ABSTRACT
The incidence of human papillomavirus (HPV)-associated head and neck cancers is rising, particularly among men. Whether observed epidemiological differences in sex are explained by differences in sexual exposure and/or by immune response is unclear. In this cross-sectional, multi-institutional study, seroprevalence of antibodies to HPV L1 capsid antigen was compared by patient characteristics among 374 adult patients without cancer. A significantly higher seroprevalence was observed among women compared with men for HPV16 (OR = 2.96, 95% CI = 1.21–7.21) and HPV18 (OR = 2.84, 95% CI = 1.06–7.60) L1 antibodies. This difference persisted for HPV16 after controlling for lifetime and recent sexual behavior. After controlling for sex, HPV16 and HPV18 L1 seroprevalence was also significantly associated with higher number of lifetime (HPV16 OR = 1.05, 95% CI = 1.01–1.08; HPV18 OR = 1.04, 95% CI = 1.01–1.08) and recent (HPV16 OR = 1.54, 95% CI = 1.15–2.07; HPV18 OR = 1.40, 95% CI = 1.07–1.82) oral but not vaginal sexual partners. These findings potentially suggest a more robust immune response to HPV16/18 among women compared with men that may not be explained by differences in number of sexual partners, and thereby presumably HPV exposure. The independent association of HPV16/18 L1 seroprevalence with higher number of oral sexual partners suggests a possible role for site of mucosal exposure in the HPV immune response.
KEYWORDS: Human papillomavirus, seroepidemiologic studies, sexual partners, head and neck neoplasms
Introduction
The incidence of human papillomavirus (HPV)-associated head and neck cancer is rising in North America.1 Among men, the incidence is more than twice that of women, although the prevalence of cancers attributable to HPV is rising among both sexes.2 Though the male predominance in head and neck cancer incidence is attributed to a higher prevalence of tobacco and alcohol use,3 the reasons for the sex differences in HPV-associated cancer remain unclear. Hypothesized explanations for male predominance of HPV-related cancers have been attributed to men having a greater number of vaginal and oral sexual partners. However, research suggests that higher numbers of lifetime sexual partners only partially explain the sex difference 16–18% of the sex difference in prevalence of oral HPV,4,5 supporting a role for other co-factors beyond behavior. The prevalence of oral HPV infection is greatest among men4-6 who smoke cigarettes and have at least five lifetime oral sexual partners.7
This higher prevalence of oral HPV among men is hypothesized to be due to differences in immune response among men and women which, in turn, may contribute to the observed differences in HPV-associated head and neck cancer incidence. Average time to clearance of oral HPV16 infection is more than twice as long among men compared with women.8 Furthermore, oral HPV incidence9 and prevalence4,5 is more strongly associated with a higher number of sexual partners among men than women, suggesting per partner risk of oral HPV acquisition may be less for women than men.
Consistent with the more robust humoral and cell-mediated immune response to other viral antigens among women compared with men,10 multiple prior studies have demonstrated higher seroprevalence to HPV L1 capsid antibody among women. However, many of these studies are limited as they were performed in young cohorts with high-risk exposure to sexually transmitted infections (including Human Immunodeficiency Virus [HIV]) and were conducted over a decade ago. Only one of these studies had data on both oral and vaginal sex partners to consider analytically.11-16 It remains unknown whether the sex differences in HPV antibody response previously reported in at-risk populations are observed among older healthy individuals, and if differences in seroprevalence are explained by oral and/or vaginal sexual exposure.
Methods
Study population
The study population for this cross-sectional analysis was comprised of individuals with no history of head and neck cancer or radiation that were enrolled as age and sex matched clinic controls for a multicenter, case-control study of incident head and neck cancer. Adult patients seeking care at outpatient clinics for Otology, Audiology, and Laryngology between May 2014 to May 2017 for chief complaints that did not include or result in a diagnosis of cancer were eligible for this study. Further eligibility criteria included serum availability, no history of HPV vaccination (6 vaccinated people were excluded as potentially not at risk), and less than 100 oral or vaginal sexual partners (24 people with >100 partners excluded as non-representative). Study sites included Johns Hopkins Hospital (Baltimore, MD), Greater Baltimore Medical Center (Baltimore, MD), University of California – San Francisco Medical Center (San Francisco, CA), and Mount Sinai (New York, NY). Written informed consent was given by all enrollees, and institutional review boards at each study site approved the protocol.
Sample collection
Behavioral data were collected by computer-assisted self-interview. Subjects were asked about race/ethnicity, demographic data, substance use, and sexual behaviors. The wording of survey questions was adapted from NHANES 2011–2012.17 A 6 milliliter peripheral blood sample was collected in a non-heparinized Vacutainer tube and serum was separated by centrifugation. Samples were transported on dry ice and stored at −20 degrees C until processing.
