Abstract
Objective:
Elevated body mass index (BMI), tobacco use, and sleep disturbance are common health concerns among women with gynecologic cancers. The extent to which these factors are associated with systemic inflammation in gynecologic cancers is unknown. This is a significant literature gap given that (1) chronic, systemic inflammation may mediate relationships between behavioral health factors and cancer outcomes, and (2) elevated BMI, tobacco use, and sleep disturbances can be modified via behavioral interventions. This study examined IL-6 relations with BMI, tobacco use history, and sleep disturbances in patients undergoing surgery for suspected gynecologic cancer.
Methods:
Participants were 100 women (M age =58.42 years, SD =10.62 years) undergoing surgery for suspected gynecologic cancer. Smoking history was determined by participant self-report. Sleep quality/disturbance was assessed via the Pittsburgh Sleep Quality Index. BMI was abstracted from electronic health records. Presurgical serum IL-6 concentrations were determined using Enzyme-Linked Immunosorbent Assay.
Results:
Controlling for the cancer type and stage, regression analyses revealed higher BMI, β = 0.258, p = .007, and former/current smoking status, β = 0.181, p = .046, were associated with higher IL-6. IL-6 did not differ between former and current smokers, β = 0.008, p = .927. Global sleep quality, sleep latency, and sleep efficiency were not associated with IL-6.
Conclusions:
Higher BMI and any history of tobacco use predicted higher IL-6 among women undergoing surgery for suspected gynecologic cancers. Cognitive-behavioral interventions targeting primary and secondary obesity and tobacco use prevention may reduce systemic inflammation and optimize cancer outcomes in this population.
Keywords: Psychoneuroimmunology, nterleukin-6, tobacco use, obesity, sleep, gynecologic oncology
Introduction
Epidemiology of Gynecologic Cancers
Gynecologic cancers account for significant morbidity and mortality among women in the United States. Incidence and mortality rates vary by cancer site. Endometrial cancer is one of the most common cancers among women, with an incidence rate of 26.2 per 100,000. However, mortality rates are relatively low, with only 4.6 deaths per 100,000. In comparison, ovarian cancer affects fewer women than endometrial cancer (i.e., incidence rate of 11.3 per 100,000); however, it has a higher mortality rate than endometrial cancer, resulting in 7.2 deaths per 100,000 (U.S. Cancer Statistics Working Group, 2017). Indeed, ovarian cancer tends to be diagnosed at later stages, demonstrate resistance to treatment, have high recurrence rates, and have relatively low 5-year survival rates (American Cancer Society, 2017; Jemal et al., 2008; National Cancer Institute, 2017). Overall, these statistics suggest that gynecologic cancers represent a significant public health problem in the United States. As such, it is important to identify biobehavioral factors that contribute to the pathogenesis of these cancers.
Inflammation and Cancer Pathogenesis
Chronic inflammatory responses at systemic and tumor microenvironmental levels promote cancer initiation, progression, recurrence, metastasis, and treatment resistance, including in gynecologic cancers (Taniguchi & Karin, 2014). IL-6 is one of the most potent promoters of inflammation and tumorigenesis in humans. IL-6 is a cytokine produced by immune cells, fibroblasts, and epithelial/malignant cells. It activates signaling pathways that stimulate the proliferation, survival, differentiation, and chemo-resistance of cancer cells. In addition, IL-6 is capable of inducing an epithelial-mesenchymal transition (EMT), which can promote tumor invasion, migration, and metastasis. IL-6 also promotes the supply of blood to tumor cells, a process known as angiogenesis. Consistent with these known biological mechanisms, IL-6 is high in many cancers, including gastrointestinal, breast, genitourinary, and gynecologic cancers (Taniguchi & Karin, 2014).
In ovarian cancer, IL-6 is a known inflammatory mediator of tumor initiation, progression, angiogenesis, metastasis, and chemo-resistance (Savant, Sriramkumar, & O’Hagan, 2018). Ovarian epithelial cells, ovarian cancer cells, macrophages, mesothelial cells, and ascites (malignant fluid surrounding the tumor) secrete IL-6, which then promotes a pro-inflammatory tumor microenvironment, tumor angiogenesis, and tumor metastasis (Savant, Sriramkumar, & O’Hagan, 2018). In ovarian cancer, higher levels of IL-6 are linked to faster disease progression (Kotowicz, Fuksiewicz, Jonska-Gmyrek, Bidzinski, & Kowalska, 2016; Lane, Matte, Rancourt, & Piché, 2011; Zakrzewska & Poznanski, 2001), faster tumor growth, and shorter survival (Guo, Xu, Lu, Duan, & Zhang, 2012; Scambia et al., 1995). Elevated IL-6 is also associated with non-home discharge after primary debulking of advanced stage ovarian cancer, surgical complications, and longer hospital stay. Similar associations exist in endometrial and cervical cancers. Serum IL-6 is associated with cervical cancer progression (Chopra, Dinh, & Hannigan, 1998) and presence of poor prognosis endometrial cancer subtypes (Bellone et al., 2005). In summary, the link between elevated IL-6 and poorer cancer outcomes is robust enough that researchers are beginning to explore whether blocking IL-6 signaling pathways is an effective cancer treatment (Taniguchi & Karin, 2014).
Behavioral Health Concerns, Inflammation, and Gynecologic Cancers
Furthering knowledge about modifiable predictors of inflammation may be a crucial component of comprehensive cancer treatment approaches. This research may have the greatest utility by targeting behavioral health factors that are (a) common in cancer, (b) associated with clinically-significant cancer outcomes, and (c) potentially modifiable via behavioral intervention strategies. Three of the most common behavioral health concerns among those with cancer are obesity (Calle, Rodriguez, Walker-Thurmond, & K., 2003), tobacco use (Kelemen, Warren, Koziak, Kobel, & Steed, 2016; Kim et al., 2017), and sleep disturbance/poor sleep quality (Sandadi et al., 2011; Savard, Ivers, Villa, Caplette-Gingras, & Morin, 2011), all of which meet the criteria above for critical targets for future research.
The Obesity Medicine Association defines obesity as “a chronic, relapsing, multifactorial, neurobehavioral disease” that is marked by an increase in body fat and adipose tissue dysfunction and results in negative metabolic and psychosocial health outcomes (Obesity Medicine Association, 2017). Obesity is commonly operationalized as body mass index (BMI), which represents an individual’s weight in kilograms divided by height in square meters. Overweight is defined as a BMI between 25.0 and 29.9 kg/m2, while obesity is defined as a BMI over 30.0 kg/m2. Obesity is a well-established cause of chronic, systemic inflammation. Adipose tissue releases IL-6 into the body (Fain, Madan, Hiler, Cheema, & Bahouth, 2004; Orban, Remaley, Sampson, Trajanoski, & Chrousos, 1999), which may be one mechanism through which obesity is associated with elevated risk of endometrial cancer (Calle & Kaaks, 2004; Reeves et al., 2007) and higher risk of death from endometrial cancer (Calle, Rodriguez, Walker-Thurmond, & Thun, 2003). Notably, the relationship between elevated BMI and higher IL-6 may be moderated by other maladaptive behavioral health factors, such as greater sleep disturbances (Arnardottir et al., 2012; Prather et al., 2009; Vgontzas et al., 1997).
Another behavioral health factor that has been consistently related to greater systemic inflammation is smoking (Aldaham, Foote, Chow, & Hakim, 2015; Bermudez, Rifai, Buring, Manson, & Ridker, 2002; Frost-Pineda et al., 2011; Liu et al., 2011; Panoulas et al., 2009; Wannamethee et al., 2005). Cigarette smoke contains immunomodulatory toxins, such as nicotine, carbon monoxide, acrolein, and reactive oxidant substances (Lee, Taneja, & Vassallo, 2012). These toxins cause a cascade of effects that result in inflammatory gene activation and chronic inflammation. Cigarette smoke also suppresses innate immunity, as well as T-helper (Th) 1 adaptive immunity, and impairs immune responses to infections (Arnson, Shoenfeld, & Amital, 2010; Cui & Li, 2010; Lee et al., 2012; Sopori, 2002). Notably, tobacco use is associated with higher levels of IL-6 even decades after quitting usage (Yanbaeva, Dentener, Creutzberg, Wesseling, & Wouters, 2007).
