Abstract
The mechanism of arsenic-induced skin carcinogenesis is not yet fully understood. Chromosomal instability contributes to aneuploidy and is a driving force in carcinogenesis. Arsenic causes mitotic arrest and induces aneuploidy. hsa-miR-186 overexpression is associated with metastatic cancers as well as arsenic-induced squamous cell carcinoma and is reported to target several mitotic regulators. Decreased levels of these proteins can dysregulate chromatid segregation contributing to aneuploidy. This work investigates the potential aneuploidogenic role of hsa-miR-186 in arsenic carcinogenesis. Clones of immortalized human keratinocytes (HaCaT) stably transfected with a hsa-miR-186 expression or empty vector were isolated. Three clones with high and low hsa-miR-186 expression determined by RT-qPCR were selected for further analysis and cultured with 0 or 100 nM NaAsO2 for 8 weeks. Analysis of mitoses revealed that chromosome number and structural abnormalities increased in cells overexpressing hsa-miR-186 and were further increased by arsenite exposure. Double minutes were the dominant structural aberrations. The peak number of chromosomes also increased. Cells with >220 to >270 chromosomes appeared after 2 months in hsa-miR-186 overexpressing cells, indicating multiple rounds of endomitosis had occurred. The fraction of cells with increased chromosome number or structural abnormalities did not increase in passage matched control cells. Levels of selected target proteins were determined by western blot. Expression of BUB1, a predicted hsa-miR-186 target was suppressed in hsa-miR-186 overexpressing clones, but increased with arsenite exposure. CDC27 remained constant under all conditions. These results suggest that overexpression of miR-186 in arsenic exposed tissues likely induces aneuploidy contributing to arsenic-induced carcinogenesis.
Keywords: arsenic, carcinogenesis, skin cancer, miR-186, chromosomal instability, keratinocytes
1. Introduction
Arsenic is a pervasive natural water pollutant contaminating drinking water in both developing and developed countries (Polya, 2016). In central and western China, ground water and surface water are widely contaminated with arsenic (arsenic level in groundwater as high as 969 μg/L) and various arsenic metabolites were detected in local resident’s urine samples (Fu et al., 2014; Guo et al., 2016). Nearly twenty million Chinese residents consume arsenic-contaminated groundwater (>10 μg/L) (Rodriguez-Lado et al., 2013). As many as 70 million people living in the Ganges River delta region of Bangladesh and West Bengal, India are exposed to arsenic in drinking water exceeding 50 ppb (Rahman et al., 2001). In the U.S., it is estimated that as many as 3 million people are at risk of arsenic exposure from unregulated private well water with arsenic concentrations > 10 μg/L (Ayotte et al., 2017) and >200 million people are affected by arsenic-contaminated drinking water worldwide (Naujokas et al., 2013).
Skin is a major target organ for arsenic and skin lesions are specific signs of chronic arsenic poisoning, with clinical symptoms initiating within a few years of exposure. Arsenic-induced skin lesions usually begin with pigmentation anomalies, including hyperpigmentation, hypopigmentation, raindrop lesions and café au lait spots (Sarma, 2016). These non-malignant lesions are followed by pre-malignant hyperkeratosis, and then malignant basal cell carcinoma, Bowen’s disease and squamous cell carcinoma (Karagas et al., 2015). Epidemiological studies from different countries and regions worldwide have confirmed that arsenic exposure via drinking water causes skin cancers (Fu et al., 2014; Hsu et al., 2015; Karagas et al., 2015; Cheng et al., 2016). In rodents, chronic arsenic exposure in drinking water alone will not induce skin cancer, but will enhance carcinogenesis in two-stage models using dimethylbenzanthracene (DMBA) (Palmieri and Molinari, 2015) or ultraviolet light (Rossman et al., 2004) as initiators (Tokar et al., 2010). Thus, lacking animal models that recapitulate the human disease, one must use in vitro systems to elucidate the molecular mechanisms of arsenic-induced skin cancer.
The proposed modes of action in arsenic carcinogenesis include inhibition of DNA repair, co-mutagenicity, altered epigenetics (DNA methylation, miRNA expression), oxidative stress, and aneuploidy (reviewed in (States, 2015)). Aneuploidy results from chromosome instability, which can be either numerical or structural chromosomal abnormalities. Chromosome instability is not only an important driving force in carcinogenesis, but also an inherent feature of cancer genomes (Heng et al., 2013; Tanaka and Hirota, 2016; Sansregret and Swanton, 2017). Oncogene activation or tumor suppressor gene inactivation resulting from chromosomal abnormalities contributes to carcinogenesis (Gordon et al., 2012). The National Research Council reports on arsenic in drinking water (Council, 1999; Council, 2001) concluded that aneuploidy is a likely mechanism of arsenic carcinogenesis. However, aneuploidy as a major driver of arsenic-induced carcinogenesis has received little attention. Several investigations showed that arsenic induced aneuploidy in diploid fibroblasts and lymphocytes (Vega et al., 1995; Yih et al., 1997; Salazar et al., 2010). Arsenic induces mitotic arrest and arsenic-induced aneuploidy is associated with long mitotic delay in diploid human fibroblasts (Yih et al., 1997). A long standing conundrum is how an agent that causes mitotic arrest can induce cancer, which is a disease of uncontrolled cell proliferation (States, 2015). In some cancers, dysregulated miRNAs promote genomic and chromosomal instability by targeting the mitotic checkpoint or DNA damage repair components (Durrbaum et al., 2018). miRNAs are evolutionarily conserved non-coding small RNAs that regulate cell growth, development, differentiation and apoptosis through post-transcriptional regulation, and are important physiological and pathological regulatory molecules. It is well known that miRNAs play an important role in carcinogenesis acting as oncogenes or tumor suppressors, and are biomarkers of carcinogen exposure, hazard identification, and cancer development (Verma et al., 2015; Pogribny et al., 2016). Arsenic exposure can significantly change miRNA expression both in vitro and in vivo (Sturchio et al., 2014; Al-Eryani et al., 2017; Al-Eryani et al., 2018a; Al-Eryani et al., 2018b). Recently, hsa-miRNAs-21, −145, −155, and −191 were found increased in patients with skin arsenicism caused by coal-burning in China (Sun et al., 2017). Chronic arsenic exposure induced hsa-miR-21 expression and enhanced the invading potential by down-regulation of Programmed Cell Death 4 (PDCD4) in the malignant transformation of human bronchial epithelial cells (Luo et al., 2015). Additionally, Chen et al., (Chen et al., 2017) suggested that hsa-miR-155 may play a role in NRF2 signaling pathway regulation during arsenite-induced malignant transformation of human bronchial epithelial cells. We also found elevated expression of many miRNAs in squamous cell carcinoma induced by chronic arsenic exposure via drinking water, relative to non-malignant hyperkeratosis (Al-Eryani et al., 2018a). Interestingly, hsa-miR-186 was highly induced in some squamous cell carcinomas (States, 2015). hsa-miR-186 may serve a critical role in various biological processes, including cell development, proliferation and apoptosis, and may act as an oncogenic or tumor suppressor miRNA, depending on the tissue and context (Su et al., 2018). Securin is a target of hsa-miR-186 (Li et al., 2013), suggesting a possible mechanism to overcome arsenic inhibition of chromatid segregation (States, 2015). Abnormal expression of hsa-miR-186 occurs in metastatic melanoma (Qi et al., 2014) and metastatic pancreatic cancer tissues (Zhang et al., 2009; Zhang et al., 2015). Elevated hsa-miR-186 was found in the blood of melanoma (Leidinger et al., 2010) and oral squamous cell carcinoma (Ries et al., 2014) patients. A miRNA signature discriminating metastatic cancers from primary colon, bladder, breast and lung tumors included hsa-miR-186 (Baffa et al., 2009). Clearly, hsa-miR-186 has a likely role in carcinogenesis.
