ABSTRACT
High-risk human papillomavirus (HPV) types are responsible for nearly all cases of cervical cancers. Cervarix® and Gardasil® 9 are the current prophylactic vaccines available that protect against the majority of HPVs associated with cancer. Although these vaccines are highly effective, HPV vaccine implementation has been slow, particularly in low-and-middle income countries. Major barriers to the widespread availability of the HPV vaccines is its cost and the requirement for continuous refrigeration (2–8°C). Here, we used spray drying along with stabilizing excipients to formulate a thermostable Gardasil® 9 vaccine. We evaluated the immunogenicity and protective efficacy of the vaccine in mice immediately after spray drying and following storage for three months at 4°C, 25°C, and 40°C. The immunogenicity studies were performed using Gardasil® 9 as a whole antigen, and not individual HPV types, for ELISA. At the dose tested, the spray dried vaccine conferred protection against HPV following storage at temperatures up to 40°C. In addition to the spray-dried vaccine, our studies revealed that the Gardasil® 9 vaccine, as currently marketed, may be stored and transported at elevated temperatures for up to 3 months without losing efficacy, especially against HPV16. This study is critical, as a thermostable vaccine will decrease vaccine cost associated with cold-chain maintenance and could increase vaccine access and coverage, especially in remote regions of the world.
KEYWORDS: Gardasil® 9 vaccine, spray drying, thermostable, human papillomavirus, cervical cancer, cold-chain
1. Introduction
Human papillomavirus (HPV) is the most common sexually transmitted pathogen worldwide. Most HPV infections are resolved within 2 years of the infection but some can persist and advance to cancers.1 Approximately, 19 HPV types (HPV16, 18, 26, 31, 33, 35, 39, 45, 51–53, 56, 58, 59, 66, 68, 70, 73, and 82) can cause cancers; these HPV types are known as high-risk HPV types. High-risk HPVs account for nearly 5% of all cancers, primarily causing cancers of the cervix, penis, vulva, anus, vagina and head & neck squamous cell carcinomas (oral, oropharyngeal, and laryngeal cancers).2,3 High-risk HPVs cause virtually all cases of cervical cancer and two HPV types (16 and 18) account for ~70% of these cases.4,5 Two prophylactic vaccines, all comprised of virus-like particles (VLPs) assembled from recombinant L1, the HPV major capsid protein, are available commercially that protect against the HPV types associated with a high percentage of cancers. Cervarix® (bivalent, GlaxoSmithKline, London, UK) protects against HPV16 and HPV18 while Gardasil® 9 (nonavalent HPV, Merck & Co., NJ, USA) protects against HPV6, 11, 16, 18, 31, 33, 45, 52, and 58. It is predicted that Gardasil® 9 will provide protection against the HPV types that cause ~90% of cervical cancer cases.6
More than 500,000 women are diagnosed with cervical cancer annually, with nearly half dying from the disease;7 further, a staggering 85–95% of all cervical cancer cases are reported in low-and-middle-income countries (LMICs).8,9 The lack of national screening and immunization programs, poor infrastructure, a paucity of trained medical personnel, and the high cost of HPV vaccines are some of the barriers to HPV vaccines in LMICs. One major barrier is the cost; two doses of the vaccine costs between $250 and $400.10 Gardasil® was available to Global Alliance for Vaccines and Immunizations (GAVI)-eligible countries, which accounts for less than 50 countries, for a fraction of the cost ($4.50 per dose); however, even this price was not affordable to many countries.11,12 The actual manufacturing costs of Gardasil® are estimated to be in the range of $0.48–$0.59 per dose,11 suggesting that the requirement for continuous refrigeration could be a major contributor to the final vaccine cost. According to the World Health Organization (WHO), greater than 50% of the total vaccine cost potentially goes toward maintaining a reliable cold-chain infrastructure.13 Currently, Cervarix®and Gardasil® 9 must be refrigerated (2–8°C) during transport and storage to prevent loss in vaccine efficacy.14,15 This has potentially led to slow implementation of the HPV vaccine in national immunization programs, especially in LMICs. Therefore, the formulation of heat-stable and affordable vaccines, which can be adapted in LMICs immunization programs, has the potential to increase vaccine access and coverage.
One promising approach to reduce vaccine costs and improve accessibility is to eliminate the dependence on cold-chain by formulating thermostable vaccines. Although manufacturing techniques such as lyophilization have existed for many decades to formulate vaccines, spray drying has recently been considered to be an attractive option owing to its ease of use and the ability to adapt to large-scale manufacturing.16–19 In addition to spray drying, excipients such as sugars, surfactants, proteins, and polymers play an important role in stabilizing the vaccine during manufacture, storage, and transportation.20–22 In this study, we spray-dried the commercially available Gardasil® 9 vaccine with stabilizing excipients. Gardasil® 9 is made up of nine VLPs (derived from HPV6, 11, 16, 18, 31, 33, 45, 52, and 58) absorbed on an aluminum adjuvant (amorphous aluminum hydroxyphosphate sulfate). We evaluated the immunogenicity and protective efficacy of the vaccine in mice, immediately after spray drying and following storage for three months at 4°C, 25°C, and 40°C.