Human papillomavirus serology
Sera were tested for HPV major capsid protein (L1) antibodies to oncogenic HPV types 16, 18, 31, 33, 35, 45, 52, and 58 and non-oncogenic HPV types 6 and 11. Testing was performed at the German Cancer Research Center (DKFZ, Heidelberg, Germany) using multiplex serology, an antibody detection method based on glutathione S-transferase (GST) capture ELISA, in combination with fluorescent bead-based technology, in a procedure that has been previously described.18 Median fluorescence intensity (MFI) values were dichotomized as antibody positive or negative, using standardized cutoff values.19,20
Statistical analyses
Demographics and behavioral exposures of male and female participants were compared. For some analyses, HPV16 or HPV18 L1 seropositive participants were compared to seronegative. Age of participant and number of sexual or open mouth kissing partners were considered as continuous variables and compared by a two-sided t-test. Recent sexual partners were defined as sexual partners in the last 12 months. The following variables were treated as binary: serostatus (seropositive, seronegative), race/ethnicity (white non-Hispanic, nonwhite), relationship status (widowed/divorced/separated/never married, married/living with a partner), history of genital warts, current smoking status, current alcohol use (≥15 drinks/month, <15 drinks/month), illegal drug use (ever, never), coitarche at <18 years, type of first sexual act (yes/no for oral sex, oral sex on a woman, oral sex on a man, vaginal sex, vaginal sex with a woman, vaginal sex with a man), sexual orientation (homosexual/bisexual/other, heterosexual). Regular marijuana use was categorized as none, 1–2 times/week, >2 times/week. Categorical variables were compared by Pearson’s χ2 or Fisher’s exact test when samples were small.
Logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (95% CI) for the associations between exposure variables and HPV L1 seroprevalence. Variables significant in univariate analysis based on p-value <0.05 or of interest given existing literature were evaluated in a multiple logistic regression model.
Results
374 participants met the eligibility criteria. As described in Table 1, the median age of the study population was 60 years (IQR 52–68), most participants were men (281, 75%), white non-Hispanic (287, 77%), college graduates (230, 65%) and enrolled at Johns Hopkins (251, 67%). Men and women were similar by age and education, although women were more likely to be nonwhite (p = 0.001), and to report a lower annual family income (p = 0.007). The most frequent reason for clinic visit was hearing loss (125, 33%), followed by other otologic complaints (117, 31%), and benign lesions of the head and neck (69, 18%).
Table 1.
Demographics of study population. Bolding indicates statistical significance.
| Sex |
||||
|---|---|---|---|---|
| Total N = 374, No. (%) |
Men n = 281, No. (%) |
Women n = 93, No. (%) |
p-value | |
| Median age (IQR) | 60 (52–68) | 60 (52–67) | 62 (51–70) | p = 0.57 |
| Race | p = 0.001 | |||
| White non-Hispanic | 287 (77) | 227 (81) | 60 (64) | |
| Nonwhite | 87 (23) | 54 (19) | 33 (35) | |
| Income | p = 0.007 | |||
| Less than $15,000 | 14 (4) | 6 (2) | 8 (10) | |
| $15,000-$29,999 | 25 (8) | 13 (5) | 12 (15) | |
| $30,000-$49,999 | 28 (9) | 19 (8) | 9 (11) | |
| $50,000-$74,999 | 46 (14) | 37 (15) | 9 (11) | |
| $75,000-$99,999 | 44 (14) | 34 (14) | 10 (12) | |
| $100,000-$149,999 | 63 (20) | 47 (20) | 16 (20) | |
| $150,000-$199,999 | 45 (14) | 35 (15) | 10 (12) | |
| $200,000-$399,999 | 45 (14) | 39 (16) | 6 (7) | |
| Greater than $400,000 | 11 (3) | 10 (4) | 1 (1) | |
| Education | p = 0.81 | |||
| Less than high school | 10 (3) | 8 (3) | 2 (2) | |
| High school | 41 (12) | 29 (11) | 12 (13) | |
| GED or equivalence | 14 (4) | 12 (5) | 2 (2) | |
| Some college | 60 (17) | 43 (16) | 17 (19) | |
| College graduate | 110 (31) | 79 (30) | 31 (34) | |
| Advanced/professional | 120 (34) | 92 (35) | 28 (30) | |
| degree | ||||
| Study Site | p = 0.09 | |||
| JHH | 251 (67) | 198 (70) | 53 (57) | |
| GBMC | 17 (5) | 12 (4) | 5 (5) | |
| UCSF | 74 (20) | 48 (17) | 26 (28) | |
| MS | 32 (9) | 23 (8) | 9 (10) | |
IQR, interquartile range; JHH, Johns Hopkins Hospital; GBMC; Greater Baltimore Medical Center; UCSF, University of California – San Francisco Medical Center; MS, Mount Sinai
Overall, 122 (33%) of participants were seropositive for any HPV L1 type, 95 (25%) for any oncogenic HPV L1 type, 21 (6%) for HPV16 L1, and 17 (5%) for HPV18 L1.