HaCaT cells are a spontaneously immortalized human keratinocyte line that is a well-established model used in the study of human skin cancer (Fusenig and Boukamp, 1998; Boukamp, 2005). Chronic exposure to sodium arsenite transforms HaCaT cells and these transformed cells are capable of forming malignant squamous cell carcinomas when transplanted into nude mice (Pi et al., 2008; Vlachos et al., 2015). We have adapted this model to investigate the potential aneuploidogenic role in arsenic carcinogenesis of hsa-miR-186 by overexpression in immortalized human keratinocytes (HaCaT). We show that ectopic expression of hsa-miR-186 in HaCaT cells induces marked increase in chromosome number and in structural abnormalities that are further enhanced with chronic arsenite exposure, and suppresses expression of BUB1 Mitotic Checkpoint Serine/Threonine Kinase (BUB1), a predicted hsa-miR-186 target.
2. Methods and Materials
2.1. Cell culture and arsenite exposure
HaCaT cells were the kind gift of the Dr. TaiHao Quan (University of Michigan Ann Arbor). The identity of the cells as HaCaT before and at the end of the experiments was confirmed by STR mapping (Genetica DNA Laboratories/LabCorp, Burlington, NC). HaCaT cells were cultured in alpha modification of minimal essential medium (α-MEM, Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (characterized, HyClone, Logan, UT, USA), 1% glutamine and 1% penicillin-streptomycin solution (GIBCO, Invitrogen, Carlsbad, CA, USA) (complete α-MEM) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Quadruplicates of HaCaT (with 0 or 100 nM sodium arsenite (CAS 7784-46-5, ThermoFisher Scientific, Waltham, MA, USA)) were maintained for 28 weeks and used for cytogenetic analyses (Fig. 2). HaCaT cells transfected with pEP-hsa-miR-186 Expression Vector or empty vector control were maintained with puromycin (0.9 μg/mL, P7255, Sigma-Aldrich, St Louis, MO, USA). The clones were isolated and characterized for hsa-miR-186 expression. We selected the three hsa-miR-186 transfectants with highest expression and the three empty vector transfectants with lowest expression for further study. Clones were then propagated independently for 8 weeks in complete α-MEM with 0 or 100 nM sodium arsenite.
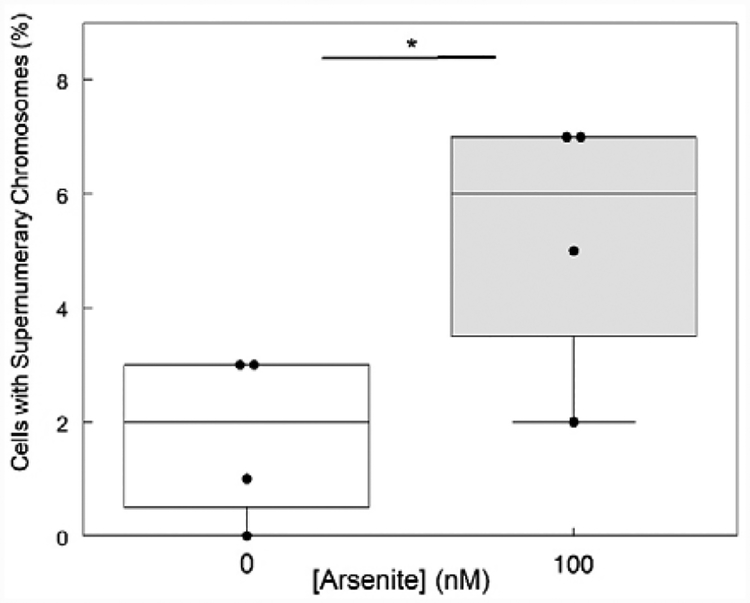
Fig. 2.
Aneuploidy increases in HaCaT cells exposed to sodium arsenite for 28 weeks. Quadruplicate HaCaT cultures incubated with 0 or 100 nM sodium arsenite for 28 weeks were processed for cytogenetic analysis as described in methods. Chromosome numbers were determined in 100 mitoses for each sample. Cells with >83 chromosomes were scored as supernumerary. * denotes significant difference (p < 0.05 by t-test).
2.2. Plasmid construction and cell transfection
MiRNASelect™ pEP-hsa-miR-186 Expression Vector was purchased from Cell Biolabs, Inc. (San Diego, CA, USA). Empty vector control was prepared by digestion of pEP-hsa-miR-186 with BamH1 and Nhe1, polishing the ends with T4 DNA polymerase and ligation with T4 DNA ligase. NEB 5-alpha Competent E. coli cells were transformed with plasmids following 5 Minute Transformation protocol (C2987H/C2987I). Individual colonies were isolated by culturing on LB-ampicillin plates and expanded in liquid LB culture overnight. Plasmid DNAs were purified using QIAGEN Plasmid Midi Kits according to the manufacturer’s instructions. Plasmid DNA concentrations were determined using NanoDrop® Spectrophotometer ND-1000 (ThermoFisher Scientific).
Cell transfection was conducted according to the Amaxa Cell line Nucleofector Kit V Protocol (Lonza, Basel, Switzerland). Briefly, cells (1×106) were seeded in a 15-cm dish. When ~80% confluent, cells were harvested and 1×106 cells were centrifuged at 100×g for 10 min at room temperature. Supernatant was removed and the cell pellet was resuspended carefully in 100 μL Nucleofector solution. Three micrograms of plasmid DNA of pEP-hsa-miR-186 Expression Vector or empty vector control were added to the above cell suspension. The cell/DNA suspension was transferred into a cuvette and Nucleofector Program U-020 was run. When the program was finished, 500 μL of complete α-MEM pre-equilibrated at 37°C were added to the cuvette immediately to collect the transfected cells and cells were seeded in 6-well plates, each well with ~66,000 cells. After 48 hr incubation, the media were replaced with complete α-MEM supplemented with 0.9 μg/mL puromycin to select stable clones transfected with hsa-miR-186 overexpression or empty vector. A single clone containing more than 64 cells was isolated from each well of the plates, propagated and characterized.
2.3. RNA extraction and real time reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using the miRVana™ miRNA Isolation Kit (Ambion, ThermoFisher Scientific) according to the manufacturer’s instructions. cDNA was synthesized with 10 ng of total RNA using the TaqMan miRNA Reverse Transcription Kit (Applied Biosystems), and expression of hsa-miR-186 was quantified with hsa-miR186-specific TaqMan miRNA Assay kit (002285) using StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). U6 snRNA (001973) levels were used for normalization.
RT-qPCR analysis of RNA extracted from laser capture microdissected keratinocytes from hyperkeratosis and squamous cell carcinoma samples was as described previously (Al-Eryani et al., 2018a).
2.4. Cytogenetic analysis
Cells were seeded into 10-cm dishes (8×105/dish) with 7 mL complete α-MEM. Cells were allowed to acclimate for 48 hr and re-enter normal cell cycle pattern. Demecolchicine solution (#D1925, Sigma, St. Louis, MO, USA) was added to each dish to make the final concentration 0.2 μg/mL. The cultures were then incubated at 37°C for 1 hr. Subsequently, cells were washed with phosphate buffered saline (PBS), dissociated with trypsin, transferred to 15 mL tubes and centrifuged to pellet cells at 180 × g for 5 min at 4°C. The pellets were resuspended and incubated in 10 mL 0.075 M KCl solution for 17 min at room temperature. At the end of 17 min, 1 mL of freshly mixed methanol-glacial acetic acid (3:1, v:v) was added to the tubes and centrifuged at 200 × g for 5 min. Pellets were suspended gradually in 10 mL methanol-acetic acid (3:1) and incubated at room temperature for 20 min, collected by centrifugation and resuspended in methanol-acetic acid. The cells were then pipetted onto microscope slides to give a suitable density of metaphases for microscopic analysis. The slides were stained with 5% Giemsa for 5 min and then washed several times in deionized water, dried overnight and sealed with microscope cover glass. For chromosome analysis, 100 metaphase cells were analyzed for each clone. Cells with >83 chromosomes were scored as supernumerary.