2. Results
2.1. Formulation, yield, and loading of Gardasil® 9 dry powder
Gardasil® 9 vaccine suspension (alum-absorbed) was spray-dried using the excipients mannitol, dextran, L-leucine, polyvinylpyrrolidine (PVP), trehalose, and myo-inositol. We have previously shown that these excipients, when used in varying concentrations, provide stability to different vaccine types by decreasing the hygroscopicity and crystallinity of the formulations.20,22–25 Further, the spray drying parameters were optimized from our previous spray drying studies for various vaccines.20,22 A dry powder yield of 91.52% (w/w) was obtained after spray drying the Gardasil® 9 vaccine; further, a VLPs loading of ~7 µg (initial VLPs loading prior to spray drying ~10 µg) per mg of dry powder (70% VLPs loading efficiency) was achieved. The higher amount of excipients in our vaccine formulation was necessary for ease of handling during in vivo delivery.
2.2. In vivo studies
2.2.1. Freshly spray-dried Gardasil® 9 dry powder
2.2.1.1. Immunogenicity
The immunogenicity of freshly spray-dried Gardasil® 9 powder was assessed in mice that were vaccinated twice (separated by 3 weeks) with dry powder Gardasil® 9 (reconstituted in PBS) or PBS alone. For comparison, another group of mice were immunized with a similar dose (10 µg) of liquid Gardasil® 9 suspension (as purchased from the manufacturer and stored at 4°C). As shown in Figure 1, mice immunized with both liquid and spray-dried Gardasil® 9 vaccine had similar antibody responses (> 104 titers) when this dose was used. However, we did not evaluate varying dose comparison between the spray-dried and liquid Gardasil® 9 vaccine.
Figure 1.
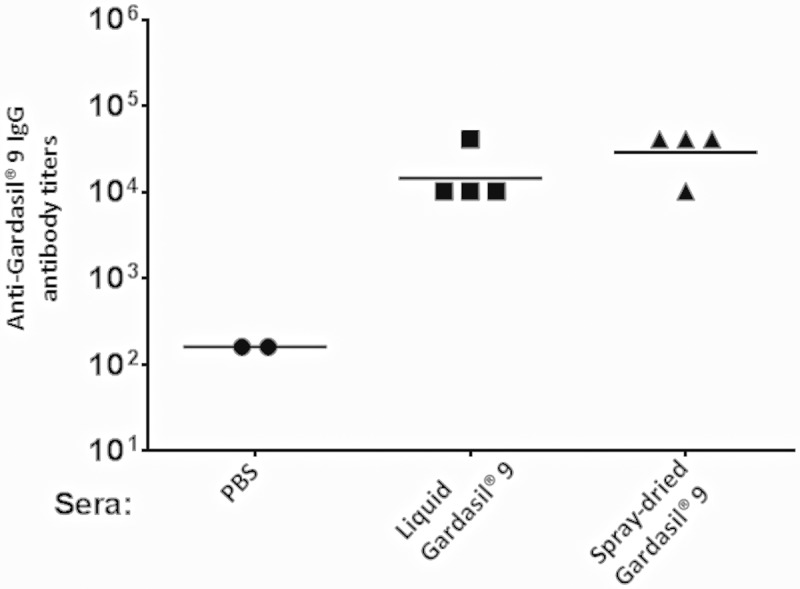
Anti-Gardasil® 9 antibody titers in mice. Mice were immunized with liquid (non-spray-dried), freshly spray-dried Gardasil® 9, or with PBS (control) and boosted with the same dose three-weeks after the first immunization. Sera were collected three weeks after the boost immunization and anti-Gardasil® 9 IgG titers were determined by ELISA.
2.2.1.2. Spray-dried Gardasil® 9 vaccine protects against vaginal infection with diverse HPV pseudovirus (PsV) types
Protection from genital infection was assessed by using a mouse HPV PsV challenge model.26 Immunized mice were vaginally infected with four different high-risk HPV PsV types (16, 18, 31, and 45). A bioluminescence signal in the genital region of mice 48 h post-PsV challenge signifies infectivity. We compared the protective efficacy of Gardasil® 9 dry powder vaccine (resuspended in saline) to the liquid Gardasil® 9 suspension. As shown in Figures 2 and 3, mice immunized with both Gardasil® 9 dry powder and liquid suspension (using a 10 µg dose) were significantly protected from HPV infection with PsV 16 and 45 (p < 0.01), PsV31, and PsV 18 (p < 0.05) compared to mice immunized with PBS alone (control). Moreover, we did not observe any statistically significant differences in protection in mice immunized with dry powder and liquid Gardasil® 9 after infection with PsV 16, 18, 31, and 45. We therefore demonstrate that the Gardasil® 9 vaccine remained immunogenic and was protective after spray drying.
Figure 2.

IVIS images: bioluminescence signal in the genitals of mice immunized with (I) phosphate buffered saline (PBS), (II) liquid Gardasil® 9 suspension, or (III) fresh spray-dried Gardasil® 9 powder and infected with HPV (A) PsV16, (B) PsV18, (C) PsV31, and (D) PsV45.
Figure 3.
