Abstract
Objectives
To determine and compare the clinical and immunologic outcomes for HIV-infected women initiated on antiretroviral therapy, with and without previous exposure to single dose nevirapine in the MTCT plus program-Kampala, Uganda from 2003 to2011.
Methods
This was a retrospective comparison of prospectively collected programmatic data of clinical and immunologic treatment outcomes among HIV-infected Ugandan women, with and without prior exposure to sdNVP, who received NNRTI-based antiretroviral therapy for a median follow-up of six years.
Results
Of the 408 women in the program, 289(70.8%) were started on ART, of whom 205 (70.9 %) had prior exposure to sdNVP. Clinical, immunologic, and combined (clinical and or immunological) treatment failure occurred in 29(10.0%), 132(45.7%) and 142(49.1%) women, respectively. There was no significant difference in the distribution of time to immunologic failure for women by exposure to sdNVP (log-rank p=0.98). In Cox proportional hazard modeling, exposure to sdNVP was not associated with immunologic failure [adjusted hazard ratio (HR) = 0.89, 95% confidence interval (CI): 0.61–1.30]. CD4 count>100 cells/mm3 at initiation was associated with reduced incidence of immunologic failure in adjusted analyses [HR=0.32, 95% CI: 0.22–0.48].
Conclusions
HIV infected Ugandan women initiated on a NVP based ART regimen had similar immunological treatment outcomes irrespective of previous NVP exposure. CD4 cell count prior to initiating HAART was a key prognostic factor for successful long term immunologic treatment outcomes. In resource limited settings, regular follow-up of patients on HAART with adequate counselling to promote adherence and safe disclosure may promote low clinical failure rates.
Introduction
The Uganda’s prevention of mother to child transmission (PMTCT) of HIV guidelines have evolved over time. More efficacious ART regimens have been introduced, replacing sdNVP which was emphasized during 2000–2005 (MOH Uganda 2009). However progress toward full coverage of PMTCT has been slow; by the end of 2009 only 53% of women and their infants accessed PMTCT services and only half of these received combination short-course ART (WHO/UNAIDS/UNICEF (2010)). The low PMTCT coverage, coupled with late first antenatal visit and drug stock-out, may result in some women receiving only sdNVP instead of more efficacious regimens recommended for PMTCT.
Exposure to sdNVP prior to ART initiation has been associated with poor treatment outcomes in some but not all studies, particularly when women initiate treatment within the first 12–18 months after sdNVP exposure (Jourdain. G, et al., 2004; Lockman, et al., 2007). Chi et al. (2007), found no difference in mean CD4 cell change by exposure to NVP at 6 and 12 months after HAART initiation(Benjamin H Chi, et al., 2007). A related study in Côte d’Ivoire also showed similar increases in CD4+ T cell count after 36 months of follow-up in women irrespective of sdNVP exposure (Ekouevi D. K., et al., 2010).
We analyzed data for women on ART enrolled in the MTCT plus family care program at Mulago National Referral Hospital-Kampala to observe the impact of sdNVP on long-term maternal immunologic and clinical treatment outcomes within the Ugandan context where sdNVP has been widely used. Many women received sdNVP for PMTCT, and others did not, providing an opportunity to study differences in treatment outcomes associated with sdNVP exposure. The main objective was to determine the immunologic failure and combined clinical and immunologic failure after initiating ART treatment.
Methods
Study design
This was a retrospective analysis using data from a longitudinally followed Uganda cohort of HIV-infected women to evaluate the clinical, immunologic and combined ART treatment outcomes among HIV-infected women in an HIV program within a context of scaling up of PMTCT 2003–2011.
Study setting and population
At Mulago Hospital HIV-infected women identified through the PMTCT program or perinatal studies, were referred to the MTCT Plus program for HIV/AIDS care. The program was run by the Makerere University-Johns Hopkins University (MU-JHU) Research Collaboration with technical support from Columbia University, USA (Toro P. L., et al., 2012). From March-2003 to October-2007, HIV-infected women and their families were enrolled into the MTCT plus program to receive long-term HIV/AIDS care. At enrolment demographic, social, clinical, and laboratory data were collected. For this analysis, women had to have initiated ART by December 31, 2010. We evaluated immunologic and clinical outcomes of 289 women during the follow-up period March 2003 to December 31, 2011.
