Abstract
Objective:
Coronary artery disease (CAD) patients with co-morbid depression show an increase in mortality compared to cardiac patients without depression, but the mechanisms mediating this effect remain obscure. One possible explanation for this finding is that depressed patients with CAD exhibit an increased vulnerability to stress. The purpose of this study was to assess the effects of stress and depression on brain function and to explore its relationship with myocardial ischemia in CAD patients.
Methods:
Patients with CAD and depression (N=13) and CAD without depression (N=15) underwent imaging of the brain with positron emission tomography and [O-15] water and imaging of the heart with single photon emission computed tomography (SPECT) and [Tc-99m] sestamibi under mental stress task and control conditions.
Results:
CAD patients with depression compared to non-depressed showed decreased function with mental stress in the rostral anterior cingulate, hippocampus, parts of dorsolateral temporal, and parietal cortex, and cerebellum, and uncus, with increased blood flow in parahippocampus, visual association cortex, and posterior cingulate. Depressed CAD patients who became ischemic during mental stress task had relative decreases in caudal and posterior cingulate, orbitofrontal cortex, and cerebellum, and increased activation in parietal cortex and precuneus/visual association cortex compared to non-ischemic depressed CAD patients.
Conclusions:
These findings are consistent with dysfunction in a network of brain regions involved in the stress response in patients with comorbid CAD and depression that has direct and indirect links to the heart, suggesting a pathway by which stress and depression could lead to increased risk of heart disease related morbidity and mortality.
Keywords: Stress, depression, cardiovascular disease, cortisol, single photon emission tomography, PET, SPECT
INTRODUCTION
Coronary artery disease (CAD) is the leading cause of death in the United States (Hennekens, 1998). There is increased interest in behavioral risk factors for CAD, which in addition to lifestyle factors like physical activity, smoking, and diet, include stress, emotional reactivity, and personality style (Burg, Jain, Soufer, Kerns, & Zaret, 1993; Burg & Soufer, 2014; Lane, Waldstein, Chesney, et al., 2009; Lichtman et al., 2008; Lichtman et al., 2014; Sheps et al., 2002; Vaccarino & Bremner, 2014). This has led to an appreciation of the fact that mental disorders related to stress, including major depression and posttraumatic stress disorder (PTSD), are associated with an increased risk for CAD and worse outcomes when present in CAD patients (Carney & Freedland, 2017; Freedland & Carney, 2013; Lichtman, et al., 2014; Vaccarino & Bremner, 2014; Vaccarino et al., 2013; Vaccarino et al., 2009).
The lifetime prevalence rate of major depression in the United States is about 17% (Kessler et al., 2003). An important risk factor for the development of depression is exposure to stress, especially in early childhood (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995), and a recent stressful life event is a risk factor for the onset of depression (Kendler, Thornton, & Gardner, 2000). Stress can affect cardiac function (Burg & Soufer, 2014; Wittstein et al., 2005), and has also been shown to induce myocardial ischemia in coronary artery disease (CAD) patients (Arri, Ryan, Redwood, & Marber, 2016; Hammadah, Alkhoder, et al., 2017; Ramadan et al., 2013; Vaccarino et al., 2014; Wei, Pimple, et al., 2014; Wei, Rooks, et al., 2014). There is also evidence that patients with depression are more vulnerable to stress-induced myocardial ischemia (Burg et al., 2014; Wei, Pimple, et al., 2014).
Alterations in brain and neurohormonal systems in patients with depression represent a possible mechanism by which stress and depression can induce and/or maintain CAD (Carney, Freedland, & Veith, 2005; Vaccarino & Bremner, 2014). The hypothalamic-pituitary-adrenal (HPA) and noradrenergic systems play critical roles in the stress response. Stress is associated with activation of the HPA axis with release of cortisol (Yehuda, 2002, 2006) and increased noradrenergic activity (Bremner, Krystal, Southwick, & Charney, 1996a, 1996b; Tanaka, Yoshida, Emoto, & Ishii, 2000). Depression is associated with an increased in cortisol and norepinephrine (Carroll, Curtis, Davies, Mendels, & Sugarman, 1976; Charney, Sklar, Buxbaum, & Nestler, 2014; Delgado & Moreno, 2000; Gold et al., 1986; Otte et al., 2004; Traskman et al., 1980; Young, Haskett, & Grunhaus, 1994). Depression is also associated with altered function in brain areas that are sensitive to stress and have direct outputs to neurohormonal and cardiovascular systems, including the medial prefrontal cortex (anterior cingulate and orbitofrontal cortex) (Bremner, 2002; Cheng et al., 2016; Drevets et al., 1997; Drysdale et al., 2016; George et al., 1997; George, Ketter, & Post, 1994; Mayberg et al., 1997; Price & Drevets, 2012; Sheline et al., 2009; Willner, Scheel-Krüger, & Belzungc, 2013). Functional alterations in these brain regions in patients with CAD and depression could act through neurohormonal systems to affect cardiovascular function (Frysztak & Neafsey, 1994; Lane, Waldstein, Critchley, et al., 2009; Soufer, 2004; Thayer & Lane, 2009). Thus, stress and depression could converge through biological pathways to create a particularly acute risk for CAD.
Standardized protocols have been developed for the induction of stress in the laboratory. Mental stress protocols involve performing difficult tasks under time pressure and/or with negative feedback and social pressure from an attendant performing the protocol. Tasks can include mental arithmetic which involves performing difficult arithmetic tasks including addition, subtraction, and multiplication with difficulty titrated to the individual (Bremner et al., 2018; Bremner et al., 2009; Bremner, Vythilingam, Vermetten, et al., 2003; Burg, et al., 1993; Burg, et al., 2014), and public speaking tasks in which the individual has to create a speech to deliver in the context of an interpersonal conflict situation (Hammadah, Al Mheid, et al., 2017; Hammadah, Alkhoder, et al., 2017; Vaccarino et al., 2018; Vaccarino et al., 2016). These mental stress methods are associated with increases in heart rate, blood pressure (Bremner, et al., 2018; Bremner, Vythilingam, Vermetten, et al., 2003; Burg, et al., 1993; Burg, et al., 2014; Hammadah, Alkhoder, et al., 2017; Vaccarino, et al., 2018; Vaccarino, et al., 2016), subjective distress (Bremner, et al., 2018), peripheral vasoconstriction (Bremner, et al., 2018; Hammadah, Alkhoder, et al., 2017) and cortisol (Bremner, et al., 2009; Bremner, Vythilingam, Vermetten, et al., 2003), and to induce myocardial ischemia in some vulnerable patients with CAD as measured with Single Photon Emission Computed Tomography (SPECT) and other methods (Bremner, et al., 2018; Bremner, et al., 2009; Bremner, Vythilingam, Vermetten, et al., 2003; Burg, et al., 1993; Burg, et al., 2014; Hammadah, Al Mheid, et al., 2017; Hammadah, Alkhoder, et al., 2017; Ramachandruni et al., 2006; Ramadan, et al., 2013; Soufer et al., 1998; Sullivan et al., 2018; Vaccarino, et al., 2018; Vaccarino, et al., 2016). Mental stress-induced myocardial ischemia (MSI) can occur in patients without exercise-induced ischemia, and does not require diseased coronary arteries or increased heart rate or blood pressure, suggesting that it may be related to coronary vasospasm and/or peripheral vasoconstriction (Hammadah, Alkhoder, et al., 2017; Ramadan, et al., 2013; Sullivan, et al., 2018). Although the mechanisms by which this occurs are not fully elucidated, brain pathways involved in stress and emotion including limbic areas (medial prefrontal cortex/anterior cingulate, amygdala, insula, hippocampus, parietal cortex) are likely involved. This is supported by studies of the relationship between cardiovascular function and brain blood flow as measured with Positron Emission Tomography (PET) and functional Magnetic Resonance Imaging (fMRI) (Critchley, Corfield, Chandler, Mathias, & Dolan, 2000; Critchley, Mathias, & Dolan, 2001; Gianaros et al., 2005; Gianaros, Jennings, Sheu, Derbyshire, & Matthews, 2007; Gianaros, May, Siegle, & Jennings, 2005; Gianaros, Sheu, Jennings, Manuck, & Hariri, 2008; Gianaros, Van der Veen, & Jennings, 2004; Jennings, Van der Molen, & Tanase, 2009; Lane, McRae, et al., 2009; Lane, Waldstein, Critchley, et al., 2009; Pollatos, Schandry, Auer, & Kaufmann, 2007; Tanida, Sakatani, Takano, & Tagai, 2004). Studies in CAD patients are consistent with this idea (Bremner, et al., 2018; Rosen et al., 1996; Soufer, et al., 1998). MSI has also been linked increased symptoms of depression in patients with CAD (Burg, et al., 2014; Wei, Pimple, et al., 2014).
