ABSTRACT
Human papillomavirus (HPV) causes a number of cancers that disproportionally affect Latinos yet there is a paucity of research on interventions to increase HPV vaccination among this population. We sought to evaluate the efficacy of a web-based, individually customizable intervention, called CHICOs (Combatting HPV Infection and Cancers, tailored intervention) for its impact on HPV vaccine utilization. We conducted a three-armed, randomized, controlled trial in the waiting rooms of five family medicine practices from June 2014-February 2016 where CHICOS was compared to an iPad-based version of the Vaccine Information Sheet from the Centers for Disease Control and Prevention (untailored intervention), and usual care. Pair-wise comparisons between study arms of 6 different measures of HPV vaccine uptake were assessed, with analyses stratified by adolescents versus young adults. Of the 1,294 participants enrolled in the study, 1,013 individuals could be assessed for vaccination. Across study arms, 265 adolescents, but only 18 young adults, received an HPV vaccine dose during the study period. In both intention-to-treat and per-protocol analyses there were essentially no differences between the CHICOS and untailored arms in any vaccination measure, or between the untailored or CHICOS arms and usual care. Our study suggests that a tailored educational intervention may not be effective for increasing HPV vaccine uptake among Latino adolescents or young adults. However, the higher than expected baseline levels of positive vaccination attitudes of study participants could have diminished the statistical power of the study.
Trial Registration Number: ClinicalTrials.gov (NCT02145156).
KEYWORDS: Human papillomavirus, health communication, clinical trial, Latino, vaccination
Introduction
More than 14 million people are newly infected with human papillomavirus (HPV) in the US each year, exacting a significant emotional, financial and medical toll.1–4 Though HPV infection is similarly prevalent in nearly all race and ethnicity groups, significant disparities in HPV-associated cancers exist for Latinos. For example, Latinas have the highest risk of contracting cervical cancer compared to all other population groups in the US, and Latinos have the highest risk of developing penile cancer.1
Although an effective vaccine against HPV has been recommended by the Centers for Disease Control and Prevention (CDC) for the last several years for those ages 9–26 years, vaccination rates continue to remain low in the US.5 As of 2016, it was estimated that only 49.5% of 13–17 year old girls and only and 37.5% of 13–17 year old boys nationally were up to date with HPV vaccination.6 Though vaccination rates are generally slightly higher among Latino adolescents than Caucasians7,8 they are still far below that of other adolescent vaccines, and well below the national vaccination target level of 80% coverage.8,9 HPV vaccination rates among young adults are even lower. Without significant increases in HPV vaccination, especially among high-risk populations like Latinos, disparities in HPV-related cancers are likely to continue.
Numerous barriers to HPV vaccination have been described in the literature,10–16 including barriers specific to the Latino population such as a perception that HPV infection and cervical cancer is caused by physical trauma or poor hygiene, increased stigma associated with both HPV infection and vaccination compared to other populations, and concerns about uncovering undocumented status when trying to seek preventive care for cervical cancer (i.e. Pap testing or vaccination).16–22 Thus, while the reasons why individuals in general, and the Latino population specifically, do not get the HPV vaccine have been well described, there is a paucity of information on effective interventions to mitigate these issues. Creation and evaluation of interventions to improve HPV vaccination among Latinos, as well as among other populations, has become a high public health priority.23 However, these efforts are hindered by the fact that barriers to HPV vaccination are numerous, can vary significantly from individual to individual, and often cannot be adequately discussed in the short amount of time typically allotted to clinical encounters.
To address this knowledge gap, we developed an individually customized, web-based intervention about HPV vaccination. This intervention, called CHICOs (Combatting HPV Infections and CancerS), was developed specifically for the Latino population and created with significant community and end-user input, as described previously.24 CHICOS was based on the concept of “tailored messaging” in which informational materials are crafted so as to reflect each person’s own unique questions, experiences, attitudes and beliefs, which in turn improves message saliency and internalization. Tailored communication strategies have been shown to be highly effective for increasing patient adherence to a wide variety of recommended health behaviors but has not been extensively applied previously to the topic of vaccination.25,26 Thus, we conducted a randomized controlled trial to test the hypothesis that Latino participants exposed to the tailored CHICOs intervention would have significantly higher HPV vaccine utilization compared to those receiving untailored information about the vaccine or usual care.
Results
Study recruitment
As shown in Figure 1, there were 1294 participants randomized (intention to treat sample). Table 1 depicts the demographic characteristics of these individuals. Although sex was not used as an eligibility criterion, it was notable was that no male young adults enrolled in the study. There were 281 participants in the intention to treat sample who could not be matched to any vaccination or EMR records at the end of the study period and had vaccination data imputed.
Figure 1.