A third behavioral health factor associated with greater systemic inflammation is sleep difficulty. Sleep difficulties occur among 30 – 50% of individuals with newly diagnosed cancer (Savard & Morin, 2001). Women with gynecologic cancers are among the most affected by clinical insomnia (29 – 44%) and sub-threshold insomnia symptoms (39%) at the point of diagnosis (Palesh et al., 2010; Savard et al., 2011). Importantly, sleep disturbance and poor sleep quality are associated with higher IL-6 levels in both healthy and cancer samples. In healthy samples, higher levels of IL-6 are seen among those with clinical sleep disorders characterized by excessive daytime sleepiness (Vgontzas et al., 1997; Zisapel, 2007), as well as among individuals without clinical disorders who still suffer from sleep loss and shorter sleep duration (Mullington, Simpson, Meier-Ewert, & Haack, 2010). Within cancer populations, improvement in overall sleep (e.g., sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances) is significantly associated with decreases in IL-6 at pre-surgery and at 1-year follow-up in women diagnosed with ovarian cancer (Clevenger et al., 2012). Similar findings in head and neck cancers have been observed. Specifically, better Medical Outcomes Study (MOS) scores (Hays, Sherbourne, & Mazel, 1995), which assess global sleep quality, are significantly associated with lower circulating IL-6 (Duffy et al., 2013).
Very few studies have explored how multiple behavioral factors are associated with IL-6 in cancer samples. This is a significant gap in the literature due to the fact that poor health behaviors are often comorbid with each other in individuals affected by cancer. For instance, Duffy and colleagues (Duffy et al., 2013) examined obesity, tobacco use, and IL-6 in head and neck cancer patients. They found that current and former smokers had higher IL-6 than never smokers. In addition, while BMI was associated with higher IL-6 in univariate analyses, this relationship was no longer significant in multivariate analyses controlling for demographic and health-related variables. This latter study, in particular, highlights the importance of modeling the effects of multiple, comorbid behavioral risk factors on IL-6 in cancer.
Purpose of the Present Study
The present study examined the extent to which BMI, smoking history, and sleep disturbance/poor sleep quality were associated with serum IL-6 in women undergoing surgery for suspected gynecologic cancers. It was hypothesized that higher BMI and greater sleep disturbance/poorer sleep quality would be associated with higher IL-6. It was also hypothesized that current and former smokers would have higher IL-6 than never smokers, while current smokers would have higher IL-6 than former smokers. In line with research suggesting an interaction between sleep disturbances and BMI on IL-6 (Arnardottir et al., 2012; Prather et al., 2009; Vgontzas et al., 1997), an exploratory aim evaluated whether greater sleep disturbances moderated the relationship between higher BMI and higher IL-6.
Methods
Participants
Participants were women with suspected gynecologic cancers enrolled in one of two biobehavioral oncology research projects at the University of Florida. The first parent study was a nonexperimental, longitudinal study (N = 134) of PNI relationships in endometrial cancer conducted from 2004 to 2009. The second parent study was a randomized clinical trial (RCT) (N = 115) examining psychological intervention effects on sleep, pain, mood, cortisol, and cytokines in gynecologic cancers; this study was conducted from 2009 to 2017 (ClinicalTrials.gov Identifier: ).
Inclusion criteria for enrollment at pre-surgery across both studies were: (a) suspected primary gynecologic cancer1 scheduled to undergo surgical intervention, (b) at least 18 years of age, and (c) fluency in English. Exclusion criteria for enrollment at pre-surgery across both studies were: (a) recurrent gynecologic cancer, (b) metastases to the reproductive organs from another primary cancer site, (c) pre-operative chemotherapy or radiotherapy, and/or (d) severe psychopathology. An additional inclusion criterion for the RCT was a positive screen for sleep disturbance/poor sleep quality at pre-surgery. An additional exclusion criterion for the RCT was a medical record documented history of seizure disorder and/or obstructive sleep apnea (OSA), conditions for which a behavioral intervention for insomnia would be unsafe or ineffective. Participants meeting pre-surgical eligibility criteria were included in the current analyses if they had (a) surgically confirmed primary gynecologic cancer, borderline ovarian tumor, or complex endometrial hyperplasia with atypia (endometrial pre-cancer), and (b) viable serum at pre-surgery for quantitation of IL-6. Forty-four women from the nonexperimental, longitudinal study, and 65 women from the RCT (total N = 109) met these criteria. One woman from the nonexperimental study had medical record documented OSA, while five women from the RCT were ultimately diagnosed with OSA. These participants were excluded from analyses. Of the remaining 103 participants, 3 had missing tobacco use data. Therefore, analyses were performed on 100 participants (nonexperimental study: N = 40, 40.00%; RCT: N = 60, 60.00%).
Procedures
All participants from both parent studies were recruited from the Gynecologic Oncology Clinic at the University of Florida. Patients who were potentially eligible based on medical record review were identified during their preoperative consultation visit with their oncology surgeon and nursing staff. Interested potential participants met in a private room with a study team member who provided an overview of the study. All study procedures were conducted according to the rules and regulations set forth by the University of Florida Institutional Review Board (IRB).
Following consent, participants underwent a brief psychiatric screening to rule out current psychotic symptoms and current/recent suicidal ideation, intent, or plan. If the assessment was negative, a preoperative psychosocial assessment was scheduled and questionnaires were provided. After completing study interviews and written questionnaires, participants in both studies completed a preoperative blood draw by a certified phlebotomist.
Measures
Psychological Screening Measures.
To be enrolled in either of the two parent studies, patients were required to complete two types of screeners. First, patients completed a brief assessment for severe psychopathology as evaluated by the Structured Clinical Interview for DSM Disorders (SCID) (First, 1997) and the Beck Suicide Scale (BSS) (A. T. Beck, Steer, & Ranieri, 1988). The SCID is a multimodal, interview-based survey that was used to evaluate potential participants in both parent studies for the presence of current or previous mania, hypomania, and/or psychotic symptoms. Patients with these disorders were excluded from participation. The BSS is a 21-item questionnaire designed to measure respondents’ current and/or history of suicidal ideation, planning, and/or suicide attempts. Patients with any current suicidal ideation were more fully assessed by the study team to evaluate immediate patient safety and provide referrals if needed. These patients were not eligible to participate in the parent studies. Patients with no current suicidal ideation but a history of ideation or attempt were also further evaluated. Those with a remote history of suicidality who, based on the clinical judgment of the Study PI, a licensed psychologist, were determined to be low risk for future suicide attempt, were retained in the parent studies.
Sleep Quality/Disturbance Assessment.
The PSQI was used to assess sleep quality/disturbance. The PSQI provides a global score, as well as seven subscale scores (e.g., subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction). Higher scores indicate greater self-reported sleep dysfunction.
The PSQI has been used among diverse clinical groups, including cancer populations (S. L. Beck, Schwartz, Towsley, Dudley, & Barsevick, 2004; Carpenter & Andrykowski, 1998). The PSQI had good internal consistency in two samples of heterogeneous cancer patients (Cronbach’s alpha=0.770–0.808) (S. L. Beck et al., 2004), which is consistent with results obtained in the original psychometric validation study (Cronbach’s alpha=0.83) (Buysse et al., 1989). Similar results were found in bone marrow transplant and breast cancer patients (Cronbach’s alpha=0.80) (Carpenter & Andrykowski, 1998), which offers further evidence that the PSQI is a valid and reliable measure of sleep disturbance/sleep quality in cancer populations.
As noted previously, RCT participants were required to screen positive for sleep disturbances/poor sleep quality using a Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989) global score > 5 and/or a brief insomnia screener based on the American Academy of Sleep Medicine’s clinical research diagnostic criteria for insomnia (Edinger, 2004). Participants in the nonexperimental study completed the PSQI at pre-surgery, as well. However, a PSQI Global Index Score > 5 was not required for enrollment.
