ABSTRACT
HPV vaccination coverage in the United States (US) falls short of the Healthy People 2020 goal of 80% coverage among 13–15 year-old adolescents. Pharmacies are a promising alternative vaccine delivery site that may increase access to HPV vaccination. Our objective was to assess pharmacists’ insights into HPV vaccination provision to adolescents. We recruited 40 licensed pharmacists in eight states with different pharmacy vaccination laws: Alabama, California, Indiana, Kentucky, Maine, Tennessee, Texas, and Washington. Eligible pharmacists either previously provided or were currently providing HPV, tetanus-diphtheria-pertussis, or meningococcal vaccines to adolescents aged 9–17 years. Pharmacists were administered a semi-structured survey to explore insights into HPV vaccination provision. Forty-five percent of surveyed pharmacies offered HPV vaccination to adolescents. Pharmacists’ reported challenges to providing HPV vaccination were parental consent (28%), tracking and patient recall (17%), perceived stigma of vaccination (17%), and education about or promotion of vaccination (17%). Pharmacists offering HPV vaccination sent patient reminders for vaccines with multiple doses (89%) and utilized telephone reminders (72%). Pharmacists informed patients’ primary care providers of HPV vaccination doses most commonly through fax (72%) and updating electronic medical records (22%). One-third of pharmacists reported vaccination provision using the state immunization information system (IIS). Seventy-five percent reported vaccination rates could be increased at their respective pharmacy. Pharmacies are underutilized, although highly accessible, for HPV vaccination in the US. National efforts should expand educational programs to improve public awareness of in-pharmacy HPV vaccination, and improve the utilization of state IIS for reporting immunization coverage of adolescents by pharmacists.
Keywords: Pharmacy, HPV vaccination, adolescents, facilitators, barriers
Introduction
Human papillomavirus (HPV) vaccination is recommended by the Advisory Committee on Immunization Practices (ACIP) to prevent HPV-associated diseases, including cancers of the cervix, anus, and oropharynx.1 Universal HPV vaccination at ages 11 or 12 years has been recommended by the ACIP since 2006 for females, and since 2011 for males.1 Despite national guidelines and proven health benefits,1 national coverage of HPV vaccination remains below other vaccines recommended for adolescents (i.e. meningococcal conjugate vaccine (MenACWY) and tetanus-diphtheria-acellular pertussis (Tdap) vaccine.2 In 2016, HPV vaccination initiation coverage (≥1 dose) among adolescents aged 13–17 years was relatively low: 65% among females and 56% among males.3,4 In contrast, MenACWY and Tdap vaccine initiation among adolescents aged 13–17 years met the Healthy People 2020 target of 80% during this same year,3,4 indicating a missed opportunity for HPV vaccination in the adolescent target population.
High-quality healthcare provider recommendation is the strongest predictor of HPV vaccination uptake and a potential solution to improving adolescent vaccine coverage in the U.S.5 However, provider recommendation for vaccination occurs in primary care settings, which are visited by adolescents less frequently than younger children.6 In the U.S., almost half of adolescents, aged 12–17 years, do not have reliable access to comprehensive primary care.6,7 Regional differences in HPV vaccination uptake,2 are likely attributed to scarce or unavailable primary care services in rural and low-resource areas.8,9 Expanded vaccine delivery sites for adolescents are needed to address the gap in primary care delivery and improve adolescent coverage of HPV vaccination in the U.S.
The Centers for Disease Control and Prevention (CDC) and the American Cancer Society (ACS), have endorsed pharmacies as an alternative vaccination delivery site.10 Pharmacists are uniquely positioned to address the gap in HPV vaccination coverage due to their convenient accessibility.11,12 In fact, an estimated 95% of U.S. residents live within 5 miles of a pharmacy.11 Previous studies have assessed parental acceptance of providing HPV vaccination to adolescents in pharmacies.13-15 Additionally, two prior studies were conducted to assess pharmacists’ attitudes and perceived barriers to HPV vaccination in Utah16 and Alabama.17 Further data are needed from pharmacists’ perspectives on insights into the facilitators and barriers of HPV vaccination delivery within pharmacies located in a variety of states and vaccine policy environments. Such data will be important to assess the feasibility of improving access to and receipt of HPV vaccination through pharmacies. In 2017, we presented data on pharmacists’ perspective of opportunities and challenges of in-pharmacy vaccine administration to adolescents and adults.18 Here, we focus on HPV vaccination among adolescents, and explore pharmacists’ insights into barriers and facilitators of providing HPV vaccination in pharmacy settings.
Results
Background characteristics
The mean age of pharmacists was 39.3 years (Range: 25–65). Most pharmacists were white (75%), female (55%), and had a Doctorate in Pharmacy (70%) (Table 1). The majority of pharmacists offered the following vaccinations to adolescents: influenza (100%), Tdap (80%), and MenACWY (60%). Forty-five percent of pharmacists offered HPV vaccination to adolescents. Most (78%) pharmacists were employed at chain pharmacies.
Table 1.
Characteristics of U.S. pharmacists* and pharmacies providing vaccination in eight states.
| Total (n = 40) |
|
|---|---|
| n (%) | |
| Pharmacist | |
| Age (years)† | |
| 25–34 | 16 (40) |
| 35–44 | 13 (33) |
| ≥ 45 | 11 (28) |
| Race | |
| White | 30 (75) |
| Asian | 6 (15) |
| Black | 2 (5) |
| Multiracial | 2 (5) |
| Sex | |
| Female | 22 (55) |
| Male | 18 (45) |
| Highest Degree Earned† | |
| PharmD | 28 (70) |
| BSPharm or BS | 11 (28) |
| MS – Pharmacy Administration | 1 (3) |
| Vaccinations Offered to Adolescents | |
| Influenza | 40 (100) |
| TDAP | 32 (80) |
| MenACWY | 24 (60) |
| HPV | 18 (45) |
| Pneumococcal | 14 (35) |
| Hepatitis B | 14 (35) |
| Hepatitis A | 11 (28) |
| Tetanus and diphtheria | 7 (18) |
| Type of pharmacy practice† | |
| Chain | 31 (78) |
| Independent | 5 (13) |
| Grocery | 2 (5) |
| Big box retailer | 2 (5) |
| Pharmacy | |
| Number of pharmacists currently administering vaccines† | |
| 2–3 | 31 (78) |
| 4–5 | 7 (18) |
| ≥ 6 | 2 (5) |
| Number of years pharmacy has provided adolescent vaccination† | |
| 1–3 | 8 (20) |
| 4–6 | 18 (45) |
| ≥ 7 | 9 (23) |
| Don’t know | 5 (13) |
| Methods used to administer vaccines‡ | |
| Walk-In | 40 (100) |
| Off-site | 31 (78) |
| Appointments during business hours | 21 (53) |
| Mass clinics | 15 (38) |
| Appointments during advertised date | 12 (30) |
| Type of payment accepted by pharmacy‡ | |
| Out of pocket/Self Pay | 40 (100) |
| Private Insurance | 40 (100) |
| Medicare | 40 (100) |
| Medicaid | 39 (98) |
| Tricare | 26 (65) |
| Children’s Health Insurance Program | 24 (60) |
| English-speaking patient population† | |
| <80 % | 3 (8) |
| 80–94 % | 8 (20) |
| ≥95 % | 29 (73) |
| Vaccination capacity | |
| Could vaccinate more people | 30 (75) |
| Pharmacy at capacity | 8 (20) |
| Pharmacy over capacity | 2 (5) |
* Includes 18 pharmacists who did provide HPV vaccination to adolescents aged 9–18 years, and 22 pharmacists who did not provide HPV vaccines but provided Tdap or tetanus and diphtheria, and/or meningitis (MenACWY) vaccines
† Totals may exceed 100% due to rounding
‡ Totals may exceed 100%, multiple answers possible
Abbreviations: Human papillomavirus (HPV), United States (U.S.), Doctor of Pharmacy (PharmD), Bachelor of Science in Pharmacy (BSPharm), Bachelor of Science (BS), TDAP = tetanus, diphtheria and pertussis; TD = tetanus and diphtheria; HPV = human papillomavirus; MenACWY = meningococcal vaccine American Pharmacists Association (APhA), Basic Life Support (BLS), Occupational Safety and Health Administration (OSHA), Continuing Pharmacy Education (CPE)
Each pharmacist provided background details of their respective pharmacy. Over three-quarters of pharmacies had 2–3 pharmacists currently administering vaccines, and about half of pharmacies offered adolescent vaccinations for the past 4–6 years. Most pharmacies offered vaccinations to adolescents by walk-in (100%), off-site (78%), and appointments during business hours (53%). All surveyed pharmacies accepted out-of-pocket payment, and private insurance for vaccination in pharmacies. Ninety-eight percent of pharmacies accepted Medicaid. A small proportion (8%) of pharmacies served a patient population in which less than 80% were English-speaking. Three-quarters of pharmacists reported their pharmacy had the capacity to vaccinate more clients.
