels in obese pubertal individuals. J Pineal Res 40:214–218. [DOI] [PubMed] [Google Scholar]
- Koyama H, Nakade O, Takada Y, Kaku T, Lau KH. 2002. Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J Bone Miner Res 17:1219–1229. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. 2000. CDC Growth Charts: United States. Adv Data 8:1–27. [PubMed] [Google Scholar]
- Laakso ML, Porkka-Heiskanen T, Alila A, Stenberg D, Johansson G. 1990. Correlation between salivary and serum melatonin: Dependence on serum melatonin levels. J Pineal Res 9:39–50. [DOI] [PubMed] [Google Scholar]
- Nishiyama K, Yasue H, Moriyama Y, Tsunoda R, Ogawa H, Yoshimura M, Kugiyama K. 2001. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am Heart J 141:E9. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. 2006. Melatonin: Nature’s most versatile biological signal? FEBS J 273:2813–2838. [DOI] [PubMed] [Google Scholar]
- Tamarkin L, Abastillas P, Chen HC, McNemar A, Sidbury JB. 1982. The daily profile of plasma melatonin in obese and Prader–Willi syndrome children. J Clin Endocrinol Metab 55:491–495. [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Kristensen K, Bruun JM, Ostergaard JR, Heickendorff L, Mosekilde L, Richelsen B. 2004. Reduced bone mineral density and increased bone turnover in Prader–Willi syndrome compared with controls matched for sex and body mass index—A cross-sectional study. J Pediatr 144:614–619. [DOI] [PubMed] [Google Scholar]
- von Bahr C, Ursing C, Yasui N, Tybring G, Bertilsson L, Rojdmark S. 2000. Fluvoxamine but not citalopram increases serum melatonin in healthy subjects—An indication that cytochrome P450 CYP1A2 and CYP2C19 hydroxylate melatonin. Eur J Clin Pharmacol 56:123–127. [DOI] [PubMed] [Google Scholar]
- Willig RP, Braun W, Commentz JC, Stahnke N. 1986. Circadian fluctuation of plasma melatonin in Prader–Willi’s syndrome and obesity. Acta Endocrinol Suppl (Copenh) 279:411–415. [DOI] [PubMed] [Google Scholar]