2.5. Western blot analysis
Western blotting was performed according to standard procedures. Briefly, total protein was extracted from the cells with lysis solution (10 mM Tris – HCl pH 7.4, 1 mM Na2- EDTA, 0.1 % SDS, 10 mM Na-Vanadate, 1 mM PMSF and 1 X protease inhibitor cocktail (Complete, Roche, Mannheim, Germany). Aliquots (50 μg) of total protein extracts were resolved by electrophoresis in sodium dodecyl sulfate 7% polyacrylamide gels (SDS-PAGE) and electroblot transferred to polyvinylidene fluoride membranes (PVDF, Millipore). The membranes were then incubated with antibodies against BUB1 (1:1000; #ab195268, Abcam, Cambridge, United Kingdom), CDC27 (1:1000; #ab129085, Abcam) and β-Actin (1:20000, A5441, Sigma-Aldrich). Subsequently, the membranes were incubated with either anti-rabbit (1:1000; #7074, Cell Signaling Technology, Danvers, MA, USA) or anti-mouse (1:10000; #7076, Cell Signaling Technology) HRP-linked antibodies. Signals were detected using the enhanced chemiluminescence western blotting system (ThermoFisher Scientific). Densitometry was performed using Image Studio Lite Software version 5.2.5.
2.6. Statistical analysis
Relative expression of hsa-miR186 measured in terms of ΔCT, which is relatively better normally distributed than fold change, was analyzed using a full ANOVA model along with interaction with time points. SAS 9.4 software was used for the statistical analysis. Results are declared statistically significant at alpha <0.05. ANOVA, t-test and Fishers Exact (http://www.socscistatistics.com/tests/fisher/Default2.aspx) tests were used as indicated in other figure legends to analyze the differences. P < 0.05 was considered statistically significant.
3. Results
3.1. hsa-miR-186 expression in arsenic-induced squamous cell carcinomas and hyperkeratoses
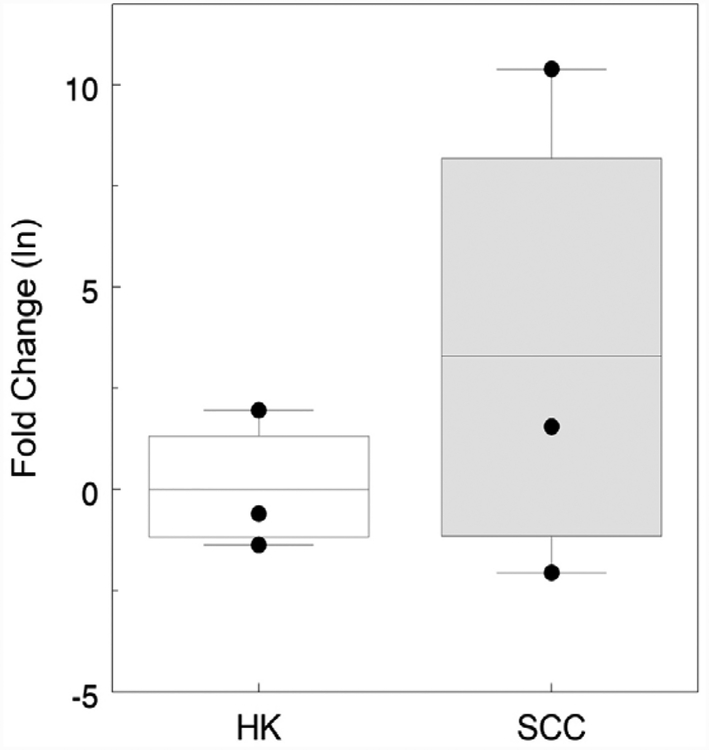
Aberrant expression of hsa-miR-186 has been reported in a variety of cancers. We determined hsa-miR-186 expression in arsenic-induced squamous cell carcinomas and hyperkeratoses as part of a larger study (Al-Eryani et al., 2018a). Expression of hsa-miR-186 was elevated in two arsenic-induced squamous cell carcinomas whereas in two premalignant hyperkeratoses, hsa-miR-186 expression was lower (Fig. 1). One squamous cell carcinoma had extremely high hsa-miR-186 expression.
Fig. 1.
Expression of hsa-miR-186 in keratinocytes laser capture microdissected from hyperkeratoses (HK) and squamous cell carcinomas (SCC). Data shown are extracted from analysis of multiple miRNAs using an RT-qPCR array (Al-Eryani et al., 2018a) and expressed as the natural logarithm of the fold change relative to the mean level in hyperkeratoses samples. Difference between HK and SCC is not statistically significant due to high variability in the SCC samples.
3.2. Chronic arsenite exposure induces aneuploidy in HaCaT cells
Arsenic exposure is known to induce aneuploidy (Vega et al., 1995; Yih et al., 1997; Salazar et al., 2010). Therefore, cytogenetic analyses were performed to determine whether aneuploidy also was increased in HaCaT cells chronically exposed to arsenite for 28 weeks. As shown in Fig. 2, the fraction of cells with supernumerary chromosomes was significantly increased in arsenite-exposed cells compared to unexposed cells. Thus, increased aneuploidy is associated with chronic arsenite exposure in vitro.
3.3. Ectopic expression of hsa-miR-186 in HaCaT cells
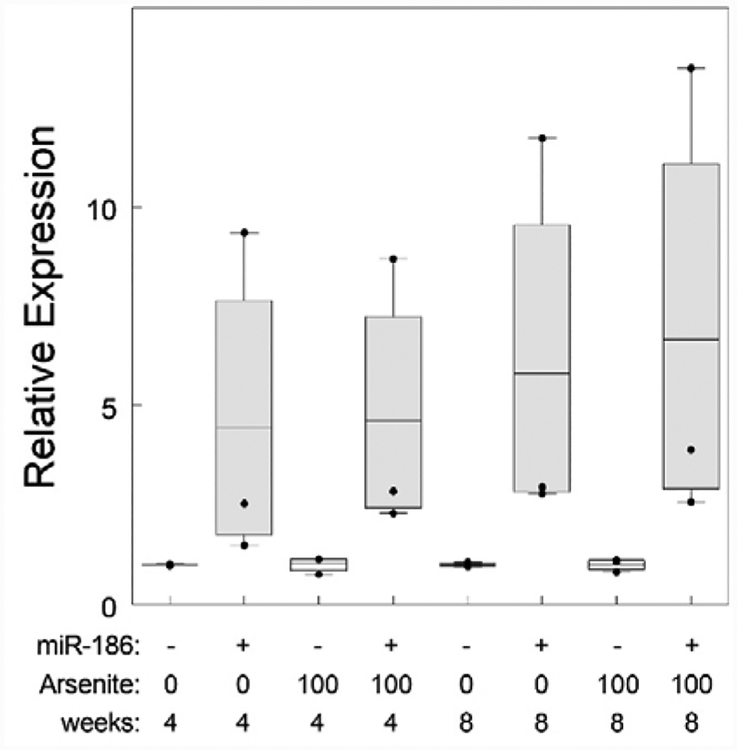
Arsenite exposure has myriad effects on cells. One effect is induction of chromosomal instability and aneuploidy (Vega et al., 1995; Yih et al., 1997; Salazar et al., 2010) and hsa-miR-186 has the potential to mediate this effect (States, 2015). We sought to understand the impact of hsa-miR-186 overexpression both with and without concurrent arsenite exposure. One clone of hsa-miR-186 transfectants had much higher hsa-miR-186 expression than the other two. After 4 weeks, hsa-miR-186 transfected cells had on average 3.3-fold higher hsa-miR-186 expression than empty vector transfected cells (Fig. 3). Incubation with 100 nM sodium arsenite increased expression to nearly 4-fold higher than empty vector control cells. Continued culture to 8 weeks further enhanced expression in hsa-miR-186 transfected cells to 4.5-fold and 5.1-fold empty vector control transfected cells incubated with 0 and 100 nM sodium arsenite respectively. Thus, hsa-miR-186 expression was maintained in an elevated state over 8 weeks and was slightly higher in cells exposed to sodium arsenite.
