ABSTRACT
The HPV vaccine is an important vaccine for childhood cancer survivors because of their risks of second cancers, yet few survivors receive it. We examined HPV vaccine knowledge among caregivers of childhood cancer survivors, whether their child had received the vaccine, and their intentions to vaccinate. Eligible participants were caregivers (mostly parents) whose child finished cancer treatment at Primary Children’s Hospital in Salt Lake City, Utah 3 to 36 months prior to the start of the study (N = 145). Additional analyses were done among caregivers whose child was age-eligible for the HPV vaccine (ages 11 and up; N = 61). We ran descriptive statistics and fit multivariable generalized linear models to identify factors associated with intention to vaccinate and HPV vaccination uptake. Among caregivers whose child had not yet gotten the HPV vaccine, approximately 30% stated they were not likely to get the vaccine for their child and the most commonly cited reason was not enough information (25.2%). Provider discussion about vaccines and side effects (relative risk (RR) = 1.85, 95% CI 1.16–2.94), along with recommendations regarding vaccines after cancer treatment (RR = 1.35, 95% CI 1.06–1.72), led to greater caregiver intention to get the HPV vaccine for their child with cancer. Approximately 40% of age-eligible survivors had gotten at least one dose of the HPV vaccine. Our findings demonstrate a need for oncology-focused interventions to educate families of childhood cancer survivors about the importance of the HPV vaccine after cancer therapy.
KEYWORDS: Childhood cancer survivors, HPV vaccination, follow-up care, prevention, survivorship, provider recommendations
Introduction
The over 400,000 survivors of childhood cancer in the U.S. represent a growing population with unique human papillomavirus (HPV)-related health risks.1,2 Survivors of childhood cancer face a substantial risk of developing second cancers,3,4 including HPV-related cancers. Nationally, the relative excess of HPV-associated malignancies was 40% among female survivors of childhood cancer and 150% among male survivors compared to the general population.5 HPV vaccination is recommended as part of routine immunization schedule for all adolescents.6 In addition, the Children’s Oncology Group guidelines recommend administration of the HPV vaccine to reduce the risk for HPV-related cancers for all eligible childhood cancer survivors.7,8 However, research to date demonstrates low uptake and completion of the three-dose HPV vaccination series among survivors.9 Most recently, a study of five pediatric oncology institutions found that HPV vaccine initiation rates are much lower in childhood cancer survivors than the general population (24% vs. 41%).10
HPV vaccines and other adolescent vaccines including Tdap (Tetanus, Diphtheria, Pertussis), meningococcal, and yearly influenza are typically provided at a primary care provider (PCP) office or at local health agencies. While most childhood cancer survivors eventually transition to a PCP for the majority of their healthcare,11 many will only see their oncologist for several months to years after completing therapy. This could be a barrier to HPV vaccination as oncology providers often do not provide vaccines during cancer follow-up care because vaccines are typically considered a service provided by PCPs. As a result, childhood cancer survivors may be less likely to have had a provider recommend the HPV vaccine or to initiate or complete the HPV vaccine series.10
Here we report on findings from a clinic-based survey of primary caregivers (typically parents) of childhood cancer survivors – that is, patients who have finished therapy and are in remission – conducted at Primary Children’s Hospital (PCH) in Salt Lake City, Utah. PCH is the only pediatric oncology clinic for the state of Utah and provides oncology care to the five state Mountain West region (Utah, Nevada, Idaho, Wyoming, and Montana). These states all have low to moderate rates of HPV vaccination in the general population.12 Our study aim was to understand HPV vaccine knowledge and beliefs among caregivers of childhood cancer survivors as they make medical decisions for their child regarding vaccines. We also evaluated the likelihood that caregivers intended to have their child with cancer receive the HPV vaccine. Additionally, among caregivers whose child is age-eligible for the HPV vaccine, we report on factors associated with HPV vaccination, including whether their providers had discussed vaccination strategies after the end of cancer treatment. Provider recommendations for the HPV vaccine have been shown to be the most effective strategy for improving HPV vaccine uptake in the general population.13 Our goal is to inform future interventions to increase uptake of the HPV vaccine among childhood cancer survivors through developing educational materials for survivors, their parents, and clinicians.
Results
In Table 1, caregiver demographics and vaccine experiences are shown for the overall sample (N = 145) and the two subsamples of caregivers: those whose child was age eligible for the HPV vaccine (N = 61) and those whose child was not age eligible (N = 84). For the full sample, caregivers tended to be in their 30s at the time of the survey (54.9%) and mothers (80.6%). Almost half were college educated (44.4%) and most were married or living as married (88.2%). When we examined demographic differences by HPV age eligibility, caregivers whose child was age eligible for the HPV vaccine tended to be older with 41.7% in their 40s compared to 16.7% of the non-eligible sample (overall p < .001). No other statistically significant differences existed by HPV vaccine age eligibility.
Table 1.