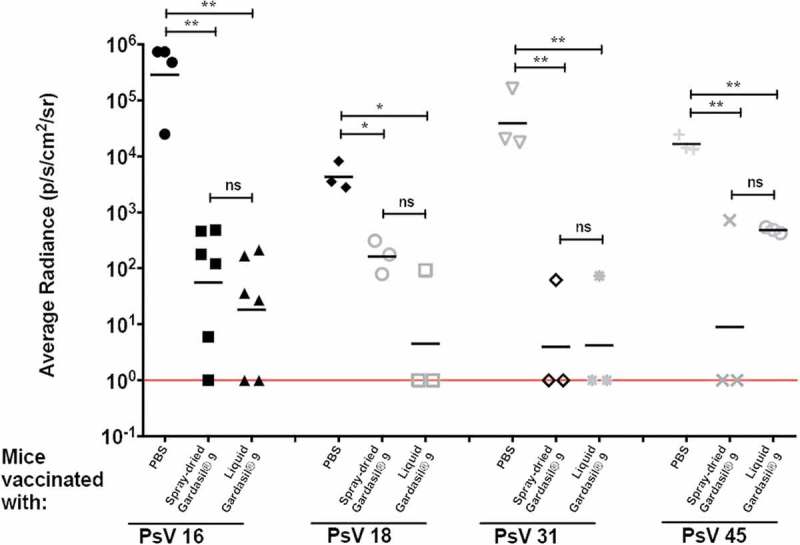
Protection against HPV PsVs 16, 18, 31, and 45. Groups of 3–6 BALB/c mice were immunized i.m. twice with 10 µg of either liquid or fresh spray-dried Gardasil® 9 (equivalent to 2.22 µg PsV16 VLPs, 1.48 µg PsV18 VLPs, 0.74 µg PsV31 VLPs, and 0.74 µg PsV45 VLPs). Three weeks after the boost immunization, mice were vaginally challenged sequentially with various HPV PsV types [PsV16 (8.76 × 106 IU), PsV18 (3 × 105 IU), PsV31 (2.5 × 107 IU), and PsV45 (9.3 × 106 IU)]. Two days later, 0.4 mg of luciferin was instilled vaginally and images were taken 3 minutes post-luciferin instillation. The average radiance (p/s/cm2/sr) of luciferase expression at the genitals was determined using Living Image 4.1 software. Background radiance (determined by gating on another region of the mouse) was subtracted from this value. Each datum represents the radiance value of an individual mouse and the lines represent the average geometric mean for each group. Statistical analysis was done using one-way ANOVA, Tukey’s multiple comparison test; ns – not significant, *p < 0.05 and **p < 0.01. Red line indicates limit of detection.
2.2.2. Immunogenicity and protective efficacy of spray-dried Gardasil® 9 vaccine stored for 3 months
2.2.2.1. Immunogenicity
The immunogenicity of spray-dried Gardasil® 9 powder stored for three months at 4°C, 25°C/60% RH, and 40°C/75% RH was assessed. Mice were given two doses (10 µg each) of stored Gardasil® 9 dry powder (reconstituted in PBS). For comparison, another group of mice were immunized with liquid Gardasil® 9 suspension stored at similar conditions (4°C, 25°C/60% RH, and 40°C/75% RH). As shown in Figure 4, mice immunized with Gardasil® 9 dry powder and liquid vaccine, at all storage conditions, elicited high-titer IgG antibody responses (>103) compared to PBS control (=40).
Figure 4.
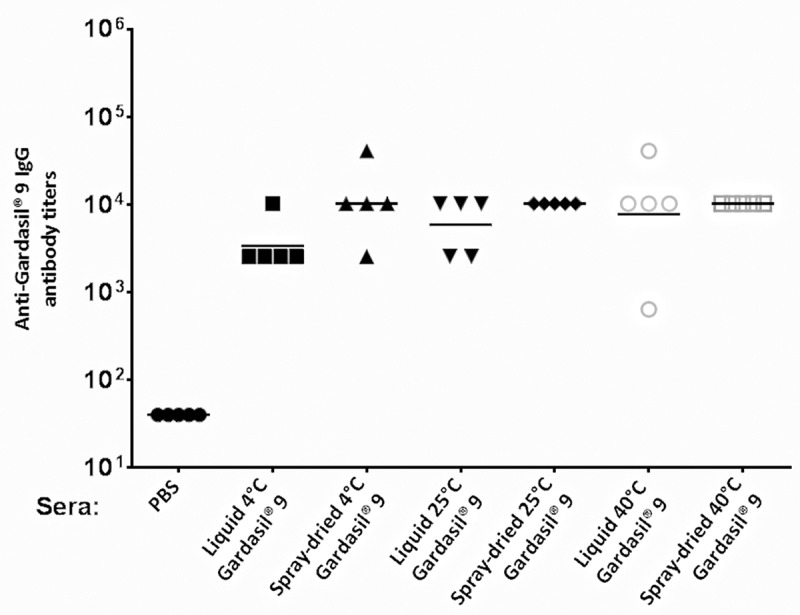
Anti-Gardasil® 9 antibody titers in mice after immunization with Gardasil® 9 stored for 3 months at different temperatures. Mice were immunized twice with either liquid or spray-dried Gardasil® 9 stored at 4°C, 25°C/60% RH, and 40°C/75% RH or with PBS (control) at three-week intervals. Sera were collected three weeks after the last immunization and anti-Gardasil® 9 IgG titers were determined by ELISA. Each datum represents the IgG antibody value of an individual mouse.
2.2.2.2. Protection against HPV PsV challenge
Protection from genital infection with HPV was assessed by vaginally infecting immunized mice with HPV PsV type 16. The protective efficacy of Gardasil® 9 dry powder vaccine stored for three months at 4°C, 25°C/60% RH, and 40°C/75% RH was compared to liquid Gardasil® 9 vaccine stored at similar conditions, or PBS alone. As shown in Figure 5, mice immunized with either Gardasil® 9 dry powder or liquid suspension, at all storage conditions, were significantly protected from infection with HPV PsV 16 (p < 0.0001) when compared to mice immunized with PBS alone (control). Moreover, we did not observe any statistically significant differences in protection between the group of mice that received Gardasil® 9 dry powder and liquid suspension, at all storage conditions. This suggests that the Gardasil® 9 dry powder and liquid vaccine were able to confer protection against HPV PsV16 infection even after three months of storage at 25°C or 40°C.
