Abstract
IMPORTANCE
To facilitate comparative clinical outcome research in low vision rehabilitation, we must use patient-centered measurements that reflect clinically meaningful changes in visual ability.
OBJECTIVE
To quantify the effects of currently provided low vision rehabilitation (LVR) on patients who present for outpatient LVR services in the United States.
DESIGN, SETTING, AND PARTICIPANTS
Prospective, observational study of new patients seeking outpatient LVR services. From April 2008 through May 2011, 779 patients from 28 clinical centers in the United States were enrolled in the Low Vision Rehabilitation Outcomes Study. The Activity Inventory, a visual function questionnaire, was administered to measure overall visual ability and visual ability in 4 functional domains (reading, mobility, visual motor function, and visual information processing) at baseline and 6 to 9 months after usual LVR care. The Geriatric Depression Scale, Telephone Interview for Cognitive Status, and Medical Outcomes Study 36-Item Short-Form Health Survey physical functioning questionnaires were also administered to measure patients’ psychological, cognitive, and physical health states, respectively, and clinical findings of patients were provided by study centers.
MAIN OUTCOMES AND MEASURES
Mean changes in the study population and minimum clinically important differences in the individual in overall visual ability and in visual ability in 4 functional domains as measured by the Activity Inventory.
RESULTS
Baseline and post-rehabilitation measures were obtained for 468 patients. Minimum clinically important differences (95% CIs) were observed in nearly half (47% [95% CI, 44%–50%]) of patients in overall visual ability. The prevalence rates of patients with minimum clinically important differences in visual ability in functional domains were reading (44% [95% CI, 42%–48%]), visual motor function (38% [95% CI, 36%–42%]), visual information processing (33% [95% CI, 31%–37%]), and mobility (27% [95% CI, 25%–31%]). The largest average effect size (Cohen d = 0.87) for the population was observed in overall visual ability. Age (P = .006) was an independent predictor of changes in overall visual ability, and logMAR visual acuity (P = .002) was predictive of changes in visual information processing.
CONCLUSIONS AND RELEVANCE
Forty-four to fifty percent of patients presenting for outpatient LVR show clinically meaningful differences in overall visual ability after LVR, and the average effect sizes in overall visual ability are large, close to 1 SD.
Clinically meaningful outcome measures are needed to advance low vision rehabilitation (LVR) and provide effective treatment for patients with vision impairment. A recent review1 described 47 different LVR outcome measures used by 52 different studies. Early LVR studies incorporated patient satisfaction measures and assessed the frequency of use of vision-assistive equipment.2–4 A transition then occurred to using fixed-item Likert-scale visual function questionnaires and clinic-based performance measures (eg, reading speed and navigation accuracy).5–13 Over time, however, the flaws in Likert scaling were recognized, and there was a transition to interval scaling of visual function questionnaire responses using Rasch analysis.14–20 In addition, it became evident that the meaningfulness of clinic-based performance measures is limited because they typically are measures of efficacy, which reflect how well an intervention works under ideal conditions, rather than measures of effectiveness, which reflect the benefits of intervention under “real-world” conditions.
Because LVR is a patient-centered type of rehabilitation, it is necessary to use a patient-reported outcome measure (PROM) that is relevant to the treatment objective, viz, to improve the ability of patients to perform activities that depend on vision, a latent variable that we will call visual ability. In contrast to medical or surgical treatment that improves vision (eg, cataract surgery), for which the improvement in visual acuity improves visual ability for all activities, LVR intervenes at the task level, primarily by modifying an activity and making it easier to perform without improving the patient’s visual acuity.21,22 Because LVR is a goal-directed intervention and, therefore, governed by individual patient preferences, the PROM must be adaptive rather than a “one-size-fits-all” questionnaire. The PROM for LVR must allow patients to self-identify their rehabilitation goals by determining what is individually important to them, must contain only items that have room for improvement (and therefore are appropriate targets of intervention), and must place all patients on the same scale in the same units, regardless of their goals.23
Given the complexity of the individualized nature of rehabilitation, the variability of the sample, and the random variation associated with measurement error, the PROM must have enough precision to detect a minimum meaningful change in visual ability at the individual level, not just overall mean changes for the population. The Cohen effect size (d = change score/standard deviation of the change score) offers a well-accepted index for scaling the magnitude of the average overall effects of intervention for the sample (assuming homoscedasticity of estimation errors in the sample). At the individual level, a minimum clinically important difference (MCID) is one in which the change score significantly exceeds the estimation error for the individual’s measure.24 Measuring the effect of the intervention at the individual level is important because even groups with negligible mean changes could include individual patients whose improvements are significant.
The aim of the Low Vision Rehabilitation Outcomes Study is to measure the effectiveness of usual outpatient LVR across the United States using a well-validated, adaptively administered measure of the effects of goal-directed interventions. Overall visual ability (50 goals), as measured by the Activity Inventory (AI), an adaptive visual function questionnaire, was the primary outcome measure, whereas the 4 functional domains (reading, mobility, visual motor function, and visual information processing) in the AI were the secondary outcome measures.