Caregiver demographics and vaccine experiences among the full sample and among caregivers whose child is age eligible vs. not age eligible for the HPV vaccine.
| Age eligibility for HPV vaccine |
|||||||
|---|---|---|---|---|---|---|---|
| Overall Sample (N = 145) |
Child age eligible for HPV vaccine (N = 61)* |
Child not age eligible for HPV vaccine (N = 84)* |
|||||
| Demographics | N | % | N | % | N | % | P value |
| Age at survey (years) | |||||||
| 18–29 | 14 | 9.7 | 2 | 3.3 | 12 | 14.3 | <0.001 |
| 30–39 | 79 | 54.9 | 22 | 36.7 | 57 | 67.9 | |
| 40–49 | 39 | 27.1 | 25 | 41.7 | 14 | 16.7 | |
| 50–59 | 12 | 8.3 | 11 | 18.3 | 1 | 1.2 | |
| Relationship with survivor | |||||||
| Mother | 116 | 80.6 | 49 | 81.7 | 67 | 79.8 | 0.63 |
| Father | 25 | 17.4 | 9 | 15.0 | 16 | 19.1 | |
| Grandparent/Legal guardian | 3 | 2.1 | 2 | 3.3 | 1 | 1.2 | |
| Gender | |||||||
| Female | 116 | 81.1 | 48 | 81.4 | 68 | 80.1 | >0.99 |
| Male | 27 | 18.9 | 11 | 18.6 | 16 | 19.1 | |
| Education | |||||||
| High school | 23 | 16.2 | 11 | 18.6 | 12 | 14.5 | 0.67 |
| Some college/tech | 56 | 39.4 | 21 | 35.6 | 35 | 42.2 | |
| College graduate | 63 | 44.4 | 27 | 45.8 | 36 | 43.4 | |
| Race | |||||||
| White | 129 | 90.9 | 57 | 95.0 | 72 | 87.8 | 0.24 |
| Other | 13 | 9.2 | 3 | 5.0 | 10 | 12.2 | |
| Ethnicity | |||||||
| Non-Hispanic | 124 | 89.2 | 52 | 91.2 | 72 | 87.8 | 0.59 |
| Hispanic | 15 | 10.8 | 5 | 8.8 | 10 | 12.2 | |
| Annual household income | |||||||
| <$20,000 | 10 | 7.3 | 6 | 10.3 | 4 | 5.0 | 0.11 |
| $20,000-$39,999 | 20 | 14.5 | 10 | 17.2 | 10 | 12.5 | |
| $40,000-$59,999 | 26 | 18.8 | 10 | 17.2 | 16 | 20.0 | |
| $60,000-$79,999 | 22 | 15.9 | 4 | 6.9 | 18 | 22.5 | |
| $80,000-$99,999 | 21 | 15.2 | 8 | 13.8 | 13 | 16.3 | |
| >$100,000 | 39 | 28.3 | 20 | 34.5 | 19 | 23.8 | |
| Insurance status | |||||||
| Insured | 133 | 93.7 | 55 | 93.2 | 78 | 94.0 | >0.99 |
| Uninsured | 9 | 6.3 | 4 | 6.8 | 5 | 6.0 | |
| Marital status | |||||||
| Married/Living as married | 127 | 88.2 | 49 | 81.7 | 78 | 92.9 | 0.06 |
| Divorced/Separated/Never married | 17 | 11.8 | 11 | 18.3 | 6 | 7.1 | |
| Rurality of Residence | |||||||
| Urban | 116 | 80.6 | 47 | 78.3 | 69 | 82.1 | 0.67 |
| Rural | 28 | 19.4 | 13 | 21.7 | 15 | 17.9 | |
| Experiences with Vaccines | |||||||
| Providers give enough information on vaccines/potential side effects | |||||||
| Yes | 107 | 76.4 | 46 | 76.7 | 61 | 76.3 | >0.99 |
| No | 33 | 23.6 | 14 | 23.3 | 19 | 23.8 | |
| Cancer care team discussed when to restart vaccinations | |||||||
| Yes | 66 | 48.5 | 15 | 25.9 | 51 | 65.4 | <0.001 |
| No | 70 | 51.5 | 43 | 74.1 | 27 | 34.6 | |
| Oncologist or PCP recommended catch-up or booster vaccines | |||||||
| Yes | 74 | 51.0 | 21 | 34.4 | 53 | 63.1 | 0.001 |
| No | 71 | 49.0 | 40 | 65.6 | 31 | 36.9 | |
Some columns do not sum to total sample sizes due to missing data.
*Age eligible: child is age 11 or older at survey; Not age eligible: child is younger than age 11 at survey.
Overall, more than 75% of caregivers reported that providers gave them enough information on vaccine side effects, and this did not differ by HPV age eligibility. However, caregivers whose child was age-eligible for the HPV vaccine were less likely to report that the cancer team discussed when to restart vaccines (25.9% vs. 65.4%, p < .001) and that their oncologist/PCP recommended catch-up or booster vaccines (34.4% vs. 63.1%, p = .001) compared to caregivers whose child was not age eligible.
Among survivors in the overall sample (Table 2), most were ages 5–10 years at the time of the caregiver survey (40.7%), followed by 24.1% ages 11–15. Among the HPV age-eligible group, there were fewer female survivors (32.8% vs. 60.7% in the age ineligible sample, p = .001). Leukemia was the most common cancer in the overall sample (37.2%), although the distribution of cancers differed between the HPV age eligible and not age eligible subsamples (overall p < .001).
Table 2.
