Abstract
In the unique supply chain of cellular therapies, preservation is important to keep the cell product viable. Many factors in cryopreservation affect the outcome of a cell therapy: 1) formulation and introduction of a freezing medium, 2) cooling rate, 3) storage conditions, 4) thawing conditions, and 5) post-thaw processing. This article surveys clinical trials of cellular immunotherapy that utilized cryopreserved regulatory, chimeric antigen receptor or gamma delta T cells; dendritic cells; or natural killer cells. Several observations are summarized from the given information. The aforementioned cell types have been similarly frozen in media containing 5–10% DMSO with plasma, serum or human serum albumin. Two common freezing methods are an insulated freezing container such as Nalgene Mr. Frosty and a controlled-rate freezer at a cooling rate of −1 °C/min. ~37 °C water bath has been universally used for thawing. Post-thaw processing of cryopreserved cells varied greatly: some studies infused the cells immediately upon thawing; some diluted the cells in a carrier solution of varying formulation before infusion; some washed cells to remove cryoprotective agents; others recultured cells to recover cell viability or functionality lost due to cryopreservation. Emerging approaches to preserving cellular immunotherapies are also described. DMSO-free formulations of the freezing media have demonstrated improved preservation of cell viability in T lymphocytes and of cytotoxic function in NK cells. Saccharides are a common type of molecule used as an alternative cryoprotective agent to DMSO. Improving methods of preservation will be critical to growth in the clinical use of cellular immunotherapies.
Keywords: cell therapies, cryopreservation, dendritic cells, NK cells, T cells
Introduction
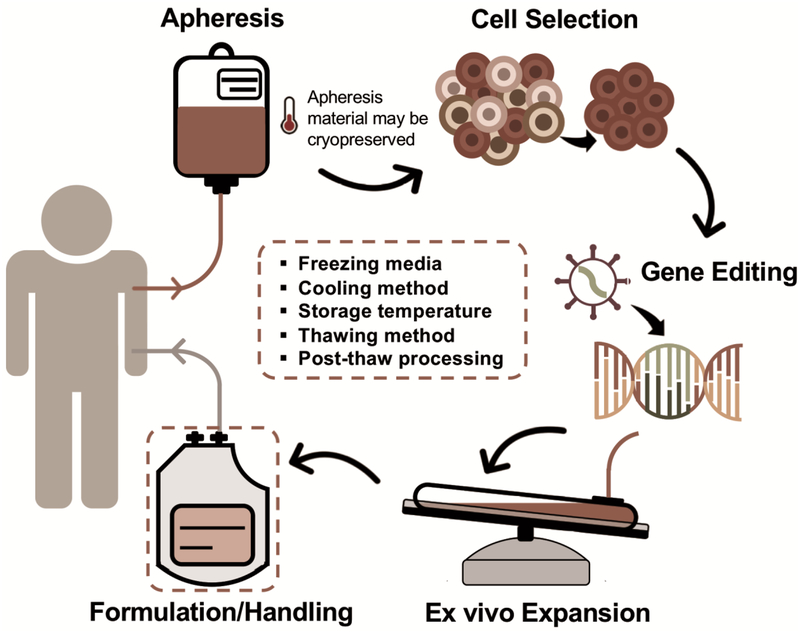
The supply chain of cellular therapy is unique and distinct from that of other medical therapies. Cells are harvested from a living donor typically in a clinical location and then sent to a processing facility for selection, expansion, genetic modification, etc. before they are returned to a clinical location for administration (Figure 1). The cells must remain viable and functional along this complex supply chain. Ineffective methods of preservation limit growth in the use of cell therapies and contribute significantly to their cost. Effective methods of cryopreservation permit coordination of the therapy with patient care and completion of safety and quality control testing.
Figure 1.
A schematic of the unique supply chain of cellular immunotherapy where the 5 factors of cryopreservation play central role to keep cells viable. Cells are harvested from a donor (and sometimes cryopreserved at this point), subpopulations of cells selected; they may be genetically modified or antigen-based treatment to express the desired biological properties; expanded in vitro to achieve the desired cell dose, a cryopreservation solution introduced and the cells cryopreserved.
This paper reviews the common methods of preservation for cell-based immunotherapies used for clinical trials. The survey was conducted using publicly available databases: the WHO International Clinical Trials Registry Platform, the U.S. National Library of Medicine Clinical Trials Registry, the E.U. Clinical Trials Register, the (Japan) UMIN Clinical Trials Registry and the (Republic of Korea) Clinical Research Information Service. Certain cell therapy trials did not provide enough information to determine the state of the cells being administered to patients (fresh or cryopreserved). Cell types covered here are T lymphocytes, including chimeric antigen receptor (CAR) T cells, T regulatory cells (Tregs), and gamma delta (γδ) T cells, dendritic cells (DCs), and natural killer (NK) cells. Mesenchymal stromal cells (MSCs) are another cell type involved in a large number of clinical trials, where they are employed for their immunomodulatory effect. Recent reviews have already described methods of preserving MSCs[1,2], and thus this cell type has been omitted.
Factors that influence preservation of cellular therapies
The most common descriptions of cell preservation for clinical trials of cell therapies include the cryopreservation solution and the cooling rate. This section will provide a brief overview of the critical factors that can influence post-thaw recovery of a cellular therapy. More details on these processes can be found in a recent book[3]. Several factors affect the outcome of cellular therapy: 1) formulation and introduction of a freezing medium, 2) cooling rate, 3) storage conditions, 4) thawing conditions, and 5) post-thaw processing.
Formulation and introduction of a freezing medium:
Cells that are cryopreserved must be suspended in a specialized solution containing agents that help the cells survive the stresses of freezing and thawing (e.g. cryoprotective agents, CPAs). The most commonly used cryoprotective agents are dimethyl sulfoxide (DMSO)[4] and glycerol. Cryopreservation solutions are typically hypertonic, and cell losses can result from introduction of the solution (osmotic stress)[5]. Certain CPAs (i.e. DMSO) are toxic to cells, and cell losses are observed as a function of time of exposure (biochemical toxicity).
As a result, the introduction process must be optimized to minimize cell losses from osmotic stress and biochemical toxicity. The window of manufacturing both pre-freeze and post-thaw can be influenced by biochemical toxicity. For example, duration in which cells are exposed to DMSO-containing cryopreservation solutions is limited to 30 minutes pre-freeze and post-thaw[6]. This restriction complicates the workflow considerably at both the site of manufacture and the site of use. DMSO-free multi-osmolyte solutions have effectively preserved MSCs[7] and T lymphocytes[8] and have provided improved cell stability. Therefore, these CPAs have prolonged the window of manufacturing both pre-freeze and post-thaw.
Cooling rate:
The rate of change of temperature with time from the freezing temperature of the solution to roughly −50 °C is the cooling rate of interest during freezing. Studies have demonstrated that for a wide range of cell types, the post-thaw recovery is strongly influenced by the cooling rate[9]. The prevalence of controlled rate freezing for cell therapy products demonstrates the importance of the cooling rate on post-thaw survival.
Storage conditions:
Once the freezing process is complete, samples are placed into storage. Cell therapy products may be stored for days, weeks or even decades. The scientific basis for selection of a storage temperature is beyond the scope of this review article, but more information on this issue can be found in Hubel et al. [10]. Best practices for storage suggest that cell therapy products be stored at temperatures < −150 °C[3]. The stability of a cell therapy product in cryogenic storage is of interest. As with any biospecimen, transient warming of a frozen sample, which occurs when the storage unit is accessed to remove or add samples, is a potential cause of degradation[11]. Proper training or the use of robotic sample retrieval technologies can reduce the potential for degradation of the samples in storage.
Thawing conditions:
Samples are not used in the frozen state and are only useful after thawing. Therefore, the same temperature range traversed during cooling has to be traversed during warming. Standard practice has been to immerse frozen cellular products in a 37 °C water bath and swirl the product to enhance the thawing rate. Recently, controlled thawing devices have been developed that do not use a water bath, reduce the potential for contamination and improve the consistency of the thawing process. Not surprisingly, the proper thawing rate is influenced by the cooling rate used[9]. The thawing process has been studied far less than the cooling process, and a recent study suggests that warming rates are not critical for slowly cooled samples[12].
Post-thaw processing:
Cells that are frozen and thawed may be infused directly, diluted, or washed and suspended in a carrier solution. Cryopreservation solutions are typically hypertonic, and post-thaw wash solutions are typically designed to reduce the osmotic stress associated with dilution or washing, as cells are more sensitive to expansion than contraction[13]. The product infused into the patient reflects the nature of post-thaw processing (diluted, washed or non-manipulated cells).