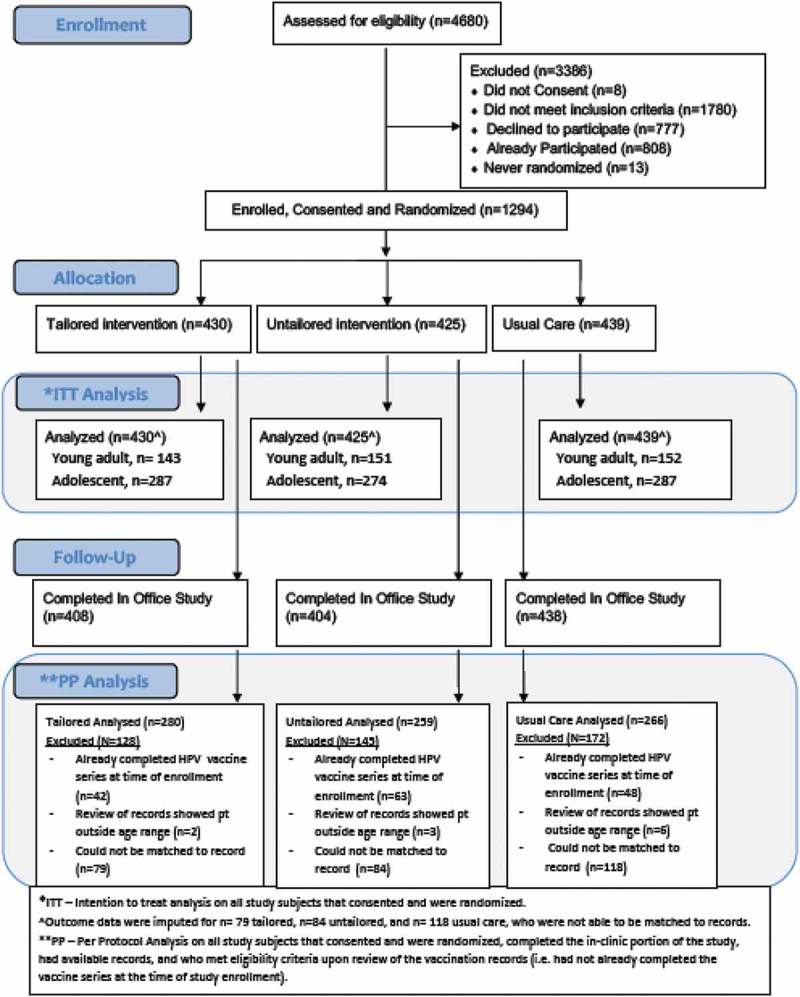
CHICOS consort diagram for vaccination outcomes.
Table 1.
Demographic characteristics of participants.
| Arm Assignment |
|||||
|---|---|---|---|---|---|
| Participant Characteristics | Usual Care % (n) | Untailored % (n) | Tailored % (n) | All % (N) |
|
| Young Adults | 34.6% (152) | 35.5% (151) | 33.3% (143) | 34.5% (446) | |
| Parents | 65.4% (287) | 64.55 (274) | 66.7% (287) | 65.5% (848) | |
| TOTAL (100% column) | 100% (439) | 100% (425) | 100% (430) | 100% (1294) | |
| Gender | |||||
| Young Adults | Male | 0% (0) | 0% (0) | 0% (0) | 0% (0) |
| Female | 100% (152) | 100% (151) | 100% (143) | 100% (446) | |
| TOTAL | |||||
| Parents (Adolescent Gender) | Male | 51.6% (148) | 51.5% (141) | 50.2% (144) | 51.1% (433) |
| Female | 48.4% (139) | 48.5% (133) | 49.8% (143) | 48.9% (415) | |
| TOTAL | 100% (287) | 100% (274) | 1005 (287) | 100% (848) | |
| Race/Ethnicity | |||||
| Young Adults | Hispanic | 85.5% (130) | 84.1% (127) | 86.0% (123) | 85.2% (380) |
| White, Non-Hispanic | 12.5% (19) | 13.2% (20) | 11.9% (17) | 12.6% (56) | |
| Other, Non-Hispanic | 2.0% (3) | 2.6% (4) | 2.1% (3) | 2.2% (10) | |
| TOTAL | 100% (152) | 100% (151) | 100% (143) | 100% (446) | |
| Parents (Adolescent Race/Ethnicity) | Hispanic | 93.7% (269) | 92.7% (254) | 92.7% (266) | 93% (789) |
| White, Non-Hispanic | 5.9% (17) | 6.9% (19) | 6.3% (18) | 6.45 (54) | |
| Other, Non-Hispanic | 0.3% (1) | 0.4% (1) | 1.0% (3) | 0.6% (5) | |
| |
TOTAL |
100% (287) |
100% (274) |
100% (287) |
100% (848) |
| Age at Baseline |
Median Age (MED) |
Average |
|||
| Young Adults | 23 | 23 | 22 | 22 | |
| Parents (Adolescent Age) | 12 | 12 | 12 | 12 | |
In the per protocol analyses we removed the 281 individuals with imputed vaccination data (CHICOS n = 79, Untailored n = 84, Usual Care n = 118). Also removed in the per protocol sample were participants whose age recorded in the EMR was outside the range for HPV vaccination (i.e. <9 years or >26 years), and participants who EMR data indicated that they had already completed the HPV vaccine series. This left 805 participants for the per-protocol analysis.
