Abstract
Influenza virus is a major respiratory pathogen and vaccination is the main method of prophylaxis. In 2012, the trivalent live attenuated influenza vaccine (LAIV) was licensed in Europe for use in children. Vaccine-induced antibodies directed against the main viral surface glycoproteins, haemagglutinin (HA) and neuraminidase (NA), play important roles in limiting virus infection. The objective of this study was to dissect the influenza-specific antibody responses in children and adults, and T cells responses in children induced after LAIV immunization to the A/H1N1 viruses. Blood samples were collected pre- and at 28 and 56 days post-vaccination from 20 children and 20 adults. No increase in microneutralization (MN) antibodies against A/H1N1 were observed after vaccination. A/H1N1 stalk specific neutralizing and NA-inhibiting (NI) antibodies were boosted in children after LAIV. Interferon γ producing T cells increased significantly in children, and antibody-dependent cellular-mediated cytotoxic (ADCC) cell activity increased slightly in children after vaccination, although this change was not significant. The results indicate that the NI assay is more sensitive to qualitative changes in serum antibodies after LAIV. There was a considerable difference in the immune response in children and adults after vaccination, which may be related to priming and previous influenza history. Our findings warrant further studies for evaluating LAIV vaccination immunogenicity.
Keywords: Influenza A/H1N1, Children, Adults, Immune response, LAIV
Introduction
Influenza virus is a major respiratory pathogen causing annual epidemics leading globally to approximately 3–5 million severe infections, with 300,000–650,000 estimated deaths [1]. Influenza is a vaccine preventable disease and two main types of vaccines are available, the inactivated influenza vaccine (IIV) and the live attenuated influenza vaccine (LAIV). In 2012, the LAIV was licensed in Europe for use in children 2–17 years old, but not for adults. Trivalent live attenuated influenza vaccine (LAIV) has previously been shown to provide broader protection against influenza A infection [2–4]. LAIV mimics natural infection and both the innate and the adaptive immune responses are activated after LAIV [5–7].
The influenza virus has two major surface glycoproteins; the hemagglutinin (HA) and neuraminidase (NA), and antibodies directed towards both glycoproteins play an important role in protection against influenza virus. The haemagglutinin comprises an immunodominant head domain (composed of the majority of the HA1 subunit) and a stalk domain (mainly the HA2 subunit and the N- and C-terminal ends of HA1). Antibodies to the HA head inhibit viral attachment to the host cell receptors and can be measured by the hemagglutination inhibition (HI) assay. HI antibodies are measured as a surrogate correlate of protection [8–9] with an HI titer of 40 providing a 50% protective threshold in adults. The micro-neutralization assay also measures mainly HA head specific antibodies that can neutralize the influenza virus [11]. The HA head specific antibodies do not reflect the whole spectrum of protective antibodies and the HA stalk-specific antibodies can also confer protection [12]. The stalk domain is conserved and provides broad cross-protection by neutralizing antibody and through antibody-dependent cellular-mediated cytotoxicity (ADCC) by interaction of antibodies and FcγR on natural killer cells [13–16]. The hemagglutinins of the influenza A viruses are divided into groups based on their amino acid sequence of mainly the head region, the more conserved stalk region may be a promising target for universal vaccine development. A recent animal model study identified broadly protective anti-HA stalk antibodies that were induced after LAIV vaccine expressing chimeric HA [17]. Furthermore, neuraminidase inhibition (NI) antibodies can also provide protection from influenza [18]. NA is an important viral surface glycoprotein with enzymatic activity cleaving sialic acid on the lumen of mucosal epithelial cells of the respiratory tract. NA is important in the infection process, clearing a path for infection, lowering the pH at the cell surface, and releasing of progeny virus from infected cells. Blocking NA activity is an effective way to inhibit infection and viral shedding [19,20].
During the 2014–15 season, the USA experienced unexpectedly low protection from the H1Npdm09 strain in the LAIV, which was not observed with IIV. This led the US Advisory Committee on Immunization Practices (ACIP) to withdraw its earlier preferential recommendation of LAIV in children. However, moderate protection was observed to the H1N1 strain in Europe, with 41.5% vaccine effectiveness in the UK and 47.9% in Finland, although remaining lower than IIV [21,22]. A study in Senegal found that LAIV failed to protect against H1N1pdm09 in young children [23], whereas protection was found in a similar study in Bangladesh [24]. The reason for these differences is currently unknown but could be due to the vaccines were based on different backbones, or the populatiońs exposure history [25,26]. The LAIV manufacturer reported that the A/H1N1 strain had a temperature sensitive mutation rendering it heat instable providing a possible explanation for the reduced protection observed, and has since updated the vaccine [27]. Importantly, exposure history to influenza will influence protection and differences in vaccine recommendations could possibly affect protection observed after LAIV. The USA recommends influenza vaccination for everybody from > 6 months, while the European vaccine recommendations primarily focus on risk groups.
In our previous study, we found no increase in HI antibodies to the H1N1 strain after LAIV, however most children had pre-existing antibodies [28]. However, a slight trend of rising H1N1 stalk specific antibodies was observed in children after LAIV, whereas adults had already higher levels of H1-stalk specific IgG antibodies pre-vaccination and no further increase was observed after vaccination. We suggested that this was due to preexisting antibodies present after vaccination or infection during the 2009 pandemic. The aim of this study was to further dissect the antibody responses, focusing on the functional immune response after LAIV immunization against H1N1.
Materials and Methods
Patients & Samples
Forty subjects (20 children (3–17 years old) and 20 adults (21–59 years old)) were intranasally immunized with 0.1 mL per nostril of the seasonal LAIV (Fluenz, Astra Zeneca, Liverpool, UK). LAIV (Fluenz) contained 107 fluorescent focus units (FFU) of A/California/7/2009(H1N1)pdm09-like and A/Victoria/361/2011(H3N2)-like strains in both 2012/13 and 2013/14 seasons, and either B/Wisconsin/1/209-like or B/Massachusetts/2/2012-like influenza B virus strains in the 2012/13 or 2013/14 seasons, respectively. The study was approved by the ethical and regulatory authorities (EUDRACT2012–002848-24, www.clinicaltrials.gov; ). Children received one (≥9 years old, n=6) or two doses (<9 years old, n=14) of LAIV in 2012 at a four-week interval, whereas adults received a single dose in 2013–14 season [5]. Blood samples were collected at day 0 (pre-vaccination), 28- and 56-days post-vaccination and plasma aliquoted and frozen for use in the serological assays, as previously described [28]. In children, cell preparation tubes (CPT, BD) were used to separate peripheral blood mononuclear cells (PBMCs) for the ELISpot assay.
Viruses & Antigens
Viruses were propagated in the allantoic cavity of 10 day old embryonated hen’s eggs. Allantoic fluid was harvested, clarified and frozen at −80ºC until used in the assays as described below. The reassortant A/California/7/2009(H1N1) virus (X-179A) was used for the micro-neutralization (MN) assay, the chimeric cH9/1N3 virus containing the HA stalk from A/California/7/2009(H1N1) and head from A/guinea fowl/Hong Kong/WF10/99 for the virus neutralization (VN) assay, and the reverse genetics H7N1 virus (NIBRG-127 containing the NA from A/California/7/2009(H1N1) and HA from the equine A/Prague/56 (H7N7) strain) for enzyme-linked lectin assay (ELLA). The wild type A/California/7/2009(H1N1) virus was used for the antibody dependent cellular cytotoxicity (ADCC) assay.