As described above, a variety of factors influence the post-thaw recovery of cells. In describing the freezing process used for a clinical trial, the most common method is to describe the composition of the cryopreservation solution and the cooling rate, and the review given below will use that common notation. Including a brief description of the post-thaw processing, so that it is clear the nature of the product being infused into the patient, is also helpful.
Cryopreserved T lymphocytes in clinical trials
Regulatory T-cell therapy
Hematopoietic stem cell transplantation (HSCT) has been implemented successfully for the treatment of hematological malignancies since the late 1950s[14]. However, the incidence of graft-versus-host disease (GvHD) following allogeneic stem cell transplantation is associated with various adverse effects in patients and results in a decrease in survival. Regulatory T cells (Tregs) as a subpopulation of lymphocytes have been explored to mitigate the severity of GvHD due to their capabilities of modulating immune responses and maintaining immunological self-tolerance and immune homeostasis[15,16]. In addition to adoptive transfer of Tregs as a prophylaxis against acute GvHD[17,18] in the setting of HSCT, Tregs have also attracted much research interest in the field of organ transplantation and autoimmune disease treatment[19].
The majority of current immunotherapies rely on the enrichment of Tregs derived from cord blood or peripheral blood and subsequent infusion of fresh Tregs to patients. Only a few clinical studies used cryopreserved Tregs with documentation of the freezing medium and protocols (see Table 1). Some studies only utilized cryopreserved cells when necessary due to patient difficulties and delay[20,21]. 10% DMSO in intravenous infusion solution with a varying concentration of human serum albumin (HSA) was most frequently used as the freezing medium in these studies. Cryopreserved cells were further stored in liquid nitrogen (LN). The freezing method and thawing method were not mentioned in most studies. After thawing, Tregs were either diluted with dextran and HSA solution before infusion or directly infused to patients. In one study, non-expanded Tregs were cryopreserved and after thawing, cells were washed into culture media and expanded for 7 days for a second infusion of Tregs[22]. In another study, cryopreserved Tregs were found to maintain their phenotype and suppressive function after being stored for 3 months in the vapor phase of LN[23].
Table 1.
Methods of T-cell preservation for clinical trials
| Study ID | Phase | Cell type | Disease | Freezing medium | Freezing method | Storage | Thawing method | Post-thaw processing | Institution | Ref.1 |
|---|---|---|---|---|---|---|---|---|---|---|
| I | Tregs | GvHD prophylaxis | Plasma-Lyte A, 10% DMSO, HSA | (unknown) | (unknown) | (unknown) | Diluted in 5% albumin, 10% dextran 40 and infused | University of Minnesota | [20,21] | |
| EK 206082008 | I | Tregs | GvHD prophylaxis | 10% DMSO, 200g/L HSA | (unknown) | LN | (unknown) | Diluted and recultured | Technische Universitat Dresden | [22] |
| I/II | Tregs | Liver transplant | CryoStor CS10 | Controlled-rate freezer | Vapor phase LN | (unknown) | (unknown) | Guy’s and St. Thomas’ NHS Foundation Trust | [23] | |
| EudraCT2006-004712-44 | I/II | Ovalbumin-specific Tregs | Refractory Crohn’s disease | (unknown) | (unknown) | Vapor phase LN | (unknown) | Infused immediately upon thawing | Huriez Hospital | [67]* |
| I | CD19 CAR T cells | Leukemia, lymphoma | (unknown) | (unknown) | (unknown) | (unknown) | Infused immediately upon thawing | Baylor College of Medicine | [68]* | |
| I | κ light chain CAR T Cells | Lymphoma, myeloma, leukemia | (unknown) | (unknown) | (unknown) | (unknown) | (unknown) | Baylor College of Medicine | [69]* | |
| I/II | CD19 CAR T cells | Lymphoma | Cryostor CS5 | Nalgene Mr. Frosty | (unknown) | (unknown) | (unknown) | City of Hope Medical Center | [31] | |
| I | CD19 CAR T cells | Multiple myeloma | 31.25% Plasma-Lyte A, 31.25% dextrose, 0.45% NaCl, 7.5% DMSO, 1% dextran 40, 5% HSA | (unknown) | (unknown) | 36 to 38 °C water bath | Infused immediately upon thawing | University of Pennsylvania | [30] | |
| I | CD19 CAR T cells | Leukemia, lymphoma | 31.25% Plasma-Lyte A, 31.25% dextrose, 0.45% NaCl, 7.5% DMSO, 1% dextran 40, 5% HSA | (unknown) | (unknown) | 36 to 38 °C water bath | Infused immediately upon thawing | University of Pennsylvania | [33,70–72] | |
| I | CD19 CAR T cells | Leukemia, lymphoma | 50% HSA, 40% PlasmaLyte, 10% DMSO | Controlled-rate freezer (Planer) | Vapor phase LN | (unknown) | (unknown) | M.D. Anderson Cancer Center | [32,73,74] | |
| I | CD20 CAR T cells | Leukemia, lymphoma | Plasma-Lyte A, 5% HSA, 10% DMSO | (unknown) | (unknown) | (unknown) | Infused immediately upon thawing | Fred Hutchinson Cancer Research Center | [35,75] | |
| I | Her2 CAR T cells | HER2-positive sarcoma | (unknown) | (unknown) | (unknown) | (unknown) | (unknown) | Baylor College of Medicine | [76]* | |
| I | GD-2 CAR T cells | Neuroblastoma | (unknown) | (unknown) | (unknown) | (unknown) | (unknown) | Baylor College of Medicine | [77]* | |
| I | Mesothelin CAR T cells | Malignant pleural mesothelioma | (unknown) | (unknown) | (unknown) | (unknown) | Infused immediately upon thawing | University of Pennsylvania | [28,29]* | |
| I | 3Ra2-redirected CD8CAR T Cells | Recurrent glioblastoma | (unknown) | (unknown) | (unknown) | (unknown) | Recultured | City of Hope Medical Center | [78]* | |
| I | Anti-CEA T cells | Liver metastases | PlasmaLyte, 20% HSA, 10% DMSO, IL2 | (unknown) | LN | (unknown) | Infused immediately upon thawing | Roger Williams Medical Center | [79] |
denotes clinical trials that used cryopreserved cells but did not provide clear information of the freezing media.
CAR T-cell therapy
Chimeric antigen receptor (CAR) T-cell therapy is another novel immunotherapy in which T cells from patients are genetically modified followed by ex vivo expansion and infusion [24].
Two immunotherapies based on CD19-targeted CAR T cells have been approved by the FDA to treat two types of hematologic cancers: acute lymphoblastic leukemia (KYMRIAH) and diffuse large B-cell lymphoma (YESCARTA and KYMRIAH)[25]. Other B-cell antigens such as CD20, CD22, and κ light chain have also been targeted in clinical studies for the treatment of different types of lymphoma and leukemia, and studies using CAR T cells targeting B-cell maturation antigen have revealed very promising clinical outcomes for the treatment of multiple myeloma[26]. In addition to hematologic cancers, T cells have also been genetically modified to target antigens of solid tumors such as prostate cancer[27] and malignant pleural mesothelioma[28,29], although the successful development of CAR T-cell therapy for solid tumors has its unique challenges[25].
Several clinical studies have used cryopreserved CAR T cells, some of which reported the details of freezing medium and protocols (see Table 1). Similar to Tregs, CAR T cells were most frequently frozen in 10% DMSO in intravenous infusion solution with a varying concentration of HSA. Plasma-Lyte A was the most common intravenous infusion solution used in these studies. Some studies have lowered the concentration of DMSO in the freezing medium from 10% to 7.5% or 5% [30,31]. In addition to DMSO, some clinical trials also included dextran and dextrose in the freezing medium[30]. The use of dextran could prevent aggregation of DNA and proteins from lysed cells as well as swelling of cells during thawing. Similar to Tregs, the majority of CAR T-cell therapy clinical studies did not clearly state the freezing and thawing methods. A few studies mentioned using a controlled-rate freezer to freeze cells[23,32] and a 36 to 38 °C water bath to thaw cryopreserved cells[30,33]. Unlike Tregs, the vast majority of CAR T-cell studies infused the cryopreserved cells immediately upon thawing. It is noteworthy that in two clinical trials[34,35], only a portion of the patients received cryopreserved cells, while the rest of the patients received fresh cells.
