ABSTRACT
Background: Genotype distribution and prevalence of human papillomavirus (HPV) among women vary from different regions and crowds, prophylactic HPV vaccin could prevent some diseases related to HPV, which include cervical precancerous lesions and cancer. Baseline surveys prior to mass HPV vaccination are critical to determine vaccine efficacy and detect changes in HPV type after vaccination.
Objective: The aim of this study is to study the HPV type-specific prevalence in 698 women with cytological abnormalities, aging from 18 to 77 years old. Additionally, the association between HPV infection and cervical disease was investigated as well.
Methods: A total of 698 cervical specimens of cytological abnormalities were collected from the First Affiliated Hospital of Xinjiang Medical University. The Thinprep liquid-based cytologic test (TCT) was performed and the cytological status was classified according to Bethesda 2001. The samples were tested HPV genotype by the PCR-based hybridization gene chip assay.
Results: Overall, the HPV prevalence was 54.87%, and it was shown to be age dependent, and with the decreasing and zigzag prevalence until the age of 55 years. 204 patients (53.26%) were infected with pure high-risk HPV, 139 (36.30%) with pure low-risk HPV, and 40 (10.44%) with mixed HPV types. HPV16 was the most common type (35.36%), followed by HPV58 (13.62%) and HPV52 (9.15%). In this study, 386 (55.30%) were affected by ASCUS, 11 (1.58%) by ASC-H, 137 (19.63%) by L-SIL and 151 (21.63%) by H-SIL. Women with a cytology result of ASCUS, ASC-H, L-SIL and H-SIL had the infection of HPV 39.12%, 54.17%, 70.80% and 80.79% respectively.
Conclusions: In conclusion, this study presents the first investigation about the prevalence of HPV infection and HPV genotype distribution in Xinjiang women who have abnormal cytological tests. Prior to HPV immunization in Xinjiang’s population, our results could be baseline data and validation set, which provide robust available estimates of the prevalence of type-specific HPV.
KEYWORDS: Human papillomavirus, genotype distribution and prevalence, cervical cytological abnormalities, Xinjiang
Introduction
Human papillomavirus is the most common sexually transmitted pathogen in both men and women. Accumulating epidemiological evidence could support a strong association between HPV and genital warts as well as cancer of the cervix, vulva, vagina, anus, and penis.1-3 Cervical cancer (CC) is one of the most common cancers in women. It is estimated that approximately 500,000 new cases of cervical cancer are diagnosed each year, with the mortality rate as high as 56% in developing countries.3,4 As the world’s most populous developing country, China also faces serious disease burden caused by cervical cancer, especially in medical resource limited rural areas where more than 70% population of the country reside.5 The crude incidence of cervical cancer was estimated to be about 8.7–11.3/100,000 in females in China, of whom 45.0% died.6,7 Screening results have indicated that the incidence and mortality rate of cervical cancer in women who resided in Xinjiang were 459–590/100,000 and 17.78/100,000, respectively.8 These numbers are obviously higher than those reported of other ethnic groups.
HPV16 (4.82%), HPV52 (4.52%), and HPV58 (2.74%) represented the most prevalent high-risk HPV types of HPV infection among the general population in China.9 In our previous study, we described that the total positive rate of HPV in normal cytology females was 14.02%, the most prevalent genotypes of women who hadn’t been vaccinated were HPV 16 (3.79%), HPV 52 (2.47%), HPV 58 (1.76%), HPV 53 (1.35%) and HPV 31 (0.72%). However, there were no results about cervical cytological abnormalities in our previous study, and no good characterizations of HPV infection in women with cytological abnormalities in Xinjiang. Hence, another independent cohort including cytological abnormalities is required to confirm whether HPV-16, HPV-52, HPV-58, HPV 53 and HPV 31 were the most frequent HPV types that have a substantial impact on cervical cytological abnormalities and cervical cancer in Xinjiang’s population.
A good management of CC depends on an early detection of the disease and efficient prophylactic vaccines.10 The oncogenic potential of different HPV types highlights the importance of the detection and typing of different HPV isolates.11 Currently, there are three prophylactic HPV vaccines available (Gardasil, Gardasil 9, and Cervarix) that are approved for use in many countries.12–14 All three vaccines provide protection against infection with HR-HPV16 and HR-HPV18, Gardasil also includes prevention against LR-HPV6 and LR-HPV11, and the Gardasil 9 which has been approved recently makes protection further by adding five additional HR-HPV types (31, 33, 45, 52, and 58).12-14 Using this panel, additional 4.2%-18.3% of cervical cancers in USA and 12–19% across four countries including Brazil, Mexico, India, and China had been prevented with targeting HPV-31/HPV-33/HPV-45/HPV-52/HPV-58.15
WHO did not make any biased recommendation on the three vaccines,16 believing that “there is no difference in the efficacy of HPV16/18 related cervical cancer prevention, and most cancers can be prevented”. According to the epidemiological data described in the literature, it seemed that the protective effect of 9vHPV in the Asian population can only reach the global protection level of 2vHPV or 4vHPV (about 70%). Unfortunately, first, the clinical trial of 9vHPV vaccine has not been fully carried out in China. Second, its current price on the market in China is 1,298 RMB/dose nearly twice time than that in the first two vaccines, and it takes about 3,900 RMB to complete the whole vaccination, which is unattainable.17
Two-, four-, nine-valent HPV vaccines might not be “enough.” However, more HPV valence numbers do not mean the better, strong pertinence could provide effective protection. Vaccine research and development could be more targeted only while there are sufficient epidemiological data, and the cost-effectiveness of vaccination for Chinese people could be improved. Therefore, understanding the HPV type distribution is important for tailoring regional screening programs. Furthermore, describing the baseline HPV type distribution before the introduction of vaccination programs facilitates further evaluation of potential impact of HPV vaccines on people in Xinjiang .