The PSQI global score and the PSQI sleep latency and sleep efficiency subscales were selected as predictors for the current analyses for several reasons. First, the PSQI sleep latency subscale was selected because prolonged sleep latency is a common feature of insomnia and maps closely onto the required criterion “A” for insomnia in the Diagnostic and Statistical Manual of Mental Disorders (DSM 5) (Diagnostic and statistical manual of mental disorders: DSM-5, 2013). In addition, sleep onset latency (SOL) and wake after sleep onset (WASO) comprise the construct of total wake time (TWT), which is a major target of behavioral sleep treatments. Second, the PSQI sleep efficiency subscale was selected, as sleep efficiency percentage (total sleep time/time in bed × 100) is a major target of behavioral sleep treatments.
Smoking History Assessment.
Participants were classified as never smokers (“0”), former smokers (“1”), or current smokers (“2”). Former smokers with very low exposure to cigarette smoking equivalent to a pack year history equal to 0 (e.g., 1 cigarette per year for 8 years) were considered to have negative lifetime histories of smoking.
Sociodemographic Assessment.
Information regarding participants’ race, ethnicity, education, marital status, and other demographic factors was captured via the MacArthur Sociodemographic Questionnaire (MSQ; (Adler, Epel, Castellazzo, & Ickovics, 2000)). The MSQ is a self-report survey developed by the MacArthur Network on SES and Health to assess subjective social status, educational attainment, occupational status, income, assets, and racial/ethnic background. Participants completed the MSQ during the preoperative baseline assessment.
BMI Assessment.
BMIs (in kg/m2) were abstracted from post-surgical discharge summaries.
Control Variable Assessment.
Presence of ovarian cancer or fallopian tube cancer (poor prognosis cancer types) and presence of Stage III or IV (advanced) cancer were selected as a priori control variables based upon the known relationship between these cancer types and greater inflammation/higher IL-6 (Anglesio et al., 2011; Macciò & Madeddu, 2012; Scambia et al., 1995; Tempfer et al., 1997). These variables were abstracted from post-surgical discharge summaries.
IL-6 Assessment.
IL-6 was obtained via a pre-operative blood draw completed by a hospital phlebotomist. Samples were centrifuged at 1000 × g for 15 minutes, aliquoted, and stored at − 80°C until assaying. Standardized ELISA assays were used for quantification of IL-6 concentrations (R&D Systems, Minneapolis, MN) at the University of Florida’s Institute for Wound Research laboratory under the direction and supervision of the Institute’s Director (G. Schultz). Values were expressed in pg/mL.
Statistical Analyses
Analyses were conducted using IBM SPSS Version 25. Descriptive statistics were performed to assess normality assumptions for parametric analyses. IL-6 was non-normally distributed and Blom-transformed to normalize the data. The smoking history variable was then recoded as two orthogonal vectors using Helmert coding under the condition of unequal smoking history subgroup sizes. Vector 1 tested whether IL-6 was higher among former and current smokers compared to never smokers (never versus former/current smoking status). Vector 2 tested whether IL-6 was higher among current smokers compared to former smokers (former versus current smoking status). Bivariate correlations were conducted to explore univariate relationships among the variables of interest. Then, three multiple linear regression analyses were performed with IL-6 as the criterion. In each equation, Block 1 included the a priori control variables. Block 2 included the predictors of interest: BMI, both smoking variables, and then one of the following: (Equation 1) PSQI global score, (Equation 2) PSQI sleep latency subscale score, or (Equation 3) PSQI sleep efficiency subscale score. For the exploratory aim, the product of PSQI global scores and BMI values was added to Equation 1, Block 2.
Results
Participants were primarily middle-aged (M age = 58.42 years, SD = 10.62 years), married (N = 54, 54.0%), non-Hispanic (N = 67, 67.0%), and Caucasian (N = 82, 82.0%) women with some college education (M education = 13.63 years, SD = 2.94 years). Most participants had endometrial cancer (N = 72, 72.0%) and early-stage (Stage I-II) disease (N = 71, 71.0%). Following receipt of tumor pathology reports, nine participants (9.0%) had confirmed complex endometrial hyperplasia with atypia (a benign but precancerous condition of the endometrium); these participants were retained in the analyses. Non-transformed IL-6 levels ranged from 0 pg/mL to 22.00 pg/mL (M = 3.10 pg/mL, SD = 3.68 pg/mL). Further information on demographic and disease characteristics for our sample is reported in Table 1.
Table 1.
Sample Demographics and Health Characteristics
| Variable | M | SD | N | % |
|---|---|---|---|---|
| Age (Years) | 58.42 | 10.62 | ||
| Education (Years) | 13.63 | 2.94 | ||
| Race | ||||
| Caucasian | 82 | 82 | ||
| African-American/Black | 12 | 12 | ||
| Asian | 2 | 2 | ||
| American Indian/Native Alaskan | 2 | 2 | ||
| Missing | 2 | 2 | ||
| Ethnicity | ||||
| Non-Hispanic | 67 | 67 | ||
| Hispanic | 10 | 10 | ||
| Missing | 23 | 23 | ||
| Marital Status | ||||
| Married | 54 | 54 | ||
| Never Married | 9 | 9 | ||
| Separated | 3 | 3 | ||
| Divorced | 17 | 17 | ||
| Widowed | 11 | 11 | ||
| Missing | 6 | 6 | ||
| Cancer Type | ||||
| Ovarian/Fallopian Tube, Invasive | 10 | 10 | ||
| Endometrial, Invasive | 72 | 72 | ||
| Squamous Cell Carcinoma of the | 5 | 5 | ||
| Vulva, Cervix, or Vagina, Invasive | ||||
| Complex Endometrial Hyperplasia | 9 | 9 | ||
| Borderline Ovarian Tumor | 4 | 4 | ||
| Tumor Stagea | ||||
| Precancerb | 9 | 9 | ||
| Stage I | 55 | 55 | ||
| Stage II | 16 | 16 | ||
| Stage III | 18 | 18 | ||
| Stage IV | 2 | 2 | ||
| BMI (kg/m2) | 35.33 | 10.37 | ||
| Underweight | 1 | 1 | ||
| Normal Weight | 15 | 15 | ||
| Overweight | 21 | 21 | ||
| Obese | 63 | 63 | ||
| Smoking History | ||||
| Never Smoker | 53 | 53 | ||
| Former Smoker | 34 | 34 | ||
| Current Smoker | 13 | 13 | ||
| PSQI Global Score | 9.03 | 4.22 | ||
| PSQI Global score > 5 (Buysse et al., 1989) | 86 | 86 | ||
| PSQI Global score > 8 (Carpenter & Andrykowski, 1998) | 46 | 46 | ||
| PSQI Sleep Latency Subscale Score | 1.34 | 1.15 | ||
| PSQI Sleep Efficiency Subscale Score | 1.39 | 1.19 | ||
| IL-6 (pg/mL)c | 3.10 | 3.68 |
Tumor staging applies to invasive tumors and borderline ovarian tumors.
Complex endometrial hyperplasia.
Mean and standard deviation of Blom-transformed scores = 0.00 and 1.00, respectively.
Forty-seven (47.0%) of participants endorsed a history of smoking, 13 of whom (13.0% of entire sample) were current smokers. BMI values ranged from 17.85 kg/m2 (Underweight) to 72.62 kg/m2 (Class III Obesity) (M = 35.33 kg/m2, SD = 10.37 kg/m2). Sixty-three (63.0%) of participants had BMI values in the obese range (≥ 30 kg/m2) (Table 1). Mean PSQI global score was 9.03 (SD = 4.22). Consistent with differing eligibility criteria for the two parent studies, participants in the RCT had significantly higher PSQI global index scores (M = 10.23, SD = 3.91) than those in the nonexperimental study (M = 7.22, SD = 4.07), F(1,98) = 13.753, p <.001, . However, PSQI global index scores did not differ significantly by study origin among participants screening positive for sleep disturbance/poor sleep quality using either a cutoff of 5 (Buysse et al., 1989), F(1,86) = .934, p = .337, , or a modified cutoff of 8 suggested for cancer patients (Carpenter & Andrykowski, 1998), F(1,46) = 3.234, p = .079, . In the total sample, mean PSQI sleep latency and sleep efficiency subscale scores were 1.34 (SD = 1.15) and 1.39 (SD = 1.19), respectively.