Exposure to single dose nevirapine
Uganda PMTCT guidelines evolved during this study. The exposed women received a 200mg tablet of sdNVP for PMTCT at onset of labor either 1) alone when HIV-infection was identified during labor, 2) after receiving short-course ZDV (300mg bd), starting at 28–36 weeks’ gestation, 3) after receiving of short-course ZDV plus 3TC(150mg bd), initiated from 33 weeks’ gestation and continued until 7 days post-delivery (MOH(a) Uganda 2003; MOH(c) Uganda 2008). Details on the procedure of how sdNVP was administered in the PMTCT program has been previously described (Namukwaya, et al., 2011).
Antiretroviral initiation
Participants initiated antiretroviral therapy in accordance with World Health Organization (WHO) and Ugandan Ministry of Health (MOH) ART eligibility criteria(MOH(a) Uganda 2003): 1) WHO stage 4 disease regardless of CD4+ T-lymphocyte count, or 2) WHO stage 3 plus CD4+ T-lymphocyte count<350 cells/mm3, or 3) CD4+ T-lymphocyte count<200 cells/mm3, or 4) Tuberculosis and CD4 cell count between 200–350/mm3. First line HIV treatment were a backbone of 1) zidovudine (ZDV) (300mg) and lamivudine (3TC) (150mg) bd or 2) 3TC (150mg) and stavudine (D4T) (30mg) twice daily, plus NVP (200mg) od for two weeks escalated to bd) or efavirenz (EFV) (600mg) od. Twenty-five (8.7%) patients started on D4T switched to ZDV or tenofovir (TDF) (300mg) od in accordance with WHO and Ugandan MOH revised guidelines for ART treatment(MOH(a) Uganda 2003; MOH(c) Uganda 2008)
Follow-up
At monthly visits, patients were seen by a nurse for vital signs, a counselor to discuss psychosocial support, disclosure status and adherence. At the same visit, the physician would obtain an interval history, and perform a physical examination, record new diagnoses, and encourage the patients to return in case of inter-current illness or side-effects. Data was recorded in patient charts and entered into a central database by trained clerks after each visit. For women who missed visits, telephone calls or home visits were made by health visitors to establish reasons. If a death occurred outside the hospital, the health visitor would conduct a verbal autopsy. The CD4 cell counts were enumerated at enrolment and every six months thereafter using the BD FACS Caliber instrument (Becton, Dickinson & Company). HIV viral load and drug resistance testing were not performed in MTCT-Plus program. All laboratory tests were performed at MU-JHU Core Laboratory, Uganda, which is certified by the College of American Pathologists.
Adherence
Adherence was assessed by patient self-report of number of pills ingested during the last six days prior to clinic visit. Adherence was reinforced with patient targeted, pre-approved unannounced home visits. The majority of clients had high (≥95% of pills) adherence documented during the earlier phase of the program (Byakika-Tusiime, et al., 2009). Patients with poor adherence (<95%) were offered special adherence counseling to supplement routine counselors’ visits.
Study outcomes and other measurements
The primary outcomes were clinical, immunologic and combined treatment failure. Clinical failure was defined as a new OI of WHO stage 3 or 4 , and/or death at least six months after initiating ART with assumption of good adherence (MOH(a) Uganda 2003; World Health Organization 2007) Death was ascertained from verbal autopsy or hospital records. Immunologic failure was defined as: 1) fall of CD4 cell count to baseline; 2) 50% fall from on-treatment peak, or 3) persistent CD4 cell count100 cells/mm3 at least six months after ART initiation. Combined failure was defined as having either or both clinical and immunologic failure.
Statistical analysis
Patients’ characteristics were compared between groups (sdNVP-exposed verses non-exposed) using Pearson’s chi-square (or Fishers’ exact test for sparse data) for categorical variables and t-tests (Wilcoxon rank sum test if non-normal distributions) for continuous variables. Kaplan-Meier estimates were used to describe distributions of time to event endpoints. If a patient experienced more than one event, time was counted to the first occurrence. All available follow-up data was included, censoring at loss to follow-up, transfer out of the program, or on December 31, 2011, whichever came first. Hazard ratio estimates from Cox regression models were used to quantify differences in outcomes by receipt of sdNVP at labor/delivery for PMTCT, in both unadjusted and multiple regression models. Variables with p-value<0.2 or previously known to have clinical association to treatment failure were considered for multiple Cox regression analysis (previous sdNVP exposure, baseline CD4 count, WHO stage, parity and disclosure to partner). All testing was two-sided at 5% significance level. Hazard ratios were reported with 95% confidence intervals (CI). Analyses were performed in SAS version 9.1 and Intercooled STATA version 10.