Seroprevalence by sex
Women were more likely than men to be seropositive for HPV16 (OR = 2.96, 95% CI = 1.21–7.21) and HPV18 (OR = 2.84, 95% CI = 1.06–7.60) L1 (Figure 1). A similar magnitude of difference in seroprevalence was observed by sex for HPV33 (OR = 4.16, 95% CI 0.91–18.96, p = 0.06) and HPV11 (OR = 2.40, 95% CI 0.98–5.90, p = 0.05), although these were marginally statistically significant. For the six other HPV L1 types examined, the seroprevalence was also higher among females, however the difference was not statistically significant (p = 0.09–0.90; Supplemental Table 1). When analysis was restricted to 23 men and 20 women who reported never having had oral sex, women remained at higher odds of HPV16 L1 seropositivity than men (15.0% vs. 4.3%; OR = 3.88, 95% CI = 0.37–40.71), although this was no longer statistically significant.
Figure 1.
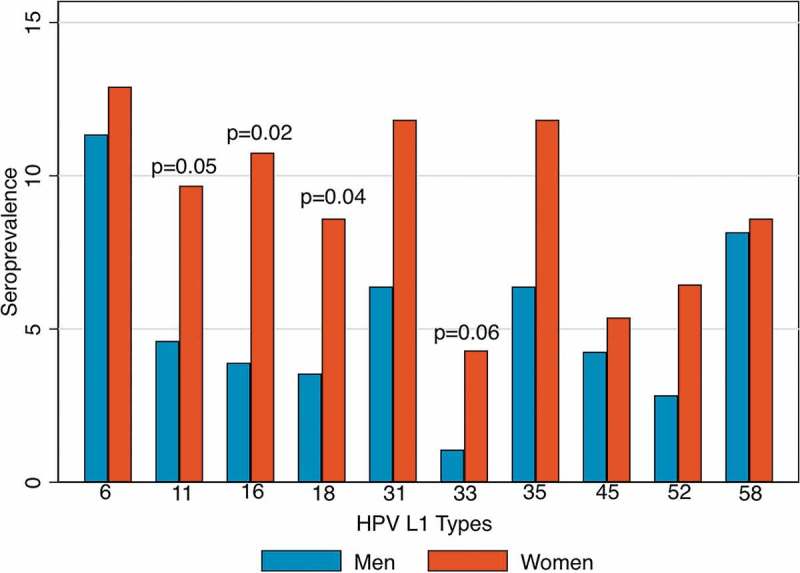
Seroprevalence of HPV L1 antibodies by sex and HPV type, compared by logistic regression.
Prevalence of behaviors of interest was compared by sex to explore whether differences in HPV seroprevalence may be attributed to differences in sexual exposure, relationship status or substance use (Table 2). Compared with women, men were more likely to have a higher number of lifetime oral sexual partners (p = 0.005), and recent vaginal sexual partners (p = 0.03). Men were more likely to have had coitarche at age <18 (p = 0.008), and were more likely to be married or living with a partner (75% vs. 61%, p = 0.01). Substance use was similar in men and women except that men were more likely to drink ≥15 drinks/month (24% vs. 9%, p = 0.001).
Table 2.
Differences in behavior by sex and HPV16 and HPV18 L1 seroprevalence. Bolding indicates statistical significance. IQR, interquartile range.
| Men n(%) | Women n(%) | p-value | HPV16 (+) n(%) | HPV16 (-) n(%) | p-value | HPV18 (+) n(%) | HPV18 (-) n(%) | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| 281 (75) | 93 (25) | 21 (6) | 353 (94) | 17 (5) | 357 (95) | ||||
| Sexual Exposure | |||||||||
| First sexual act as: | |||||||||
| Oral sex | 39 (15) | 8 (9) | 0.14 | 2 (10) | 45 (14) | 1.0 | 2 (12) | 45 (13) | 1.0 |
| Oral sex on a woman | 32 (12) | 1 (1) | 0.001 | 0 (0) | 33 (10) | 0.24 | 0 (0) | 33 (10) | 0.38 |
| Oral sex on a man | 8 (3) | 7 (8) | 0.06 | 2 (10) | 13 (4) | 0.21 | 2 (12) | 13 (4) | 0.14 |
| Vaginal sex | 189 (74) | 67 (80) | 0.30 | 13 (68) | 243 (76) | 0.46 | 11 (73) | 245 (76) | 0.77 |
| Vaginal sex with a woman | 189 (74) | 0 (0) | <0.001 | 7 (37) | 182 (57) | 0.09 | 6 (40) | 183 (56) | 0.21 |
| Vaginal sex with a man | 0 (0) | 67 (81) | <0.001 | 6 (32) | 61 (19) | 0.18 | 5 (33) | 62 (19) | 0.18 |