Inter-correlations among a priori control variables, predictors, and IL-6 revealed that, as hypothesized, higher IL-6 was significantly associated with presence of ovarian/fallopian tube cancer, r(100)= 0.380, p < .001, and advanced cancer (Stage III-IV), r(100) = 0.340, p = .001 (Table 2). There were nonsignificant, small-to-medium effect size relationships between IL-6 and the following predictors: BMI, r(100) = 0.154, p = .127; never smoker versus former/current smoker status, r(100) = 0.136, p = .177; PSQI global index scores, r(100) = 0.155, p = .125; and PSQI sleep latency subscale scores, r(100) = 0.113, p = .267. IL-6 was unrelated to former versus current smoker status, r(100) = −0.022, p = .830 and PSQI sleep efficiency subscale scores, r(100) = .081, p = .423 (Table 2).
Table 2.
Summary of Inter-correlation Matrix among Control Variables, Predictors, and Presurgical IL-6 in Women Undergoing Surgery for Suspected Gynecologic Cancer
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1.Ovarian/ Fallopian Tube Cancer | -- | 0.500*** | −0.139 | −0.114 | −0.090 | 0.117 | 0.104 | 0.116 | 0.380** |
| 2.Advanced Cancer | 0.500*** | -- | −0.282** | −0.120 | 0.005 | 0.080 | 0.091 | 0.089 | 0.340** |
| 3.BMI | −0.139 | −0.282** | -- | 0.068 | −0.059 | 0.085 | −0.001 | −0.085 | 0.154 |
| 4.Never Smoker Vs. Former and Current Smokers | −0.114 | −0.120 | 0.068 | -- | 0.000 | 0.084 | 0.004 | −0.141 | 0.136 |
| 5. Former Vs. Current Smokers | −0.090 | 0.005 | −0.059 | 0.000 | -- | 0.161 | 0.157 | 0.034 | −0.022 |
| 6.PSQI Global Score | 0.117 | 0.080 | 0.085 | 0.084 | 0.161 | -- | 0.706** | 0.632** | 0.155 |
| 7. PSQI Sleep Latency Subscale Score | 0.104 | 0.091 | −0.001 | 0.004 | 0.157 | 0.706** | -- | 0.399** | 0.113 |
| 8. PSQI Sleep Efficiency Subscale Score | 0.116 | 0.089 | −0.085 | −0.141 | 0.034 | 0.632** | 0.399** | -- | 0.081 |
| 9. IL-6 (Blom– Transformed ) | 0.380** | 0.340** | 0.154 | 0.136 | −0.022 | 0.155 | 0.113 | 0.081 | -- |
p ≤ .050,
p ≤ .010,
p ≤ .001.
Hierarchical linear regression analyses were used to predict IL-6 with a priori control variables in step 1 and predictors of interest in step 2. Three equations were tested. Equation 1 included PSQI global index scores as the sleep quality/disturbance variable of interest, while Equations 2 and 3 included PSQI sleep latency and PSQI sleep efficiency subscale scores, respectively.
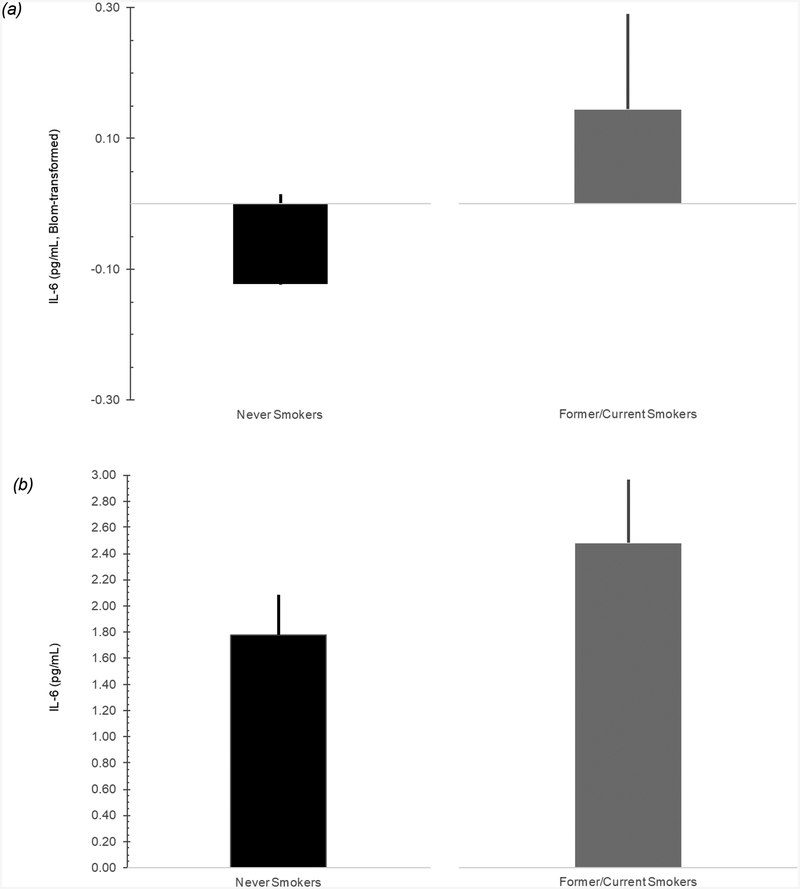
In the first equation (Table 3), step 1 revealed that presence of ovarian/fallopian tube cancer was significantly associated with higher IL-6, β = 0.280, p = .010, while presence of advanced cancer was marginally associated with higher IL-6, β = 0.201, p = .063. These variables accounted for 17.5% of the variance in IL-6, F of ΔR2 = 10.265, p < .001. In step 2, higher BMI, β = 0.258, p = .007, and never smoker versus former/current smoker status, β = 0.181, p = .046, were associated with higher IL-6. As depicted in Figure 1, IL-6 estimated marginal means were higher among former/current smokers than never smokers. However, neither former versus current smoker status, β = 0.008, p = .927, nor PSQI global scores, β = 0.060, p = .513, were associated with IL-6. Step 2 accounted for 10.5% of the variance in IL-6 above and beyond a priori control variables, F of ΔR2 = 3.396, p = .012.
Table 3.
BMI, Smoking History, and Sleep Quality/Disturbances Predicting IL-6 in Women Undergoing Surgery for Suspected Gynecologic Cancer
| Predictor | IL-6 | ||||||
|---|---|---|---|---|---|---|---|
| B | SE B | β |
95% CI for B |
P | ΔR2 | ||
| Step 1 | .175*** | ||||||
| Ovarian/Fallopian Tube Cancer | 0.912 | .347 | 0.280 | [0.223–1.601] | .010 | ||
| Advanced Cancer | 0.490 | .260 | 0.201 | [−0.027–1.007] | .063 | ||
| Step 2 | .105* | ||||||
| BMI | 0.024 | .009 | 0.258 | [0.007–0.042] | .007 | ||
| Never Vs. Former/Current Smokers | 0.004 | .002 | 0.181 | [0.000 – 0.007] | .046 | ||
| Former Vs. Current Smokers | 0.001 | .006 | 0.008 | [−0.012 – 0.013] | .927 | ||
| PSQI Global Score | 0.014 | .021 | 0.060 | [−0.028–0.056] | .513 | ||
| R2 | .280** | ||||||
| F | 6.023** | ||||||
| N | 100 | ||||||
p <.050,
p < .010,
p < .001.
Figure 1. Relationship between IL-6 and Smoking Status in Women Undergoing Surgery for Suspected Gynecologic Cancer.