Fig. 3.
Over-expression of miR-186 in clones of HaCaT cells transfected with miR-186 expression plasmid or empty vector. Expression of miR-186 was determined by RT-qPCR of RNA isolated from 3 clones stably transfected with pEP-hsa-miR-186 Expression Vector (186) and 3 clones transfected with empty vector control plasmid (Ctrl) and incubated for 4 or 8 weeks with 0 or 100 nM sodium arsenite as indicated. The means and 25/75% confidence intervals are indicated by the line and box. Individual values are shown as dots. One pEP-hsa-miR-186 transfected clone expressed miR-186 at much higher level than the other two and lies above the confidence interval. The empty vector transfected clones all expressed miR-186 at about the same level (often overlapping) and lower than any of the pEP-hsa-miR-186 transfected clones. All values are normalized to the mean of the control cells without arsenite at 8 weeks.
3.4. Overexpression of miR-186 induces increased chromosome numbers
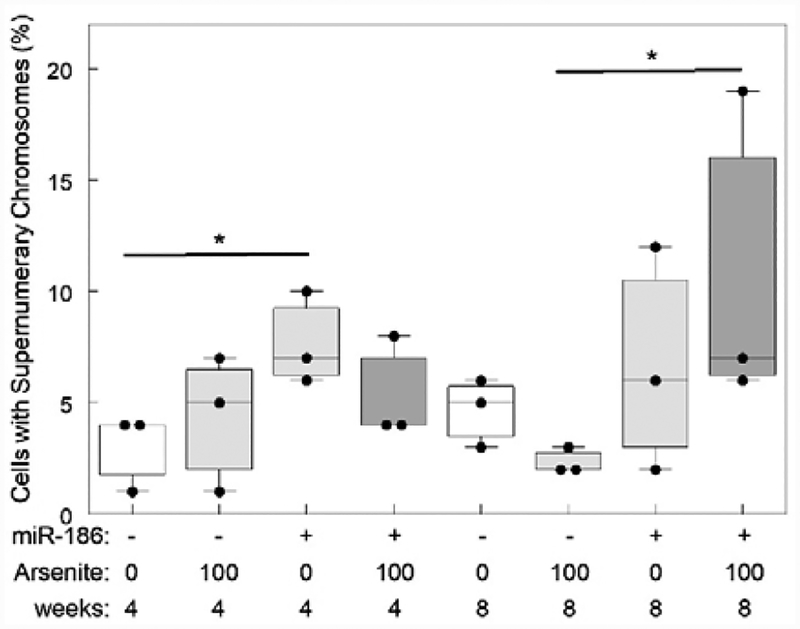
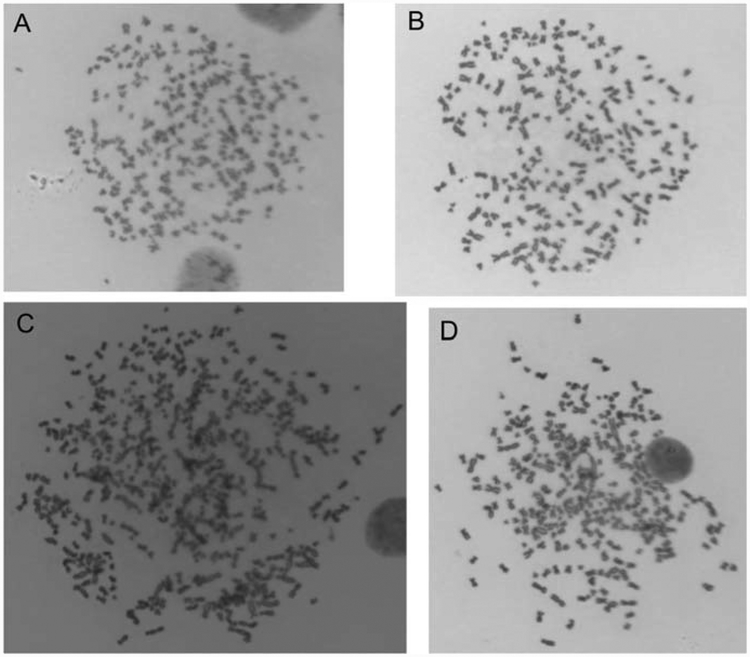
Cytogenetic assays revealed that cells with increased chromosomal number are more abundant in clones overexpressing hsa-miR-186 (Fig. 4). The fraction of cells with more than 83 chromosomes significantly increased from 3 to 7.7% when cells were transfected with hsa-miR-186 at 4 weeks. The number of mitosis with supernumerary chromosomes was around 4 times higher in cells exposed to arsenite and overexpressing hsa-miR-186 at 8 weeks. In addition, some metaphase cells with more than 200 (>10N) chromosomes occurred after incubation for 8 weeks with or without arsenic exposure (Fig. 5), suggesting hsa-miR-186 overexpression is inducing endomitosis.
Fig. 4.
Cells with increased chromosomal number are more abundant in clones overexpressing hsa-miR-186. 100 metaphase cells were analyzed for each clone. After 4 weeks, hsa-miR-186 was sufficient to increase chromosomal number. At 8 weeks, arsenite exposure and hsa-miR-186 overexpression were required to induce supernumerary chromosomes. Comparisons were performed using Fisher’s Exact Test (FET) and t-test. (* indicates p<0.05).
Fig. 5.
Mitotic figures with more than 200 chromosomes in cells overexpressing hsa-miR-186 indicating endomitosis. Mitotic figures of cells after 8 weeks incubation: A. Clone 1 cell without arsenite showing >240 chromosomes; B. Clone 2 cell with arsenite showing >220 chromosomes; C. Clone 3 cell without arsenite showing >270 chromosomes; D. Clone 1 cell with arsenite showing >240 chromosomes.