Figure 5.
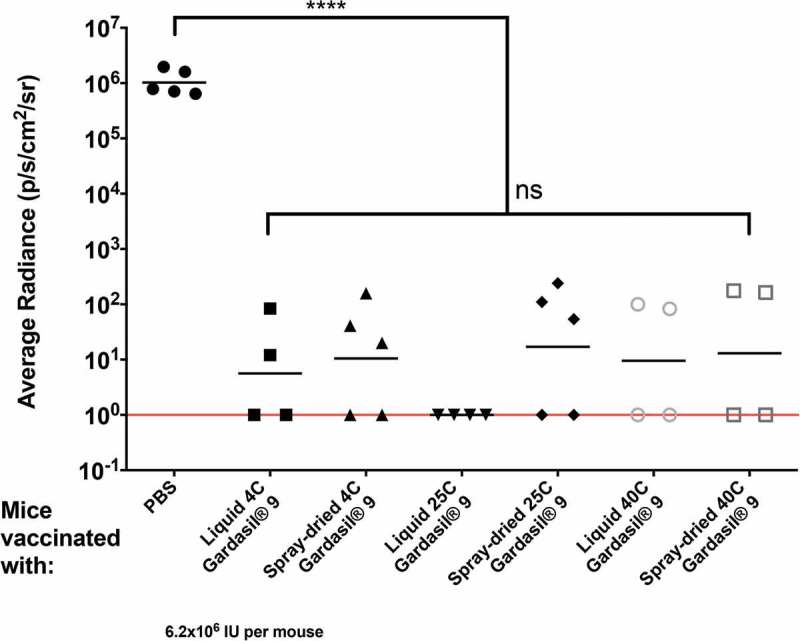
Mice immunized with spray-dried Gardasil® 9 dry powder stored for three months at 4°C, 25°C/60% RH, and 40°C/75% RH confers protection against HPV PsV16. Groups of 4–5 female BALB/c mice were immunized i.m. twice with 10 µg of either liquid or fresh spray-dried Gardasil® 9 (equivalent to 2.22 µg PsV16 VLPs). Three weeks after the boost immunization, mice were vaginally challenged with HPV PsV16 6.2 × 106 IU. Two days later, 0.4 mg of luciferin was instilled vaginally and images were taken 3 minutes post-luciferin instillation. The average radiance (p/s/cm2/sr) of luciferase expression at the genitals was determined using Living Image 4.1 software. Background radiance (determined by gating on another region of the mouse) was subtracted from this value. Each datum represents the radiance value of an individual mouse and the lines stand for the average geometric mean for each group. Statistical analysis was done using one-way ANOVA, Tukey’s multiple comparison tests; ns – not significant, ****p < 0.0001. Red line indicates limit of detection.
3. Discussion
Cervical cancer is the second most common cancer in women and HPV infection is the most significant risk factor in its etiology.27 Further, cervical cancer is highly preventable in high-income countries where HPV vaccines are easily available. There are two FDA approved vaccines, among which, Gardasil® 9 potentially offers broader protection as it contains antigens against HPV types that cause the most cancers. Like most other vaccines, Gardasil® 9 has to be refrigerated (2–8°C) during storage and transport to prevent any loss in efficacy.14 It is estimated that 20–65% of the final vaccine cost goes toward maintaining the cold-chain, with 50% vaccines wasted due to excursions outside the recommended temperature.13,28,29 The majority of this wastage can be attributed to the poor cold-chain infrastructure to store and transport these vaccines.30 Unfortunately, vaccine wastage due to breaches in cold-chain is only predicted to get worse. The existing cold-chain infrastructure is overburdened and cannot keep pace with the constantly changing landscape of national immunization programs. Thus, the bottleneck caused by the dependence on the cold-chain infrastructure in LMICs results in vaccine shortages, avoidable wastage, and administration of ineffective vaccines, all of which have considerable performance and cost implications.31,32
Currently, only a single exposure of Gardasil® 9 to up to 25°C for less than 72 h is allowed.14 However, it can be difficult to comply with this requirement in countries where the temperatures reach as high as 40°C for prolonged periods during the summer. Further, 72 h may not be sufficient to traverse the remote regions in LMICs where the cold-chain infrastructure is the most fragile. Availability, affordability, vaccine cost, and lack of cold-chain infrastructure in LMICs are therefore a huge impediment to achieving universal vaccination against HPV and toward reducing the global burden of cervical cancer.33 An alternative that has been well researched by us and others is to formulate vaccines into a dry form.20–25,34,35 An optimum vaccine formulation in a dry form with stabilizing excipients will eliminate the need for uninterrupted cold-chain and significantly reduce the overall cost of a vaccine. Such a thermostable vaccine could potentially facilitate the goal of universal vaccination against HPV. In this study, we formulated the liquid Gardasil® 9 suspension into a dry form by spray drying with stabilizing excipients to eliminate the need for cold-chain. We were successful in formulating Gardasil® 9 vaccine into a heat-stable dry powder form, which conferred protection against HPV immediately after spray drying. We further demonstrated the stability of Gardasil® 9 dry powders after three months of storage at temperatures up to 40°C. The antibody titers achieved in immunized mice were similar between fresh and stored spray-dried Gardasil® 9 at the dose tested. However, there is a possibility that differences in immunogenicity could have been observed if lower doses (closer to the ED50) were compared between the liquid and spray-dried vaccine. Future studies should consider a dose-ranging comparison at equivalent doses that lead to 50% seroconversion in immunized mice. Further, there is a possibility of protein (VLPs) denaturing after spray drying or during storage; however, based on our previous studies this is unlikely. We have previously spray-dried VLPs and evaluated its integrity by agarose gel electrophoresis and transmission electron microscopy (TEM); no degradation of VLPs was observed after spray drying or during storage.22,24
Our control group, the liquid Gardasil® 9 suspension, as supplied by the manufacturer, was also stable at 25°C/60% RH and 40°C/75% RH for up to 3 months and conferred protection against HPV PsV16. This is in contrary to what the label suggests, where it states that the vaccine is only allowed a single excursion up to 72 h at 25°C. We observed high-antibody titers from groups of mice immunized with both liquid and spray-dried Gardasil® 9 vaccine with no differences between the groups at different storage conditions indicating the inherent thermal stability of the vaccine. A limitation with our study is that we used Gardasil® 9 as a whole antigen for ELISA, which contains VLPs from nine different HPV types. Since individual HPV VLP types were not used as target antigens for ELISA, it was not feasible to compare the antibody response to each of the nine antigens. Thus, it is difficult to elucidate which of the nine VLPs elicited the immune responses reported here for either the spray dried Gardasil® 9 or liquid Gardasil® 9 (stored for 3 months). There is a possibility that either the spray-dried vaccine or the liquid vaccine could possibly have one or more specific HPV types compromised during storage (at extreme conditions). Our studies therefore report the overall immunogenicity of the vaccine based on the anti-Gardasil® 9 antibodies detected using ELISA.