To the best of our knowledge, epidemiological studies in Xinjiang regarding the distribution of HPV types in women with cytological abnormalities are still lacking. The purpose of this study was to confirm the description of HPV prevalence and type-specific distribution in cervical cytological abnormalities of people living in Xinjiang. Importantly, the data presented here could provide a valuable baseline for assessing the impact of the newly introduced vaccination programme in the future.
Results
HPV prevalence and age distribution
Among the 698 tested women, `the mean age of these women was 43 ± 10.71 yrs, 383 women (54.87%) were positive for at least one HPV type. The prevalence among age groups was listed in Table 1. Overall, the HPV prevalence was age dependent, with the decreasing and zigzag prevalence until the age of 55 years. The highest prevalence of HPV was detected in women between 26 and 30 years old(65.67%), and the lowest prevalence of HPV infection (46.03%) was found in women between 51 and 55 years. The HPV infection rate increased after the age of 56, reaching 56.18%, which was close to the HPV infection rate (56.64%) of women aged 31–35. (Table 1 and Figure 1).
Table 1.
Results of HPV detection by age and cytology.
| Outcomes of HPV detection and typing, N/% (95% C.I) |
||||||||
|---|---|---|---|---|---|---|---|---|
| HPV- | HPV+ | Single | Multiple | Pure-HR | Pure-LR | Mixed | Total(%) | |
| Total | 315(45.13%) | 383(54.87%) | 305(79.63%) | 78(20.37%) | 204(53.26%) | 139(36.30%) | 40(10.44%) | 698 |
| (41.39–48.91) | (51.09–58.61) | (75.25–83.56) | (16.44–24.75) | (48.13–58.35) | (31.47–41.33) | (7.57–13.95) | (100%) | |
| Age | ||||||||
| 0–25 | 7(46.67%) | 8(53.33%) | 8(100%) | 0(0%) | 4(50%) | 4(50%) | 0(0%) | 15 |
| (21.27–73.41) | (26.59–78.73) | (63.01–100) | (<0.000–36.94) | (15.70–84.30) | (15.70–84.30) | (<0.000–36.94) | (100%) | |
| 26–30 | 23(34.33%) | 44(65.67%) | 36(81.82%) | 8(18.18%) | 23(52.27%) | 17(38.64%) | 4(9.09%) | 67 |
| (23.15–46.94) | (53.06–76.85) | (67.29–91.81) | (8.19–32.71) | (36.69–67.54) | (24.36–54.50) | (2.53–21.67) | (100%) | |
| 31–35 | 49(43.36%) | 64(56.64%) | 53(82.81%) | 11(17.19%) | 40(62.50%) | 15(23.44%) | 9(14.06%) | 113 |
| (34.07–53.01) | (46.99–65.93) | (71.32–91.09) | (8.91–28.68) | (49.51–74.30) | (13.75–35.69) | (6.64–25.02) | (100%) | |
| 36–40 | 50(42.74%) | 67(57.26%) | 51(76.12%) | 16(23.88%) | 40(59.70%) | 20(29.85%) | 7(10.45%) | 117 |
| (33.63–52.21) | (47.79–66.37) | (64.14–85.69) | (14.31–35.86) | (47.00–71.51) | (19.28–42.27) | (4.3–20.35) | (100%) | |
| 41–45 | 61(49.19%) | 63(50.81%) | 51(80.95%) | 12(19.05%) | 30(47.62%) | 27(42.86%) | 6(9.52%) | 124 |
| (40.11–58.32) | (41.68–59.89) | (69.09–89.75) | (10.25–30.91) | (34.88–60.59) | (30.46–55.95) | (3.58–19.59) | (100%) | |
| 46–50 | 52(47.27%) | 58(52.73%) | 44(75.86%) | 14(24.14%) | 28(48.28%) | 22(37.93%) | 8(13.79%) | 110 |
| (37.68–57.02) | (42.98–62.32) | (62.83–86.13) | (13.87–37.17) | (34.95–61.78) | (25.51–51.63) | (6.15–25.38) | (100%) | |
| 51–55 | 34(53.97%) | 29(46.03%) | 20(68.97%) | 9(31.03%) | 16(55.17%) | 10(34.49%) | 3(10.34%) | 63 |
| (40.94–66.61) | (33.39–59.06) | (49.17–84.72) | (15.28–50.83) | (35.69–73.55) | (17.94–54.33) | (2.19–27.35) | (100%) | |
| 56+ | 39(43.82%) | 50(56.18%) | 42(84.00%) | 8(16.00%) | 23(46.00%) | 24(48.00%) | 3(6%) | 89 |
| (33.32–54.75) | (45.25–6.68) | (70.89–92.83) | (7.17–29.11) | (31.81–60.68) | (33.66–62.58) | (1.26–16.55) | (100%) | |
| Cytology | ||||||||
| ASC-US | 235(60.88%) | 151(39.12%) | 123(81.46%) | 28(18.54%) | 91(60.27%) | 50(33.11%) | 10(6.62%) | 386 |
| (55.81–65.78) | (34.22–44.19) | (74.33–87.31) | (12.69–25.67) | (51.99–68.13) | (25.68–41.23) | (3.22–11.84) | (100%) | |
| ASC-H | 11(45.83%) | 13(54.17%) | 12(92.31%) | 1(7.69%) | 5(38.46%) | 8(61.54%) | 0(0%) | 24 |
| (25.55–67.18) | (32.82–74.45) | (63.97–99.81) | (0.19–36.03) | (13.86–68.42) | (31.58–86.14) | (<0.000–24.71) | (100%) | |
| L-SIL | 40(29.20%) | 97(70.80%) | 78(80.41%) | 19(19.59%) | 65(67.01%) | 22(22.68%) | 10(10.31%) | 137 |
| (21.75–37.57) | (62.43–78.25) | (71.11–87.78) | (12.22–28.89) | (56.73–76.22) | (14.79–32.30) | (5.06–18.14) | (100%) | |
| H-SIL | 29(19.21%) | 122(80.79%) | 92(75.41%) | 30(24.59%) | 43(35.25%) | 59(48.36%) | 20(16.39%) | 151 |
| (13.26–26.40) | (73.60–86.74) | (66.79–82.75) | (17.25–33.21) | (26.81–44.41) | (39.22–57.58) | (10.31–24.18) | (100%) | |
Figure 1.