Impact of the intervention of HPV vaccination intention
There were 752 participants across the CHICOS and Untailored arms of the study that completed baseline and post-intervention assessments of vaccination intention. A high proportion of both young adult (49%) and parent (72%) participants indicated prior to viewing the intervention materials that they were Very Likely to receive the vaccine at that visit. This increased to 60% and 77%, respectively, in the post-intervention survey. When the responses of “Very Likely” or “Somewhat Likely” were combined, positive vaccination intention was uniformly high (>90%) at baseline and at post intervention for both groups. Regardless of how it was analyzed, there were no differences between study arms in vaccination intention at baseline or post-intervention for either parents or young adults (data not shown).
Impact of the intervention on HPV vaccination levels
Amongst all study arms combined, 265 adolescents, but only 18 young adults received an HPV vaccine dose during the study period. Among young adults, there were no statistically significant differences between any two study arms in any of the vaccination uptake measures in the intention to treat analysis (Table 2) or the per-protocol analysis (data not shown).
Table 2.
Two way comparisons of adolescent vaccination between study arms.
| Outcome | Comparison Arms | Odds Ratio, 95% CI |
|---|---|---|
| Young Adults | ||
| Got Any Dose of HPV During Study | Tailored vs. Usual Care | 2.3 (0.6, 8.3) |
| Untailored vs. Usual Care | 2.4 (0.7, 8.9) | |
| Tailored vs. Untailored | 0.9 (0.3, 2.6) | |
| Initiated the Vaccine Seriesb | Tailored vs. Usual Care | 2.3 (0.6, 8.8) |
| Untailored vs. Usual Care | 1.00 (0.2, 4.6) | |
| Tailored vs. Untailored | 2.3 (0.6, 8.6) | |
| Initiated the Vaccine, Series not Completedc | Tailored vs. Usual Care | 5.8 (0.3, 112.8) |
| Untailored vs. Usual Care | 12.4 (0.2, 722.4) | |
| Tailored vs. Untailored | 0.5 (0.0, 22.4) | |
| Completed the Vaccine Series, Among All Eligibled | Tailored vs. Usual Care | 0.7 (0.1, 5.3) |
| Untailored vs. Usual Care | 1.7 (0.3, 8.3) | |
| Tailored vs. Untailored | 0.4 (0.1, 2.6) | |
| Completed the Vaccine Series Among Those Already Initiated at Study Starte | Tailored vs. Usual Care | 1.1 (0.0, 60.2) |
| Untailored vs. Usual Care | 9.3 (0.5, 193.9) | |
| Tailored vs. Untailored | 0.1 (0.0, 2.4) | |
| Completed the Vaccine Series, Among Those Who Initiated During the Studyf | Tailored vs. Usual Care | 0.2 (0.0, 3.4) |
| Untailored vs. Usual Care | 0.1 (0.0, 4.7) | |
| Tailored v. Untailored | 2.2 (0.0, 103.5) | |
| Adolescents | ||
| Got Any Dose of HPV During Study | Tailored vs. Usual Care | 1.05 (0.7, 1.5) |
| Untailored vs. Usual Care | 1.1 (0.7, 1.7) | |
| Tailored vs. Untailored | 0.9 (0.7, 1.3) | |
| Initiated the Vaccine Seriesb | Tailored vs. Usual Care | 1.2 (0.7, 2.0) |
| Untailored vs. Usual Care | 1.3 (0.7, 2.2) | |
| Tailored vs. Untailored | 0.9 (0.5, 1.5) | |
| Initiated the Vaccine, Series not Completedc | Tailored vs. Usual Care | 1.3 (0.4, 4.1) |
| Untailored vs. Usual Care | 2.3 (0.7, 7.3) | |
| Tailored vs. Untailored | 0.6 (0.2, 1.8) | |
| Completed the Vaccine Series, Among All Eligibled | Tailored vs. Usual Care | 1.2 (0.7, 2.1) |
| Untailored vs. Usual Care | 0.8 (0.5, 1.5) | |
| Tailored vs. Untailored | 1.5 (0.9, 2.5) | |
| Completed the Vaccine Series Among Those Already Initiated at Study Starte | Tailored vs. Usual Care | 1.6 (0.8, 3.2) |
| Untailored vs. Usual Care | 0.8 (0.4, 1.6) | |
| Tailored vs. Untailored | 2.0 (1.1, 3.8) | |
| Completed the Vaccine Series, Among Those Who Initiated During the Studyf | Tailored vs. Usual Care | 0.7 (0.2, 2.2) |
| Untailored vs. Usual Care | 0.4 (0.1, 1.4) | |
| Tailored vs. Untailored | 1.7 (0.6, 5.0) | |
aP-value for the % vaccinated among the two arms denoted
bDescribes those who had 0 doses at study enrollment and received at least one dose during the study period.