Insights into HPV vaccination delivery
Pharmacists who provided HPV vaccination (n = 18) were surveyed on challenges and facilitators to HPV vaccination provision within their pharmacy. The commonly reported challenges to providing adolescent HPV vaccination were parental consent (28%); tracking and recall of patients for multiple-dose vaccines; perceived stigma about the HPV vaccination among parents of adolescents; and education or promotion of vaccination (each at 17%) (Table 2). Twenty-seven percent of pharmacists implemented strategies to overcome challenges to providing HPV vaccination to adolescents. These strategies included: providing support or education to patient and patient’s guardian (22%), and a reminder system for patients of upcoming doses (6%). Clear guidelines from pharmacy or corporate management (22%) was the most common facilitator for HPV vaccination provision success, followed by state legislative authority to provide vaccination, promotion outside pharmacy, and education/promotion within pharmacy (each at 17%). However, approximately 22% reported the HPV vaccination program in their pharmacy was relatively unsuccessful due to higher cost of obtaining HPV vaccination at the pharmacy compared to the doctor’s office (n = 2) and little demand despite having the vaccine in stock (n = 2). Over three-quarters of pharmacists reported to feel “very comfortable” administering HPV vaccination to adolescents.
Table 2.
Semi-quantitative data on U.S. pharmacist insights into HPV vaccination in eight states among HPV vaccine providers (n = 18).
| n (%) | |
|---|---|
| Challenges to providing HPV vaccines to adolescents* | |
| Parental consent | 5 (28) |
| Tracking and recall of patients | 3 (17) |
| Education/promotion of vaccination | 3 (17) |
| Stigma about vaccination among parents of adolescents | 3 (17) |
| Cost of vaccination | 2 (11) |
| Potential adverse reactions | 2 (11) |
| State laws | 1 (6) |
| Don’t know | 1 (6) |
| None | 3 (17) |
| Strategies to overcome challenges of providing HPV vaccination † | |
| Providing education or support to patient and patient’s guardian | 4 (22) |
| Reminder system for patients of upcoming doses | 1 (6) |
| Facilitators towards success of HPV vaccination program in pharmacy* | |
| Clear guidelines from pharmacy/corporate management | 4 (22) |
| State legislative authority to provide vaccination | 3 (17) |
| Promotion outside pharmacy | 3 (17) |
| Education or promotion within pharmacy | 3 (17) |
| Reimbursement of adolescent vaccines | 2 (11) |
| Designated space for vaccination | 1 (6) |
| Student vaccination | 1 (6) |
| Having vaccines in stock | 1 (6) |
| Not applicable‡ | 4 (22) |
| Don’t know | 3 (17) |
| Comfort level administering HPV vaccines to adolescents§ | |
| Very comfortable | 14 (78) |
| Somewhat comfortable | 3 (17) |
| Somewhat uncomfortable | 1 (6) |
* Totals may exceed 100%, multiple answers possible
† Only 27% (n = 5) of pharmacists reported ways to overcome challenges
‡ Pharmacists described their HPV vaccination program as unsuccessful
§ Pharmacists were given the following options: very comfortable, somewhat comfortable, neutral, somewhat uncomfortable, and very uncomfortable
Abbreviations: Human papillomavirus (HPV), United States (U.S.)
Among all pharmacists, the most common advantages to offering HPV vaccination in pharmacies were greater access (60%), improved vaccine coverage (30%), and financial benefit to the providing pharmacy (20%) (Figure 1). Almost 60% of pharmacists (58%) reported no disadvantages to offering HPV vaccination. The most commonly reported disadvantage was tracking and recall difficulties for patients requiring more than one vaccination dose (18%).
Figure 1.
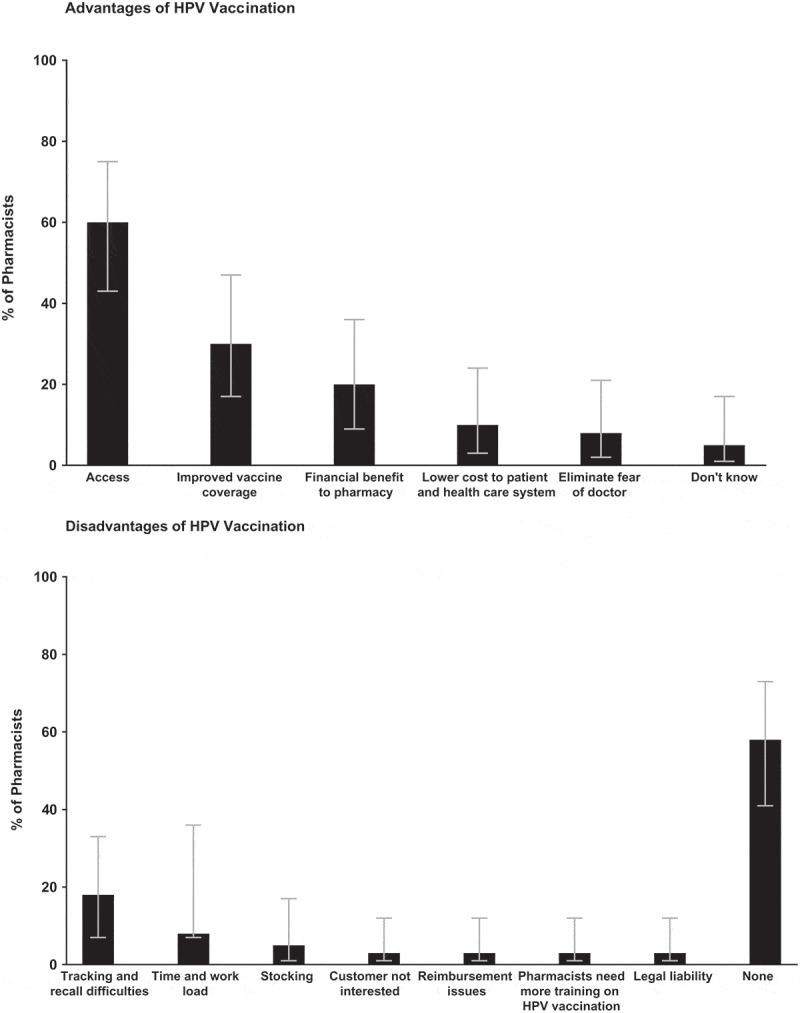
U.S. pharmacist insights into advantages and disadvantages of human papillomavirus (HPV) vaccination in eight states.
Vaccination operations and communication procedures
Pharmacists were surveyed on immunization-related operational procedures, as well as, communication practices with patients and their primary care providers. Operationally, pharmacists cited pharmacy management or corporate notifications as the most common method to identify new national vaccination recommendations (73%) (Table 3). For prescriptions, almost half of pharmacists reported their patients were not required to present with a prescription for HPV vaccination, per state law authorization. About one-third of pharmacists, however, required a prescription for HPV vaccination when the patient’s age was outside the vaccination’s recommended age range per state regulations. Most pharmacists required adolescents to have only parental consent (68%) or consent of both the parent and the adolescent (28%) to obtain a vaccination (Table 3). Ninety-seven percent of pharmacists accepted a parental written consent document for an adolescent to obtain vaccination at their pharmacy.
Table3.