Gamma delta (γδ) T-cell therapy
Another small subset of T cells, gamma delta (γδ) T cells, has also been investigated for safety and efficiency in immunotherapy due to its broad functionality including production of cytokines and interaction with infected cells or other immune cells[36]. Unlike most T cells, with T-cell receptors (TCRs) composed of an alpha and a beta chain, γδ T cells express TCRs composed of a gamma and a delta chain, making the activation of γδ T cells MHC-independent. The clinical application of γδ T cells has evolved into two basic methods: stimulation of γδ T cells by drugs in vivo and adoptive transfer of enriched γδ T cells population[37]. For the second method, γδ cells are purified from peripheral blood mononuclear cells (PBMCs), and expanded ex vivo to obtain sufficient cells before infused into patients. As the majority of γδ T-cell clinical trials used fresh cells, information regarding cryopreservation of gamma-delta cells for clinical trials is rare. A research study found that γδ T cells expanded from umbilical cord blood (UCB) and cryopreserved in the complete medium with 10% DMSO maintained tumor-killing capacity showing a potential for clinical application[38].
Cryopreserved dendritic cells in clinical trials
Dendritic cells (DCs), a type of antigen presenting cell, function to process antigens and present them on the cell membrane to T cells. DCs act as a bridge between the innate and adaptive immune systems[39]. DCs emerged in immunotherapy with a first clinical trial in 1996 and gained a first and so far only FDA product approval in 2010. DC-based immunotherapy aims to treat various types of cancer or in some cases[40,41], human immunodeficiency virus (HIV) infection by eliciting and boosting a patient’s immunity against diseased cells, and hence they are often called DC vaccines. Production of a DC therapy frequently involves harvesting monocytes from the patient, differentiating them into mature DCs and then loading the cells with tumor antigens, resulting in a final product[42]. Loading of the cells with disease antigens occurs by a variety of different payload delivery methods: incubation with lysed or apoptotic cancer cells; fusion with live, irradiated cancer cells; and electroporation with disease antigen peptide or its encoding mRNA[42]. Cells at different stages of production may be cryopreserved.
Current methods of cryopreserving DCs that are found in practice of DC-based immunotherapy are listed in Table 2. Most clinical trials cryopreserved fully-processed, mature, antigen-loaded DCs at the end of the workflow. There were few exceptions of cryopreserving intermediate, immature or non-loaded DCs while providing freshly cultured DC vaccines to patients. All clinical trials used at least 5% DMSO as a CPA in the freezing media, most of which were formulated in HSA, serum or plasma. Two of the most common freezing modalities were freezing containers (e.g. Nalgene, Mr. Frosty) and controlled-rate freezers (e.g. Planer), both used with a desired cooling rate of 1 °C/min. Long-term storage temperatures at the end of the freezing process varied slightly within the cryogenic range, from −150 °C (vapor phase LN) to – 196 °C (liquid phase LN). A 37 °C water bath was used by all to thaw cryopreserved DCs. However, post-thaw processing varied among the different trials. CPAs were removed by washing or simply diluted in a carrier solution prior to administration of DC vaccines, and the composition of the carrier solution varied.
Table 2.
Methods of dendritic cell preservation for clinical trials
| Study ID | Phase | Disease | Cell type2 | Freezing cell concentration | Freezing medium | Freezing method | Storage | Thawing method | Post-thaw processing | Institution | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | Acute myeloid leukemia | DC (R) | 1e7 cells/ml | 10% DM SO, 2% glucose in serum | Nalgene Mr. Frosty in −80 °C freezer | (unknown) | 37 °C water bath | (unknown) | Antwerp University Hospital | [80] | |
| I | HIV | DC (P) | 3e7 cells/ml | 10% DM SO, 5% dextrose, 10% Plasma-Lyte A in serum | Controlled-rate freezer | −180 °C vapor phase LN | (unknown) | Diluted 10-fold in saline and infused | Baylor Research Institute | [41] | |
| I/II | Melanoma | DC (A) | 3e7 cells/ml | 10% DM SO, 5% dextrose, 10% Plasma-Lyte A in serum | Controlled-rate freezer (Planer), followed by 4–24 hours in −80 °C freezer | LN | (unknown) | Washed, resuspended in Ringer’s lactate solution and infused | Baylor Research Institute | [81] | |
| II | Acute myeloid leukemia | DC (F) | 5e6 cells/vial | 10% DM SO in plasma | (unknown) | Vapor phase LN | (unknown) | Irradiated at 30cGy and infused | Beth Israel Deaconess Medical Center | [82] | |
| II | Multiple myeloma | DC (F) | 0.5–5e6 cells/vial per cell yield | 10% DM SO in plasma | (unknown) | Vapor phase LN | (unknown) | Irradiated at 30cGy and infused | Beth Israel Deaconess Medical Center | [83] | |
| I | Multiple myeloma | DC (F) | 1, 2, 4e6 cells/vial per dose ranging | 10% DM SO in plasma | (unknown) | Vapor phase LN | (unknown) | Irradiated at 30cGy and infused | Beth Israel Deaconess Medical Center | [84] | |
| I/II | Melanoma | DC (L) | 1–1.5e6 cells/vial | (unknown) | (unknown) | LN | (unknown) | (unknown) | Cancer Insight, LLC | [85] | |
| I | Glioblasto ma | DC (P) | 1e7 cells/ml | 10% DM SO in serum | Nalgene Mr. Frosty in −80 °C freezer | Vapor phase LN | 37 °C water bath | Infused immediately upon thawing | Cedars Sinai Medical Center | [86] | |
| I/II | Chronic myeloid leukemia | DC (P) | 2e6 cells/ml | 10% DMSO, 40% HSA, 5% FCS in RPMI | Controlled-rate freezer (Consartic) built-in PBMC program | LN | 37 °C water bath | Washed, resuspended and recultured | Charite University | [87,88] | |
| II | Colorectal carcinoma hepatic metastasis | DC (L) | 3e7 cells/aliquot | 5% DMSO in serum | Programmable step down freezer (Carburos Metalicos) | −120 °C | (unknown) | Resuspended in saline and infused | Clinica Universidad de Navarra, Universidad de Navarra | [89,90] | |
| II | Metastatic cancer | DC (L) | 3e7 cells/aliquot | 5% DMSO in serum | Programmable step down freezer (Carburos Metalicos) | −120 °C | (unknown) | Resuspended in saline and infused | Clinica Universidad de Navarra, Universidad de Navarra | [90] | |
| I/II | Acute myeloid leukemia | DC (A) | (unknown) | 10% DMSO (Cryostor CS10) | (unknown) | (unknown) | (unknown) | Infused immediately upon thawing | DCPrime BV | [91] | |
| I | Metastatic cancer | DC (R) | 1–1.4e7 cells/vial | 10% DMSO in serum | (unknown) | (unknown) | (unknown) | (unknown) | Duke University | [92] | |
| I | Multiple sclerosis | DC (P) | 1e7 cells/vial | 10% DM SO in serum | Nalgene Mr. Frosty in −80 °C freezer | LN | 37 °C water bath | Washed with PBS and infused | Fundacio Institut Germans Trias i Pujol | [93] | |
| EudraCT2007-007596-16 | II | Breast cancer | DC (P) | minimum 5e6 cells/vial | 10% DM SO, 5% glucose in serum | Controlled-rate freezer (Planer) | (unknown) | (unknown) | Washed, resuspended and loaded with peptides | Herlev Hospital | [94] |
| I | Breast cancer | DC (P) | 1e7 cells/aliquot | 10% DM SO, 5% glucose in serum | Controlled-rate freezer (Planer) | (unknown) | (unknown) | Washed, resuspended and loaded with peptides | Herlev Hospital | [95] | |
| II | Melanoma | DC (L) | 0.5–1.5e7 cells/aliquot | Unknown formulation in saline | Automated freezer | (unknown) | (unknown) | Washed, resuspended in saline and infused | Istituto Scientifico Romagnolo per lo Studio e la cura dei Tumor | [96] | |
| (unknown) | I | Leukemia | DC (A) | 1–2e7 cells/ml | 10% DM SO in plasma | Controlled-rate freezer (Planer) | −150 °C | (unknown) | Washed, resuspended in saline and infused | Karolinska University Hospital | [97,98] |
| UMIN000001 024 | I | Acute myeloid leukemia | DC (A) | (unknown) | 12% hydroxymethyl starch, 10% DMSO, 8% HSA in saline | (unknown) | −150 °C | (unknown) | Mixed with 1 KE OK-432 | Kyoto University Hospital | [99] |
| I | Ovarian cancer | DC (R) PROCURE® | 2.6e7 cells/ml | 10% DM SO, 5% glucose in saline | (unknown) | (unknown) | (unknown) | (unknown) | Life Research Technologies GmbH | [100] | |
| I | Recurrent, progressive malignant glioma | DC (S) | 6.67–8e8 cells/vial | 10% DMSO | (unknown) | −150 °C vapor phase LN | (unknown) | Recultured with tumor lysate | Masonic Cancer Center, University of Minnesota | [101] | |
| I/II | HIV | DC (R) | (unknown) | (unknown) | (unknown) | (unknown) | (unknown) | (unknown) | Massachusetts General Hospital | [40] | |
| UMIN000011 423 | I | Adult T cell leukemia/lymphoma | DC (P) | 5e6 cells/vial | 10% DMSO, 50% serum, 40% RPMI 1640 | Bicell biofreezing vessel (Nihon) −1 °C/min to −135 °C | (unknown) | 37 °C water bath | Washed and infused | National Hospital Organization Kyushu Cancer Center | [102,103] |