Methods
Study Methods
The Low Vision Rehabilitation Outcomes Study was designed to evaluate the effectiveness of usual outpatient LVR for new patients. This study was conducted using a network of 28 out-patient centers providing LVR. The study protocol and the obtaining of oral informed consent were approved by the Johns Hopkins University institutional review board and adhered to the tenets of the Declaration of Helsinki. In addition, all study sites complied with the requirements of the Health Insurance Portability and Accountability Act and, when required, also obtained separate institutional review board approval for their participating centers. All patients provided oral informed consent for their study participation.
Study Populations
Participants were new low vision patients seeking outpatient LVR services at 1 of the 28 collaborating clinical centers. The study design, the participants’ traits, and the data collection methods were described in a previous report.25 Eligible patients were 18 years of age or older and were new to the physician (ie, defined as new to the physician by not having received LVR services from the clinical center within the past 3 years). Exclusion criteria were non-English speaking and hearing loss so severe that questions could not be heard over the telephone. There were no visual acuity, visual field, or diagnosis requirements because the aim of the study was to observe outcomes of typical low vision patients receiving usual LVR care.
Protocol Design
New patients scheduled for outpatient LVR services at each of the 28 clinical centers were recruited to participate in the Low Vision Rehabilitation Outcomes Study. Enrolled patients were administered several health status questionnaires over the telephone by research assistants at the coordinating center prior to their clinical evaluation. Questionnaires included the AI to measure overall visual ability and its different functional domains,21,22,26,27 the Telephone Interview for Cognitive Status (TICS),28 the Geriatric Depression Scale (GDS),29 the physical functioning subscale of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36),30 and a standardized “check-box” intake survey that acquired a patient’s trait and history information.25 Patient interviews were conducted over 1 or 2 sessions, depending on a patient’s availability. The average interview time was 62 minutes.
The AI is a well-validated 510-item visual function questionnaire that is administered adaptively by computer-assisted interview. The hierarchical structure of the questionnaire organizes the interview into 50 goals and 460 tasks surrounding daily living, social, and recreation-related activities. The patient is asked to rate the importance of each goal. If the goal is “not important,” then the interviewer moves onto the next goal; otherwise, the patient is asked to rate the difficulty of the goal. If a goal is at least slightly important and at least slightly difficult, then the patient is asked to rate the difficulty of subsidiary tasks (using the same difficulty ratings) or identify the task as not applicable.
Prior to the patient’s appointment, questionnaire results were provided to the physician. Included were summaries of the intake history, TICS and GDS raw scores with questionnaire-suggested cutoff scores for screening disorders, and both itemized and summarized AI results. Results of the SF-36 were not included in the report because there are no defined cutoff values for this instrument. Five AI summary scores were included in the report: overall visual ability (estimated from difficulty ratings of AI goals), reading, mobility, visual information processing, and visual motor functions (estimated from difficulty ratings of different subsets of AI tasks). In addition to the numeric scoring (logits), the report included a listing of the specific AI goals and tasks that were identified by the study participant as at least “somewhat important” and “somewhat difficult” to perform. If the goal was not important or if the task was not difficult, then it was not listed in the physician report because it would not be included in the individualized rehabilitation plan.
Participating centers included university-based clinics, private practices, and multidisciplinary rehabilitation centers. Usual LVR services, which included evaluation of visual function by the physician (optometrist or ophthalmologist), were provided. Depending on an individual clinic’s practices, the evaluation and/or treatment by an occupational therapist, vision rehabilitation therapist, ophthalmic technician, social worker, and/or orientation and mobility instructor also may have been provided on the first visit. Subsequent follow-up visits may have occurred with any member of the rehabilitation team, depending on the usual practices of the clinical site and the patient’s needs. Clinical findings from the initial evaluation, including visual acuity, contrast sensitivity, visual field test results, and disorder diagnosis, were entered online along with details of how each test was performed.25
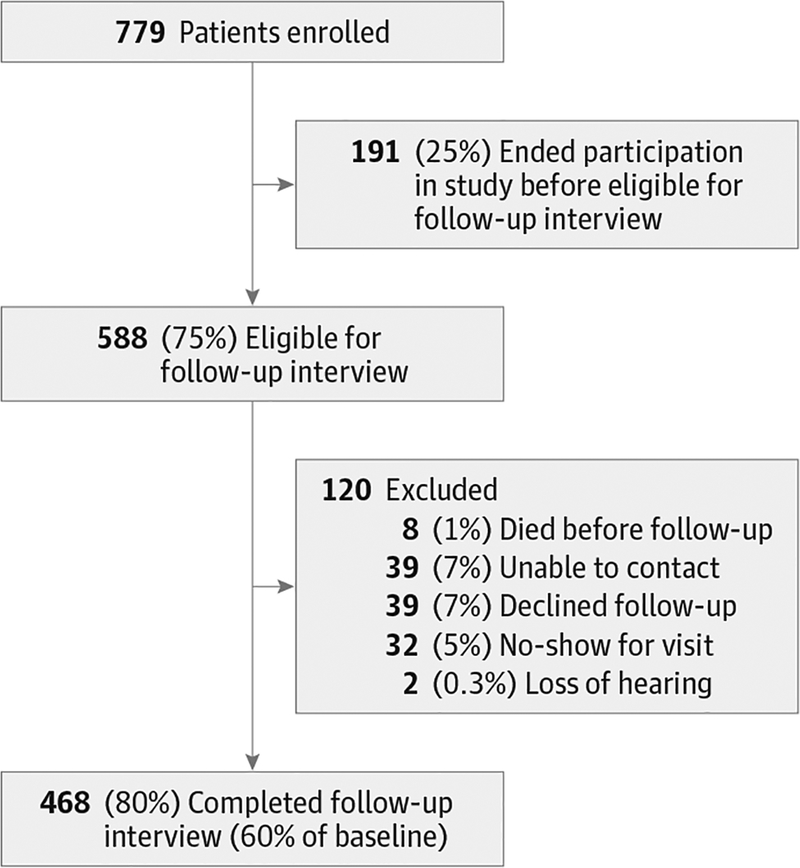
Low vision rehabilitation outcomes were assessed by telephone 6 to 9 months after initial evaluation through the administration of the AI. Post-rehabilitation interviews were completed for 468 of 779 patients. Figure 1 illustrates a breakdown of patient attrition in a CONSORT (Consolidated Standards of Reporting Trials)–type diagram. Baseline patient characteristics, visual acuity, and visual ability measures were compared between patients who did and patients who did not participate in post-rehabilitation interviews.