Micro-neutralization assay (MN)
The microneutralization assay was conducted as previously described [28]. Briefly, plasma and control sheep serum samples (NIBSC, UK) were 2-fold serially diluted from 1:10 in flat-bottom 96-well cell culture plates (Nunclone Delta surface, USA) before incubation with one-hundred 50% tissue culture infectious does (TCID50)/50µl/well of X-179A for 1 h at room temperature. Then, 1.5×105 MDCK (Mardin Darby Canine Kidney) cells/ml were added and incubated for 16–18 hours at 37°C. The propagation of influenza virus was detected using antibody to the nucleoprotein and TMB (3,3′, 5,5′-Tetramethylbenzidine; Thermo Fisher Scientific, USA) before reading at 450nm and 620nm to obtain the final optical density (OD). The microneutralization titers (IC50) were calculated using the Reed and Muench method [29].
Virus neutralization assay (VN)
The virus neutralization assay was conducted with the cH9/1 virus using a 3-day incubation period [30,31]. Briefly, cell culture plates (Flat-bottom 96-well Nunclone Delta surface, USA) were seeded with 1.5×104 MDCK cells/well and incubated at 37°C overnight. Next, heat-inactivated plasma samples were diluted to 1:10 and 2-fold serially diluted before incubation with cH9/1N3 (100 TCID50/50 µl) for 1h at 37°C. MDCK cells were washed with phosphate buffered saline (PBS), and plasma/virus dilutions were added and incubated for 1 h at 37°C. The mixture was removed, cells were washed with PBS, and 50 µl of serially diluted plasma plus 50 µl infection medium (DMEM medium containing 2.5µg/ml tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Worthington Biomedical, USA), PSA (100 IU/ml penicillin, 100mg/ml streptomycin and 0.25 μg fungizone; Lonza, Switzerland) and 0.14% bovine serum albumin (Sigma-Aldrich, USA) were added to each well before incubation at 37°C for 72 h. The virus neutralization titers were measured by haemagglutination assay using the supernatant (50 µl) and 50 µl of 0.7% human red blood cells and read after 30 min of incubation at room temperature. The highest dilution of plasma giving complete haemagglutination was read as the neutralizing antibody titer.
Enzyme-linked lectin assay (ELLA)
The ELLA was used to measure antibodies inhibiting the ability of neuraminidase to cleave sialic acid as previously described [32–34] using the H7N1 virus containing the HA from an equine influenza virus strain and NA from A/California/07/09. Ninety-six well flat bottom Maxisorb plates (VWR, USA) were coated overnight at 4°C with 100µl coating solution containing 0.25µg fetuin per well (Sigma-Aldrich, USA; KPL; Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA). Heat-inactivated (56°C for 45–60min) serially diluted serum and H7N1 virus were added and incubated at 37°C for 16–18 hours. The virus was diluted to a titer giving 90% neuraminidase activity. The plates were developed by adding 0.1µg/100µl/well horseradish peroxidase (HRP)- conjugated peanut agglutinin (PNA) (Sigma-Aldrich, USA) and incubation at room temperate for 2 hours, followed by adding o-phenylenediamine dihydochloride (OPD) (Sigma-Aldrich, USA) substrate (0.5mg/ml) in citrate buffer to all wells. After 10 min incubation at room temperature in the dark, the reaction was stopped with 100 μl 1M sulphuric acid. The plates were read with a microplate reader by spectrophotometry at OD 490 nm. The anti-NA antibody titres (reported as 50% inhibition concentration, IC50) in the plasma samples were calculated as the reciprocal dilution of plasma which gave OD values equal to 50% of total OD (OD virus control + OD blank) in four-parameter non-linear regression analysis using GraphPad Prism.
Antibody dependent cellular cytotoxicity (ADCC) assay
The ADCC Reporter Bioassay (Core Kit G7010/G7018, Promega, USA) was used to quantify pre- and post-vaccination ADCC antibodies [15]. MDCK cells as ‘target cells’ were seeded in 96 F-well white tissue culture plates (VWR, USA) at 1.5×104 cells/well. Between 18–24 hours later, cells were infected with wild type A/California/7/2009(H1N1)pdm09 virus at a multiplicity of infection (MOI) of 3. On the day of assay, the medium was replaced with assay buffer (RPMI 1640 with 4% (vol/vol) low IgG fetal bovine serum (FBS) (Lonza, Switzerland) followed by the addition of 5-fold (starting at 1:10) serial dilutions of plasma. The infected cells and antibodies were incubated at 37 °C for 30 mins. The effector cells (Jurkat) at 7.5×104 cells/well were added to the assay plates. After 6 hours incubation at 37°C, the Bio-Glo Luciferase Assay Reagent (Promega, USA) system was used to quantify using a plate reader with glow-type luminescence.
ELISpot Assay
Antigen-specific interferon gamma (IFN-γ) cytokine-secreting T cells were quantified at the single-cell level by the ELISpot assay (Mabtech) [35]. Optimized libraries of peptides represented unique T cell epitopes from four of the initial H1N1pdm09 circulating strains for CD4+ (originating from HA, NA, M1, NP, PB2) and CD8+ (M1, NA, PA and NS2) responses (Table S1). Briefly, 400 000 PBMCs per well were stimulated with CD4+ and CD8+ conserved peptide pools (2 μg/mL) anti-CD3 T-cell activator (positive control), or lymphocyte medium alone (negative control) [36]. Plates were incubated overnight at 37°C with 5% CO2. After developing the following day, the plates were read using an automated reader (Advanced Imaging Devices) and spot-forming units (SFUs) were counted. The background values were subtracted from the influenza virus-specific response.
Statistics
The statistical tests and graphs were made using GraphPad Prism; v.7.0d for Mac (GraphPad Software, USA), where, P<0.05 was considered significant. Immune responses within each patient group, children or adults, were analysed using Friedman test (One-Way ANOVA, non-parametric, paired test). Comparison between children and adults were performed using Mann-Whitney test (t-Test, non-parametric, unpaired). Correlation analysis was performed using linear regression and Spearman correlation.
Results
Twenty children (3–17 years old) and twenty adults (21–59 years old) were intranasally vaccinated with seasonal LAIV. The children received one (≥9 years old, n=6) or two doses (<9 years old, n=14) of LAIV in 2012 at a four-week interval, whilst adults received one dose of vaccine. In this study, the objective was to analyze the functional HA and NA H1N1 specific responses in children and adults, and the T cellular immune response in children.