| I | Melanoma | DC (P) | 0.1–1.5e7 cells/ml | 10% DMSO in plasma | Controlled-rate freezer (Planer) −1 °C/min | LN | 37 °C water bath | Placed on ice | New York University School of Medicine | [104] | |
| III | Glioblasto ma | DC (L) DCVax ®-L | 0.4, 2, 4e6 cells/ml per dose escalation | (unknown) | (unknown) | (unknown) | (unknown) | (unknown) | Northwest Biotherapeutics | [43,105,106] | |
| I/II | Melanoma | DC (R) | 0.5–2e7 cells/vial | 10% DMSO, 50% HSA in CellGro DC medium | Controlled-rate freezer −1 °C/min to −40°C, 1–2°C/min to −80°C | LN | 37 °C water bath | Washed, resuspended in PB S and infused | Oslo University Hospital | [107,108] | |
| I/II | Prostate cancer | DC (R) | 2e7 cells/vial | (unknown) | Unknown method to −80 °C | LN | 37 °C water bath | Washed, resuspended in PB S and infused | Oslo University Hospital | [109] | |
| I/II | Acute myeloid leukemia | DC (R) | 1–1.25e7 cells/ml | 10% DMSO, 5% glucose in HSA | Nalgene Mr. Frosty in −80 °C freezer | Vapor phase LN | (unknown) | (unknown) | Oslo University Hospital | [110] | |
| II/III | Glioblasto ma | DC (R) DEN-STEM | (unknown) | (unknown) | (unknown) | Vapor phase LN | (unknown) | (unknown) | Oslo University Hospital | [111] | |
| I | Melanoma | DC (L) | 5e7 cells/dose | (unknown) | Nalgene Mr. Frosty in −80 °C freezer | −130 °C | (unknown) | Resuspended in 0.9% NaCl and infused | PrimeVax Immuno-Oncology Inc. | [112] | |
| NTR1086 | I | Multiple myeloma | DC (R) | (unknown) | (unknown) | (unknown) | (unknown) | (unknown) | Washed twice with 0.9% NaCl and 5% HSA and infused | Radboud University Nijmegen Medical Centre | [113] |
| I | Melanoma | DC (P) | 0.3, 1, 3e6 cells/vial per dose ranging | 10% DMSO in unknown basal formulation | (unknown) | (unknown) | (unknown) | Loaded with peptides | Radboud University Nijmegen Medical Centre | [114] | |
| KCT0000008 | II | Hepatocel lular carcinoma | DC (P) CreaVax-HCC | 3e7 cells/vial | 5% DMSO in HSA or plasma | Ultralow freezer for >12h | Below −150 °C | (unknown) | Infused immediately | Seoul National University Hospital | [115,116] |
| KCT0000427 | I/II | Hepatocel lular carcinoma | DC (P) CreaVax-HCC | 3.3e7 cells/ml | 5% DMSO in HSA or plasma | Ultralow freezer for >12h | Below −150 °C | (unknown) | Infused immediately | Seoul National University Hospital | [116,117] |
| I/II | Prostate cancer | DC (A) | (unknown) | 10% DMSO (CryoStor CS10) | (unknown) | LN | (unknown) | (unknown) | SOTIO a.s. | [118] | |
| (unknown) | I/II | Prostate cancer, renal cell carcinoma | DC (L) | 3e6 cells/ml | 12% DMSO, 44% FCS, 44% RPMI 1640 | Nalgene Mr. Frosty in −70 °C freezer | LN | 37 °C water bath | Washed, resuspended and recultured | St. George’s Hospital Medical School | [119,120] |
| I/II | Melanoma | DC (P) | 1e7 cells/ml | 10% DMSO, 5% glucose +/− serum | Nalgene Mr. Frosty in −80 °C freezer | Vapor phase LN | (unknown) | Loaded with peptides | University Hospital Erlangen | [121–123] | |
| I | Melanoma | DC (P) | 1e7 cells/ml | 10% DMSO, 5% glucose +/− serum | Nalgene Mr. Frosty in −80 °C freezer | Vapor phase LN | (unknown) | Loaded with peptides | University Hospital Erlangen | [122,123] | |
| I | Type 1 diabetes | DC (R) | 1e7 cells/aliquot | (unknown) | (unknown) | (unknown) | (unknown) | Diluted to 1e6 cells/ml in PBS and infused | University of Pittsburgh | [124] | |
| I | Melanoma | DC (P) | 1.5e7 cells/ml | 10% DMSO, 40% AIM V, 50% serum | Nalgene Mr. Frosty in −80 °C freezer | LN | 37 °C water bath | Diluted in saline and 5% HSA and infused | University of Southern California | [125] |
(A) denotes DCs pulsed with apoptotic cancer cells; (F) denotes DCs fused with irradiated cancer cells; (L) denotes DCs pulsed with tumor lysate; (P) denotes DCs pulsed with disease antigen peptides; (R) denotes DCs pulsed with disease antigen-encoding mRNAs; (S) denotes DCs pulsed with tumor stem cells.
While the first dose of each multi-dose treatment commonly was freshly harvested DC vaccine, cryopreservation has been used for the remaining doses. The surveyed cryopreserved DC-based clinical trials were also primarily in phase I and II (except for DCVax®-L[43] in phase III and DEN-STEM recruiting in phase II/III), so the supply chain was shortened by in-house manufacturing and on-site administration. As these DC vaccines progress into later phases of clinical investigation and multi-site studies, centralized manufacturing and distribution will become necessary. The cryopreservation methods of these vaccines will be more stringently challenged in their effectiveness and reproducibility upon the introduction of longer storage times and transportation distances.
Cryopreserved natural killer cells in clinical trials
Natural killer (NK) cells are considered to be a critical part of the innate immune system, given their ability to kill tumor and infected cells without prior exposure to them[44]. Naïve NK cells can undergo activation in the presence of stressed, non-self cells with or without antibodies present. The activation is determined by a balance between responses from the inhibitory receptors and the activating receptors on the NK cell membrane[45]. Activated NK cells can act as effector cells releasing cytokines and chemokines to recruit and activate B and T lymphocytes. They are also cytolytic in a nonspecific manner, releasing cytotoxic granules containing perforin and granzymes that cause the target cells to lyse. It was recently discovered that NK cells have a more direct role in the adaptive immune system than initially assumed. In the presence of certain viruses, NK cells can act as memory cells that recognize viral glycoproteins and contribute to adaptive immunity similarly to the antigen recognition by other immune cells[46].
NK-cell immunotherapies have been used to treat a variety of malignancies, especially leukemias. While initial NK-cell studies focused on the treatment of acute myeloid leukemia, NK cells have also demonstrated effective treatment of solid tumors, including melanoma[47]. Before the emergence of NK cellular therapy, IL-2 was commonly administered to treat cancers. IL-2 stimulates endogenous NK cells and enhances their immunity[48] and, as the mechanism of action became better understood, called attention to NK cells as a potential cellular therapy[47]. Both autologous and allogenic NK cells have been clinically used to treat cancers[49–52].
Current protocols for obtaining, expanding and administering NK cells are variable. NK cells are traditionally isolated from allogenic PBMCs or UCB by removing all or some of the other cell types in the sample[53]. The isolated NK cells may then be expanded to obtain a clinically relevant cell quantity using a variety of growth factors and other activating molecules[54]. Allogeneic haploidentical NK cells have shown efficacy on tumors without inducing rejection or severe side effects in patients[55]. NK cells can also be derived from embryonic or induced pluripotent stem cells[56]. Robust allogeneic cell lines such as NK-92 have been used to develop cellular therapy products and can be more reliable than primary NK cells to achieve consistent product and large-scale production, though efficacy remains to be determined[54,57].
Of over 600 clinical trials involving NK cells in the US registry, less than 15 entries mentioned the use of cryopreserved NK cells. The use of fresh versus cryopreserved cells is likely due to observed poor post-thaw viability and functionality of NK cells[49,50,58,59]. In one study directly comparing fresh NK cells and NK cells that had been frozen, thawed and recultured, it was shown that the cryopreserved cells did not show proliferation in the patients who received the cellular therapy[50]. It is noteworthy that cryopreserved NK cells were shown to have fair viability (70%) but poor cytotoxicity after thawing[60]. The cytotoxicity could be regained by reculturing the cryopreserved cells in media containing IL-2, but significant cell losses were also observed in the post-thaw culture over time. For this reason, most clinical trials have opted for the use of fresh NK cells in their therapies. The use of fresh, non-cryopreserved cells creates a critical problem for NK-cell therapies that administer multiple doses, as infusing fresh cells for all doses would require either multiple, timed collections of donor cells or continuous maintenance of donor cell culture to span the timeframe of the multi-dose treatment. Other clinical trials have opted for using fresh cells for the first infusion and cryopreserved cells for the rest, where it is challenging to provide consistent dosage and efficacy to patients between the fresh and cryopreserved cells[52]. Many of the clinical trials that did use cryopreserved NK cells referenced cell banks but made no mention of freezing medium formulation, freezing method, etc.[49,51,59,61].