This observation that Gardasil® 9 vaccine is stable for 3 months ‘as is’ could allow for a ‘Controlled Temperature Chain (CTC)’ approval for at least 3 months, and potentially have a huge impact on HPV vaccination in LMICs. Currently, the recommended storage condition for Gardasil® 9 vaccine is between 2°C and 8°C, with the total time out of refrigeration (at temperature between 8°C and 25°C only) not exceeding 72 h.14 A CTC approval will be significant in LMICs since most HPV vaccinations are administered outside cold-chain monitored facilities such as schools.12 The findings here suggest that Gardasil® 9 may not require cold-chain for up to 3 months to remain efficacious. This will significantly reduce the cost of the vaccine and allow for increased vaccine access and coverage in hard to reach regions around the world.
Future studies should replicate these preclinical findings in clinical trials for Gardasil® 9 vaccine to be evaluated in a CTC for up to 3 months. In addition, limitations reported in the study must be addressed in future studies. A dose-range study will enable to establish a dose–response curve (and a dose closer to the ED50) that will allow for a better understanding of any degradation occurring between the liquid and spray-dried vaccine. Further, an ELISA specific to each virus-type present in the vaccine has to be performed to determine specific antibody titers against those antigens, and thus establish the susceptibility of the nine HPV types during spray drying and storage. We expect the outcome of this study will have significant health and economic benefits for many countries, including LMICs in which more than 85% of cervical cancers occur and expected to rise to 95% by the year 2030.8,9 Further, we could reduce the burden placed on the existing cold-chain infrastructure, that is already overburdened due to the addition of new vaccines that require 2–8°C storage, with the development of vaccines that can withstand high-temperature exposures.
4. Materials and methods
4.1. Materials
Gardasil® 9 (alum-absorbed vaccine), nonoxynol-9 (N-9), and Depo-Provera were purchased from the University of New Mexico hospital pharmacy. Dextran and trehalose dihydrate were purchased from MP Biomedicals, Solon, OH. Mannitol and myo-Inositol were purchased from EMD Millipore, Billerica, MA. L-Leucine (>98%) and phosphate buffered saline (PBS) was purchased from Sigma–Aldrich, Milwaukee, WI. Polyvinylpyrrolidine (PVP) was purchased from ACROS Organics.
4.2. Formulation of Gardasil® 9 dry powder
A Büchi Mini Spray Dryer B-290 with a standard two-fluid nozzle (0.7 mm diameter; Büchi Corporation, Flawil, Switzerland) was used to prepare Gardasil® 9 dry powder. The parameters for spray drying were as follows: inlet temperature 145 ± 10°C, outlet temperature of 47 ± 3°C, nitrogen flow rate of 742 L/h, aspirator rate of 100%, and feed rate of 10% corresponding to 4 mL/min. The total solids content in the spray drying suspension was 1% w/v. The spray drying suspension comprised of 50% w/w L-Leucine, 35% w/w Mannitol, 5% w/w each of trehalose and myo-inositol, 2.5% w/w each of dextran and PVP, and 1% w/w of Gardasil® 9 vaccine. As a control, solution of excipients without Gardasil® 9 was spray-dried.
4.3. Yield and loading
The spray drying dry powder yield was calculated as the difference in the weight of the sample vial before and after product collection. The weight difference was compared to the initial total dry mass (sum of total solids content (1% w/v; vaccine and excipients added) and excipients already present in the Gardasil® 9 liquid suspension, 12.8 mg per 0.5 mL dose) and reported as yield in % (w/w). The loading of VLPs (Gardasil® 9) per milligram of dry powder was determined using the initial vaccine content (1% w/w) in total solids and after spray drying.