| Lifetime sexual partners, median (IQR): | |||||||||
| Any | 8 (3–19) | 5 (2–13) | 0.31 | 9 (4–23) | 7 (3–15) | 0.22 | 9 (5–20) | 6 (3–15) | 0.11 |
| Oral | 4 (1–8) | 2 (1–4) | 0.005 | 4 (2–13) | 3 (1–7) | 0.004 | 5 (2–10) | 3 (1–7) | 0.006 |
| Vaginal | 7 (3–15) | 5 (2–12) | 0.08 | 8 (4–15) | 6 (3–15) | 0.98 | 8 (4–15) | 6 (2–15) | 0.98 |
| Open mouth kissing | 10 (5–23) | 8 (3–20) | 0.10 | 15 (5–35) | 10 (4–20) | 0.48 | 15 (6–30) | 10 (4–20) | 0.10 |
| Recent sexual partners, median (IQR): | |||||||||
| Any | 1 (0–1) | 1 (0–1) | 0.41 | 1 (0–2) | 1 (0–1) | 0.17 | 1 (0–1) | 1 (0–1) | 0.24 |
| Oral | 1 (0–1) | 0 (0–1) | 0.07 | 1 (0–2) | 1 (0–1) | <0.001 | 1 (0–1) | 1 (0–1) | 0.002 |
| Vaginal | 1 (1–1) | 1 (0–1) | 0.03 | 1 (1–1) | 1 (0–1) | 0.76 | 1 (0–1) | 1 (0–1) | 0.47 |
| Homosexual/Bisexual/Other | 17 (7) | 11 (12) | 0.09 | 24 (7) | 4 (20) | 0.06 | 4 (25) | 24 (7) | 0.03 |
| History of genital warts | 13 (5) | 4 (4) | 1.0 | 1 (5) | 16 (5) | 1.0 | 1 (7) | 16 (5) | 0.53 |
| Coitarche at <18 years | 124 (48) | 28 (32) | 0.008 | 8 (40) | 144 (45) | 0.69 | 5 (31) | 147 (45) | 0.28 |
| Relationship status | |||||||||
| Widowed/divorced/separated | 0.01 | 0.10 | 0.84 | ||||||
| /never married | 67 (25) | 36 (39) | 9 (45) | 94 (28) | 5 (31) | 98 (29) | |||
| Married/living with a partner | 196 (75) | 56 (61) | 11 (55) | 241 (72) | 11 (69) | 241 (71) | |||
| Substance use | |||||||||
| Current smoker | 18 (7) | 6 (6) | 0.92 | 2 (10) | 22 (7) | 0.63 | 21 (6) | 3 (19) | 0.08 |
| ≥ 15 drinks/month | 67 (24) | 8 (9) | 0.001 | 5 (24) | 70 (20) | 0.66 | 5 (29) | 70 (20) | 0.32 |
| Ever used illegal drugs | 163 (62) | 51 (56) | 0.28 | 15 (75) | 199 (60) | 0.18 | 12 (75) | 202 (60) | 0.30 |
| Regular marijuana use: | 0.09 | 0.73 | 0.63 | ||||||
| Yes, ≤ 2 times/week | 33 (20) | 6 (12) | 2 (13) | 37 (19) | 1 (8) | 38 (19) | |||
| Yes, >2 times/week | 33 (20) | 6 (12) | 4 (27) | 35 (18) | 3 (25) | 36 (18) |
To better understand the association between sex and HPV seroprevalence, other risk factors for HPV16 and HPV18 L1 seroprevalence were examined by univariate analysis (Table 3). Interestingly, number of lifetime (OR = 1.04, 95% CI = 1.01–1.07) and recent (OR = 1.46, 95% CI = 1.10–1.93) oral sexual partners, but not lifetime or recent vaginal sexual partners were associated with increased odds of HPV16 L1 seroprevalence. These results were similar for HPV18 L1 seroprevalence. Increasing decade of age was negatively associated with HPV16 L1 seroprevalence (OR = 0.68, 95% CI = 0.49–0.95).
Table 3.
Logistic regression of HPV16 and HPV18 L1 seroprevalence on demographic factors and behavioral exposure. Bolding indicates statistical significance.