cDescribes those who enrolled in the study with 0 doses, had enough time in the study period after the most recent dose to complete the series, but did not complete the series during the study period.
dDescribes those who enrolled in the study with 0, 1 or 2 doses, had enough time in the study period after the most recent dose to complete the series, and completed the series during the study period.
eDescribes those who enrolled in the study with 1 or 2 doses, had enough time in the study period after the most recent dose to complete the series, and completed the series during the study period.
fDescribes those who enrolled in the study with 0 doses, had enough time in the study period after the most recent dose to complete the series, and completed the series during the study period.
Bolded p-values highlight significant results.
Similarly, there were few differences among adolescents in two-way intention to treat (Table 2) and per protocol (data not shown) analyses. The one exception was completion of the series among adolescents who entered the study with at least one dose in the intention to treat analysis. In this analysis the CHICOS group performed significantly better than the untailored group (Odds ratio [OR] 2.0, 95% Confidence Interval [CI] 1.1–3.8) but there were no statistically significant differences in series completion among this cohort when the CHICOS intervention was compared to usual care (OR 1.6, 95% CI 0.8–3.2). A similar lack of difference between study arms was also found in the 3-way comparisons, for both young adults and adolescent cohorts (Supplemental Materials).
Discussion
In this study we found no evidence that exposure to CHICOS, a tailored educational intervention about HPV vaccination for Latinos, lead to significant increases in HPV vaccine utilization compared to an Untailored intervention or Usual Care. This was true for both adolescents and young adults. Moreover, while vaccination intention improved after reading the educational material in both the CHICOS and Untailored groups, there were no differences in this improvement between the two study arms. From this we conclude that CHICOS is not likely more effective than usual care to improve HPV vaccination among the Latino population targeted for the study.
Increasing HPV vaccination, particularly among adolescents for whom the vaccine is preferentially recommended, is a national health priority.23,27–32 Many groups have recently sought to develop and test interventions to improve this outcome and several effective interventions targeted at the provider or clinic level have been identified. For example, in a 2010 study, Fiks et al. showed that electronic medical record prompts plus parent reminders increased HPV vaccine initiation among females (males were not in the study) by 9% percentage points compared to control (from 16% to 25%, p < 0.001).33 In another study by Perkins et al. of a provider-focused intervention that provided a combination of provider education and feedback on vaccination rates, and quality improvement incentives, a 10%-20% increase (depending on month of analysis) in adolescent HPV vaccine initiation was found compared to controls.34 Interventions targeted to parents have been less successful as the vast majority of these studies use parental intention for adolescent vaccination as the outcome of interest rather than actual adolescent HPV vaccine receipt.35,36 Yet, it remains unclear the degree to which these improvements translate into actual increases in vaccination. In our study both parents and young adults had high levels of vaccination intention, but actual vaccine receipt was much lower. However, it was clear that vaccination intention did not translate well into vaccination behaviors in our study and suggests that researchers should use caution when interpreting results of intervention studies that use vaccination intention as a proxy measure for vaccine uptake. We are currently assessing paradata from the different study websites to determine if there were differences in the level of patient engagement with the interventions (i.e. how much time was spent, and on which intervention webpages) to see if this may have moderated the outcomes we assessed. It is possible that with some improvements in the user interface the intervention may have more significant impacts.
Low levels of vaccination (2%-6%) in our young adult population, despite high vaccination intentions, suggests significant and systematic barriers that could not be overcome by the information the interventions provided. In our study, lack of insurance coverage was likely a contributing factor. Adolescents without health insurance are able to get vaccinated free of charge under the Vaccines For Children program from the U.S. government.37 However, a similar program does not exist for young adults which means that uninsured individuals over the age of 18 who want the HPV vaccine generally must pay for it out of pocket. Many of the young adults seen in the study clinics had no insurance coverage, and were low-income, which likely reduced the incidence of vaccination among this population.
A surprising outcome in our study was that no young adult men enrolled in our study. In contrast, there was nearly equal representation of male and female adolescents among parents enrolled in the study. This low level of adult male participation may reflect young adults’ misperceptions that HPV is a “woman’s problem” and thus not very relevant to males. Consistent with this is the finding that, among adolescents in the study, vaccination was more likely to occur among girls than boys across all study arms. Lack of adult male participation in the trial was somewhat disappointing as a concerted effort was made to highlight in CHICOS the risk of HPV infection and the need for HPV vaccination among people of both sexes. Without this perspective, one cannot make definitive conclusions about the efficacy of our intervention for young adult males. However, given the low uptake of the vaccine among young adult males nationally38 and the similarities in attitudinal barriers between males and females,39 we suspect it would have similarly low efficacy as for young adult females.