4.4. Storage stability conditions
Powders formulated immediately after spray drying and those stored for three months at different storage conditions were evaluated. For storing, the spray-dried powders were loaded into hydroxypropyl methylcellulose (HPMC) capsules (size 3) and placed in amber-colored serum bottles (referred to as storage bottles) at 4°C, 25°C (RT – room temperature), and 40°C, as described previously.20 As a control, Gardasil® 9 liquid suspension was also stored at the above storage conditions in storage bottles. PharmaKeep® KD-20 packets (combined antioxidant and moisture protectant; Mitsubishi Gas Chemical Company, Inc.) were placed in the storage bottles for protection from moisture and oxygen scavengers. Further, the bottles were purged with an inert gas (N2). The storage bottles were placed in incubators (4°C, 25°C/60% relative humidity (RH), and 40°C/75% RH) with RH and temperature monitored using a wireless miniature temp/RH logger (Omega Engineering Inc., USA). To assess the efficacy of the vaccine, samples were removed from the storage bottles just prior to immunization, resuspended in PBS, and evaluated in mice for protection against HPV PsV challenge.
4.5. In vivo studies
4.5.1. Immunization studies
The immunogenicity of the Gardasil® 9 dry powder, in comparison to Gardasil® 9 suspension, was evaluated by immunizing mice and assessing protection against HPV PsV challenge. All animal studies were performed in accordance with the University of New Mexico Institutional Animal Care and Use Committee (UNM IACUC) guidelines and were approved by the UNM IACUC (protocol #16-200498-HSC).
Gardasil® 9 powders were first evaluated immediately after spray drying. Female 3-weeks old BALB/c mice (10 per group) were immunized intramuscularly (i.m.) with 10 µg Gardasil® 9 dry powder VLPs resuspended in PBS, 10 µg Gardasil® 9 VLPs liquid suspension, and PBS alone. Three weeks after immunization, a booster dose of similar concentration was administered. The concentration of each HPV VLP type in the immunized dose was: 2.22 µg HPV16 VLPs, 1.48 µg HPV18 VLPs, 0.74 µg HPV31 VLPs, and 0.74 µg HPV45 VLPs. Three weeks after the booster dose, 4 mice per group (2 mice in PBS group) were sacrificed and sera collected to evaluate the antibody titers against Gardasil® 9. Remaining immunized mice were challenged sequentially with HPV PsV16, PsV18, PsV31, and PsV45 (high-risk HPV types) to assess protection. Similarly, for samples stored for three months at different storage conditions, 5 mice per group were immunized i.m. with 10 µg Gardasil® 9 dry powder (resuspended in PBS) or liquid VLPs stored at 4°C, 25°C/60% RH, and 40°C/75% RH, and PBS alone. Three weeks after immunization, a booster dose of similar VLPs concentration was administered. Three weeks later, blood (sera) was collected from mice by retro-orbital bleeds for measuring antibody titers. Further, mice were challenged with HPV PsV16 only and protection assessed.
4.5.2. Antibody titers
Sera from all experimental groups were collected three weeks after the booster dose (six weeks after the first immunization) and analyzed for IgG titers against Gardasil® 9 by ELISA.36 Briefly, ELISA plates (96-well) were coated with 0.5 µg of Gardasil® 9 (alum-absorbed antigen). The wells were blocked and serial dilutions of serum from immunized mice were added. The plates were incubated for 2 h at room temperature, washed, and 1:5000 dilution of HRP-conjugated goat anti-mouse IgG antibodies was added. The plates were incubated for 1 h at the same temperature, washed and were developed by adding 3, 3ʹ, 5, 5ʹ-tetramethylbenzidine (TMB) and the reactivity was determined at OD450. Antibody titers were determined by considering the reciprocal of the highest serum dilution with an OD450nm greater than two-fold compared to the control sera at the same dilution.
4.5.3. Pseudovirus production and purification
HPV PsV 16, 18, 31, and 45, encapsidating a reporter plasmid (pClucf) encoding both luciferase and green fluorescence protein (GFP), were produced in 293TT cells (human fibroblast cells), purified, and PsV titers estimated as previously described by us.37 The HPV PsVs were then used in in vivo challenge studies described below.
4.5.4. Cervicovaginal HPV PsV challenge
The HPV PsV challenge studies were performed as previously described.37 Briefly, three weeks after the booster dose, mice were administered 3 mg of Depo-Provera (Pharmacia Corp) subcutaneously. Five days later, mice received Nonoxynol-9 treatment vaginally 4 h prior to challenge; mice were challenged vaginally with 3 × 105–2.5 × 107 infectious units (IU) of PsVs (16, 18, 31, and 45). Two days post-challenge, mice were administered with 0.4 mg of luciferin (Caliper Life Sciences) vaginally. Three minutes later, images of mice were captured with a 5-min exposure using a Caliper IVIS Lumina II (Caliper Life Sciences). Average radiance (p/s/cm2/sr) was determined from the images by drawing equal-sized regions of interests (ROI) surrounding the site of PsV instillation. Statistical significance was calculated using one-way ANOVA with Tukey’s multiple comparison tests in GraphPad Prism 6.
Funding Statement
This research was funded in part by the Clinical & Translational Science Center (CTSC) at the University of New Mexico Health Sciences Center (#CTSC006-8), the US National Institute of Dental & Craniofacial Research of the National Institutes of Health, grant number 1R15 DE025812-01A1, and by a cooperative agreement from the US National Institutes of Health (National Institute of Allergy and Infectious Diseases) establishing the Epidemiology and Prevention Interdisciplinary Center for Sexually Transmitted Diseases (U19 AI113187).
Disclosure of potential conflicts of interest
E Tumban and B Chackerian are inventors of HPV L2 phage VLPs-related HPV vaccine patent application licensed to Agilvax Biotech. Interactions with Agilvax are managed by the University of New Mexico in accordance with its conflict of interest policies.