Note. Figure 1a depicts Blom-transformed IL-6 estimated marginal means and 95% confidence intervals for Never Smokers and Former/Current Smokers. To facilitate interpretation of the results presented in Figure 1a, Blom-transformed IL-6 values were back-transformed to approximate, raw IL-6 values and plotted for Never Smokers and Former/Current Smokers. These results are depicted in Figure 1b for illustrative purposes.
Similar relationships emerged in Equations 2 and 3, which substituted PSQI sleep latency and sleep efficiency subscale scores for PSQI global scores. Neither PSQI sleep latency subscale scores in Equation 2, β = 0.053, p = .561, nor PSQI sleep efficiency subscale scores in Equation 3, β = 0.072, p = .423, predicted IL-6 (not shown).
For the exploratory aim, the product of PSQI global scores and BMI values was added to Equation 1, Block 2. However, the interaction term did not significantly predict IL-6, β = 0.012, p = .901 (not shown).
Discussion
The current study examined whether BMI, smoking history, and sleep disturbance/poor sleep quality were associated with IL-6 above and beyond the presence of poor prognosis cancer types (e.g., ovarian/fallopian tube cancers) and advanced cancer (i.e., Stages III-IV) in women undergoing surgery for suspected gynecologic cancer. Results revealed that, as hypothesized, IL-6 was significantly associated with presence of ovarian/fallopian tube cancer and marginally associated with the presence of Stage III-IV cancer. Together, these variables predicted 17.5% of the variance in IL-6. These findings are consistent with previous research implicating advanced stage (Esfandi, Mohammadzadeh Ghobadloo, & Basati, 2006; Guo et al., 2012; Kaminska et al., 2005) and cancer type (Anglesio et al., 2011; Macciò & Madeddu, 2012; Scambia et al., 1995; Tempfer et al., 1997) as important factors in elevated IL-6 levels.
Consistent with hypotheses, greater BMI and former/current smoking status were significantly associated with higher IL-6. Obesity and tobacco use represent behavioral health factors that: (a) tend to co-occur (Grandner et al., 2016; Hussaini, Nicholson, Shera, Stettler, & Kinsman, 2011; Kauffman, Farris, Alfano, & Zvolensky, 2017), (b) increase risk for cancer and cardiovascular disease (Koene, Prizment, Blaes, & Konety, 2016), and (c) are modifiable via evidence-based behavioral health interventions (Kushner & Ryan, 2014; Morin, 2006; Sallit, Ciccazzo, & Dixon, 2009). Recently, overweight and obesity have been associated with higher risk of at least 13 cancer types, with these cancers accounting for 40% of all cancers diagnosed in 2014. Endometrial and ovarian cancers are two of these 13 types. Every 1 kg/m2 increase in BMI is associated with an 8% increase in risk for endometrial cancer and a 1% increase in risk for ovarian cancer (Steele et al., 2017). In our sample of 100 women undergoing surgery for suspected gynecologic cancer, 63% of participants had BMI values in the obese range. The high prevalence of obese women in our sample is consistent with the prevalence of overweight and obese women (66.9%) in the United States population (National Institute of Diabetes and Digestive and Kidney Diseases, 2017).
The American College of Obstetricians and Gynecologists (ACOG) has recognized the high prevalence and negative consequences of obesity among gynecology patients. As such, they recently created physician toolkits which offer specific guidelines and resources for managing obesity (Crowe, Gregg, & DeFrancesco, 2016). While publications and guidelines such as these may be useful if implemented, a recent study examining attitudes toward weight management among gynecologic cancer survivors found that only a small percentage of patients received weight management counseling, even though the vast majority of patients felt that it was appropriate for their oncologist to discuss behavioral health and weight loss topics with them (Zaleta, Neff, McCann, O’Malley, & Carpenter, 2017). This is significant for several reasons. First, obesity may soon be associated with more cancer deaths than tobacco use (Ligibel et al., 2014). Second, existing guidelines and empirically supported lifestyle programs exist for weight management among cancer patients (Rutledge, 2016) and the general population (Jensen et al., 2014). Third, as suggested by the current study, obesity is associated with higher IL-6, which may have deleterious downstream consequences on perioperative and other cancer outcomes (He, Wang, Bian, Deng, & Wang, 2017; Huo, Smith, Giordano, Reece, & Tina Shih, 2016).
Slightly less than half of participants (47.0%) reported a positive lifetime history of tobacco use. This is relatively consistent with other studies which have shown that 52.8% of women with ovarian cancer report a history of cigarette use (Rossing, Cushing-Haugen, Wicklund, & Weiss, 2008). The health risks of cigarette smoke have received considerable attention since being identified as a cause of lung cancer. While this line of research has developed significant inquiry and important knowledge into direct mechanisms through which smoking is associated with cancer outcomes, particularly in lung and head and neck cancers, more research is needed on the indirect pathways through which tobacco use may affect treatment outcomes and prognoses among individuals with other types of cancers.
It is well known that tobacco cessation upon cancer diagnosis confers immediate health benefits (Sitas et al., 2014), while, in contrast, continued tobacco use is associated with negative outcomes such as tumor progression, decreased response to chemotherapy, worse quality of life, and greater pain-related functional impairment (Florou, Gkiozos, Tsagouli, Souliotis, & Syrigos, 2014). However, the current study found that any lifetime history of smoking was associated with higher IL-6 in women with gynecologic cancers, suggesting that both primary and secondary prevention of tobacco use should be key public health priorities for optimizing health outcomes in gynecologic cancers. Decades of research on tobacco prevention and cessation have resulted in strong evidence for the efficacy of individual (Lancaster & Stead, 2017) and group-based (Stead, Carroll, & Lancaster, 2017) behavioral interventions. Furthermore, ACOG has developed toolkits for smoking cessation in gynecology patients (Crowe et al., 2016) which allows known interventions to be tailored to the specific needs of women with gynecologic cancers.
Contrary to hypotheses, current smokers did not have significantly greater IL-6 than former smokers. This finding may be attributed to several factors. The current study did not have complete information on pack year smoking history or length of time between quitting tobacco use and assessment of tobacco use in this study. It is possible that individuals who were categorized as past smokers had quit smoking fairly recently and therefore may have appeared more similar to the current smoker group in terms of IL-6 levels. Previous findings have shown that at the time of cancer diagnosis, approximately 20–40% of individuals are current smokers (Burke, Miller, Saad, & Abraham, 2009; Schnoll et al., 2003; Walker, Larsen, Zona, Govindan, & Fisher, 2004). Burke and colleagues found that, among the 20% of patients who were smoking at the time of diagnosis, 44% reported smoking cessation following their cancer diagnosis (Burke et al., 2009). In patients with newly diagnosed cancer, it is likely important to assess cumulative tobacco exposure among current and former smokers, as well as length of abstinence among former smokers.
In addition, contrary to hypotheses, PSQI measures of global sleep quality/disturbance, sleep latency, and sleep efficiency were unrelated to IL-6 in this sample. The PSQI assesses sleep disturbance/poor sleep quality rather than presence or severity of clinical insomnia. It is possible that it is the construct of insomnia per se and/or the degree of insomnia severity that is associated with elevated IL-6 in cancer, rather than simply poor sleep quality. In addition, the current study excluded individuals diagnosed with other sleep disorders marked by excessive daytime sleepiness, such as obstructive sleep apnea (Vgontzas et al., 1997; Zisapel, 2007). Maintaining a more homogeneous sleep-disordered sample may have contributed to discordant findings between the current study and prior published research.
While the current study modeled the effects of behavioral health factors on IL-6, it is important to note that the relationship between health behaviors and inflammation is likely to be bidirectional. IL-6 and other pro-inflammatory cytokines may induce subjective feelings of illness and so-called “sickness behaviors,” such as depressed mood, sleep disturbances, impaired appetite, and fatigue (Dantzer, 2009). In a study of ovarian cancer patients, Costanzo and colleagues found that greater IL-6 was associated with compromised physical and functional well-being and lower social support (Costanzo et al., 2005). Similarly, in another study examining the relationship between IL-6 and mood in ovarian cancer patients at pre-surgery, 6 month, and 1 year follow-up, declines in IL-6 were associated with reductions in depression and fatigue across time (Schrepf et al., 2013). Therefore, while obesity and tobacco use may have effects on IL-6, elevated IL-6 may have indirect effects on obesity and tobacco use through poorer mood and quality of life.
