ABSTRACT
Pharmacies are promising alternative settings for human papillomavirus (HPV) vaccination because of their population reach, convenience, and existing infrastructure for vaccine delivery. However, pharmacies in the US are rarely used for adolescent HPV vaccination. We sought to document challenges and opportunities of implementing pharmacy-located HPV vaccination services in five US states by mapping process evaluation results onto key implementation science constructs: service penetration, acceptability, appropriateness, feasibility, fidelity, adoption, and sustainability. Pilot projects were planned in North Carolina (k = 2 pharmacies), Michigan (k = 10), Iowa (k = 2), Kentucky (k = 1), and Oregon (no pharmacy recruited) with varying procedures and recruitment strategies. Sites had open enrollment for a combined 12 months. Despite substantial efforts in these states, only 13 HPV vaccine doses were administered to adolescents and three doses to age-eligible young adults. We identified two major reasons for these underperforming results. First, poor outcomes on service penetration and appropriateness pointed to engagement barriers: low parent demand and engagement among pharmacy staff. Second, poor outcomes on feasibility, adoption, and sustainability appeared to result from administrative hurdles: lacking third party reimbursement (i.e., billing commercial payers, participation in Vaccines for Children program) and limited integration into primary care systems. In summary, pilot projects in five states all struggled to administer HPV vaccines. Opportunities for making pharmacies a successful setting for adolescent HPV vaccination include expanding third party reimbursement to cover all vaccines administered by pharmacists, increasing public awareness of pharmacists’ immunization training, and improving care coordination with primary care providers.
KEYWORDS: HPV vaccine, pharmacies, pharmacists, alternative vaccination settings, scope of practice, implementation science
Introduction
Up-to-date human papillomavirus (HPV) vaccination in the US has increased since the vaccine’s introduction over a decade ago to 49% of adolescents ages 13–17 in 2017.1 However, vaccination coverage remains far below the Healthy People 2020 goal of 80% for adolescents ages 13–15.1,2 As a strategy to improve uptake, the President’s Cancer Panel3,4 and the National Vaccine Advisory Committee5 have recommended expanding HPV vaccine provision in pharmacies.
Pharmacies can play a meaningful role in increasing HPV vaccination for several reasons. First, pharmacies are geographically accessible to most families, including many rural communities.6 Most U.S. residents (89%) live within five miles of a pharmacy.7 Second, pharmacies have extended hours of operation and normally give vaccinations without an appointment.8,9 Third, 48 US states and the District of Columbia allow pharmacists to administer HPV vaccination (the exceptions are New York and New Hampshire).10 Fourth, many pharmacists are trained immunizers and already administer vaccines.
Pharmacists’ ability to increase HPV vaccine uptake is limited by their scope of practice to vaccinate age-eligible adolescents, which varies greatly by state.10 For instance, state laws may limit vaccination practices to certain ages or the arrangement under which pharmacists can administer HPV vaccine (e.g., independent authority, collaborative practice agreement, or by prescription only).11 Recent national surveys of primary care physicians and parents show that most supported HPV vaccination of adolescents by trained pharmacists.12–14
Pharmacists administer millions of vaccine doses every year,8,15 but HPV vaccines represent a vanishingly small fraction of those doses delivered.8 Although several authors have noted challenges and barriers related to establishing pharmacy-located HPV vaccination programs,13,16–19 no study we are aware of has documented the experiences of implementing HPV vaccination programs in real-world pharmacy settings. Our study evaluated the implementation of HPV vaccination services in community pharmacies through pilot projects in several states, mapping this process onto constructs from implementation science (Table 1).
Table 1.
Definitions and example measures of implementation outcomes assessed in pilot projects.
| Construct | Definitiona | Example measure |
|---|---|---|
| Service Penetration | Extent to which an evidence-based practice is integrated within a service setting and its subsystems. Also known as “reach.” | Number of HPV vaccine doses given to vaccine eligible patients contacted during the pilot project |
| Acceptability | Extent to which implementation stakeholders perceive a service to be agreeable, palatable, or satisfactory | Parents’ satisfaction with the waiting time, privacy, pharmacy’s appearance, and interactions with staff |
| Appropriateness | Perceived fit, relevance, or compatibility of the evidence-based practice for a given practice setting, provider, or consumer | Pharmacy staff’s support for HPV vaccination |
| Feasibility | Extent to which a new practice can be successfully used or carried out within a given setting | Number of health insurance plans covering HPV vaccine doses given at a pharmacy |
| Fidelity | Degree to which an implementation strategy was delivered as prescribed in the original protocol | Percentage of protocol items implemented as planned |
| Adoption | Intention, initial decision, or action to try or employ an evidence-base practice in a service setting. Also called “uptake.” | Number of physicians referring patients to the pharmacy |
| Sustainability | Extent to which a recently implemented practice is maintained and institutionalized within a service setting‘s ongoing operations | Number of pharmacies applying and obtaining for VFC program designation |
a Definitions adapted from Gerke D, Lewis E, Prusaczyk B, Hanley C, Baumann A, Proctor E. Eight toolkits related to Dissemination and Implementation. Implementation Outcomes. 2017. [accessed 2018 Nov 28]. https://sites.wustl.edu/wudandi/.