Presence of neutralizing serum antibodies by microneutralisation assay (MN)
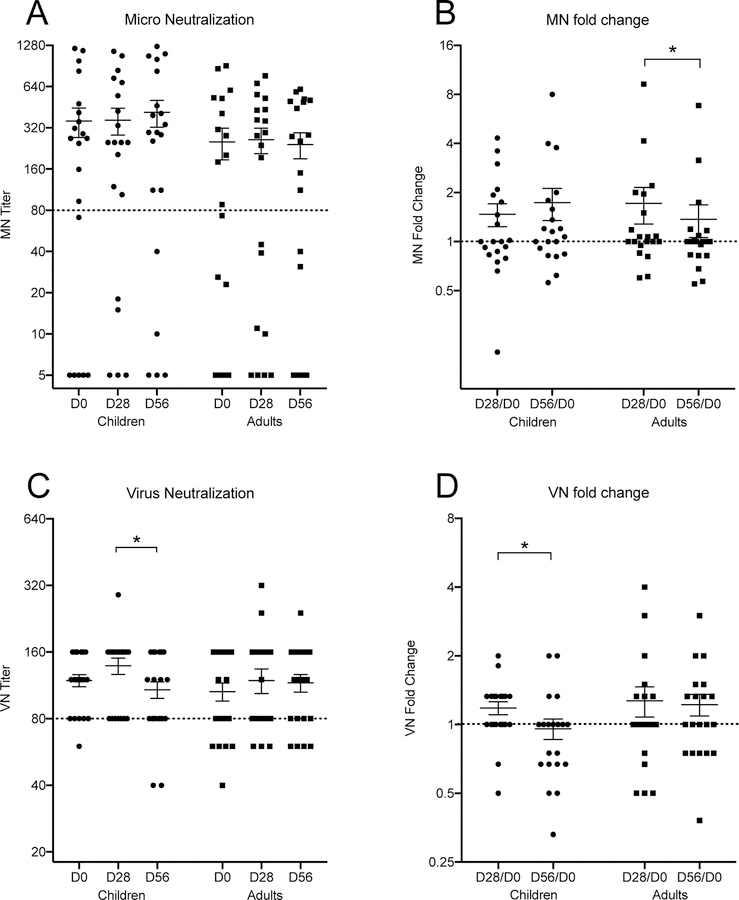
A MN titer of 80 is considered to protect 50% of individuals from disease [37]. Sixty percent of children had protective pre-vaccination MN titers (Figure 1A). Four children (20%) were boosted after 1st dose of vaccine and one of these children (5%) seroconverted after LAIV to a protective antibody titer. Only three children (15%) had no detectable MN antibodies both pre- and post-vaccination. Eleven adults (55%) had protective pre-vaccination MN titers of whom 25% increased post-vaccination (Figure 1A) and one (5%) adult sero-converted to protective antibody titers after LAIV. Of the adults with antibodies below the protective MN threshold before vaccination, 30% remained sero-negative post-vaccination. After vaccination MN antibody titers increased <2 fold in both children and adults at day 28 and decreased in adults at day 56 (Figure 1B). Children had higher MN titers than the adults both pre- and post -vaccination. As expected, both pre-and post-vaccination HI and MN titers correlated well (pre-vaccination: children- R=0.5546, adults- R=0.7514; post-vaccination: children- R=0.839, adults- R=0.3267; Figure S2). In summary, LAIV did not significantly boost micro-neutralizing antibody titers in adults or children against the H1N1 vaccine strain.
Figure 1.
Dot plot of the microneutralization (MN, X-179A) and virus neutralization (VN, cH9/1N3) assays. Each dot represents data from one subject. The panels A to D are; MN, the fold change of MN, VN and fold change of VN respectively. The titers and fold change are indicated on the y-axis. The groups on the horizontal axis are children (circles) and adults (squares), sampled at day zero, and 28 and 56 after vaccination. The mean value +/− SEM of each group is indicated in all panels. In panels A and C, a horizontal dotted line indicates a protective threshold titer. A similar dotted line is indicating no titer change (fold change = 1). A significant difference in median values are indicated with asterisks above the group columns, p <0.05.
Assessing the stalk specific neutralizing serum antibodies by ELISA
We have previously shown that only children, and not adults showed a slight trend of an increase in stalk specific antibodies measured by ELISA using chimeric HA proteins after LAIV vaccination [28]. We extended these observations by investigating the HA stalk specific virus neutralizing antibody responses using a chimeric cH9/1 virus. Nineteen (95%) children had high VN titer ≥ 80 pre-vaccination (geometric mean titer GMT: 114.2). H1N1 stalk-specific VN antibodies increased slightly after 1st dose of LAIV in 50% of children, (GMT=129.3) but decreased after the second dose (GMT=99.7) (Figure 1C). Fifteen adults (75%) had pre-vaccination stalk specific VN titers ≥ 80. No significant boost in stalk-specific VN antibodies was observed after LAIV, although 35% of adults had increases in VN antibodies (Figure 1C). In summary, neutralizing stalk antibodies showed a slight trend of an increase after LAIV vaccination in children after first 1st LAIV dose but were not maintained after the 2nd dose (Figure 1D). Of note, the method used for these assays is different than the standard MN assay used for wild type virus and is – in combination with the cH9/1N3 virus - more sensitive than standard MN assays, leading to a higher baseline as well.
Neuraminidase Inhibiting Assay (NI) showed increases titers in children and adults
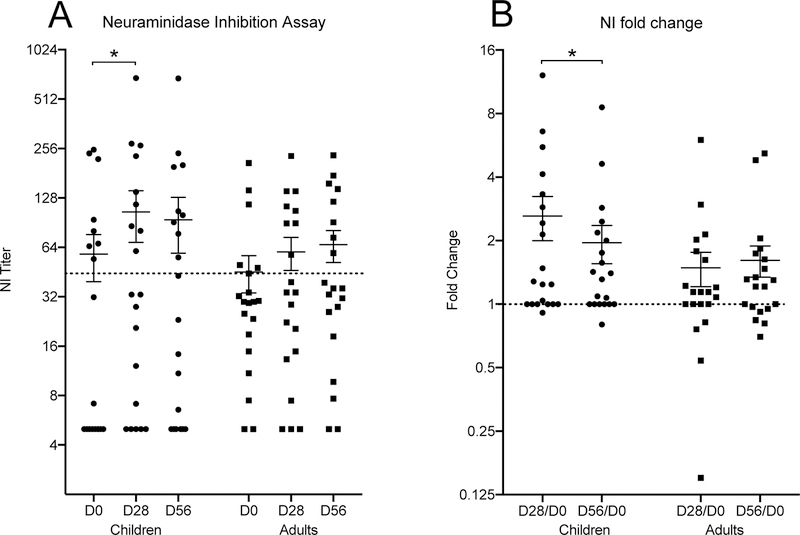
Neuraminidase inhibiting antibodies also play an important protective role in influenza virus infection. A neuraminidase inhibition (NI) titer of ≥40 reduced influenza illness in the human challenge model and has been recommended as protective titer [38,39]. Eight children had pre-vaccination NI titers above the protective titer, two children had low titers, and the remaining 10 children did not have detectable titers (<10) (Figure 2A). The median NI titer increased significantly at 28 days post vaccination in children, and most children responded with higher NI titers, although not all responses were ≥40.