3.5. Overexpression of miR-186 induces chromosome structural abnormalities
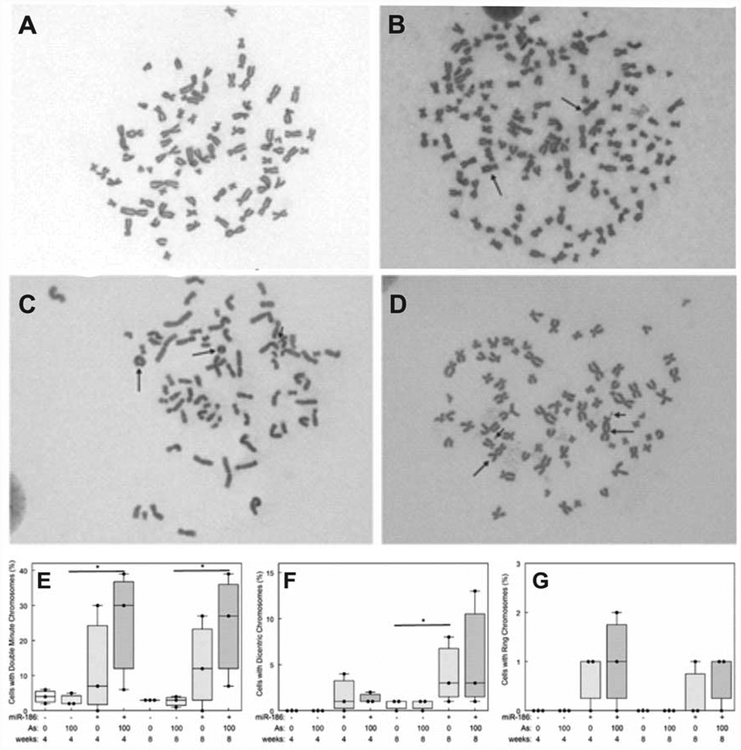
Chromosomal structural abnormalities were increased in hsa-miR-186 overexpressing clones (Fig. 6). Example metaphase spreads from vector control transfected HaCaT cells showing no structural abnormalities (Fig. 6A) can be compared with metaphase spreads of cells from hsa-miR-186 overexpressing clones showing supernumerary chromosomes (152 chromosomes) including dicentrics (Fig. 6B), ring chromosomes and double minutes (Fig. 6C), and dicentrics and double minutes (Fig. 6D). A greater percentage of hsa-miR-186 overexpressing cells had double minutes and the difference became significant with arsenite exposure (Fig. 6E). Likewise, a greater percentage of hsa-miR-186 overexpressing cells had dicentric chromosomes (Fig. 6F). We observed ring chromosomes only in hsa-miR-186 overexpressing cells, but the numbers were between one and two (Fig. 6G).
Fig. 6.
Overexpression of miR-186 induced chromosomal structural abnormalities in HaCaT cells. A, B, C and D are representative pictures of mitoses showing chromosomal abnormalities. (A) Vector control clone with 77 chromosomes; (B) hsa-miR-186 clone with 152 chromosomes and 2 dicentrics (arrows); (C) hsa-miR-186 clone with 74 chromosomes, 2 rings and 1 double minute (arrows); (D) hsa-miR-186 clone with 71 chromosomes, 2 dicentrics and 2 double minutes (arrows). In E, F and G the abnormalities were quantified. (E) Double minutes; (F) Dicentric; (G) Ring. Statistical analysis by 2-way ANOVA (* indicates p<0.05).
3.5. Expression of hsa-miR-186 target proteins
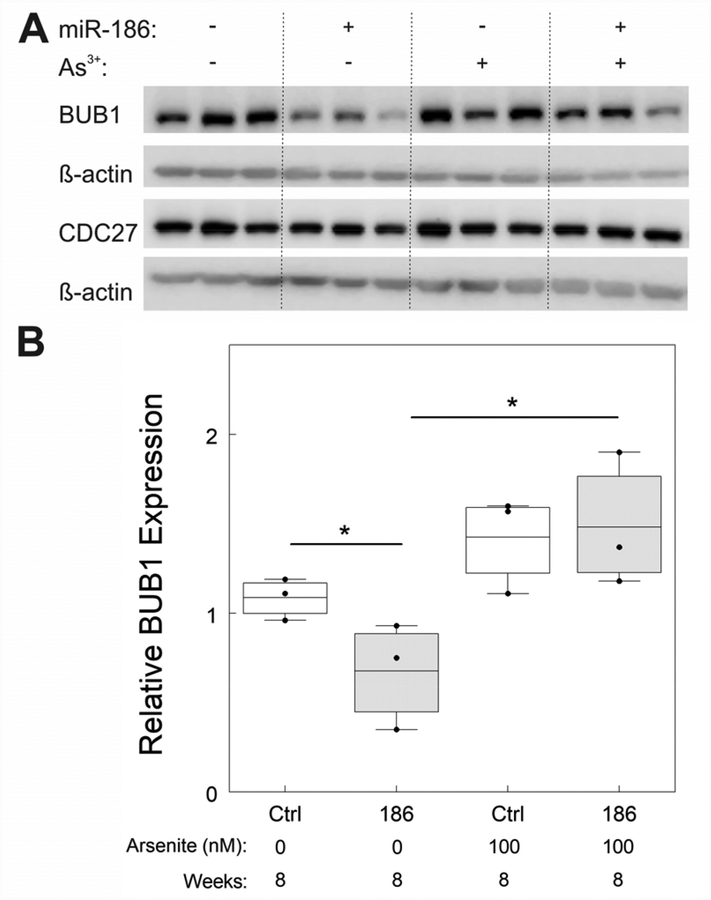
While published literature shows securin (aka PTTG1) to be a hsa-miR-186 target (Li et al., 2013), recent bioinformatic predictions by MirPath V3 indicate that BUB1 and CDC27 are also hsa-miR-186 targets (more precisely, hsa-miR-186–5p) (Vlachos et al., 2015). BUB1 and CDC27 are of interest because they are involved in regulation of spindle assembly checkpoint (SAC) (Manic et al., 2017) and mitotic progression (Pines, 2009). We found that securin was barely expressed, if at all in HaCaT cells before or after stable transfection (data not shown). After 8 weeks, decreased BUB1 expression was found in hsa-miR-186 overexpressing clones without arsenite exposure, but arsenite exposure increased BUB1 expression in both empty vector transfected control clones and hsa-miR-186 overexpressing clones (Fig. 7). CDC27 levels were essentially the same in all clones regardless of hsa-miR-186 expression or arsenite exposure (Fig. 6).
Fig. 7.
BUB1 levels respond to hsa-miR-186 overexpression and to arsenite exposure. (A) Western blots of BUB1, CDC27 and β-Actin in hsa-miR-186 overexpressing clones +/− 100 nM arsenite for 8 weeks. (B) Quantitation of BUB1 relative to β-Actin. Western blot and quantitation showing decreased expression of BUB1 in hsa-miR-186 overexpressing clones, arsenite increases BUB1 suppression. Statistical analysis by 2-Way ANOVA.
4. Discussion
miRNAs, as tumor promoters or suppressors, play an important role in carcinogenesis and are being studied as biomarkers of carcinogen exposure, hazard identification, and cancer development (States et al., 2014; Pogribny et al., 2016). Changes in miRNA expression could alter physiological processes and can lead to disease outcomes. Previous studies revealed that arsenic exposure significantly induced differential miRNA expression profiles both in vitro and in vivo (Sturchio et al., 2014; Al-Eryani et al., 2017; Al-Eryani et al., 2018a; Al-Eryani et al., 2018b), and that miRNA changes likely contribute to malignant transformation (Beezhold et al., 2011; Luo et al., 2015).