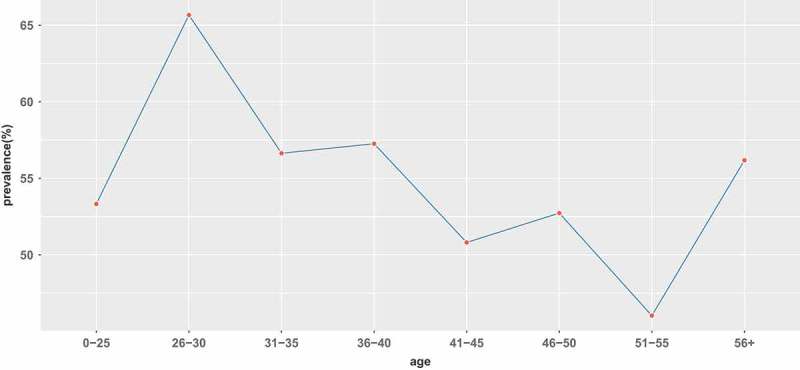
Prevalence of all HPVs infection in each age group.
HPV genotype distribution
The 383 samples identified to be positive for HPV DNA were further analyzed to identify the infecting HPV genotype. In general, regardless of patient age, the pure HR-HPV types (including single and multiple HR-HPV infections) were the most frequent (53.26% of the overall HPV prevalence), with the highest prevalence (62.50%) in women between 31 and 35 years old. The prevalence of pure HR-HPV types declined marginally as age increased up to the group of women ≥36 years old (0–25: 50%; 26–30: 52.27%; 31–35: 62.50%; 36–40:59.70%; 41–45: 47.62%; 46–50: 48.28%; 51–55: 55.17%; ≥56: 46%). The age distribution of pure LR-HPV infection (including single and multiple LR-HPV infections) presented a bimodal curve, with the first peak at group ≤ 25 years old(50%) and the second peak at group ≥ 56 years old (48%). Mixed HPV infection (including HR-HPV and LR-HPV mixed infection) peaked at 31–35 years old(14.06%) and then gradually declined. (Table 1 and Figure 2)
Figure 2.
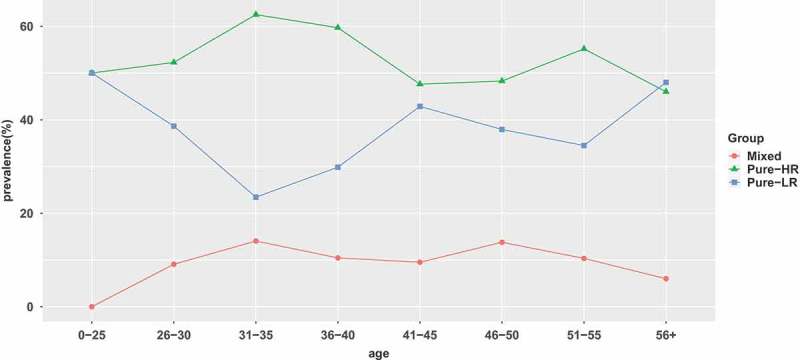
Prevalence of high-risk (HR), low-risk (LR), and mixed HPV infection in each age group.
Detailed analysis of the prevalence of individual HPV genotypes in the whole cohort of samples revealed that HPV16 was the most common type (35.36%), followed by HPV58 (13.62%) and HPV52 (9.15%) (Figure 3). The remaining HPV types were detected in order of decreasing prevalence were HPV18 (8.30%), HPV53 (8.09%), HPV33 (4.04%), HPV56 (2.98%), HPV31 (2.77%), HPV51 (2.77%), HPV39 (2.55%), HPV66 (1.91%), HPV68 (1.49%) (Figure 3). Overall, the frequencies of infections with LR-HPV were lower than the occurrence of HR-HPV infections, with the most common LR- HPV types being HPV81 (2.34%) (Figure 3).
Figure 3.
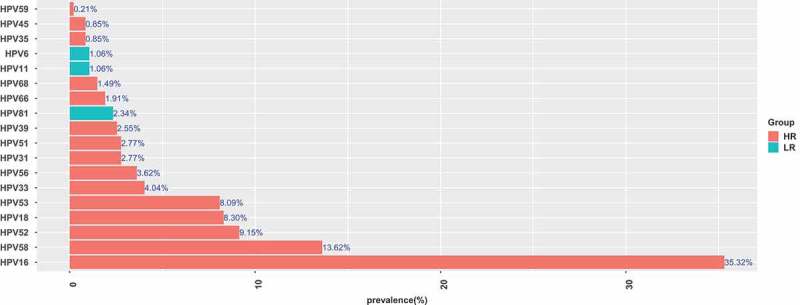
Prevalence and genotype distribution of HPV (HR-HPV and LR-HPV).