Vaccination operations and communication procedures of U. S. pharmacies in eight states.
| Total (n = 40) |
|
|---|---|
| n (%) | |
| Methods of obtaining new vaccine recommendations* | |
| Pharmacy management or corporate notification | 29 (73) |
| CDC/ACIP recommendations | 8 (20) |
| Local/state government alerts | 6 (15) |
| Practice or professional society newsletter | 4 (10) |
| Other† | 4 (10) |
| Requires patients to present with prescription for HPV vaccination ‡ | |
| Yes | 2 (5) |
| No | 18 (47) |
| Depends on vaccine | 3 (8) |
| Required if outside of age range per state protocol | 13 (34) |
| All except flu vaccine | 3 (8) |
| Pharmacy requires minor permission or parental consent or both to immunize adolescents | |
| Yes, parental consent only | 27 (68) |
| Yes, both | 11 (28) |
| Yes, minor assent only | 1 (3) |
| Don’t know | 1 (3) |
| Methods utilized to obtain parental consent* | |
| Written consent document | 37 (97) |
| Verbal consent to pharmacist | 3 (8) |
| Electronic consent form | 1 (3) |
| Requires pharmacists be under collaborative practice agreement with clinical prescriber§ | |
| Yes | 38 (97) |
| Depends on vaccine | 1 (3) |
| Pharmacy provides patients with vaccination record to give to their provider | 36 (90) |
| Methods used to report vaccination records to primary care providers* | |
| Fax | 31 (78) |
| Electronic medical records | 7 (18) |
| Handled by corporate office of pharmacy | 2 (5) |
| Paper documentation given to patient | 1 (3) |
| None | 2 (5) |
| Utilizes state immunization information system | |
| Yes | 13 (33) |
| No | 19 (48) |
| Don’t know | 8 (20) |
| Procedures used for patient reminders of multiple-dose vaccines* | |
| Telephone reminders | 22 (55) |
| Mail reminders | 3 (8) |
| Email reminder | 1 (3) |
| Other | 5 (13) |
| None | 5 (13) |
| Don’t know | 10 (25) |
* Totals may exceed 100%, multiple answers possible
† Email, chart, phone application, district supervisor updates pharmacists every few weeks by tracking patient eligibility
‡ Two responses missing or not applicable (n = 38)
§ One missing response (n = 39)
Abbreviations: Human papillomavirus (HPV), United States (U.S.), Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices (ACIP)
Almost all pharmacists were required to be under a collaborative practice agreement with an authorized clinical prescriber to administer vaccines. Most pharmacists had strong communication practices with primary care providers: ninety percent of pharmacists provided patients with vaccination records to give to their primary care provider. Fax (78%) was the most common method used to report vaccination records to primary care providers. Only 5% of pharmacists had no method to report vaccination records to a patient’s primary care provider.
One-third of pharmacists reported utilizing the state immunization information system (IIS) to record administered vaccines. Pharmacists most commonly used telephone reminders (55%) to notify patients of upcoming doses of multiple-dose vaccines. Pharmacists who provided HPV vaccination were more likely to have a procedure to track the number of vaccination doses (89%), as compared to those who did not provide HPV vaccination (59%) (Figure 2). Both pharmacists who provided and did not provide HPV vaccination utilized common methods to track number of vaccination doses, including, electronic records (28%, 14% respectively), print or tracking records by hand (28%, 14%), and patient self-report (28%, 14%).
Figure 2.
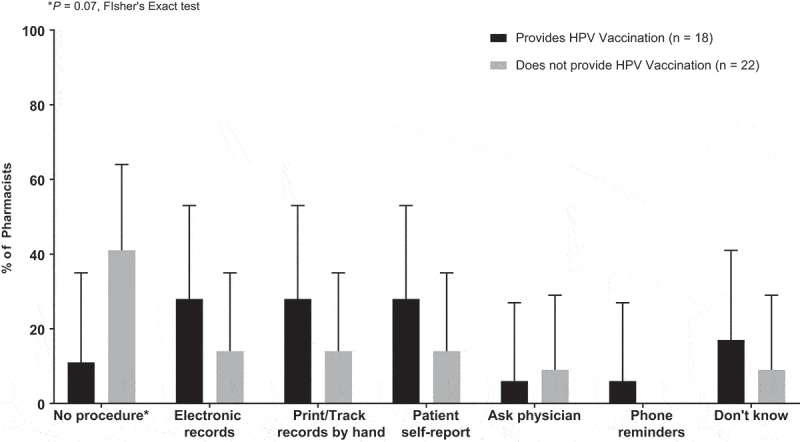
U.S pharmacy procedures for tracking number of doses of multiple-dose vaccines stratified by current HPV vaccination provision status.
Discussion
In this survey of pharmacists providing vaccination in eight states, access and improved vaccine coverage were the most commonly cited advantages to providing adolescent HPV vaccination in pharmacies. Almost all pharmacists had strong communication procedures with physicians, and regularly provided vaccination reports to their patients’ primary care providers through fax or electronic medical records. However, only one-third of pharmacists utilized their state’s immunization information system (IIS), indicating a need to improve reporting practices of vaccination administration to state registries.
Our study identified improved access and vaccine coverage as the most important advantages to providing HPV vaccination in pharmacies. This finding is consistent with prior studies which found that the availability of community pharmacists generally increased the coverage of vaccinations administered.19-23 For example, based on national-level data published by the Behavioral Risk factors Surveillance System (BRFSS), influenza vaccination rates steadily improved among young adults following the implementation of state-level policies to allow pharmacy-based influenza vaccination.23 Community pharmacies offer consistent hours and days of operation, and more flexible scheduling than physicians’ offices or clinics.20,24 Given that pharmacists can administer vaccines to patients that may have difficulty accessing traditional physicians’ offices, pharmacists’ flexibility allows for improved access to immunization services. In-pharmacy HPV vaccination can improve disparities in vaccination coverage among certain groups, such as rural populations or adolescents from low socioeconomic backgrounds with limited access to primary health care services.25 Indeed, implementation of supermarket chain pharmacy immunization programs in rural areas has improved access and visibility of in-pharmacy vaccination and led to an increased uptake of influenza and pneumococcal immunization.24,26
Despite the success of pharmacy-based vaccination programs for influenza27 and herpes zoster,18,26 the uptake of HPV vaccination in pharmacies continues to lag. In 2013, the American Pharmacists Association (APhA) reported that while 88% and 75% of pharmacies administered influenza and pneumococcal vaccines, respectively, only 37% administered HPV vaccination.28 Similarly, we found that less than half of pharmacists offered HPV vaccination at their respective pharmacy, however, all pharmacists offered influenza vaccination to adolescents. The proportion of HPV vaccination providers in our survey may be influenced by state authority to administer HPV vaccination in pharmacies. For example, at the time of this survey, pharmacists from Maine were unable to provide HPV vaccination to any age groups. Each state has its own mandate on appropriate age range for HPV vaccination administration in pharmacies.29 Although state laws allowing in-pharmacy vaccination was not a major challenge identified by pharmacists included in this survey, expanding pharmacists’ ability to provide HPV vaccination to adolescent age range could have a meaningful impact on immunization rates.29
State laws authorizing pharmacists to administer HPV vaccination to adolescents will not alone improve HPV vaccination coverage in the US.30 Among pharmacists who did offer HPV vaccination in our study, roughly 25% described their HPV vaccination program efforts as unsuccessful, as they were generally unable to identify patients interested in obtaining in-pharmacy vaccination. Similarly, a recent study conducted among pharmacists in Alabama identified insufficient patient demand as a barrier to HPV vaccination uptake at pharmacies.17 The lack of demand, and in turn, poor uptake of in-pharmacy HPV vaccination, may be due to relatively low public awareness of the availability of vaccinations at pharmacies. Additionally, perspectives among pharmacists about the suitability of adolescent in-pharmacy HPV immunization should be considered. Although not specifically addressed in this study, not all pharmacists may be proponents of HPV vaccination despite institutional recognition of its importance. Sub-optimal support of HPV vaccination among pharmacists may directly translate into ineffective promotion of adolescent vaccinations by pharmacists or other staff.16
Our study provides unique insights into operational procedures for vaccine delivery in pharmacies. We found that only one-third of pharmacists utilized their state’s immunization information system (IIS), also known as “immunization registries.” IIS is a CDC-recommended population-based vaccination registry for participating providers to record administered vaccination doses.31 The immunization registry consolidates fragmented immunization records of patients who seek care from multiple providers, and provides clear guidance on the latest immunization recommendations.32 Barriers to effective use of IIS have been identified by the American Immunization Registry Association (AIRA),33 including ISS system restrictions on the types of data collection methods to document administered vaccinations, and variations in the IIS reporting forms and reporting timelines between states.33 Variations in IIS specifications by state require substantial efforts of pharmacy staff to configure, compile, and transmit pharmacy data on a weekly basis.33 Although we were unable to assess barriers to IIS utilization among our sample of pharmacists, the low uptake of IIS may reflect similar experiences among participating pharmacies in our study.