Childhood cancer survivor demographics among the full sample and among caregivers whose child is age eligible vs. not age eligible for the HPV vaccine.
| Age eligibility for HPV vaccine |
|||||||
|---|---|---|---|---|---|---|---|
| Overall Sample (N = 145) |
Child age eligible for HPV vaccine (N = 61)* |
Child not age eligible for HPV vaccine (N = 84)* |
|||||
| N | % | N | % | N | % | P value | |
| Age at survey (years) | |||||||
| 0–4 | 25 | 17.3 | – | – | 25 | 29.8 | n/a |
| 5–10 | 59 | 40.7 | – | – | 59 | 70.3 | |
| 11–15 | 35 | 24.1 | 35 | 57.4 | – | – | |
| 16–20 | 26 | 17.9 | 26 | 42.6 | – | – | |
| Gender | |||||||
| Female | 71 | 51.0 | 20 | 32.8 | 51 | 60.7 | 0.001 |
| Male | 74 | 49.0 | 41 | 67.2 | 33 | 39.3 | |
| Race | |||||||
| White | 129 | 89.6 | 56 | 91.8 | 73 | 88.0 | 0.58 |
| Other | 15 | 10.4 | 5 | 8.2 | 10 | 12.1 | |
| Ethnicity | |||||||
| Non-Hispanic | 120 | 85.7 | 48 | 82.8 | 72 | 87.8 | 0.47 |
| Hispanic | 20 | 14.3 | 10 | 17.2 | 10 | 12.2 | |
| Insurance status | |||||||
| Insured | 142 | 97.9 | 59 | 98.3 | 82 | 97.6 | >0.99 |
| Uninsured | 3 | 2.1 | 1 | 1.7 | 2 | 2.4 | |
| Age at diagnosis (years) | |||||||
| 0–4 | 53 | 36.6 | – | – | 53 | 63.1 | na |
| 5–9 | 39 | 26.9 | 8 | 13.1 | 31 | 36.9 | |
| 10–14 | 33 | 22.8 | 33 | 54.1 | – | – | |
| 15–17 | 20 | 13.8 | 20 | 32.8 | – | – | |
| Time since diagnosis | |||||||
| 3 months to <1 year | 77 | 53.1 | 36 | 59.0 | 41 | 48.8 | 0.07 |
| 1 year to <2 years | 57 | 39.3 | 18 | 29.5 | 39 | 46.4 | |
| 2 years to <3 years | 11 | 7.6 | 7 | 11.5 | 4 | 4.8 | |
| Diagnosis | |||||||
| Leukemia | 54 | 37.2 | 14 | 23.0 | 40 | 47.6 | <0.001 |
| Brain/Central Nervous System | 21 | 14.5 | 5 | 8.2 | 16 | 19.1 | |
| Lymphoma | 26 | 17.9 | 19 | 31.2 | 7 | 8.3 | |
| Other | 44 | 30.3 | 23 | 37.7 | 21 | 25.0 | |
Some columns do not sum to total sample sizes due to missing data.
*Age eligible: child is age 11 or older at survey; Not age eligible: child is younger than age 11 at survey.
Caregiver knowledge and beliefs regarding the HPV vaccine are shown in Table 3. Among the overall sample, most caregivers had heard of HPV (87.6%) and the HPV vaccine (84.1%). Medical providers – either a doctor or nurse – were the most common ways caregivers had heard about the HPV vaccine (42.1% for the overall sample). There were differences between the HPV vaccine-eligible groups, with caregivers whose child was age eligible for the vaccine less likely to hear about the vaccine from TV (19.7% vs. 39.3%, p = .009) and more likely to indicate hearing about the vaccine from the health department (26.2% vs. 11.9%, p = .02) than those not age eligible.
Table 3.
Caregiver knowledge and beliefs regarding the HPV vaccine among the full sample and among caregivers whose child is age eligible vs. not age eligible for the HPV vaccine.
| Age eligibility for HPV vaccine |
|||||||
|---|---|---|---|---|---|---|---|
| Overall Sample (N = 145) |
Child age eligible for HPV vaccine (N = 61)* |
Child not age eligible for HPV vaccine (N = 84)* |
|||||
| N | % | N | % | N | % | P value | |
| Have you heard of HPV? | |||||||
| Yes | 127 | 87.6 | 54 | 88.5 | 73 | 86.9 | 0.49 |
| No | 18 | 12.4 | 7 | 11.5 | 11 | 13.1 | |
| Have you heard of the HPV vaccine? | |||||||
| Yes | 116 | 84.1 | 48 | 81.4 | 68 | 86.1 | 0.30 |
| No | 22 | 15.9 | 11 | 18.6 | 11 | 13.9 | |
| Where did you hear about the HPV vaccine? (select all that apply) | |||||||
| Doctor or nurse | 61 | 42.1 | 29 | 47.5 | 32 | 38.1 | 0.17 |
| TV | 45 | 31.0 | 12 | 19.7 | 33 | 39.3 | 0.009 |
| Family/friends | 32 | 22.1 | 14 | 23.0 | 18 | 21.4 | 0.49 |
| Health department | 26 | 17.9 | 16 | 26.2 | 10 | 11.9 | 0.02 |
| Social Media | 25 | 17.2 | 11 | 18.0 | 14 | 16.7 | 0.50 |
| Websites other than social media | 17 | 11.7 | 7 | 11.5 | 10 | 11.9 | 0.58 |
| School | 14 | 9.7 | 6 | 9.8 | 8 | 9.5 | 0.58 |
| Radio | 10 | 6.9 | 3 | 4.9 | 7 | 8.3 | 0.52 |
| Newspapers | 7 | 4.8 | 3 | 4.9 | 4 | 4.8 | 0.63 |
| Other | 5 | 3.5 | 4 | 6.6 | 1 | 1.2 | 0.10 |
| Community health worker | 3 | 2.1 | 3 | 4.9 | 0 | 0 | 0.07 |
| Church | 1 | <1 | 0 | 0 | 1 | 1.2 | 0.58 |
| Insurance company | 1 | <1 | 0 | 0 | 1 | 1.2 | 0.58 |
| In general, do you think children ages 11–12 should get the HPV vaccine? | |||||||
| Yes | 96 | 69.1 | 43 | 72.9 | 53 | 66.3 | 0.26 |
| No | 43 | 30.9 | 16 | 27.1 | 27 | 33.8 | |