The purpose of the current study was to measure myocardial perfusion with SPECT and brain function with PET during exposure to a mental stress task in CAD patients with and without depression. We hypothesized that CAD patients with depression would exhibit decreased activity in the medial prefrontal cortex / anterior cingulate during stress, and that greater changes would be observed in CAD patients with depression who developed stress-induced myocardial ischemia.
METHODS
Subjects
Subjects were recruited with stable coronary artery disease (CAD) from Emory University-affiliated hospitals and clinics and by newspaper advertisement. Subjects were selected with CAD based on standard criteria for history of previous CAD which were all verified through medical records. Criteria for CAD included: 1) abnormal coronary angiography or intravascular ultrasound demonstrating atherosclerosis with at least luminal irregularities, 2) previous percutaneous or surgical coronary revascularization, 3) documented myocardial infarction, or 4) positive exercise or pharmacological nuclear stress test or electrocardiographic exercise stress test.
Subjects were included in the CAD with depression group with the diagnosis of CAD and the categorical diagnosis of current major depression based on the Structured Clinical Interview for DSM-IV (SCID). Subjects were included in the CAD non-depressed group with the diagnosis of CAD who did not have a current or past history of major depression or other affective or major psychiatric disorder and did not have a score of greater than 9 on the Hamilton Depression Scale (Ham-D) (Hamilton, 1960). Subjects were excluded with a neurological disorder, unstable angina, history of myocardial infarction in the past month, or major medical illness other than CAD based on history and laboratory testing, schizophrenia, bipolar disorder, current alcohol and/or substance abuse or dependence or with the past six months based on the SCID, or current treatment with steroids or psychotropic medications including neuroleptics, benzodiazepines, sedative hypnotic, or barbiturates. Subjects who were currently receiving antidepressant treatment were not excluded if they remained symptomatic as confirmed by a current diagnosis of major depression based on the SCID and a Ham-D score of greater than 9. Subjects on cardiac medications were not excluded. Cardiac medications were held on the morning of the study. All subjects provided written informed consent for participation. This study was approved by the Emory University Institutional Review Board.
Forty seven subjects were initially screened for the study. Of those, 14 were not interested or did not follow-up, two were excluded for medical or neurological conditions, and three were excluded based on the psychiatric assessments, leaving 28 subjects who completed the study, including CAD patients with (N=13) and without (N=15) depression. Demographic characteristics of the sample are displayed in Table 1.
Table 1.
Risk Factors and Treatments
| Variable | CAD with Depression (N=13) |
CAD without Depression (N=15) |
|---|---|---|
| Age | 58 (9 SD) | 60 (5 SD) |
| Gender | 11 M/2 F | 13 M/2 F |
| Race | 11 C/2 AA | 14 C/1 AA |
| HAM-D Score | 14 (4 SD) | 2 (2 SD)* |
| Hypercholesterolemia | 69% | 87% |
| Hypertension | 69% | 67% |
| Diabetes | 27% | 33% |
| Smoking | 20% | 27% |
| Statins | 62% | 80% |
| Beta blockers | 69% | 53% |
| ACE inhibitors | 77% | 60% |
| Calcium channel blockers |
23% | 13% |
| Diuretics | 23% | 13% |
| Antidepressants | 77% | 7% |
p<0.001
Demographics, risk factors, and medication usage in Coronary Artery Disease (CAD) patients with and without depression. SD=Standard Deviation; F=Female; M=Male; C=Caucasian; AA=African American; HAM-D= Hamilton Depression Scale;ACE=angiotensin converting enzyme inhibitor;
Psychometric Assessments
All subjects were assessed with the Structured Clinical Interview for DSMIV (SCID) (First, Spitzer, Williams, & Gibbon, 1995). Severity of depressive symptoms was measured with the 21-item Hamilton Depression Rating Scale (Ham-D) (Hamilton, 1960). History of childhood physical and/or sexual abuse was measured with the Early Trauma Inventory (Bremner, Bolus, & Mayer, 2007). Analogue ratings of nervousness and fearfulness (scale of 0 to 4, 4 being most severe) were collected by self-report during rest and after mental stress (Bremner et al., 1999). At the beginning of the study, subjects were shown the analogue scale, which consists of a line marked at five points from 0−4, with adjectives of 0=not at all, 1=slightly, 2=moderately, 3=considerably, 4=extremely. Subjects were then asked to rate separately their current feelings of fear and nervousness using the analogue scale during rest and stress.
Single Photon Emission Computed Tomography
Subjects underwent Single Photon Emission Computed Tomography (SPECT) measurement of myocardial perfusion at rest and with mental stress. Subjects underwent a baseline SPECT scan of the heart at rest following intravenous injection of 8 mCi [Tc-99m]sestamibi, a radiopharmaceutical used for the measurement of myocardial blood flow. Subjects then underwent mental stress in conjunction with imaging of the brain (see below), at the end which they were injected with 20 mCi [Tc-99m]sestamibi followed repeat imaging of the heart. Since [Tc-99m]sestamibi is taken up in the heart with little wash-out, subjects could undergo cardiac imaging of the heart 30 minutes later to obtain an image of the heart at the time of the mental stress challenge, thereby allowing simultaneous cardiac and brain imaging with stress.
Positron Emission Tomography
Subjects underwent Positron Emission Tomography (PET) imaging of the brain with radiolabeled (O−15) water in conjunction with control and mental stress tasks. PET imaging was performed on a CTI ECAT Exact camera. The ECAT has a voxel size of 1.7 × 1.7 × 3.4 mm and an intrinsic resolution of approximately 6.2 mm. The subject was placed in the scanner with their head held in a holder to minimize motion and positioned with the cantho-meatal line parallel to an external laser light. An intravenous line was inserted in the antecubital fossa for administration of [150]H20. Following positioning within the camera gantry, a transmission scan of the head was obtained, in order to correct emission data for attenuation due to overlying bone and soft tissue. Ten seconds before administration of [150]H20 subjects received instructions regarding the task. This was followed by the counting control or mental stress task which was 90 seconds in duration. At the beginning of the task subjects received a bolus injection of 30 mCi of [150]H20 followed ten seconds later by a PET scan acquisition which was 90 seconds in length. The onset of the PET scan acquisition was timed to correspond to the point of maximum rate of increase in uptake of tracer into the brain.
