Abstract
Since the first description of Seasonal Affective Disorder (SAD) by Rosenthal et al. in the 1980s, treatment with daily administration of light, or Bright Light Therapy (BLT), has been proven effective and is now recognized as a first-line therapeutic modality. More recently, studies aimed at understanding the pathophysiology of SAD and the mechanism of action of BLT have implicated shifts in the circadian rhythm and alterations in serotonin reuptake. BLT has also been increasingly used as an experimental treatment in non-seasonal unipolar and bipolar depression and other psychiatric disorders with known or suspected alterations in the circadian system. This review will discuss the history of SAD and BLT, the proposed pathophysiology of SAD and mechanisms of action of BLT in the treatment of SAD, and evidence supporting the efficacy of BLT in the treatment of non-seasonal unipolar major depression, bipolar depression, eating disorders, and ADHD.
HISTORY OF SAD
The first formal description of Seasonal Affective Disorder (SAD), the most well-known psychiatric condition associated with seasonality in humans, was introduced in the mid-1980s by Rosenthal et al. (Rosenthal et al., 1984) who described a group of 29 patients living in a temperate climate who experienced depressive episodes characterized by hypersomnia, hyperphagia, and weight gain in the fall or winter, and whose symptoms remitted by the next spring or summer. SAD was incorporated into the Diagnostic and Statistical Manual (DSM) of Mental Disorders III-R (American Psychiatric Association. & American Psychiatric Association. Work Group to Revise DSM-III., 1987) when “seasonal pattern” was introduced as a specifier for Major Depression and Bipolar Disorders: “the seasonal pattern is used when there has been a regular temporal relationship between a Mood Disorder and a particular 60 day period of the year.” Subsequent revision in DSM-IV described SAD as “a regular temporal relationship between the onset of Major Depressive Episodes in Bipolar I (BPI) or Bipolar II (BPII) Disorder or Major Depressive Disorder (MDD), recurrent, and a particular time of the year” (American Psychiatric Association. & American Psychiatric Association. Task Force on DSM-IV., 1994). Since the last article on SAD and light therapy published in this journal (Padeh, 1999), the psychiatric nomenclature used to describe the disorder in DSM-5 (American Psychiatric Association. & American Psychiatric Association. DSM-5 Task Force., 2013) has remained as a course specifier – “with seasonal pattern” – applied to recurrent major depressive episodes within MDD or BP disorder. While the DSM-5 does not include SAD as a diagnosis separate from that of major depression, the term SAD is still widely used.
Today, SAD, or MDD with seasonal pattern, is defined as recurrent episodes of major depression that meet the following criteria: at least two consecutive years where the onset and offset of depressive symptoms occur at characteristic times with no non-seasonal episodes, a temporal relationship between onset of symptoms and time of year, a temporal relationship between remission of symptoms and time of year, and an outnumbering of seasonal compared to non-seasonal episodes throughout the lifetime of the patient (American Psychiatric Association. & American Psychiatric Association. DSM-5 Task Force., 2013). As in the initial description of SAD, more recent studies have supported the notion that features generally considered to be part of atypical depression, including hypersomnia, hyperphagia (especially carbohydrate craving), and weight gain, are more common in SAD than in non-seasonal unipolar depression (Tam et al., 1997). Although the definition of SAD allows for depressive symptoms to be present in either fall/winter or spring/summer, winter or “typical SAD” is far more common than summer or “reverse SAD” (3% vs. 0.1%) (Mersch, Middendorp, Bouhuys, Beersma, & van den Hoofdakker, 1999) and will be the main focus of this discussion.
HISTORY OF BRIGHT LIGHT THERAPY
Clues to the pathophysiology of SAD were first revealed in the initial study by Rosenthal et al., which demonstrated that subjecting patients with winter SAD to bright white light, thereby lengthening their photoperiod (daily exposure to light), reduced their depressive symptoms (Rosenthal et al., 1984). More specifically, patients were subjected to bright white full-spectrum fluorescent light of 2,500 lux for 3 hours at dawn and 3 hours at dusk for two weeks. The authors observed a statistically significant antidepressant effect as measured by the Hamilton Depression Rating Scale (HDRS), with effects generally seen within the first 3–7 days of treatment. Interestingly, when patients were then crossed over to a dim yellow light treatment of 100 lux, a significant degree of relapse occurred, suggesting that the antidepressant effect is specific to light characteristics such as illuminance and/or spectrum.
Over the years, the conditions for administration of bright light therapy (BLT) for SAD have been optimized. The current strategy for initiating treatment with BLT for patients with SAD has been summarized by Pail et al. (Pail et al., 2011). In general, BLT is performed early in the morning using a device known as a light box. The initial parameters for treatment are outlined in Table 1.
Table 1. Initial parameters for BLT.