| Among those who have not received the HPV vaccine: | |||||||
| How likely are you to get the HPV vaccine for your child?** | |||||||
| Very likely to somewhat likely | 80 | 70.2 | 22 | 66.7 | 58 | 71.6 | 0.38 |
| Unlikely to very unlikely | 34 | 29.8 | 11 | 33.3 | 23 | 28.4 | |
| Why wouldn’t you get the HPV vaccine for your child? (select all that apply)** | |||||||
| Not enough information | 30 | 25.2 | 12 | 33.3 | 18 | 21.7 | 0.25 |
| Not the right age | 27 | 22.7 | 8 | 22.2 | 19 | 22.8 | >0.99 |
| Side effects | 26 | 21.9 | 7 | 19.4 | 19 | 22.9 | 0.81 |
| No specific reason | 23 | 19.3 | 6 | 16.7 | 17 | 20.5 | 0.80 |
| Not sexually active | 16 | 13.5 | 8 | 22.2 | 8 | 9.6 | 0.08 |
| Unnecessary | 10 | 8.4 | 0 | 0 | 10 | 12.5 | 0.03 |
| Not recommended | 9 | 7.6 | 0 | 0 | 9 | 10.8 | 0.06 |
| I don’t vaccinate my kids | 4 | 3.4 | 0 | 0 | 4 | 4.8 | 0.31 |
| Costs | 2 | 1.7 | 1 | 2.8 | 1 | 1.2 | 0.52 |
| It will promote sexual activity | 0 | 0 | 0 | 0 | 0 | 0 | na |
| Other reason | 5 | 4.2 | 1 | 2.8 | 4 | 4.8 | >0.99 |
Some columns do not sum to total sample sizes due to missing data.
*Age eligible: child is age 11 or older at survey; Not age eligible: child is younger than age 11 at survey.
**Among parents whose child with cancer has not received any doses of the HPV vaccine (N = 119 among the overall sample; N = 36 among the age-eligible sample and N = 83 among the not age-eligible sample who responded to this question).
Overall, approximately 31% of caregivers did not agree that children age 11–12 years should get the HPV vaccine and this did not differ by HPV age eligibility. When asked how likely they were to get the HPV vaccine for their child with cancer, 29.8% of the full sample said they were unlikely to very unlikely. The top reason endorsed regarding why they wouldn’t get the HPV vaccine for their child included not having enough information on the HPV vaccine (25.2%), followed by their child not being the right age (22.7%) and worries about side effects (21.9%). Among those caregivers whose child had not yet gotten the HPV vaccine but were age eligible, 33.3% were unlikely to very unlikely to get it for their child.
In multivariable regressions (Table 4), caregivers who reported that providers give enough information on vaccines and side effects (RR = 1.78, 95% CI 1.15–2.77) and who reported discussing with their cancer care team when to restart vaccinations (RR = 1.21, 95% CI 0.98–1.49) were more likely to indicate that children ages 11–12 years should get the HPV vaccine than caregivers who did not report provider input on vaccines. Similarly, both of these factors were associated with being more likely to get the HPV vaccine for the child (providers give enough information RR = 1.85, 95% CI 1.16–2.94; cancer care team discussed when to restart vaccinations RR = 1.35, 95% CI 1.06–1.72). In addition, caregivers whose child was further from diagnosis (≥1 year) were less likely to say they would get the HPV vaccine for their child (RR = 0.78, 95% CI 0.62–0.98) than those 3 months to <1 year.
Table 4.
Multivariable regressions of factors associated with HPV vaccination intentions reported by caregivers.
| In general, do you think children ages 11–12 should get the HPV vaccine?* |
How likely are you to get the HPV vaccine for your child?** |
|||||
|---|---|---|---|---|---|---|
| Relative Risk | 95% CI | p-value | Relative Risk | 95% CI | p-value | |
| Child’s gender | ||||||
| Male (ref) | 1 | 0.84 | 1 | 0.71 | ||
| Female | 0.98 | 0.79–1.22 | 0.96 | 0.76–1.21 | ||
| Caregiver age at survey | ||||||
| <40 years (ref) | 1 | 0.25 | 1 | 0.98 | ||
| ≥40 years | 1.13 | 0.92–1.40 | 0.98 | 0.77–1.25 | ||
| Time from diagnosis | ||||||
| 3 months to 1 year (ref) | 1 | 0.38 | 1 | 0.04 | ||
| ≥1 year | 0.91 | 0.73–1.13 | 0.78 | 0.62–0.98 | ||
| Providers give enough information on vaccines/potential side effects | ||||||
| No (ref) | 1 | 0.01 | 1 | 0.009 | ||
| Yes | 1.78 | 1.15–2.77 | 1.85 | 1.16–2.94 | ||
| Cancer care team discussed when to restart vaccinations | ||||||
| No (ref) | 1 | 0.08 | 1 | 0.02 | ||
| Yes | 1.21 | 0.98–1.49 | 1.35 | 1.06–1.72 | ||
*Among all caregivers (N = 126 due to missing data).
**Sub-sample of caregivers whose child with cancer has not received any doses of the HPV vaccine (N = 104 due to missing data).
