Abstract
Background:
There is limited data about the impact of narrowband ultraviolet B phototherapy on patient-reported measures of health-related quality of life (HRQoL).
Objective:
To evaluate the impact of adalimumab and phototherapy on HRQoL.
Methods:
We examined patient-reported outcomes (PROs) from a multicenter, randomized placebo-controlled trial (). Dermatology Life Quality Index (DLQI) and EQ-5D-3L were evaluated every 4 weeks.
Results:
We enrolled 97 patients: 30.9% female, mean (SD) age 43.5(14.0) years, median (interquartile range) PASI 16.7(13.9–21.6). At week 12, patients being treated with adalimumab (OR: 2.88, 95%CI:1.02, 8.17) and phototherapy (OR:8.83, 95%CI:2.47, 31.57) were more likely to achieve the minimal clinically important difference (MCID) in DLQI compared to placebo. There were higher odds of achieving the MCID for the EQ-5D-3L Index score when comparing phototherapy versus placebo (OR:9.78, 95%CI:2.99, 31.95) and phototherapy versus adalimumab (OR:4.07, 95%CI:1.42, 11.70).
Limitations:
small sample size, secondary analysis, generalizability
Conclusion:
Phototherapy and adalimumab both improve skin-related quality of life and overall health related quality of life compared to placebo in patients with psoriasis, however, phototherapy treated patients achieved more improvement in overall health quality of life compared to patients treated with adalimumab.
Keywords: patient-reported outcomes, DLQI, EQ5D, randomized controlled trials, psoriasis, phototherapy, adalimumab
INTRODUCTION
The treatment of psoriasis has been revolutionized by the development of biologic antibodies that target cytokines central to the pathophysiology of psoriasis. Despite these advances, narrowband ultraviolet B (nbUVB) phototherapy, which has been used since the 1980s, remains a preferred treatment by both dermatologists and patients.1, 2 Multiple placebo-controlled studies involving thousands of patients have shown that novel biologics improve both physician-reported and patient-reported outcomes (PROs),3–5 but the effects of nbUVB phototherapy are less well-established in rigorous clinical trials.
A meta-analysis combining data from nine randomized controlled trials, with a total of 293 patients, concluded the mean PASI 75 for nbUVB monotherapy was 62% (95% CI: 45–79).6 However, the trials included in this analysis were small (N = 10–55), all were single center, and data on patient reported outcomes was limited. Additionally, there is minimal data comparing phototherapy, as monotherapy, to other active comparators. There is also relatively limited data about the impact of nbUVB on patient reported measures of health-related quality of life (HRQoL) in psoriasis patients and to our knowledge, no controlled trials comparing phototherapy alone to modern biologic treatments.7 We recently published the results of the Vascular Inflammation in Psoriasis trial, demonstrating the rate of achieving PASI 75 at week 12 was nearly identical in patients receiving adalimumab and phototherapy8 Therefore, the purpose of this study was to evaluate the impact of both adalimumab and phototherapy compared to each other and compared to placebo injections on commonly used measures of HRQoL.
METHODS
Patient and Study Design
Briefly, this was a multicenter, 3-arm, randomized placebo-controlled 12-week trial designed to enroll 97 patients with 1:1:1 allocation at baseline to adalimumab injections or placebo injections, or narrowband ultraviolet B phototherapy () as previously described.8 All participants were aged 18 years or older, had a body surface area ≥10% and PASI of ≥12 and were not receiving any concurrent prescription psoriasis treatment. Patients were also excluded if they had a previous adverse event from or lack of response to a TNF-alpha antagonist and/or UV phototherapy that led to discontinuation of either of these therapies. Adalimumab (or corresponding placebo injections) therapy was administered in a double-blind manner as subcutaneous injection with an initial 80mg dose at baseline followed by maintenance doses of 40mg every other week throughout the study.