| HPV L1 type | n(%) seropositive | Univariate | Bivariate | ||||
|---|---|---|---|---|---|---|---|
| HPV16 | 21 (6) | OR (95%CI) | |||||
| Female sex | 2.96 (1.21–7.21) | 3.29 (1.25–8.68) | 3.59 (1.21–10.58) | 2.84 (1.05–7.68) | 1.70 (0.49–5.95) | ||
| Lifetime oral sexual partners | 1.04 (1.01–1.07) | 1.05 (1.01–1.08) | |||||
| Recent oral sexual partners | 1.46 (1.10–1.93) | 1.54 (1.15–2.07) | |||||
| Lifetime vaginal sexual partners | 1.00 (0.96–1.04) | 1.00 (0.96–1.05) | |||||
| Recent vaginal sexual partners | 0.86 (0.34–2.17) | 0.91 (0.37–2.24) | |||||
| Increasing decade of age | 0.68 (0.49–0.95) | ||||||
| Nonwhite race | 1.71 (0.67–4.37) | ||||||
| Married or living with a partner | 0.48 (0.19–1.19) | ||||||
| Younger age at coitarche | 0.83 (0.33–2.08) | ||||||
| Regular alcohol use | 1.26 (0.45–3.57) | ||||||
| Homosexual/Bisexual/Other | 3.19 (0.99–10.29) | ||||||
| HPV18 | 17 (5) | OR (95%CI) | |||||
| Female sex | 2.84 (1.06–7.60) | 3.07 (1.05–9.01) | 2.29 (0.71–7.32) | 2.70 (0.87–8.34) | 2.39 (0.71–7.98) | ||
| Lifetime oral sexual partners | 1.04 (1.00–1.07) | 1.04 (1.01–1.08) | |||||
| Recent oral sexual partners | 1.36 (1.05–1.76) | 1.40 (1.07–1.82) | |||||
| Lifetime vaginal sexual partners | 1.00 (0.95–1.05) | 1.00 (0.96–1.05) | |||||
| Recent vaginal sexual partners | 0.67 (0.23–1.89) | 0.76 (0.28–2.07) | |||||
| Increasing decade of age | 0.84 (0.58–1.23) | ||||||
| Nonwhite race | 1.01 (0.32–3.20) | ||||||
| Married or living with a partner | 0.89 (0.30–2.64) | ||||||
| Younger age at coitarche | 0.56 (0.19–1.64) | ||||||
| Regular alcohol use | 1.71 (0.58–5.01) | ||||||
| Homosexual/Bisexual/Other | 4.31 (1.29–14.37) | ||||||
Bivariate models including vaginal and oral sexual exposure were then considered. Adjusting for number of lifetime (aOR = 3.29, 95% CI = 1.25–8.68) or recent (aOR = 3.59, 95% CI = 1.21–10.58) oral sexual partners, women remained at significantly increased odds of HPV16 L1 seroprevalence compared with men (Table 3). Lifetime oral sexual partners (aOR = 1.05, 95% CI = 1.01–1.08) and to a greater extent recent oral sexual partners (aOR = 1.54, 95% CI = 1.15–2.07) were independently associated with HPV16 L1 seroprevalence, after adjustment for sex. Results were similar after adjustment for decade of age (results not shown).
HPV18 L1 seroprevalence was associated with lifetime (aOR = 1.04, 95% CI 1.01–1.08) and recent (aOR = 1.40, 95% CI = 1.07–1.82) number of oral sexual partners, adjusting for female sex (aOR = 3.07, 95% CI = 1.05–9.01 and aOR = 2.29, 95% CI = 0.71–7.32, Table 3), similar to the associations seen for HPV16 L1. Homosexual/bisexual/other sexual orientation was also associated with increased odds of HPV18 L1 in univariate analysis and when the bivariate analyses of HPV18 seroprevalence were adjusted for sexual orientation, resulting strengths of association were similar in magnitude to unadjusted, but no longer significant (results not shown).
Sensitivity analyses including 11 participants with a high number (100–200) of oral or vaginal sexual partners showed similar results.
Examining interaction in sexual behavior and sex on HPV seropositivity
When men and women were considered separately in univariate models, number of lifetime or recent oral sexual partners remained strongly associated with HPV16 L1 seroprevalence, and was statistically significant for the 281 men (lifetime p = 0.017, recent p = 0.007) and marginally significant among the 93 women (lifetime p = 0.07, recent p = 0.06). Indeed, when interaction between female sex and number of oral and vaginal lifetime as well as oral and vaginal recent sexual partners on HPV16 L1 seroprevalence was explored, no significant interaction was found (pinteraction>0.10). Similarly, no interaction between sexual partners and sex was detected for HPV18 L1 seroprevalence and numbers of sexual partners (pinteraction>0.10).
Other HPV types
In contrast to what was observed for HPV16 and 18, female sex was not significantly associated with HPV L1 seroprevalence for other HPV types tested (HPV6, 11, 31, 33, 35, 45, 52, 58), or when HPV seroprevalence to any type was considered. Number of oral sex partners was associated with increased seropositivity to any HPV L1 type and to HPV11, HPV45, or HPV52 (as well as HPV16 and 18), but was not associated with seropositivity to other HPV L1 types (Supplemental Table 1).