Results
Service penetration
All sites experienced low or no service penetration of pharmacy-located HPV vaccination for adolescents. In North Carolina, five HPV vaccine doses were given to adolescents; four doses were administered by nursing staff using the standing order protocol and one by a pharmacist who was a clinical pharmacist practitioner (CPP). In Michigan, pharmacists administered no HPV vaccine doses to adolescents and three doses to adults (first dose for a female and third doses for two males). In Kentucky, three vaccine doses were delivered to adolescents. Five HPV vaccine doses were given by a pharmacist in Iowa, while none were administered in Oregon whose program did not successfully recruit a pharmacy site.
Acceptability
Parents who got HPV vaccine for their children in participating pharmacies found the service highly acceptable. In North Carolina, for example, parents completed a satisfaction survey (while being monitored for potential post-vaccination side effects) and a follow-up survey (mailed 3 months after vaccination). The parents of the five adolescents who received HPV vaccine at a pharmacy in North Carolina reported that it was convenient, the waiting time was acceptable, the privacy and confidentiality were satisfactory, the pharmacy’s appearance was what they expected from a place that provides quality health care, and that they would recommend other parents get HPV vaccine for their children at a pharmacy. Parents in North Carolina also reported that the pharmacists and nurses who educated about and administered HPV vaccine communicated clearly, spent enough time with them, made their children feel comfortable during vaccination, and gave the vaccination safely.
Appropriateness
Appropriateness of pharmacy-located HPV vaccination varied by pharmacy site and stakeholder. Participating pharmacists were knowledgeable about vaccines in general not just HPV vaccine, had the training to immunize adolescents, and were able to report vaccines administered to state immunization registries. In addition, in Michigan, the study team conducted four stakeholder surveys with providers, parents, patients, and pharmacists about HPV vaccination in pharmacies. The appropriateness of delivering HPV vaccines in pharmacies was supported by a majority of those interviewed. However, some pharmacy staff exhibited resistance to offering HPV vaccination, which challenged the implementation of the pilot projects. Three pharmacy locations in Michigan, for example, had pharmacy staff who did not fully engage in the project because they did not approve of the vaccine. The Iowa project also encountered some reluctance from a physician who had concerns about the potential revenue loss caused by sending existing patients to complete dose series at a pharmacy. This concern was alleviated during recruitment of clinics into the study when the clinic manager expressed interest in participating in the project. Limited time was also a significant challenge for researchers and pharmacy teams. In Iowa, Kentucky, and Oregon, investigators faced delays in funding disbursement that impeded their ability to commence planned recruitment of adolescents and young adults to get HPV vaccination.
In addition, pilot project offerings were not completely aligned with pharmacies patient demographics. In Iowa, for example, one of the two pharmacies was in a rural town of 6,000 people, yielding a reduced pool of eligible adolescents available for participation. Additionally, some participating pharmacies in Michigan and Iowa served populations that speak little English (e.g., French, Swahili). Promotional and educational materials were only available in English and Spanish, and pharmacists were not readily able to counsel these patients in their native language.
Feasibility
The pilot projects faced substantial challenges related to feasibility. Third party reimbursement for HPV vaccination within the pharmacy was a major challenge for all sites. During the pilot period, most commercial health insurance plans did not cover the cost of HPV vaccine administered in the pharmacy. Pharmacies can bill the medical benefit in some instances, but there is no guarantee of reimbursement, which would result in the patient receiving a bill if the plan did not cover the cost of the vaccine. As many health insurers cover HPV vaccination for adolescents through the medical benefit when administered in a physician’s office, convincing patients to pay out-of-pocket for the same service in a pharmacy was a significant barrier. In Iowa and Kentucky, for example, some parents turned away vaccination in the pharmacy due to insurance not covering the benefit in that alternative setting.