Figure 1. Flowchart of Study Participants.
Assessment of Outcomes
The primary outcome measure was the change in overall visual ability, estimated from a participant’s difficulty ratings of AI goals at baseline and at 6 to 9 months after the initial evaluation. Secondary outcome measures were reading, mobility, visual information processing, and visual motor function estimated from the patient’s difficulty ratings of respective subsets of tasks in the AI. Item measures (visual ability demanded by each goal and task in the AI) and structure calibrations (response thresholds for each difficulty rating category) were calibrated from the responses of 3177 low vision patients at baseline, as described elsewhere.31 Visual ability was estimated from the participant’s responses to items that were reported to be important and at least slightly difficult at baseline because these items were in need of rehabilitation and therefore eligible to be targeted by the plan of care. Items identified as “not important” or “not difficult” at baseline were filtered out and did not contribute to the outcome measures.
Statistical Analyses
Rasch analysis was used to estimate interval-scaled measures from AI difficulty ratings.32,33 Using the Andrich Rating Scale Model (Winsteps statistical software, version 3.65), we performed Rasch analysis on a larger data set of baseline AI responses (described elsewhere)31 to anchor item measures and response category thresholds to calibrated values for the low vision population. Using these calibrated values, we performed Rasch analysis on filtered baseline and post-rehabilitation AI difficulty ratings to estimate 5-person measures for each patient (overall visual ability and visual ability in each of the 4 functional domains). Mean values and standard deviations were calculated for the respective distributions of changes in overall visual ability measures and changes in each of the 4 functional domains from baseline to post-LVR follow-up. To evaluate whether changes in each patient’s visual ability measures were clinically meaningful, an MCID was calculated for each measure from the ratio of the change in visual ability to 1.95 SEs of the estimated change (ie, the ratio represents the change as a fraction of the 95% CI on the estimated change). To assess potential bias and provide a pragmatic estimate of the benefit of a change in visual ability, an intention-to-treat analysis was performed presuming an effect size of 0 for the 12% of total eligible patients who declined a follow-up interview or who did not show for the clinic visit.
Rasch analysis was performed on the responses to the GDS and SF-36 to estimate continuous interval-scaled measures for depressed mood and physical ability, respectively. Rasch analysis was not performed on the TICS responses because the raw scores were heavily skewed toward the ceiling; therefore, raw scores were used as the continuous variables for cognitive ability, which is expected to be a monotonic function of an interval measure that would be estimated from Rasch analysis.34
One-way analysis of variance (ANOVA) was performed with change in overall visual ability as the dependent variable and categorical baseline traits as independent variables. Multivariable predictive regression models for changes in overall visual ability and changes in each functional domain were tested using continuous variables that previously were observed to be predictors of baseline visual ability (SF-36 physical, GDS, logMAR, and TICS) and significant from the ANOVA (age) to determine if any combination of measures were predictors of primary or secondary outcome measures.31
Results
Patient characteristics, visual impairment measures, and baseline ability were obtained for 779 patients. Table 1 shows the comparison of baseline characteristics of patients with AI follow-up data (n = 468) and patients without (n = 311) using the t test and odds ratios (ORs). The t test showed no differences between groups in mean logMAR visual acuity (0.69 [Snellen equivalent, 20/100] for patients with AI follow-up data vs 0.66 [Snellen equivalent, 20/100] for patients without), mean baseline visual ability score (1.01 vs 0.93), mean baseline reading ability score (0.82 vs 0.78), mean baseline visual motor function score (0.84 vs 0.67), mean SF-36 score (0.56 vs 0.60), and mean TICS score (38.59 vs 37.86). Compared with the patients without follow-up data, the patients with follow-up data were older (mean age, 73.9 years vs 69.36 years), had better mobility function (mean score, 0.61 vs 0.40), had better visual information processing (mean score, 0.79 vs 0.60), and lower depressed mood (mean score, −2.29 vs −1.85) at baseline. However, the magnitudes of the differences were small. The ORs showed that patients with follow-up data were more likely to be female (OR, 1.42) and to drive (OR, 1.40).