According to the results, regardless of patient age, single HPV infections were the most frequent, with the highest prevalence (84%) in women ≥56 years old. Co-infection with two or more HPV types was observed in 78 of the 383 HPV-positive samples (20.37%). Dual infections accounted for 18.02% of all positive samples, with three viruses detected in 2.35% of positive samples (Table 2).
Table 2.
Prevalence of single and multiple HPV infection.
| Genotype | Frequency | Prevalence |
|---|---|---|
| Single | 305 | 79.63% |
| Double | 69 | 18.02% |
| Triple | 9 | 2.35% |
| total | 383 |
Prevalence of HPV types stratified by the cervical cytological result
In this cohort, 386 (55.30%) were affected by ASCUS, 11 (1.58%) by ASC-H, 137 (19.63%) by L-SIL and 151 (21.63%) by H-SIL (Table 1). Among women with a cytology result of ASCUS, ASC-H, L-SIL and H-SIL, 39.12%, 54.17%, 70.80% and 80.79% were infected with HPV, respectively (Table 1). Among the women with cytological abnormality, HPV infection was mainly single infection, with multiple infection rate of 18.54% (ASCUS), 7.69% (ASC-H), 19.59% (LSIL) and 24.59% (HSIL), respectively.
The prevalence of pure HR-HPV, pure LR-HPV and Mixed HPV types among HPV-positive samples diagnosed with ASC-US, ASC-H, L-SIL and H-SIL was also listed in Table 1. HR-HPV genotypes were detected with the highest prevalence in three cytology classes (ASCUS: 66.89%, L-SIL: 77.32%, H-SIL: 51.64%) and except ASC-H group (38.46%). The positive rate of HR-HPV in SIL (63.01%, 138/219) was equivalent to that in ASC (64.63%,106/164). HPV16, HPV58, HPV52, HPV18 and HPV53 accounted for 91.38% (350/383) of all cervical cytology abnormalities. Specifically, HPV16 had the highest frequency in all classes,and the frequency of ASCUS, ASC-H, LSIL, and HSIL was 35.10%,61.54%,27.84% and 63.93%,respectively, while HPV58 was the second common type in ASC-US (17.88%) and LSIL (25.77%). Notably, HPV18 was the second common type in HSIL (22.95%) and HPV53 was the third common type in LSIL (16.49%). The distribution and proportion of the HPV genotypes in different classes of cytological abnormalities were presented in Table 3.
Table 3.
Distribution and proportion of HPV genotypes in cytology abnormalities.
| Cytology result, N(%) |
|||||
|---|---|---|---|---|---|
| Genotypes | ASCUS(N = 151) | ASC-H(N = 13) | LSIL(N = 97) | HSIL(N = 122) | Total(%) |
| HR-HPV | |||||
| HPV16 | 53 (35.10%) | 8 (61.54%) | 27 (27.84%) | 78 (63.93%) | 166(43.34%) |
| HPV58 | 27 (17.88%) | 3 (23.08%) | 25 (25.77%) | 9 (7.38%) | 64(16.71%) |
| HPV52 | 22 (14.57%) | 0 (0%) | 11 (11.34%) | 10 (8.20%) | 43 (11.23%) |
| HPV18 | 5 (3.31%) | 0 (0%) | 6 (6.19%) | 28 (22.95%) | 39 (10.18%) |
| HPV53 | 14 (9.27%) | 1 (7.69%) | 16 (16.49%) | 7 (5.74%) | 38 (9.92%) |
| HPV33 | 7 (4.64%) | 0 (0%) | 4 (4.12%) | 8 (6.56%) | 19 (4.96%) |
| HPV56 | 6 (3.97%) | 0 (0%) | 9 (9.28%) | 2 (1.64%) | 17 (4.44%) |
| HPV31 | 6 (3.97%) | 0 (0%) | 2 (2.06%) | 5 (4.10%) | 13 (3.39%) |
| HPV51 | 7 (4.64%) | 0 (0%) | 5 (5.15%) | 1 (0.82%) | 13 (3.39%) |
| HPV39 | 6 (3.97%) | 1 (7.69%) | 4 (4.12%) | 1 (0.82%) | 12 (3.13%) |
| HPV66 | 6 (3.97%) | 0 (0%) | 2 (2.06%) | 1 (0.82%) | 9 (2.35%) |
| HPV68 | 4 (2.65%) | 0 (0%) | 1 (1.03%) | 2 (1.64%) | 7 (1.83%) |
| HPV45 | 3 (1.99) | 0 (0%) | 0 (0%) | 1 (0.82%) | 4 (1.04%) |
| HPV35 | 2 (1.32%) | 0 (0%) | 0 (0%) | 2 (1.64%) | 4 (1.04%) |
| HPV59 | 0 (0%) | 0 (0%) | 1 (1.03%) | 0 (0%) | 1 (0.26%) |
| LR-HPV | |||||
| HPV6 | 4 (2.65%) | 1 (7.69%) | 0 (0%) | 0 (0%) | 5 (1.31%) |
| HPV11 | 3 (1.99) | 0 (0%) | 1 (1.03%) | 1 (0.82%) | 5 (1.31%) |
| HPV81 | 6 (3.97) | 0 (0%) | 5 (5.15%) | 0 (0%) | 11 (2.87%) |
Discussion
Accurate epidemiological information on HPV infections, including genotype-specific prevalence, is essential for achieving further progress in prevention, such as evaluation of potential impact of HPV vaccines, and type-specific monitoring. International research data showed that bivalent and tetravalent HPV vaccines could prevent about 70% of cervical cancer, and the nine-valent vaccine HPV type coverage rate was as high as 92%. All three vaccines were designed and verified mainly based on the epidemiological background of western populations, and the protection ratio of Asian populations was relatively lower than that of western populations.18 Vaccine development should be supported by epidemiological data, otherwise it would be difficult to achieve the desired results. However, it should be noted that inadequate data was a unified challenge for epidemiological studies in different countries, which also brought uncertainty and risks to the effectiveness and strategies of existing vaccines in different countries.19 Based on the cumulative percentage of HPV type prevalence in cervical cancer in China, it was predicted that the protective effect of 2vHPV and 4vHPV on cervical cancer in Chinese women was about 55.4% and 57.4%, while the protective effect of 9vHPV was about 75.4%. Both were lower than the current globally recognized rates of 71% and 90%, and also lower than the 9vHPV prediction of 87.7% protection for Asian women based on global data.20
Notably, almost in all studies or reviews of epidemiological investigations of HPV types in China and other Asian countries, the authors have consistently called for more systematic and large-scale epidemiological studies in the future. The challenges of such research are considerable, especially for China, a country with unbalanced regional development, large population mobility and rapidly changing society. The analysis showed a study about the rate of HPV detection and type distribution in samples of women who were referred because of an abnormal TCT in Xinjiang region. As we know, this work is the first of its kind in this population.