Some pharmacies’ inability to participate in the federal Vaccines for Children program, which is administered differently by each state, was another major feasibility challenge. Medicaid patients up through age 18 can receive vaccines through the program, but most states do not yet include pharmacies as qualified providers. In Kentucky and North Carolina, the study pharmacies were not enrolled as providers in the Vaccines for Children program and were deterred from doing so due to time constraints and programmatic requirements. In contrast, one participating pharmacy in Iowa and another in Michigan were able to be recognized as Vaccines for Children program providers.
Limited pharmacy staffing was another challenge. Some participating pharmacies were understaffed to perform their typical daily workload, so offering a new service like adolescent HPV vaccination added too great of a burden on existing staff. In Michigan, this issue was aggravated when several pharmacies experienced large staff turnover during the project time period. When they hired new staff, adding project training on top of their basic job training was not possible. Another challenge that hampered the feasibility of the projects was low consumer awareness of HPV vaccine availability in pharmacies. Despite displaying an impressive array of community-oriented advertisements promoting in-pharmacy vaccination offering, many parents were not aware of pharmacists’ immunization training and the availability of adolescent vaccination in pharmacies. In Iowa, for example, multiple parents were surprised to learn their children can get HPV vaccination in pharmacies.
Fidelity
Excluding the challenges noted above, across sites, pharmacies implemented the study protocols with high fidelity. In North Carolina, for example, the study team developed an 18-item fidelity checklist to help pharmacists and nurses implementing all aspects of the protocol when enrolling a patient in the study (online supplement). The investigators used the fidelity checklist to calculate the percentage of protocol items implemented as planned. With five patients getting HPV vaccine, the fidelity score was 100%. Other sites also developed a range of workflow materials to support pharmacies implementing study protocols, which are available online.20
Adoption
Pharmacy study sites had low adoption of HPV vaccination services. Protocols and procedures were not well integrated into pharmacy workflow. Pharmacists were not successful in integrating into the broader primary care ecosystem. Pharmacists across sites required a physician agreement authorizing them to give HPV vaccine to adolescents, greatly limiting their integration into the “medical neighborhood.” Some sites struggled finding physician partners for their projects. In Michigan, for example, SpartanNash pharmacies had an existing standing order protocol for adult patients but the signing physician was not willing to provide a similar protocol for adolescents. For their pilot project, the pharmacists found another physician who provided the needed standing order protocol for HPV vaccination for adolescents. Michigan found that some physicians were very interested in referring patients to pharmacies because they could not afford to stock HPV vaccines. However, the process of referring patients to a limited number of pilot pharmacies and not knowing whether the pharmacy could bill for vaccination services precluded many Michigan physician offices to agree on a standing order protocol.
Similarly, the research team in Kentucky was not successful in engaging primary care providers to sign for a standing order protocol, but the outreach efforts opened the opportunity to partnering with the local health department which readily agreed. This type of pharmacist-physician agreement also restricts the ability of pharmacists to give HPV vaccine to children not covered by protocols or prescriptions. In North Carolina, the researchers employed a combination of CPPs for handling physician referrals and registered nurses for giving vaccine to patient walk-ins. This is a model most pharmacies could not integrate within their existing service infrastructure due to budgetary and space requirements, though.
Sustainability
The pilot projects did not establish sustainable HPV vaccination practices. Only one of the pharmacies continued providing HPV vaccine to patients after the project ended. Many challenges presented above, like limited third party reimbursement, inability to participate in the Vaccines for Children program, and low parent demand, were also reported as major challenges for maintaining stable HPV vaccination services in pharmacies.
Discussion
The 2018 President’s Cancer Panel report on HPV vaccination reaffirmed their 2014 recommendation to use pharmacies as one way to improve access to vaccination.4 Despite pharmacies’ noted conveniences and accessibility to patients,4 pharmacies continue to be underused for HPV vaccination of adolescents. The multistate pilot projects struggled in distributing vaccinations to adolescents, and our evaluation of mapping these challenges onto implementation science constructs identified two broad reasons for the poor performance of pharmacy-located HPV vaccination. First, poor outcomes on service penetration and appropriateness point to engagement barriers: low parent demand and commitment from pharmacy staff. Second, poor outcomes related to feasibility, adoption, and sustainability appeared to be the result of administrative hurdles: lacking payer reimbursement and limited integration into clinic systems. These findings also highlight the importance of including concepts from implementation science to improve program adoption at pharmacies.