Table 1.
Baseline Characteristics and Visual Ability Measures in LVROS Patients With or Without Follow-up Data
| Follow-up Data | No Follow-up Data | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unit Value | Total No. | Unit Value | Total No. | ||||||
| Age, mean (SD), y | 73.9 (14.0) | 441 | 69.4 (17.8) | 278 | −4.53 | 3.60 | 487.38 | <.001 | |
| Male sex, No. (%) | 135 (30.6) | 441 | 107 (38.5) | 278 | NP | NP | NP | NP | 0.70 (0.51–0.97) |
| Symptomatic comorbidities, mean (SD), No. | 3.2(1.8) | 441 | 2.9(1.7) | 278 | −0.28 | 2.15 | 605.82 | .03 | |
| Living alone, No. (%) | 175 (39.7) | 441 | 101 (36.3) | 278 | NP | NP | NP | NP | 1.15 (0.85–1.57) |
| Support provided, No. (%) | 378 (85.7) | 441 | 232 (83.5) | 278 | NP | NP | NP | NP | 1.19 (0.79–1.80) |
| Driving, No. (%) | 143 (32.4) | 441 | 71 (25.5) | 278 | NP | NP | NP | NP | 1.40 (1.00–1.96) |
| Visual acuity | |||||||||
| logMAR, mean (SD) | 0.69 (0.45) | 388 | 0.66 (0.48) | 204 | −0.03 | 0.85 | 393.30 | .39 | |
| Snellen equivalent | 20/100 | 20/100 | |||||||
| Baseline functional ability score,a mean (SD), logits | |||||||||
| Goals or overall visual ability | 1.01 (1.18) | 468 | 0.93 (1.38) | 311 | −0.08 | 0.81 | 650.57 | .42 | |
| Reading | 0.82 (1.28) | 427 | 0.78 (1.47) | 312 | −0.03 | 0.33 | 612.96 | .74 | |
| Mobility | 0.61 (1.10) | 331 | 0.40 (1.14) | 243 | −0.21 | 2.24 | 508.83 | .03 | |
| Visual information processing | 0.79 (1.04) | 403 | 0.60 (1.16) | 297 | −0.19 | 2.21 | 595.54 | .03 | |
| Visual motor function | 0.84(1.06) | 423 | 0.67 (1.15) | 305 | −0.16 | 1.93 | 621.97 | .05 | |
| GDS Rasch score,b mean (SD) | −2.26 (1.69) | 441 | −1.85 (1.81) | 259 | 0.37 | 2.71 | 511.66 | .01 | |
| SF-36 physical functioning Rasch score, mean (SD) | 0.56 (2.36) | 445 | 0.6 (2.49) | 313 | 0.04 | 0.21 | 649.28 | .83 | |
| TICS raw score,c mean (SD) | 38.59 (6.45) | 441 | 37.86 (7.22) | 306 | −0.72 | 1.40 | 607.15 | .16 | |
Abbreviations: GDS, Geriatric Depression Scale; LVROS, Low Vision Rehabilitation Outcomes Study; NP, not performed in sample; OR, odds ratio; SF-36, Medical Outcomes Study 36-Item Short Form Health Survey; TICS, Telephone Interview for Cognitive Status.
Higher score indicates better ability or less difficulty performing activities.
Higher score indicates greater risk of depression.
Raw scores greater than 30 indicate normal cognition.
Table 2 details the mean changes in visual ability after rehabilitation at the goal and domain levels and the percentage of patients with MCID ratios greater than 1.0, for patients with follow-up data and for patients in the intention-to-treat group. Mean changes in visual ability score were greatest at the goal level (0.89), followed by visual motor function (0.54), mobility (0.50), reading (0.45), and visual information processing (0.42). After rehabilitation, nearly half (47% [95% CI, 44%–50%]) of patients exhibited changes in overall visual ability that exceeded the MCID criterion. The prevalence of patients with MCID ratios greater than 1.0 in 4 functional domains were as follows: reading (44% [95% CI, 42%–48%]), visual motor function (38% [95% CI, 36%–42%]), visual information processing (33% [95% CI, 31%–37%]), and mobility (27% [95% CI, 25%–31%]). The intention-to-treat analysis showed that the prevalence of patients exceeding the MCID criteria was 42% in overall visual ability, 40% in reading, 34% in visual motor function, 30% in visual information processing, and 25% in mobility.
Table 2.
Effects of Low Vision Rehabilitation Intervention
| AI Change Score, Logits | MCID Criteria,a % | ||||
|---|---|---|---|---|---|
| Mean (SD) | ITT Sample | Achieved (95% CI) | Achieved (ITT Sample) | ||
| Goals/overall visual ability (n = 468) | 0.89(1.02) | 0.79 | 0.87 (0.82) | 47(44–50) | (41) |
| Reading (n = 422) | 0.45 (1.12) | 0.40 | 0.40 (0.38) | 44 (42–48) | (40) |
| Mobility (n = 269) | 0.50(1.25) | 0.45 | 0.40 (0.38) | 27 (25–31) | (25) |
| Visual information processing (n = 393) | 0.42 (0.83) | 0.38 | 0.51 (0.46) | 33 (31–37) | (30) |
| Visual motor function (n = 398) | 0.54(1.13) | 0.48 | 0.48 (0.45) | 38 (36–42) | (34) |
Abbreviations: AI, activity inventory; ITT, intention-to-treat;
MCID, minimum clinically important difference.