References
- 1.World Health Organization Human papillomavirus and cervical cancer. Reprod Health Matters [Internet]. 2000;8:190 [accessed 2018 May31] http://linkinghub.elsevier.com/retrieve/pii/S0968808000902593. [Google Scholar]
- 2.Roden RBS, Stern PL.. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer [Internet]. 2018;18:240–54. [accessed 2018 May31] http://www.nature.com/doifinder/10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai L, Tumban E. Gardasil-9: a global survey of projected efficacy. Antiviral Res [Internet]. 2016;130:101–09. [accessed 2018 May31] https://www.sciencedirect.com/science/article/pii/S0166354216300377?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- 4.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, et al. The global burden of cancer 2013. JAMA Oncol [Internet]. 2015;1:505–27. [accessed 2018 May31] http://www.ncbi.nlm.nih.gov/pubmed/26181261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJF, Meijer CJLM. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med [Internet]. 2003;348:518–27. [accessed 2018 May31] http://www.nejm.org/doi/abs/10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 6.Merck FDA approves Merck’s HPV vaccine, GARDASIL®9, to prevent cancers and other diseases caused by nine HPV types – including types that cause about 90% of cervical cancer cases [Internet]. 2014. [accessed 2018 May31] http://www.mrknewsroom.com/news-release/prescription-medicine-news/fda-approves-mercks-hpv-vaccine-gardasil9-prevent-cancers-an.
- 7.Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, Brotons M, Mena M, Cosano R, Muñoz J, et al. ICO Information Centre on HPV and cancer (HPV Information Centre). Human papillomavirus and related diseases in [Botswana] [Internet]. 2014. [accessed 2019 April 3] http://www.hpvcentre.net/statistics/reports/XWX.pdf.
- 8.Cervical Cancer Action Progress in cervical cancer prevention: the CCA report card [Internet]. 2011. [accessed 2019 April 3] http://www.paho.org/hq/dmdocuments/2011/CCA_reportcard_low-res.pdf.
- 9.Gallagher KE, Erio T, Baisley K, Lees S, Watson-Jones D. The impact of a human papillomavirus (HPV) vaccination campaign on routine primary health service provision and health workers in Tanzania: a controlled before and after study. BMC Health Serv Res [Internet]. 2018;18:173 [accessed 2018 June8] http://www.ncbi.nlm.nih.gov/pubmed/29530042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC CDC vaccine price list [Internet]. CDC. 2015. p. 1–7. [accessed 2018 June5] http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/.
- 11.Clendinen C, Zhang Y, Warburton RN, Light DW. Manufacturing costs of HPV vaccines for developing countries. Vaccine [Internet]. 2016;34:5984–89. [accessed 2018 June8] https://www.sciencedirect.com/science/article/pii/S0264410X16308568. [DOI] [PubMed] [Google Scholar]
- 12.MSF The right shot: bring down barriers to affordable and adapted vaccines. 2015. Geneva, Switzerland: MSF Access Campaign. [Google Scholar]
- 13.World Health Organization (WHO) Vaccines and biologicals. The Department of Immunization. Monitoring vaccine wastage at country level – guidelines for programme managers [Internet]. Geneva. 2003. [accessed 2019 April 3]https://apps.who.int/iris/bitstream/handle/10665/68463/WHO_VB_03.18.Rev.1_eng.pdf.
- 14.Merck Gardasil-9, human papillomavirus 9-valent vaccine, recombinant-storage and handling [Internet]. 2018. [accessed 2018 May31] https://www.merckvaccines.com/Products/Gardasil9/storage/temperature-excursions.
- 15.World Health Organization Controlled Temperature Chain (CTC) [Internet]. 2016. [accessed 2018 May 13] http://www.who.int/biologicals/areas/vaccines/controlledtemperaturechain/en/.
- 16.Kunda NK, Somavarapu S, Gordon SB, Hutcheon GA, Saleem IY. Nanocarriers targeting dendritic cells for pulmonary vaccine delivery. Pharm Res [Internet]. 2013;30:325–41. doi: 10.1007/s11095-012-0891-5. [DOI] [PubMed] [Google Scholar]
- 17.Kanojia G, Willems G-J, Frijlink HW, Kersten GFA, Soema PC, Amorij J-P. A design of experiment approach to predict product and process parameters for a spray dried influenza vaccine. Int J Pharm [Internet]. 2016;511:1098–111. doi: 10.1016/j.ijpharm.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Afkhami S, LeClair DA, Haddadi S, Lai R, Toniolo SP, Ertl HC, Cranston ED, Thompson MR, Xing Z. Spray dried human and chimpanzee adenoviral-vectored vaccines are thermally stable and immunogenic in vivo. Vaccine. 2017;35:2916–24. doi: 10.1016/j.vaccine.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Kanojia G, ten Have R, Soema PC, Frijlink H, Amorij J-P, Kersten G. Developments in the formulation and delivery of spray dried vaccines. Hum Vaccin Immunother. 2017;13:2364–78. doi: 10.1080/21645515.2017.1356952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunda NK, Wafula D, Tram M, Wu TH, Muttil P. A stable live bacterial vaccine. Eur J Pharm Biopharm. 2016;103:109–17. doi: 10.1016/j.ejpb.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Contreras L, Wong Y-L, Muttil P, Padilla D, Sadoff J, DeRousse J, Germishuizen WA, Goonesekera S, Elbert K, Bloom BR, et al. Immunization by a bacterial aerosol. Proc Natl Acad Sci [Internet]. 2008;105:4656–60. [accessed 2019 April 3] http://www.pnas.org/cgi/doi/10.1073/pnas.0800043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saboo S, Tumban E, Peabody J, Wafula D, Peabody DS, Chackerian B, Muttil P. Optimized formulation of a thermostable spray-dried virus-like particle vaccine against human papillomavirus. Mol Pharm [Internet]. 2016;13:1646–55. [accessed 2018 June1] http://www.ncbi.nlm.nih.gov/pubmed/27019231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price DN. Pulmonary BCG vaccination for uniform protection against tuberculosis in environmental mycobacteria endemic regions. 2016. [Google Scholar]