The first set of challenges that resulted in underperforming vaccination programs were related to engagement barriers with vaccine provision. Low parent demand for HPV vaccines in pharmacies was a major challenge. The observed finding may be explained by parents’ limited knowledge that pharmacists can vaccinate adolescents.12,13 Unfamiliarity with obtaining health care for children outside the traditional medical office may leave parents unaware, or even hesitant, to get vaccination at alternative settings. Studies with parents of adolescents show that those who have had previous experience with vaccinating their children in pharmacies are more willing to get HPV vaccine from pharmacists.12 Pharmacies recruited in our pilot projects had no prior experience administering Tdap or meningococcal vaccines to adolescents, which also left parents unfamiliar with adolescent vaccinations at these settings. Pharmacies interested in implementing new or expanding upon existing immunization programs to include adolescent HPV vaccination may benefit by educating their clients and the general public about pharmacists’ scope of practice and immunization qualifications. Parent demand may also increase by knowing how administered vaccines will be communicated back to children’s primary care providers.
Low engagement of pharmacy staff was another significant barrier for some sites. We noticed that for pharmacies with existing immunization programs, it was less disruptive to incorporate adolescent HPV vaccination into their workflow. However, asking for additional study documentation and reporting may have created disengagement among some staff. Researchers need to minimize new burdens on pharmacies’ daily operations when implementing study protocols. When allowable by state law, implementation of adolescent vaccination programs should involve pharmacy technicians or student pharmacists. Low engagement was also observed when some pharmacies were incapable of fully involving staff due to their personal beliefs about HPV vaccination. Successful implementation of an innovation (e.g., practice, service) may be a function of intervention-values fit.21 It may be important to distinguish between unenthusiastic implementation, which occurs when a novel intervention fits poorly with staff values, and committed implementation, which occurs when an intervention fits well with staff values.21 Pharmacies interested in HPV vaccination should work to stimulate an organizational climate that makes it more appealing to the staff to support and implement this new offering. In such a way, the climate for implementation would reinforce the intervention-values fit for pharmacist-delivered HPV vaccination.
The second set of challenges that resulted in underperforming vaccination programs related to administrative barriers. Lack of medical insurance reimbursement along with limited participation in public programs such as Vaccines for Children, severely hindered pharmacies from maximizing their participation in these pilot projects. For instance, although participating pharmacy sites in Michigan were able to become Vaccines for Children program, providers, time and resource constraints on the Michigan State Department of Health to conduct mandatory inspections only allowed one pharmacy to participate in this public program. Variable vaccination coverage through the pharmacy benefits also deterred parents from seeking HPV vaccination for their adolescent children in pharmacies. Several empirical studies and reviews have noted this as a chief reason for pharmacies not adopting vaccination platforms.9,15,17,18,22 Fragmented coverage between pharmacy benefits and medical benefits from insurance providers need to be reconciled and better aligned to the current changes in pharmacy practice, notably around the expanded role pharmacists play in vaccination. Future projects may benefit from partnering with pharmacies in states (e.g., Washington) where pharmacists are recognized providers by third party insurers and can bill for vaccinations and participate in Vaccines for Children program.
Limited integration into primary care systems was also a significant administrative challenge for pharmacy-located HPV vaccinations. Due to each state’s differing pharmacy practice laws, most of the pilot projects had to use some form of a collaborative practice agreement to allow pharmacists to vaccinate adolescents, ranging from more restrictive CPP agreements (North Carolina) to more open standing order protocols (Michigan). However, establishing collaborative practice agreements can be labor intensive for pharmacists, requiring buy-in from physicians in many circumstances and regulatory oversight by state medical and pharmacy boards. It was encouraging to find that pilot pharmacies could use diverse methods for vaccination dose reporting and verification. By reporting doses to their state immunization information systems, pharmacists facilitate vaccination management and accountability, and help providers identifying missed opportunities for vaccinations.23
These pilot studies highlighted the importance of using constructs from implementation science to evaluate how well the projects were implemented in diverse pharmacy sites; specifically, we assessed well-established implementation outcomes. Implementation outcomes serves as indicators of the implementation success, proximal indicators of implementation processes, and key intermediate outcomes for intervention effectiveness.24 Only by mapping our process evaluation findings onto this set of implementation outcomes we identified the main factors that drove the limited outcomes of the pilot projects and, at the same time, the potential solutions to overcome similar challenges in future projects. As such, when implementing new immunization services in pharmacies, practitioners and researchers must include a catalog of implementation science constructs to evaluate the mechanisms and results of their implementation strategies. Although no precise guidelines exist to guide the choice of implementation constructs to assess in studies, some authors24,25 suggest taking into account the potential barriers and opportunities to implementation, the novelty of the evidence-based practice to be implemented, the setting in which implementation will occur, the resources for evaluation, the unit of analysis, and the stage of implementation (e.g., early vs. later implementation).