Figure 2.
Neuraminidase inhibition assay (NIBRG-127), tested at day zero and days 28 and 56 after LAIV vaccination in children (circles) and adults (squares). Each dot represents an individual test-point. The titer and fold change is indicated on the vertical axis and the groups on the horizontal axis. The mean value +/−SEM are shown for each group. A dotted line indicates the protective threshold in the left panel (A) and a no fold change in the right panel (B). A significant difference in median values are indicated with asterisks above the group columns, p <0.05.
In adults, thirty percent had protective NI antibody titers pre-vaccination, whilst 60 percent had low NI titers and ten percent had non-detectable NI titers (<10) before vaccination (Figure 2A). Vaccination boosted the NI titers in most of the adults, and the number of adults with protective level increased to forty percent. Overall the NI titers increased in most of the subjects, and very few had a decrease in titer (Figure 2B).
Children had stronger Antibody Dependent Cellular Cytotoxity (ADCC) levels than adults after LAIV
We examined the ability of antibodies to elicit ADCC activity in children and adults to the H1N1pdm09 virus after LAIV vaccination. Adults had higher ADCC antibody titers compared to children both pre- and post-vaccination. The mean ADCC titer was 95 pre-vaccination in children and increasing to 156 after 28 days, but this change was not significant (Figure 3A). The adults had a mean ADCC titer of 271 pre-vaccination and this dropped significantly to 147 after 28 days (Figure 3A). Significantly higher fold changes were found in children compared to adults after 1st dose of vaccine (Figure 3B).
Figure 3.
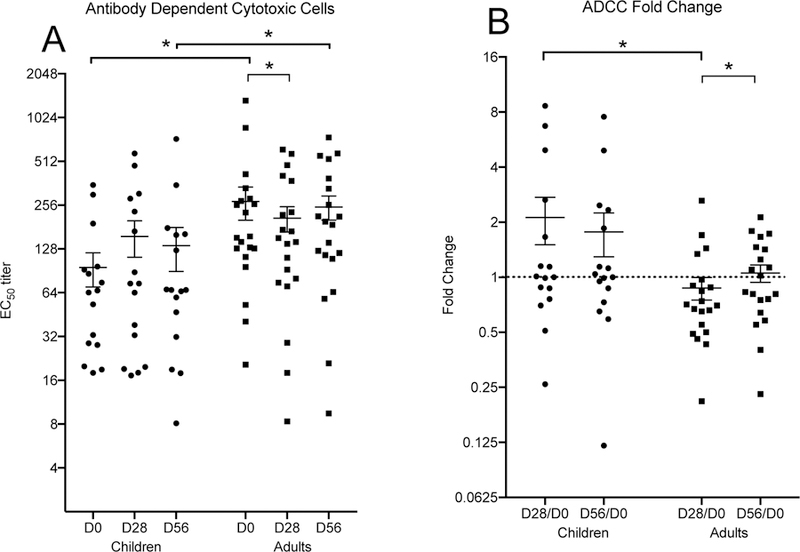
The antibody-dependent cytotoxic cell (ADCC, A/California/7/2009(H1N1) ) activity in children (circles) and adults (squares) after LAIV vaccination, each dot represents an individual test point. Panel A (left) shows the ADCC activity and panel B (right) shows the fold change in the ADCC activity. The groups; children and adults, and the time point after vaccination is shown on the horizontal axis. The ADCC titer and fold change are indicated on the vertical axis. The mean value and SEM is indicated for each group. A dotted horizontal line is shown in panel B, indicating a no fold change level. A significant change in median values are indicated with asterisks above the group columns, p <0.05.
LAIV elicited CD4+T cell activation in children but not CD8+ cell activation
As only children responded after LAIV we further extended this work by investigating the ability of LAIV vaccine to elicit IFN-γ producing T-cells in children using H1N1pdm09 strain specific CD4 or CD8 peptides. These CD4+ and CD8+ peptides (Table S1) represented unique T cell epitopes from four of the initial H1N1pdm09 circulating strains. Previous work has shown that 100 spot forming units (SFUs)/106 PBMCs provided protection from laboratory confirmed influenza after LAIV immunization in children [40]. Although a recent population based study in the UK showed that T-cell responses to NP ≥20 SFU/106 PBMC were associated with protection in adults [41].
Most children had less then 100 IFN-γ secreting CD4+100 SFUs per million PBMCs and only 1 child (5%) had above this level before vaccination. LAIV significantly boosted the CD4+ IFN-γ response (Figure 4A) with twenty percent of children had more then 100 IFN-γ CD4+ T-cells at 28 days post-vaccination. Two of these children (10%) maintained high IFN-γ secreting CD4+ T-cell after 2nd dose of LAIV. Two further children had increases in IFN-γ to >100 SFUs/106 PBMCs after the 2nd dose of LAIV. However, we observed no changes after LAIV in IFN-γ secreting H1N1 specific CD8+ T-cells, except one (5%) child who showed an increase at day 56 (Figure 4B).
Figure 4.
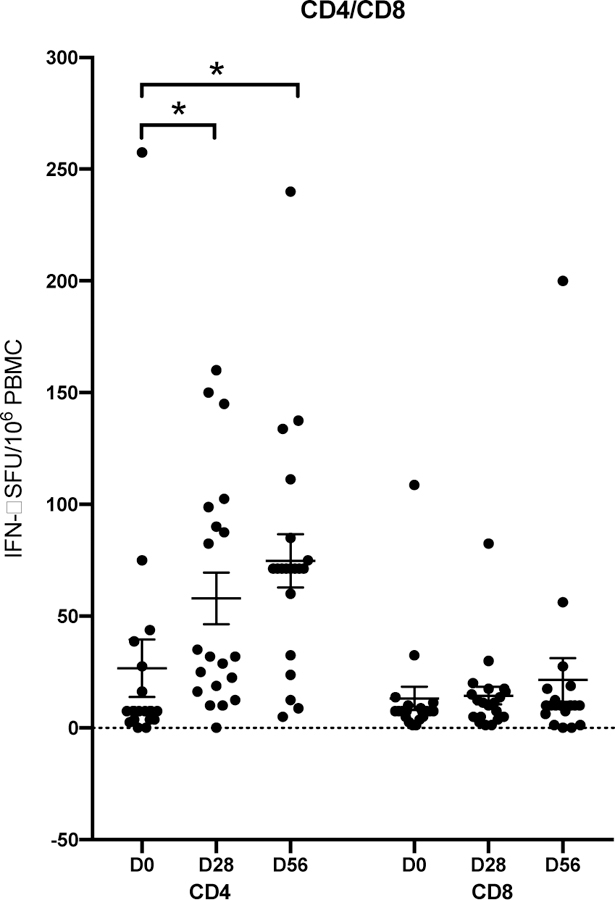
CD4+ and CD8+ IFN γ positive T-cell responses to influenza A H1N1 virus strains after live attenuated influenza vaccine (LAIV) in children. IFN γ + T-cell specific antibody response is shown in children per 106 peripheral blood mononuclear cells (PBMCs) after LAIV vaccination. The columns represent the CD4+ and CD8+ T-cell responses, and the timepoints after LAIV vaccination. Each symbol represents an individual child, and bars represent the means with standard errors of the mean. Significant change in median values are indicate with asterisks above the group columns, p <0.05.