Narrowband ultraviolet B phototherapy was administered 3 times weekly. Blinding of phototherapy (i.e., such as the use of sham treatment) was not performed. Dosing was based on an estimated minimal erythema dose (MED) and Fitzpatrick skin type using a modified protocol published by Zanolli and Feldman.9 Subjects with skin types 1–2, 3–4, and 5–6 respectively received 300, 500, and 800 mJ/cm2 as initial doses. After that, dosing was adjusted at each treatment visit allowing for increases as a percentage of MED based on patient reaction to the previous treatment. Patients presenting with 1) transient erythema lasting <24 hours following treatment had a 20% dose increase; 2) persistent erythema for 24–48 hours had the same dose held until the erythema lasts <24 hours; 3) persistent erythema for >48 hours resulted in no treatment on that day and a return to the last lower dose that did not cause persistent erythema. The primary outcomes were aortic vascular inflammation measured by 18-FDG-PET/CT and blood-based cardiovascular biomarkers. Sample size calculations were based on the primary outcome. Details of the power analyses, randomization and blinding methods were reported in a previous manuscript.8 Here, we report secondary outcomes measuring HRQoL.
The Institutional Review Board at the University of Pennsylvania or respective local Institutional Review Board when indicated, approved the study, and the study was conducted in accordance with the principles of Declaration of Helsinki, good clinical practice and the Belmont Report. All study participants provided written informed consent. The randomized placebo-controlled trial was overseen by an independent data monitoring committee.
Patient-Reported Outcomes (PROs)
The DLQI is a dermatology-specific QoL questionnaire that has been used extensively in psoriasis clinical trials.10 The 10 DLQI questions are scored on a 4-point scale (0 to 3). The total DLQI score ranges from 0 to 30. The higher the score, the more impaired the HRQoL.11
The EQ-5D-3L is a widely used measure of generic health status.12 The first part of the tool involves rating the extent of having difficulties in each of the five dimensions: mobility, self-care, usual activities, pain, and anxiety. Each dimension is ranked according to three levels as follows: “having no problems,” “having some or moderate problems,” or “being unable to do/having extreme problems.” These domains can be converted to an EQ-5D Index utility score where ‘0’ corresponds to death and ‘1’ corresponds to perfect health.13, 14 The second part of the EQ-5D-3L is a visual analogue scale (EQ-VAS) where the patient marks their health status on a 10 cm vertical scale that ranges from 0–100, with a higher score corresponding to a better HRQoL.
Statistical Analyses
All data were summarized using descriptive statistics and graphical techniques. The mean EQ-5D VAS and mean DLQI responses were plotted longitudinally over weeks 0, 4, 8, and 12. Multiple imputation with bootstrapping was used to account for missing data that was greater than 10% at week 4 in the EQ-5D VAS and DLQI. A change score for each PROs was calculated as the difference between the week 12 and baseline values. Between group comparisons were conducted using one way analysis of variance (ANOVA), with Tukey correction for multiple comparisons. The continuous DLQI and EQ-5D-3L, overall and subcomponent scores, were converted to 2 different dichotomous variables to identify patients who reported their skin disease had “no effect” on their quality of life and to identify individuals who achieved the previously established minimal clinically important difference (MCID) for the PRO.15 For DLQI, a score of <2 at 12 weeks signified symptoms had “no effect” on quality of life (A score >2 suggests any effect.). Achievement of the MCID is defined as a 4 point decrease in DLQI score at week 12.16 . For EQ-5D-3L, scores less than or equal to 1 represented “no problem/effect on quality of life”, and scores greater than one suggested any problem.17 Achievement of MCID was considered at a 0.05 point increase in score at week 12.18 Using these dichotomized outcomes, logistic regression was used to compare odds of achieving “no effect on quality of life” or the MCID for each treatment as compared to placebo and each other. Various sensitivity analyses were conducted: imputing patients who dropped out as failures, excluding patients with a history of phototherapy, history of biologic drug use, and those with psoriatic arthritis (data not shown). We also performed a multivariable logistic regression model adjusting for comorbidities that effect HRQol as a sensitivity analysis to ensure that any potential unbalanced covariates which can occur during randomization of trials with smaller sample sizes did not impact the results (data not shown). Stata 15.1 (StataCorp LLC, College Station, TX) was used for analysis.
RESULTS
After screening 179 patients for eligibility, 97 were randomized. The baseline characteristics were similar for the three treatment groups (Table 1 and previously described).8 Study subjects were on average 43.5 years old, 69.1% male, and had a median PASI of 16.7. At baseline, the mean DLQI was 12.13, 13.67, and 12.79 in the placebo, adalimumab, and phototherapy groups, respectively. The mean EQ-5D Index was 0.80, 0.78, and 0.72 in the placebo, adalimumab, and phototherapy group respectively. The mean EQ-VAS was: 67.58, 53.38, and 54.86 in the placebo, adalimumab and phototherapy groups, respectively.