- 24.Peabody J, Muttil P, Chackerian B, Tumban E. Characterization of a spray-dried candidate HPV L2-VLP vaccine stored for multiple years at room temperature. Papillomavirus Res [Internet]. 2017;3:116–20. [accessed 2018 June1] https://www.sciencedirect.com/science/article/pii/S2405852116301173?via%3Dihub#s0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tumban E, Muttil P, Escobar CAA, Peabody J, Wafula D, Peabody DS, Chackerian B. Preclinical refinements of a broadly protective VLP-based HPV vaccine targeting the minor capsid protein, L2. Vaccine [Internet]. 2015;33:3346–53. [accessed 2015 July13] https://www.sciencedirect.com/science/article/pii/S0264410X15006374?via%3Dihub#sec0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med [Internet]. 2007;13:857–61. [accessed 2018 June22] http://www.nature.com/articles/nm1598. [DOI] [PubMed] [Google Scholar]
- 27.Daniyal M, Akhtar N, Ahmad S, Fatima U, Akram M, Asif HM. Update knowledge on cervical cancer incidence and prevalence in Asia. Asian Pacific J Cancer Prev [Internet]. 2015;16:3617–20. [accessed 2018 June18] http://www.ncbi.nlm.nih.gov/pubmed/25987011. [DOI] [PubMed] [Google Scholar]
- 28.Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals [Internet]. 2014;42:237–59. [accessed 2015 May13] http://www.ncbi.nlm.nih.gov/pubmed/24996452. [DOI] [PubMed] [Google Scholar]
- 29.Lydon P, Zipursky S, Tevi-Benissan C, Djingarey MH, Gbedonou P, Youssouf BO, Zaffran M. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad. Bull World Health Organ [Internet]. 2014;92:86–92. [accessed 2018 June 8] http://www.who.int/entity/bulletin/volumes/92/2/13-123471.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaffran M, Vandelaer J, Kristensen D, Melgaard B, Yadav P, Antwi-Agyei KO, Lasher H. The imperative for stronger vaccine supply and logistics systems. Vaccine [Internet]. 2013;31:B73–80. [accessed 2018 June5] https://www.sciencedirect.com/science/article/pii/S0264410X12016295?via%3Dihub#bib0100. [DOI] [PubMed] [Google Scholar]
- 31.WHO Immunization Practices Advisory Committee Immunization supply chain and logistics: a neglected but essential system for national immunization programmes [Internet]. Geneva. 2014. [accessed 2019 April 3] http://www.who.int/immunization/sage/meetings/2014/april/presentations_background_docs/en/.
- 32.Kristensen DD, Lorenson T, Bartholomew K, Villadiego S. Can thermostable vaccines help address cold-chain challenges? Results from stakeholder interviews in six low- and middle-income countries. Vaccine [Internet]. 2016;34:899–904. [accessed 2018 June5] 10.1016/j.vaccine.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dairo DM, Osizimete OE. Factors affecting vaccine handling and storage practices among immunization service providers in Ibadan, Oyo State, Nigeria. Afr Health Sci [Internet]. 2016;16:576–83. [accessed 2018 June5] http://www.ncbi.nlm.nih.gov/pubmed/27605974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunda NK, Alfagih IM, Miyaji EN, Figueiredo DB, Gonçalves VM, Ferreira DM, Dennison SR, Somavarapu S, Hutcheon GA, Saleem IY. Pulmonary dry powder vaccine of pneumococcal antigen loaded nanoparticles. Int J Pharm [Internet]. 2015;495:903–12. [accessed 2015 September29] http://www.sciencedirect.com/science/article/pii/S0378517315302325. [DOI] [PubMed] [Google Scholar]
- 35.Lu D, Garcia-Contreras L, Muttil P, Padilla D, Xu D, Liu J, Braunstein M, McMurray DN, Hickey AJ. Pulmonary immunization using antigen 85-B polymeric microparticles to boost tuberculosis immunity. AAPS J [Internet]. 2010;12:338–47. [accessed 2018 June 8] http://www.springerlink.com/index/10.1208/s12248-010-9193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhai L, Peabody J, Pang YYS, Schiller J, Chackerian B, Tumban E. A novel candidate HPV vaccine: MS2 phage VLP displaying a tandem HPV L2 peptide offers similar protection in mice to Gardasil-9. Antiviral Res [Internet]. 2017;147:116–23. [accessed 2018 June8] https://www.sciencedirect.com/science/article/pii/S0166354217305168?via%3Dihub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One [Internet]. 2011;6:e23310 [accessed 2018 June1] http://dx.plos.org/10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Merck FDA approves Merck’s HPV vaccine, GARDASIL®9, to prevent cancers and other diseases caused by nine HPV types – including types that cause about 90% of cervical cancer cases [Internet]. 2014. [accessed 2018 May31] http://www.mrknewsroom.com/news-release/prescription-medicine-news/fda-approves-mercks-hpv-vaccine-gardasil9-prevent-cancers-an.