In this study, a total of 18 HPV genotypes were found and among them HR-HPVs were 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66 and 68, LR-HPVs included HPV6, 11 and 81. HR-HPV26, 73, 82 and LR-HPV70 were not detected in the samples with cytological abnormalities.(HR-HPV26, 73, 82 and LR-HPV70 in the samples were detected without cytological abnormalities) Overall, the prevalence of HPV in the entire group (n = 698) was 54.87% (383 of 698). This result is consistent with previous studies of similar nature conducted in some European countries that reported HPV prevalence ranging from 35.3% to 88.9% among women with cytological abnormalities.21,22 Variations between studies were likely to reflect the differences in population studied with respect to risk factors exposing to HPV and the methods used for analysis and triage for HPV testing by gynaecologists.23
Age is one of the most important risk factors of HPV infection.24 Similar to a previous report,25 a bimodal curve for HPV prevalence versus age was also observed in our study, however, the first peak of HPV infection was 5 years later than reported in the literature. As this study was an analysis of HPV infection in women with cytological abnormalities, so the young women had positive HPV with normal cytology were not included. The second peak of HPV infection occurred after age 56. This result might because that menopausal hormones had obvious fluctuations resulting from the decline in ovarian function, and because of this, the immune response of human physiological properties was decreased with aging and the function of immune system was in a disorderly manner, and the ability to eliminate and inhibit the virus was diminished, further the susceptibility to form a persistent infection or to activate the virus during the incubation period was enhanced, and the chances to form a multiple infection increased.26 Therefore, our findings suggested that the Chinese government, especially in resource-poor communities, should pay more attention to the population age of women older than 56 years old for cervical cancer screening and HPV genotyping.
In the present study, a spectrum of 22 genotypes were identified, with pure HR-HPV types being more frequently detected than pure LR-HPV types and mixed HPV types. Fifteen HR-HPV genotypes were detected in the sample cohort and accounted for 95.54% of HPV-positive samples, in accordance with other studies carried out in European populations.21 HPV16 together with HPV18 were known to be responsible for approximately 70% of the cervical cancer cases worldwide.27 The prevalence of HPV18 had been reported in other studies, ranging between 1.8% and 16.3%.21 In agreement with the aforementioned studies, the prevalence of HPV18 in the present study was 8.30%. Furthermore, HPV-52 which was the highest type in Thai women had been reported and even was common in Asians.28 Recently, a preliminary study suggested that the high attribution of disease to HPV-52 in Asia was due to the high prevalence of lineage B named “Asian lineage”.29 In this study, HPV52 ranked third, while the first and second was HPV16 and 58, respectively.
Our study showed that at least 20.37% of women were infected with two or three HPV genotypes, in accordance with other studies reporting the prevalence of multiple infections was in 11% to 50% of cases.30 The popular opinion of many reports indicated that i) multiple HPV infections were associated with the grades of cervical abnormalities, and ii) the cervical cancer risk of patients who suffered from multiple HPV infections was higher than those with single HPV infection. The clinical significance of co-infection with multiple HPV genotypes remained uncertain, with some reports showing that the clearance rate in immunocompetent women was not dependent on the number of genotypes involved in the infection.31 In this study, the multiple HPV infection rates in ASC-US, ASC-H, L-SIL and H-SIL were 18.54%, 7.69%, 19.59% and 24.59%, respectively. Hence, the incidence of multiple HPV infections indicated that multiple HPV infection was not related to the grades of cytological abnormalities.
An increasing trend in the overall prevalence of HPV infection was observed in parallel with an increase in the degree of cervical cytological abnormalities (ASCUS: 39.12%, ASC-H: 54.17%, L-SIL: 70.80%, H-SIL: 80.79%). Guan P et al.32 reported an overall HPV prevalence was 84% and 85% in H-SIL cases in Europe and globally, respectively. Our data was similar to this result. The prevalence of HR-HPV genotypes has been reported to increase with the grade of cytological lesions in some published studies33 but not all of them.34 In present work, such an association was not observed, the group was classified according to pure high-risk, pure low-risk and mixed HPV infection, both of which (pure high-risk and mixed groups) substantially included high-risk HPV infection. Therefore, the distribution of high-risk HPV in cytological abnormalities was 66.89% (ASCUS), 38.45% (ASC-H), 77.32% (LSIL) and 51.64% (HSIL). The reasons for this might as follows: i) the low number of HPV-positive L-SIL and H-SIL cases that were included for analysis; ii) lacking of histopathological results to finally identify the level of cervical lesions;35 as previously reported, almost 40% of women with an ASCUS diagnosis could be histologically confirmed as high-grade cervical neoplasia;19 iii) the differences of the geographical environment and the selection criteria for the samples.35 Nevertheless, HR-HPV infection still occured more frequently than LR-HPV, so the management of patients with HR-HPV infection needed to be strengthened in clinical practice.