Discussion
Earlier, the LAIV showed poor protective efficacy against the H1N1pdm09 strain and was therefore not recommended used by the ACIP, however in the 2018/19 season the vaccine was reinstated [42]. Several European studies showed other results and therefore the EU continued to recommend use of LAIV in children [43]. We have previously shown that LAIV immunization did not boost H1N1 HI titers in adults or children [28]. Although children had significantly higher H1N1 HI titers than adults prior to and up to 56 days after vaccination, probably due to previous infection or the pandemic vaccination during the 2009 mass vaccination campaign. In this study, we evaluated the humoral (children and adults) and the cellular immune response (children only) after LAIV immunization to further understand the lower immune responses to H1N1pdm09. Our results demonstrate that the majority of children had relatively high pre-vaccination MN titers, perhaps due to either infection from circulating H1N1pdm09 or the previous pandemic vaccination. Although no boosting in MN antibodies was found after vaccination, in agreement with a previous report [44], the children had low but significant increases in neuraminidase specific antibody levels and CD4+ T cell responses, supporting findings that LAIV induces a broad immune response, which could have a protective effect.
No boost in H1N1 specific MN antibodies in children or adults after LAIV
The immune response to LAIV is multifaceted and the HI titer underestimates protection achieved from LAIV [45] Overall, no significant changes in MN antibodies were observed after LAIV, although a trend of an increase in MN titers was observed in both adults and children. Children have experienced less influenza virus exposure in their life-time compared to adults and therefore may have a stronger recall response to antigenically matched strains [46–48], perhaps explaining the higher MN antibody observed in the children (Figure 1C & 1D). Our study reveals that HI titers correlated well with the MN titers in both children and adults, pre- and 28 days post vaccination, with adults and children with HI titers > 40 also having higher MN titers. Previously the MN assay has been shown to have a higher sensitivity to H1N1pdm09 than the HI assay [37]. Interestingly, HI titers of 40 were associated with the geometric mean of MN titers over 160 in both children and adults in agreement with a previous study that suggested that MN titers of 160 are associated with protective HI titres [47], but this threshold may be different from study to study.
During the 2009 pandemic, Norway conducted a mass vaccination campaign with AS03 adjuvanted pandemic vaccine and nearly half of the population was vaccinated. In our study, half of the children and adults were previously vaccinated with adjuvanted pandemic vaccine and these vaccinated subjects had higher MN antibody titers compared to the unvaccinated group, in agreement with previous findings [50]. It has been shown that H1N1pdm09 specific HI antibodies persist after a single adjvuanted pandemic vaccination beyond the 2012 and 2013 seasons [51]. Interestingly, the adults vaccinated with the pandemic vaccine had significantly higher MN titres than unvaccinated adults, whereas this was not observed in the children many of whom may have had H1N1pdm09 as their first influenza virus infection which circulated in Norway in the 2009/10 and 2010/11 seasons (Figure S3). In children, influenza imprinting by early exposure to influenza virus influences subsequent influenza immunity [52,53], but whether the imprinting is specific to the first infection, or a result of the cumulative effect of repeated exposures and boosting, remains unclear. Although a child’s first encounter with influenza virus (HA imprinting) has a large impact on future immune status [54,55].
An elevated H1N1 stalk specific neutralizing antibody titer were observed in children post LAIV vaccination
MN antibodies prevent infection therefore are a direct measure of protection, although HA stalk-specific antibodies may also confer protection [12] through neutralization or by ADCC through Fc–FcγR interactions [13]. We have previously reported a slight trend of an increase in group 1 HA stalk specific IgG in children after LAIV, but not in adults [28]. Stalk specific neutralizing antibodies also showed a slight boost after the first dose in children suggesting that LAIV could be used as a priming vaccine for inducing broadly cross-reactive stalk responses. However, no change in stalk neutralizing responses after LAIV vaccination was found in adults, irrespective of previous vaccination history. Furthermore, the stalk specific VN antibody response did not correlates with HI titer in either children or adults and confirm the different specificity of the assays (data not shown).
Overall children had more head specific neutralizing MN antibodies, whereas adults had higher stalk specific virus neutralizing antibodies (data not shown). Interestingly, we found both children and adults previously vaccinated with AS03 pandemic vaccination had elevated head specific MN antibodies, whereas elevated stalk specific VN antibody responses were observed in non-vaccinated children and adults (Figure S5). Infection do generally stimulate stronger antibody responses to stalk antigens than vaccination. This suggests induction of stalk specific neutralizing antibodies depends on previous exposure history but is not related age related [56,57].
LAIV boosts neuraminidase inhibition (NI) response in children
NI antibodies can reduce release of new viruses from infected cells [58,59] and NA specific antibodies may be a correlate of protection [38,39] in man, independently of HA antibodies. Here, we found significant increases in NI antibodies after LAIV in children although titres often remained below the protection level (<40). In contrast, in the UK no increase in NI antibodies was found after LAIV4 perhaps because of the H1N1pdm09 having lowest replication of the four LAIV strains [44,60]. Here, LAIV boosted the NI response after the 1st dose in children which continued to increase after the 2nd dose in the previously vaccinated group, whereas, antibodies only increased after 1st dose in the previously unvaccinated group. Further analysis demonstrated that both children and adults with high HI or MN antibody titers (> 40) also had protective NI antibody titers, although these NI antibodies were only boosted significantly in children. Surprisingly, the NI antibody fold change after LAIV seemed to increase in children with increasing age, whilst decreasing in adults [18].
No boost in ADCC inducing antibodies
We and others have previously shown that A(H1N1)pdm09 vaccination and infection induced HA-specific ADCC mediating antibodies, with the stalk-specific antibodies better at mediating ADCC than head-specific antibodies [30]. In the current study, we evaluated the ADCC activity to wild type H1N1pdm09 with the aim of detecting both H1 stalk and NA antibodies. Half of the children were vaccinated with AS03 pandemic vaccine in 2009 and these children had high stalk specific functional antibodies, as hypothesized previously [61–63]. But we also found high titers of stalk specific VN antibodies in the unvaccinated children who may have experience natural infection with H1N1pdm 09 (Figure S4). Higher pre-existing ADCC inducing antibodies may limit replication of the LAIV strains and therefore limiting the ability of the vaccine to boost antibody responses [64]. Interestingly, our study illustrated that both children and adults had pre-existing ADCC antibodies, although the children had significantly lower antibody titers than adults, perhaps explaining the lower efficacy seen after LAIV in adults. Concurrently the adults had lower HA (HI and MN) and NA specific (NI) antibody titers than the children despite having higher ADCC titers pre and post vaccination. No significant changes in ADCC reporter activity were found after LAIV, in agreement with an earlier study [65].