Adapted from Praschak-Rieder & Willeit, 2003, Pail et al., 2011, and “Center for Environmental Therapeutics,” 2016.
| Parameter | Description |
|---|---|
| Light source | Fluorescent light box with diffusion screen. |
| Light intensity | Standard Treatment: 10,000 lux for 30 minutes Alternative Treatment: 2,500 lux for 1–2 hours |
| Wavelengths | Full-spectrum visible light. |
| Light source position | The light box is angled ~30° from the line of gaze. The user does not stare directly into the light. As the illuminance (in lux) delivered to the cornea is dependent on distance, the user sits at a distance specified by the device (usually 12–24”). |
| Daily treatment | Morning treatment with time varying depending on intensity of light. (i.e. 30 mins for 10,000 lux, 1–2h for 2,500 lux). |
| Length of treatment | Unclear, though data suggest symptoms should begin to remit within the first week of therapy and cessation of therapy may lead to relapse. One recommendation for SAD patients: to begin in the autumn and continue through the spring. |
| Treatment variations (not as effective as traditional BLT) |
Dawn simulation: Gradual increase in bedside light in the AM after awakening. Evening treatment. For some with normal awakening: 1–2 hours in the evening ending 1 hour before bedtime. |
| Side effects | Minimal: Headache, eye strain, nausea. Patients with glaucoma, cataracts, or retinopathy should be under the supervision of an ophthalmologist. Caution is recommended with a photosensitive medical condition (e.g. SLE) or when taking photosensitizing medications (e.g. some antibiotics) Monitor for emergence of hypomania/mania |
BLT is now widely considered the first-line treatment of SAD. Specifically, two meta-analyses of eight randomized blinded and controlled studies (n=703 total) were consistent in demonstrating that BLT showed efficacy in treatment of SAD when compared to a control condition (Golden et al., 2005; Martensson, Pettersson, Berglund, & Ekselius, 2015). A study by Eastman et al. demonstrated the magnitude of light therapy’s effect (Eastman, Young, Fogg, Liu, & Meaden, 1998). In this study, the majority of patients with SAD (61% of 33 patients) who received bright light therapy during the 4 week study period reached symptom remission using strict two-part criteria: patients had to demonstrate a reduction of at least 50% from their initial score on the Structured Interview Guide for the Hamilton Depression Rating Scale, SAD Version (SIGH-SAD), and a final overall score of 8 or lower on the same instrument. In contrast, only 32% of the 31 patients receiving placebo achieved remission during the study, and the difference was statistically significant (difference in remission rate between bright morning light and morning placebo at 3 weeks: z=2.55, p<0.05). Thus, BLT is proven not only to be an effective treatment in SAD, but also powerful enough to bring patients with depression into remission.
Reimbursement for light boxes has historically been difficult (Kanofsky, Aspengren, & Watts, 2003). However, an increasing number of private insurance companies view light therapy with a high intensity light box as “medically appropriate” for patients who meet the criteria for SAD (“Excellus BlueCross BlueShield Health Insurance Plans,” 2015). Nevertheless, it is unlikely Medicaid will provide coverage for the device in the near future (personal communication with officer of the Medicaid Medical Devices section). A clinically tested light box currently sells for approximately $150 on the internet (“Center for Environmental Therapeutics,” 2016).
PATHOPHYSIOLOGY OF SAD
To date, the pathophysiology of SAD is unclear. Early research into the mechanism of SAD focused on day length, or photoperiod. This hypothesis posited that shorter days in winter, possibly mediated by longer duration of nocturnal melatonin secretion, leads to depressed mood in susceptible individuals (Wehr et al., 2001). To date, there is little data to support this hypothesis. Furthermore, given that bright light in the evening has not been found to be as effective as that given in the morning (Lewy, Sack, Miller, & Hoban, 1987), it now seems unlikely that photoperiod is the underlying pathological mechanism of SAD.
Although some animal studies have implicated a direct effect of light on the midbrain (Miller, Miller, Obermeyer, Behan, & Benca, 1999; Miller, Obermeyer, Behan, & Benca, 1998), the most prominent hypothesis driving human studies involves disruption of circadian rhythms. Research on the role of serotonin is also active.
Circadian Rhythm
A circadian rhythm refers to the approximately 24-hour cycle of physiological processes present in humans and other animals. This cycle is governed via clock gene expression by the suprachiasmatic nucleus (SCN), the master pacemaker located within the anterior hypothalamus. Though circadian oscillations are endogenously generated by the SCN, they need to be entrained to the 24-hour day by external cues. Light exposure is the most important synchronizing agent of endogenous circadian rhythms (Roenneberg & Foster, 1997). As opposed to the more well-known opsin-containing rod and cone cells, this photic entrainment is regulated largely through melanopsin-containing retinal cells known as intrinsically photosensitive Retinal Ganglion Cells (ipRGCs) that transduce light signals with maximum absorption in the blue wavelength range and deliver them directly to the SCN via the retinohypothalamic tract (Hattar et al., 2003). However, though mice lacking melanopsin have altered circadian rhythms, they can still be entrained by light (Panda et al., 2002), suggesting a collateral role for rods or cones in the modulation of ipRGCs. In addition, the SCN is regulated by neuromodulatory signals, including melatonin from the pineal gland. Melatonin acts to transduce daily and seasonal photoperiod information (Moore & Speh, 2004; Stehle, von Gall, & Korf, 2003), with melatonin peaking during the night and returning to baseline trough levels during the day.