Table I:
Baseline Characteristics of the Study Population
| Placebo N = 31 | Adalimumab N = 33 | Phototherapy N = 33 | |
|---|---|---|---|
| Age, in years, mean (SD) | 44.3 (14.5) | 44.2 (14.0) | 42.0 (14.0) |
| Male, N (%) | 20 (60.5) | 24 (72.7) | 23 (69.7) |
| Body Mass Index, mean (SD) | 32.0 (7.7) | 30.9 (7.4) | 32.6 (8.7) |
| Alcohol Mean Standardized Units/week | 2.53 (3.54) | 3.30 (4.50) | 2.59 (3.79) |
| Current Smoker, N (%) | 9 (29.03) | 10 (30.30) | 5 (15.15) |
| Psoriasis duration, in years | 19.3 (13.6) | 15.0 (14.7) | 15.9 (13.6) |
| Psoriatic arthritis, N (%) | 2 (6.5) | 4 (12.1) | 3 (9.1) |
| History of Biologic treatment, N (%) | 11 (35.5) | 10 (30.3) | 8 (24.2) |
| History of Phototherapy, N (%) | 11 (35.5) | 5 (15.2) | 13 (39.4) |
| Psoriasis Assessment, baseline | |||
| BSA, mean (SD) | 25.7 (15.0) | 23.4 (14.5) | 23.0 (13.4) |
| PASI, median (IQR) | 15 (13.3–20.6) | 17.4 (15.4–22) | 16.8 (14.5–21) |
| PGA, mean (SD) | 3.2 (0.6) | 3.4 (0.6) | 3.3 (0.7) |
| Health-related quality of life, baseline | |||
| DLQI, mean (SD) | 12.13 (6.75) | 13.67 (5.90) | 12.79 (7.10) |
| EQ-5D-3L Index, mean (SD) | 0.80 (0.13) | 0.78 (0.14) | 0.72 (0.21) |
| EQ VAS, mean (SD) | 67.58 (23.78) | 53.38 (31.07) | 54.86 (34.55) |
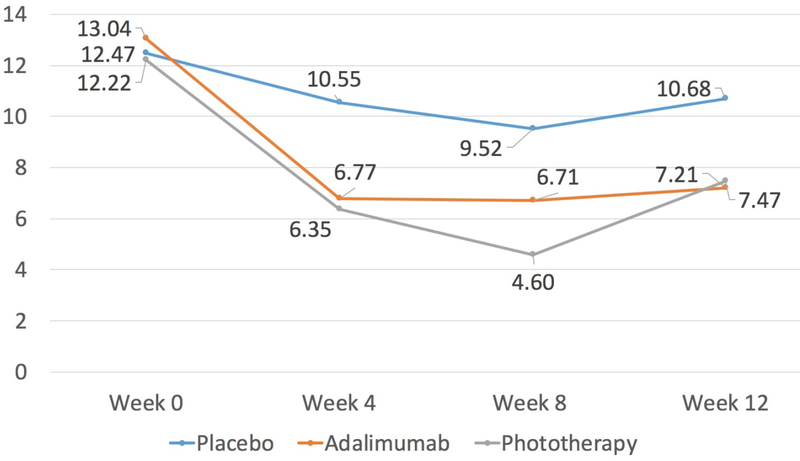
After 12 weeks, patients in all three treatment groups achieved statistically significant improvements in skin-related HRQoL compared to baseline, as measured by DLQI (Figure 1 and Table II). However, the difference of the mean change score was higher when comparing the change in the adalimumab group, vs the change in the placebo group (−3.80, 95% CI: −7.64, 0.04) and the change in the phototherapy group vs the change in the placebo group (−4.80, 95% CI: −8.67, −0.93) (Table II). There was no difference in the change score when comparing phototherapy to adalimumab (–1.0, 95% CI: −4.81, 2.81). When using dichotomous outcomes, patients were more likely to report “no effect” versus any effect as determined by DLQI in the adalimumab and phototherapy groups compared to placebo and in the phototherapy group compared to adalimumab. However, statistical significance of “no effect” was observed only in the phototherapy versus placebo comparison (OR:7.41, 95% CI: 1.85, 29.66). Both active treatment groups were more likely to reach a clinically meaningful improvement in DLQI as compared to placebo (adalimumab vs placebo OR: 2.88, 95% CI: 1.02, 8.17 and phototherapy vs placebo OR: 8.83, 95% CI: 2.47, 31.57). When comparing phototherapy to adalimumab, patients receiving phototherapy were more likely to achieve the MICD, but this result was not statistically significant (OR: 3.07, 95% CI: 0.85, 11.13) (Table 3).