LAIV boosts CD4 T cells but not CD8 T cells in children
T-cells play an important role in coordinating the immune response and are also critical for the control of influenza infection. Early human studies showed an inverse correlation between influenza virus shedding and CD8+ T cells in sero-negative adults [66,67]. The importance of CD8 cells was confirmed during the 2009 pandemic when pre-existing late-effector CD8+ IFN-γ T-cells with lung-homing and cytotoxic potential were associated with milder symptoms and less severe illness [68] in the absence of H1N1pdm09 specific antibody. In human challenge studies, pre-existing CD4+ T-cells resulted in lower virus shedding and less severe influenza symptoms in sero-negative subjects [69]. We have previously shown that LAIV induces durable increases in IFN-γ positive T cells to H1N1pmd09 in children and that pre-existing cross-reactive CD8 T cells are boosted in young children [70]. Here, we aimed to further understand the T cell response in children using optimized libraries of CD4 and CD8 peptides representing unique T cell epitopes from the H1N1pdm09 virus. In the children, we found that only H1N1pdm09 specific CD4 responses were boosted after LAIV and no change in CD8 responses was observed [71].
Our results suggest that the H3N2 LAIV strain may be responsible for our earlier observation of boosting of CD8 cross protective responses but will need to be confirmed using conserved CD8 peptides. In earlier LAIV studies we found that 25% had significant increases in virus specific T-cell responses after vaccination although no increase in serum responses was detected [5]. This study further extends these findings to show that although the children did not respond by traditional MN antibodies, they responded with increase in CD4 T-cells, which may provide clinical protection. LAIV has been shown to induce broader protection in ferrets [72] and in children when used in a school setting [73]. Furhermore, the inclusion of LAIV in the childhood vaccination programme in the UK has shown the benefits of herd immunity, which, are thought to be mediated by T-cells [74,75]. Therefore, the use of LAIV in children could be an important step in the protection against novel drifted or pandemic strains.
This study has focused on the functional systemic immune responses. The LAIV vaccine is intended to mimic a natural infection and stimulate the local immune response. We have in a previous study, shown that LAIV induces local influenza specific IgA production [71]. As there are some degree of compartmentalization of the immune system, and we cannot expect to observe the same degree of immune response systemically as with parenteral administered vaccines. Using established immune response thresholds validated for systemic vaccines may have to be used with caution when evaluating LAIV. This warrant further work and development of improved evaluation tools for mucosal vaccines.
In conclusion, children and adults have different previous exposure histories to the influenza virus through infection and vaccination and this can impact upon the generation of immune responses after LAIV. The LAIV vaccine is administered mucosally requiring replication of the vaccine viruses, therefore pre-existing antibodies may limit replication and subsequent induction of immune responses as observed in the adults with relatively high pre-vaccination antibody levels. Although the children also had high MN antibodies they responded with significant increase in NI and CD4 T cells after vaccination. This indicate that the NI assay may be more sensitive to detect boosting in antibody responses after mucosal LAIV vaccination which needs further investigation. The multifaceted immune response after LAIV in children supports the continued recommendation of LAIV for use in children.
Supplementary Material
Acknowledgement
We thank the children and their parents and adults who participated altruistically in this study; all of the staff at the Ear, Nose, and Throat Department; and the Children’s clinical trial unit at Haukeland University Hospital and the Influenza Centre for assistance with the clinical trial. Thanks to Dr. Fredrik Oftung at the Norwegian Institute for Public Health, Oslo, for providing the peptides used in this study. The study was supported intramurally by the Influenza Centre at the University of Bergen. The Influenza Centre is funded by the Ministry of Health and Care Services, Norway, the European Union (Univax 601738, EU IMI115672 FLUCOP), Helse Vest, and the K.G. Jebsen Centre for Influenza Vaccine Research. Support for the Krammer laboratory came from the NIAID Centers of Excellence for Influenza Virus Research and Surveillance (CEIRS) contract HHSN272201400008C
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- [1].Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowson MA, Bresee JS; Global Seasonal Influenza-associated Mortality Collaborator Network. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018. March 31;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. Epub 2017 Dec 14. Erratum in: Lancet. 2018 Jan 19;:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Atmar RL, Keitel WA, Cate TR, Quarles JM, Couch RB. Comparison of trivalent cold-adapted recombinant (CR) influenza virus vaccine with monovalent CR vaccines in healthy unselected adults. J Infect Dis 1995;172:253–7. [DOI] [PubMed] [Google Scholar]
- [3].Belshe RB, Van Voris LP, Bartram J, Crookshanks FK. Live attenuated influenza A virus vaccines in children: results of a field trial. J Infect Dis 1984;150:834–40. [DOI] [PubMed] [Google Scholar]
- [4].Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr., Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis 1994;169:68–76. [DOI] [PubMed] [Google Scholar]
- [5].Mohn KG, Bredholt G, Brokstad KA, Pathirana RD, Aarstad HJ, Tøndel C, Cox RJ. Longevity of B-cell and T-cell responses after live attenuated influenza vaccination in children. J Infect Dis 2015. May 15;211(10):1541–9. doi: 10.1093/infdis/jiu654. Epub 2014 Nov 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hoft DF, Lottenbach KR, Blazevic A, Turan A, Blevins TP, Pacatte TP, et al. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin Vaccine Immunol 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Forero A, Fenstermacher K, Wohlgemuth N, Nishida A, Carter V, Smith EA, Peng X, Hayes M, Francis D, Treanor J, Morrison J, Klein SL, Lane A, Katze MG, Pekosz A. Evaluation of the innate immune responses to influenza and live-attenuated influenza vaccine infection in primary differentiated human nasal epithelial cells. Vaccine 2017. October 27;35(45):6112–6121. doi: 10.1016/j.vaccine.2017.09.058. Epub 2017 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ng S, Fang VJ, Ip DKM, Chan KH, Leung GM, Peiris JSM, et al. Estimation of the Association Between Antibody Titers and Protection Against Confirmed Influenza Virus Infection in Children. Journal of Infectious Diseases 2013;208:1320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. Bmc Med Res Methodol 2010;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Verschoor CP, Singh P, Russell ML, Bowdish DM, Brewer A, Cyr L, Ward BJ, Loeb M. Microneutralization assay titres correlate with protection against seasonal influenza H1N1 and H3N2 in children. PLoS One 2015. June 24;10(6):e0131531. doi: 10.1371/journal.pone.0131531. eCollection 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nachbagauer R, Choi A, Izikson R, Cox MM, Palese P, Krammer F. Age Dependence and Isotype Specificity of Influenza Virus Hemagglutinin Stalk-Reactive Antibodies in Humans. MBio 2016;7:e01996–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 2014;20:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Vries RD, Nieuwkoop NJ, van der Klis FRM, Koopmans MPG, Krammer F, Rimmelzwaan GF. Primary Human Influenza B Virus Infection Induces Cross-Lineage Hemagglutinin Stalk-Specific Antibodies Mediating Antibody-Dependent Cellular Cytoxicity. J Infect Dis 2017;217:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].He W, Tan GS, Mullarkey CE, Lee AJ, Lam MM, Krammer F, et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc Natl Acad Sci U S A 2016;113:11931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jegaskanda S, Laurie KL, Amarasena TH, Winnall WR, Kramski M, De Rose R, et al. Age-associated cross-reactive antibody-dependent cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. J Infect Dis 2013;208:1051–61. [DOI] [PubMed] [Google Scholar]