Criterion is met if the change score exceeds 1.96 (standard errors of the change score estimate).
Table 3 displays the results of 1-way ANOVA of change in patients’ overall visual ability (dependent variable) and patients’ characteristics (independent variables). Because of ceiling effects, many of the trait distributions were not normal; therefore, continuous independent variables were categorized into quartiles for this analysis. Significant main effects were seen only with age and visual acuity as the independent variables, but after correction for post hoc multiple comparisons, neither achieved the 5% α level.
Table 3.
Association of Overall Visual Ability With Patient Characteristics: Analysis of Variancea
| Variable | Sum of Squares | df | Mean Square | F Ratio | P Value |
|---|---|---|---|---|---|
| Ageb | 9.582 | 3 | 3.194 | 3.051 | .03 |
| Sex | 0.107 | 1 | 0.107 | 0.1 | .75 |
| logMAR visual acuityc | 8.78 | 3 | 2.927 | 2.908 | .04 |
| Logarithm of contrast sensitivityd | 1.148 | 2 | 0.574 | 0.542 | .58 |
| Depressed | 0.033 | 1 | 0.033 | 0.031 | .86 |
| Geriatric Depression Scalee | 0.646 | 3 | 0.215 | 0.201 | .90 |
| No. of symptomatic comorbidities | 6.944 | 9 | 0.772 | 0.723 | .69 |
| SF-36 physical componentf | 1.477 | 3 | 0.492 | 0.464 | .71 |
| Telephone Interview for Cognitive Statusg | 2.382 | 3 | 0.794 | 0.744 | .53 |
| Support services needed (eg, none or family) | 0.716 | 1 | 0.716 | 0.702 | .40 |
| Living alone | 1.666 | 1 | 1.666 | 1.571 | .21 |
| Driving status | 0.016 | 1 | 0.016 | 0.015 | .90 |
Abbreviation: SF-36, Medical Outcomes Study 36-Item Short Form Health Survey.
Incorporating a correction for multiple comparisons, we found that P < .004 is statistically significant for a criterion of α < .05.
Age in years is categorized into 66 years of age or younger, from older than 66 years of age to 76 years of age or younger, from 76 years of age to 84 years of age or younger, and older than 84 years.
Visual acuity is categorized into mild, moderate, severe, and profound vision loss.
Contrast sensitivity is defined as log contrast sensitivity categorized into normal (0.8–1.6) and abnormal (<0.8).
Depression is defined as a Geriatric Depression Scale continuous variable made categorical into quartiles.
Physical is defined as an SF-36 physical component continuous variable made categorical into quartiles.
Cognition is defined as a Telephone Interview for Cognitive Status continuous variable made categorical into quartiles.
Table 4 displays regression model coefficients for continuous independent variables previously identified as having independent effects on overall visual ability measures at baseline and for variables that were potential predictors from the ANOVA (age and visual acuity).31 Regression model coefficients were displayed for the same continuous independent variables when functional domain measures were dependent variables. After Bonferroni correction, age (P = .006) was an independent predictor of changes in overall visual ability at the goal level, and logMAR visual acuity (P = .002) was predictive of changes in visual information processing. Despite the independent effects of these variables, the β coefficients were small. No variable was predictive of changes in reading ability or visual motor function.
Table 4.
Regression Analysis of Overall Visual Ability and Domains
| Effect | r2 Coefficient (SE) | β Coefficient | t Value | 2-Tailed P Value |
|---|---|---|---|---|
| Goals | 0.027 | |||
| Constant | −0.041 (0.487) | 0 | −0.084 | .93 |
| SF-36 physical | 0.047 (0.024) | 0.106 | 1.927 | .06 |
| GDS | 0.058(0.033) | 0.094 | 1.766 | .08 |
| logMAR | −0.17 (0.103) | −0.08 | −1.648 | .10 |
| TICS | 0.011 (0.009) | 0.058 | 1.141 | .26 |
| Age | 0.01 (0.004) | 0.14 | 2.74 | .006a |
| Reading | 0.004 | |||
| Constant | −0.093 (0.586) | 0 | −0.158 | .88 |
| SF-36 physical | 0.023 (0.03) | 0.047 | 0.777 | .44 |
| GDS | 0.055 (0.039) | 0.081 | 1.405 | .16 |
| logMAR | 0.082 (0.125) | 0.035 | 0.656 | .51 |
| TICS | 0.007 (0.011) | 0.032 | 0.588 | .56 |
| Age | 0.005 (0.004) | 0.06 | 1.07 | .29 |
| Mobility | 0.007 | |||
| Constant | 2.351 (0.842) | 0 | 2.793 | .006 |
| SF-36 physical | 0.029 (0.404) | 0.056 | 0.724 | .47 |
| GDS | 0.046 (0.052) | 0.065 | 0.894 | .37 |
| logMAR | −0.225 (0.175) | −0.086 | −1.285 | .200 |
| TICS | −0.034(0.016) | −0.147 | −2.054 | .04 |
| Age | −0.005 (0.006) | −0.057 | −0.778 | .44 |
| Visual information | 0.008 | |||
| Constant | 0.417 (0.445) | 0 | 0.938 | .35 |
| SF-36 physical | 0.008 (0.022) | 0.022 | 0.356 | .72 |
| GDS | 0.033 (0.03) | 0.066 | 1.11 | .27 |
| logMAR | −0.298 (0.095) | −0.17 | −3.145 | .002a |
| TICS | 0 (0.009) | 0 | −0.005 | .10 |
| Age | 0.004 (0.003) | 0.075 | 1.31 | .19 |
| Visual motor | 0.001 | |||
| Constant | 0.33 (0.619) | 0 | 0.533 | .59 |
| SF-36 physical | 0.021 (0.031) | 0.041 | 0.669 | .50 |
| GDS | −0.012 (0.041) | −0.018 | −0.295 | .77 |
| logMAR | 0.022 (0.132) | 0.009 | 0.169 | .87 |
| TICS | 0 (0.012) | −0.001 | −0.013 | .99 |
| Age | 0.003 (0.005) | 0.037 | 0.636 | .53 |
Abbreviations: GDS, Geriatric Depression Scale; SF-36, Medical Outcomes Study 36-Item Short Form Health Survey; TICS, Telephone Interview for Cognitive Status.