A significant limitation of our study was that there was a high proportion of enrolled participants with missing data, which required imputation. Missingness was due primarily to the fact that a substantial proportion of individuals could not be matched to any vaccination record. This mostly impacted adolescents whose name and birthdate were provided by the parent yet could not be matched to the immunization registry or the clinic’s EMR. Moreover, in the per-protocol analysis a large number of additional individuals were eliminated because they erroneously reported that they/their adolescent had not completed the three-dose series at the time of study enrollment (when in fact they had, as judged by their vaccination records that were reviewed at the end of the study period), and/or that they were in the eligible age range for the study when they actually weren’t. The removal of so many participants from the per protocol analysis was not expected and hindered our statistical power to detect differences between study arms in the per-protocol analysis where outcome data were more reliable than in the intention to treat analysis.
Another limitation is that because Research Assistants approached patients in the waiting room in order to enroll them in the study, the sample may have been biased towards those with already fairly positive attitudes toward HPV vaccination. This could account for the high levels of HPV vaccination intention reported in both the CHICOS and untailored arms at baseline. Secondly, many participants requested Research Assistant help in using the intervention. Because of this, social desirability may have influenced our results, particularly related to vaccination intention, as study subjects might not have felt comfortable expressing hesitancy or ambiguous intentions about the HPV vaccine directly to the Research Assistant. Finally, our CHICOs intervention was clinic-based and therefore captured patients who were already in the clinic and thus had access to the vaccine. It is not known how effective our intervention would be in high-risk populations that do not routinely access primary care.
Conclusions
The tailored CHICOs intervention, which was developed in close collaboration with the Latino community, did not appear to be any more effective than an Untailored web-based intervention or Usual Care in improving HPV vaccination rates among Latino adolescents or young adults. While both the CHICOS and Untailored interventions significantly improved parents’ and young adults’ HPV vaccination intentions, this did not appear to translate into improvements in actual HPV vaccine uptake. This was particularly notable in the young adult population which had very low levels of HPV vaccine utilization across all three study arms, likely reflecting substantial systematic barriers to vaccination that could not be overcome by the information provided. Ongoing research is needed to identify effective mechanisms to improve HPV vaccination among Latinos, which are a high-risk group for HPV-related cancers and other diseases.
Materials and methods
The CHICOS intervention was developed collaboratively with members of the Latino community, as described previously.24 A detailed description of the CHICOS intervention, study enrollment and consent processes, and study procedures are provided in the associated Supplemental Materials to this manuscript.
Study design overview
This was a 3-armed randomized controlled trial comparing CHICOS to an untailored intervention or to usual care. Two groups of participants were enrolled – Latino young adults ages 18–26 years who were making the vaccination decision for themselves, and Latino parents of adolescent 9–17 years old who were making vaccination decisions for their adolescent child. The intervention took place in 5 primary care clinics’ waiting rooms during a single clinic visit. Vaccination assessments occurred at the end of a 21-month study period which included 16 months where the intervention was actively implemented in the clinics, plus a 5 month “follow up” period to allow for vaccine doses to be given. The study was registered at ClinicalTrials.gov (NCT02145156). All study activities were approved by the Colorado Multiple Institutional Review Board.
Description of study interventions
Tailored intervention (CHICOS)
Those in the tailored intervention received an iPad from a Research Assistant with the CHICOS intervention programmed onto it. CHICOS was written at a 6th grade reading level and available in English or Spanish and provided in the clinics’ waiting rooms. The intervention commenced with a short baseline survey that collected information about the participants’/participants’ adolescent’s name and birthday (to allow matching to vaccination records), attitudes and beliefs about HPV infection and vaccination, demographics, and self-reported/parent-reported vaccination status. These data were then used to individually customize information in CHICOS that was provided directly on the iPad immediately following completion of the survey. Participants viewed the CHICOS information at their own pace for as long as they wished. The Supplemental Material shows screen shots of the baseline survey and the CHICOS intervention. Following this, they were asked by the Research Assistant to complete a short “post-intervention” survey that reassessed their vaccination intentions for the visit. The Research Assistant was present throughout this process to help navigate the iPad or answer questions.
Untailored intervention
Those in the untailored intervention also initiated the study with the same iPad-based baseline survey as in the CHICOS intervention. However, this information was not used to customize information. Instead, upon completion of the baseline survey the participant was provided with information from the Centers for Disease Control and Prevention’s (CDC) HPV Vaccine Information Sheet that had been transcribed verbatim and shown over a series of seven webpages. The Supplemental Material provides screen shots of the untailored intervention. As with the CHICOS intervention, a Research Assistant was present throughout this process and a short post-intervention survey was provided.