Figure 1:
Mean DLQI Score over time, by treatment group
Table II:
Change Between Week 12 and Baseline Scores of each PRO, by treatment group
| Change Score, by group (Week 12 – baseline) | Difference of Mean Change Score (95% Cl)e | |||||
|---|---|---|---|---|---|---|
| Placebo Mean (SD), p-valued | Adalimumab Mean (SD), p-valued | Phototherapy Mean (SD), p-valued | Adalimumab vs Placebo | Phototherapy vs Placebo | Phototherapy vs Adalimumab | |
| DLQIa | −3.26 (5.38), 0.002 | −7.06(7.71), <0.001 | −8.06 (5.58), <0.001 | −.80 (−7.64, 0.04) | −4.80 (−8.67, −0.93) | −1.00 (−4.81,2.81) |
| EQ-5D Indexb | −0.002(0.17), 0.95 | 0.07(0.14), 0.01 | 0.15(0.21), <0.001 | 0.07 (−0.04,0.18) | 0.15 (0.04, 0.26) | 0.80 (−0.02,0.18) |
| EQ VASc | −7.26(23.97), 0.11 | 11.73(31.94), 0.046 | 10.06(36.76), 0.14 | 18.99 (−0.04,38.01) | 17.32 (−1.85,36.50) | −1.66 (−20.53, 17.21) |
DLQI is scored from 0 to 30, where a higher score signifies more impaired health-related quality of life (HRQoL). A negative change score is associated with improvement in QoL over time. A change score of +/− 4 is associated with a clinically meaningful change in QoL.
EQ-5D Index Score is a measure of utility where 0 corresponds to death and 1 corresponds to perfect health. A positive change score is associated with improvement in HRQoL over time. A change of 0.05 represents a clinically meaningful improvement in HRQoL for patients.
The EQ VAS is a visual analog scale from 0–100 where a higher score corresponds to improved quality of life. A positive change score is associated with improvement in HRQoL over time. The minimally important difference has been estimated to be between 7–8, depending on the disease being examined.25, 26
P-value based on paired t-test
95% CI based on the post-hoc group comparisons with Tukey adjustment
Table III.
The Odds of Reporting Standard Improvements in Patient Reported Outcomes, by treatment group
| Placebo N (%) | Adalimumab N (%) | Phototherapy N (%) | Adalimumab vs Placebo OR (95% Cl) | Phototherapy vs Placebo OR (95% Cl) | Phototherapy vs Adalimumab OR (95% Cl) | |
|---|---|---|---|---|---|---|
| “No Impairment” in Quality of Lifea | ||||||
| DLQIa | 3 (10.0) | 8 (25.0) | 14 (45.16%) | 3.00 (0.71, 12.62) | 7.41 (1.85,29.66) | 2.47 (0.85,7.19) |
| EQ-5D-3Lb | ||||||
| Mobility Dimension | 25 (83.33) | 26 (81.25) | 26 (83.87) | 0.87 (0.23, 3.20) | 1.04 (0.27,4.03) | 1.20 (0.33,4.43) |
| Self-care Dimension | 27 (90.0) | 31 (96.88) | 29 (93.55) | 3.44 (0.34, 35.09) | 1.61 (0.25, 10.39) | 0.47 (0.04, 5.42) |
| Activity Dimension | 21 (70.0) | 25 (78.12) | 27 (87.10) | 1.53 (0.49,4.81) | 2.89 (0.78, 10.71) | 1.89 (0.49,7.24) |
| Pain Dimension | 7 (23.33) | 15 (46.88) | 20 (64.52) | 2.90 (0.97, 8.66) | 5.97 (1.95,18.33) | 2.06 (0.75, 5.67) |
| Anxiety Dimension | 17 (56.67) | 20 (62.50) | 23 (74.19) | 1.27 (0.46,3.52) | 2.20 (0.75, 6.48) | 1.73 (0.59,5.06) |
| Achievement of the Minimal Clinically Important Differenceb | ||||||
| DLQI | 13 (43.33) | 22 (68.75) | 27 (87.1) | 2.88 (1.02, 8.17) | 8.83 (2.47, 31.57) | 3.07 (0.85, 11.13) |