In terms of strengths, our pilot projects were in five states with a range of pharmacy sites, yielding data for a range of capacities to implement HPV vaccination programs. These pilots also collected implementation outcome data using mixed methods, offering a comprehensive look at the research question and study findings from the perspective of many stakeholders (pharmacists, pharmacy staff, parents, primary care providers, and researchers). The study limitations include the brief study period for many of the projects, which was inadequate to fully implement the proposed activities. Given the limited timeline, it was also not possible to formulate conclusions about the sustainability or cost of these pilots in the long run. It is also important to acknowledge that these projects were in states where pharmacists do not have independent authority to administer HPV vaccine to adolescents. As such, experiences of pharmacists in states where no prior approval from an accredited prescriber is required may differ from those we reported. Finally, not all pilot projects collected the same implementation data nor used the same instruments, making some head-to-head comparisons among projects not possible.
Conclusion
Pharmacies have great potential for vaccinating adolescents in the US as demonstrated by their participation in adult vaccination. However, these diverse pilot projects suggest that much of the unrealized potential is due to significant challenges in implementation barriers related to administration and engagement. Our findings suggest that pharmacists interested in implementing effective HPV vaccination programs should focus on increasing parent demand, gaining support from their staff, expanding payer reimbursement, and improving integration into clinic systems. Future studies that look to deliver HPV vaccines in pharmacies would benefit greatly by assessing implementation outcomes that would facilitate the timely evaluation of implementation processes. Because any new interventions will not be effective if they are not implemented well, the promise of pharmacy-located HPV vaccination cannot be advanced without attention to implementation science. Future studies should also identify those pharmacies already delivering HPV vaccination to many of their clients to document the elements that predict successful implementation.
Methods
Pilot project sites were in North Carolina, Michigan, Iowa, Kentucky, and Oregon, US with different study and vaccination protocols at each site. Activities completed in Iowa, Kentucky, and Oregon are presented together as their project was sponsored as a single study with multiple sites. We outlined the study protocols used for the different pilot projects, describing provider recruitment and training and patient recruitment and vaccination protocols. We then evaluated the success of the pilot projects by mapping reported results to key constructs from implementation science: service penetration, acceptability, appropriateness, feasibility, fidelity, adoption, and sustainability.25 These implementation constructs are commonly used in the literature to appraise how well an evidence-based practice, like HPV vaccination, was implemented into a service setting.24 We provided a definition for each individual construct alongside an example on how the constructs were evaluated from pilot projects in Table 1. For pilot results from North Carolina, we relied on study records not published elsewhere. For the results from the pilot projects conducted in Michigan, Iowa, Kentucky, and Oregon, we relied on reports publicly available on the website of the National HPV Vaccination Roundtable, which provided funding for these projects.20
North Carolina
Provider recruitment and training
The University of North Carolina partnered with two independent pharmacies in Durham, NC to conduct the Vaccination in Pharmacies study. North Carolina pharmacy practice laws allow pharmacists to administer HPV vaccines to adolescents if pharmacists are designated as CPP. As a CPP, a pharmacist enters into a collaborative practice agreement with a state-licensed physician to provide specific health care services for a referred patient. The collaborative practice agreements can be conducted as individual practice agreements (one pharmacist and physician) or collectively as a group (multiple pharmacists and physicians). No CPP for adolescent HPV vaccination existed in North Carolina prior to this project. The investigators recruited a family medicine doctor in a local primary care clinic, who had an existing relationship with one of the pharmacy sites, to serve as the prescriber on the CPP. They obtained CPP approval for two pharmacists, one at each pharmacy site, from the North Carolina Board of Pharmacy and the North Carolina Medical Board, after extensive consultation with the boards and advocacy by the University’s legal counsel. The CPP agreement included protocols for administering adolescent vaccines, documenting vaccines given, communicating patients’ immunization status to their primary care providers, and managing short-term side effects and other adverse events. Since the CPPs were limited to seeing only referred patients to the pharmacy, the investigators also employed registered nurses at each pharmacy, for up to 10 hours per week, to give HPV vaccines to walk-ins through a standing order protocol supervised by a pediatrician.
Prior to launching the project, pharmacists and nursing staff received in-person educational training on study procedures, including obtaining parental consent, administering the vaccines, managing potential side effects, billing insurers, using the North Carolina Immunization Registry to document vaccines delivered, documenting vaccine administration using study-specific process evaluation instruments, and administering study surveys to parents. The study team worked with participating pharmacists to integrate these protocols into the pharmacy’s clinical and administrative workflow.