Studies in our laboratory investigating differential miRNA expression in squamous cell carcinoma (SCC) caused by chronic arsenic exposure in drinking water relative to that of premalignant lesions found that the miRNA expression profiles were dramatically different (Al-Eryani et al., 2018a) and that hsa-miR-186 was elevated in some but not all SCC (Fig. 1). It was reported that hsa-miR-186 suppressed securin (PTTG1) expression (Li et al., 2013). Securin regulates chromatid segregation allowing anaphase progression (Uhlmann, 2001). Arsenic induced mitotic arrest is associated with securin stabilization (McNeely et al., 2008). We suggested that hsa-miR-186 overexpression in chronic arsenic exposure could mitigate the securin stabilization and contribute to escape from arsenic induced mitotic delay (States, 2015). Furthermore, dysregulated securin could lead to chromatid segregation defects contributing to aneuploidy, which in turn contributes to carcinogenesis. However, securin levels in HaCaT cells before or after stable transfection were very low (data not shown), suggesting that other mRNA components of the cell cycle targeted by hsa-miR-186 might play a role in arsenic induced carcinogenesis. Thus, we investigated whether hsa-miR-186 over-expression could lead to aneuploidy in human keratinocytes by dysregulating components of SAC and anaphase promoting complex/cyclosome (APC/C).
hsa-miR-186 was overexpressed in HaCaT cells by transfection with an expression plasmid and aneuploidy was assessed in hsa-miR-186 overexpressing clones exposed to 0 or 100 nM sodium arsenite. hsa-miR-186 expression in transfected HaCaT cells increased slightly with chronic arsenite exposure (Fig. 3) consistent with the hypothesis that the arsenite exposure induced hsa-miR-186 expression. Overall, aneuploidy was increased in cells overexpressing hsa-miR-186 and the aneuploidy was further increased with chronic arsenite exposure (Figs. 4 and 6). At 4 weeks, hsa-miR-186 overexpression alone enhanced chromosomal number, but not at 8 weeks (Fig.4), suggesting that HaCaT cells might accumulate changes over time. Arsenite alone was sufficient to induce the same changes in chromosomal number as those observed when arsenite was added to hsa-miR-186 overexpressing cells at 4 weeks. However, at 8 weeks, arsenite and hsa-miR-186 overexpression were required to induce chromosome numerical changes (Fig.4). Thus, these results support the hypothesis that elevated hsa-miR-186 expression plays an essential role in arsenic-induced skin carcinogenesis.
hsa-miR-186 is predicted to interact with mRNAs of proteins that regulate the cell cycle, including BUB1 (Vlachos et al., 2015). Our results show decreased BUB1 protein levels in hsa-miR-186 overexpressing clones at 8 weeks, whereas arsenite exposure induced BUB1 levels regardless of hsa-miR-186 overexpression (Fig. 7). BUB1 expression is known to be cell cycle dependent and a target of the anaphase promoting complex/cyclosome as are cyclin B and securin (Qi and Yu, 2007). Arsenite exposure can increase the proportion of cells in G2 or M phase while stabilizing cyclin B in a fibroblast model (McNeely et al., 2006) and securin in a melanoma model (McNeely et al., 2008). Thus, the arsenite induced BUB1 expression may be due to an increase in G2 or M phase cells via inhibition of the anaphase promoting complex/cyclosome. BUB1 is key to high fidelity chromosome segregation, binding to the kinetochore and delaying mitosis in response to spindle disruption (Su et al., 2018). Mutations and deletion of BUB1 have been identified in some aneuploid tumor cell lines and primary tumors (Sasaki et al., 2006). Deficiency in cell cycle regulation is one of the causes of chromosomal instability (Thompson et al., 2010).
CDC27 is a component of the anaphase promoting complex/cyclosome (APC/C) (Sansregret et al., 2017) and a predicted target of hsa-miR-186 (DIANA miRPath V3.0, (Vlachos et al., 2015)). However, CDC27 levels were stable in all clones regardless of hsa-miR-186 expression or arsenite exposure (Fig. 6). CDC27 expression is reported to be essential for preventing high levels of CIN. Thus, suppression of CDC27 expression would be inconsistent with the increased CIN observed in hsa-miR-186 overexpressing cells. Hence, either the predicted targeting of CDC27 mRNA by hsa-miR-186 is incorrect, or alternative mechanisms such as increased RNA expression must be in play to maintain CDC27 expression.
A role for hsa-miR-186 in carcinogenesis through regulation of cell cycle is suggested by previous studies with seemingly contradictory findings. In non-small cell lung cancer (NSCLC), down-regulation of hsa-miR-186 promoted cell cycle progression and cell proliferation, leading to adverse prognosis, while overexpressed hsa-miR-186 targeted cyclin D1 (CCND1), cyclin-dependent kinases 2 (CDK2) and 6 (CDK6) causing G1/S cell cycle arrest and inhibited growth of the lung cancer cells. On the other hand, hsa-miR-186 suppressed expression of PTTG1 (aka securin), and inhibited NSCLC cell invasion and metastasis in nude mice (Li et al., 2013). hsa-miR-186 suppressed protein phosphatase PPM1B to promote G1/S cell cycle transition thus driving cell cycle progression in bladder cancer (Yang et al., 2016). In transformation of fibroblasts, hsa-miR-186 regulated glycolysis to lead to cell cycle arrest, and increased phosphorylation of cyclin dependent kinase 2 (CDK2) to activate cell cycle checkpoints (Sun et al., 2014). hsa-miR-186 expression was increased in metastatic vs primary cutaneous melanoma (Qi et al., 2014). Thus, the effect of hsa-miR-186 induction may be context specific.
Genetic instability including both numerical and structural chromosomal abnormalities is a hallmark of cancer (Gordon et al., 2012). Our cytogenetic assays revealed that some metaphase cells overexpressing hsa-miR-186 have 280 (around 12N) or 380 (around 16N) chromosomes after incubation for 8 weeks with arsenite, suggesting overexpressing hsa-miR-186 induced multiple rounds of endomitosis by arresting cells in M-phase in the absence of cytokinesis. Chromosomal number variation (aneuploidy) was also observed in fibroblasts and lymphocytes exposure to arsenic (Vega et al., 1995; Yih et al., 1997; Salazar et al., 2010). A karyotypic theory that carcinogenesis is a form of speciation suggests that cancers are generated from normal cells by random karyotypic rearrangements and selection for cancer-specific reproductive autonomy (Heng et al., 2013; Sturchio et al., 2014; Horne et al., 2015). Overexpression of hsa-miR-186 induced multiple endomitoses and caused chromosomal number variation, which result in new karyotypes and thus likely contribute to arsenic carcinogenesis.
Oncogene activation or tumor suppressor gene inactivation resulting from abnormalities of chromosomal number or structure are important causes of carcinogenesis (Gordon et al., 2012). The association of gene amplification with double minutes (DMs) has been known for decades (Horns et al., 1984; Benner et al., 1991). DMs are small fragments of extrachromosomal DNA that can harbor oncogenes, and the amplification of oncogenes is associated with the onset of certain types of carcinogenesis (Hayes and Li, 2015). DMs have been observed in a large number of human tumors including breast, lung, ovary, colon and neuroblastoma (Barker, 1982). In the current work, DMs are the dominant abnormality of chromosomal structure rather than dicentrics and ring chromosomes in hsa-miR-186 overexpressing clones. Arsenite exposure induced DMs only in hsa-miR-186 overexpressing clones and not in empty vector transfected clones. This result suggests a potential synergy between induced hsa-miR-186 expression and continued arsenite exposure contributing to arsenic carcinogenesis.
The present studies suggest that hsa-miR-186 is involved in carcinogenesis as a mitosis disruptor, resulting in chromosomal abnormalities. Future work is needed focusing on the molecular mechanism for hsa-miR-186 induction of chromosomal abnormalities, especially for endomitosis and DMs, and the role that aneuploidy plays in skin cancer induced by chronic arsenic exposure in drinking water.
In summary, we show that chronic arsenite exposure in human keratinocytes increases aneuploidy. Furthermore, we show that ectopic expression of hsa-miR-186 expression induces aneuploidy in human keratinocytes. Additionally, chronic arsenite exposure for 4 or 8 weeks increases aneuploidy in hsa-miR-186 overexpressing cells but not in the absence of hsa-miR-186 overexpression. This increase in aneuploidy includes induction of DMs suggesting that gene amplification, possibly of an oncogene, is occurring. Finally, dysregulation of cell cycle components such as BUB1 by hsa-miR-186 overexpression likely lead to chromosomal instability. These results strongly suggest that induction of hsa-miR-186 likely plays a causative role in arsenic-induced skin cancer by inducing endomitosis and aneuploidy.