| EQ-5D Index | 6 (20.00) | 12 (37.50) | 22 (70.97) | 2.40 (0.76, 7.55) | 9.78 (2.99, 31.95) | 4.07 (1.42, 11.70) |
Univariable logistic regression comparing the odds of reporting no effect of quality of life (<2) vs any effect (≥2) for DLQI and no problem (≤1) vs any problem (>1) for each of the EQ-5D dimensions
Univariable logistic regression comparing the odds of achieving MCID for DLQI (4-point decrease in DLQI score at week 12) and EQ-5D Index (0.05-point increase in EQ-5D Index value at week 1
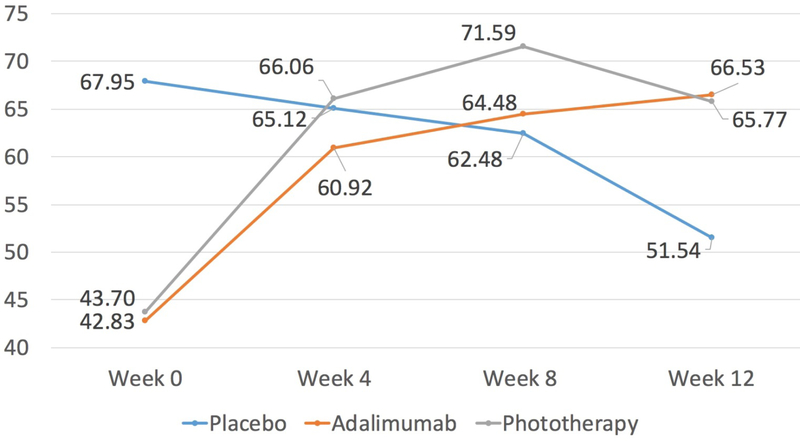
Phototherapy and adalimumab groups also had improved generic HRQoL change scores at week 12 compared to baseline as measured by the EQ-5D Index and EQ-VAS (Figure 2). Both active treatment groups performed better than placebo as determined by the EQ-5D Index and EQ-VAS change scores. Only the change score in the phototherapy group as compared to the placebo group was statistically significant (p=0.004; adalimumab vs placebo p=0.36; Table II). There was no difference in the mean change score between phototherapy vs adalimumab using the EQ-5D Index (0.80, 95% CI: −0.02, 0.18) or EQ VAS (−1.66, 95% CI: −20.53, 17.21). In general, patients treated with both adalimumab and phototherapy scored higher in the individual EQ-5D-3L domains as compared to placebo but only phototherapy compared to placebo achieved statistical significance in the pain domain (odds ratio of having “no problems” vs “any problems” 5.97, 95% CI: 1.95, 18.33; Table III). There were higher odds of achieving the MCID for the EQ-5D Index score when comparing phototherapy versus placebo (OR: 9.78, 95%CI 2.99, 31.95) and phototherapy versus adalimumab (OR: 4.07, 95% CI: 1.42, 11.70). Statistical significance of achieving the MCID for the EQ-5D Index score was not observed in the adalimumab versus placebo (OR: 2.40, 95% CI: 0.76, 7.55). The results were robust across a variety of sensitivity analyses (data not shown) with the exception that significance in the EQ-5D pain domain was not seen in phototherapy versus placebo when patients with a history of phototherapy were excluded.
Figure 2:
Mean EQ Visual Analogue Scale scores over time, by treatment group
DISCUSSION
Both adalimumab and phototherapy are well-established treatments for psoriasis, and the results from this randomized control trial confirm that treatment with both adalimumab and phototherapy are also associated with a statistically significant improvement in PROs, compared to placebo, after 12 weeks of treatment. Additionally, patients treated with phototherapy for 12 weeks were more likely to report that psoriasis had no effect on QoL compared to patients in the placebo group, as measured by DLQI (OR:7.41, 95% CI:1.85, 29.66). They were also more likely to report no problems with pain, as measured by EQ-5D-3L (OR: 5.97, 95%CI: 1.95, 18.33). The results of this study are important in that they allow for a direct comparison of the patient-reported benefits of these first-line treatments in a rigorous randomized controlled study.