Mental Stress
Subjects underwent a mental stress task during simultaneous imaging of the brain and heart. Control and mental stress tasks were performed in fixed order. All subjects underwent both control and stress tasks.
For the control task subjects counted out loud for two minutes while undergoing PET imaging of the brain. This was repeated twice. Next they underwent a mental stress task for two minutes in conjunction with PET imaging of the brain, again repeated twice. For mental stress, subjects performed stressful mental arithmetic tasks (serial subtraction, addition, multiplication and division) under time pressure with negative feedback from a research fellow. Difficulty was titrated to individual ability to perform the task. For example, if subjects got two or more correct in a row a more difficult problem was posed until the subject made a mistake.
These mental stress methods, which have been described in detail elsewhere (Bremner, et al., 2018), have been shown to be associated with significant increases in heart rate, systolic and diastolic blood pressure (Bremner, et al., 2018; Bremner, Vythilingam, Vermetten, et al., 2003; Burg, et al., 1993; Burg, et al., 2014), subjective distress (Bremner, et al., 2018), peripheral vasoconstriction (Bremner, et al., 2018) and cortisol (Bremner, et al., 2009; Bremner, Vythilingam, Vermetten, et al., 2003), and to induce myocardial ischemia in some vulnerable patients with CAD ischemia (Bremner, et al., 2018; Bremner, et al., 2009; Bremner, Vythilingam, Vermetten, et al., 2003; Burg, et al., 1993; Burg, et al., 2014; Soufer, et al., 1998), with the degree of nervousness induced by stress correlating with ischemia (Bremner, et al., 2009).
Two minutes into the second stressful cognitive challenge, subjects were intravenously injected with 20 mCi [Tc-99m]sestamibi followed by another minute of stressful cognitive challenge. Subject were then removed from the PET camera and accompanied to the SPECT camera where they underwent SPECT imaging of the myocardium 30 minutes after completion of the task (but with an image of myocardial perfusion at the time of stress). Gated images were also obtained for measurement of ejection fraction and evaluation of regional wall motion abnormalities. Myocardial images from baseline, exercise and mental stress were reconstructed in short axis, vertical long axis and horizontal long axis views.
Data Analysis
Categorical data was analyzed using chi-square and all other data by analysis of variance (ANOVA). Significance was defined as p<0.05.
Cardiac data were analyzed using the Emory Toolbox, a validated instrument for display and quantitation of cardiac SPECT imaging data,(Garcia et al., 2007; Van Train et al., 1994) and were additionally scored by a Nuclear Medicine physician blinded to subject diagnosis using a 20-segment bull’s eye diagram of the heart. This diagram is used to rate perfusion abnormalities at rest and stress on a scale of 0 (normal) to 4 (absent perfusion) for each of 20 segments of the heart and to develop a quantitative index of perfusion with rest and stress. The difference between scores at rest and stress was defined as the myocardial perfusion score. Subjects with a score of 3 or greater were defined as having MSI.
PET brain mages were reconstructed and analyzed on a SunSparc Workstation using statistical parametric mapping (spm02) (www.fil.ion.ucl.ac.uk/spm) and Matlab software (Mathworks, Natick MA). Images for each patient set were realigned to the first scan of the study session. The data underwent transformation into a common anatomical space and was smoothed with a 3-dimensional Gaussian filter to 10 mm full-width half maximum. This yielded an image set with 2 × 2 × 2 mm voxels. Regional blood flow with global blood flow as a covariate was compared in hypothesized regions (anterior cingulate, amygdala, hippocampus) between the two scans in the control condition and the two scans in the active condition (mental stress) in patients with depression and controls using the general linear model. Additional regions were examined for comparison purposes. First, contrasts were performed comparing blood flow during the active and control tasks within the non-depressed and depressed CAD patient groups. Then the interaction between group (depression vs controls) and condition (control versus mental stress) was examined to assess greater increases and decreases in blood flow in depressed versus non-depressed CAD patients. Statistical analyses yielded image data sets in which the values assigned to individual voxels correspond to t statistic (Friston, 1994; Friston, Frith, Liddle, & Frackowiak, 1991). Statistical images were displayed with values of z score units. An uncorrected threshold z score of 2.67 (p<0.005) and a minimum cluster of 44 voxels as determined by spm02 was used to examine areas of activation. A p value of <0.005 has been shown to represent the best trade-off between Type I and Type II errors (Reiman et al., 1997). Locations of areas of activation were identified as the distance from the anterior commissure in mm, with x-, y- and z- coordinates, based on the Montreal Neurological Institute (MNI) template (see Tables). Areas of activation in the brain were identified using a standard stereotaxic atlas (Talairach & Tournoux, 1988).
RESULTS
There were no differences between the groups of CAD subjects with and without depression in demographic factors or risk factors for heart disease, including age, sex and race, hypercholesterolemia, diabetes, or smoking (Table 1). Subjects with CAD and a diagnosis of major depression had significantly greater scores on the Ham-D than subjects without current major depression (Table 1). Of the CAD subjects with current depression 1 (8%) had a history of past alcohol dependence, 3 (23%) past alcohol abuse, 1 (8%) had a history of past marijuana abuse, 2 (14%) had current PTSD, 1 (8%) panic disorder without agoraphobia, 1 (8%) panic disorder with agoraphobia, 1 (8%) current obsessive-compulsive disorder, 1 (8%) current social anxiety disorder, 1 (8%) generalized anxiety disorder. Five (38%) reported a history of exposure to childhood physical and/or sexual abuse based on the ETI. Of the 15 CAD subjects without current depression, 1 (7%) had a history of past alcohol abuse, and one (7%) past marijuana abuse. None reported a history of childhood physical and/or sexual abuse.
CAD patients with depression had higher self-rated nervousness at both rest and stress conditions when compared to CAD patients without depression (main effect diagnosis; 16.45; df 1,50; p=0.0002) with no interaction between control or stress condition and diagnosis interaction. Five of the CAD patients with depression (38%) had MSI.
The CAD patients without depression when examined alone exhibited increased activity during stress compared to control tasks in bilateral superior frontal gyri, left middle and inferior frontal gyri, right superior temporal gyrus and left middle temporal inferior temporal gyri, left parietal cortex (supramarginal gyrus and inferior parietal lobule), cerebellum, right medial orbital gyrus, left hippocampus, and midbrain (Table 2). They also exhibited stress-induced decreases in bilateral superior frontal gyrus, right inferior frontal gyrus, bilateral postcentral gyrus, posterior cingulate, precuneus, left superior temporal gyrus, and cerebellum.
Table 2.