Patient recruitment and vaccination protocol
Patient recruitment occurred from July 13, 2015 through December 16, 2015. Recruitment efforts included: direct mailing to all families with one or more age-eligible children using the pharmacies’ patient databases, physician referrals to CPPs, print newspaper advertisements, posting fliers in local libraries, grocery stores, and laundromats, promotions at community events (e.g., Boys and Girls Clubs), and in-pharmacy promotion using posters, bag stuffers, and roadside signs. Additionally, online recruitment occurred using paid social media advertisements (e.g., Facebook) and parent listserv at local elementary schools. Per protocol, when a parent expressed interest in immunizing their adolescent child, a pharmacist or nurse screened the patient for adolescent vaccine eligibility and reviewed the adolescent’s immunization registry record to determine vaccination needs. After providing parental consent, the adolescent received the vaccine and a pharmacist or nurse monitored the patient for 15 minutes for potential post-vaccination side effects while parents completed a survey questionnaire. The pharmacists then updated the adolescent’s immunization registry record and faxed a copy of the patient’s record to his/her primary care provider. A simplified flow diagram of this study protocol is depicted in Figure 1.
Figure 1.
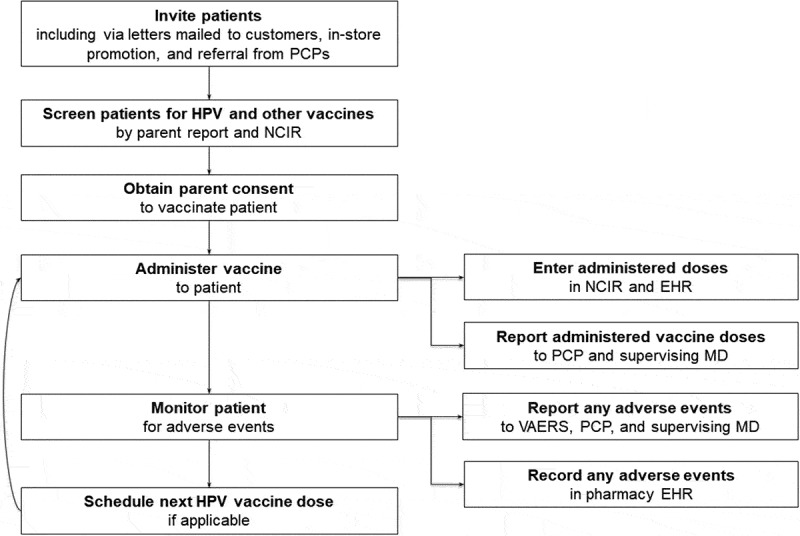
Study protocol from North Carolina pilot project. Abbreviations: NCIR = North Carolina Immunization Registry; EHR = Electronic health records; VAERS = Vaccine Adverse Event Reporting System; PCP = Primary care provider; MD = Physician.
Michigan
Provider recruitment and training
The Michigan Pharmacists Association partnered with a regional chain, SpartanNash Pharmacies, to conduct their pilot project in 10 pharmacy locations. Five pharmacies were in the Grand Rapids metropolitan area (Kent County) and each of the other five pharmacies was in one of five rural counties (Ottawa, Barry, Clare, Cass and Tuscola). In Michigan, state law allows pharmacists to administer HPV vaccines through standing orders from collaborating physicians. SpartanNash pharmacies had an existing adult standing order protocol in place for HPV vaccination for patients aged 18 to 26 years. For this project, a second physician in Grand Rapids provided a new adolescent standing order protocol for HPV vaccination for patients aged 11 to 17 years. Prior to launching the project, pharmacists and pharmacy technicians at each of the 10 participating pharmacies completed an educational training program on HPV and HPV vaccination, either in a live classroom session or a hybrid session combining videos and follow-up conference calls.
Patient recruitment and vaccination protocol
The recruitment period was from May 1, 2016 through July 31, 2016. Based on an initial assessment of the workflow at one of the pharmacies, a screening tool was developed to identify potential patients. Two versions were created, one for adolescents and another for adults. The screening tool was placed in the patients’ prescription bag alongside an age-appropriate vaccination education handout. When the patient came into the pharmacy to pick up their medication, they were asked to complete the screening tool during their prescription transaction. Of the 2,342 screening tools distributed, 429 (18%) were returned. The pharmacist then reviewed the screening tool and discussed HPV vaccination options with the parent of the adolescent patient or the adult patient using a resource guide to aid vaccination discussions. For patients interested in getting the vaccine whose insurance covered administration in the pharmacy or who were willing to pay cash, the pharmacist administered the vaccine. As a part of the screening for vaccination eligibility and dose documentation process, pharmacists used the Michigan Care Improvement Registry, an electronic statewide system for tracking childhood and adolescent vaccination.