Usual care
Participants in this arm received care routinely provided by the clinician and did not interact with or have access to the iPad. Based on our pre-study informational interviews with study practices, usual care typically consisted of bringing up the need for vaccine during “routine physicals” (i.e. not illness visits) and providing a written version of the Vaccine Information Sheet for HPV at the time the vaccine was administered. However, these activities were completely at provider discretion and were not tracked as part of the study. The usual care arm did not receive a pre-intervention survey. The post-intervention survey was provided to participants by the Research Assistant immediately after the visit, in paper format.
Study setting
The study took place in the waiting rooms of the 5 primary care clinics in central Colorado that serve low-income, primarily Latino, medically underserved clientele and were all part of a single health system. The intervention took place from June of 2014 and September of 2015 (with a vaccination follow up period extending to February 2016).
Participants
Participants were eligible for the study if they reported that they were either the parent of a child aged 9–17 years (parent participant) or were themselves aged 18–26 years (young adult participant), that they were/their adolescent was a patient of the clinic and that they could read and converse in English or Spanish. Participants who consented to the study and completed the baseline (CHICOS and Untailored arms only) and a post-intervention survey that occurred immediately after the visit (all arms) received a $10 cash incentive.
Randomization
Following consent, the iPad used an internal randomization program to assign participants to one of the three study arms in a 1:1:1 ratio (Figure 1). Due to potential clustering of patient responses, language (Spanish/English), clinical site (5 possible sites), type of participant (parent/young adult), and self-reported prior HPV doses (none/some), were used as stratification variables in the randomization process to ensure even distribution across study arms. The Research Assistants administering the intervention were aware of which study arm participants were assigned to, but the participants, providers at the clinic and study analysts were blinded to group allocation. Research assistants did not provide any vaccination counseling to participants – their role was to help participants sign up for the study and navigate the iPad (if in the tailored or untailored arms.)
Outcome measures
The primary outcomes of interest were six measures of HPV vaccine utilization assessed 21 months after the study commenced. All analyses were stratified by young adults versus adolescents based on assumptions that vaccination barriers and rates would be different between the two groups. The 6 vaccination measures included: 1) Receipt of any needed dose of HPV vaccine during the study period; 2) Initiation of the series among those who entered the study with 0 doses; 3) Initiation but not completion of the series during the study period among those who entered the study with 0 doses; 4) Completion of the vaccine series among anyone in the study; 5) Completion of the series specifically among those entering the study with 1–2 doses; and 6) Completion of the series specifically among those entering the study with 0 doses. Appropriate minimal time intervals between vaccination doses were considered when counting valid doses. Denominators for the six vaccination outcome measures varied based on receipt of prior valid doses and remaining time left in the study period. Vaccination data were derived from triangulation of the clinics’ electronic medical records and the statewide immunization registry, called CIIS (Colorado Immunization Information System).
Vaccination intention before vs. after viewing either the CHICOS or untailored materials on the iPad was assessed as a secondary outcome using responses to the questions “Now that you have read a bit about HPV infection and vaccination, how likely would you be to get a dose of the HPV vaccine today if the doctor recommended it?” Response choices included Very likely, Somewhat likely, Somewhat unlikely, Very unlikely. Based on the distribution of responses, response categories were collapsed into Very Likely vs. All Other Responses for analysis. Vaccination intention was not assessed in the Usual Care arm.
Data analysis
The main analyses were three, separate, pair-wise (2-way) analyses comparing Usual Care to either the CHICOS or Untailored interventions, or the CHICOS intervention to the Untailored intervention. Sample size calculations indicated that we would need at 573 parents and 426 young adults participating in the study to be able to detect a 15% or greater difference in HPV vaccination use between any 2 arms. A 3-way analyses where all groups were simultaneously considered were also performed (Appendix). Both per-protocol and intention-to-treat frameworks were used for the analysis. However, because conclusions from both analyses were the same, only intention-to-treat analyses are presented.
Imputation methods were used to address missingness of data in the intention to treat analyses. Of the 1294 participants, 22% were missing outcome data (could not be matched to any clinical or CIIS record), and 11.4% were missing gender, age, and/or race-ethnicity. These variables plus site of recruitment, enrollment date, and arm assignment were used to develop multiple imputation models40 separately for young adults and adolescents. Nominal variables were imputed with binary or multinomial logistic regression, and quantitative variables were imputed using predictive mean matching. The imputation was carried out with the SAS® MI procedure using the Fully Conditional Specification (FCS) method.41 For the adolescent data the number of imputations was set at 30 using the percentage missing as a guide.42 For young adults the percent missing method yielded only 12 imputations, so the number was reset to the default of 25.41 The resulting data sets were analyzed individually with logistic regression and a combined analysis was performed with the SAS MIANALYZE procedure. Many of the outcomes were very rare in the young adult group so Firth’s penalized maximum likelihood estimation41 was used to guard against potential bias.