Areas of Greater Increases and Decreases in Blood Flow with Stress Challenge in CAD Patients without Depression (N=15)
| Increased Blood Flow | Decreased Blood Flow | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z Score* |
Voxel Number* |
Talairach Coordinates | Z Score* |
Voxel Number** |
Talairach Coordinates | ||||||
| x | y | z | Brain Region | x | y | z | Brain Region | ||||
| 5.92 | 5026 | −8 | 24 | 64 | L. Sup Frontal Gyrus (6) | 7.33 | 9775 | 52 | −6 | 14 | R Inf Frontal Gyrus (44) |
| 5.12 | −50 | 28 | 18 | L. Middle/Inferior Frontal Gyrus (45,46) | 7.31 | 58 | −6 | 14 | |||
| 4.95 | −48 | 20 | 34 | L. Middle Frontal Gyrus (9) | 6.99 | 62 | −22 | 20 | R. Postcentral Gyrus (43) | ||
| 4.67 | 1199 | −66 | −42 | −10 | L. Middle Temporal Gyrus (21) | 4.40 | 4449 | −6 | −6 | 44 | Posterior Cingulate |
| 4.26 | −54 | −40 | −18 | L. Inf. Temporal Gyrus (20) | 4.35 | 2 | −44 | 54 | Precuneus (7) | ||
| 3.79 | −55 | −52 | 2 | L. Middle Temporal Gyrus (21) | 4.34 | 8 | −10 | 64 | R. Sup Frontal Gyrus (6) | ||
| 4.53 | 1343 | −60 | −52 | 40 | L Parietal-Supramarginal Gyrus (40) | 7.32 | 4730 | −52 | −10 | 22 | L. Postcentral Gyrus (32) |
| 4.51 | −30 | −66 | 50 | L Parietal-Inf Parietal Lobule (40) | 4.81 | −34 | 0 | 4 | L Sup Temp Gyrus (42) | ||
| 3.77 | −35 | −50 | 34 | 4.67 | −30 | −26 | 12 | ||||
| 3.74 | 132 | 72 | −30 | −12 | R Middle Temporal Gyrus (20, 21) | 3.90 | 408.00 | 22 | −62 | −26 | cerebellum |
| 3.39 | 68 | 58 | 10 | −22 | R. Sup Temp. Gyrus (38) | 3.69 | 20 | −55 | −18 | ||
| 2.82 | 50 | 6 | −34 | 3.66 | 466.00 | −10 | −66 | −20 | cerebellum | ||
| 3.28 | 20 | 34 | −12 | R Sup Frontal Gyrus (11) | 3.65 | −24 | −52 | −28 | |||
| 4.32 | 288 | 52 | 28 | −18 | R Sup Temp Gyrus (38) | 3.63 | 163.00 | 20 | −32 | −16 | cerebellum |
| 4.06 | 34 | 26 | −30 | 3.13 | 24 | −4 | −44 | ||||
| 3.82 | 44 | 26 | v24 | 3.10 | 6 | −14 | −40 | ||||
| 2.87 | 10 | 26 | 48 | R Sup Frontal Gyrus (8) | |||||||
| 4.11 | 283 | 24 | 54 | −16 | R Sup Frontal Gyrus (11) | ||||||
| 3.74 | 24 | 48 | −22 | R Medial Orbital Gyrus (11,47) | |||||||
| 3.28 | 20 | 34 | −12 | R Sup Frontal Gyrus (11) | |||||||
| 3.46 | 127 | 16 | 28 | 54 | R Sup Frontal Gyrus (8) | ||||||
| 2.87 | 10 | 26 | 48 | ||||||||
| 3.56 | 52 | −30 | −26 | −10 | L. Hippocampus | ||||||
| 3.26 | 70 | 4 | −28 | −16 | midbrain | ||||||
| 4.15 | 683 | 8 | −88 | −34 | cerebellum | ||||||
| 3.55 | 38 | −70 | −44 | ||||||||
| 3.26 | 40 | −68 | −36 | ||||||||
Z Score>2.67, p<.005
Number of voxels within the activated cluster
Areas in bold represent the voxel with greatest activation in a contingent group of voxels; non-bold areas are other areas of activation within the same group
The CAD patients with depression when examined alone exhibited increased activity during stress compared to control tasks in bilateral visual association cortex and cuneus, left middle frontal gyrus, fornix, cerebellum and right inferior parietal lobule (Table 3), and decreases in left inferior and right superior frontal gyrus, bilateral precentral gyrus, bilateral superior and left inferior temporal gyrus, dorsal anterior cingulate, precuneus, pons, and cerebellum.
Table 3.
Areas of Greater Increases and Decreases in Blood Flow with Stress Challenge in CAD Patients with Depression (N=13)
| Increased Blood Flow | Decreased Blood Flow | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z Score* |
Voxel umber |
Talairach Coordinates | Z Score* |
Voxel Number** |
Talairach Coordinates | ||||||
| x | y | z | Brain Region | x | y | z | Brain Region | ||||
| 4.27 | 1872 | −28 | −68 | 24 | L. Visual Association Ctx (19) | 6.53 | 3760 | −52 | −6 | 12 | L. Inf. Frontal Gyrus (45) |
| 3.97 | −24 | −54 | 38 | 4.35 | −56 | −12 | 42 | L. Precentral Gyrus (6) | |||
| 3.81 | −30 | −66 | 38 | 4.20 | −46 | −14 | 36 | L. Sup. Temporal (22) | |||
| 3.62 | 900 | 2 | −92 | 26 | R. Visual Association Ctx (19) | 5.92 | 8090.00 | 64 | −32 | 18 | R. Sup Temp Gyrus (22) |
| 3.43 | 4 | −84 | 24 | 5.71 | 62 | 4 | 10 | R. Precentral Gyrus (6) | |||
| 3.12 | −2 | −78 | 12 | Cuneus (18) | 5.53 | 52 | −10 | 34 | R Precentral Gyrus (6) | ||
| 3.57 | 235 | −32 | 24 | 20 | L. Middle Frontal Gyrus (46) | 4.60 | 744.00 | 0 | 42 | −10 | Rostral Anterior Cingulate (24,32) |
| 3.21 | −28 | 22 | 28 | 4.31 | 1363.00 | 10 | −8 | 76 | R. Sup Frontal Gyrus (6) | ||
| 3.48 | 596 | −36 | 4 | 52 | L. Middle Frontal Gyrus (8) | 3.74 | 6 | −18 | 52 | ||
| 3.48 | −46 | 4 | 52 | 3.00 | 15 | −22 | 80 | ||||
| 3.36 | −46 | 16 | 40 | 3.90 | 170.00 | 8 | −56 | 70 | Precuneus (7) | ||
| 3.39 | 58 | 26 | −30 | −8 | Fornix | 3.35 | −2 | −52 | 58 | ||
| 3.20 | 68 | 8 | −88 | −28 | cerebellum | 3.81 | 117.00 | −48 | −12 | −42 | L. Inf. Temporal Gyrus (20) |
| 2.97 | 105 | 30 | −52 | 46 | R. Inferior Parietal Lobule (40) | 3.73 | 339.00 | 54 | −28 | −28 | |
| 2.79 | 34 | −52 | 38 | 3.70 | 145.00 | −28 | −2 | −50 | |||
| 3.66 | 296.00 | −18 | −22 | 78 | L. Precentral Gyrus (6) | ||||||
| 3.49 | −22 | −10 | 74 | ||||||||
| 2.99 | −20 | 0 | 72 | ||||||||
| 3.62 | 283.00 | 10 | −32 | −40 | Pons | ||||||
| 3.23 | 46.00 | −24 | −70 | −18 | cerebellum | ||||||
Z Score>2.67, p<.005
Number of voxels within the activated cluster
Areas in bold represent the voxel with greatest activation in a contingent group of voxels; non-bold areas are other areas of activation within the same group
When CAD patients without depression were compared to those with depression (i.e. interaction of diagnosis and task), they exhibited relative increases in blood flow with stress versus control tasks in the bilateral middle frontal gyrus, left superior frontal gyrus, caudal anterior cingulate, bilateral middle and left superior temporal gyrus, left fusiform gyrus, hippocampus, uncus, and right inferior parietal lobule, and cerebellum (Table 4, Figure 1). CAD patients without depression compared to those with depression had relative decreases (greater increases in depression) in blood flow in right parahippocampal gyrus, cuneus, posterior cingulate, and left visual cortex (Table 4).