As advantages, community chain pharmacies have the potential to establish the necessary infrastructure for effective vaccination delivery. In 2007, a study of eight pharmacy chains in California found pharmacies had appropriate procedures to support vaccination of adolescents.34 Chain pharmacies have the necessary patient screening forms, require parental consent for minors, distribute vaccine information statements, and have procedures in place for follow-up and reporting of adverse reactions following vaccination.34 However, challenges of in-pharmacy vaccination include barriers in technology and lack of systems to track multiple doses.35,36 Computerized tracking of patient records in pharmacies has the potential to improve communication between vaccine providers, reduce immunization errors such as incorrect dosing or providing already administered vaccines, and improve follow-up for completion of dosage administration.37 In our study, pharmacists who provided HPV vaccination had procedures for patient reminders of multiple doses, including telephone and mail reminders. However, pharmacists who did not administer HPV vaccination often did not have adequate procedures or systems to track the number of doses administered to their adolescent patients, which is crucial to ensuring dose completion. It is important to note that pharmacies without procedures to track dosage may not regularly administer multiple-dose vaccinations and, as such, had not found it necessary to have such systems in place. Procedures to track vaccination dosage used by pharmacies who do administer HPV vaccination varied widely, and some even relied on patient self-report for dose recall. Self-report and hand records are prone to human error and may lead to inaccurate dosage records. Improvements to tracking and recall of patient vaccination dosage in pharmacies could aid for successful immunization program implementation.
Among study strengths, our survey interviews included questions to elicit open-ended and in-depth responses on barriers and facilitators to HPV vaccination administration in pharmacies. We provide novel data on operational factors of in-pharmacy vaccination, including communication practices between pharmacists and physicians, parental consent practices to obtain HPV vaccination, and systems for patient reminders of multiple-dose vaccines. Our study was conducted in 8 US states to provide a sample of pharmacist views towards on-site vaccination from a range of policy environments. To our knowledge, we present the first data on pharmacist views of HPV vaccination in pharmacies from the following states: California, Indiana, Kentucky, Maine, Tennessee, Texas, and Washington. However, our main study limitation is the small sample size, which limited our ability to conduct stratified analyses, although this is consistent with sample sizes of qualitative methods research. Additionally, we used a convenience sample of pharmacists from eight states identified through a voluntary database of vaccine providers, which may impact the generalizability of our findings. Generalizability of findings from this study may have been improved if a random sample of all pharmacists, including both vaccine providers and non-providers, were included.
In our study, we did not examine pharmacists’ competency to provide HPV vaccination, although one prior study conducted in Utah found that despite high knowledge of HPV and the benefits of HPV vaccination, only one-third of pharmacists actually recommended HPV vaccination to adolescents.”16 In the coming years, recent updates to the doctor of pharmacy (Pharm D) curriculum may improve the competence of pharmacists to provide in-pharmacy HPV vaccination. As of 2016, Pharm D training programs are now required to include training on immunization across the lifespan, including HPV vaccination.35 With required training, pharmacist’s attitude and knowledge may improve and in turn improve vaccination recommendation practices among pharmacists.
In conclusion, our study findings provide insights into the role of pharmacists in vaccination and potential strategies to improve adolescent in-pharmacy vaccination uptake. Lessons learned through these pharmacist interviews could inform implementation of in-pharmacy HPV vaccination programs.
Methods
Participants
Between June to November 2014, we recruited a convenience sample of 40 pharmacists from eight states: Alabama, California, Indiana, Kentucky, Maine, Tennessee, Texas, and Washington. Pharmacists were eligible to participate if they previously administered or were currently administering1 any type of vaccine to adults (≥18 years of age) and2 HPV, Tdap or TD (tetanus and diphtheria), or MenACWY vaccines to adolescents (≥9 and <18 years of age).
We recruited a convenience sample of pharmacists using HealthMap Vaccine Finder (vaccinefinder.org), a free, searchable, opt-in database of vaccine providers across the US.38 HealthMap Vaccine Finder included 38,813 pharmacy locations across all 50 states, the District of Columbia, and Puerto Rico at the end of the 2012–2013 influenza season.39 In June 2014, we obtained all pharmacy data from the HealthMap research investigators for pharmacies providing HPV, Tdap/TD or MenACWY vaccinations. Information provided by HealthMap included store number, site or clinic name, vaccines provided, start and end dates for vaccine provision, address, phone number, and hours of operation. Using the pharmacy data, we created a sampling list stratified by all states across the US. Next, we randomly selected pharmacies to contact by telephone from the sampling list, stratified by state. Utilizing a standardized script, we identified eligible pharmacists available at the pharmacy location and were willing to participate by responding to questions concerning their pharmacy.
A total of 40 licensed pharmacists were recruited, five in each of the eight states, as previously described.18 A broad geographic distribution of states was included to obtain lessons learned from pharmacists under varied state vaccination laws. These laws varied based on the authority that states granted pharmacists to administer vaccines, including HPV, Tdap and MenACWY.29 In Alabama and Indiana, pharmacists were allowed to administer vaccines to all age groups, yet only with a prescription from a primary care physician.29 Similar policies applied in Kentucky and Texas for vaccinations administered to those 9 and 12 years of age.29 Additionally, pharmacists from Kentucky and Texas were able to administer vaccines to adults (19 years and above) upon the signing of a collaborative agreement with a prescriber,29 which allowed pharmacists to administer vaccines to patients regardless of their primary care doctor. In California, Tennessee and Washington, pharmacists were able to administer vaccines to any age-group, also based on a collaborative agreement with a physician prescriber.29 State policies on vaccination authority in Maine did not permit pharmacists to administer the HPV or MenACWY vaccines to adolescents over 12 years of age and adults,29 however, pharmacists were authorized to provide Tdap with a prescription from a provider.29
Procedures and measures
Study methods were previously published in a survey on in-pharmacy adolescent and adult immunizations in general.18 Here, we focus specifically on into facilitators and barriers to adolescent HPV vaccination in pharmacies. In brief, enrolled pharmacists completed a semi-structured interview administered by telephone. Study interviewers were trained on survey implementation, and used a standardized script to administer the survey. The survey included 52 close-ended questions, and 24 open-ended questions to assess pharmacist insights into administering adolescent and adult vaccines within pharmacies (Appendix 1). Participants were able to provide open-ended responses on any number of challenges or facilitators to adolescent vaccinations which they experienced as practicing pharmacists. Questions covered pharmacy contextual information; demographics of clients served; pharmacist education and training; pharmacy operational issues related to vaccination provision; vaccination provision within pharmacies (i.e., whether a vaccine is offered at the pharmacy for administration); insurance reimbursement; healthcare communication; and minor consent procedures. We specifically probed into facilitators and barriers to HPV vaccination in pharmacies using the following questions: (1) What is the most important challenge to providing the HPV vaccine to adolescents in your pharmacy? (2) What has helped make HPV vaccination more successful in your pharmacy? (3) What, if any, advantages do you see to offering HPV vaccination in pharmacies? and (4) What, if any, disadvantages do you see to offering HPV vaccination in pharmacies? Interviews lasted 30–60 minutes.
Pharmacists received an incentive of $50 for completing the interview. The UNC Institutional Review Board reviewed the study protocol and deemed the study to be non-human subjects’ research.
Data analyses
Semi-qualitative responses were recorded verbatim, transcribed by a trained interviewer, and entered into the study database as free text. A qualitative data analyst reviewed the free text fields and combined semi-qualitative responses with similar themes to create response categories. A thematic analysis framework was used to code the data. Additional codes were added as they emerged from the data through an iterative process. We summarized coded response categories using descriptive frequency statistics.