While extensive data has evaluated the impact of adalimumab on DLQI and EQ-5D-3L, similar data are limited for phototherapy.19–21 Our study demonstrates that phototherapy alone not only improves skin-related quality of life (i.e., DLQI) in psoriasis patients but also improves general HRQoL (i.e., EQ-5D-3L). Of special interest, our results show that patients treated with phototherapy were more likely to report no problem with pain than patients treated with placebo (OR:5.97, 95% CI:1.95,18.33). Patients treated with adalimumab also were more likely to report no problem with pain compared to placebo; however, this finding was not statistically significant (OR: 2.90, 95% CI: 0.97, 8.66). The instruments ask questions about overall pain and do not differentiate between skin pain and joint pain, which may explain the improvement seen after treatment with phototherapy. An exact mechanism for phototherapy improving overall pain is not well understood, however, previous studies suggest that the skin generates opioid p-endorphins in response to ultraviolet radiation, which ameliorates pain signals.22
Patients treated with phototherapy were more likely to achieve a clinically significant improvement in the EQ-5D Index (OR: 4.07, 95% CI: 1.42,11.70) compared to patients treated with adalimumab. Also, patients treated with phototherapy were more than twice as likely as patients treated with adalimumab to achieve no impairment on the DLQI (OR: 2.47, 95% CI: 0.85, 7.19) and no pain on the EQ-5D-3L (OR: 2.06, 95% CI: 0.75, 5.67); however, these findings were not statistically significant. These results suggest that phototherapy may outperform or at least achieve similar results to adalimumab on PROs in patients with psoriasis. Indeed, previously, we demonstrated in a multicenter routine clinical practice study that psoriasis patients treated with phototherapy had a similar HRQoL based on the DLQI to patients treated with biologics including adalimumab and ustekinumab.20
Our study has certain limitations. First, the relatively small sample size resulted in imprecise estimates of our measurements of HRQoL. Similarly, HRQoL was a secondary outcome for our trial and the study was not specifically designed to test the hypothesis of superiority or non-inferiority of adalimumab compared to phototherapy on measures of HRQoL. Of special importance, the trial was designed such that after the placebo-controlled period, all patients crossed over to start or continue adalimumab for 52 weeks. As a result, patients who may not have been ideal candidates for phototherapy (e.g., due to having extensive scalp or genital disease) may have enrolled given that they would eventually be treated with an approved biologic and thus we may have underestimated the benefit of phototherapy in patients who are better candidates for this treatment approach. Additionally, there was no sham treatment for phototherapy, so we are unable to ascertain the degree to which the improvements observed are related to the efficacy of phototherapy as opposed to the benefits of being seen regularly by phototherapy staff. Finally, our study found lower response rates, as determined by PASI-75 than what is typically expected, to adalimumab (46.88%8 vs >71%)5, 23 and nbUVB (46.67%8 vs 62%6).
In summary, the results of this multi-center, randomized, placebo-controlled trial suggest that nbUVB phototherapy treatment of psoriasis achieves similar improvements in HRQoL to adalimumab, with higher improvements in specific measures. Surprisingly, phototherapy also significantly improved symptoms of pain in patients with psoriasis, a new finding for a treatment that has been used for decades. Unfortunately, phototherapy is limited in both its distribution (90% of counties in the US have no physicians that offer phototherapy) and its inconvenience given the modern-day difficulties for patients who do not live near a physician whom offers phototherapy to travel to the office for regular treatments.24 We are, therefore, conducting a large-scale pragmatic trial of home vs office based narrowband phototherapy for the treatment of psoriasis (the LITE study ) to further advance our knowledge of how to use this treatment in a manner that is most safe, effective, and patient-centered.
Capsule Summary:
There are limited multi-center, head-to-head trials with phototherapy monotherapy evaluating patient-reported outcomes.
Phototherapy and adalimumab both improve skin-related and overall health related quality of life in patients with psoriasis. Phototherapy treated patients achieved more improvement in overall health quality of life compared to patients treated with adalimumab.
Funding Sources:
Supported in part by a grant from NIH 5P30AR069589–03, NIH/NHLBI R01-HL111293 (JMG), a grant from AbbVie for VIP (JMG), NIAMS K23-AR073932 (MHN) and a medical dermatology fellowship from the National Psoriasis Foundation (MTW).