Table 4.
Areas of Greater Increases and Decreases in Blood Flow with Stress Challenge in CAD Patients without Depression (N=15) Compared to CAD Patients with Depression (N=13)
| Increased Blood Flow | Decreased Blood Flow | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z Score* |
Voxel Number** |
Talairach Coordinates | Z Score* |
Voxel Number** |
Talairach Coordinates | ||||||
| x | y | z | Brain Region | x | y | z | Brain Region | ||||
| 5.14 | 249 | −8 | 24 | 64 | L. Sup. Frontal Gyrus (6) | 4.18 | 275 | 22 | −32 | −12 | R. Parahippocampal Gyrus (36) |
| 3.30 | 88 | −24 | −10 | 74 | L. Superior Frontal Gyrus (6) | 3.36 | 279 | 6 | −80 | 26 | Cuneus (19) |
| 3.00 | −24 | 5 | 68 | 3.20 | 104 | 4 | −24 | 32 | Posterior Cingulate (31) | ||
| 2.93 | −26 | −2 | 70 | 3.06 | 49 | −26 | −84 | 12 | L. Visual Cortex (18) | ||
| 3.13 | 45 | 24 | 58 | −14 | R. Middle Frontal Gyrus (11) | ||||||
| 3.29 | 162 | −2 | 42 | −6 | Rostral Anterior Cingulate (32) | ||||||
| 3.43 | 119 | 70 | −34 | −4 | R. Middle Temporal Gyrus (21) | ||||||
| 4.50 | 102 | 66 | −50 | 20 | R. Sup. Temporal Gyrus (21) | ||||||
| 3.35 | 56 | 32 | 22 | −28 | R. Sup. Temporal Gyrus (38) | ||||||
| 3.52 | 202 | −40 | 38 | −20 | L. Fusiform Gyrus (20) | ||||||
| 3.13 | −36 | 44 | −6 | L. Middle Frontal Gyrus (10) | |||||||
| 2.83 | −34 | 34 | −24 | L. Sup. Temporal Gyrus (38) | |||||||
| 3.40 | 107 | −64 | −54 | 4 | L. Middle Temporal Gyrus (21) | ||||||
| 4.14 | 344 | −46 | −18 | −10 | L. Middle Temporal Gyrus (21) | ||||||
| 3.75 | −30 | −28 | −8 | Hippocampus | |||||||
| 3.73 | 113 | −48 | −12 | −42 | Uncus | ||||||
| 3.59 | 122 | 52 | −62 | 38 | R. Inf Parietal Lobule (40) | ||||||
| 2.92 | 56 | −52 | 52 | ||||||||
| 3.35 | 226 | −52 | −36 | −24 | L. Fusiform Gyrus (20) | ||||||
| 3.14 | −48 | −44 | −38 | Cerebellum | |||||||
Z Score>2.67, p<.005
Number of voxels within the activated cluster
Figure 1.
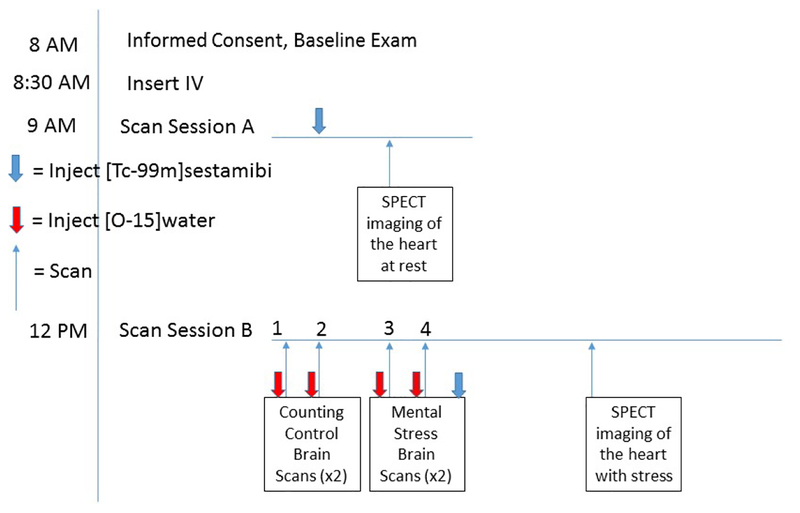
Imaging protocol for Single Photon Emission Computed Tomography (SPECT) imaging of the heart and Positron Emission Tomography (PET) imaging of the brain. Subjects initially undergo injection of [Tc-99m]sestamibi followed by SPECT imaging of the heart at rest, followed by PET imaging of the brain with counting control and mental stress tasks following injection of [O–15]water. At the end of the second mental stress task subjects are injected with [Tc-99m]sestamibi followed by SPECT imaging of the heart with stress. The pharmacodynamics of sestamibi permit delayed imaging of the heart that provides a picture of myocardial activity at the time of stress.
Depressed CAD patients with MSI showed a failure of activation relative to depressed CAD patients without MSI in posterior and caudal anterior cingulate, superior and middle temporal gyrus, insula, cerebellum, midbrain/Periaqueductal gray (PAG) and orbitofrontal cortex (Table 5, Figure 2). Depressed CAD patients with MSI showed a relative increase in precuneus, right inferior parietal lobule, and visual association cortex (Table 5).
Table 5.