Discussion
We demonstrate for the first time, to our knowledge, that the number of oral sexual partners but not vaginal sexual partners is independently associated with HPV16 and HPV18 L1 seroprevalence among men and women. In addition, we use a population demographically similar to those with incident HNSCC to show that women have approximately three times the odds of seropositivity to HPV16 L1 capsid antibody compared with men after controlling for number of vaginal or oral sex partners. Our findings suggest oral sex may be an important exposure for HPV16 and 18 L1 seroconversion, and potentially suggest a more robust response to HPV exposure among women compared with men. These findings are of particular significance given the dramatic rise in oropharyngeal cancers due to HPV in many geographic regions worldwide.21
HPV16 is thought to cause at least 70% of oropharyngeal cancers and ~59% of cervical cancers, and HPV18 is the second most common HPV type implicated in cervical cancers.22-24 Compared to other HPV types, HPV16 is thought to have a more developed immune escape mechanism.25 Cervical HPV16 and 18 infections are able to reach a higher copy number26 than other HPV types and persist longer, and persistence is associated with positive serology.27 Consistent with prior analyses, we did not observe a higher seroprevalence of HPV16 compared with other types. However, our finding that only the sex differences in HPV16 and HPV18 L1 reached statistical significance is intriguing, and may imply these types have improved immune evasion compared with other types examined, particularly among men. Prior analyses similarly show the greatest sex difference for HPV16 L1 seroprevalence among other HPV L1 types.15,19 Although not significant, qualitatively higher seroprevalence by female sex among all types, particularly among HPV33 (OR = 4.16) and HPV11 (OR = 2.40), implies either an easier seroconversion by vaginal exposure, or a stronger response among women to exposure to all HPV types examined.
The finding that women are three times more likely to be HPV16 L1 seropositive compared with men is consistent with existing literature.12,14,15 In the one available previous study controlling for lifetime sexual behavior, higher HPV16 L1 seroprevalence among women persisted (aOR = 2.1, 95% CI = 1.6–2.8) after adjustment,14 corroborating our finding that the sex difference in HPV16 L1 seroprevalence is not explained by number of sexual partners (Table 3). Though these prior studies included patients at high risk of STI, significantly higher HPV seroprevalence among women has also been reported in population-based studies in the US,5 the Netherlands,28 England29 and Australia.30 Notably, the majority of patients in these prior analyses were young (<50 years). In addition, no studies known to the authors with a comparison of HPV antibodies by sex are available on cohorts assembled after 2005. As the prevalence of oral HPV infection appears to be rising,6 our analysis is a valuable contemporary contribution to understanding evolving HPV epidemiology.
The contribution of vaginal and oral sexual exposure on HPV L1 seroprevalence has also been previously examined. Though some prior seroepidemiological analyses found higher HPV16 L1 seroprevalence was associated with higher number of lifetime sexual partners,11,13 others found no association.14,19 It has been suggested that vaginal HPV exposure may be protective of subsequent oral infection,9 although it is well-established that oral sexual exposure increases risk for prevalent and incident oral HPV infection.4,5,9,31 Therefore, it is important to analyze seroprevalence by anatomic site of sexual exposure, even though the collinearity of these exposures makes drawing conclusions about the independent effect of each exposure challenging. Only one prior study to our knowledge examined vaginal and oral sexual exposure separately, and found no association with seroprevalence.15 Our finding that oral sexual partners, rather than vaginal, is associated with HPV16 and HPV18 L1 seroprevalence is novel, and aligns with prior studies showing that oral rather than vaginal8 or any type31 of recent sexual exposure increases risk for incident oral HPV infection.
The association of seroprevalence with oral sexual exposure is consistent with the idea that HPV exposure at a mucosal (oral, vaginal, anal) rather than keratinized (penile) anatomic site increases seroconversion. Indeed, prior studies have found that men who have sex with men have a high seroprevalence.15,28 Likewise, greater likelihood of seropositivity in response to HPV exposure among women suggested by this study and others has been thought to be partly due to the greater immune surveillance in the cervical mucosal epithelium compared with penile keratinized squamous epithelium.12,32 Although further investigation is necessary, our analysis did not support this as vaginal exposure was not associated with seroprevalence when women were considered separately. However, almost all (97%) participants in the present analysis report having had vaginal sex, limiting our ability to detect the role of a single vaginal exposure in determining seroconversion, in contrast to a possible per-partner increase in seroprevalence for oral exposure. A prior study, for example, found that though HPV16 and HPV18 L1 seroprevalence was similar and low among virginal and sexually active male college students, a significantly higher seroprevalence (10%) was found among sexually active female students.19 Whether receptive vaginal exposure drives the observed epidemiologic sex differences, differential responses to viral challenge are likely to play a role. More robust responses among women compared with men have been well-described for antigen challenge from a range of infections including influenza A, hepatitis B, and human immunodeficiency virus, 33 possibly due to genetic and hormonal factors.