Differences in vaccination intention before vs. after viewing iPad materials were assessed using Bhapkar’s tests of marginal homogeneity and repeated measures binomial regression as vaccination intention measurement occurred twice per individual (in the post-intervention and follow-up surveys).
Funding Statement
This work was supported by the Patient Centered Outcomes Research Institute under grant #1455
Acknowledgments
The authors wish to thank the Research Assistants who helped to implement the study: Catia Chavez, Carol Gorman, Briana Lopez and Maria Garcia, and the members of the Community Advisory Board who worked so hard to develop the CHICOS intervention: Jalene Salazar, Vanessa Vasquez, Reina Reyes, Evelinn Borrayo, Lupe Ruiz, Sonja Gutierrez, Rosalva Yesces, Vilma Cavero, Estibaliz Salinas, Alejandra Mendoza, and Oyuke Fernandez
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- 1.Jemal A, Simard EP, Dorell C, Noone A-M, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, et al. Annual report to the nation on the status of cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MCB, Su J, Xu F, Weinstock H.. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 3.Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41(11):660–664. doi: 10.1097/OLQ.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30(42):6016–6019. doi: 10.1016/j.vaccine.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014 - United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 6.Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–882. doi: 10.15585/mmwr.mm6633a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Curtis CR, MacNeil J, Markowitz LE, Singleton JA. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 8.Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Singleton JA, Curtis CR, MacNeil J, Markowitz LE, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh HK, Blakey CR, Roper AY. Healthy people 2020: a report card on the health of the nation. Jama. 2014;311(24):2475–2476. doi: 10.1001/jama.2014.6446. [DOI] [PubMed] [Google Scholar]
- 10.Wong CA, Berkowitz Z, Dorell CG, Anhang Price R, Lee J, Saraiya M. Human papillomavirus vaccine uptake among 9- to 17-year-old girls: national health interview survey, 2008. Cancer. 2011;117(24):5612–5620. doi: 10.1002/cncr.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constantine NA, Jerman P. Acceptance of human papillomavirus vaccination among Californian parents of daughters: a representative statewide analysis. J Adolesc Health. 2007;40(2):108–115. doi: 10.1016/j.jadohealth.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117(5):1486–1493. doi: 10.1542/peds.2005-1381. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey AF, Abraham LM, Dalton V, Ruffin M. Understanding the reasons why mothers do or do not have their adolescent daughters vaccinated against human papillomavirus. Ann Epidemiol. 2009;19(8):531–538. doi: 10.1016/j.annepidem.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel DA, Zochowski M, Peterman S, Dempsey AF, Ernst S, Dalton VK. Human papillomavirus vaccine intent and uptake among female college students. J Am Coll Health. 2012;60(2):151–161. doi: 10.1080/07448481.2011.580028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liddon NC, Hood JE, Leichliter JS. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: findings from the 2006-2008 national survey of family growth. Vaccine. 2012;30(16):2676–2682. doi: 10.1016/j.vaccine.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Bastani R, Glenn BA, Tsui J, Chang LC, Marchand EJ, Taylor VM, Singhal R. Understanding suboptimal human papillomavirus vaccine uptake among ethnic minority girls. Cancer Epidemiol, Biomarkers Prev. 2011;20(7):1463–1472. doi: 10.1158/1055-9965.EPI-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobetz E, Kornfeld J, Vanderpool RC, Finney Rutten LJ, Parekh N, O’Bryan G, Menard J. Knowledge of HPV among United States Hispanic women: opportunities and challenges for cancer prevention. J Health Commun. 2010;15 Suppl 3(Suppl 3):22–29. doi: 10.1080/10810730.2010.522695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casillas A, Singhal R, Tsui J, Glenn BA, Bastani R, Mangione CM. The impact of social communication on perceived HPV vaccine effectiveness in a low-income, minority population. Ethn Dis. 2011;21(4):495–501. [PubMed] [Google Scholar]
- 19.Flores K, Bencomo C. Preventing cervical cancer in the Latina population. J Women’s Health (2002). 2009;18(12):1935–1943. doi: 10.1089/jwh.2008.1151. [DOI] [PubMed] [Google Scholar]