Areas of Greater Increases and Decreases in Blood Flow with Stress Challenge in CAD Patients with Depression without (N=8) Compared to those with (N=5) Stress Induced Myocardial Ischemia
| Increased Blood Flow | Decreased Blood Flow | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z Score* |
Voxel Number** |
Talairach Coordinates | Z Score* |
Voxel Number** |
Talairach Coordinates | ||||||
| x | y | z | Brain Region | x | y | z | Brain Region | ||||
| 4.18 | 303 | 10 | −32 | 42 | Posterior Cingulate (31) | 3.42 | 382 | −18 | −52 | 42 | L. Precuneus (7) |
| 3.99 | 1039 | 36 | −12 | 12 | R. Sup Temporal Gyrus(22)/Insula | 2.93 | −14 | −56 | 72 | ||
| 3.58 | 60 | 8 | −2 | R. Sup Temporal Gyrus(22) | 2.73 | −26 | −46 | 50 | |||
| 3.43 | 58 | 12 | −10 | R. Sup Temporal Gyrus (12,38) | 3.32 | 304 | 34 | 30 | 24 | R. Middle Frontal Gyrus (46,9) | |
| 3.99 | 785 | −58 | 14 | −8 | L. Sup Temporal Gyrus (12,38) | 3.10 | 26 | 16 | 34 | ||
| 3.61 | −54 | −8 | 8 | L. Sup Temporal Gyrus (22) | 2.79 | 34 | 24 | 30 | |||
| 3.13 | −50 | −12 | −2 | L. Sup Temporal Gyrus (22) | 3.25 | 272 | 40 | −50 | 40 | R. Inf. Parietal Lobule (40) | |
| 3.17 | 132 | −66 | −18 | −6 | L. Middle Temporal Gyrus (21) | 2.96 | −8 | −48 | 38 | L. Precuneus (7) | |
| 2.51 | −66 | −12 | 0 | 2.94 | −4 | −54 | 26 | L. Precuneus (7) | |||
| 2.96 | 260 | 46 | 46 | −12 | R. Middle Frontal Gyrus (11) | 3.03 | 179 | −6 | −74 | 44 | R. Precuneus (7) |
| 2.91 | 54 | 34 | −14 | 2.73 | −30 | −68 | 40 | Visual Association Ctx (19) | |||
| 2.81 | 44 | 44 | −2 | 2.61 | 4 | −60 | 36 | ||||
| 2.86 | 63 | −20 | −72 | −16 | Cerebellum | 2.91 | 67.00 | 38 | −32 | 80 | Precentral/Postcentral (1,2,3,4 |
| 2.77 | 53 | 0 | 34 | 26 | Caudal Anterior Cingulate (32) | ||||||
| 2.71 | 83 | −10 | −36 | −18 | Midbrain/PAG | ||||||
| 2.77 | 44 | 2 | 38 | −16 | Orbitofrontal (11) | ||||||
Z Score>2.67, p<.005
Number of voxels within the activated cluster
Areas in bold represent the voxel with greatest activation in a contingent group of voxels; non-bold areas are other areas of activation within the same group
Figure 2.
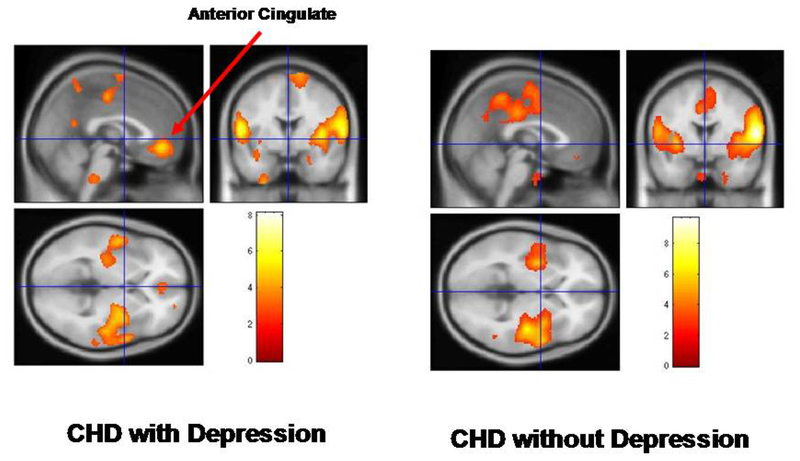
Areas of decreased mental stress-induced brain function in depressed (N=13) compared to non-depressed CAD patients (N=15). There were decreases in bilateral rostral anterior cingulate in depressed CAD patients (left) not seen in non-depressed patients (yellow area indicated by arrow; numbers next to the color bar refer to z scores and areas represented correspond to areas with p<.005 and z score>2.67)
DISCUSSION
When compared to CAD patients without depression, CAD patients with depression showed a failure of activation in brain areas known to be involved in emotion and cognition, including the rostral anterior cingulate (medial prefrontal cortex), hippocampus, and parts of the dorsolateral prefrontal, parietal and temporal cortex, and cerebellum. The depressed CAD patients tended to activate different brain regions during stress, including visual association cortex, posterior cingulate and parahippocampal gyrus. When depressed CAD patients with mental stress-induced myocardial ischemia (MSI) were compared to those without MSI, they showed a pattern of relative failure of activation of the caudal anterior cingulate and parts of the medial prefrontal cortex (orbitofrontal cortex), insula, temporal cortex, and cerebellum. The MSI patients preferentially activated precuneus, visual association and parietal cortex.
In the current study depressed patients with CAD showed a failure of activation in brain areas involved in emotion, including medial prefrontal cortex (rostral anterior cingulate) and hippocampus. In the subgroup of depressed CAD patients with MSI, however, there was a failure of activation with MSI in caudal anterior cingulate, which plays more of a role in attention, and the orbitofrontal cortex (Kringelbach & Rolls, 2004; Vogt, Finch, & Olson, 1992). Previous studies have shown a decrease in orbitofrontal function in depression (Bremner et al., 1997; Bremner, Vythilingam, Ng, et al., 2003; Cheng, et al., 2016; Drevets, et al., 1997; Drysdale, et al., 2016; George, et al., 1997; Mayberg, et al., 1997; Sheline, et al., 2009). Alterations in these brain regions could act through neurohormonal systems to affect cardiovascular responses to stress. We have previously shown an increase in activation in the rostral anterior cingulate with MSI in a sample of CAD patients not selected for the diagnosis of depression (Bremner, et al., 2018). The medial prefrontal cortex, which includes anterior cingulate and orbitofrontal cortex, is involved in emotion and cognition. Activation of the medial prefrontal cortex represents the neural mechanism involved in the extinction of fear (Devinsky, Morrell, & Vogt, 1995; Kosson et al., 2006; Luo et al., 2007; Marsh, Blair, Vythilingam, Busis, & Blair, 2007; Milad & Quirk, 2002; Milad, Rauch, Pitman, & Quirk, 2006; Pardo, Pardo, Janer, & Raichle, 1990; Quirk, 2002; Vogt, et al., 1992; Whalen et al., 1998). This area has inhibitory inputs to the amygdala (Quirk, Garcia, & Gonzalez-Lima, 2006), which plays a central role in the stress response. Activation of the medial prefrontal cortex represents the neural mechanism involved in the extinction of fear (Milad & Quirk, 2002; Milad, et al., 2006; Quirk, 2002). Altered function in this areas could therefore modulate peripheral cardiovascular responses, which could in turn be associated with an increased risk of stress-induced myocardial ischemia (Soufer, 2004; Soufer, Jain, & Yoon, 2009; Vaccarino & Bremner, 2013; Vaccarino & Bremner, 2014, 2015; Vaccarino & Bremner, 2017). The findings suggest that an aberrant brain response to stress could put depressed CAD patients at risk for increased mortality through myocardial ischemia or ventricular abnormality.
In depressed CAD patients with MSI, mental stress was associated with increased activation in the parietal lobe, precuneus and visual association cortex. These brain areas are involved in the processing of visual information and visuospatial memory. The parietal cortex is additionally involved in the perception of the self in space and time (Bremner, Krystal, Southwick, & Charney, 1995; Jonides et al., 1993; Pardo, Fox, & Raichle, 1991; Petersen, Fox, Posner, Mintun, & Raichle, 1988; Zandbelt, Bloemendaal, Neggers, Kahn, & Vink, 2013) as well as modulation of peripheral cardiovascular responses to stress (de Morree, Szabo, Rutten, & Kop, 2013). These processes play a key role in vigilance during stress (Bremner, 2003; Bremner, et al., 1995). Prior studies have shown that altered parietal cortex function is associated with risk for cardiovascular disease (Chuang et al., 2014). Our findings suggest brain areas involved in visual and spatial processing that are involved in the stress response could underlie stress-induced ischemia in some vulnerable patients with depression and CAD.