Among individual HPV types, HPV16 was the most frequently identified type in all cytological abnormalities, and the infection rate in ASCUS, ASC-H, LSIL and HSIL was 35.10%, 61.54%, 27.84% and 63.93%, respectively, confirming that HPV16 was the most frequent HPV type associated with cervical lesions, as was previously reported.36 Due to the high carcinogenicity of HPV16 and 18, current guidelines in most countries recommend for HPV16 and/or 18 positive patients for closer monitoring and surveillance. In this study, the positive rate of HPV18 was 6.19% in LSIL and 22.95% in HSIL. George et al.23 reported that the prevalence detected in the H-SIL group (5.3%) was followed by the ASCUS group (4.3%) and the L-SIL group (3.3%). Clifford et al.36 showed the prevalence of HPV18 in L-SIL and H-SIL cases was 8.6% and 7% respectively. Nonetheless, it is interesting to note that the short time of progression to invasive cancer as a result of infection by HR-HPV types, such as HPV18, with or without transition through the pre-invasive cases, which might be an additional contributor to the low prevalence of HPV18 in L-SIL and H-SIL cases in some studies.
Guzalnur Abliz8 reported that HPV-16, HPV-58, and HPV-39 were the most prevalent
genotypes in Uyghur women in Karasay (a township of Hotan area in Xinjiang). However, in Guzalnur Abliz’s study, there were only 40 patients with cytological abnormalities, and the author did not analyze the HPV prevalence and distribution in patients with cytological abnormalities alone, so it was not comparable with the results of our study. Yuanzhi Wang35 showed that the most frequently observed HPV genotypes were HPV16, 53, 52, 58 and 35 in cervical histological abnormalities, there was no studies have addressed the prevalence of HPV in women with abnormal cytology in the geographical region in Xinjiang, so we can only compare the data with those from some areas of China. Patients with abnormalities on cervical cytology in Liaocheng of Shandong province had a high infection rate of HPV16, 52, 58, 33 and 18.37 The most prevalent HPV type was HPV-16 (16.85%, 226/1341) in all of the cervical cytology abnormalities, followed by HPV-52 (9.55%, 128/1341) and HPV-58 (7.83%, 105/1341) in Shantou of Guangdong province.38 In this study, the top five HR-HPVs were HPV16 (35.36%), HPV58 (13.62%), HPV52 (9.15%), HPV18 (8.30%) and HPV53 (8.09%). Our results were not entirely consistent with the above studies. These discrepancies might be attributed to the differences in study populations and designs as well as due to the different methodologies used for sample analysis. It is worth noting that HPV53 was a non-vaccine type, but in this study it was the most common high-risk HPV type at the top five among patients with cytological abnormalities.
The current results should be evaluated under the background of the strengths and limitations of the study. A major advantage of this study is that it provides the first estimates of the prevalence of HPV and its type-specific distribution among women from Xinjiang diagnosed with cytological abnormalities by Pap test. Certainly, the study also has limitations. First, this study is a single-center study in the context of opportunistic screening, so the sample size of cytological abnormality is relatively small. Second, these data cannot be considered regionally representative because the Xinjiang women included in the study might not be a representative sample of all the Xinjiang women living in this province.
Method and material
Study design and population
This was a retrospective and cross-sectional study composed of three kinds of participants, who proceeded to routine screening from Health Management Center, who were referred for opportunistic screening and for evaluation of HPV-associated lesions from the Outpatient and Inpatient Gynecological Department of the First Affiliated Hospital of Xinjiang Medical University from November 2013 to July 2018. Participants meeting the following criteria were included: participants who were permanent residents of Xinjiang, not pregnant, didn’t have vaccinated against HPV, no undergone hysterectomy, no history of cervical surgery, and had never suffered from pelvic radiation therapy. All participants included in the study gave their written informed consent. This study was approved by First Affiliated Hospital of Xinjiang Medical University ethics committee.
Cervical specimen collection and HPV DNA extraction
In this study, Pap test based cervical specimens were collected from exfoliated cell. Cervical exfoliated cell specimens were obtained by a gynecologist as part of routine investigative procedures. Two separated cervical exfoliated cell specimens were collected independently for liquid-based cytological diagnosis and HPV DNA genotyping assays, respectively. Cervical slides were prepared using a liquid-based cytology method. Cytological classifications of disease grade were performed according to the Bethesda 2001 criteria (TBS2001),39 including atypical squamous cells of undetermined significance (ASC-US), atypical squamous cells that couldn’t exclude high-grade squamous intraepithelial lesion (ASC-H), low-grade squamous intraepithelial lesion (L-SIL), and high-grade squamous intraepithelial lesion (H-SIL). For HPV DNA extraction, exfoliated cells were stored at a specimen transport medium. The samples were placed in a cell preservation solution and stored at 2–8°C until HPV DNA extraction and genotyping could be performed. The HPV genotype testing of the samples was completed within a week.
HPV DNA extraction, PCR amplification and genotyping
Multi-asymmetrical PCR combined with general chip (Tag array) was used to detect and classify common HPV types. To extract genomic DNA of human cervical epithelial cells as a template (cell genomic DNA extraction kit, 340011, CapitalBio Corporation, China), HPV detection and genotyping were performed by Human Papillomavirus Genotyping Detection Kit (Microarray) (Crystal Core®, 340010, CapitalBio Corporation, China), a PCR-based flow-through hybridization and gene chip system. Specific primers with Tag sequences were used to amplify and fluoresce labeling the HPV genotypes, and then hybridization with generic gene chips that recognized the corresponding tag sequences, finally, the chip was scanned (LuxScanTM 10K-B Microarray chip scanner, CapitalBio Corporation, China) and the data was analyzed to obtain the results. All specific primers and operating procedures followed the manufacturer’s instructions. Twenty-two type-specific probes, which recognized 18 HR-HPV genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73and 82) and four LR-HPV genotypes (6, 11, 70 and 81). HPV negative and positive controls provided in the Kit were simultaneously detected in every test.