Statistically significant after Bonferroni correction.
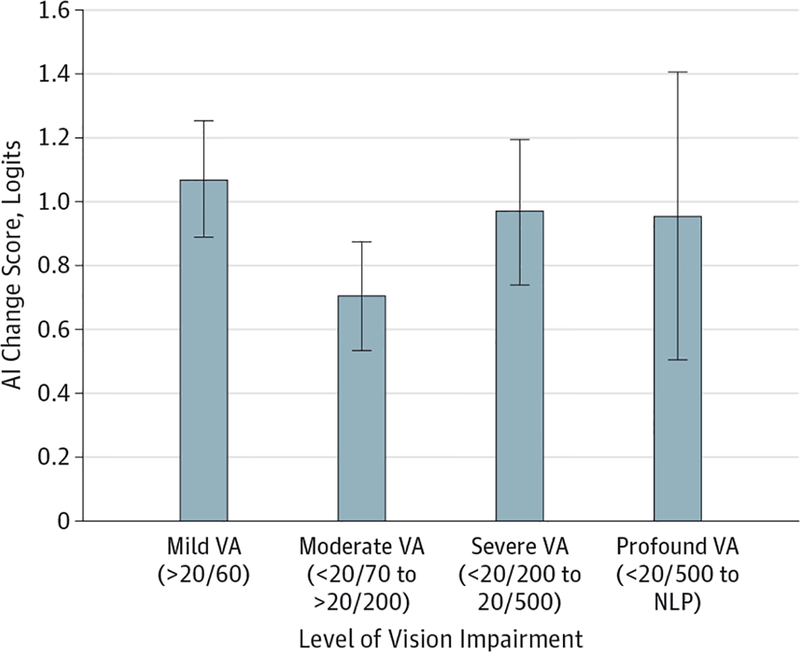
logMAR visual acuity was a significant predictor of visual information processing (P < .002) but not overall visual ability (P = .10) in the multivariable regression model, and the effect of logMAR on visual ability (P = .04) was minimized in the ANOVA once a correction was made for post hoc comparisons. Figure 2 shows changes in overall visual ability by logMAR visual acuity categorized as mild, moderate, severe, and profound. Similar magnitude changes in visual ability were seen across all categories of visual impairment with the exception of moderate visual impairment, for which the visual ability change score was smaller (95% CI, 0.54–0.88).
Figure 2. Change in Overall Visual Ability by Degree of Visual Impairment.
Patients with a visual acuity (VA) of better than 20/60 or worse than 20/200 show the greatest effects of low vision rehabilitation. Smaller effects are observed in patients with moderate loss of VA (<20/70 to >20/200). AI indicates Activity Inventory; NLP, no light perception. Error bars indicate 95% CIs.
Discussion
We learned that outpatient LVR services across the United States were effective in improving overall visual ability in nearly half (47%) of patients, with large average effect sizes (Cohen d = 0.87). At the domain level, the effect sizes were moderate (Cohen d = 0.40–0.51), with 28% to 45% of patients meeting the MCID change criteria. The visual ability effect sizes from this study slightly exceeded those of prior outpatient studies (Cohen d = 0.25–0.75).16,18,35 However, they did not achieve the very large effects (Cohen d = 1.96)15 seen in the Department of Veterans Affairs Blind Rehabilitation Center, which offers an intense 4- to 6-week inpatient LVR program with full coverage of vision-assistive equipment costs.