Acknowledgements:
This work was supported by NIH Grants R21ES023627 and R01ES027778, the University of Louisville Executive Vice President for Research and Innovation Competitive Enhancement Grant, and the Scholarship Fund of China Scholarship Council (No. 201408440133). Dr. Rai was partially supported by Wendell Cherry Chair in Clinical Trial Research.
Footnotes
Competing financial interests: None by any author other than grants listed in acknowledgements.
References
- Al-Eryani L, Jenkins SF, States VA, Pan J, Malone JC, Rai SN, Galandiuk S, Giri AK, States JC, 2018a. miRNA expression profiles of premalignant and malignant arsenic-induced skin lesions. PLoS One 13, e0202579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Eryani L, Waigel S, Jala V, Jenkins SF, States JC, 2017. Cell cycle pathway dysregulation in human keratinocytes during chronic exposure to low arsenite. Toxicol Appl Pharmacol 331, 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Eryani L, Waigel S, Tyagi A, Peremarti J, Jenkins SF, Damodaran C, States JC, 2018b. Differentially Expressed mRNA Targets of Differentially Expressed miRNAs Predict Changes in the TP53 Axis and Carcinogenesis-Related Pathways in Human Keratinocytes Chronically Exposed to Arsenic. Toxicol Sci 162, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayotte JD, Medalie L, Qi SL, Backer LC, Nolan BT, 2017. Estimating the High-Arsenic Domestic-Well Population in the Conterminous United States. Environ Sci Technol 51, 12443–12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffa R, Fassan M, Volinia S, O’Hara B, Liu CG, Palazzo JP, Gardiman M, Rugge M, Gomella LG, Croce CM, Rosenberg A, 2009. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol 219, 214–221. [DOI] [PubMed] [Google Scholar]
- Barker PE, 1982. Double minutes in human tumor cells. Cancer Genet Cytogenet 5, 81–94. [DOI] [PubMed] [Google Scholar]
- Beezhold K, Liu J, Kan H, Meighan T, Castranova V, Shi X, Chen F, 2011. miR-190-mediated downregulation of PHLPP contributes to arsenic-induced Akt activation and carcinogenesis. Toxicol Sci 123, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner SE, Wahl GM, Von Hoff DD, 1991. Double minute chromosomes and homogeneously staining regions in tumors taken directly from patients versus in human tumor cell lines. Anticancer Drugs 2, 11–25. [DOI] [PubMed] [Google Scholar]
- Boukamp P, 2005. UV-induced skin cancer: similarities--variations. J Dtsch Dermatol Ges 3, 493–503. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang X, Gu S, Zhang Z, 2017. MicroRNA-155 regulates arsenite-induced malignant transformation by targeting Nrf2-mediated oxidative damage in human bronchial epithelial cells. Toxicol Lett 278, 38–47. [DOI] [PubMed] [Google Scholar]
- Cheng PS, Weng SF, Chiang CH, Lai FJ, 2016. Relationship between arsenic-containing drinking water and skin cancers in the arseniasis endemic areas in Taiwan. J Dermatol 43, 181–186. [DOI] [PubMed] [Google Scholar]
- Council NR, 1999. Arsenic in Drinking Water, Washington DC. [Google Scholar]
- Council NR, 2001. Arsenic in Drinking Water: 2001 Update, Washington (DC). [Google Scholar]
- Durrbaum M, Kruse C, Nieken KJ, Habermann B, Storchova Z, 2018. The deregulated microRNAome contributes to the cellular response to aneuploidy. BMC Genomics 19, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Wu J, Li Y, Liu Y, Gao Y, Yao F, Qiu C, Song L, Wu Y, Liao Y, Sun D, 2014. Urinary arsenic metabolism in a Western Chinese population exposed to high-dose inorganic arsenic in drinking water: influence of ethnicity and genetic polymorphisms. Toxicol Appl Pharmacol 274, 117–123. [DOI] [PubMed] [Google Scholar]
- Fusenig NE, Boukamp P, 1998. Multiple stages and genetic alterations in immortalization, malignant transformation, and tumor progression of human skin keratinocytes. Mol Carcinog 23, 144–158. [DOI] [PubMed] [Google Scholar]
- Gordon DJ, Resio B, Pellman D, 2012. Causes and consequences of aneuploidy in cancer. Nat Rev Genet 13, 189–203. [DOI] [PubMed] [Google Scholar]
- Guo H, Jia Y, Wanty RB, Jiang Y, Zhao W, Xiu W, Shen J, Li Y, Cao Y, Wu Y, Zhang D, Wei C, Zhang Y, Cao W, Foster A, 2016. Contrasting distributions of groundwater arsenic and uranium in the western Hetao basin, Inner Mongolia: Implication for origins and fate controls. Sci Total Environ 541, 1172–1190. [DOI] [PubMed] [Google Scholar]
- Hayes M, Li J, 2015. An integrative framework for the identification of double minute chromosomes using next generation sequencing data. BMC Genet 16 Suppl 2, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng HH, Bremer SW, Stevens JB, Horne SD, Liu G, Abdallah BY, Ye KJ, Ye CJ, 2013. Chromosomal instability (CIN): what it is and why it is crucial to cancer evolution. Cancer Metastasis Rev 32, 325–340. [DOI] [PubMed] [Google Scholar]
- Horne SD, Pollick SA, Heng HH, 2015. Evolutionary mechanism unifies the hallmarks of cancer. Int J Cancer 136, 2012–2021. [DOI] [PubMed] [Google Scholar]
- Horns RC Jr., Dower WJ, Schimke RT, 1984. Gene amplification in a leukemic patient treated with methotrexate. J Clin Oncol 2, 2–7. [DOI] [PubMed] [Google Scholar]
- Hsu LI, Wu MM, Wang YH, Lee CY, Yang TY, Hsiao BY, Chen CJ, 2015. Association of Environmental Arsenic Exposure, Genetic Polymorphisms of Susceptible Genes, and Skin Cancers in Taiwan. Biomed Res Int 2015, 892579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas MR, Gossai A, Pierce B, Ahsan H, 2015. Drinking Water Arsenic Contamination, Skin Lesions, and Malignancies: A Systematic Review of the Global Evidence. Curr Environ Health Rep 2, 52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidinger P, Keller A, Borries A, Reichrath J, Rass K, Jager SU, Lenhof HP, Meese E, 2010. High-throughput miRNA profiling of human melanoma blood samples. BMC Cancer 10, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yin C, Zhang B, Sun Y, Shi L, Liu N, Liang S, Lu S, Liu Y, Zhang J, Li F, Li W, Liu F, Sun L, Qi Y, 2013. PTTG1 promotes migration and invasion of human non-small cell lung cancer cells and is modulated by miR-186. Carcinogenesis 34, 2145–2155. [DOI] [PubMed] [Google Scholar]
- Luo F, Ji J, Liu Y, Xu Y, Zheng G, Jing J, Wang B, Xu W, Shi L, Lu X, Liu Q, 2015. MicroRNA-21, up-regulated by arsenite, directs the epithelial-mesenchymal transition and enhances the invasive potential of transformed human bronchial epithelial cells by targeting PDCD4. Toxicol Lett 232, 301–309. [DOI] [PubMed] [Google Scholar]
- Manic G, Corradi F, Sistigu A, Siteni S, Vitale I, 2017. Molecular Regulation of the Spindle Assembly Checkpoint by Kinases and Phosphatases. Int Rev Cell Mol Biol 328, 105–161. [DOI] [PubMed] [Google Scholar]
- McNeely SC, Taylor BF, States JC, 2008. Mitotic arrest-associated apoptosis induced by sodium arsenite in A375 melanoma cells is BUBR1-dependent. Toxicol Appl Pharmacol 231, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely SC, Xu X, Taylor BF, Zacharias W, McCabe MJ Jr., States JC, 2006. Exit from arsenite-induced mitotic arrest is p53 dependent. Environ Health Perspect 114, 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA, 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri MA, Molinari BL, 2015. Effect of Sodium Arsenite on Mouse Skin Carcinogenesis. Toxicol Pathol 43, 704–714. [DOI] [PubMed] [Google Scholar]