Statistical analysis
All statistical analyses were performed with statistical software including Python version 3.6.1, R Software 3.5.1 and Excel 2011. The prevalence of HPV infection, genotype distribution, single and multiple HPV infections, pure HR, LR infection and mixed infection were analyzed separately. The chi-square test was used to compare HPV prevalence across groups. Data were considered to be statistically significant when the P value was <0.5.
Conclusions
In conclusion, this study presents the first investigation about the prevalence of HPV infection and HPV genotype distribution in Xinjiang women with abnormal cytological tests. Our results could be the baseline data and validation set, which provide the most robust available estimates of the prevalence of type-specific HPV, prior to HPV immunization in Xinjiang’s population.
Funding Statement
This work was supported by the an open project of the Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia [SKL-HIDCA-2017-7];Xinjiang uygur autonomous region health and family planning commission young science and technology talent special projects [2016Y09];
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Bosch FX, Manos MM, Munoz N, Sherman M, AM Jansen, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV.. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) study group. J Natl Cancer Inst. 1995;87:796–802. PMID: 7791229. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. PMID:11919208. PMCID: PMC1769629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641 PMID:12571259. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107 PMID:21296855. [DOI] [PubMed] [Google Scholar]
- 5.Wen C. China’s plans to curb cervical cancer. Lancet Oncol. 2005;6:139–41. doi: 10.1016/S1470-2045(05)01761-4 PMID: 15737830. [DOI] [PubMed] [Google Scholar]
- 6.Kim K, Zang R, Choi S-C, Ryu S-Y, Jae WK. Current status of gynecological cancer in China. J Gynecol Oncol. 2009. June;20(2):72–76. doi: 10.3802/jgo.2009.20.2.72 PMID:19590717. PMCID: PMC2705001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo P, Huang ZL, Yu P, Li K. Trends in cancer mortality in China: an update. Annals Oncol. 2012. October;23(10):2755–62. doi: 10.1093/annonc/mds069 PMID: 22492700. [DOI] [PubMed] [Google Scholar]
- 8.Mijit F, Ablimit T, Abduxkur G, Abliz* G. Distribution of human papillomavirus (HPV)genotypes detected by routine pap smear in Uyghur-Muslim Women from Karasay Township Hotan (Xinjiang, China). J Med Virol PMID: 26081269. PMCID: PMC5033003 2015;87:1960–65. doi: 10.1002/jmv.24240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Rong, Guo Xiao-lei, Wisman G. Bea. A., Ed Schuuring, Wen-feng Wang, Zheng-yu Zeng, Hong Zhu, Shang-wei Wu. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC Infect Dis. 2015. July 4;15:257. doi: 10.1186/s12879-015-0998-5 PMID: 26142044.PMCID: PMC4491249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assoumou Z, Ndjoyi Mbiguino A, Mabika Mabika B, Nguizi Ogoula S, El Mzibri M, Khattabi A, Ennaji MM. Human papillomavirus genotypes distribution among Gabonese women with normal cytology and cervical abnormalities. Infect Agent Cancer. 2016;11:2. doi: 10.1186/s13027-016-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith, Krashias AM, Smouse SL, Tau NP, Bamford C, Moodley VM, Jacobs C, McCarthy KM, Lourens A, Keddy KH. HPV prevalence and type distribution in Cypriot women with cervical cytological abnormalities. BMC Infect Dis. 2017;17:346. doi: 10.1186/s12879-017-2439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen O-E, Olsson S-E, Høye J, Steinwall M, Riis-Johannessen G, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–66. doi: 10.1038/sj.bjc.6603469 PMID: 17117182. PMCID: PMC2360730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli MCM, Jenkins D, Schuind A, Costa CSA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0 PMID: 16631880. [DOI] [PubMed] [Google Scholar]
- 14.Kirby T. FDA approves new upgraded Gardasil 9. Lancet Oncol. 2015;16:e56. doi: 10.1016/S1470-2045(14)71191-X PMID: 25532625. [DOI] [PubMed] [Google Scholar]
- 15.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst . 2015. April 29;107(6):djv086. doi: 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Human papillomavirus vaccines: WHO position paper, May 2017. PMID:28596091 DOI: 10.1016/j.vaccine.2017.05.069 [DOI]
- 17.Zhou H-L, Zhang W, Zhang C-J, Wang S-M, Duan Y-C, Wang J-X, Yang H, Wang X-Y. Prevalence and distribution of humanpapillomavirus genotypes in Chinese women between 1991 and 2016: A systematicreview. J Infect. 2018. doi: 10.1016/j.jinf.2018.02.008 pii: S0163-4453(18)30061-6. PMID: 29477803. [DOI] [PubMed] [Google Scholar]
- 18.Huh WK, Joura EA, Giuliano AR, Iversen O-E, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safetyanalyses of a nine-valent human papillomavirus vaccine in women aged 16–26years: final analyses of a randomised, double-blind trial. Lancet. 2017;390(10108):2143–59. doi: 10.1016/S0140-6736(17)31821-4 PMID: 28886907 [DOI] [PubMed] [Google Scholar]
- 19.Xu Qiu-Xiang, Zhang ZY. High-risk human papillomavirus genotypes incervical lesions and vaccination challenges in China. Asian Pac J Cancer Prev. 2015;16:2193–97. PMID: 25824736. [DOI] [PubMed] [Google Scholar]