The regression analysis demonstrated that there were no consistent, strong predictors of LVR outcomes. Our findings showed that age was predictive of improved overall visual ability (measured at the goal level) and that visual acuity was predictive of improved visual information processing. However, neither showed strong effects, and there were no interactive effects between the 2 variables. This observation agrees with anecdotal reports of the difficulty predicting which patients will benefit from LVR, and it supports prior study results showing poor agreement between physician predictions of outcomes and observed changes in patients.36
Patients with a visual acuity of greater than 20/60 or less than 20/200 showed the greatest effects from LVR. Smaller effects were observed for patients with moderate loss of visual acuity (<20/70 to >20/200) and may be related to the patients not obtaining the necessary vision-assistive equipment (eg, because it was not covered by their insurance), the need for more intensive LVR training, or the lack of effective rehabilitation solutions for this subgroup. The beneficial changes seen in patients with greater than 20/60 visual acuity may result from simpler interventions, such as an increase in reading spectacle power and lighting enhancement. Patients with severe to profound loss of visual acuity may have had better LVR out- comes because they may have had time to adapt to their gradually deteriorating vision, and they may have had prior experience with rehabilitation strategies and vision-assistive equipment. Because visual acuity is not a good predictor of improvement in visual ability and because patients with visual acuity better than 20/60 show the greatest mean improvement after rehabilitation, these results suggest that visual acuity should not be the only basis for a referral to LVR services.
Conclusions
The strength of the MCID is that it provides a meaningful clinical end point to determine when beneficial effects from LVR are observed. The ability to quantify the percentage of patients who benefit from outpatient LVR services across the United States lays the foundation to test new surgical, electronic, and optical rehabilitation solutions. A limitation of our research is that it is an observational study without the presence of a control group. A randomized controlled clinical trial is still necessary to provide incontrovertible evidence of the effectiveness of outpatient LVR. With a well-validated, clinically meaningful PROM available, we are positioned to conduct such trials and develop and test new LVR treatments to improve visual ability in the 53% of patients for whom no clinically meaningful benefit was observed and to further increase the effectiveness of LVR for all patients.
Funding/Support:
This work was supported by grants EY012045 and EY018696 from the National Eye Institute, National Institutes of Health, Bethesda, Maryland, and a grant from Reader’s Digest Partners for Sight Foundation.
Role of the Funder/Sponsor: The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and Dr Massof reports serving as a consultant on patient-reported outcome measures for Alcon. No other disclosures are reported.
REFERENCES
- 1.Binns AM, Bunce C, Dickinson C, et al. How effective is low vision service provision? a systematic review. Surv Ophthalmol. 2012;57(1): 34–65. [DOI] [PubMed] [Google Scholar]
- 2.McIlwaine GG, Bell JA, Dutton GN. Low vision aids—is our service cost effective? Eye (Lond). 1991; 5(pt 5):607–611. [DOI] [PubMed] [Google Scholar]
- 3.Harper R, Doorduyn K, Reeves B, Slater L. Evaluating the outcomes of low vision rehabilitation. Ophthalmic Physiol Opt. 1999;19(1): 3–11. [DOI] [PubMed] [Google Scholar]
- 4.Rees G, Keeffe JE, Hassell J, Larizza M, Lamoureux E. A self-management program for low vision: program overview and pilot evaluation. Disabil Rehabil. 2010;32(10):808–815. [DOI] [PubMed] [Google Scholar]
- 5.Scott IU, Smiddy WE, Schiffman J, Feuer WJ, Pappas CJ. Quality of life of low-vision patients and the impact of low-vision services. Am J Ophthalmol. 1999;128(1):54–62. [DOI] [PubMed] [Google Scholar]
- 6.McCabe P, Nason F, Demers Turco P, Friedman D, Seddon JM. Evaluating the effectiveness of a vision rehabilitation intervention using an objective and subjective measure of functional performance. Ophthalmic Epidemiol. 2000;7(4):259–270. [DOI] [PubMed] [Google Scholar]
- 7.Hinds A, Sinclair A, Park J, Suttie A, Paterson H, Macdonald M. Impact of an interdisciplinary low vision service on the quality of life of low vision patients. Br J Ophthalmol. 2003;87(11):1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamoureux EL, Hassell JB, Keeffe JE. The determinants of participation in activities of daily living in people with impaired vision. Am J Ophthalmol. 2004;137(2):265–270. [DOI] [PubMed] [Google Scholar]
- 9.Reeves BC, Harper RA, Russell WB. Enhanced low vision rehabilitation for people with age related macular degeneration: a randomised controlled trial. Br J Ophthalmol. 2004;88(11):1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Grow SJ. The effectiveness of comprehensive low vision services for older persons with visual impairments in New Zealand. J Vis Impair Blind. 2004;98(11):679–692. http://eric.ed.gov/?id=EJ683813. Accessed March 3, 2015. [Google Scholar]