Ethical clearance
After removing patient identifiers from collected clinical care data, the study was exempted from human subject review by the Uganda National Council of Science and Technology and University of Medicine and Dentistry of New Jersey Institutional Review Board.
Results
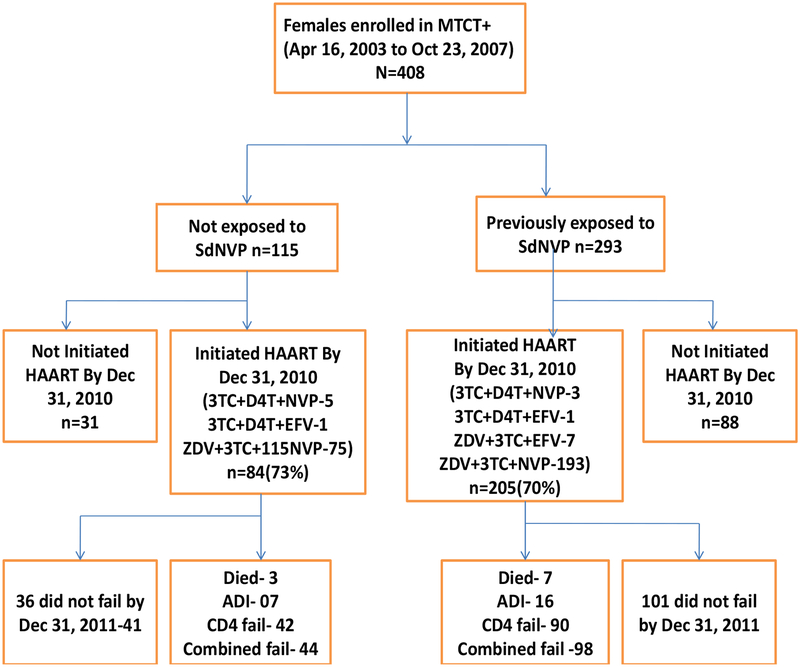
From 2003, 408 women were followed up in MTCT plus program; and by December 31, 2010, 289 women had initiated ART and were followed for median duration of 6.0 years (interquartile range (IQR) 3.6–7.1). The majority, 205(71%) of women on ART were previously exposed to sdNVP for PMTCT and 84 (29%) were not (Figure 1). Fourteen (3.4 %) women were lost to follow-up during the study period. Women exposed to sdNVP were younger than women who were not exposed (p=0.003). More unexposed women were classified as WHO stage 3&4 compared to sdNVP-exposed women (p=0.009). There were no significant differences in sdNVP-exposed and unexposed women’s baseline CD4 cell counts and other baseline characteristics (Table 1). At ART initiation, women exposed to sdNVP, had a median (IQR) duration of 27.9 (9.0–56.8) months since NVP exposure; 93 (45.4%) women had ≤24 months and 112 (54.6%)>24 months since last exposure. The vast majority (96.9%) of women initiated NVP-based ART regimens.
Figure 1:
Flow chart showing disposition of women over the six years of the study
Table 1:
Baseline characteristics for HIV-infected women (N=289) enrolled in the Mulago MTCT plus program, April, 2003 to October 2007 and initiated on antiretroviral therapy by December, 2010
| Baseline Characteristics | Exposed to Nevirapine N=205 | Non Exposed to Nevirapine N=84 | Total N=289 |
P-value |
|---|---|---|---|---|
| Age years: median (IQR) | 28.5(26–32) | 30(28–34) | 29(26–33) | 0.005 |
| Baseline CD4+ cells/mm3: median (IQR) | 175.5(128–198) | 175.5(106–208) | 175.5(124–208) | 0.56 |
| Type of ART regimen initiated: n (%) | ||||
| Started on NVP based regimen | 197(96.1) | 83(98.8) | 280(96.9) | 0.23 |
| Started on EFV based regimen | 8(3.8) | 1(1.2) | 9(3.1) | |
| WHO stage: n (%) | ||||
| 1 | 42(20.5) | 14(16.7) | 56(19.4) | 0.009 |
| 2 | 100(48.8) | 32(38.0) | 132(45.7) | |
| 3 | 55(26.8) | 26(31) | 81(28.0) | |
| 4 | 8(3.9) | 12(14.3) | 20(6.9) | |
| Disclosure status to partner (n=286) : n (%) | ||||
| Yes | 137(66.8) | 54(66.7) | 191(66.8) | 0.98 |
| No | 68(33.2) | 27(33.3) | 95(33.2) | |