We assessed the frequency of pharmacy and pharmacist characteristics, pharmacists’ insight into vaccination delivery, and operational practices in U.S. pharmacies. We calculated the percent and exact 95% confidence intervals (CI) of pharmacists’ insight into advantages and disadvantages of HPV vaccination delivery and procedures for tracking dosage of multiple-dose adolescent vaccinations. Fisher’s exact test was conducted to evaluate statistical differences in procedures for tracking number of doses of multiple-dose vaccines by HPV vaccination provision status. We used SAS version 9.1 (SAS Institute, Cary, NC) to perform statistical analyses.
APPENDIX 1: Pharmacist Questionnaire
1. How many pharmacists work at your pharmacy? This includes floating or part-time pharmacists. ______ [Record number]
2. How many pharmacists at your pharmacy have training that allows them to administer vaccines?
__________ [Record number of pharmacists]
88 = Don’t know
3. How many pharmacists at your pharmacy site are currently administering vaccines?
__________ [Record number of pharmacists]
88 = Don’t know
4. What other types of health care providers, if any, administer vaccines at your pharmacy?
1 = Nurses
2 = Nurse practitioners
3 = Doctors
1 = No other types
99 = Other
5. What vaccines does your pharmacy currently provide to adolescents?
[Select all that apply]
Just to confirm, your pharmacy does not provide (read vaccines not listed). Is this correct?
For (list vaccine), what age range do you vaccinate?
| Immunization | Age Range | |
| Influenza | □ | |
| Pneumococcal | □ | |
| Tdap (adolescent/adult tetanus, diphtheria & pertussis) | □ | |
| Td (tetanus, diphtheria) | □ | |
| Hepatitis A | □ | |
| Hepatitis B | □ | |
| HPV (Human Papillomavirus) | □ | |
| MCV4 (meningococcal) | □ | |
| Zoster (shingles) | □ | |
| Travel vaccines (Yellow fever, Japanese Encephalitis, polio, typhoid, and rabies) | □ |
6. What vaccines does your pharmacy currently provide to adults?
[Select all that apply]
Just to confirm, your pharmacy does not provide (read vaccines not listed). Is this correct?
| Immunization | |
| Influenza | □ |
| Pneumococcal | □ |
| Tdap (adolescent/adult tetanus, diphtheria & pertussis) | □ |
| Td (tetanus, diphtheria) | □ |
| Hepatitis A | □ |
| Hepatitis B | □ |
| HPV (Human Papillomavirus) | □ |
| MCV4 (meningococcal) | □ |
| Zoster (shingles) | □ |
| Travel vaccines (Yellow fever, Japanese Encephalitis, polio, typhoid, and rabies) | □ |
7. Are there any vaccines that your pharmacy does not currently administer that your pharmacy previously administered?
1 = Yes
1 = No [Skip to Q10]
8. What vaccines did your pharmacy previously provide to adolescents?
[Select all that apply]
| Immunization | |
| Influenza | □ |
| Pneumococcal | □ |
| Tdap (adolescent/adult tetanus, diphtheria & pertussis) | □ |
| Td (tetanus, diphtheria) | □ |
| Hepatitis A | □ |
| Hepatitis B | □ |
| HPV (Human Papillomavirus) | □ |
| MCV4 (meningococcal) | □ |
| Zoster (shingles) | □ |
| Travel vaccines (Yellow fever, Japanese Encephalitis, polio, typhoid, and rabies) | □ |
9. What vaccines did your pharmacy previously provide to adults?
[Select all that apply]
| Immunization | |
| Influenza | □ |
| Pneumococcal | □ |
| Tdap (adolescent/adult tetanus, diphtheria & pertussis) | □ |
| Td (tetanus, diphtheria) | □ |
| Hepatitis A | □ |
| Hepatitis B | □ |
| HPV (Human Papillomavirus) | □ |
| MCV4 (meningococcal) | □ |
| Zoster (shingles) | □ |
| Travel vaccines (Yellow fever, Japanese Encephalitis, polio, typhoid, and rabies) | □ |
10. How many years has your pharmacy been providing any type of vaccine to adolescents or adults? ______ [Record number of years]
888 = Don’t Know
11. How would you describe your pharmacy practice type?
1 = Community – chain
2 = Community – independent
3 = Hospital/clinic located
88 = Don’t Know
99 = Other, please specify ____________________________
12 How many injections do you estimate that your pharmacy gave in the past year? _____ [Record number]
8888 = Don’t Know
13 Would you say your pharmacy could vaccinate more people, you are right at capacity, or you are having to turn people away??
1 = Could vaccinate more people
2 = At Capacity
3 = Have to turn people away
88 = Don’t know
14 Does your pharmacy plan to maintain, expand or reduce vaccines offered in the future?
1 = Maintain the same offerings
2 = Expand the vaccination offerings
3 = Reduce the vaccination offerings
88 = Don’t know
15 What type of training does your pharmacy require that pharmacists complete in order to administer immunizations? [Check all that apply]
1 = APhA Pharmacy-based Immunization Program
2 = Other Accreditation Council for Pharmacy Education approved certificate program
3 = Class/elective in pharmacy school
4 = BLS certification
0 = None
88 = Don’t know
99 = Other, please specify ____________________________
16 Does your pharmacy management require that pharmacists obtain continuing education on immunizations?
1 = Yes
1 = No
88 = Don’t know
17 How does your pharmacy track current and new recommendations for adolescent and adult vaccine types and doses? [Select all that apply]
1 = Pharmacy management notice
2 = Practice or professional society newsletter
3 = Alert from local/state government
99 = Other, please specify ________________________
18 How are vaccine administration records maintained at the pharmacy? [Select all that apply]
1 = Vaccine Administration Record (VAR) entered into the pharmacy computer
2 = Paper records are maintained in the pharmacy
3 = Pharmacy updates patient’s personal immunization record or issues a new one
99 = Other, please specify ____________________________
19 Do you update patient records in the state immunization information system (IIS)?
2 = Yes
1 = No
88 = Don’t know
20 Some pharmacists are concerned about maintaining their records on vaccine coverage, including tracking the number of doses. What have you done to address this challenge?
____________________________ [Record open-ended]
21 Once patients receive one dose of a vaccine that requires 2 or more doses, do you remind them to come in for the additional doses?
1 = Yes
1 = No [Skip to Q22]
88 = Don't know [Skip to Q22]
22 What do you do to remind patients?
1 = Mail reminders
2 = Email reminders
3 = Telephone reminders
99 = Other
23 What are the three most important challenges to providing immunizations to adolescents in your pharmacy?
1 = State laws that prevent it
2 = Staff support
3 = Time constraints
4 = Legal liability
5 = Pharmacy can’t handle an adverse reaction
6 = Low reimbursement
7 = Not being able to bill insurance
8 = Access to Vaccines for Chidren (VFC)
9 = Availability of administration area
10 = Availability/cost of vaccine storage
11 = State's reporting requirements
12 = Changing/confusing vaccine recommendations
13 = Parental consent/consent issues
88 = Don’t know
99 = Other, please specify ____________________________
24 Which, if any, of these challenges has your pharmacy found a way to overcome?
1 = State laws that prevent it
2 = Staff support
3 = Time constraints
4 = Legal liability
5 = Pharmacy can’t handle an adverse reaction
6 = Low reimbursement
7 = Not being able to bill insurance
8 = Access to Vaccines for Chidren (VFC)
9 = Availability of administration area
10 = Availability/cost of vaccine storage
11 = State's reporting requirements
12 = Changing/confusing vaccine recommendations
13 = Parental consent/consent issues
88 = Don’t know
99 = Other, please specify ____________________________
25 [If any selected in 24] How has your pharmacy overcome these challenges? [List each challenge and then explain how it was overcome] __________________________________
26 What do you think is required to make a successful adolescent vaccination program in a pharmacy? __________________________________ [Record open-ended]
27 What has helped make vaccination of adolescents successful for your pharmacy? [Select all that apply]
1 = State legislative authority to provide vaccine
2 = Clear guidelines from pharmacy/corporate management
3 = Promotion outside of the pharmacy (television/radio/internet)
4 = Promotion within the pharmacy (signs/brochures)
5 = Better reimbursement of adolescent vaccines
6 = Other staff who can provide vaccines (nurses, nurse practitioners)
7 = Designated space for vaccination (or patient care)
88 = Don’t know
99 = Other (please specify)____________________________
28 What are the three most important challenges to providing immunizations to adults in your pharmacy?
1 = State laws that prevent it
2 = Staff support
3 = Time constraints
4 = Legal liability
5 = Pharmacy can’t handle an adverse reaction
6 = Low reimbursement
7 = Not being able to bill insurance
8 = Access to Vaccines for Chidren (VFC)
9 = Availability of administration area
10 = Availability/cost of vaccine storage
11 = State's reporting requirements
12 = Changing/confusing vaccine recommendations
13 = Parental consent/consent issues
88 = Don’t know
99 = Other, please specify ____________________________
29 [If any selected in 28] Which, if any, of these challenges has your pharmacy found a way to overcome?