- 11.de Boer MR, Twisk J, Moll AC, Völker-Dieben HJ, de Vet HC, van Rens GH. Outcomes of low-vision services using optometric and multidisciplinary approaches: a non-randomized comparison. Ophthalmic Physiol Opt. 2006;26(6):535–544. [DOI] [PubMed] [Google Scholar]
- 12.Court H, Ryan B, Bunce C, Margrain TH. How effective is the new community-based Welsh low vision service? Br J Ophthalmol. 2011;95(2):178–184. [DOI] [PubMed] [Google Scholar]
- 13.Ryan B, Khadka J, Bunce C, Court H. Effectiveness of the community-based Low Vision Service Wales: a long-term outcome study. Br J Ophthalmol. 2013;97(4):487–491. [DOI] [PubMed] [Google Scholar]
- 14.Smith HJ, Dickinson CM, Cacho I, Reeves BC, Harper RA. A randomized controlled trial to determine the effectiveness of prism spectacles for patients with age-related macular degeneration. Arch Ophthalmol. 2005;123(8):1042–1050. [DOI] [PubMed] [Google Scholar]
- 15.Stelmack JA, Szlyk JP, Stelmack TR, et al. Measuring outcomes of vision rehabilitation with the veterans affairs low vision visual functioning questionnaire. Invest Ophthalmol Vis Sci. 2006;47(8):3253–3261. [DOI] [PubMed] [Google Scholar]
- 16.Lamoureux EL, Pallant JF, Pesudovs K, Rees G, Hassell JB, Keeffe JE. The effectiveness of low-vision rehabilitation on participation in daily living and quality of life. Invest Ophthalmol Vis Sci. 2007;48(4):1476–1482. [DOI] [PubMed] [Google Scholar]
- 17.Stelmack JA, Tang XC, Reda DJ, Rinne S, Mancil RM, Massof RW; LOVIT Study Group. Outcomes of the veterans affairs low vision intervention trial (LOVIT). Arch Ophthalmol. 2008;126(5):608–617. [DOI] [PubMed] [Google Scholar]
- 18.Pearce E, Crossland MD, Rubin GS. The efficacy of low vision device training in a hospital-based low vision clinic. Br J Ophthalmol. 2011;95(1):105–108. [DOI] [PubMed] [Google Scholar]
- 19.Stelmack JA, Tang XC, Wei Y, Massof RW; Low-Vision Intervention Trial Study Group. The effectiveness of low-vision rehabilitation in 2 cohorts derived from the Veterans Affairs Low-Vision Intervention Trial. Arch Ophthalmol. 2012;130(9):1162–1168. [DOI] [PubMed] [Google Scholar]
- 20.Velozo CA, Warren M, Hicks E, Berger KA. Generating clinical outputs for self-reports of visual functioning. Optom Vis Sci. 2013;90(8):765–775. [DOI] [PubMed] [Google Scholar]
- 21.Massof RW, Hsu CT, Baker FH, et al. Visual disability variables, I: the importance and difficulty of activity goals for a sample of low-vision patients. Arch Phys Med Rehabil. 2005;86(5):946–953. [DOI] [PubMed] [Google Scholar]
- 22.Massof RW, Hsu CT, Baker FH, et al. Visual disability variables, II: the difficulty of tasks for a sample of low-vision patients. Arch Phys Med Rehabil. 2005;86(5):954–967. [DOI] [PubMed] [Google Scholar]
- 23.Massof RW, Stelmack JA. Interpretation of low-vision rehabilitation outcome measures. Optom Vis Sci. 2013;90(8):788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein JE, Massof RW, Deremeik JT, et al. ; Low Vision Research Network Study Group. Baseline traits of low vision patients served by private outpatient clinical centers in the United States. Arch Ophthalmol. 2012;130(8):1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massof RW. A systems model for low vision rehabilitation, I: basic concepts. Optom Vis Sci. 1995;72(10):725–736. [DOI] [PubMed] [Google Scholar]
- 27.Massof RW, Ahmadian L, Grover LL, et al. The Activity Inventory: an adaptive visual function questionnaire. Optom Vis Sci. 2007;84(8):763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manly JJ, Schupf N, Stern Y, Brickman AM, Tang MX, Mayeux R. Telephone-based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68(5):607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients: a comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997; 157(4):449–454. [PubMed] [Google Scholar]
- 30.Cooper JK, Kohlmann T, Michael JA, Haffer SC, Stevic M. Health outcomes: new quality measure for Medicare. Int J Qual Health Care. 2001;13(1):9–16. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein JE, Chun MW, Fletcher DC, Deremeik JT, Massof RW; Low Vision Research Network Study Group. Visual ability of patients seeking outpatient low vision services in the United States. JAMA Ophthalmol. 2014;132(10):1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bond TG, Fox CM. Applying the Rasch Model: Fundamental Measurement in the Human Sciences. 2nd ed Routledge: New York, NY; 1977:314. [Google Scholar]
- 33.Massof RW. Understanding Rasch and item response theory models: applications to the estimation and validation of interval latent trait measures from responses to rating scale questionnaires. Ophthalmic Epidemiol. 2011;18(1):1–19. [DOI] [PubMed] [Google Scholar]
- 34.Zelinski EM, Gilewski MJ. Effects of demographic and health variables on Rasch scaled cognitive scores. J Aging Health. 2003;15(3):435–464. [DOI] [PubMed] [Google Scholar]
- 35.Wolffsohn JS, Cochrane AL. Design of the Low Vision Quality-of-Life Questionnaire (LVQOL) and measuring the outcome of low-vision rehabilitation. Am J Ophthalmol. 2000;130(6):793–802. [DOI] [PubMed] [Google Scholar]
- 36.Chan TL, Goldstein JE, Massof RW; Low Vision Research Network Study Group. Comparison of clinician-predicted to measured low vision outcomes. Optom Vis Sci. 2013;90(8):776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]