Strengths of the study include the fact that the sample was comprised of participants from two large, NIH-funded, longitudinal studies with well-characterized samples. Participants represented a wide range of gynecologic pathologies and treatment interventions, which allows for enhanced generalizability to the overall gynecologic oncology population.
However, study results must be interpreted in light of several limitations. Our sample was cross-sectional and somewhat homogenous in terms of sociodemographic characteristics. Patients were mostly married, well-educated, middle-aged, and Caucasian. Additionally, both parent studies were conducted at an academic medical center serving a large rural population. It is possible that regional differences in patterns of behavioral health and/or inflammation may limit our ability to generalize results more broadly. Second, there are several important limitations of using BMI as a measure of abnormal fat accumulation. BMI does not discern among fat, muscle, and bone mass or provide information on body fat distribution, such as central adiposity. Future research, particularly with post-menopausal women, should use methods such as dual-energy x-ray absorptiometry, which provides a direct and objective measure of body fat (Banack, Wactawski-Wende, Hovey, & Stokes, 2018). Third, given some inconsistencies in smoking history data (e.g., some participants reported different smoking histories at different study time points and/or did not complete all parts of the self-report smoking history questionnaire), we did not have pack-year history data and thus could not examine how cumulative tobacco exposure was associated with IL-6. Relatedly, 28.26% of non-smoking adult cancer survivors are exposed to secondhand smoke (95% CI: 24.97%–31.55%), with even higher rates occurring among individuals (a) from racial/ethnic minority groups, (b) affected by poverty, and (c) with a smoking history (Akinboro et al., 2017). This highlights the importance of understanding the relationship between passive smoking and inflammation among current, former, and never smokers. Fourth, we had a relatively small number of current smokers, and it is possible that low statistical power precluded the ability to detect significant differences between current smokers and former smokers. Fifth, the current study capitalized on the commonalities between the eligibility criteria of two NCI-funded parent studies. Using a PSQI global index score cutoff of 8 recommended by Carpenter and Andrykowski (Carpenter & Andrykowski, 1998) for cancer patients, the resultant sample contained almost equivalent numbers of individuals with and without clinically-significant sleep disturbance/poor sleep quality. While this was a strength of the study, it is possible that there are differences between the parent study samples that confounded the emergence of a true relationship between sleep and IL-6. Lastly, the current study did not assess chronotype or individual sleep-wake preferences ranging from morningness to eveningness (Horne & Ostberg, 1976; Roennenberg, 2012). Chronotype impacts sleep behavior and a growing body of research has started to explore the ways in which chronotype, along with disruptions in natural circadian rhythm, may be associated with cancer risk and outcomes (Cash et al., 2015; Innominato et al., 2012; Papantoniou et al., 2015).
In summary, future research should continue to examine the bidirectional associations between behavioral health factors and inflammation in gynecologic cancers. Behavioral health factors should include pre-surgical BMI, tobacco use and secondhand smoke exposure, and insomnia symptoms/severity. In addition, future research should explore the extent to which these behavioral health factors are associated with clinical outcomes, such as post-surgical complications, treatment resistance, cancer recurrence, and survival in women with gynecologic cancers. In particular, this research should explore the extent to which cognitive-behavioral interventions for primary and secondary obesity and tobacco use prevention may reduce systemic inflammation and optimize cancer outcomes in this population.
Acknowledgments
This work was supported by the National Cancer Institute (NCI; R03 CA117480; PI Deidre B. Pereira, PhD) and the National Institutes of Health (NIH; R01 CA138808; PI Deidre B. Pereira, PhD). The authors report no financial disclosures or conflicts of interest.
Footnotes
The non-experimental study was limited to endometrial cancers, while the RCT was open to all gynecologic cancers. The majority of gynecologic cancers in the RCT were endometrial cancers.
Contributor Information
Elizabeth L. Kacel, Department of Clinical and Health Psychology, University of Florida
Janae L. Kirsch, Department of Clinical and Health Psychology, University of Florida
Timothy S. Sannes, Department of Clinical and Health Psychology, University of Florida;
Seema Patidar, Department of Clinical and Health Psychology, University of Florida;.
Rachel Postupack, Department of Clinical and Health Psychology, University of Florida.
Sally Jensen, Department of Clinical and Health Psychology, University of Florida.
Shan Wong, Department of Clinical and Health Psychology, University of Florida.
Stephanie Garey, Department of Clinical and Health Psychology, University of Florida.
Stacy Dodd, Department of Clinical and Health Psychology, University of Florida.
Chantel M. Ulfig, Department of Clinical and Health Psychology, University of Florida
Christina S. McCrae, Department of Clinical and Health Psychology, University of Florida
Michael E. Robinson, Department of Clinical and Health Psychology, University of Florida
Jacqueline Castagno, Department of Obstetrics and Gynecology, University of Florida.
Gregory Schultz, Department of Obstetrics and Gynecology, University of Florida.
Deidre B. Pereira, Department of Clinical and Health Psychology, University of Florida
References
- Adler NE, Epel ES, Castellazzo G, & Ickovics JR (2000). Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol, 19(6), 586–592. [DOI] [PubMed] [Google Scholar]
- Akinboro O, Olorunfemi O, Basak P, Phillips E, Pomerantz D, Bernhardt B,Ostroff JS. (2017). Secondhand Smoke Exposure Among Community-Dwelling Adult Cancer Survivors in the United States: 1999–2012. Cancer Epidemiology and Prevention Biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaham S, Foote JA, Chow HH, & Hakim IA (2015). Smoking Status Effect on Inflammatory Markers in a Randomized Trial of Current and Former Heavy Smokers. Int J Inflam, 439396(10), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. (2017). Get the Facts about Gynecologic Cancer, from Centers for Disease Control and Prevention https://www.cdc.gov/cancer/knowledge/pdf/cdc_gyn_comprehensive_brochure.pdf
- Anglesio MS, George J, Kulbe H, Friedlander ML, Rischin D, Lemech C,House CM (2011). Il6-Stat3-Hif signalling and therapeutic response to the angiogenesis inhibitor, sunitinib, in ovarian clear cell cancer. Clinical cancer research, clincanres. 3314–2010.. [DOI] [PubMed] [Google Scholar]
- Arnardottir ES, Maislin G, Schwab RJ, Staley B, Benediktsdottir B, Olafsson I,Pack AI (2012). The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep, 35(7), 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnson Y, Shoenfeld Y, & Amital H (2010). Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun, 34(3), J258–265. doi: 10.1016/j.jaut.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Banack HR, Wactawski-Wende J, Hovey KM, & Stokes A (2018). Is BMI a valid measure of obesity in postmenopausal women? Menopause, 25(3), 307–313. doi: 10.1097/gme.0000000000000989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Ranieri WF (1988). Scale for Suicide Ideation: psychometric properties of a self-report version. J Clin Psychol, 44(4), 499–505. [DOI] [PubMed] [Google Scholar]
- Beck SL, Schwartz AL, Towsley G, Dudley W, & Barsevick A (2004). Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage, 27(2), 140–148. doi: 10.1016/j.jpainsymman.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Bellone S, Watts K, Cane S, Palmieri M, Cannon MJ, Burnett A,Santin AD (2005). High serum levels of interleukin-6 in endometrial carcinoma are associated with uterine serous papillary histology, a highly aggressive and chemotherapy-resistant variant of endometrial cancer. Gynecol Oncol, 98(1), 92–98. doi: 10.1016/j.ygyno.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Bermudez EA, Rifai N, Buring JE, Manson JE, & Ridker PM (2002). Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol, 89(9), 1117–1119. [DOI] [PubMed] [Google Scholar]
- Burke L, Miller LA, Saad A, & Abraham J (2009). Smoking behaviors among cancer survivors: an observational clinical study. J Oncol Pract, 5(1), 6–9. doi: 10.1200/jop.0912001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Calle EE, & Kaaks R (2004). Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer, 4(8), 579–591. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, & K., T, M. J (2003). Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. New England Journal of Medicine, 348(17), 1625–1638. doi: 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, & Thun MJ (2003). Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- Facts Cancer and Figures 2017. (2017). from https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html