The higher seroprevalence by female sex may give insight into the role of natural immunity in sex differences of HPV-associated head and neck cancer incidence. There is precedent that positive HPV L1 serology confers natural immunity as evidenced by protection from future cervical infections among women;32 however, similar serotiters do not appear to prevent anal infections among men.34 An adequately powered study to examine natural immunity against incident oral infection among women compared with men has not yet been performed. However, should natural immunity play a more significant role for women, this may partly explain the higher incidence of HPV-related cancers among men. Vaccination results in greater than 50 times the HPV16 L1 serotiter of natural immunity, and could theoretically overwhelm sex differences in immunity.35 Importantly, a recent NHANES study found a significant decrease in oral prevalence of type-specific HPV infections among both men and women who had received at least one dose of the HPV vaccine.36
We acknowledge a limited population size with low overall HPV L1 seroprevalence in this study compared with previously published analyses, which is likely a reflection of the older age of our study population.15 The cross-sectional design limited the ability to determine the response to sexual exposure over time. The low seroprevalence barred additional subgroup examination of the effect of types of sexual behavior. In addition, we acknowledge that seroprevalence is an imperfect marker for immune response by sex, as routes of sexual exposure are largely distinct by sex; thus, routes of sexual exposure could not be controlled for. It is possible that some of the variation in seroprevalence observed by sex, is due to differences in viral exposure. For example, there could be more HPV exposure when performing oral sex on a woman than on a man. Finally, oral HPV DNA was unavailable for this analysis as an adjunct marker of exposure, and it is possible that the serology assay used may not have efficiently detected HPV L1 antibodies at low titers.
The authors show for the first time to our knowledge that oral sexual exposure, but not vaginal, is independently associated with HPV16 and HPV18 L1 seroprevalence. The higher seroprevalence among females compared with males in this contemporary cohort was not explained by sexual behavior. Instead, higher female seroprevalence is potentially a reflection of innate immune differences by sex or by route of exposure. Further study of HPV seroconversion and natural immunity are required in order to better characterize if this sex difference in seroprevalence contributes to the observed male predominance in HPV-associated head and neck cancer.
Funding Statement
This work was supported by the National Cancer Institute [P30CA006973];National Institute of Dental and Craniofacial Research [P50DE019032];National Institute of Dental and Craniofacial Research [R35DE026631 and NIH 5T32DC000027-29].
Disclosure of potential conflicts of interest
No potential conflict of interest were disclosed.
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Souza G, Westra WH, Wang SJ, van Zante A, Wentz A, Kluz N, Rettig E, Ryan WR, Ha PK, Kang H, et al. Differences in the prevalence of Human Papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3(2):169–177. doi: 10.1001/jamaoncol.2016.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541-50. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillison ML, Broutian T, Pickard RKL, Tong Z-Y, Xiao W, Kahle L, Graubard BI, Chaturvedi AK.. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307(7):693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong Z-Y, Xiao W, Kahle L, Gillison ML. NHANES 2009–2012 findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res. 2015;75(12):2468–77. doi: 10.1158/0008-5472.CAN-14-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam S, Fu S, Xu L, Krause KJ, Lairson DR, Miao H, Sturgis EM, Dahlstrom KR. The epidemiology of oral human papillomavirus infection in healthy populations: A systematic review and meta-analysis. Oral Oncol. 2018;82:91–99. doi: 10.1016/j.oraloncology.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 7.D'Souza G, McNeel TS, Fakhry C, McNeel TS, Fakhry C. Understanding personal risk of oropharyngeal cancer: risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol. 2017:28;3065–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Souza G, Wentz A, Kluz N, Zhang Y, Sugar E, Youngfellow RM, Guo Y, Xiao W, Gillison ML. Sex differences in risk factors and natural history of oral human papillomavirus infection. J Infect Dis. 2016;213(12):1893–96. doi: 10.1093/infdis/jiw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199(9):1263–69. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink AL, Klein SL. the evolution of greater humoral immunity in females than males : implications for vaccine efficacy. Curr Opin Psychol. 2018;5:16–20. doi: 10.1016/j.cophys.2018.03.010 6:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svare EI, Kjaer SK, Nonnenmacher B, Worm A-M, Moi H, Christensen RB, van Den Brule AJC, Walboomers JMM, Meijer CJLM, Hubbert NL, et al. Seroreactivity to human papillomavirus type 16 virus-like particles is lower in high-risk men than in high-risk women. J Infect Dis. 1997;176:876–883. doi: 10.1086/jid.1997.176.issue-4. [DOI] [PubMed] [Google Scholar]
- 12.Strickler HD, Kirk GD, Figueroa JP, Ward E, Braithwaite AR, Escoffery C, Drummond J, Goebel B, Waters D, McClimens R, et al. HPV 16 antibody prevalence in Jamaica and the United States reflects differences in cervical cancer rates. Int J Cancer. 1999;80:339–44. [DOI] [PubMed] [Google Scholar]