1 = State laws that prevent it
2 = Staff support
3 = Time constraints
4 = Legal liability
5 = Pharmacy can’t handle an adverse reaction
6 = Low reimbursement
7 = Not being able to bill insurance
8 = Access to Vaccines for Chidren (VFC)
9 = Availability of administration area
10 = Availability/cost of vaccine storage
11 = State's reporting requirements
12 = Changing/confusing vaccine recommendations
13 = Parental consent/consent issues
88 = Don’t know
99 = Other, please specify ____________________________
30 How has your pharmacy overcome these challenges? [List each challenge and then explain how it was overcome] __________________________________
31 What do you think is required to make a successful adult vaccination program in a pharmacy? _____________________________________ [Record open-ended]
32 What has helped make vaccination of adults successful in your pharmacy? [Select all that apply]
1 = State legislative authority to provide vaccines
2 = Clear guidelines from pharmacy management/corporate office
3 = Promotion outside of the pharmacy (television/radio/internet)
4 = Promotion within the pharmacy (signs/brochures)
5 = Better reimbursement of adolescent vaccines
6 = Other staff who can provide vaccines (nurses, nurse practitioners)
7 = Designated space for vaccination (or patient care)
88 = Don’t know
99 = Other (please specify)____________________________
33 Is there any vaccine that you find especially difficult compared to others to administer to adolescents? [Select all that apply]
1 = Influenza
2 = Pneumococcal
3 = Tdap (adolescent/adult tetanus, diphtheria & pertussis)
4 = Td (tetanus, diphtheria)
5 = Hepatitis A
6 = Hepatitis B
7 = HPV (Human Papillomavirus)
8 = MCV4 (meningococcal)
9 = Travel vaccines (Yellow fever, Japanese Encephalitis, polio, typhoid, and rabies)
88 = Don’t know
34 Is there any vaccine that you find more difficult than others to administer to adults? [Select all that apply]
1 = Influenza
2 = Pneumococcal
3 = Tdap (adolescent/adult tetanus, diphtheria & pertussis)
4 = Td (tetanus, diphtheria)
5 = Hepatitis A
6 = Hepatitis B
7 = HPV (Human Papillomavirus)
8 = MCV4 (meningococcal)
9 = Zoster
10 = Travel vaccines (Yellow fever, Japanese Encephalitis, polio, typhoid, and rabies)
88 = Don’t know
35 What, if anything, makes it difficult to administer these vaccines? [Select all that apply]
1 = Staff support
2 = Time constraints
3 = Legal liability
4 = Pharmacy can’t handle an adverse reaction
5 = Reimbursement Issues
6 = Availability of administration area
7 = Availability/cost of vaccine storage
8 = State's reporting requirements
0 = Nothing
88 = Don’t know
99 = Other, please specify ___________________
36 Which of the following factors do you perceive as benefits to providing immunization services within your pharmacy? [Read responses aloud. Select all that apply]
1 = Increases access to vaccine for patients
2 = More convenient for patients
3 = Allows me to have more direct interactions with patients
4 = Brings financial benefit to my pharmacy
5 = Increases professional satisfaction
0 = No benefits
88 = Don’t know
37 [Q1 = Stopped providing 1 or more immunizations]: What caused you or your pharmacy to stop administering vaccines? [Select all that apply]
1 = Too little staff support
2 = Time constraints
3 = Pharmacy can’t handle adverse reaction
4 = Management did not want to handle legal liability
5 = Reimbursement issue
6 = Unavailability of administration area
7 = Unavailability/cost of vaccine storage
8 = State's reporting requirements
9 = Changing/confusing vaccine recommendations
88 = Don’t know
99 = Other, please specify ____________________________
38 What are the challenges or concerns in your pharmacy specific to administering vaccines that require multiple doses? [Select all that apply]
1 = Tracking and recall of patients
2 = Reporting to patients’ medical home
3 = Staff support
4 = Time constraints
6 = Refrigerator/storage space
7 = Changing/confusing vaccine recommendations
8 = Requiring multiple prescriptions/referrals from providers
0 = None
88 = Don’t know
99 = Other, please specify ____________________________
39 What kinds of concerns, if any, have you heard from patients about getting vaccines for their adolescent children in pharmacies? [Select all that apply]
1 = Insurance might not cover it
2 = Staff might not be good at giving shots
3 = I want my child’s doctor to keep track of his/her shots and other health history
4 = Getting it there would embarrass him/her
5 = I'd want to be there when he/she got it
6 = Concerns over adverse drug reactions
0 = No concerns
99 = Other, please specify _____________________
40 What kinds of concerns have you heard from adult patients about getting vaccines for themselves or adult family members in pharmacies?
1 = Insurance might not cover it
2 = Staff might not be good at giving shots
3 = I want my doctor to keep track of our family’s shots
4 = Getting it there would embarrass me/my family member
5 = Concerns over adverse drug reactions
0 = No concerns
99 = Other, please specify: _____________________
41 [If Q1 = Is currently providing HPV vaccine to adolescents] What is the most important challenge to providing the HPV vaccine to adolescents in your pharmacy?
1 = State laws that prevent it
2 = Staff support
3 = Time constraints
4 = Legal liability
5 = Pharmacy can’t handle an adverse reaction
6 = Reimbursement issues
7 = Availability of administration area
8 = Availability/cost of vaccine storage
9 = State's reporting requirements
10 = Changing/confusing vaccine recommendations
11 = Parental concent/consent issues
88 = Don’t know [Skip to Q51]
99 = Other, please specify ____________________________
42 [If Q1 = Is currently providing HPV vaccine to adolescents] Have you or your pharmacy found a way to overcome this challenge?
1 = Yes
1 = No [Skip to 51]
88 = Don’t know [Skip to 51]
43 [If Q1 = Is currently providing HPV vaccine to adolescents] How has your pharmacy overcome this challenge?__________________________________ [Record open-ended]
44 [If Q1 = Is currently providing HPV vaccine to adolescents] Do you feel very comfortable, somewhat comfortable, neutral, somewhat uncomfortable, or very uncomfortable administering the HPV vaccine to adolescents?
1 = Very comfortable [Skip to Q52]
2 = Somewhat comfortable [Skip to Q52]
3 = Neutral [Skip to Q52]
4 = Somewhat uncomfortable
5 = Very uncomfortable
88 = Don’t know [Skip to Q52]
45 [If Q1 = Is currently providing HPV vaccine to adolescents] What makes you feel uncomfortable? ___________________ [Record open-ended]
46 [If Q1 = Is currently providing HPV vaccine to adolescents or adults] What has helped make HPV vaccination more successful in your pharmacy? [Select all that apply]
1 = State legislative authority to provide vaccine
2 = Clear guidelines from pharmacy/corporate management
3 = Promotion outside of the pharmacy (television/radio/internet)
4 = Promotion within the pharmacy (signs/brochures)
5 = Better reimbursement of adolescent vaccines
6 = Other staff who can provide vaccines (nurses, nurse practitioners)
7 = Designated space for vaccination (or patient care)
88 = Don’t know
99 = Other (please specify)____________________________
47 [If Q1 = Not currently providing HPV vaccine to adolescents] How comfortable would you feel administering the HPV vaccine to adolescents?