Downstream of the SCN, a collection of systemically active neurohumoral networks transduce circadian information to the rest of the body. For instance, via projections to the paraventricular nucleus of the hypothalamus, the activation of the SCN leads to autonomic changes including cardiovascular modulation (Scheer, Kalsbeek, & Buijs, 2003), and together the central, peripheral, and autonomic nervous systems collaborate to affect systemic changes (Oldham & Ciraulo, 2014). Thus, the SCN receives information about the external day-night cycle both directly through retinofugal pathways and indirectly through neuromodulatory signaling. Circadian information is then relayed systemically through neurohumoral networks.
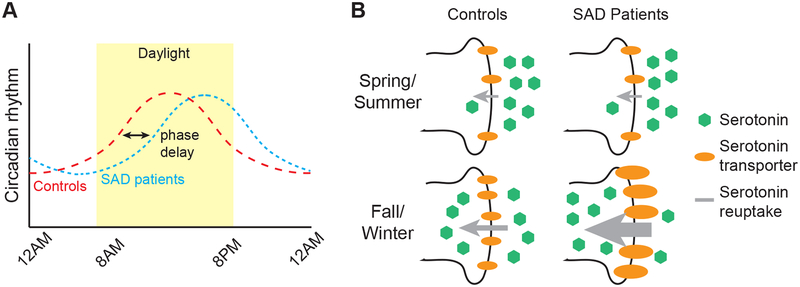
The current primary hypothesis for the pathophysiology of SAD, known as the “phase-shift hypothesis,” posits that there is an optimal relationship in the alignment of the sleep-wake cycle and the endogenous circadian rhythm. During the fall and winter, as day length shortens, the circadian rhythm begins to drift later with respect to clock time and the sleep-wake cycle. This phase delay is hypothesized to bring about mood symptoms (Figure 1A). One method to measure this circadian misalignment is known as the phase-angle difference (PAD), and is calculated as the difference in time between the dim light melatonin onset (DLMO, the time of evening rise in melatonin) and mid-sleep (Lewy, Lefler, Emens, & Bauer, 2006). A pulse of morning bright light generates a circadian phase advance, which is thought to correct the discordance between sleep and circadian phase, and thereby ameliorate depressive symptoms (Lewy et al., 2006) (Figure 2A). However, the phase-shift hypothesis would predict that the amount of phase correction required for each patient would depend on an individual’s PAD, which has not yet been proven.
Figure 1. Proposed pathological mechanisms of Seasonal Affective Disorder.
(A) The phase-shift hypothesis. Compared to control individuals (red broken line), in SAD patients (blue broken line) the daily circadian rhythm is delayed with respect to clock time, creating a discordance between the circadian rhythm and the clock time/sleep-wake cycle. The discordance, which essentially produces a winter-long jet lag for patients with SAD, is hypothesized to lead to depressive symptoms.
(B) The serotonin hypothesis. In control individuals there is greater binding potential at the pre-synaptic serotonin transporter during fall/winter compared to summer months and in SAD patients, there is even greater binding potential during fall/winter compared to healthy individuals. Greater binding potential is schematized in the figure through the use of greater number and/or thicker representations of the transporters. According to the monoamine hypothesis of depression, if greater binding at the transporter results in greater uptake of serotonin into the presynaptic neuron and less available serotonin in the synapse cleft, the outcome could be the appearance of depressive symptoms in affected individuals.
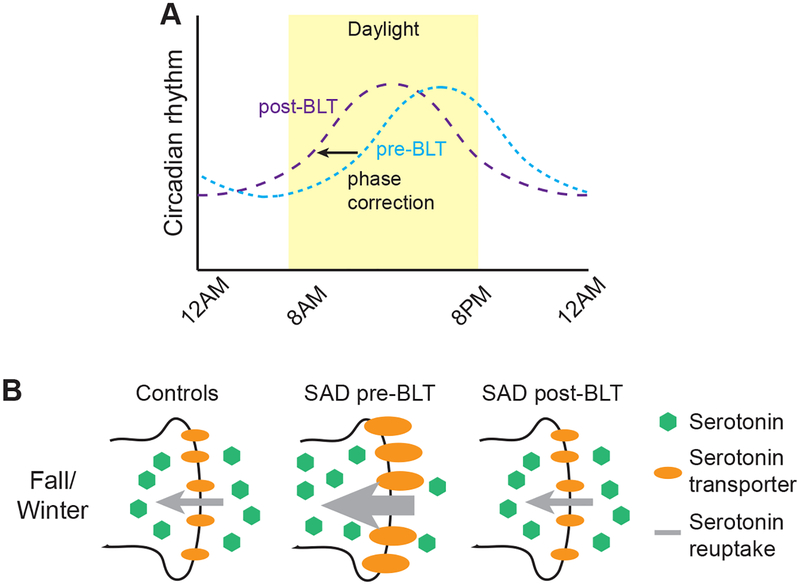
Figure 2. Mechanisms of BLT action based on proposed pathophysiology of Seasonal Affective Disorder.