- 13.Stone KM, Karem KL, Sternberg MR, McQuillan GM, Poon AD, Unger ER, Reeves WC. Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis. 2002;186(10):1396–402. doi: 10.1086/344354. [DOI] [PubMed] [Google Scholar]
- 14.Thompson DL, Douglas JM Jr, Foster M, Hagensee ME, Diguiseppi C, Barón AE, Cameron JE, Spencer TC, Zenilman J, Malotte CK, et al. Seroepidemiology of infection with human papillomavirus 16, in men and women attending sexually transmitted disease clinics in the united states. J Infect Dis. 2004;190(9):1563–74. doi: 10.1086/423817. [DOI] [PubMed] [Google Scholar]
- 15.Kreimer AR, Alberg AJ, Viscidi R, Gillison ML. Gender differences in sexual biomarkers and behaviors associated with human papillomavirus-16, −18, and −33 seroprevalence. Sex Transm Dis. 2004;31:247–56. [DOI] [PubMed] [Google Scholar]
- 16.Furniss CS, McClean MD, Smith JF, Bryan J, Nelson HH, Peters ES, Posner MR, Clark JR, Eisen EA, Kelsey KT. Human papillomavirus 16 and head and neck squamous cell carcinoma. Int J Cancer. 2007;120(11):2386–92. doi: 10.1002/ijc.22633. [DOI] [PubMed] [Google Scholar]
- 17.National Health and Nutrition Examination Survey Data (NHANES). NHANES 2011–2012. Questionnaire Instruments. Hyattsville (MD); 2012. [accessed 2018 June 27]. cdc.gov/nchs/nhanes/continuousnhanes/questionnaires.aspx?BeginYear=2011
- 18.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. Multiplex human papillomavirus serology based on in situ-purified glutathione S-transferase fusion proteins. Clin Chem. 2005;51(10):1845–53. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 19.Clifford GM, Shin HR, Oh JK, Waterboer T, Ju Y-H, Vaccarella S, Quint W, Pawlita M, Franceschi S. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1874–79. doi: 10.1158/1055-9965.EPI-07-0349. [DOI] [PubMed] [Google Scholar]
- 20.Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, Page R, Lind MJ, Peake MD, Møller H. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708–15. doi: 10.1200/JCO.2013.49.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–42. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 23.Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsagué X, Laporte L, Bosch FX, de Sanjosé S, Trottier H. HPV DNA, E6/E7 mRNA, and p16INK4adetection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–31. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 24.Guo T, Eisele DW, Fakhry C. The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer. Cancer. 2016;122(15):2313–23. doi: 10.1002/cncr.29992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strickler HD, Palefsky JM, Shah KV, Anastos K, Klein RS, Minkoff H, Duerr A, Massad LS, Celentano DD, Hall C, et al. Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J Natl Cancer Inst. 2003;95:1062–71. [DOI] [PubMed] [Google Scholar]
- 26.Swan DC, Tucker RA, Tortolero-Luna G, Mitchell MF, Wideroff L, Unger ER, Nisenbaum RA, Reeves WC, Icenogle JP. Human papillomavirus (HPV) DNA copy number is dependent on grade of cervical disease and HPV type. J Clin Microbiol. 1999;37:1030–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911–19. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 28.Heiligenberg M, Michael KM, Kramer MA, Pawlita M, Prins M, Coutinho RA, Dukers-Muijrers NHTM, Waterboer T. Seroprevalence and determinants of eight high-risk human papillomavirus types in homosexual men, heterosexual men, and women: A population-based study in Amsterdam. Sex Transm Dis. 2010;37(11):672–80. doi: 10.1097/OLQ.0b013e3181e71069. [DOI] [PubMed] [Google Scholar]
- 29.Desai S, Chapman R, Jit M, Nichols T, Borrow R, Wilding M, Linford C, Lowndes CM, Nardone A, Pebody R, et al. Prevalence of human papillomavirus antibodies in males and females in England. Sex Transm Dis. 2011;38(7):622–29. doi: 10.1097/OLQ.0b013e31820bc880. [DOI] [PubMed] [Google Scholar]
- 30.Newall AT, Brotherton JML, Quinn HE, McIntyre PB, Backhouse J, Gilbert L, Esser MT, Erick J, Bryan J, Formica N, et al. Population seroprevalence of human papillomavirus types 6, 11, 16, and 18 in men, women, and children in Australia. Clin Infect Dis. 2008;46(11):1647–55. doi: 10.1086/587895. [DOI] [PubMed] [Google Scholar]
- 31.Beachler DC, Sugar EA, Margolick JB, Weber KM, Strickler HD, Wiley DJ, Cranston RD, Burk RD, Minkoff H, Reddy S, et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am J Epidemiol. 2015;181(1):40–53. doi: 10.1093/aje/kwu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N. Natural acquired immunity against subsequent genital human papillomavirus infection: A systematic review and meta-analysis. J Infect Dis. 2016;213(9):1444–54. doi: 10.1093/infdis/jiv753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein SL. The evolution of greater humoral immunity in females than males: implications for vaccine efficacy. Current Opinion in Physiology. 2018;6:16–20. doi: 10.1016/j.cophys.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beachler D, Pinto L, Kemp TJ, Nyitray AG, Hildesheim A, Viscidi R, Giuliano AR et al. An examination of HPV16 natural immunity in men who have sex with men (MSM) in the HPV in men (HIM) study. Cancer Epidemiol Biomarkers Prev. 2018;cebp.0853.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Jansen KU et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2018;347(21). [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong Z-Y, Xiao W, Kahle L, Gillison ML. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36(3):262–67. doi: 10.1200/JCO.2017.75.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]