The cryopreservation methods that could be found for these clinical trials are summarized in Table 3. All but one of the studies used freezing media containing 10% DMSO. Plasma and serum were also common components of the freezing media. Controlled-rate freezers were used in both studies that specified freezing methods, although cooling rate was not specified. Most studies stored their NK cells in vapor phase LN, and one stored the cells in LN without specifying liquid or vapor phase. All studies used a 37°C water bath for rapid thawing, although post-thaw processing varied among them. A few groups recultured the cells after thawing, one group washed the cells to remove CPAs, and two groups immediately administered the thawed cells in the freezing medium.
Table 3.
Methods of natural killer cell preservation for clinical trials
| Study ID | Phase | Cell type | Freezing cell concentration | Disease | Freezing medium | Freezing method | Storage | Thawing method | Post-thaw processing | Institution | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (unknown) | I | NK-92 | 5e7 cells/mL vial | Advanced, treatment resistant malignancies | 10% DMSO, 40% plasma, 50% X-Vivo 10 | (unknown) | (unknown) | (unknown) | Recultured | Institute for Transfusion Medicine and Immunohematology, Germany | [126] |
| (unknown) | I | NK-92 | (unknown) | Advanced renal cell cancer and melanoma | (unknown) | (unknown) | (unknown) | (unknown) | Recultured | Rush University Medical Center | [61] |
| I | Activated NK | 2e7 cells/vial | Refractory or relapsed acute myeloid leukemia | (unknown) | (unknown) | (unknown) | (unknown) | Recultured | University of Pittsburgh | [49] | |
| EudraCT 2005006087–62 | I | CD56+ NK | (unknown) | Acute myeloid leukemia | X-Vivo10 | (unknown) | Vapor phase LN | 37°C water bath | Infused immediately upon thawing | Royal Free Hospital, UCL Medical School | [127] |
| (unknown) | IIa | Activated NK | <2e8 cells/mL | Lymphoma, metastatic breast cancer | 10% DMSO, 20% plasma, 20% Medium 199 | Controlled-rate freezer | LN | 37°C water bath | Infused immediately upon thawing | University of Pittsburgh | [128] |
| II | Expanded NK | 2e7 cells/mL | Multiple myeloma | 10% DMSO, 40% HBSS, 50% or 25% HSA | Controlled-rate freezer (CryoMed) | Vapor phase LN | (unknown) | (unknown) | University of Arkansas | [50,58] | |
| I | Invariant NK-T | (unknown) | Malignant melanoma | (unknown) | (unknown) | (Unknown) | (unknown) | (unknown) | Beth Israel Deaconess Medical Center | [51] | |
| I | mbIL21-NK | (unknown) | Myeloid malignancies | 40% PlasmaLyte, 50% serum, 10% DMSO | (unknown) | (unknown) | 37°C water bath | Washed, resuspended in 0.5% HSA in PlasmaLyte and infused | University of Texas MD Anderson Cancer Center | [52] | |
| I | Activated NK | (unknown) | High-risk solid tumors | (unknown) | (unknown) | (unknown) | (unknown) | Recultured | National Cancer Institute | [59] |
Emerging approaches
Most clinical trials of the aforementioned cell types used freezing media containing 5–10% DMSO. However, DMSO-free freezing media may be an advantageous alternative for clinical applications of cryopreserved cells in the future. One recent T-cell study found that when cryopreserved in a DMSO solution, the post-thaw recovery of CD3+CD4+ and CD3+CD8+ subpopulations were lower than that of other subpopulations in leukapheresis products[62]. Another study found that the use of 10% DMSO for cryopreservation resulted in lower post-thaw viability for most lymphocyte subpopulations, especially CD3+CD4+ and regulatory T cells, as well as growth arrest of CD3+ cells in the G2/M phase[63]. These might account for loss of functional cells in cryopreserved T-cell product for immunotherapy. In comparison, DMSO-free freezing media composed of sugars, sugar alcohols and amino acids have been demonstrated to successfully preserve Jurkat cells as a CD3+CD4+ T lymphocyte model with high post-thaw viability[8,64] and can potentially be used to cryopreserve other types of T cells. Similar to T cells, while NK cells cryopreserved in DMSO have shown significant functional loss in terms of their cytotoxicity[60], a DMSO-free freezing medium composed of poly-l-lysine, dextran and ectoine has demonstrated improved post-thaw cytotoxicity in cryopreserved NK cells[65]. Unlike T cells or NK cells, DCs have not been the focus of recent advancement in cryopreservation technologies. However, DMSO-free freezing media may improve the immunobiological function of cryopreserved DCs by avoiding DMSO-induced depolymerization of the cytoskeleton[66] essential to the antigen-receptor binding-mediated cell signaling.
As healthy cells collected from donors can be significantly expanded in vitro, allogeneic cell therapies are usually manufactured in large batch, where multiple units of cells are introduced to cryopreservation medium sequentially. In these batches, the CPA exposure time and therefore, post-thaw outcome vary greatly. As a result, the need for DMSO-free freezing medium is urgent, as cells are more stable over time in DMSO-free solutions than in DMSO solutions. In addition to the freezing medium, new technologies of freezing and thawing cells for clinical applications have been developed. For example, LN-free controlled-rate freezers as well as dry thawing devices have been developed, as alternatives to LN-based freezers and water baths to prevent contamination of the cell therapy products and provide more consistent, standardized methods for thawing.
Highlights.
Preservation technology is essential to the supply chain of cellular therapy.
Five factors in cryopreservation affect the outcome of a cell therapy.
DMSO is the most commonly used CPA to freeze cells in cell-based immunotherapy.
−1 °C/min is the most commonly used cooling rate to freeze cells for immunotherapy.
Non-DMSO CPAs have demonstrated to be better than DMSO at preserving immune cells.
Acknowledgements
This work was supported by the National Institute of Health [R01EB023880].
Abbreviations
- CAR
Chimeric Antigen Receptor
- Treg
Regulatory T Cell
- γδ
Gamma Delta T Cell
- DC
Dendritic Cell
- NK
Natural Killer Cell
- MSC
Mesenchymal Stromal Cell
- CPA
Cryoprotective Agent
- DMSO
Dimethyl Sulfoxide
- HSCT
Hematopoietic Stem Cell Transplantation
- GvHD
Graft-Versus-Host Disease
- HSA
Human Serum Albumin
- LN
Liquid Nitrogen
- TCR
T-Cell Receptor
- PBMC
Peripheral Blood Mononuclear Cell
- UCB
Umbilical Cord Blood
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interest
No competing financial interests exist.