(A) BLT corrects the phase delay. Pre- and post-BLT treatment are displayed as blue and purple broken lines respectively. Morning BLT treatment corrects the phase delay by shifting the circadian rhythm earlier (to the left) leading to less discordance between the circadian rhythm and the clock time/sleep-wake cycle.
(B) BLT increases available serotonin. BLT treatment during the fall/winter reduces the serotonin transporter binding potential, theoretically leading to less uptake, and a greater amount of available serotonin in the synaptic cleft.
Serotonin
Several studies have also proposed that serotonin is implicated in the pathophysiology of SAD, as selective serotonin reuptake inhibitors (SSRIs) appear to be effective in the treatment of SAD (Lam et al., 1995; Moscovitch et al., 2004). Supporting this hypothesis, one study used Positron Emission Tomography (PET) imaging to look at binding probability at synaptic serotonin transporters in the anteromedial prefrontal cortex, anterior cingulate cortex, caudate, putamen, thalamus, and mesencephalon in 88 normal individuals living in the temperate climate of Toronto, Canada (Praschak-Rieder, Willeit, Wilson, Houle, & Meyer, 2008). The binding probability was increased during fall and winter, as compared to warmer months, thus eliciting an inverse correlation between binding potential and duration of sunlight. Of note, the largest difference in transporter binding was found in the mesencephalon, a finding consistent with animal studies demonstrating the importance of direct effects of light to the midbrain on behavior (Miller et al., 1999; Miller et al., 1998). If increased transporter activity indicated greater reuptake of serotonin during the fall/winter, and if this resulted in lower density of cleft serotonin, then the seasonal variation in transporter activity (i.e. higher transporter efficiency in the winter) would seem to leave susceptible individuals particularly prone to mood symptoms during the darker seasons (Figure 1B). A study by Willeit et al. demonstrated increased synaptic serotonin transporter efficiency in platelets derived from drug-naïve patients with SAD compared to normal controls (Willeit et al., 2008). Moreover, following BLT, and during periods of remission in the summer months, the activity of the synaptic transporter was shown to be reduced to control levels in these patients (Willeit et al., 2008) (Figure 2B).
MECHANISM OF ACTION OF BLT
Whereas BLT has been shown to be an effective therapy for SAD, its exact mechanism of action remains unclear. Based on the proposed pathophysiologies of SAD described above, it has been hypothesized that BLT may function by either correcting the winter circadian rhythm phase delay or by increasing synaptic serotonin (Figure 2), possibly in the serotonin-rich midbrain, a target of retinofugal pathways or indeed, by both mechanisms. However, one thing that is clear is that the therapeutic effects of BLT appear to require the eyes (and light-activated retinofugal pathways), as BLT administered to the popliteal fossa in the knee of patients with SAD was shown to have no effect on symptoms (Koorengevel et al., 2001). Recent literature has also explored the possibility that other non-melanopsin and non-opsin light-absorbing molecules such as hemoglobin, biliverdin and bilirubin, and gaseous molecules such as CO and NO present in retinal blood vessels could be involved in the mechanism of action of BLT (Oren, Koziorowski, & Desan, 2013). Future research in this area should reveal the relative importance of these novel mechanisms.
BEYOND SAD
Non-seasonal Unipolar Depression
The success of BLT in SAD has prompted the investigation of its use in the treatment of other psychiatric conditions. Given its effectiveness in treating seasonal depression, the first avenue of investigation was to determine whether BLT could be beneficial for patients suffering from non-seasonal unipolar depression. Several studies have been conducted to this end and, although BLT has been shown to be efficacious in treating non-seasonal depression in most studies, critics have argued that many of these studies have been difficult to interpret due to small study sizes, difficulty in supplying adequate blinding and control conditions, and inconsistencies in therapy dosing and timing. However, a seminal meta-analysis of three randomized controlled trials, which was sponsored by the American Psychiatric Association and led to the inclusion of BLT in its Practice Guidelines for depression, showed that the effect of BLT on non-seasonal depression is as (or more) robust as the effect seen in pharmacotherapy trials (effect size=0.53, 95% CI 0.18 to 0.89) (Golden et al., 2005). Moreover, a second meta-analysis has shown that use of BLT as an adjunctive treatment with antidepressant medications leads to greater improvement than antidepressant medication alone (Even, Schroder, Friedman, & Rouillon, 2008). The most recent meta-analysis of nine trials also demonstrated the efficacy of BLT; study results demonstrated that use of BLT led to a significant reduction of non-seasonal depressive symptoms (Standard mean difference (SMD) = −0.62, 95% CI −0.88 to −0.35, p<0.001) (Al-Karawi & Jubair, 2016).