| Duration spent in school (years): median (IQR) | 8(6–11) | 9(6–11) | 8(6–11) | 0.57 |
| Parity (number of pregnancies) at enrolment: median (IQR) years | 3 (2–5) | 3(2–5) | 3(2–5) | 0.62 |
| Marital status (n=286): n (%) | ||||
| Married | 115(56.1) | 47(58.0) | 162(56.5) | |
| Not married | 90(43.9) | 34(42) | 125(43.5) | 0.77 |
| Duration since last NVP exposure to initiation of treatment (months): median (IQR) | 28(9.0–56.8) |
IQR: interquartile range
Clinical failure
Among the 84 women unexposed to sdNVP, seven (8.3%) experienced new OIs and three (3.6%) died. One woman who developed a new OI, died later; therefore nine (10.7%) sdNVP-exposed women experienced clinical failures. In comparison, among the 205 women exposed to sdNVP, 16 (7.8%) experienced new OIs and seven (3.4%) died; three of those with new OIs died, 20 (9.8%) had a clinical event. The clinical failure rates did not differ by sdNVP exposure (log-rank p=0.88). The OIs included severe bacterial infection (9, 39.1%), pulmonary tuberculosis (5, 21.7%), grade IV wasting (3, 13.0%), Kaposi’s sarcoma (2, 8.7%), extra-pulmonary tuberculosis (2, 8.8%), suspected disseminated candidiasis (1, 4.3%), and Pneumocystis jiroveci pneumonia (PCP) (1, 4.3%).
Immunologic (CD4 cell count) failure
In total 132/289 women, including 90/205 (43.9%) of sdNVP-exposed and 42/84 (50.0%) unexposed women, experienced immunologic failure (log-rank p=0.33). Five of the 10 deaths occurred subsequent to immunologic failure. Among 90 sdNVP-exposed women with immunologic failure, 62 (68.9%) resulted from CD4 count dropping to half the peak, 26 (28.9%) from CD4 count returning to baseline level, and two (2.2%) from CD4 dropping to <100 cells/mm3. Of the 42 sdNVP non-exposed women classified as failing immunologically, 29 (69.0%) failed due to CD4 cell level dropping to half the peak, eight (19.0%) from CD4 count returning to baseline level, and five (12.0%) from to CD4 count dropping to <100 cells/mm3.
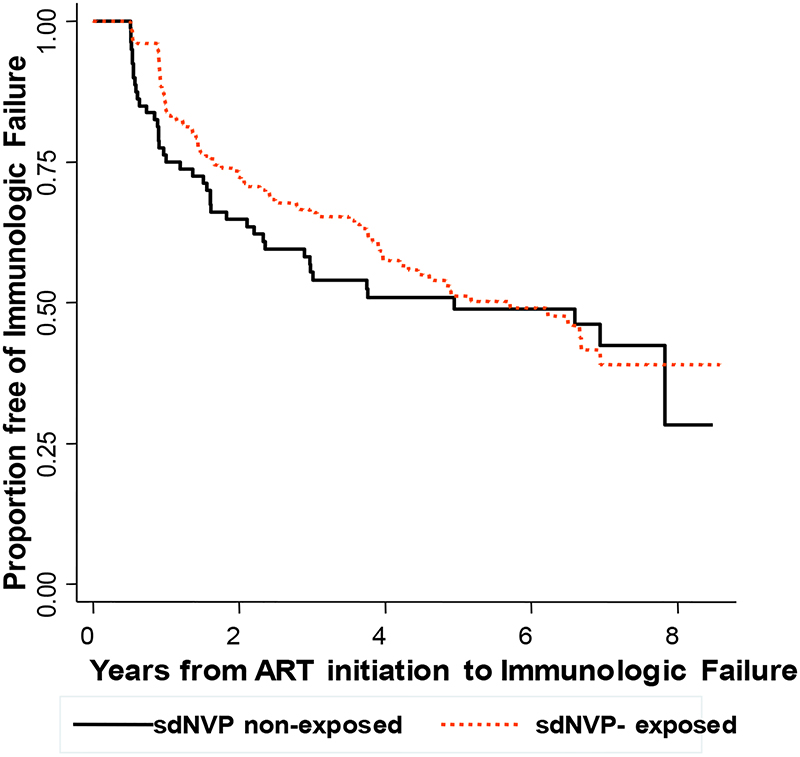
There was no significant difference in distributions of time to immunologic failure for women exposed and unexposed to sdNVP. For sdNVP-exposed women, the Kaplan-Meier estimates of the percentages with immunologic failure were 15.7%, 34.0%, 48.8%, and 51.0% at 1, 3, 5 and 6 years compared to 23.8%, 44.6%, 51.1%, and 51.1%, respectively, of unexposed women (log-rank p=0.88) (Figure 2).