References
- [1].Yong KW, Wan Safwani WKZ, Xu F, Wan Abas WAB, Choi JR, Pingguan-Murphy B. Cryopreservation of Human Mesenchymal Stem Cells for Clinical Applications: Current Methods and Challenges. Biopreserv Biobank 2015. doi: 10.1089/bio.2014.0104. [DOI] [PubMed] [Google Scholar]
- [2].Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JAW. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015;71:181–97. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- [3].Hubel A Preservation of cells: a practical manual. Hobokken, NJ: Wiley-Blackwell; 2018. [Google Scholar]
- [4].Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature 1959;183:1394–5. [DOI] [PubMed] [Google Scholar]
- [5].Levin RL, Miller TW. An optimum method for the introduction or removal of permeable cryoprotectants: isolated cells. Cryobiology 1981;18:32–48. [DOI] [PubMed] [Google Scholar]
- [6].Pollock K Algorithm optimization of cryopreservation protocols to improve mesenchymal stem cell functionality. University of Minnesota, 2016. doi: 10.1016/j.cryobiol.2016.09.078. [DOI] [Google Scholar]
- [7].Pollock K, Yu G, Moller-Trane R, Koran M, Dosa PI, McKenna DH, et al. Combinations of Osmolytes, Including Monosaccharides, Disaccharides, and Sugar Alcohols Act in Concert During Cryopreservation to Improve Mesenchymal Stromal Cell Survival. Tissue Eng Part C Methods 2016;22:999–1008. doi: 10.1089/ten.tec.2016.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pi CH, Yu G, Petersen A, Hubel A. Characterizing the “sweet spot” for the preservation of a T-cell line using osmolytes. Sci Rep 2018. doi: 10.1038/s41598-018-34638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mazur P Principles of Cryobiology In: Fuller B, Lane N, Benson E, editors. Life Frozen State, Boca Raton, FL: CRC Press; 2004, p. 3–66. [Google Scholar]
- [10].Hubel A, Spindler R, Skubitz APN. Storage of Human Biospecimens: Selection of the Optimal Storage Temperature. Biopreserv Biobank 2014;12:165–75. doi: 10.1089/bio.2013.0084. [DOI] [PubMed] [Google Scholar]
- [11].Chabot D, Tremblay T, Paré I, Bazin R, Loubaki L. Transient warming events occurring after freezing impairs umbilical cord–derived mesenchymal stromal cells functionality. Cytotherapy 2017;19:978–89. doi: 10.1016/j.jcyt.2017.04.005. [DOI] [PubMed] [Google Scholar]
- [12].Baboo J, Kilbride P, Delahaye M, Milne S, Fonseca F, Blanco M, et al. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci Rep 2019;9:3417. doi: 10.1038/s41598-019-39957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reinhart WH, Piety NZ, Deuel JW, Makhro A, Schulzki T, Bogdanov N, et al. Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion 2015;55:1872–81. doi: 10.1111/trf.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Henig I, Zuckerman T. Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med J 2014;5:e0028. doi: 10.5041/RMMJ.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sakaguchi S Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- [16].Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: How do they suppress immune responses? Int Immunol 2009. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- [17].Ramlal R, Hildebrandt GC. Advances in the use of regulatory T-cells for the prevention and therapy of graft-vs.-host disease. Biomedicines 2017;5. doi: 10.3390/biomedicines5020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Beres AJ, Drobyski WR. The Role of Regulatory T Cells in the Biology of Graft Versus Host Disease. Front Immunol 2013;4. doi: 10.3389/fimmu.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gliwiński M, Iwaszkiewicz-Grześ D, Trzonkowski P. Cell-Based Therapies with T Regulatory Cells. BioDrugs 2017. doi: 10.1007/s40259-017-0228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: Kinetics, toxicity profile, and clinical effect. Blood 2016. doi: 10.1182/blood-2015-06-653667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood 2011. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Theil A, Tuve S, Oelschlägel U, Maiwald A, Döhler D, Oßmann D, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy 2015. doi: 10.1016/j.jcyt.2014.11.005. [DOI] [PubMed] [Google Scholar]
- [23].Safinia N, Vaikunthanathan T, Fraser H, Thirkell S, Lowe K, Blackmore L, et al. Successful expansion of functional and stable regulatory T cells for immunotherapy in liver transplantation. Oncotarget 2016. doi: 10.18632/oncotarget.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Androulla MN, Lefkothea PC. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr Pharm Biotechnol 2018. doi: 10.2174/1389201019666180418095526. [DOI] [PubMed] [Google Scholar]
- [25].D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR-T cells: The long and winding road to solid tumors review-article. Cell Death Dis 2018. doi: 10.1038/s41419-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Berdeja JG, Lin Y, Raje NS, Siegel DSD, Munshi NC, Liedtke M, et al. First-in-human multicenter study of bb2121 anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: Updated results. J Clin Oncol 2017;35:3010–3010. doi: 10.1200/JCO.2017.35.15_suppl.3010.28715249 [DOI] [Google Scholar]
- [27].Junghans RP, Ma Q, Rathore R, Gomes EM, Bais AJ, Lo ASY, et al. Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: Possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate 2016. doi: 10.1002/pros.23214. [DOI] [PubMed] [Google Scholar]
- [28].Beatty GL, Haas AR, Maus MV., Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-Specific Chimeric Antigen Receptor mRNA-Engineered T Cells Induce Antitumor Activity in Solid Malignancies. Cancer Immunol Res 2018. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T Cells Expressing Chimeric Antigen Receptors Can Cause Anaphylaxis in Humans. Cancer Immunol Res 2013. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Garfall AL, Maus MV., Hwang W-T, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med 2015. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang X, Popplewell LL, Wagner JR, Naranjo A, Blanchard MS, Mott MR, et al. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood 2016. doi: 10.1182/blood-2015-12-686725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, et al. Phase i trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Invest 2016. doi: 10.1172/JCI86721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kalos M, June CH, Levine BL, Bagg A, Porter DL. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med 2011. doi: 10.1056/nejmoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhao Y, Niu C, Cui J. Gamma-delta (γδ) T Cells: Friend or Foe in Cancer Development. J Transl Med 2018. doi: 10.1186/s12967-017-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fisher JPH, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: A systematic review of clinical trials. Oncoimmunology 2014. doi: 10.4161/onci.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Berglund S, Gaballa A, Sawaisorn P, Sundberg B, Uhlin M. Expansion of gammadelta T cells from cord blood: A therapeutical possibility. Stem Cells Int 2018. doi: 10.1155/2018/8529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Steinman RM. Linking innate to adaptive immunity through dendritic cells. Novartis Found Symp 2006;279:101–9; discussion 109–13, 216–9. [PubMed] [Google Scholar]
- [40].Gandhi RT, Kwon DS, Macklin EA, Shopis JR, McLean AP, McBrine N, et al. Immunization of HIV-1-Infected Persons With Autologous Dendritic Cells Transfected With mRNA Encoding HIV-1 Gag and Nef. JAIDS J Acquir Immune Defic Syndr 2016;71:246–53. doi: 10.1097/QAI.0000000000000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cobb A, Roberts LK, Palucka AK, Mead H, Montes M, Ranganathan R, et al. Development of a HIV-1 lipopeptide antigen pulsed therapeutic dendritic cell vaccine. J Immunol Methods 2011;365:27–37. doi: 10.1016/J.JIM.2010.11.002. [DOI] [PubMed] [Google Scholar]
- [42].Anguille S, Smits EL, Bryant C, Van Acker HH, Goossens H, Lion E, et al. Dendritic Cells as Pharmacological Tools for Cancer Immunotherapy. Pharmacol Rev 2015;67:731–53. doi: 10.1124/pr.114.009456. [DOI] [PubMed] [Google Scholar]
- [43].Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med 2018;16:1. doi: 10.1186/s12967-018-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Science (80-) 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Borrego F, Larrucea S, Solana R, Tarazona R. Editorial: NK Cell-Based Cancer Immunotherapy. Front Immunol 2016;7. doi: 10.3389/fimmu.2016.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Marcus A, Raulet DH. Evidence for Natural Killer Cell Memory. Curr Biol 2013;23:R817–20. doi: 10.1016/j.cub.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med 2009;266:154–81. doi: 10.1111/j.1365-2796.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- [48].Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016;5:e1163462. doi: 10.1080/2162402X.2016.1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Boyiadzis M, Agha M, Redner RL, Sehgal A, Im A, Hou J-Z, et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 2017;19:1225–32. doi: 10.1016/j.jcyt.2017.07.008. [DOI] [PubMed] [Google Scholar]