Two more studies comparing the effect of antidepressants alone to antidepressants plus BLT treatment in non-seasonal depression have delineated similarly positive results. In the first study, 50 inpatients with severe MDD were structurally randomized, according to their baseline HDRS score, to one of two unblinded treatments: 1) venlafaxine or 2) BLT and venlafaxine. After 4 weeks of treatment, 76% of patients treated with venlaxafine plus BLT attained the target HDRS score (≤13) vs. only 44% of patients treated with venlaxafine alone (p<0.05) (Guzel Ozdemir et al., 2015). An eight-week study that was randomized and double-blinded, with placebo and sham-controls, examined 122 outpatients with at least moderate depression. The patients treated with BLT plus fluoxetine had a mean change in Montgomery-Åsberg Depression Rating Scale (MADRS) of 16.9 vs. those treated with fluoxetine alone who had a change of 8.8 (p = 0.02) (Lam et al., 2016). Of note, fluoxetine alone did not perform statistically better than placebo in this trial but the effect sizes for treatment with BLT were sizeable (effect sizes = 1.11, 95% CI 0.54 to 1.64; 0.80, 95% CI 0.28 to 1.31; and 0.24, 95% CI −0.27 to 0.74 for BLT+fluoxetine, BLT alone, and fluoxetine alone respectively). Altogether, BLT appears to be effective in treating nonseasonal unipolar depression, and even appears to have additive effects when used in combination with pharmacotherapies.
Unipolar Depression in Special Populations
The studies reviewed above mainly examined unipolar depression in the general adult population; however, use of BLT in unipolar depression in certain specific patient populations has also been analyzed. Of particular interest, a randomized, blinded, placebo-controlled study of BLT for antepartum depression (n=27) showed positive results with a response (HDRS ≥ 50% improvement from baseline) in 81% of patients treated with bright white light versus 46% of patients treated with placebo dim light (p < 0.05). Moreover, remission (HDRS ≤ 8) was attained by 69% of BLT patients versus 36% of patients receiving placebo dim light (p < 0.05) and the HDRS scores displayed a significant interaction of treatment with time (F4,92 = 2.91; p < 0.05) (Wirz-Justice et al., 2011). Likewise, a randomized controlled cross-over trial in adolescents aged 14–17 years old with non-seasonal depression (n=28) has also shown promising results with BLT performing significantly superior to the placebo control at treating depressive symptoms as measured by the Beck Depression Inventory (BDI) (p=0.014) (Niederhofer & von Klitzing, 2012). That BLT, which is non-invasive and has very few side effects, can be effective in children and pregnant women is quite appealing, since use of pharmacotherapies in these populations remains controversial. Together, these data strongly suggest that BLT is an effective treatment for depression in general and not just for patients suffering from SAD, as was originally thought.
Bipolar Depression
Earlier studies examining BLT as a treatment for bipolar depression have shown conflicting results (Dauphinais et al., 2012; Deltito, Moline, Pollak, Martin, & Maremmani, 1991). A recent meta-analysis of nine studies (n=489 patients) of the efficacy of BLT in the treatment of bipolar depression, however, has shown a reduction in disease severity as measured by the HDRS or BDI in patients treated with either BLT monotherapy or in combination with other treatments (effect size=−0.69, 95% CI −0.90 to −0.48, p < 0.001) (Tseng et al., 2016). In particular, BLT, when combined with total sleep deprivation, has been shown to lead to a more sustained antidepressant effect than total sleep deprivation alone (Colombo et al., 2000). Furthermore, in a study of 60 inpatients with bipolar depression treated with lithium or antidepressants, one week treatment with combined total sleep deprivation and BLT led to a response (50% reduction in HDRS score from baseline) in both non treatment-resistant patients and treatment-resistant (as deemed by failure of at least 1 adequate trial of 1 major class of antidepressant) patients (70% and 44% responders respectively, p = 0.045) (Benedetti et al., 2005). Furthermore, response was maintained at 9 months post-treatment in 57% and 17% of non treatment-resistant and treatment-resistant responders respectively. These data suggest an important role for BLT as a combination therapy in the treatment of bipolar depression, particularly in treatment-resistant patients.
Eating Disorders
BLT has also been investigated to a lesser extent in eating disorders. Because binge eating episodes have been observed to increase in fall and winter in some patients (Levitan, Kaplan, Levitt, & Joffe, 1994), BLT has been examined as a treatment modality for anorexia nervosa (AN) and bulimia nervosa (BN). Based on a pilot study (n=5 women) that showed BLT may have positive effects on eating pathology and depressive symptoms in patients with AN (Daansen & Haffmans, 2010), a larger study (n=24 women aged 15–20) comparing the use of Cognitive Behavioral Therapy (CBT) to CBT plus BLT in the treatment of patients with restrictive type AN and concomitant depression was conducted. Study results demonstrated no difference in body mass index (BMI) in the two groups following treatment, but did find a significant reduction in depressive symptoms as measured by the HDRS score (47% vs. 28% decrease from baseline HDRS score with CBT plus BLT vs. CBT alone, p<0.001); thus, whereas BLT may help to curb carbohydrate cravings in patients with SAD, it does not seem to lead to either weight loss or weight gain in patients with AN (Janas-Kozik et al., 2011). Moreover, this result is similar to the results of the Guzel Ozdemir et al and Lam et al. studies discussed above, which showed increased benefit to patients with addition of light therapy when compared to the benefit of antidepressants alone. Whether this indicates that antidepressants and BLT work through separate pathways to ameliorate depression is a question that remains for future research. Paradoxically, in a blinded, placebo-controlled study of BLT in patients with BN (n=34, women aged 18–50 years), those patients treated with BLT had a decreased binge frequency (Wilk’s λ=0.81, F2,28=3.31, p=0.05) with no change in their depressive symptoms or weight (Braun, Sunday, Fornari, & Halmi, 1999). Thus the effects of BLT in patients with eating disorders remain enigmatic and additional studies, including larger, randomized blinded and controlled trials, are needed to further elucidate the role of BLT in treating this patient population. Further research might also determine whether BLT would be a useful treatment in Binge-Eating Disorder, a diagnosis new to DSM-5.