1 = Very comfortable [skip to 57]
2 = Somewhat comfortable [skip to 57]
3 = Neutral [skip to 57]
4 = Somewhat uncomfortable [skip to 57]
5 = Very uncomfortable [skip to 57]
88 = Don’t know [skip to 57]
48 What, if any, advantages do you see to offering HPV vaccination in pharmacies?
___________________ [Record open-ended]
49 What, if any, disadvantages do you see to offering HPV vaccination in pharmacies?
___________________ [Record open-ended]
50 What types of payment, such as Medicare, Medicaid, private insurance, etc., does your pharmacy accept for vaccination? [Select all that apply]
1 = Medicare
2 = Medicaid
3 = Children’s Health Insurance Program (CHIP)
4 = Private insurance
5 = Tricare (Military insurance)
6 = Out of pocket / self-pay
88 = Don’t know
99 = Other
51 Is the level of reimbursement a challenge for providing adolescent or adult vaccines in your pharmacy?
1 = Yes
1 = No [Skip to Q65]
88 = Don’t know [Skip to Q65]
52 For which specific vaccines is the level of reimbursement a challenge or barrier?
1 = Influenza
2 = Pneumococcal
3 = Tdap (adolescent/adult tetanus, diphtheria & pertussis)
4 = Td (tetanus, diphtheria)
5 = Hepatitis A
6 = Hepatitis B
7 = HPV (Human Papillomavirus)
8 = MCV4 (meningococcal)
9 = Zoster (shingles)
10 = Travel vaccines (Yellow fever, Japanese Encephalitis, polio, typhoid, and rabies)
88 = Don’t know
53 Is reimbursement from private insurance enough to completely cover vaccine administration costs (your time, vaccine stocking, refrigerator space, etc.) for adolescent and adult vaccines?
1 = Yes, true for all vaccines and insurance plans
2 = Yes, for some vaccines and insurance plans only
0 = No
88 = Don’t know
54 Is reimbursement from public insurance enough to completely cover vaccine administration costs (your time, vaccine stocking, refrigerator space, etc.) for adolescent and adult vaccines?
1 = Yes, true for all vaccines and insurance plans
2 = Yes, for some vaccines and insurance plans only
0 = No
88 = Don’t know
55 Do you require patients to have a current prescription from a physician to receive a vaccine?
1 = Yes
1 = No
88 = Don’t know
56 Do you report information on vaccination of adolescents and adults back to their medical provider?
1 = Yes [Skip to Q68]
1 = No
88 = Don’t know [Skip to Q69]
57 Why don’t you report information on vaccination back to their medical provider?
1 = Time constraints [Skip to Q69]
2 = No process in place for contacting PCP[Skip to Q69]
3 = State law does not require reporting to PCP [Skip to Q69]
88 = Don’t know [Skip to Q69]
99 = Other, please specify _______________ [Skip to Q69]
58 How do you share information on the vaccination of adolescents and adults back to the patients’ medical provider? [Select all that apply]
1 = Electronic medical record
2 = Paper documenting vaccination given to patient
3 = Mailed paper documenting vaccination given
4 = Email
5 = Phone call to provider
6 = Fax
99 = Other, please specify ________________
59 Do you also provide patients with their own record to give to their primary provider?
1 = Yes
1 = No
88 = Don’t know
69 Does your pharmacy require minor permission, parental consent, or both to immunize individuals under 18 years of age?
1 = Yes, both
2 = Yes, parental consent only
3 = Yes, minor assent only
1 = No [Skip to Q75]
88 = Don’t know [Skip to Q75]
70 How do you obtain parental consent to administer vaccines to adolescents? _________________________ [Record open-ended]
71 Do you have a different or additional procedure for obtaining minor assent or parental consent to provide HPV vaccine?
1 = Yes, both
2 = Yes, parental consent only
3 = Yes, minor assent only
1 = No
88 = Don’t know
72 What is your age? ________________ [Record number]
777 = Prefer not to answer
73 What is your race or ethnicity? [Select all that apply]
1 = White
2 = Black or African American
3 = Asian
4 = Native Hawaiian or Pacific Islander
5 = American Indian or Alaska Native
99 = Other, please specify ________________
777 = Prefer not to answer
74 What is your gender?
1 = Male
2 = Female
777 = Prefer not to answer
75 What is your highest pharmacy degree?
1 = PharmD
2 = BSPharm
99 = Other (please specify) ________________
777 = Prefer not to answer
76 In what year did you earn this degree? _____ [Record year]
777 = Prefer not to answer
Funding Statement
Funding for this research was provided by Merck and Co., Inc., in Kenilworth, NJ USA (VT ID #50873). JYI was supported by a NIH NRSA individual predoctoral fellowship funded by the NCI (F31-CA210474-01A1);
Abbreviations
- ACS
American Cancer Society
- ACIP
Advisory Committee on Immunization Practices
- BRFSS
Behavioral Risk Factors Surveillance System
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- HPV
Human papillomavirus
- IRB
Institutional Review Board
- MenACWY
Meningococcal conjugate vaccine
- NIS-Teen
National Immunization Survey-Teen
- PCP
Presidential Cancer Panel
- TD
Tetanus and diphtheria vaccine
- Tdap
Tetanus, diphtheria, and pertussis vaccine
- US
United States
Acknowledgments
We would like to thank the organization HealthMap Vaccine Finder for generously providing their database of pharmacy providers for this research.
Disclosure of potential conflicts of interest
JSS has received unrestricted educational grants, consultancy, and research grants from Merck Corporation and GlaxoSmithKline (GSK) over the past 5 years. All other authors have no conflicts to declare.
References
- 1.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA, Unger ER.. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR–05):1–30. PubMed PMID: 25167164. [PubMed] [Google Scholar]
- 2.Vielot NA, Butler AM, Brookhart MA, Becker-Dreps S, Smith JS. Patterns of use of human papillomavirus and other adolescent vaccines in the United States. J Adolesc Health. 2017;61(3):281–287. doi: 10.1016/j.jadohealth.2017.05.016 PubMed PMID: 28739327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–882. doi: 10.15585/mmwr.mm6633a2 PubMed PMID: 28837546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Curtis CR, MacNeil J, Markowitz LE, Singleton JA. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850–858. doi: 10.15585/mmwr.mm6533a4 PubMed PMID: 27561081. [DOI] [PubMed] [Google Scholar]
- 5.Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine. 2016;34(9):1187–1192. doi: 10.1016/j.vaccine.2016.01.023 PubMed PMID: 26812078; PubMed Central PMCID: PMCPMC4944755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah PD, Gilkey MB, Pepper JK, Gottlieb SL, Brewer NT. Promising alternative settings for HPV vaccination of US adolescents. Expert Rev Vaccines. 2014;13(2):235–246. doi: 10.1586/14760584.2013.871204 PubMed PMID: 24405401; PubMed Central PMCID: PMCPMC4267674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strickland BB, Jones JR, Ghandour RM, Kogan MD, Newacheck PW. The medical home: health care access and impact for children and youth in the United States. Pediatrics. 2011;127(4):604–611. doi: 10.1542/peds.2009-3555 PubMed PMID: 21402643. [DOI] [PubMed] [Google Scholar]
- 8.Bodenheimer T, Pham HH. Primary care: current problems and proposed solutions. Health Aff (Millwood). 2010;29(5):799–805. doi: 10.1377/hlthaff.2010.0026 PubMed PMID: 20439864. [DOI] [PubMed] [Google Scholar]
- 9.Elster A, Jarosik J, VanGeest J, Fleming M. Racial and ethnic disparities in health care for adolescents: a systematic review of the literature. Arch Pediatr Adolesc Med. 2003;157(9):867–874. doi: 10.1001/archpedi.157.9.867 PubMed PMID: 12963591. [DOI] [PubMed] [Google Scholar]
- 10.Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, Goldie SJ, Harper DM, Kinney W, Moscicki A-B, et al. American cancer society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57(1):7–28. PubMed PMID: 17237032. [DOI] [PubMed] [Google Scholar]