Figure 2:
Kaplan-Meier estimate of the proportion of women without immunologic failure stratified by previous exposure to sdNVP
The median(IQR) CD4 cell count at six year follow-up was 466(324–614) cells/mm3 for the sdNVP-exposed group compared to 517(332–711)cells/mm3 for unexposed group (P-value=0.22).
In Cox models, exposure to sdNVP was not associated with increased risk of immunologic failure (unadjusted hazard ratio (HR), 0.86 (95% CI: 0.59–1.24) even after adjusting for baseline CD4 cell count, WHO stage, age and parity of woman, (adjusted HR 0.91 (95% CI: 0.62–1.33) (Table 2). Women with baseline CD4<100 cell/mm3 had increased risk of immunologic failure. WHO stage 3&4 and parity>3 were associated with increased hazard of immunologic failure before but not after adjustment.
Table 2:
Cox regression analysis to predict immunologic or combined clinical and immunologic outcome in HIV-infected women enrolled in the Mulago MTCT plus program, April, 2003 to October 2007 and initiated on antiretroviral therapy by December, 2010 using follow-up through December, 2011 (n=289)
| Immunologic Outcome | Combined Clinical and Immunologic Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Unadjusted | Adjusted | Unadjusted | Adjusted | ||||||||
| Hazard Ratio | [95% CI] | P-Value | Hazard Ratio | [95% CI] | P-Value | Hazard Ratio | [95% CI] | P-Value | Hazard Ratio | [95% CI] | P-Value | |
|
Previous sdNVP exposure NVP Exposed |
1(ref) | 1(ref) |
1 (ref) |
1 (ref) |
||||||||
| Non NVP Exposed | 0.85 | 0.59–1.23 | 0.39 | 0.89 | 0.61–1.3 | 0.58 | 0.88 | 0.62–1.26 | 0.45 | 0.93 | 0.64–1.34 | 0.71 |
|
Baseline CD4 (cells/mm3) ≤100 |
1(ref) | 1(ref) |
1(ref) |
1 (ref) |
||||||||
| >100 | 0.30 | 0.20–0.43 | <0.001 | 0.32 | 0.22–0.48 | <0.001 | 0.31 | 0.21–0.45 | <0.001 | 0.34 | 0.23–0.51 | <0.001 |
|
WHO stage category 1&2 |
1(ref) | 1(ref) |
1(ref) |
1 (ref) |
||||||||
| 3&4 | 1.55 | 1.10–2.20 | 0.013 | 1.33 | 0.93–1.90 | 0.23 | 1.57 | 1.10–2.20 | 0.008 | 1.36 | 0.96–1.92 | 0.18 |
|
Mother’s age in years <30 |
1(ref) |
1(ref) |
||||||||||
| ≥30 | 1.06 | 0.75–1.50 | 0.75 | 1.01 | 0.72–1.40 | 0.99 | ||||||
|
Disclosed to partner Disclosed |
1(ref) |
1(ref) |
||||||||||
| Not disclosed | 1.10 | 0.76–1.57 | 0.62 | 1.04 | 0.73–1.47 | 0.83 | ||||||
|
Marital status Married |
1(ref) | 1(ref) | ||||||||||
| Not married | 1.13 | 0.80–1.60 | 0.49 | 1.05 | 0.75–1.46 | 0.78 | ||||||
|
Education level Primary |
1(ref) |
1(ref) |
||||||||||
| Above primary | 0.97 | 0.69–1.37 | 0.88 | 0.99 | 0.72–1.39 | 0.99 | ||||||
|
Parity
0–3 |
1(ref) |
1(ref) |
||||||||||
| > 3 | 1.47 | 1.04–2.10 | 0.031 | 1.14 | 0.8–1.62 | 0.18 | 1.39 | 1.00–1.95 | 0.051 | 1.13 | 0.81–1.59 | 0.21 |
ref: reference category CI: confidence interval. We adjusted for variables with p<0.2 in bivariate analyses or those known to have association with immunologic and clinical failure.