- [50].Szmania S, Lapteva N, Garg T, Greenway A, Lingo J, Nair B, et al. Ex Vivo Expanded Natural Killer Cells Demonstrate Robust Proliferation In Vivo In High-Risk Relapsed Multiple Myeloma Patients. J Immunother 2015;38:24–36. doi: 10.1097/CJI.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Exley MA, Friedlander P, Alatrakchi N, Vriend L, Yue S, Sasada T, et al. Adoptive Transfer of Invariant NKT Cells as Immunotherapy for Advanced Melanoma: A Phase I Clinical Trial. Clin Cancer Res 2017;23:3510–9. doi: 10.1158/1078-0432.CCR-16-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ciurea SO, Schafer JR, Bassett R, Denman CJ, Cao K, Willis D, et al. Phase 1 clinical trial using mbIL21 ex vivo–expanded donor-derived NK cells after haploidentical transplantation. Blood 2017;130:1857–68. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Koepsell SA, Miller JS, McKenna DH Jr. Natural killer cells: a review of manufacturing and clinical utility: MANUFACTURING AND CLINICAL UTILITY OF NK CELLS. Transfusion 2013;53:404–10. doi: 10.1111/j.1537-2995.2012.03724.x. [DOI] [PubMed] [Google Scholar]
- [54].Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013;10:230–52. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol 2017;31:37–54. doi: 10.1016/j.smim.2017.07.009. [DOI] [PubMed] [Google Scholar]
- [56].Domogala A, Madrigal JA, Saudemont A. Natural Killer Cell Immunotherapy: From Bench to Bedside. Front Immunol 2015;6. doi: 10.3389/fimmu.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med 2012;6:56–66. doi: 10.1007/s11684-012-0177-7. [DOI] [PubMed] [Google Scholar]
- [58].Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 2012;14:1131–43. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, et al. Acute GVHD in patients receiving IL-15/4–1BBL activated NK cells following T-cell–depleted stem cell transplantation. Blood 2015;125:784–92. doi: 10.1182/blood-2014-07-592881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lapteva N, Szmania SM, van Rhee F, Rooney CM. Clinical Grade Purification and Expansion of Natural Killer Cells. Crit Rev Oncog 2014;19:121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 2008;10:625–32. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- [62].Berens C, Heine A, Müller J, Held SAE, Mayer K, Brossart P, et al. Variable resistance to freezing and thawing of CD34-positive stem cells and lymphocyte subpopulations in leukapheresis products. Cytotherapy 2016;18:1325–31. doi: 10.1016/j.jcyt.2016.06.014. [DOI] [PubMed] [Google Scholar]
- [63].Worsham DN, Reems J-A, Szczepiorkowski ZM, McKenna DH, Leemhuis T, Mathew AJ, et al. Clinical methods of cryopreservation for donor lymphocyte infusions vary in their ability to preserve functional T-cell subpopulations. Transfusion 2017;57:1555–65. doi: 10.1111/trf.14112. [DOI] [PubMed] [Google Scholar]
- [64].Pi C, Yu G, Dosa PI, Hubel A. Characterizing modes of action and interaction for multicomponent osmolyte solutions on Jurkat cells. Biotechnol Bioeng 2019;116:631–43. doi: 10.1002/bit.26880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pasley S, Zylberberg C, Matosevic S. Natural killer-92 cells maintain cytotoxic activity after long-term cryopreservation in novel DMSO-free media. Immunol Lett 2017;192:35–41. doi: 10.1016/j.imlet.2017.09.012. [DOI] [PubMed] [Google Scholar]
- [66].Ragoonanan V, Hubel A, Aksan A. Response of the cell membrane–cytoskeleton complex to osmotic and freeze/thaw stresses. Cryobiology 2010;61:335–44. doi: 10.1016/j.cryobiol.2010.10.160. [DOI] [PubMed] [Google Scholar]
- [67].Balzola F, Cullen G, Ho GT, Hoentjen F, Russell RK. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Inflamm Bowel Dis Monit 2013. doi: 10.1053/j.gastro.2012.07.116. [DOI] [Google Scholar]
- [68].Cruz CRY, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed After allogeneic stem cell transplant: A phase 1 study. Blood 2013. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O, et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest 2016. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Porter DL, Hwang WT, Frey NV., Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Maude SL, Frey N, Shaw P, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med 2014. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Singh H, Huls H, Kebriaei P, Cooper LJN. A new approach to gene therapy using Sleeping Beauty to genetically modify clinical-grade T cells to target CD19. Immunol Rev 2014. doi: 10.1111/imr.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Singh H, Figliola MJ, Dawson MJ, Olivares S, Zhang L, Yang G, et al. Manufacture of Clinical-Grade CD19-Specific T Cells Stably Expressing Chimeric Antigen Receptor Using Sleeping Beauty System and Artificial Antigen Presenting Cells. PLoS One 2013. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: Pilot clinical trial results. Blood 2012. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, et al. Human epidermal growth factor receptor 2 (HER2) - Specific chimeric antigen receptor - Modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol 2015. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV., Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res 2015. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res 2015. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Van Driessche A, Van De Velde ALR, Nijs G, Braeckman T, Stein B, De Vries JM, et al. Clinical-grade manufacturing of autologous mature mRNA-electroporated dendritic cells and safety testing in acute myeloid leukemia patients in a phase i dose-escalation clinical trial. Cytotherapy 2009;11:653–68. doi: 10.1080/14653240902960411. [DOI] [PubMed] [Google Scholar]
- [81].Palucka AK, Banchereau JF, Roberts L. Methods And Compositions For Treating Breast Cancer With Dendritic Cell Vaccines. United States Patent No. US20150368612A1, 2015.
- [82].Rosenblatt J, Stone RM, Uhl L, Neuberg D, Joyce R, Levine JD, et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci Transl Med 2016;8:368ra171. doi: 10.1126/scitranslmed.aag1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rosenblatt J, Avivi I, Vasir B, Uhl L, Munshi NC, Katz T, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res 2013;19:3640–8. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rosenblatt J, Vasir B, Uhl L, Blotta S, MacNamara C, Somaiya P, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood 2011;117:393–402. doi: 10.1182/blood-2010-04-277137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Myers JW, Herbert GS, Clifton G, Vreeland TJ, Brown TA, Peace KM, et al. A prospective, randomized, blinded, placebo-controlled, phase IIb trial of an autologous tumor lysate + yeast cell wall particles (YCWP) + dendritic cells (DC) vaccine vs unloaded YCWP + DC and embedded phase I/IIa trial with tumor lysate particle only (T. vol. 36 2018. doi: 10.1200/JCO.2018.36.5_suppl.TPS201. [DOI] [Google Scholar]
- [86].Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuño MA, et al. Phase i trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother 2013;62:125–35. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Westermann J, Kopp J, Van Lessen A, Hecker AC, Baskaynak G, Le Coutre P, et al. Vaccination with autologous non-irradiated dendritic cells in patients with bcr/abl+ chronic myeloid leukaemia. Br J Haematol 2007;137:297–306. doi: 10.1111/j.1365-2141.2007.06547.x. [DOI] [PubMed] [Google Scholar]
- [88].Westermann J, Korner IJ, Kopp J, Kurz S, Zenke M, Dorken B, et al. Cryopreservation of mature monocyte-derived human dendritic cells for vaccination: influence on phenotype and functional properties. Cancer Immunol Immunother 2003;52:194–8. doi: 10.1007/s00262-002-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rodriguez J, Castañón E, Perez-Gracia JL, Rodriguez I, Viudez A, Alfaro C, et al. A randomized phase II clinical trial of dendritic cell vaccination following complete resection of colon cancer liver metastasis. J Immunother Cancer 2018;6:96. doi: 10.1186/s40425-018-0405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Alfaro C, Perez-Gracia JL, Suarez N, Rodriguez J, Fernandez de Sanmamed M, Sangro B, et al. Pilot Clinical Trial of Type 1 Dendritic Cells Loaded with Autologous Tumor Lysates Combined with GM-CSF, Pegylated IFN, and Cyclophosphamide for Metastatic Cancer Patients. J Immunol 2011;187:6130–42. doi: 10.4049/jimmunol.1102209. [DOI] [PubMed] [Google Scholar]
- [91].van de Loosdrecht AA, van Wetering S, Santegoets SJAM, Singh SK, Eeltink CM, den Hartog Y, et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol Immunother 2018;67:1505–18. doi: 10.1007/s00262-018-2198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Morse MA, Nair SK, Boczkowski D, Tyler D, Hurwitz HI, Proia A, et al. The Feasibility and Safety of Immunotherapy with Dendritic Cells Loaded with CEA mRNA Following Neoadjuvant Chemoradiotherapy and Resection of Pancreatic Cancer. Int J Gastrointest Cancer 2002;32:1–6. doi: 10.1385/IJGC:32:1:1. [DOI] [PubMed] [Google Scholar]