Adult ADHD
Additionally, BLT has been studied in the context of adult Attention-Deficit/Hyperactivity Disorder (ADHD), where, in addition to normal ADHD symptoms, patients often have depressed mood and difficulties falling asleep, awakening on time, and maintaining arousal (Brown & McMullen, 2001). These symptoms are indicative of a possible delay in the circadian rhythm. In one open trial study (n=29 adults aged 18–60 years) with no control group, BLT was shown to be associated with decreases in ADHD symptoms (15% decrease from baseline of Brown Adult ADD Scale, p=0.001) in addition to improved mood (52% decrease from baseline of SIGH-SAD score, p=0.001) and improved circadian rhythm (11% increase from baseline of Horne-Ostberg Morningness-Eveningness Questionnaire (MEQ), p=0.016) (Rybak, McNeely, Mackenzie, Jain, & Levitan, 2006). Furthermore, multiple regression analysis, in which the overall model was statistically significant (F=5.88, df=2.24; p=0.008), showed that only the MEQ score, or an improvement in circadian rhythm, was a significant predictor of improvements in ADHD symptoms in patients treated with BLT (t=−2.35, p=0.027). A case report of symptom improvement following BLT in a child with ADHD who displayed signs of delayed sleep phase also supports the idea that BLT may be useful in treating symptoms of ADHD (Gruber, Grizenko, & Joober, 2007). Whether the pathways that subserve the improvement of mood symptoms in response to BLT are the same pathways that underlie the seemingly beneficial effects of BLT in ADHD remains to be studied. While these results are promising, further studies, preferably in the form of randomized blinded and controlled studies, will need to be performed.
SUMMARY
While its role in treating psychiatric disorders beyond SAD continues to be elucidated, BLT is an increasingly promising treatment option, particularly for those disorders that show seasonal variation in symptoms, delayed circadian phase, and depressive symptoms. In addition to more robust randomized controlled trials for the use of BLT in disorders other than SAD, further research into the physiological mechanisms of BLT is needed. The goal of such research would be to determine optimal conditions for light therapy specific to each disorder, to better predict those disorders that may respond to BLT, and to better understand the pathophysiological mechanisms of the disorders affected by BLT, as well as pathways subserving the positive effects of BLT. Nonetheless, the future of BLT in psychiatry – as an adjunct or as monotherapy – is very bright.
Acknowledgements:
This work was supported in part by the National Institutes of Health, T32-GM007288.
Footnotes
Conflict of Interests: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. They have no conflicts of interest to report.
REFERENCES
- Al-Karawi D, & Jubair L (2016). Bright light therapy for nonseasonal depression: Metaanalysis of clinical trials. J Affect Disord, 198, 64–71. doi: 10.1016/j.jad.2016.03.016 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association., & American Psychiatric Association. DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders : DSM-5 (5th ed.). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association., & American Psychiatric Association. Task Force on DSM-IV. (1994). Diagnostic and statistical manual of mental disorders : DSM-IV (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association., & American Psychiatric Association. Work Group to Revise DSM-III. (1987). Diagnostic and statistical manual of mental disorders : DSMIII-R (3rd ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Benedetti F, Barbini B, Fulgosi MC, Colombo C, Dallaspezia S, Pontiggia A, & Smeraldi E (2005). Combined total sleep deprivation and light therapy in the treatment of drug-resistant bipolar depression: acute response and long-term remission rates. J Clin Psychiatry, 66(12), 1535–1540. [DOI] [PubMed] [Google Scholar]
- Braun DL, Sunday SR, Fornari VM, & Halmi KA (1999). Bright light therapy decreases winter binge frequency in women with bulimia nervosa: a double-blind, placebo-controlled study. Compr Psychiatry, 40(6), 442–448. [DOI] [PubMed] [Google Scholar]
- Brown TE, & McMullen WJ Jr. (2001). Attention deficit disorders and sleep/arousal disturbance. Ann N Y Acad Sci, 931, 271–286. [DOI] [PubMed] [Google Scholar]
- Center for Environmental Therapeutics. (2016). Retrieved from http://www.cet.org/