- Carpenter JS, & Andrykowski MA (1998). Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res, 45(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Cash E, Sephton SE, Chagpar AB, Spiegel D, Rebholz WN, Zimmaro LA, & Dhabhar FS (2015). Circadian disruption and biomarkers of tumor progression in breast cancer patients awaiting surgery. Brain, Behavior, and Immunity, 48, 102–114. [DOI] [PubMed] [Google Scholar]
- Chopra V, Dinh TV, & Hannigan EV (1998). Circulating serum levels of cytokines and angiogenic factors in patients with cervical cancer. Cancer Invest, 16(3), 152–159. [DOI] [PubMed] [Google Scholar]
- Clevenger L, Schrepf A, Christensen D, DeGeest K, Bender D, Ahmed A,Lutgendorf SK (2012). Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav Immun, 26(7), 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, & Lubaroff DM (2005). Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer, 104(2), 305–313. doi: 10.1002/cncr.21147 [DOI] [PubMed] [Google Scholar]
- Crowe SD, Gregg LC, & DeFrancesco MS (2016). Obesity and Tobacco Cessation Toolkits: Practical Tips and Tools to Save Lives. Obstet Gynecol, 128(6), 1314–1319. doi: 10.1097/AOG.0000000000001752 [DOI] [PubMed] [Google Scholar]
- Cui W-Y, & Li MD (2010). Nicotinic modulation of innate immune pathways via α7 nicotinic acetylcholine receptor. Journal of neuroimmune pharmacology, 5(4), 479–488. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2009). Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am, 29(2), 247–264. doi: 10.1016/j.iac.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders: DSM-5. (2013). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Duffy SA, Teknos T, Taylor JM, Fowler KE, Islam M, Wolf GT,Terrell JE (2013). Health behaviors predict higher interleukin-6 levels among patients newly diagnosed with head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev, 22(3), 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Dograhmji K, Dorsey CM, Espie CA, Jamieson AO, McCall V, Morin CM, & Stepaniski EJ (2004). Derivation of Research Diagnostic Criteria for Insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep, 27(8), 1567–1596. [DOI] [PubMed] [Google Scholar]
- Esfandi F, Mohammadzadeh Ghobadloo S, & Basati G (2006). Interleukin-6 level in patients with colorectal cancer. Cancer Lett, 244(1), 76–78. doi: 10.1016/j.canlet.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Fain JN, Madan AK, Hiler ML, Cheema P, & Bahouth SW (2004). Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology, 145(5), 2273–2282. [DOI] [PubMed] [Google Scholar]
- First MB (1997). User’s guide for the Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. Washington, DC: American Psychiatric Press. [Google Scholar]
- Florou AN, Gkiozos IC, Tsagouli SK, Souliotis KN, & Syrigos KN (2014). Clinical significance of smoking cessation in subjects with cancer: a 30-year review. Respir Care, 59(12), 1924–1936. doi: 10.4187/respcare.02559 [DOI] [PubMed] [Google Scholar]
- Frassanito MA, Cusmai A, Iodice G, & Dammacco F (2001). Autocrine interleukin-6 production and highly malignant multiple myeloma: relation with resistance to drug-induced apoptosis. Blood, 97(2), 483–489. [DOI] [PubMed] [Google Scholar]
- Frost-Pineda K, Liang Q, Liu J, Rimmer L, Jin Y, Feng S,Sarkar M (2011). Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine Tob Res, 13(3), 182–193. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Alfonso-Miller P, Fernandez-Mendoza J, Shetty S, Shenoy S, & Combs D (2016). Sleep: important considerations for the prevention of cardiovascular disease. Curr Opin Cardiol, 31(5), 551–565. doi: 10.1097/HCO.0000000000000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xu F, Lu T, Duan Z, & Zhang Z (2012). Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer treatment reviews, 38(7), 904–910. [DOI] [PubMed] [Google Scholar]
- Hays R, Sherbourne C, & Mazel R (1995). User’s Manual for Medical Outcomes Study (MOS) Core measures of health-related quality of life. RAND Corporation, MR-162. [Google Scholar]
- He Y, Wang J, Bian H, Deng X, & Wang Z (2017). BMI as a Predictor for Perioperative Outcome of Laparoscopic Colorectal Surgery: a Pooled Analysis of Comparative Studies. Dis Colon Rectum, 60(4), 433–445. [DOI] [PubMed] [Google Scholar]
- Hodge DR, Hurt EM, & Farrar WL (2005). The role of IL-6 and STAT3 in inflammation and cancer. European journal of cancer, 41(16), 2502–2512. [DOI] [PubMed] [Google Scholar]
- Horne JA & Ostberg O (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol, 4(2), 97–110. [PubMed] [Google Scholar]
- Huo J, Smith BD, Giordano SH, Reece GP, & Tina Shih YC (2016). A comparison of patient-centered economic and clinical outcomes of post-mastectomy breast reconstruction between obese and non-obese patients. Breast, 30, 118–124. [DOI] [PubMed] [Google Scholar]
- Hussaini AE, Nicholson LM, Shera D, Stettler N, & Kinsman S (2011). Adolescent obesity as a risk factor for high-level nicotine addiction in young women. J Adolesc Health, 49(5), 511–517. doi: 10.1016/j.jadohealth.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, Coudert B, Iacobelli S, Tampellini M, Durando Z, Mormount M, Waterhouse J & Levi FA (2012). Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. International Journal of Cancer, 131 2684–2692. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, & Thun MJ (2008). Cancer statistics, 2008. CA Cancer J Clin, 58(2), 71–96. doi: 10.3322/CA.2007.0010 [DOI] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA,Tomaselli GF (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation, 129(25 Suppl 2), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska J, Nowacki MP, Kowalska M, Rysinska A, Chwalinski M, Fuksiewicz M,Chechlinska M (2005). Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: soluble tumor necrosis factor receptor type I--an independent prognostic factor. Tumour Biol, 26(4), 186–194. doi: 10.1159/000086951 [DOI] [PubMed] [Google Scholar]
- Kauffman BY, Farris SG, Alfano CA, & Zvolensky MJ (2017). Emotion dysregulation explains the relation between insomnia symptoms and negative reinforcement smoking cognitions among daily smokers. Addict Behav, 72, 33–40. doi: 10.1016/j.addbeh.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen LE, Warren GW, Koziak JM, Kobel M, & Steed H (2016). Smoking may modify the association between neoadjuvant chemotherapy and survival from ovarian cancer. Gynecol Oncol, 140(1), 124–130. doi: 10.1016/j.ygyno.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Rosen B, Fan I, Ivanova A, McLaughlin JR, Risch H,Kotsopoulos J (2017). Epidemiologic factors that predict long-term survival following a diagnosis of epithelial ovarian cancer. Br J Cancer, 116(7), 964–971. doi: 10.1038/bjc.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T (1989). The biology of interleukin-6. Blood, 74(1), 1–10. [PubMed] [Google Scholar]
- Koene RJ, Prizment AE, Blaes A, & Konety SH (2016). Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation, 133(11), 1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotowicz B, Fuksiewicz M, Jonska-Gmyrek J, Bidzinski M, & Kowalska M (2016). The assessment of the prognostic value of tumor markers and cytokines as SCCAg, CYFRA 21.1, IL-6, VEGF and sTNF receptors in patients with squamous cell cervical cancer, particularly with early stage of the disease. Tumour Biol, 37(1), 1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner RF, & Ryan DH (2014). Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. Jama, 312(9), 943–952. [DOI] [PubMed] [Google Scholar]
- Lancaster T, & Stead LF (2017). Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev, 31(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D, Matte I, Rancourt C, & Piché A (2011). Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC cancer, 11(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT,Hudis CA (2014). American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol, 32(31), 3568–3574. doi: 10.1200/JCO.2014.58.4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang Q, Frost-Pineda K, Muhammad-Kah R, Rimmer L, Roethig H,Sarkar M (2011). Relationship between biomarkers of cigarette smoke exposure and biomarkers of inflammation, oxidative stress, and platelet activation in adult cigarette smokers. Cancer Epidemiol Biomarkers Prev, 20(8), 1760–1769. [DOI] [PubMed] [Google Scholar]
- Macciò A, & Madeddu C (2012). Inflammation and ovarian cancer. Cytokine, 58(2), 133–147. doi: 10.1016/j.cyto.2012.01.015 [DOI] [PubMed] [Google Scholar]
- Morin C Bootzin M, Richard R, Buysse, Daniel J, Edinger, Jack D, Espie, Colin A, Lichstein, Kenneth L (2006). Psychological and Behavioral Treatment of Insomnia: Update of the recent Evidence (1998–2004). Sleep, 29(11), 1398–1414. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Simpson NS, Meier-Ewert HK, & Haack M (2010). Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab, 24(5), 775–784. doi: 10.1016/j.beem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases (2017, August). Overweight & Obesity Statistics. Retrieved December 17, 2018, from https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity.