- 11.Fava JP, Colleran J, Bignasci F, Cha R, Kilgore PE. Adolescent human papillomavirus vaccination in the United States: opportunities for integrating pharmacies into the immunization neighborhood. Hum Vaccin Immunother. 2017:1–12. doi: 10.1080/21645515.2017.1325980 PubMed PMID: 28605256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah PD, Trogdon JG, Golden SD, Golin CE, Marciniak MW, Brewer NT. Impact of pharmacists on access to vaccine providers: A geospatial analysis. Milbank Q. 2018;96(3):568–592. doi: 10.1111/1468-0009.12342 PubMed PMID: 30203603; PubMed Central PMCID: PMCPMC6131320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calo WA, Gilkey MB, Shah P, Marciniak MW, Brewer NT. Parents’ willingness to get human papillomavirus vaccination for their adolescent children at a pharmacy. Prev Med. 2017;99:251–256. doi: 10.1016/j.ypmed.2017.02.003 PubMed PMID: 28188796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McRee A-L, Reiter PL, Pepper JK, Brewer NT. Correlates of comfort with alternative settings for HPV vaccine delivery. Hum Vaccin Immunother. 2013;9(2):306–313. PubMed PMID: 23291948; PubMed Central PMCID: PMCPMC3859752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiter PL, McRee A-L, Pepper JK, Chantala K, Brewer NT. Improving human papillomavirus vaccine delivery: a national study of parents and their adolescent sons. J Adolesc Health. 2012;51(1):32–37. doi: 10.1016/j.jadohealth.2012.01.006 PubMed PMID: 22727074; PubMed Central PMCID: PMCPMC3383639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolentino V, Unni E, Montuoro J, Bezzant-Ogborn D, Kepka D. Utah pharmacists’ knowledge, attitudes, and barriers regarding human papillomavirus vaccine recommendation. J Am Pharm Assoc (2003). 2018;58(4S):S16–S23. doi: 10.1016/j.japh.2018.04.014 PubMed PMID: 29739667. [DOI] [PubMed] [Google Scholar]
- 17.Hastings TJ, Hohmann LA, McFarland SJ, Teeter BS, Westrick SC. Pharmacists’ attitudes and perceived barriers to Human Papillomavirus (HPV) vaccination services. Pharmacy (Basel). 2017;5(3). doi: 10.3390/pharmacy5030045 PubMed PMID: 28970457; PubMed Central PMCID: PMCPMC5622357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam JY, Gruber JF, Lockhart A, Kunwar M, Wilson S, Smith SB, Brewer NT, Smith JS. Opportunities and challenges of adolescent and adult vaccination administration within pharmacies in the United States. Biomed Inform Insights. 2017;9:1178222617692538. doi: 10.1177/1178222617692538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westrick SC, Owen J, Hagel H, Owensby JK, Lertpichitkul T. Impact of the RxVaccinate program for pharmacy-based pneumococcal immunization: A cluster-randomized controlled trial. J Am Pharm Assoc (2003). 2016;56(1):29–36 e1. doi: 10.1016/j.japh.2015.11.010 PubMed PMID: 26802917. [DOI] [PubMed] [Google Scholar]
- 20.Grabenstein JD. Daily versus single-day offering of influenza vaccine in community pharmacies. J Am Pharm Assoc (2003). 2009;49(5):628–631. doi: 10.1331/JAPhA.2009.08118 PubMed PMID: 19748870. [DOI] [PubMed] [Google Scholar]
- 21.Goode JV, Mott DA, Stanley DD. Assessment of an immunization program in a supermarket chain pharmacy. J Am Pharm Assoc (2003). 2007;47(4):495–498. doi: 10.1331/JAPhA.2007.06100 PubMed PMID: 17616496. [DOI] [PubMed] [Google Scholar]
- 22.Bearden DT, Holt T. Statewide impact of pharmacist-delivered adult influenza vaccinations. Am J Prev Med. 2005;29(5):450–452. doi: 10.1016/j.amepre.2005.08.003 PubMed PMID: 16376709. [DOI] [PubMed] [Google Scholar]
- 23.Chun GJ, Sautter JM, Patterson BJ, McGhan WF. Diffusion of pharmacy-based influenza vaccination over time in the United States. Am J Public Health. 2016;106(6):1099–1100. doi: 10.2105/AJPH.2016.303142 PubMed PMID: 27077353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weitzel KW, Goode JV. Implementation of a pharmacy-based immunization program in a supermarket chain. J Am Pharm Assoc (Wash). 2000;40(2):252–256. PubMed PMID: 10730026. [DOI] [PubMed] [Google Scholar]
- 25.Ernst ME, Chalstrom CV, Currie JD, Sorofman B. Implementation of a community pharmacy-based influenza vaccination program. J Am Pharm Assoc (Wash). 1997;NS37(5):570–580. PubMed PMID: 9479410. [DOI] [PubMed] [Google Scholar]
- 26.Burson RC, Buttenheim AM, Armstrong A, Feemster KA. Community pharmacies as sites of adult vaccination: A systematic review. Hum Vaccin Immunother. 2016;12(12):3146–3159. doi: 10.1080/21645515.2016.1215393 PubMed PMID: 27715409; PubMed Central PMCID: PMCPMC5215426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald TJ, Kang Y, Bridges CB, Talbert T, Vagi SJ, Lamont B, Graitcer SB. Integrating pharmacies into public health program planning for pandemic influenza vaccine response. Vaccine. 2016;34(46):5643–5648. doi: 10.1016/j.vaccine.2016.09.020 PubMed PMID: 27686834; PubMed Central PMCID: PMCPMC5206751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annual Pharmacy-Based Influenza and Adult Immunization Survey 2013. US Department of Health and Human Services, National Vaccine Program Office 2013.
- 29.Brewer NT, Chung JK, Baker HM, Rothholz MC, Smith JS. Pharmacist authority to provide HPV vaccine: novel partners in cervical cancer prevention. Gynecol Oncol. 2014;132(Suppl 1):S3–8. doi: 10.1016/j.ygyno.2013.12.020 PubMed PMID: 24361732. [DOI] [PubMed] [Google Scholar]
- 30.Trogdon JG, Shafer PR, Shah PD, Calo WA. Are state laws granting pharmacists authority to vaccinate associated with HPV vaccination rates among adolescents? Vaccine. 2016;34(38):4514–4519. doi: 10.1016/j.vaccine.2016.07.056 PubMed PMID: 27496275; PubMed Central PMCID: PMCPMC4996696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Immunization Information Systems (IIS) Centers for disease control and prevention. 2017. [accessed 2018 March 6]. https://www.cdc.gov/vaccines/programs/iis/index.html.
- 32.Martin DW, Lowery NE, Brand B, Gold R, Horlick G. Immunization information systems: a decade of progress in law and policy. J Public Health Manag Pract. 2015;21(3):296–303. doi: 10.1097/PHH.0000000000000040 PubMed PMID: 24402434; PubMed Central PMCID: PMCPMC4671281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Survey of Immunization Reporting to Immunization Information Systems by Major U.S. Pharmacies American Immunization Registry Association. 2014.
- 34.Pilisuk T, Goad J, Backer H. Vaccination delivery by chain pharmacies in California: results of a 2007 survey. J Am Pharm Assoc (2003). 2010;50(2):134–139. doi: 10.1331/JAPhA.2010.09168 PubMed PMID: 20199953. [DOI] [PubMed] [Google Scholar]
- 35.Bach AT, Goad JA. The role of community pharmacy-based vaccination in the USA: current practice and future directions. Integr Pharm Res Pract. 2015;4:67–77. doi: 10.2147/IPRP.S63822 PubMed PMID: 29354521; PubMed Central PMCID: PMCPMC5741029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dempsey AF, Zimet GD. Interventions to improve adolescent vaccination: what may work and what still needs to be tested. Am J Prev Med. 2015;49(6 Suppl 4):S445–54. doi: 10.1016/j.amepre.2015.04.013 PubMed PMID: 26272849. [DOI] [PubMed] [Google Scholar]
- 37.Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance: part 1: childhood vaccinations. P T. 2016;41(7):426–436. PubMed PMID: 27408519; PubMed Central PMCID: PMCPMC4927017. [PMC free article] [PubMed] [Google Scholar]
- 38.HealthMap Vaccine Finder 2012. http://flushot.healthmap.org/.
- 39.Huston JE, Mekaru SR, Kluberg S, Brownstein JS. Searching the web for influenza vaccines: healthmap vaccine finder. Am J Public Health. 2015;105(8):e134–9. doi: 10.2105/AJPH.2014.302466 PubMed PMID: 25880945; PubMed Central PMCID: PMCPMC4503243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Immunization Information Systems (IIS) Centers for disease control and prevention. 2017. [accessed 2018 March 6]. https://www.cdc.gov/vaccines/programs/iis/index.html.