- 20.Tao G, Yaling G, Zhan G, Pu L, Miao H. Human papillomavirus genotype distributionamong HPV-positive women in Sichuan province, Southwest China. Arch Virol. 2018;163(1):65–72. doi: 10.1007/s00705-017-3556-1 PMID: 28983744 [DOI] [PubMed] [Google Scholar]
- 21.Meloni A, Pilia R, Campagna M, Usai A, Masia G, Caredda V, Coppola RC. Prevalence and molecular epidemiology of human papillomavirus infection in italian women with cervical cytological abnormalities. J Public Health Res. 2014;3:157. doi: 10.4081/jphr.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simanaviciene V, Gudleviciene Z, Popendikyte V, Dekaminaviciute D, Stumbryte A, Rubinaite V, Zvirbliene A. Studies on the prevalence of oncogenic HPV types among Lithuanian women with cervical pathology. J Med Virol. 2015;87:461–71. doi: 10.1002/jmv.24073. [DOI] [PubMed] [Google Scholar]
- 23.Krashias G, Koptides D, Christodoulou C, Bamford C, Moodley VM, Jacobs C, McCarthy KM, Lourens A, Keddy KH. HPV prevalence and type distribution in Cypriot women with cervical cytological abnormalities. BMC Infect Dis. 2017;17:346. doi: 10.1186/s12879-017-2439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonsson A, Cornford M, Perry S, Davis M, Dunne MP, Whiteman DC. Prevalence and risk factors for oral HPV infection in young Australians. PLoS One . 2014. March 17;9(3):e91761. doi: 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruni L, Diaz M, Castellsague′ X, Ferrer E, Bosch FX, de Sanjose′ S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010. December 15;202(12):1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 26.Althoff KN, Paul P, Burke AE, Viscidi R, Sangaramoorthy M, Gravitt PE. Correlates of cervicovaginal human papillomavirus detection in perimenopausal women. J Women’s Health. 2009. September;18(9):1341–46. doi: 10.1089/jwh.2008.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin H-R, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 28.Kantathavorn N, Mahidol C, Sritana N, Sricharunrat T, Phoolcharoen N, Auewarakul C, Teerayathanakul N, Taepisitpong C, Saeloo S, Sornsamdang G, et al. Genotypic distribution of human papillomavirus (HPV) and cervical cytology findings in 5906 Thai women undergoing cervical cancer screening programs. Infect Agent Cancer. 2015;10 article 7. doi: 10.1186/s13027-015-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, Park J-S, Magdalena G, Hibbitts S, Palefsky JM, Konno R, Smith-McCune KK, Giovannelli L, Chu T-Y, Picconi MA, et al. Geographical distribution and risk association of human papillomavirus genotype 52-variant lineages. J Infect Dis. 2014;210(10):1600–04. doi: 10.1093/infdis/jiu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panotopoulou E, Tserkezoglou A, Kouvousi M, Tonusin S, Phan TG, Okitsu S, Ushijima H. Prevalence of human papillomavirus types 6, 11, 16, 18, 31, and 33 in a cohort of Greek women. J Med Virol. 2007;79:1898–905. doi: 10.1002/jmv.21025. [DOI] [PubMed] [Google Scholar]
- 31.Molano M, Van Den Brule A, Plummer M, Weiderpass E, Posso H, Arslan A, Meijer CJ, Muñoz N, Franceschi S. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486–94. doi: 10.1093/aje/kwg171 PMID:12936904. [DOI] [PubMed] [Google Scholar]
- 32.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, Clifford GM, Stram DO, Henderson BE, Kolonel LN, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–59. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 33.Dickson EL, Vogel RI, Geller MA, Downs LS Jr.. Cervical cytology and multiple type HPV infection: a study of 8182 women ages 31-65. Gynecol Oncol. 2014;133:405–08. doi: 10.1016/j.ygyno.2014.03.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moga MA, Irimie M, Oanta A, Pascu A, Burtea V. Type-specific prevalence of human papillomavirus by cervical cytology among women in Brasov. Romania. Asian Pac J Cancer Prev. 2014;15:6887–92. PMID: 25169541. [DOI] [PubMed] [Google Scholar]
- 35.Wang Lina, Wang Pengyan, Ren Yan, Jingyun Du, Jianjun Jiang, Xuesong Jia, Chuangfu Chen, Yuanzhi Wang. Prevalence of high-risk human papillomavirus (HR-HPV) genotypes and multiple infections in cervical abnormalities from Northern Xinjiang, China. PLoS One. 2016. August 5. doi: 10.1371/journal.pone.0160698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomark Prev. 2005;14:1157–64. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 37.You W, Li S, Du R, Zheng J, Shen A. Epidemiological study of high-risk human papillomavirus infection in subjects with abnormal cytological findings in cervical cancer screening. Exp Ther Med. 2018;15:412–18. doi: 10.3892/etm.2017.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Yuanyuan, Wang Shaohong, Shen Jinhui, Yanyan Peng, Lechuan Chen, Ruiqin Mai, Guohong Zhang. Genotype distribution of human papillomavirus among women with cervical cytological abnormalities or invasive squamous cell Carcinoma in a high-incidence area of esophageal carcinoma in China. Biomed Res Int. 2016;2016:1256384. doi: 10.1155/2016/1256384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, et al. The 2001 Bethesda system: terminology for reporting results of cervical cytology. J Am Med Assoc. 2002. April 24;287(16):2114–19. PMID:11966386. [DOI] [PubMed] [Google Scholar]