- Colombo C, Lucca A, Benedetti F, Barbini B, Campori E, & Smeraldi E (2000). Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiatry Res, 95(1), 43–53. [DOI] [PubMed] [Google Scholar]
- Daansen PJ, & Haffmans J (2010). Reducing symptoms in women with chronic anorexia nervosa. A pilot study on the effects of bright light therapy. Neuro Endocrinol Lett, 31(3), 290–296. [PubMed] [Google Scholar]
- Dauphinais DR, Rosenthal JZ, Terman M, DiFebo HM, Tuggle C, & Rosenthal NE (2012). Controlled trial of safety and efficacy of bright light therapy vs. negative air ions in patients with bipolar depression. Psychiatry Res, 196(1), 57–61. doi: 10.1016/j.psychres.2012.01.015 [DOI] [PubMed] [Google Scholar]
- Deltito JA, Moline M, Pollak C, Martin LY, & Maremmani I (1991). Effects of phototherapy on non-seasonal unipolar and bipolar depressive spectrum disorders. J Affect Disord, 23(4), 231–237. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Young MA, Fogg LF, Liu L, & Meaden PM (1998). Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry, 55(10), 883–889. [DOI] [PubMed] [Google Scholar]
- Even C, Schroder CM, Friedman S, & Rouillon F (2008). Efficacy of light therapy in nonseasonal depression: a systematic review. J Affect Disord, 108(1–2), 11–23. doi: 10.1016/j.jad.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Excellus BlueCross BlueShield Health Insurance Plans. (2015). Retrieved from https://www.excellusbcbs.com/wps/wcm/connect/80907d63-2a83-45e7-b23fa35d55ab2f04/mp+photo_sad+mpc3+15.pdf?MOD=AJPERES&CACHEID=80907d63-2a83-45e7-b23f-a35d55ab2f04
- Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, … Nemeroff CB (2005). The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry, 162(4), 656–662. doi: 10.1176/appi.ajp.162.4.656 [DOI] [PubMed] [Google Scholar]
- Gruber R, Grizenko N, & Joober R (2007). Delayed sleep phase syndrome, ADHD, and bright light therapy. J Clin Psychiatry, 68(2), 337–338. [DOI] [PubMed] [Google Scholar]
- Guzel Ozdemir P, Boysan M, Smolensky MH, Selvi Y, Aydin A, & Yilmaz E (2015). Comparison of venlafaxine alone versus venlafaxine plus bright light therapy combination for severe major depressive disorder. J Clin Psychiatry, 76(5), e645–654. doi: 10.4088/JCP.14m09376 [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, … Yau KW (2003). Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature, 424(6944), 76–81. doi: 10.1038/nature01761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas-Kozik M, Krzystanek M, Stachowicz M, Krupka-Matuszczyk I, Janas A, & Rybakowski JK (2011). Bright light treatment of depressive symptoms in patients with restrictive type of anorexia nervosa. J Affect Disord, 130(3), 462–465. doi: 10.1016/j.jad.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Kanofsky JD, Aspengren KL, & Watts GR (2003). Medicaid reimbursement for light therapy. Am J Psychiatry, 160(4), 796–797. [DOI] [PubMed] [Google Scholar]
- Koorengevel KM, Gordijn MC, Beersma DG, Meesters Y, den Boer JA, van den Hoofdakker RH, & Daan S (2001). Extraocular light therapy in winter depression: a double-blind placebo-controlled study. Biol Psychiatry, 50(9), 691–698. [DOI] [PubMed] [Google Scholar]
- Lam RW, Gorman CP, Michalon M, Steiner M, Levitt AJ, Corral MR, … Joffe RT (1995). Multicenter, placebo-controlled study of fluoxetine in seasonal affective disorder. Am J Psychiatry, 152(12), 1765–1770. doi: 10.1176/ajp.152.12.1765 [DOI] [PubMed] [Google Scholar]
- Lam RW, Levitt AJ, Levitan RD, Michalak EE, Cheung AH, Morehouse R, … Tam EM (2016). Efficacy of Bright Light Treatment, Fluoxetine, and the Combination in Patients With Nonseasonal Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry, 73(1), 56–63. doi: 10.1001/jamapsychiatry.2015.2235 [DOI] [PubMed] [Google Scholar]
- Levitan RD, Kaplan AS, Levitt AJ, & Joffe RT (1994). Seasonal fluctuations in mood and eating behavior in bulimia nervosa. Int J Eat Disord, 16(3), 295–299. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, & Bauer VK (2006). The circadian basis of winter depression. Proc Natl Acad Sci U S A, 103(19), 7414–7419. doi: 10.1073/pnas.0602425103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson B, Pettersson A, Berglund L, & Ekselius L (2015). Bright white light therapy in depression: A critical review of the evidence. J Affect Disord, 182, 1–7. doi: 10.1016/j.jad.2015.04.013 [DOI] [PubMed] [Google Scholar]
- Mersch PP, Middendorp HM, Bouhuys AL, Beersma DG, & van den Hoofdakker RH (1999). The prevalence of seasonal affective disorder in The Netherlands: a prospective and retrospective study of seasonal mood variation in the general population. Biol Psychiatry, 45(8), 1013–1022. [DOI] [PubMed] [Google Scholar]