Our final set of analyses was limited to caregivers whose child was age-eligible for the HPV vaccine. In Figure 1, 40.9% of caregivers with an eligible child stated that their child had received at least one dose of the HPV vaccine. Parents ages 40 and older were more likely to indicate that their child had received the HPV vaccine (50.0% vs. 25.0%, p = .06). Caregivers who endorsed that most children should get the HPV vaccine were more likely to report HPV vaccination (48.8% vs. 18.8%, p = .04). Figure 2 includes HPV vaccine receipt by provider and clinical factors. Although no factors met statistical significance at p < .05, several trends were observed, with higher HPV vaccination levels among caregivers who had received a provider recommendation for getting catch-up or booster vaccines, and among survivors ≥1 year from diagnosis. In a multivariable regression (Table 5), no factors were statistically significantly associated with HPV vaccination.
Figure 1.
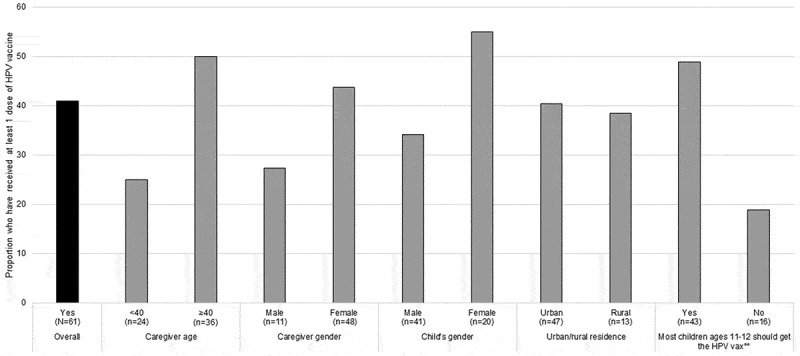
HPV vaccine receipt by caregiver and child factors reported by caregivers whose child is age eligible for the HPV vaccine (N = 61)*.
*Sub-sample of full sample, limited to caregivers whose child with cancer was age 11 or older at survey; Ns for each variable do not sum to 61 due to missing data. **Statistically significant at p < .05.
Figure 2.
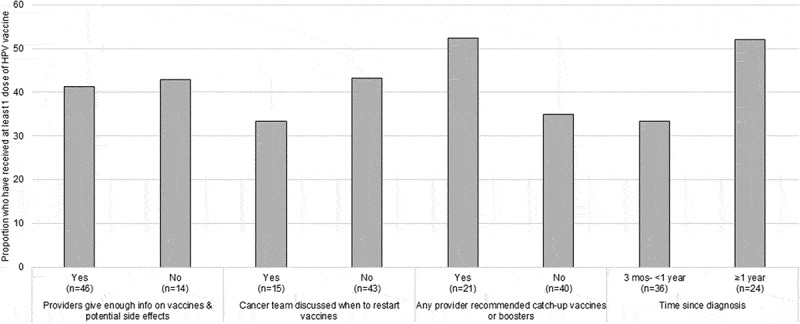
HPV vaccine receipt by provider and clinical factors reported by caregivers whose child is age eligible for the HPV vaccine (N = 61)*.
*Sub-sample of full sample, limited to caregivers whose child with cancer was age 11 or older at survey; Ns for each variable do not sum to 61 due to missing data.
Table 5.
Multivariable regression of factors associated with HPV vaccination reported by caregivers whose child is age eligible for the HPV vaccine (N = 56)*.
| Relative Risk | 95% CI | p-value | |
|---|---|---|---|
| Child’s gender | |||
| Male (ref) | 1 | 0.17 | |
| Female | 1.51 | 0.84–2.73 | |
| Caregiver age at survey | |||
| <40 years (ref) | 1 | 0.06 | |
| ≥40 years | 2.05 | 0.96–4.37 | |
| Time from diagnosis | |||
| 3 months to 1 year (ref) | 1 | 0.67 | |
| ≥1 year | 1.14 | 0.62–2.09 | |
| Providers give enough information on vaccines/potential side effects | |||
| No (ref) | 1 | 0.79 | |
| Yes | 1.12 | 0.49–2.54 | |
| Cancer care team discussed when to restart vaccinations | |||
| No (ref) | 1 | 0.81 | |
| Yes | 0.91 | 0.44–1.92 |
* Sub-sample is limited to caregivers whose child with cancer was age 11 or older at survey with no missing data.
Discussion
The HPV vaccine represents an important, but underutilized, resource to prevent second cancers among childhood cancer survivors. In this survey of childhood cancer caregivers, we found that most caregivers have heard of the HPV vaccine and they received this information most often from a health-care provider. However, almost one-third of caregivers stated that they were unlikely to get the HPV vaccine for their child who had cancer, and only 40.9% of those with an age-eligible child had received at least one dose of HPV vaccine. While some parents reported worries about vaccine safety or that their child was not age-eligible as reasons they would not get the HPV vaccine for their child, the most common reason cited for not getting the vaccine was lack of information. However, caregivers who stated the cancer care team had discussed vaccinations and felt that providers gave them adequate information on vaccines and side effects were much more likely to state that they would get the HPV vaccine for their child who had cancer. Together these results demonstrate the importance of oncology providers, and medical providers in general, in providing recommendations about vaccination after cancer treatment. Moreover, providers are uniquely positioned to ensure that parents and survivors understand that the HPV vaccine is essential preventive health care as they transition into survivorship.