Among sdNVP-exposed women, the risk of immunologic failure was reduced in the 113 women with remote (>24months) exposure to sdNVP compared to more recent exposure [HR= 0.56 (95% CI: 0.36–0.87), p=0.009]. This risk of failure was less significant (HR=0.71 (95% CI: 0.45–1.12), p=0.14) after adjusting for baseline CD4 cell count.. Similar findings were observed when using 12 and 18 months as cutoff values for defining remote exposure.
Combined failure
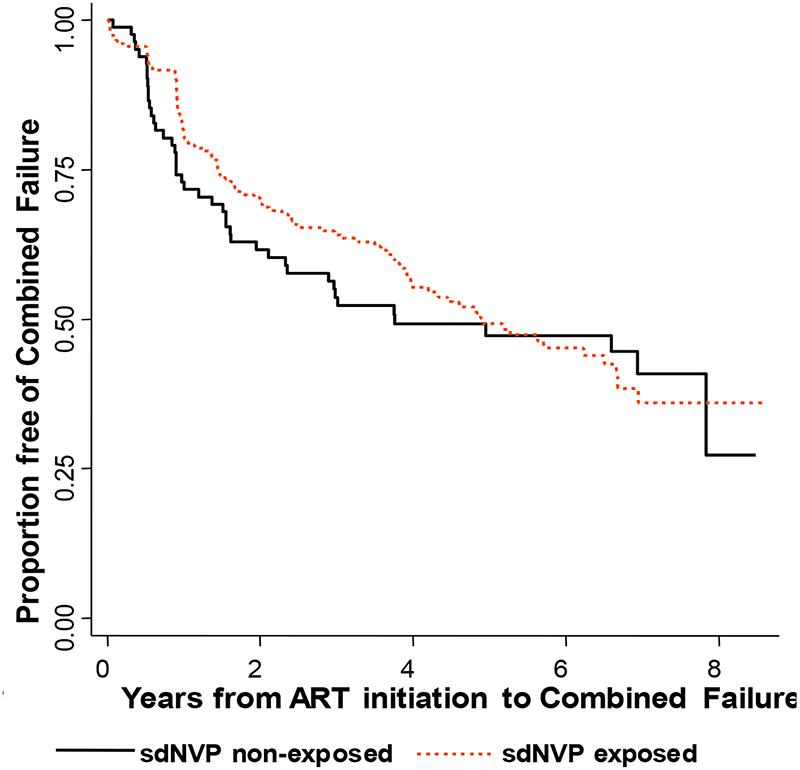
A total of 142/289 women failed treatment based on either immunological or clinical failure which only included 10 additional endpoints over the immunologic failure endpoint. Among the sdNVP-exposed women, 90/205 had combined failure compared to 42/84 unexposed (log-rank p=0.35For sdNVP-exposed women the Kaplan-Meier estimates of the percentage with combined failure were 19.3%, 35.8%, 50.7%, and 54.7% compared to 27.1%, 46.4%, 52.8%, and 52.8% of unexposed women at 1, 3, 5, and 6 years, respectively (log-rank p=0.98) (Figure 3). Cox modeling showed no significant difference in combined outcomes between groups (Table 2).
Figure 3:
Kaplan-Meier estimate of the proportion of women without combined failure (death or AIDS defining illness or immunological failure) stratified by previous exposure to sdNVP
For sdNVP-exposed women, risk of combined failure was lower in those who had remote exposure (>24month) to sdNVP, versus women with more recent exposure [HR= 0.66 (95% CI: 0.44–0.99), p=0.048] but not after adjusting for baseline CD4 cell count [HR=0.71(95%CI: 0.55–1.32), p=0.48].
Discussion
About half of the women initiated on a NNRTI- containing regimen, experienced treatment failure six years after ART initiation by immunologic or combined immunologic and clinical criteria. Failure rates and distributions were similar using the two outcomes because of the small numbers of women who failed clinically without first failing immunologically. There was no difference in the distributions of immunologic or combined failure in women by previous exposure to sdNVP for PMTCT. Our findings are similar to previous studies that found no difference in rates of failure by sdNVP-exposure after one year of ART (Benjamin H. Chi, et al., 2007; Ekouevi D. K., et al., 2010).