- Miller AM, Miller RB, Obermeyer WH, Behan M, & Benca RM (1999). The pretectum mediates rapid eye movement sleep regulation by light. Behav Neurosci, 113(4), 755–765. [DOI] [PubMed] [Google Scholar]
- Miller AM, Obermeyer WH, Behan M, & Benca RM (1998). The superior colliculuspretectum mediates the direct effects of light on sleep. Proc Natl Acad Sci U S A, 95(15), 8957–8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, & Speh JC (2004). Serotonin innervation of the primate suprachiasmatic nucleus. Brain Res, 1010(1–2), 169–173. doi: 10.1016/j.brainres.2004.02.024 [DOI] [PubMed] [Google Scholar]
- Moscovitch A, Blashko CA, Eagles JM, Darcourt G, Thompson C, Kasper S, & Lane RM (2004). A placebo-controlled study of sertraline in the treatment of outpatients with seasonal affective disorder. Psychopharmacology (Berl), 171(4), 390–397. doi: 10.1007/s00213-003-1594-8 [DOI] [PubMed] [Google Scholar]
- Niederhofer H, & von Klitzing K (2012). Bright light treatment as mono-therapy of nonseasonal depression for 28 adolescents. Int J Psychiatry Clin Pract, 16(3), 233–237. doi: 10.3109/13651501.2011.625123 [DOI] [PubMed] [Google Scholar]
- Oldham MA, & Ciraulo DA (2014). Bright light therapy for depression: a review of its effects on chronobiology and the autonomic nervous system. Chronobiol Int, 31(3), 305–319. doi: 10.3109/07420528.2013.833935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren DA, Koziorowski M, & Desan PH (2013). SAD and the not-so-single photoreceptors. Am J Psychiatry, 170(12), 1403–1412. doi: 10.1176/appi.ajp.2013.13010111 [DOI] [PubMed] [Google Scholar]
- Padeh Y (1999). Seasonal Affective Disorder: The Theories and Future of Light Therapy. Einstein Quart. J. Biol. Med, 16, 78–83. [Google Scholar]
- Pail G, Huf W, Pjrek E, Winkler D, Willeit M, Praschak-Rieder N, & Kasper S (2011). Bright-light therapy in the treatment of mood disorders. Neuropsychobiology, 64(3), 152–162. doi: 10.1159/000328950 [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, … Kay SA (2002). Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science, 298(5601), 2213–2216. doi: 10.1126/science.1076848 [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, & Willeit M (2003). Treatment of seasonal affective disorders. Dialogues Clin Neurosci, 5(4), 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, Willeit M, Wilson AA, Houle S, & Meyer JH (2008). Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry, 65(9), 1072–1078. doi: 10.1001/archpsyc.65.9.1072 [DOI] [PubMed] [Google Scholar]
- Roenneberg T, & Foster RG (1997). Twilight times: light and the circadian system. Photochem Photobiol, 66(5), 549–561. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, … Wehr TA (1984). Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry, 41(1), 72–80. [DOI] [PubMed] [Google Scholar]
- Rybak YE, McNeely HE, Mackenzie BE, Jain UR, & Levitan RD (2006). An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry, 67(10), 1527–1535. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Kalsbeek A, & Buijs RM (2003). Cardiovascular control by the suprachiasmatic nucleus: neural and neuroendocrine mechanisms in human and rat. Biol Chem, 384(5), 697–709. doi: 10.1515/bc.2003.078 [DOI] [PubMed] [Google Scholar]
- Stehle JH, von Gall C, & Korf HW (2003). Melatonin: a clock-output, a clock-input. J Neuroendocrinol, 15(4), 383–389. [DOI] [PubMed] [Google Scholar]
- Tam EM, Lam RW, Robertson HA, Stewart JN, Yatham LN, & Zis AP (1997). Atypical depressive symptoms in seasonal and non-seasonal mood disorders. J Affect Disord, 44(1), 39–44. [DOI] [PubMed] [Google Scholar]
- Tseng PT, Chen YW, Tu KY, Chung W, Wang HY, Wu CK, & Lin PY (2016). Light therapy in the treatment of patients with bipolar depression: A meta-analytic study. Eur Neuropsychopharmacol, 26(6), 1037–1047. doi: 10.1016/j.euroneuro.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Willeit M, Sitte HH, Thierry N, Michalek K, Praschak-Rieder N, Zill P, … Singer EA (2008). Enhanced serotonin transporter function during depression in seasonal affective disorder. Neuropsychopharmacology, 33(7), 1503–1513. doi: 10.1038/sj.npp.1301560 [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Bader A, Frisch U, Stieglitz RD, Alder J, Bitzer J, … Riecher-Rossler A (2011). A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry, 72(7), 986–993. doi: 10.4088/JCP.10m06188blu [DOI] [PubMed] [Google Scholar]