The current findings support prior findings that the medial prefrontal cortex and limbic brain areas are involved in stress-induced cardiovascular responses. Studies in healthy human subjects have correlated cardiovascular responses to stress with activation in the amygdala (Gianaros, et al., 2008; Gianaros, et al., 2004; Lane, McRae, et al., 2009), hippocampus (Gianaros, et al., 2004; Lane, McRae, et al., 2009), prefrontal cortex (Jennings, et al., 2009; Pollatos, et al., 2007; Tanida, et al., 2004), medial prefrontal / anterior cingulate and posterior cingulate (Bremner, et al., 2018; Gianaros, Derbyshire, et al., 2005; Gianaros, et al., 2007; Gianaros, May, et al., 2005; Gianaros, et al., 2004; Lane, McRae, et al., 2009), insula (Critchley, et al., 2000; Critchley, et al., 2001; Gianaros, Derbyshire, et al., 2005; Gianaros, et al., 2004; Lane, McRae, et al., 2009) temporal cortex (Critchley, et al., 2000), and cerebellum (Critchley, et al., 2000; Gianaros, Derbyshire, et al., 2005). These brain areas have also been implicated in CAD patients with stress (Bremner, et al., 2018; Soufer, et al., 1998) and silent ischemia (Rosen, et al., 1996). In summary, studies to date suggest a role for anterior cingulate, amygdala, insula and hippocampus in stress-induced myocardial ischemia.
Patients with CAD and depression showed a decrease in function with mental stress in the cerebellum, and this effect was greater for patients with depressed CAD patients with MSI. In addition to coordination of movement, the cerebellum has an under-recognized role in social and emotional processing and regulation (Adamaszek et al., 2017; Hoche, Guell, Sherman, Vangel, & Schmahmann, 2016; Leiner, Leiner, & Dow, 1989; Schutter & van Honk, 2009). Cerebellar activation has also been correlated with increased blood pressure and heart rate during mental stress (Critchley, et al., 2000). A failure in this brain region to mount a successful response to stress may contributes to maladaptive cardiovascular responses to stress.
There are several limitations to the current study. No age-matched healthy volunteers without CAD and depression were included for comparison, therefore conclusions about the effects of stress on the brain that are specific to patients with CAD cannot be made. More of the CAD patients with depression were on antidepressants than those without depression. However patients had not responded to antidepressants, and our prior studies have shown that antidepressants increase anterior cingulate function, not decrease it, as in the current study (Bremner, Vythilingam, Vermetten, & Charney, 2007). The current study was designed to study patients with CAD and current clinically significant symptoms of depression, therefore given the fact that most were on antidepressants they represent an essentially treatment-resistant population. This sample was older and had more men than typical samples of depressed patients, and therefore the results are not generalizable to all typical populations of patients with depression. CAD is seen in older populations and is more common in men than women, however, so the current sample is more representative of the subpopulation of depressed patients with co-morbid CAD. There was a high co-morbidity of depression with other psychiatric disorders, particularly anxiety disorders and past histories of substance abuse. This is likely due to the fact that CAD patients had severe depression and many had abuse histories, both of which are associated with increased co-morbidity (Bauer et al., 2005; Blanchard, Buckley, Hickling, & Taylor, 1998; Kessler, et al., 2003; Kessler, Chiu, Demler, Merikangas, & Walters, 2005; Sareen et al., 2007). Additionally, cardiovascular disease is associated with increased rates of both depression and anxiety disorders (Huang, Su, Chen, Chou, & Bai, 2009; Kendler, Gardner, Fiske, & Gatz, 2009). Eliminating co-morbidities might have created a population which is not representative of patients with severe depression in the community. This study had a limited sample size, and since there were only a small number of CAD patients without depression who had MSI, it was not possible to assess the effects of MSI on brain function in patients both with and without depression. Additionally, the number of depressed patients with MSI was limited. Future studies with larger sample sizes are needed to assess the relationship between MSI and brain changes in CAD patients with depression. The aims of this study were to assess the effects of stress on the brain. Difficulty of mental stress was adjusted to patient ability in order to maximize stress. Since previous studies have shown that patients with depression show deficits in cognitive function (Bremner, Vythilingam, et al., 2007; Burt, Zembar, & Niederehe, 1995; Cohen, Weingartner, Smallberg, Pickar, & Murphy, 1982; Danion et al., 1991; Sternberg & Jarvik, 1976; Vythilingam et al., 2004) it is possible that patients performed worse on the cognitive tests, and that differences in neural responses to cognitive stress tasks contributed to differences in group results. This was a pilot study and we did not include study groups of depressed patients without heart disease or without either condition. Future studies should make such comparisons. Future studies should also look at the effects of stress reduction interventions on brain response.
In conclusion, CAD patients with depression showed stress-induced alterations in brain areas involved in stress and emotion, including a failure of rostral anterior cingulate activation. Depressed CAD patients with MSI compared to those without showed decreased activation in caudal anterior cingulate and posterior cingulate. Altered brain response to stress in patients with CAD and depression could act through cardiovascular and neurohormonal pathways, thereby increasing the risk of stress-induced myocardial ischemia in these patients. Furthermore, based on comparisons with our prior studies, brain mechanisms of MSI in CAD patients with depression may differ from those in non-depressed populations of CAD patients.
Figure 3.
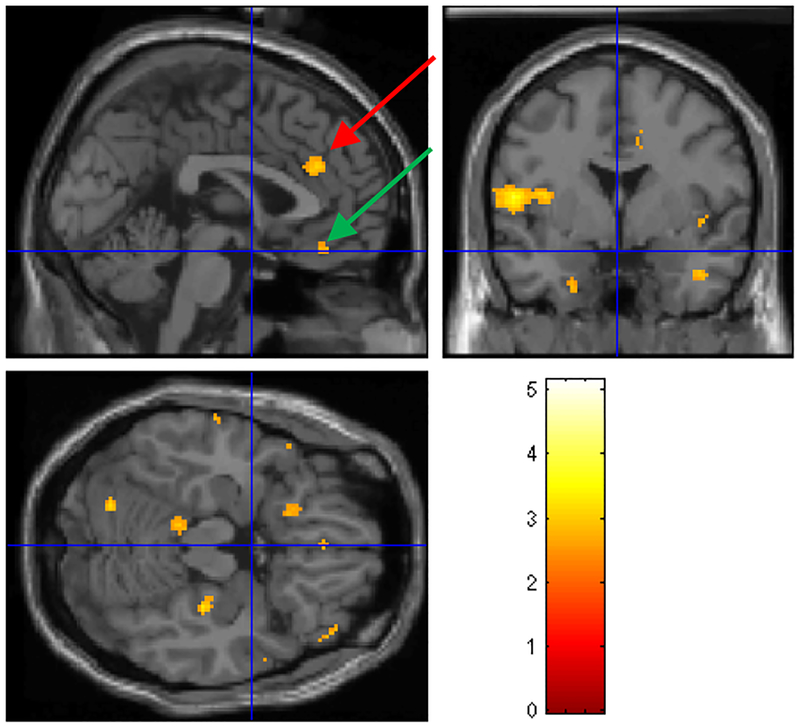
Brain activation with stress in depressed patients without stress induced myocardial ischemia (N=8) compared to depressed patients with stress induce myocardial ischemia (N=5). There was a relative failure of activation (yellow areas) in depressed CAD patients with stress-induced myocardial ischemia in bilateral caudal anterior cingulate (yellow area indicated by red arrow), superior temporal gyrus, orbitofrontal cortex (yellow area indicated by green arrow), and cerebellum. Numbers next to the color bar refer to z scores and areas represented correspond to areas with p<.005 and z score>2.67.