Iowa, Kentucky, and Oregon
Provider recruitment and training
A group of three academic institutions that were members of the Centers for Disease Control and Prevention (CDC)-funded Cancer Prevention and Control Research Network (CPCRN) in Iowa, Kentucky, and Oregon conducted the pilot project in their respective states. The institutions were the University of Iowa, the University of Kentucky, and Oregon Health & Science University in collaboration with the Northwest Portland Area Indian Health Board. The investigators developed a common protocol for local pharmacies to partner with healthcare clinics to implement coordinated delivery of HPV vaccines to patients ages 11–18 years. The investigators then approached pharmacies for study participation. Interested pharmacies provided contact information for a clinic they wished to collaborate with; the investigators then contacted clinics for recruitment. In Iowa, two local independent pharmacies, Towncrest Pharmacy (Iowa City) and Osterhaus Pharmacy (Maquoketa), partnered with Southeast Iowa City Clinic and the Medical Association of Maquoketa, respectively. In Kentucky, Total Care Pharmacy, a pharmacy belonging to a local chain with six locations, partnered with the district health department.26 In Oregon, six tribes with pharmacies were approached to discuss project participation but none were recruited. Prior to launching the project, investigators offered in-person training for pharmacy-clinic partners that included review of study protocol, distribution of project and HPV educational materials, discussion of roles and responsibilities, enrollment in the state’s Vaccines for Children program, and an assessment of the compatibility of electronic medical record systems.
Patient recruitment and vaccination protocol
The recruitment period in Kentucky was from July 2016 through September 2016; Iowa started recruiting in September 2016; and Oregon was not able to start their project. Patient recruitment efforts varied by site but overall, activities included: displaying CDC posters in pharmacies, direct mailing to existing customers, running 237 30-second ads through local radio stations, biweekly newspaper advertising, and promotions at back-to-school events. Per study protocol, clinics administered the first dose of the HPV vaccine series, and transmitted prescription orders for second and third doses to partnering pharmacies. Pharmacies then scheduled doses with patients; after three failed attempts to contact patients, pharmacies notified the health clinic of non-response. Pharmacies transmitted vaccination information to corresponding state immunization registries and sent immunization information to the patient’s healthcare provider via fax or electronically. The protocol was tailored as necessary to match the local needs at respective pharmacy-clinic partners and investigators’ institutions.
Funding Statement
This study was funded by Merck Sharp & Dohme Investigator Studies Program (grant #50928 to North Carolina) and the American Cancer Society (grant #32608 to Iowa, Kentucky and Oregon). Authors’ time were supported in part by training or career development awards from the National Cancer Institute (K22 CA186979 to Gilkey and R25 CA116369 to Calo) and the Agency for Healthcare Research and Quality (T32 HS000032 to Shah). Funders played no role in: 1) study design; 2) the collection, analysis, and interpretation of data; 3) the writing of the report; or 4) the decision to submit the manuscript for publication; American Cancer Society [#32608]; Merck Sharp and Dohme [#50928].
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website.
Disclosure of potential conflicts of interest
Dr. Brewer has received commercial research grants from Merck and Pfizer and served as a paid advisory board member for Merck. He is chair of the National HPV Vaccination Roundtable which is funded by CDC and hosted by the American Cancer Society. The other authors have no financial disclosures or potential conflicts of interest to report.
References
- 1.Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Mbaeyi SA, Fredua B, Stokley S.. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(33):909–17. doi: 10.15585/mmwr.mm6733a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Singleton JA, Curtis CR, MacNeil J, Markowitz LE, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years–United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.President’s Cancer Panel Accelerating HPV vaccine uptake: urgency for action to prevent cancer. A report to the president of the United States from the president‘s cancer panel. Bethesda (MD): President’s Cancer Panel; 2014. [Google Scholar]
- 4.President’s Cancer Panel HPV vaccination for cancer prevention: progress, opportunities, and a renewed call to action. A report to the president of the United States from the chair of the president’s cancer panel. Bethesda (MD): President’s Cancer Panel; 2018. [Google Scholar]
- 5.National Vaccine Advisory Committee Overcoming barriers to low HPV vaccine uptake in the United States: recommendations from the national vaccine advisory committee: approved by the national vaccine advisory committee on June 9, 2015. Public Health Rep. 2016;131(1):17–25. doi: 10.1177/003335491613100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanderpool RC, Stradtman LR, Brandt HM. Policy opportunities to increase HPV vaccination in rural communities. Hum Vaccin Immunother. 2019. January 4 [accessed 2019 January8]. doi: 10.1080/21645515.2018.1553475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Association of Chain Drug Stores Home page. [accessed 2018. December 1]. https://www.nacds.org/.