While our HPV vaccine report of 40% is higher than other studies of childhood cancer survivors,10,14 it is still well below the HealthyPeople 2020 goal of 80% series completion by age 15 and lower than the most recently reported rates for Utah of 58.8%.12 Recently, there has been considerable effort to improve HPV vaccination uptake in our region15 and in the United States through provider and health-care team training, practice level changes for utilization of reminder/recall systems, and parent/caregiver education.16 These initiatives could explain, at least to a certain extent, our study’s higher HPV vaccination level than earlier analyses among childhood cancer survivors. Moreover, these provider- and systems-level strategies that have worked to improve HPV vaccination in primary care settings should also be deployed to improve HPV vaccination among survivors being cared for in oncology settings.
In addition, our study is consistent with other studies on the HPV vaccine that demonstrate how important health-care providers are in prompting the vaccine for their patients. A health-care provider recommendation for HPV vaccination is associated with a higher uptake of the vaccine both among the general population and among childhood cancer survivors.10,13,17 While we did not specifically ask about provider recommendations of the HPV vaccine, we found that caregivers who reported that the cancer care team discussed when to restart vaccinations were more likely to indicate they would get the vaccine for their child. In addition, our analyses suggest that the timing of these discussions may be very important. In our multivariable model, caregivers whose child was between 3 months to 1 year from diagnosis were more likely to endorse the HPV vaccine for their child. This demonstrates the importance of discussing vaccine earlier rather than later after therapy, when parents may be primed to do everything possible to keep their child healthy and cancer-free.
Our findings point to a strong need to develop interventions to improve HPV vaccination among childhood cancer survivors and to address worries and knowledge gaps reported by caregivers. Almost one-third of surveyed caregivers reported that they were unlikely to get the HPV vaccine for their child and that lack of information was the biggest reason. Oncology-based interventions could be an important avenue for improving this gap. After cancer treatment ends, oncologists provide patients and their families with a care strategy going forward for survivorship care. For cancer patients transitioning off therapy, their oncology teams often play the role of a PCP, although most eventually transition to a PCP.18 Patients and their families may trust their oncology teams more than their PCPs,19,20 which provides an important opportunity for improving caregivers’ comfort with the HPV vaccine.
To reach the HealthyPeople 2020 goal of 80% series completion by age 15 for this high-risk population, it is critical that oncologists and other providers stress the importance of HPV vaccination along with other immunizations to their patients. Less than 50% of caregivers we surveyed had heard about the HPV vaccine from a health-care provider. The trust built over repeated interactions during cancer treatment presents an opportunity to develop oncology-focused interventions to stress the importance of HPV vaccination and other recommended immunizations for survivors. Vaccinations after cancer – including making a strong recommendation for the HPV vaccine – should be a seamless part of follow-up care. Moreover, for patients transitioning back to primary care, efforts must be made to educate PCPs on recommending the HPV vaccine to their cancer survivor patients.
This study has certain limitations. Our assessment was conducted in one pediatric oncology clinic in the Mountain West, albeit one that serves the largest geographic catchment area in the continental United States.21 Despite this, we have limited ethnic diversity in our sample. In addition, our survey was designed to be a broad assessment of vaccination practices after cancer therapy; while we asked specific questions on HPV vaccination, we were limited in our ability to ascertain more detailed information on caregiver worries about the vaccine or to capture how many doses of the HPV vaccine survivors have received. We were also not able to validate self-report of HPV vaccination with medical records. Finally, our rate of HPV vaccination was higher than earlier assessments of childhood cancer survivors. As our survey recruitment specified that this was a survey on vaccinations and participants received a modest incentive ($20 gift card), our sample could have been biased towards parents more likely to vaccinate or who participated due to the incentive. However, as a substantial proportion of our sample (approximately 30%) reported being unlikely to get the HPV vaccine for their child, we still had a strong range of parental vaccine beliefs represented in this assessment.
In summary, we found that provider discussion about vaccines and safety, along with recommendations regarding vaccines after cancer treatment, are key predictors of caregiver intent to get the HPV vaccine for their child with cancer. Caregivers cited not having enough information on the HPV vaccine as the main reason they might forego the vaccine for their child. Caregivers who were older were more likely to report that their child with cancer had received the HPV vaccine. Follow-up care for children with cancer provides an important opportunity for providers to give recommendations for the HPV vaccine as most patients see their oncologist regularly – up to five or six times a year – for the first two years after treatment ends.8 As such, promoting the HPV vaccine through oncology-based interventions is an essential future step towards improving health outcomes for childhood cancer survivors.
Materials and methods
This study was part of a larger project on vaccination after pediatric and adolescent cancer at PCH, which is part of Intermountain Healthcare, a large system of hospitals in Utah and Idaho. The study was approved by the University of Utah’s institutional review board.
Participants
Eligible participants included English-speaking caregivers ages 18 and older, who had a child diagnosed with cancer ages 17 or younger and had completed cancer treatment 3–36 months prior at PCH. This timeframe was selected as most survivors attend regular follow-up care appointments (typically every 4 to 8 weeks) for the first two years after cancer treatment ends. We identified eligible cancer survivors through chart review in the electronic medical record, which included screening for diagnosis, age at diagnosis, end of therapy date, and next appointment.