- Obesity Medicine Association (2017, August 29). Definition of Obesity. Retrieved November 19, 2018, from https://obesitymedicine.org/definition-of-obesity/.
- Orban Z, Remaley AT, Sampson M, Trajanoski Z, & Chrousos GP (1999). The differential effect of food intake and beta-adrenergic stimulation on adipose-derived hormones and cytokines in man. J Clin Endocrinol Metab, 84(6), 2126–2133. [DOI] [PubMed] [Google Scholar]
- Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S,Morrow GR (2010). Prevalence, demographics, and psychological associations of sleep disruption in patients with cance: University of Rochester Cancer Center-Community Clinical Oncology Programr. J Clin Oncol, 28(2), 292–298. doi: 10.1200/jco.2009.22.5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panoulas VF, Stavropoulos-Kalinoglou A, Metsios GS, Smith JP, Milionis HJ, Douglas KM, Kitas GD (2009). Association of interleukin-6 (IL-6)-174G/C gene polymorphism with cardiovascular disease in patients with rheumatoid arthritis: the role of obesity and smoking. Atherosclerosis, 204(1), 178–183. [DOI] [PubMed] [Google Scholar]
- PDQ Adult Treatment Editorial Board. Ovarian Epithelial, Fallopian Tube, and Primary Peritoneal Cancer Treatment (PDQ®): Health Professional Version 2018. July 19 In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK66007/ [PubMed] [Google Scholar]
- Papantoniou K, Castaño Vinyals G, Espinosa A, Aragonés N, Pérez Gómez B, Burgos J, & Arredondo F (2015). Night shift work, chronotype and prostate cancer risk in the MCC ‐S pain case‐control study. International journal of cancer, 137 (5), 1147–1157. [DOI] [PubMed] [Google Scholar]
- Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, & Manuck SB (2009). Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol, 82(1), 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, & Bull D (2007). Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Bmj, 335(7630), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roennenberg T (2012). What is chronotype? Sleep and Biological Rhythms, 10, 75–76. [Google Scholar]
- Rossing MA, Cushing-Haugen KL, Wicklund KG, & Weiss NS (2008). Cigarette smoking and risk of epithelial ovarian cancer. Cancer Causes Control, 19(4), 413–420. [DOI] [PubMed] [Google Scholar]
- Rutledge L, Denmark-Wahnefried Wendy. (2016). Weight management and exercise for cancer survivors. Clinical Journal of Oncology Nursing, 20(2), 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallit J, Ciccazzo M, & Dixon Z (2009). A cognitive-behavioral weight control program improves eating and smoking behaviors in weight-concerned female smokers. [Randomized Controlled Trial]. J Am Diet Assoc, 109(8), 1398–1405. [DOI] [PubMed] [Google Scholar]
- Sandadi S, Frasure HE, Broderick MJ, Waggoner SE, Miller JA, & von Gruenigen VE (2011). The effect of sleep disturbance on quality of life in women with ovarian cancer. Gynecol Oncol, 123(2), 351–355. doi: 10.1016/j.ygyno.2011.07.028 [DOI] [PubMed] [Google Scholar]
- Savant SS, Sriramkumar S, & O’Hagan HM (2018). The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers (Basel), 10(8). doi: 10.3390/cancers10080251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard J, Ivers H, Villa J, Caplette-Gingras A, & Morin CM (2011). Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol, 29(26), 3580–3586. doi: 10.1200/jco.2010.33.2247 [DOI] [PubMed] [Google Scholar]
- Savard J, & Morin CM (2001). Insomnia in the Context of Cancer: A review of a neglected problem. Journal of Clinical Oncology, 19(3), 895–908. doi: 10.1200/JCO.2001.19.3.895 [DOI] [PubMed] [Google Scholar]
- Scambia G, Testa U, Panici PB, Foti E, Martucci R, Gadducci A,Mancuso S (1995). Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. British journal of cancer, 71(2), 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, James C, Malstrom M, Rothman RL, Wang H, Babb J,Goldberg M (2003). Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Ann Behav Med, 25(3), 214–222. doi: 10.1207/s15324796abm2503_07 [DOI] [PubMed] [Google Scholar]
- Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A,Lutgendorf SK (2013). Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: Relationships with depression, fatigue, and disability. Brain, Behavior, and Immunity, 30, Supplement, S126–S134. doi: 10.1016/j.bbi.2012.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitas F, Weber MF, Egger S, Yap S, Chiew M, & O’Connell D (2014). Smoking Cessation After Cancer. Journal of Clinical Oncology, 32(32), 3593–3595. [DOI] [PubMed] [Google Scholar]
- Sopori M (2002). Effects of cigarette smoke on the immune system. Nat Rev Immunol, 2(5), 372–377. doi: 10.1038/nri803 [DOI] [PubMed] [Google Scholar]
- Stead LF, Carroll AJ, & Lancaster T (2017). Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev, 31(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T,Richardson LC (2017). Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep, 66(39), 1052–1058. doi: 10.15585/mmwr.mm6639e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, & Karin M (2014). IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol, 26(1), 54–74. [DOI] [PubMed] [Google Scholar]
- Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, & Kainz C (1997). Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecologic oncology, 66(1), 27–30. [DOI] [PubMed] [Google Scholar]
- Cancer US Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999–2015): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, June 2018. [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, & Chrousos GP (1997). Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab, 82(5), 1313–1316. [DOI] [PubMed] [Google Scholar]
- Walker MS, Larsen RJ, Zona DM, Govindan R, & Fisher EB (2004). Smoking urges and relapse among lung cancer patients: findings from a preliminary retrospective study. Prev Med, 39(3), 449–457. doi: 10.1016/j.ypmed.2004.04.035 [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, & Whincup PH (2005). Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J, 26(17), 1765–1773. [DOI] [PubMed] [Google Scholar]
- Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, & Wouters EF (2007). Systemic effects of smoking. Chest, 131(5), 1557–1566. [DOI] [PubMed] [Google Scholar]
- Zakrzewska I, & Poznanski J (2001). [Changes of serum il-6 and CRP after chemotherapy in patients with ovarian carcinoma]. Pol Merkur Lekarski, 11(63), 210–213. [PubMed] [Google Scholar]
- Zaleta AK, Neff R, McCann GA, O’Malley DM, & Carpenter KM (2017). Perceptions of weight management counseling among gynecologic cancer survivors: opportunities for enhancing survivorship care. Support Care Cancer, 25(5), 1537–1545. doi: 10.1007/s00520-016-3552-0 [DOI] [PubMed] [Google Scholar]
- Zisapel N (2007). Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci, 64(10), 1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]