- [17].Isakova-Sivak I, Korenkov D, Smolonogina T, Kotomina T, Donina S, Matyushenko V, et al. Broadly protective anti-hemagglutinin stalk antibodies induced by live attenuated influenza vaccine expressing chimeric hemagglutinin. Virology 2018;518:313–23. [DOI] [PubMed] [Google Scholar]
- [18].Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, et al. Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model. MBio 2016;7:e00417–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Webster RG, Laver WG. Preparation and properties of antibody directed specifically against the neuraminidase of influenza virus. J Immunol 1967;99:49–55. [PubMed] [Google Scholar]
- [20].Monto AS, Kendal AP. Effect of neuraminidase antibody on Hong Kong influenza. Lancet 1973;1:623–5. [DOI] [PubMed] [Google Scholar]
- [21].Nohynek H, Baum U, Syrjanen R, Ikonen N, Sundman J, Jokinen J. Effectiveness of the live attenuated and the inactivated influenza vaccine in two-year-olds - a nationwide cohort study Finland, influenza season 2015/16. Euro Surveill 2016;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Euro Surveill 2016;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Victor JC, Lewis KD, Diallo A, Niang MN, Diarra B, Dia N, et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among children in Senegal: a randomised, double-blind, placebo-controlled trial. Lancet Glob Health 2016;4:e955–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brooks WA, Zaman K, Lewis KD, Ortiz JR, Goswami D, Feser J, et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among young children in Bangladesh: a randomised, double-blind, placebo-controlled trial. Lancet Glob Health 2016;4:e946–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Penttinen PM, Friede MH. Decreased effectiveness of the influenza A(H1N1)pdm09 strain in live attenuated influenza vaccines: an observational bias or a technical challenge? Euro Surveill 2016;21(38):30350 DOI: 10.2807/1560-7917.ES.2016.21.38.30350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ambrose CS, Bright H, Mallory R. Letter to the editor: Potential causes of the decreased effectiveness of the influenza A(H1N1)pdm09 strain in live attenuated influenza vaccines. Euro Surveill 2016;21(45):30394 DOI: 10.2807/1560-7917.ES.2016.21.45.30394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cotter CR, Jin H, Chen Z. A single amino acid in the stalk region of the H1N1pdm influenza virus HA protein affects viral fusion, stability and infectivity. PLoS Pathog 2014. January;10(1):e1003831. doi: 10.1371/journal.ppat.1003831. Epub 2014 Jan 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Islam S, Mohn KG, Krammer F, Sanne M, Bredholt G, Jul-Larsen A, et al. Influenza A haemagglutinin specific IgG responses in children and adults after seasonal trivalent live attenuated influenza vaccination. Vaccine 2017;35:5666–73. [DOI] [PubMed] [Google Scholar]
- [29].Reed LJ, Muench H A simple method of estimating fifty percent endpoints. Am. J. Hygiene (1938); 27: 493. [Google Scholar]
- [30].Tete SM, Krammer F, Lartey S, Bredholt G, Wood J, Skrede S, et al. Dissecting the hemagglutinin head and stalk-specific IgG antibody response in healthcare workers following pandemic H1N1 vaccination. NPJ Vaccines 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gauger PC, Vincent AL. Serum virus neutralization assay for detection and quantitation of serum-neutralizing antibodies to influenza A virus in swine. Methods Mol Biol 2014;1161:313–24. [DOI] [PubMed] [Google Scholar]
- [32].Gauger PC, Loving CL, Vincent AL. Enzyme-linked immunosorbent assay for detection of serum or mucosal isotype-specific IgG and IgA whole-virus antibody to influenza A virus in swine. Methods Mol Biol 2014;1161:303–12. [DOI] [PubMed] [Google Scholar]
- [33].Rajendran M, Nachbagauer R, Ermler ME, Bunduc P, Amanat F, Izikson R, et al. Analysis of Anti-Influenza Virus Neuraminidase Antibodies in Children, Adults, and the Elderly by ELISA and Enzyme Inhibition: Evidence for Original Antigenic Sin. MBio 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gao J, Couzens L, Eichelberger MC. Measuring Influenza Neuraminidase Inhibition Antibody Titers by Enzyme-linked Lectin Assay. J Vis Exp 2016. [DOI] [PMC free article] [PubMed]
- [35].Trieu MC, Zhou F, Lartey S, Jul-Larsen A, Mjaaland S, Sridhar S, et al. Long-term Maintenance of the Influenza-Specific Cross-Reactive Memory CD4+ T-Cell Responses Following Repeated Annual Influenza Vaccination. J Infect Dis 2017;215:740–9. [DOI] [PubMed] [Google Scholar]
- [36].Savic M, Dembinski JL, Kim Y, Tunheim G, Cox RJ, Oftung F, et al. Epitope specific T-cell responses against influenza A in a healthy population. Immunology 2016;147:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Veguilla V, Hancock K, Schiffer J, Gargiullo P, Lu X, Aranio D, et al. Sensitivity and specificity of serologic assays for detection of human infection with 2009 pandemic H1N1 virus in U.S. populations. J Clin Microbiol 2011;49:2210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, Niño D, Belmont JW. Antibody Correlates and Predictors of Immunity to Naturally Occurring Influenza in Humans and the Importance of Antibody to the Neuraminidase. J Infect Dis 2013. March 15; 207(6): 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, et al. Antibody to Influenza Virus Neuraminidase: An Independent Correlate of Protection. J Infect Dis 2015;212:1191–9. [DOI] [PubMed] [Google Scholar]
- [40].Forrest BD, Pride MW, Dunning AJ, Capeding MR, Chotpitayasunondh T, Tam JS, et al. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol 2008;15:1042–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hayward AC, Wang L, Goonetilleke N, Fragaszy EB, Bermingham A, Copas A, et al. Natural T Cell-mediated Protection against Seasonal and Pandemic Influenza. Results of the Flu Watch Cohort Study. Am J Respir Crit Care Med 2015;191:1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Grohskopf Lisa A., Sokolow Leslie Z., Fry Alicia M., Walter Emmanuel B., Jernigan Daniel B.. Update: ACIP Recommendations for the Use of Quadrivalent Live Attenuated Influenza Vaccine (LAIV4) — United States, 2018–19 Influenza Season Weekly / June 8, 2018. / 67(22);643–645. https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a5.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pebody R, McMenamin J, Nohynek H. Live attenuated influenza vaccine (LAIV): recent effectiveness results from the USA and implications for LAIV programmes elsewhere. Arch Dis Child 2018;103:101–5. [DOI] [PubMed] [Google Scholar]