Of importance, the data demonstrate high rate of immunologic and combined failure which is similar to that shown in a large antiretroviral treatment program in Nigeria (Rawizza, et al., 2011), but differs from other studies that demonstrated only mild declines in CD4+ counts (Lifson, et al., 2011; Mugyenyi, et al., 2010). The difference noted in our study may have arisen from the variation in study populations, ART regimen/previous exposure to sdNVP, and definition of immunologic treatment outcomes. For example, unlike in the DART trial (Mugyenyi, et al., 2010), our participants were all female. In addition, few(3%) of the DART participants had previous exposure to sdNVP, only 7% initiated NVP-based regimens, with the majority (85%) on ZDV, 3TC plus tenofovir disoproxil fumarate or abacavir, compared to our study where over 71% were sdNVP-exposed, 96% initiated on NVP-based ART regimen and none started on PI or triple NRTI based ARVs. Despite all these differences, our results of clinical outcomes were similar to those in DART trial. Our study is unique in that we have substantially longer follow-up compared to most of the previous studies.
NNRTI treatment failure seen among HIV-infected women exposed to sdNVP has been shown to depend in part on the time interval since exposure to ART initiation (Benjamin H. Chi, et al., 2007). Women starting NNRTI-based regimens shortly after sdNVP exposure are reported to have increased risk of immunologic failure compared to those who delayed initiation (Benjamin H. Chi, et al., 2007; Ekouevi D. K., et al., 2010).The prolonged interval between sdNVP exposure and treatment initiation (median of 26 months in our study) may be responsible for lack of a difference in failure rates between the groups, since waning of resistance mutations occurs over time. Reduction in risk of immunologic failure in women with remote exposure (≥24 months) may have resulted from the strong confounding effect of the CD4 count at ART initiation.
Our findings are consistent with previous studies conducted in HIV-infected women starting HAART after exposure to sdNVP and followed for shorter durations (≤2 years) (Benjamin H Chi, et al., 2007; Ekouevi D. K., et al., 2010; Jourdain. G, et al., 2004). Currently WHO adult HIV treatment guidelines recommend initiation of NNRTI-based ART in women exposed to sdNVP, with or without an NRTI tail, only if the duration since prior exposure is > 12 months and then monitoring with viral load testing at six months(WHO (2009)). In case viral load testing is not available, WHO recommends continued monitoring using clinical criteria plus CD4 cell count. Because viral load testing was not routinely conducted in Uganda during the study timeframe, we cannot ascertain when failing women might have first demonstrated evidence of virologic failure. Nevertheless, our long term data demonstrate that women who initiate NNRTI-based ART after sdNVP exposure, with remote duration since sdNVP exposure, may have similar longer term clinical and immunologic outcomes as unexposed women. Even after adoption of the WHO-PMTCT option B-plus, PMTCT coverage in Uganda falls short of nationwide coverage. Hence, these results are useful in Uganda, where a large number of HIV-infected women may have received only sdNVP, with or without NRTI tail, for PMTCT and later initiate a NNRTI-based ART.
There were certain limitations to this study. It was observational and non-randomized, so there may have been differences in risk factors for disease progression that could have affected the results. We tried to address this concern by adjusting for known risk factors using Cox regression models when comparing women who did or did not receive sdNVP. Also, integrity of recorded data collected mainly for patient management may be less robust than clinical trial data due to less strict quality control of recorded data, and/or ascertainment of outcomes. Further, given the sample size of women available over the median six years of follow-up, comparison of treatment outcomes between sdNVP-exposed and unexposed women may have been underpowered to detect small differences; the sample size of 289 had a 76% power of detecting hazard ratio of 2.0 between groups, at the 5% significance level.
Given these caveats there were a number of strengths to the study including longer follow-up compared to previous studies, high self-reported adherence to taking ARVs as well as high retention seen in Mulago MTCT Plus program. We attribute this high retention and adherence to the MTCT-plus family model of care which has been observed in other settings to enhance disclosure and provide strong family support for women. This study also provides insight into long term treatment outcomes in cohorts of HIV-infected women in resource limited settings such as Uganda.
Summary and Conclusions
After a median of six years follow-up after initiation of NNRTI-based treatment regimens, half of the women enrolled in a family model of HIV care, failed immunologically, with similar failure rates irrespective of the women’s prior exposure to sdNVP. This study also highlights that CD4 cell count prior to initiating ART is a key prognostic factor for long term HAART treatment outcomes. Use of viral load as it becomes more available will be useful in elucidating reasons for the high failure rates in this and similar cohorts. With viral load and ART resistance testing for monitoring treatment outcomes still limited in resource constrained settings, ART programs should have regular follow-up of patients on HAART, have adequate counselling to promote treatment adherence and safe disclosure to minimize the high failure rates.