Procedures
Research staff approached caregivers of eligible survivors in person at follow-up appointments at PCH. Eligible caregivers completed the informed consent process and were enrolled in the study. In addition, we emailed or mailed surveys to caregivers who were unable to complete the survey at the time of their child’s appointment. Of the 652 unique off-therapy patients seen during our study timeframe (October 2017 to December 2018), 94 were less than 3 months off of therapy and ineligible, 353 were more than 3 years off of therapy and ineligible, and 9 did not speak English. Of the remaining 196, 43 declined to participate. A total of 153 were consented and completed surveys either via REDCap in clinic or through mailed surveys. Seven participants were later found to be ineligible and one skipped the majority of survey items, which led us to analyze 145 completed surveys (participation rate of 73.9%). We stored survey data electronically in REDCap. Participants received a $20 gift card.
Survey design
We drafted the survey based on literature review and input from experts in childhood cancer and vaccinations, including obtaining feedback from two pediatric oncologists, a pediatric infectious disease specialist, two pediatricians, and an expert in HPV vaccination. We then pilot-tested the survey with seven caregivers of childhood cancer survivors in a focus group. Prior to the focus group, caregivers received the survey, and then, as a group, discussed their notes and questions about the survey. Using both the provider and focus group feedback, we revised the survey to clarify question wording and to eliminate four questions on guidelines and eligibility related to HPV vaccination that were deemed unnecessarily detailed by the focus group participants. We then obtained additional expert feedback from pediatric oncologists and pediatricians to finalize the items. The final implemented survey included 65-items on the following domains: HPV vaccination practices, knowledge and beliefs; vaccine preferences; vaccine experiences with providers; caregiver and survivor demographics; and cancer factors.
Survey domains
Caregiver demographics: These included caregiver age at survey (18–29, 30–39, 40–49, 50–59 years), relationship with the survivor (mother, father, grandparent, legal guardian), gender (female, male), education (high school, some college/technical school, college graduate or more), race (White, Black/African American, Asian/Asian American, American Indian/Alaska Native, Pacific Islander/Native Hawaiian, Other), and ethnicity (Non-Hispanic, Hispanic). Other measures included annual household income (<$20,000, $20,000-$39,999, $40,000-$59,999, $60,000-$79,999, $80,000-$99,999, >$100,000), insurance status (insured, uninsured), and marital status (married/living as married, divorced/separated/never married). We defined rurality of caregiver residence (urban, rural) by classifying residential zip codes using Rural Urban Commuting Area codes, which are based on population density, commuting time, and urbanization in U.S. census tracts.22
Childhood cancer survivor demographics and cancer factors: We obtained information on current age (0–4, 5–10, 11–15, 16–20 years), gender (female, male), race (White, Black/African American, Asian/Asian American, American Indian/Alaska Native, Pacific Islander/Native Hawaiian, Other), and ethnicity (Non-Hispanic, Hispanic).23 Insurance status (insured, uninsured), age at diagnosis (0–4, 5–9, 10–14, 15–17 years), and diagnosis (leukemia, brain/central nervous system tumors, lymphoma, and other solid tumors) were also collected. Time since diagnosis was calculated based on the difference from the date the caregiver completed the survey and the date that treatment ended as reported by the caregiver, with confirmation of end of therapy dates from medical records as needed. We then grouped this into a categorical variable: 3 months to <1 year, 1 to <2 years, 2 to <3 years.
HPV vaccination factors: We included items on caregiver awareness of HPV and HPV vaccination, preferences and intentions for HPV vaccination for their child with cancer, whether they think children ages 11–12 should get the HPV vaccine, whether their child with cancer had received at least one dose of the HPV vaccine, where they heard about the HPV vaccine, and why they would not get the HPV vaccine for their child (e.g., not the right age; the vaccine is unnecessary, etc.; participants could select more than one option).
Experiences with vaccines factors: Caregivers indicated whether they had received a provider recommendation for catch-up/booster vaccines from their child’s oncologist/cancer care team (yes/no) or from a primary care provider (yes/no), which we combined into an overall provider recommendation variable (yes to either item vs. no for both items) for our analyses. Participants also reported whether their oncologist/cancer care team had discussed when to restart their child’s vaccine schedule (yes vs. no/my child did not need catch-up vaccines). We also ascertained whether caregivers felt that providers gave them enough information on vaccines and potential side effects (yes/no).
Statistical analysis
We calculated summary statistics for caregiver demographics, experiences with vaccines, survivor demographics and cancer factors, and HPV vaccination factors for the full sample (N = 145) and among caregivers whose child was age-eligible for the HPV vaccine (ages 11 and up; N = 61) or not yet age-eligible (under age 11; N = 84). While the HPV vaccine can be provided as early as 9 years of age, the ages of 11–12 years are the standard age for HPV vaccination, which is why we used age 11 as our threshold for these analyses. Then, to investigate factors associated with whether the caregiver endorsed that age-eligible children should get the HPV vaccine and their intention to get the HPV vaccine for their child who had cancer, we fit two generalized linear models (GLM) with a log link and binomial family to estimate relative risks (RR) and 95% Confidence Intervals (CI) among the full sample. In addition, we examined bivariate associations of caregiver, child, provider, and clinical factors associated with receipt of the HPV vaccine among the age-eligible sample. We then examined selected factors associated with HPV vaccine receipt in a multivariable GLM. Missing values were excluded from the analyses. Statistical analyses were performed in Stata 14.2.
Funding Statement
The authors would like to acknowledge funding from the American Cancer Society Institutional Research Grant (PI: Don Ayer) as well as funding from the Huntsman Cancer Institute/Huntsman Cancer Foundation. Additional support was provided by P30CA042014 from the National Cancer Institute (PI: Mary Beckerle).