- 20.Bair RM, Mays RM, Sturm LA, Zimet GD. Acceptability of the human papillomavirus vaccine among Latina mothers. J Pediatr Adolesc Gynecol. 2008;21(6):329–334. doi: 10.1016/j.jpag.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Lechuga J, Swain GR, Weinhardt LS. The cross-cultural variation of predictors of human papillomavirus vaccination intentions. J Women’s Health (2002). 2011;20(2):225–230. doi: 10.1089/jwh.2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechuga J, Swain GR, Weinhardt LS. Impact of framing on intentions to vaccinate daughters against HPV: a cross-cultural perspective. Ann Behav Med. 2011;42(2):221–226. doi: 10.1007/s12160-011-9273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute President’s cancer panel annual report, accelerating HPV vaccine uptake: urgency for action to prevent cancer. A Report to the President of the United States from the President’s Cancer Panel. Bethesda (MD); 2014. [Google Scholar]
- 24.Maertens JA, Jimenez-Zambrano AM, Albright K, Dempsey AF. Using community engagement to develop a web-based intervention for Latinos about the HPV vaccine. J Health Commun. 2017;22(4):285–293. doi: 10.1080/10810730.2016.1275890. [DOI] [PubMed] [Google Scholar]
- 25.Kreuter MW, Wray RJ. Tailored and targeted health communication: strategies for enhancing information relevance. Am J Health Behav. 2003;27(Suppl 3):S227–232. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins RP, Kreuter M, Resnicow K, Fishbein M, Dijkstra A. Understanding tailoring in communicating about health. Health Educ Res. 2008;23(3):454–466. doi: 10.1093/her/cyn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Vaccine Advisory Committee. The promise and challenge of adolescent immunization. Am J Prev Med. 2008;35(2):152–157. doi: 10.1016/j.amepre.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 28.ACOG committee opinion no 558: integrating immunizations into practice. Obstet Gynecol. 2013;121(4):897–903. doi: 10.1097/01.AOG.0000428788.74725.90. [DOI] [PubMed] [Google Scholar]
- 29.Zimet GD. Health care professionals and adolescent vaccination. A call for intervention research. Hum Vaccin Immunother. 2014;10(9):2629–2630. doi: 10.4161/hv.28525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Center for Disease Control and Prevention NCfIaRD, Health Communications Office You are the key to HPV cancer prevention: communicating about HPV. 2014. [accessed 2014 September 30] http://www.hhs.gov/opa/pdfs/you-are-the-key.pdf.
- 31.Vanderpool RC, Crosby RA, Stradtman LR. Protecting a new generation against HPV: are we willing to be bold? Hum Vaccin Immunother. 2014;10(9):2559–2561. doi: 10.4161/21645515.2014.970068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Academy of Pediatrics Give a strong recommendation for HPV vaccine to increase uptake. 2015. [accessed 2016 January 1] http://www.immunize.org/letter/recommendhpvvaccination.pdf.
- 33.Fiks AG, Grundmeier RW, Mayne S, Song L, Feemster K, Karavite D, Hughes CC, Massey J, Keren R, Bell LM, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114–1124. doi: 10.1542/peds.2012-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkins RB, Zisblatt L, Legler A, Trucks E, Hanchate A, Gorin SS. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33(9):1223–1229. doi: 10.1016/j.vaccine.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Dempsey AF, Zimet GD. Interventions to improve adolescent vaccination: what may work and what still needs to be tested. Vaccine. 2015;33(Suppl 4):D106–113. doi: 10.1016/j.vaccine.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Zimet GD, Buffler P. Prevention of human papillomavirus-related diseases: impediments to progress. Prev Med. 2013;57(5):407–408. doi: 10.1016/j.ypmed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Obaro SK, Palmer A. Vaccines for children: policies, politics and poverty. Vaccine. 2003;21(13–14):1423–1431. [DOI] [PubMed] [Google Scholar]
- 38.Du P, Camacho F, McCall-Hosenfeld J, Lengerich E, Meyers CM, Christensen ND. Human papillomavirus vaccination among adults and children in 5 US states. J Public Health Manage Pract. 2015; doi: 10.1097/PHH.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaJoie AS, Kerr JC, Clover RD, Harper DM. 2018. Influencers and preference predictors of HPV vaccine uptake among US male and female young adult college students. Papillomavirus Res. 5:114–121. doi: 10.1016/j.pvr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fairclough D. Design and Analysis of Quality of Life Studies in Clinical Trials. 2nd ed. Boca Raton, FL: Chapman & Hall/CRCTaylor & Francis Group; 2010. [Google Scholar]
- 41.SAS/STAT ® 14.1 user’s guide. Cary (NC:): SAS Institute Inc; [computer program] 2015. https://support.sas.com/documentation/cdl/en/statug/68162/HTML/default/viewer.htm#statug_chap0_sect002.htm [Google Scholar]
- 42.Von Hippel P. 2009. How to impute interactions, squares, and other transformed variables. Socio-Logical Method. 39:265–291. doi: 10.1111/j.1467-9531.2009.01215.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Center for Disease Control and Prevention NCfIaRD, Health Communications Office You are the key to HPV cancer prevention: communicating about HPV. 2014. [accessed 2014 September 30] http://www.hhs.gov/opa/pdfs/you-are-the-key.pdf.