- [44].Hoschler K, Southern J, Thompson C, Warburton F, Andrews NJ, Miller E, et al. Responses to live attenuated influenza vaccine in children vaccinated previously with Pandemrix (ASO3B adjuvanted pandemic A/H1N1pdm09). Vaccine 2018;36:3034–40. [DOI] [PubMed] [Google Scholar]
- [45].Mohn KG, Smith I, Sjursen H, Cox RJ. Immune responses after live attenuated influenza vaccination. Hum Vaccin Immunother 2018;14:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009;361:1945–52. [DOI] [PubMed] [Google Scholar]
- [47].Jacobs JH, Archer BN, Baker MG, Cowling BJ, Heffernan RT, Mercer G, et al. Searching for sharp drops in the incidence of pandemic A/H1N1 influenza by single year of age. PLoS One 2012;7:e42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 2010;375:1100–8. [DOI] [PubMed] [Google Scholar]
- [49].Hsu JP, Zhao X, Chen MI, Cook AR, Lee V, Lim WY, et al. Rate of decline of antibody titers to pandemic influenza A (H1N1–2009) by hemagglutination inhibition and virus microneutralization assays in a cohort of seroconverting adults in Singapore. BMC Infect Dis 2014;14:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mohn Kristin G.-I., Jane Cox Rebecca, Tunheim Gro, Berdal Jan Erik, Hauge Anna Germundsson, Jul-Larsen Åsne, Norwegian Pandemic Group, Peters Bjoern, Oftung Fredrik, Jonassen Christine Monceyron, and Mjaaland Siri. Immune Responses in Acute and Convalescent Patients with Mild, Moderate and Severe Disease during the 2009 Influenza Pandemic in Norway. PLoS One 2015; 10(11): e0143281 Published online 2015 Nov 25. doi: 10.1371/journal.pone.0143281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Trieu MC, Jul-Larsen Å, Sævik M, Madsen A, Nøstbakken JK, Zhou F, Skrede S, Cox RJ. Antibody responses to influenza A/H1N1pdm09 virus after pandemic and seasonal influenza vaccination in healthcare workers: a five-year follow-up study. Clin Infect Dis 2018. June 9. doi: 10.1093/cid/ciy487. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kucharski AJ, Lessler J, Read JM, Zhu H, Jiang CQ, Guan Y, et al. Estimating the life course of influenza A(H3N2) antibody responses from cross-sectional data. PLoS biology 2015;13:e1002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fonville JM, Wilks SH, James SL, Fox A, Ventresca M, Aban M, et al. Antibody landscapes after influenza virus infection or vaccination. Science (New York, NY) 2014;346:996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science (New York, NY) 2016;354:722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Viboud C, Epstein SL. First flu is forever. Science (New York, NY) 2016;354:706–7. [DOI] [PubMed] [Google Scholar]
- [56].Leon PE, He W, Mullarkey CE, Bailey MJ, Miller MS, Krammer F, Palese P, Tan GS. Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact. Proc Natl Acad Sci U S A 2016. October 4;113(40):E5944–E5951. Epub 2016 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].He W, Tan GS, Mullarkey CE, Lee AJ, Lam MM, Krammer F, Henry C, Wilson PC, Ashkar AA, Palese P, Miller MS. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc Natl Acad Sci U S A 2016. October 18;113(42):11931–11936. Epub 2016 Oct 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Johansson BE, Moran TM, Bona CA, Popple SW, Kilbourne ED. Immunologic response to influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. II. Sequential infection of mice simulates human experience. J Immunol 1987;139:2010–4. [PubMed] [Google Scholar]
- [59].Wohlbold TJ, Krammer F. In the Shadow of Hemagglutinin: A Growing Interest in Influenza Viral Neuraminidase and Its Role as a Vaccine Antigen. Viruses-Basel 2014;6:2465–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hoschler Katja Andrews Nick J. Faust Saul N. Finn Adam Pollard Andrew J. Snape Matthew D. Walker Woolf T. Zambon Maria Miller Elizabeth.Administration of AS03B-adjuvanted A(H1N1)pdm09 Vaccine in Children Aged <3 Years Enhances Antibody Response to H3 and B Viruses Following a Single Dose of Trivalent Vaccine One Year Later. Clinical Infectious Diseases, Volume 58, Issue 2, 15 January 2014, Pages 181–187, 10.1093/cid/cit692 [DOI] [PubMed] [Google Scholar]
- [61].Anderson CS, Ortega S, Chaves FA, Clark AM, Yang H, Topham DJ, et al. Publisher Correction: Natural and directed antigenic drift of the H1 influenza virus hemagglutinin stalk domain. Sci Rep 2018;8:4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, et al. 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J Infect Dis 2013;207:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Thomson CA, Wang Y, Jackson LM, Olson M, Wang W, Liavonchanka A, et al. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front Immunol 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].To KK, Zhang AJ, Hung IF, Xu T, Ip WC, Wong RT, et al. High titer and avidity of nonneutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin Vaccine Immunol 2012;19:1012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jegaskanda S, Luke C, Hickman HD, Sangster MY, Wieland-Alter WF, McBride JM, et al. Generation and Protective Ability of Influenza Virus-Specific Antibody-Dependent Cellular Cytotoxicity in Humans Elicited by Vaccination, Natural Infection, and Experimental Challenge. J Infect Dis 2016;214:945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med 1983;309:13–7. [DOI] [PubMed] [Google Scholar]
- [67].Epstein SL, Price GE. Cross-protective immunity to influenza A viruses. Expert Rev Vaccines 2010;9:1325–41. [DOI] [PubMed] [Google Scholar]
- [68].Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 2013;19:1305–12. [DOI] [PubMed] [Google Scholar]
- [69].Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012;18:274–80. [DOI] [PubMed] [Google Scholar]
- [70].Mohn KGI, Zhou F, Brokstad KA, Sridhar S, Cox RJ. Boosting of Cross-Reactive and Protection-Associated T Cells in Children After Live Attenuated Influenza Vaccination. J Infect Dis 2017. May 15;215(10):1527–1535. doi: 10.1093/infdis/jix165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mohn KG, Brokstad KA, Pathirana RD, Bredholt G, Jul-Larsen Å, Trieu MC, Lartey SL, Montomoli E, Tøndel C, Aarstad HJ, Cox RJ. Live Attenuated Influenza Vaccine in Children Induces B-Cell Responses in Tonsils. J Infect Dis 2016. September 1;214(5):722–31. doi: 10.1093/infdis/jiw230. Epub 2016 May 30. Erratum in: J Infect Dis. 2016 Oct 3;:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cheng X, Zengel JR, Suguitan AL Jr., Xu Q, Wang W, J Lin, et al. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J Infect Dis 2013;208:594–602. [DOI] [PubMed] [Google Scholar]
- [73].Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir Viruses 2011;5:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pebody RG, Green HK, Andrews N, Boddington NL, Zhao H, Yonova I, et al. Uptake and impact of vaccinating school age children against influenza during a season with circulation of drifted influenza A and B strains, England, 2014/15. Euro Surveill 2015;20. [DOI] [PubMed] [Google Scholar]
- [75].Pebody RG, Sinnathamby MA, Warburton F, Andrews N, Boddington NL, Zhao H, et al. Uptake and impact of vaccinating primary school-age children against influenza: experiences of a live attenuated influenza vaccine programme, England, 2015/16. Euro Surveill 2018;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.