Acknowledgments
We thank Samantha Pannier, Akanksha Acharya, and Laura Martel for their assistance with study design.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A.. Childhood and adolescent cancer statistics. CA Cancer J Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Temkin SM, Seibel NL. Are we missing an opportunity for cancer prevention? Human papillomavirus vaccination for survivors of pediatric and young adult cancers. Cancer. 2015;121(19):3395–402. doi: 10.1002/cncr.29515. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Goddard K, Spinelli JJ, Gotay C, McBride ML. Risk of late mortality and second malignant neoplasms among 5-year survivors of young adult cancer: a report of the childhood, adolescent, and young adult cancer survivors research program. J Cancer Epidemiol. 2012;2012:103032. doi: 10.1155/2012/103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL, Neglia JP. Subsequent neoplasms in 5-year survivors of childhood cancer: the childhood cancer survivor study. J Natl Cancer Inst. 2010;102(14):1083–95. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ojha RP, Tota JE, Offutt-Powell TN, Klosky JL, Minniear TD, Jackson BE, Gurney JG, de Sanjose S. Human papillomavirus-associated subsequent malignancies among long-term survivors of pediatric and young adult cancers. PLoS One. 2013;8(8):e70349. doi: 10.1371/journal.pone.0070349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA Jr, Unger ER; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep: Morb Mortal Wkly Rep. 2014;63(Rr–05):1–30. [PubMed] [Google Scholar]
- 7.Klosky JL, Gamble HL, Spunt SL, Randolph ME, Green DM, Hudson MM. Human papillomavirus vaccination in survivors of childhood cancer. Cancer. 2009;115(24):5627–36. doi: 10.1002/cncr.24669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 5.0. Monrovia, CA: Children's Oncology Group; October 2018 www.survivorshipguidelines.org. [Google Scholar]
- 9.Klosky JL, Russell KM, Canavera KE, Gammel HL, Hodges JR, Foster RH, Parra GR, Simmons JL, Green DM, Hudson MM. Risk factors for non-initiation of the human papillomavirus vaccine among adolescent survivors of childhood cancer. Cancer Prev Res (Phila). 2013 Oct;6(10):1101–10. doi: 10.1158/1940-62071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klosky JL, Hudson MM, Chen Y, Connelly JA, Wasilewski-Masker K, Sun CL, Francisco L, Gustafson L, Russell KM, Sabbatini G, et al. Human papillomavirus vaccination rates in young cancer survivors. J Clin Oncol: Am Soc Clin Oncol. 2017; Jco2017741843. doi: 10.1200/JCO.2017.74.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, Gurney JG, Donaldson SS, Leisenring WM, Robison LL, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26(27):4401–09. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Mbaeyi SA, Fredua B, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years – United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(33):909–17. doi: 10.15585/mmwr.mm6733a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health. 2013;103(1):164–69. doi: 10.2105/AJPH.2011.300600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klosky JL, Favaro B, Peck KR, Simmons JL, Russell KM, Green DM, Hudson MM. Prevalence and predictors of human papillomavirus (HPV) vaccination among young women surviving childhood cancer. J Cancer Surviv. 2016 Jun;10(3):449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warner EL, Fowler B, Martel L, Kepka D. Improving HPV vaccination through a diverse multi-state coalition. J Community Health. 2017. doi: 10.1007/s10900-017-0334-7. [DOI] [PubMed] [Google Scholar]
- 16.Reiter PL, Gerend MA, Gilkey MB, Perkins RB, Saslow D, Stokley S, Tiro JA, Zimet GD, Brewer NT. Advancing human papillomavirus vaccine delivery: 12 priority research gaps. Acad Pediatr. 2018;18(2S):S14–S16. doi: 10.1016/j.acap.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vadaparampil ST, Kahn JA, Salmon D, Lee J-H, Quinn GP, Roetzheim R, Bruder K, Malo TL, Proveaux T, Zhao X, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11-12 year old girls are limited. Vaccine. 2011;29(47):8634–41. doi: 10.1016/j.vaccine.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchhoff AC, Montenegro RE, Warner EL, Wright J, Fluchel M, Stroup AM, Park ER, Kinney AY. Childhood cancer survivors’ primary care and follow-up experiences. Support Care Cancer. 2014;22(6):1629–35. doi: 10.1007/s00520-014-2130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsay JM, Mann K, Kaul S, Zamora ER, Smits-Seemann RR, Kirchhoff AC. Follow-up care provider preferences of adolescent and young adult cancer survivors. J Adolesc Young Adult Oncol. 2018. doi: 10.1089/jayao.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warner EL, Montenegro RE, Stroup A, Kinney AY, Kirchhoff AC. Health care concerns of rural childhood cancer survivors. J Health Care Poor Underserved. 2014;25(2):901–12. doi: 10.1353/hpu.2014.0095. [DOI] [PubMed] [Google Scholar]
- 21.Fluchel MN, Kirchhoff AC, Bodson J, Sweeney C, Edwards SL, Ding Q, Stoddard GJ, Kinney AY. Geography and the burden of care in pediatric cancers. Pediatr Blood Cancer. 2014;61(11):1918–24. doi: 10.1002/pbc.25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rural-Urban Commuting Area Codes 2016. Accessed 11/1/2018 https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/.
- 23.Institute of Medicine. Race, ethnicity, and language data: Standardization for health care quality improvement. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Rural-Urban Commuting Area Codes 2016. Accessed 11/1/2018 https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/.