Conflicts of Interest:
Dr. Armstrong has served as a research investigator or consultant to AbbVie, Janssen, Lilly, Leo, Novartis, UCB, Ortho Dermatologics, Dermira, Sanofi Genzyme, Regeneron, BMS, Dermavant, Science 37, and Modernizing Medicine.
Dr. Callis Duffin has served as a research investigator or consultant to Amgen, Abbvie, Celgene, Janssen, Lilly, Novartis, UCB, Ortho Dermatologic, Regeneron, BMS, Pfizer, and Stiefel.
Dr. Gelfand served as a consultant for BMS, Boehringer Ingelheim, GSK, Janssen Biologics, Novartis Corp, UCB (DSMB), Sanofi, and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from AbbVie, Janssen, Novartis Corp, Celgene, Ortho Dermatologics, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly, Ortho Dermatologic and Novartis. Dr. Gelfand is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma. Dr. Gelfand is a Deputy Editor for the Journal of Investigative Dermatology, receiving honoraria from the Society for Investigative Dermatology.
Dr Kalb has received grants/research funding from AbbVie, Amgen, Boehringer Ingelheim, Janssen Ortho Inc, Merck & Co, Inc, and Novartis Pharmaceuticals Corp over the last 24 months. During this time frame, he has also served as a consultant and received honoraria for Dermira, Janssen-Ortho Inc, Sun Pharmaceutical Industries Ltd, and a DSMB member honoraria for Eli Lilly and Co.
Dr. Mehta is a full-time US Government Employee and receives research grants to the National Heart, Lung, and Blood Institute (NHLBI) from AbbVie, Janssen, Celgene, and Novartis. He serves on the medical board of the National Psoriasis Foundation and is Associate Editor of the Journal of Translational Medicine.
Dr. Menter in the last 24 months has served on the advisory board for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Janssen Biotech, Inc, and LEO Pharma. He has also worked as a consultant for AbbVie, Allergan, Amgen, Eli Lilly, Galderma, Janssen Biotech, Inc, LEO Pharma, Novartis, Pfizer, Vitae, and Xenoport. Additionally, he has acted as an investigator for AbbVie, Allergan, Amgen, Anacor, Boehringer Ingelheim, Celgene, Dermira, Eli Lilly, Janssen Biotech, Inc, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Symbio/Maruho, and Xenoport. He also serves as a speaker for AbbVie, Amgen, Janssen Biotech, Inc, and LEO Pharma. He has received compensation in the form of grants from AbbVie, Allergan, Amgen, Anacor, Boehringer Ingelheim, Celgene, Dermira, Janssen Biotech, Inc, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Symbio/Maruho, and Xenoport. He has also received honoraria from AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Galderma, Janssen Biotech, Inc, LEO Pharma, Novartis, Pfizer, Vitae, and Xenoport.
Dr Simpson has served as a consultant for AbbVie, Anacor, Celgene, Dermira, Genentech, Leo, Glaxo Smith Kline, Pfizer, Regeneron, Sanofi-Genzyme, Menlo, and Eli Lilly in the last 24 months. During this time frame, he has also acted as the primary investigator for the following sponsored trials: Anacor, Celgene, Chugai, Dermira, Eli Lilly, Genentech, MedImmune, Merck, Novartis, Regeneron, Roivant, Tioga, and Vanda.
Dr. Takeshita receives a research grant from Pfizer Inc. (to the Trustees of the University of Pennsylvania) and has received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly.
Dr Tyring conducts clinical studies sponsored by the following companies: Abbvie/BI; Celgene; Coherus; Dermira; Eli Lilly; Janssen; Leo; Merck; Novartis; Pfizer; Regeneron/Sanofi; and Valeant. He is a speaker for Abbvie, Eli Lilly, Janssen, Leo, Novartis, Pfizer, Regeneron/Sanofi, and Valeant.
Dr. Van Voorhees has served on the advisory board of Celgene, Dermira, Allergan, Merck, Pfizer, Aqua, Astra Zeneca, Jannsen, Amgen, Leo, Allergan, and Lilly. For Novartis and AbbVie, Dr. Van Voorhees acts as a consultant as well as serves on the board. Dr. Van Voorhees has received a portion of ex-spouse pension from Merck.
Dr. Chiesa Fuxench, Dr. Noe, Dr. Shin, and Dr. Wan have no relevant financial disclosures with respect to the work presented herein.
This study was approved by the IRB at the University of Pennsylvania.