- [93].Mansilla MJ, Contreras-Cardone R, Navarro-Barriuso J, Cools N, Berneman Z, Ramo-Tello C, et al. Cryopreserved vitamin D3-tolerogenic dendritic cells pulsed with autoantigens as a potential therapy for multiple sclerosis patients. J Neuroinflammation 2016;13:1–11. doi: 10.1186/s12974-016-0584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Svane IM, Pedersen AE, Johansen JS, Johnsen HE, Nielsen D, Kamby C, et al. Vaccination with p53 peptide-pulsed dendritic cells is associated with disease stabilization in patients with p53 expressing advanced breast cancer; monitoring of serum YKL-40 and IL-6 as response biomarkers. Cancer Immunol Immunother 2007;56:1485–99. doi: 10.1007/s00262-007-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Svane IM, Pedersen AE, Johnsen HE, Nielsen D, Kamby C, Gaarsdal E, et al. Vaccination with p53-peptide-pulsed dendritic cells, of patients with advanced breast cancer: Report from a phase I study. Cancer Immunol Immunother 2004;53:633–41. doi: 10.1007/s00262-003-0493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ridolfi L, De Rosa F, Fiammenghi L, Petrini M, Granato AM, Ancarani V, et al. Complementary vaccination protocol with dendritic cells pulsed with autologous tumour lysate in patients with resected stage III or IV melanoma: Protocol for a phase II randomised trial (ACDC Adjuvant Trial). BMJ Open 2018;8:8–13. doi: 10.1136/bmjopen-2018-021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Palma M, Hansson L, Choudhury A, Nasman-Glaser B, Eriksson I, Adamson L, et al. Molecular data of UL24 homolog gene (ORF37) from Brazilian isolates of equine herpesvirus type 1. Cancer Immunol Immunother 2012;61:865–79. doi: 10.1007/s00262-011-1149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Adamson L, Palma M, Choudhury A, Eriksson I, Näsman-Glaser B, Hansson M, et al. Generation of a dendritic cell-based vaccine in chronic lymphocytic leukaemia using clinimacs platform for large-scale production. Scand J Immunol 2009;69:529–36. doi: 10.1111/j.1365-3083.2009.02249.x. [DOI] [PubMed] [Google Scholar]
- [99].Kitawaki T, Kadowaki N, Fukunaga K, Kasai Y, Maekawa T, Ohmori K, et al. Cross-priming of CD8+T cells in vivo by dendritic cells pulsed with autologous apoptotic leukemic cells in immunotherapy for elderly patients with acute myeloid leukemia. Exp Hematol 2011;39:424–33. doi: 10.1016/j.exphem.2011.01.001. [DOI] [PubMed] [Google Scholar]
- [100].Imhof M, Lipovac M, Angleitner-Boubenizek L, Barta J, Gomez I, Krupa AH, et al. Double-loaded mature dendritic cell (DC) therapy for non-HLA-restricted patients with advanced ovarian cancer: Final results of a clinical phase I study. Dev Ther 2013;31. doi: 10.1200/jco.2013.31.15_suppl.3052. [DOI] [Google Scholar]
- [101].Olin MR, Low W, McKenna DH, Haines SJ, Dahlheimer T, Nascene D, et al. Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recurrent malignant brain tumors induces a CD4(+)IL17(+) response. J Immunother Cancer 2014;2:4. doi: 10.1186/2051-1426-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Suehiro Y, Hasegawa A, Iino T, Sasada A, Watanabe N, Matsuoka M, et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br J Haematol 2015;169:356–67. doi: 10.1111/bjh.13302. [DOI] [PubMed] [Google Scholar]
- [103].Nagayama H, Sato K, Morishita M, Uchimaru K, Oyaizu N, Inazawa T, et al. Results of a phase I clinical study using autologous tumour lysate-pulsed monocyte-derived mature dendritic cell vaccinations for stage IV malignant melanoma patients combined with low dose interleukin-2. Melanoma Res 2003;13:521–30. doi: 10.1097/00008390-200310000-00011. [DOI] [PubMed] [Google Scholar]
- [104].O’Neill D, Bhardwaj N. Generation of autologous peptide-and protein-pulsed dendritic cells for patient-specific immunotherapy. Methods Mol Med 2005;109:97–112. [PubMed] [Google Scholar]
- [105].Polyzoidis S, Ashkan K. DCVax®-L--developed by Northwest Biotherapeutics. Hum Vaccin Immunother 2014;10:3139–45. doi: 10.4161/hv.29276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hong S, Li H, Qian J, Yang J, Lu Y, Yi Q. Optimizing dendritic cell vaccine for immunotherapy in multiple myeloma: Tumour lysates are more potent tumour antigens than idiotype protein to promote anti-tumour immunity. Clin Exp Immunol 2012;170:167–77. doi: 10.1111/j.1365-2249.2012.04642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kyte JA, Mu L, Aamdal S, Kvalheim G, Dueland S, Hauser M, et al. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther 2006;13:905–18. doi: 10.1038/sj.cgt.7700961. [DOI] [PubMed] [Google Scholar]
- [108].Mu LJ, Gaudernack G, Sæbøe-Larssen S, Hammerstad H, Tierens A, Kvalheim G. A Protocol for Generation of Clinical Grade mRNA-Transfected Monocyte-Derived Dendritic Cells for Cancer Vaccines. Scand J Immunol 2003;58:578–86. doi: 10.1046/j.1365-3083.2003.01333.x. [DOI] [PubMed] [Google Scholar]
- [109].Mu LJ, Kyte JA, Kvalheim G, Aamdal S, Dueland S, Hauser M, et al. Immunotherapy with allotumour mRNA-transfected dendritic cells in androgen-resistant prostate cancer patients. Br J Cancer 2005;93:749–56. doi: 10.1038/sj.bjc.6602761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Schendel DJ, Zobywalski A, Bigalke I. Compositions For The Preparation Of Mature Dendritic Cells. United States Patent No. US 2010/0284976 A1, 2010.
- [111].Vik-Mo E (Oslo UH. Open Label Randomized Phase II/III Trial of Dendritic Cell Immunotherapy Against Cancer Stem Cells in Glioblastoma Patients Receiving Standard Therapy (recruiting) 2018:ClinicalTrials.gov Identifier .
- [112].PrimeVax Immuno-Oncology Inc. Study of Dendritic Cells Pulsed With Tumor Lysate in Advanced Melanoma Dendritic Cells in Advanced Melanoma (not yet recruiting) 2019:ClinicalTrials.gov Identifier .
- [113].Hobo W, Strobbe L, Maas F, Fredrix H, Greupink-Draaisma A, Esendam B, et al. Immunogenicity of dendritic cells pulsed with MAGE3, Survivin and B-cell maturation antigen mRNA for vaccination of multiple myeloma patients. Cancer Immunol Immunother 2013;62:1381–92. doi: 10.1007/s00262-013-1438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EHJG, Duiveman-De Boer T, et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res 2016;22:2155–66. doi: 10.1158/1078-0432.CCR-15-2205. [DOI] [PubMed] [Google Scholar]
- [115].Lee JH, Tak WY, Lee Y, Heo MK, Song JS, Kim HY, et al. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology 2017;6:1–12. doi: 10.1080/2162402X.2017.1328335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lee Y, Kim Y-M, Kim S-Y, Han S-S, Bae Y-S. Method For Preparing Dendritic Cell, Dendritic Cell Prepared Thereby, And Use Thereof. US 2017/0196955A1, 2017. doi: 10.1037/t24245-000. [DOI] [Google Scholar]
- [117].Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa Y, Lee Y, et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol 2012;41:1601–9. doi: 10.3892/ijo.2012.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Podrazil M, Horvath R, Becht E, Rozkova D, Bilkova P, Hromadkova H, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015;6. doi: 10.18632/oncotarget.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Pandha HS, John RJ, Hutchinson J, James N, Whelan M, Corbishley C, et al. Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: A phase I/II study. BJU Int 2004;94:412–8. doi: 10.1111/j.1464-410X.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- [120].John J, Hutchinson J, Dalgleish A, Pandha H. Cryopreservation of immature monocyte-derived dendritic cells results in enhanced cell maturation but reduced endocytic activity and efficiency of adenoviral transduction. J Immunol Methods 2003;272:35–48. doi: 10.1016/S0022-1759(02)00430-1. [DOI] [PubMed] [Google Scholar]
- [121].Gross S, Erdmann M, Haendle I, Voland S, Berger T, Schultz E, et al. Twelve-year survival and immune correlates in dendritic cell–vaccinated melanoma patients. JCI Insight 2017;2. doi: 10.1172/jci.insight.91438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, et al. Rapid Induction of Tumor-specific Type 1 T Helper Cells in Metastatic Melanoma Patients by Vaccination with Mature, Cryopreserved, Peptide-loaded Monocyte-derived Dendritic Cells. J Exp Med 2002;195:1279–88. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Feuerstein B, Berger TG, Maczek C, Röder C, Schreiner D, Hirsch U, et al. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J Immunol Methods 2000;245:15–29. doi: 10.1016/S0022-1759(00)00269-6. [DOI] [PubMed] [Google Scholar]
- [124].Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 2011;34:2026–32. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lau R, Wang F, Jeffery G, Marty V, Kuniyoshi J, Bade E, et al. Phase I trial of intravenous peptide-pulsed dendritic cells in patients with metastatic melanoma. J Immunother 2001;24:66–78. [DOI] [PubMed] [Google Scholar]
- [126].Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013;15:1563–70. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- [127].Kottaridis PD, North J, Tsirogianni M, Marden C, Samuel ER, Jide-Banwo S, et al. Two-Stage Priming of Allogeneic Natural Killer Cells for the Treatment of Patients with Acute Myeloid Leukemia: A Phase I Trial. PLoS One 2015;10:e0123416. doi: 10.1371/journal.pone.0123416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lister J, Rybka WB, Donnenberg AD, deMagalhaes-Silverman M, Pincus SM, Bloom EJ, et al. Autologous Peripheral Blood Stem Cell Transplantation and Adoptive Immunotherapy with Activated Natural Killer Cells in the Immerdiate Posttransplant Period. Clin Cancer Res 1995;1:607–14. [PubMed] [Google Scholar]