Acknowledgements
We would like to thank the clients who took part in the program and whose data were presented and the entire MTCT-Plus Initiative program staff at MUJHU. Deep appreciation is extended to Columbia University for the financial and technical support provided to the MTCT-Plus Initiative program since its inception, particularly Drs. Elaine Abrams and Patricia Toro.
Our gratitude goes to Dr. James Koola and the IMPAACT International Resource Committee Program for their support of New Investigators which helped support this work.
Footnotes
Declarations of conflict of interest
The authors have no conflicting interests to declare.
References:
- Benjamin H Chi, Sinkala M, Stringer EM, Cantrell RA, Mtonga V, Bulterys M, et al. (2007). Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS, 21(11), 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byakika-Tusiime J, Crane J, Oyugi JH, Ragland K, Kawuma A, Musoke P, et al. (2009). Longitudinal antiretroviral adherence in HIV+ Ugandan parents and their children initiating HAART in the MTCT-Plus family treatment model: role of depression in declining adherence over time. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. AIDS Behav, 13 Suppl 1, 82–91. [DOI] [PubMed] [Google Scholar]
- Ekouevi DK, Coffie PA, Chaix ML, Tonwe-Gold B, Amani-Bosse C, Leroy V, et al. (2010). Immunological response to highly active antiretroviral therapy following treatment for prevention of mother to child transmission of HIV-1: a study in Cote d’Ivoire. J Int AIDS Soc, 13, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain G, Ngo-Giang-Huong N, Le Coeur S, Bowonwatanuwong C, Kantipong P, Leechanachai P, et al. (2004). Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N engl J Med, 351(3), 229–240. [DOI] [PubMed] [Google Scholar]
- Lifson AR, Krantz EM, Eberly LE, Dolan MJ, Marconi VC, Weintrob AC, et al. (2011). Long-term CD4+ lymphocyte response following HAART initiation in a U.S. Military prospective cohort. AIDS Res Ther, 8(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, et al. (2007). Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med, 356(2), 135–147. [DOI] [PubMed] [Google Scholar]
- MOH Uganda 2009. National Antiretroviral Treatment and Care Guidelines for Adults, Adolscents and Children, 3rd Edition-June 2009. [Google Scholar]
- MOH(a) Uganda 2003. National Antiretroviral Treatment and Care Guidelines for Adults and Children, 1st Edition, August 2003 - Uganda. [Google Scholar]
- MOH(c) Uganda 2008. Policy guidelines for prevention of Mother-to-Child Transmission of HIV; Revised Edition August 2006; Circular update New Guidelines on Use of ARV for Prevention of Mother to Child Transmission of HIV (PMTCT) in Uganda; December 22, 2008. Republic of Uganda, Ministry of Health. [Google Scholar]
- Mugyenyi P, Walker AS, Hakim J, Munderi P, Gibb DM, Kityo C, et al. (2010). Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet, 375(9709), 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namukwaya Z, Mudiope P, Kekitiinwa A, Musoke P, Matovu J, Kayma S, et al. (2011). The impact of maternal highly active antiretroviral therapy and short-course combination antiretrovirals for prevention of mother-to-child transmission on early infant infection rates at the Mulago national referral hospital in Kampala, Uganda, January 2007 to May 2009. J Acquir Immune Defic Syndr, 56(1), 69–75. [DOI] [PubMed] [Google Scholar]
- Rawizza HE, Chaplin B, Meloni ST, Eisen G, Rao T, Sankale JL, et al. (2011). Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis, 53(12), 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro PL, Rabkin M, Flam R, El-Sadr W, Donahue M, Chadwick E, et al. (2012). Training Multidisciplinary Teams to Deliver High-Quality HIV Care to Families in Resource-Limited Settings: The MTCT-Plus Initiative Experience. J Assoc Nurses AIDS Care. [DOI] [PubMed] [Google Scholar]
- WHO (2009). Panel on Antiretroviral Guidelines for Adults and Adolescents:Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; December 1, 2009; 1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed (January 03, 2011). [Google Scholar]
- WHO/UNAIDS/UNICEF (2010). Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector Available at http://data.unaids.org/pub/Report/2007/20070925_oms_progress_report_en.pdf. [Google Scholar]
- World Health Organization 2007. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector: progress report, April 2007. Geneva:. [Google Scholar]