Abbreviations:
- PASI 75
a 75% reduction of the Psoriasis Area & Severity Index score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinicaltrials.gov/identifier:
REFERENCES
- 1.Wan J, Abuabara K, Troxel AB, Shin DB, Van Voorhees AS, Bebo BF Jr. et al. Dermatologist preferences for treatments to compare in future randomized controlled comparative effectiveness trials for moderate to severe psoriasis. Arch Dermatol 2012;148:539–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeshita J, Armstrong A, Duffin KC, Robertson AD, Attor R, Gelfand JM. Patient Reported Priorities for Comparative Effectiveness Research in Psoriasis 4th Congress of the Psoriasis International Network. Paris, France: 2013. [Google Scholar]
- 3.Revicki DA, Willian MK, Menter A, Gordon KB, Kimball AB, Leonardi CL et al. Impact of adalimumab treatment on patient-reported outcomes: results from a Phase III clinical trial in patients with moderate to severe plaque psoriasis. The Journal of dermatological treatment 2007;18:341–50. [DOI] [PubMed] [Google Scholar]
- 4.Revicki D, Willian MK, Saurat JH, Papp KA, Ortonne JP, Sexton C et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. The British journal of dermatology 2008;158:549–57. [DOI] [PubMed] [Google Scholar]
- 5.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol 2008;58:106–15. [DOI] [PubMed] [Google Scholar]
- 6.Almutawa F, Alnomair N, Wang Y, Hamzavi I, Lim HW. Systematic review of UV-based therapy for psoriasis. American journal of clinical dermatology 2013;14:87–109. [DOI] [PubMed] [Google Scholar]
- 7.Kirby B, Richards HL, Woo P, Hindle E, Main CJ, Griffiths CE. Physical and psychologic measures are necessary to assess overall psoriasis severity. Journal of the American Academy of Dermatology 2001;45:72–6. [DOI] [PubMed] [Google Scholar]
- 8.Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC et al. Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers: A Randomized Placebo-Controlled Trial. Circ Cardiovasc Imaging 2018;11:e007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanolli Michael D., Feldman Steven R., Clark Adele R. Jr. ABF. Phototherapy treatment protocols for psoriasis and other phototherapy responsive dermatoses. New York: Parthenon Pub. Group; 2000. [Google Scholar]
- 10.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clinical and experimental dermatology 1994;19:210–6. [DOI] [PubMed] [Google Scholar]
- 11.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? The Journal of investigative dermatology 2005;125:659–64. [DOI] [PubMed] [Google Scholar]
- 12.EuroQol G EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 13.Devlin NJ, Brooks R. EQ-5D and the EuroQol Group: Past, Present and Future. Appl Health Econ Health Policy 2017;15:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson JA, Luo N, Shaw JW, Kind P, Coons SJ. Valuations of EQ-5D health states: are the United States and United Kingdom different? Med Care 2005;43:221–8. [DOI] [PubMed] [Google Scholar]
- 15.Cook CE. Clinimetrics Corner: The Minimal Clinically Important Change Score (MCID): A Necessary Pretense. J Man Manip Ther 2008;16:E82–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology (Basel, Switzerland) 2015;230:27–33. [DOI] [PubMed] [Google Scholar]
- 17.EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 18.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health and quality of life outcomes 2007;5:70-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Robaee AA, Alzolibani AA. Narrowband ultraviolet B phototherapy improves the quality of life in patients with psoriasis. Saudi medical journal 2011;32:603–6. [PubMed] [Google Scholar]
- 20.Gelfand JM, Wan J, Callis Duffin K, Krueger GG, Kalb RE, Weisman JD et al. Comparative effectiveness of commonly used systemic treatments or phototherapy for moderate to severe plaque psoriasis in the clinical practice setting. Archives of dermatology 2012;148:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koek MB, Buskens E, van Weelden H, Steegmans PH, Bruijnzeel-Koomen CA, Sigurdsson V. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ 2009;338:b1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell 2007;128:853–64. [DOI] [PubMed] [Google Scholar]
- 23.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008;158:558–66. [DOI] [PubMed] [Google Scholar]
- 24.Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol 2018;79:672–9. [DOI] [PubMed] [Google Scholar]
- 25.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanini A, Aiello M, Adamo D, Casale S, Cherubino F, Della Patrona S et al. Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respiratory care 2015;60:88–95. [DOI] [PubMed] [Google Scholar]