- 8.Goad JA, Taitel MS, Fensterheim LE, Cannon AE. Vaccinations administered during off-clinic hours at a national community pharmacy: implications for increasing patient access and convenience. Ann Fam Med. 2013;11(5):429–36. doi: 10.1370/afm.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goad J, Bach A. The role of community pharmacy-based vaccination in the USA: current practice and future directions. Integr Pharm Res Prac. 2015;4:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Pharmacists Association Immunization Center - Immunization Authority [accessed 2018 December11].https://www.pharmacist.com/immunization-center?is_sso_called=1.
- 11.Brewer NT, Chung JK, Baker HM, Rothholz MC, Smith JS. Pharmacist authority to provide HPV vaccine: novel partners in cervical cancer prevention. Gynecol Oncol. 2014;132:S3–S8. doi: 10.1016/j.ygyno.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Calo WA, Gilkey MB, Shah P, Marciniak MW, Brewer NT. Parents‘ willingness to get human papillomavirus vaccination for their adolescent children at a pharmacy. Prev Med. 2017;99:251–56. doi: 10.1016/j.ypmed.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah PD, Calo W, Marciniak MW, Gilkey MB, Brewer NT. Support for pharmacist-provided HPV vaccination: national surveys of US physicians and parents. Cancer Epidemiol Prev Biomarkers. 2018;27(8):970–78. doi: 10.1158/1055-9965.EPI-18-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah PD, Calo WA, Marciniak MW, Golin CE, Sleath BL, Brewer NT. Service quality and parents‘ willingness to get adolescents HPV vaccine from pharmacists. Prev Med. 2018;109:106–12. doi: 10.1016/j.ypmed.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burson RC, Buttenheim AM, Armstrong A, Feemster KA. Community pharmacies as sites of adult vaccination: A systematic review. Hum Vaccin Immunother. 2016;12(12):3146–59. doi: 10.1080/21645515.2016.1215393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westrick SC, Hohmann LA, McFarland SJ, Teeter BS, White KK, Hastings TJ. Parental acceptance of human papillomavirus vaccinations and community pharmacies as vaccination settings: A qualitative study in Alabama. Papillomavirus Res. 2017;3:24–29. doi: 10.1016/j.pvr.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastings TJ, Hohmann LA, McFarland SJ, Teeter BS, Westrick SCJP. Pharmacists’ attitudes and perceived barriers to human papillomavirus (HPV) vaccination services. Pharmacy (Basel). 53). 2017;5(3):45. doi: 10.3390/pharmacy5030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah PD, Gilkey MB, Pepper JK, Gottlieb SL, Brewer NT. Promising alternative settings for HPV vaccination of US adolescents. Expert Rev Vaccines. 2014;13(2):235–46. doi: 10.1586/14760584.2013.871204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McRee A-L, Reiter PL, Pepper JK, Brewer NT. Correlates of comfort with alternative settings for HPV vaccine delivery. Hum Vaccin Immunother. 2013;9:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National HPV Vaccination Roundtable Homepage. [accessed 2018. November 8]. http://hpvroundtable.org/.
- 21.Klein KJ, Knight AP. Innovation implementation: overcoming the challenge. Curr Dir Psychol Sci. 2005;14(5):243–46. doi: 10.1111/j.0963-7214.2005.00373.x. [DOI] [Google Scholar]
- 22.Islam JY, Gruber JF, Lockhart A, Kunwar M, Wilson S, Smith SB, Brewer NT, Smith JS. Opportunities and challenges of adolescent and adult vaccination administration within pharmacies in the United States. Biomed Infor Insights. 2017;9:1178222617692538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Community Preventive Services Task Force Recommendation for use of immunization information systems to increase vaccination rates. J Public Health Manag Pract. 2015;21(3):249–52. doi: 10.1097/PHH.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 24.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, Griffey R, Hensley M. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerke D, Lewis E, Prusaczyk B, Hanley C, Baumann A, Proctor E. Eight toolkits related to dissemination and implementation. Implementation Outcomes. [accessed 2018. November 28]. https://sites.wustl.edu/wudandi/.
- 26.Vanderpool R, Pilar M, Barker J, Freeman P. Increasing HPV vaccination through community pharmacy partnerships: lessons learned from a pilot project. Ky Pharm. 2017;12:33–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- National Association of Chain Drug Stores Home page. [accessed 2018. December 1]. https://www.nacds.org/.