- Pi J, Diwan BA, Sun Y, Liu J, Qu W, He Y, Styblo M, Waalkes MP, 2008. Arsenic-induced malignant transformation of human keratinocytes: involvement of Nrf2. Free Radic Biol Med 45, 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J, 2009. The APC/C: a smorgasbord for proteolysis. Mol Cell 34, 135–136. [DOI] [PubMed] [Google Scholar]
- Pogribny IP, Beland FA, Rusyn I, 2016. The role of microRNAs in the development and progression of chemical-associated cancers. Toxicol Appl Pharmacol 312, 3–10. [DOI] [PubMed] [Google Scholar]
- Polya D L. M, 2016. Geogenic and Anthropogenic Arsenic Hazard in Groundwaters and Soils: Distribution, Nature, Origin, and Human Exposure Routes In States JC, (Ed.), Arsenic: Exposure Sources, Health Risks, and Mechanisms of Toxicity. John Wiley & Sons, Inc., Hoboken, NY, pp. [Google Scholar]
- Qi M, Huang X, Zhou L, Zhang J, 2014. Identification of differentially expressed microRNAs in metastatic melanoma using next-generation sequencing technology. Int J Mol Med 33, 1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Yu H, 2007. KEN-box-dependent degradation of the Bub1 spindle checkpoint kinase by the anaphase-promoting complex/cyclosome. J Biol Chem 282, 3672–3679. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Chowdhury UK, Mukherjee SC, Mondal BK, Paul K, Lodh D, Biswas BK, Chanda CR, Basu GK, Saha KC, Roy S, Das R, Palit SK, Quamruzzaman Q, Chakraborti D, 2001. Chronic arsenic toxicity in Bangladesh and West Bengal, India--a review and commentary. J Toxicol Clin Toxicol 39, 683–700. [DOI] [PubMed] [Google Scholar]
- Ries J, Vairaktaris E, Agaimy A, Kintopp R, Baran C, Neukam FW, Nkenke E, 2014. miR-186, miR-3651 and miR-494: potential biomarkers for oral squamous cell carcinoma extracted from whole blood. Oncol Rep 31, 1429–1436. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lado L, Sun G, Berg M, Zhang Q, Xue H, Zheng Q, Johnson CA, 2013. Groundwater arsenic contamination throughout China. Science 341, 866–868. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, 2004. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol Appl Pharmacol 198, 394–404. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Miller HL, McNeely SC, Sordo M, Ostrosky-Wegman P, States JC, 2010. Suppression of p53 and p21CIP1/WAF1 reduces arsenite-induced aneuploidy. Chem Res Toxicol 23, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansregret L, Patterson JO, Dewhurst S, Lopez-Garcia C, Koch A, McGranahan N, Chao WCH, Barry DJ, Rowan A, Instrell R, Horswell S, Way M, Howell M, Singleton MR, Medema RH, Nurse P, Petronczki M, Swanton C, 2017. APC/C Dysfunction Limits Excessive Cancer Chromosomal Instability. Cancer Discov 7, 218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansregret L, Swanton C, 2017. The Role of Aneuploidy in Cancer Evolution. Cold Spring Harb Perspect Med 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma R, 2016. Skin Manifestations of Chronic Arsenicosis In States JC, (Ed.), Arsenic:Exposure Sources, Health Risks and Mechanisms of Toxicity. John Wiley & Sons, Inc., Hoboken, NJ, pp. [Google Scholar]
- Sasaki M, Sugimoto K, Tamayose K, Ando M, Tanaka Y, Oshimi K, 2006. Spindle checkpoint protein Bub1 corrects mitotic aberrancy induced by human T-cell leukemia virus type I Tax. Oncogene 25, 3621–3627. [DOI] [PubMed] [Google Scholar]
- States JC, 2015. Disruption of Mitotic Progression by Arsenic. Biol Trace Elem Res 166, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States JC, Ouyang M, Helm CW, 2014. Systems approach to identify environmental exposures contributing to organ-specific carcinogenesis. Cancer Epidemiol 38, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchio E, Colombo T, Boccia P, Carucci N, Meconi C, Minoia C, Macino G, 2014. Arsenic exposure triggers a shift in microRNA expression. Sci Total Environ 472, 672–680. [DOI] [PubMed] [Google Scholar]
- Su BB, Zhou SW, Gan CB, Zhang XN, 2018. MiR-186 inhibits cell proliferation and invasion in human cutaneous malignant melanoma. J Cancer Res Ther 14, S60–S64. [DOI] [PubMed] [Google Scholar]
- Sun B, Xue J, Li J, Luo F, Chen X, Liu Y, Wang Q, Qi C, Zou Z, Zhang A, Liu Q, 2017. Circulating miRNAs and their target genes associated with arsenism caused by coal-burning. Toxicol Res (Camb) 6, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Hu JW, Xiong WJ, Mi J, 2014. miR-186 regulates glycolysis through Glut1 during the formation of cancer-associated fibroblasts. Asian Pac J Cancer Prev 15, 4245–4250. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hirota T, 2016. Chromosomal instability: A common feature and a therapeutic target of cancer. Biochim Biophys Acta 1866, 64–75. [DOI] [PubMed] [Google Scholar]
- Thompson SL, Bakhoum SF, Compton DA, 2010. Mechanisms of chromosomal instability. Curr Biol 20, R285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Benbrahim-Tallaa L, Ward JM, Lunn R, Sams RL 2nd, Waalkes MP, 2010. Cancer in experimental animals exposed to arsenic and arsenic compounds. Crit Rev Toxicol 40, 912–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F, 2001. Secured cutting: controlling separase at the metaphase to anaphase transition. EMBO Rep 2, 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega L, Gonsebatt ME, Ostrosky-Wegman P, 1995. Aneugenic effect of sodium arsenite on human lymphocytes in vitro: an individual susceptibility effect detected. Mutat Res 334, 365–373. [DOI] [PubMed] [Google Scholar]
- Verma AM, Patel M, Aslam MI, Jameson J, Pringle JH, Wurm P, Singh B, 2015. Circulating plasma microRNAs as a screening method for detection of colorectal adenomas. Lancet 385 Suppl 1, S100. [DOI] [PubMed] [Google Scholar]
- Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG, 2015. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res 43, W460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yuan D, Li J, Zheng S, Wang B, 2016. miR-186 downregulates protein phosphatase PPM1B in bladder cancer and mediates G1-S phase transition. Tumour Biol 37, 4331–4341. [DOI] [PubMed] [Google Scholar]
- Yih LH, Ho IC, Lee TC, 1997. Sodium arsenite disturbs mitosis and induces chromosome loss in human fibroblasts. Cancer Res 57, 5051–5059. [PubMed] [Google Scholar]
- Zhang Y, Li M, Wang H, Fisher WE, Lin PH, Yao Q, Chen C, 2009. Profiling of 95 microRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J Surg 33, 698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Pratheeshkumar P, Budhraja A, Son YO, Kim D, Shi X, 2015. Role of reactive oxygen species in arsenic-induced transformation of human lung bronchial epithelial (BEAS-2B) cells. Biochem Biophys Res Commun 456, 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]