ACKNOWLEDGEMENTS:
This study was supported by the Charles A. Dana Foundation and NIH research grants to JDB R01 HL088726, K24 MH076955, T32 MH067547–01, and R01 MH56120. VV receives research funding support from NIH R01 HL66287–01 and Aetna Health Foundation.
REFERENCES
- Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, … Verhoeven J (2017). Consensus Paper: Cerebellum and Emotion. The Cerebellum, 16(2), 552–576. doi: doi: 10.1007/s12311-016-0815-8 [DOI] [PubMed] [Google Scholar]
- Arri SS, Ryan M, Redwood SR, & Marber MS (2016). Mental stress-induced myocardial ischaemia. Heart, 102, 472–480. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Altshuler L, Evans DR, Beresford T, Williford WO, & Hauger R (2005). Prevalence and distinct correlates of anxiety, substance, and combined comorbidity in a multi-site public sector sample with bipolar disorder. J Affect Disord, 85(3), 301–315. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Buckley TC, Hickling EJ, & Taylor AE (1998). Posttraumatic stress disorder and comorbid major depression: Is the correlation an illusion? Journal of Anxiety Disorders, 12, 1–37. [DOI] [PubMed] [Google Scholar]
- Bremner J, Bolus R, & Mayer E (2007). Psychometric properties of the Early Trauma Inventory-Self Report. Journal of Nervous and Mental Disease, 195(3), 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD (2002). Structural changes in the brain in depression and relationship to symptom recurrence. CNS Spectrums, 7, 129–139. [DOI] [PubMed] [Google Scholar]
- Bremner JD (2003). Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacology Bulletin, 37(2), 6–25. [PubMed] [Google Scholar]
- Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, …Vaccarino V (2018). Brain correlates of mental stress-induced myocardial ischemia. Psychosomatic Medicine, 80(6), 515–525. doi: doi: 10.1097/PSY.0000000000000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Cheema FA, Ashraf A, Afzal N, Fani N, Reed J, … Vaccarino V (2009). Effects of a cognitive stress challenge on myocardial perfusion and plasma cortisol in coronary heart disease patients with depression. Stress and Health, 25, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Salomon RM, Staib L, Ng CK, Miller HL, … Charney DS (1997). Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Archives of General Psychiatry, 54, 364–374. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS (1995). Functional neuroanatomical correlates of the effects of stress on memory. Journal of Traumatic Stress, 8, 527–554. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS (1996a). Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse, 23, 28–38. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS (1996b). Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse, 23, 39–51. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, & Charney DS (1999). Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. The American Journal of Psychiatry, 156, 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Ng CK, Vermetten E, Nazeer A, Oren DA, … Charney DS (2003). Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: implications for the neural circuitry of depression. JAMA, 289(23), 3125–3134. doi: 10.1001/jama.289.23.3125289/23/3125 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, … Charney DS (2003). Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology, 28, 733–750. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, & Charney DS (2007). Effects of antidepressant treatment on neural correlates of emotional and neutral declarative verbal memory in depression. Journal of Affective Disorders, 101(1–3), 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MM, Jain D, Soufer R, Kerns RD, & Zaret BL (1993). Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol, 22(2), 440–448. [DOI] [PubMed] [Google Scholar]
- Burg MM, Meadows J, Shimbo D, Davidson KW, Schwartz JE, & Soufer R (2014). Confluence of depression and acute psychological stress among patients with stable coronary heart disease: effects on myocardial perfusion. Journal of the American Heart Association, 3(6), e000898. doi: 10.1161/JAHA.114.000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MM, & Soufer R (2014). Psychological stress and induced ischemic syndromes. Curr Cardiovasc Risk Rep, 8(4), 377. doi: 10.1007/s12170-014-0377-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, & Niederehe G (1995). Depression and memory impairment: A meta-analysis of the association, its pattern, and specificity. Psychological Bulletin, 117, 285–305. [DOI] [PubMed] [Google Scholar]
- Carney RM, & Freedland KE (2017). Depression and coronary heart disease. Nature Reviews Cardiology, 14, 145–155. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, & Veith RC (2005). Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med, 67(Supplement_1), S29–33. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Davies BM, Mendels J, & Sugarman AA (1976). Urinary free cortisol excretion in depression. Journal of Psychology and Medicine, 6, 43–50. [DOI] [PubMed] [Google Scholar]
- Charney DS, Sklar P, Buxbaum JD, & Nestler EJ (Eds.). (2014). Neurobiology of Mental Illness. 4th Edition (4 ed.) Oxford, U.K.: Oxford University Press. [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, … Feng J (2016). Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain, 139(Pt 12), 3296–3309. [DOI] [PubMed] [Google Scholar]
- Chuang Y-F, Eldreth D, Erickson KI, Varma V, Harris G, Fried LP, … Carlson MC (2014). Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiology of Aging, 35(6), 1396–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RM, Weingartner H, Smallberg SA, Pickar D, & Murphy DL (1982). Effort and cognition in depression. Archives of General Psychiatry, 145, 164–167. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, & Dolan RJ (2000). Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. Journal of Physiology (London), 523, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, & Dolan RJ (2001). Neural correlates of first and second-order representation of bodily states. Nature Neuroscience, 4, 207–212. [DOI] [PubMed] [Google Scholar]
- Danion J-M, Willard-Schroeder D, Zimmerman M-A, Grange D, Schlienger J-L, & Singer L (1991). Explicit memory and repetition priming in depression. Archives of General Psychiatry, 48, 707–711. [DOI] [PubMed] [Google Scholar]
- de Morree HM, Szabo BM, Rutten G-J, & Kop WJ (2013). Central nervous system involvement in the autonomic responses to psychological distress. Netherlands Heart Journal, 21, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado PL, & Moreno FA (2000). Role of norepinephrine in depression. Journal of Clinical Psychiatry, 61(Supp 1), S5–12. [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, & Vogt BA (1995). Contributions of anterior cingulate to behavior. Brain, 118, 279–306. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JRJ, Todd RD, Reich T, Vannier M, & Raichle ME (1997). Subgenual prefrontal cortex abnormalities in mood disorders. Nature, 386, 824–827. [DOI] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, … Liston C (2016). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. [Article]. Nature Medicine, 23, 28. doi: 10.1038/nm.424610.1038/nm.4246https://www.nature.com/articles/nm.4246#supplementary-informationhttps://www.nature.com/articles/nm.4246#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, & Gibbon M (1995). Structured Clinical Interview for DSMIV-Patient Edition (SCID-P). Washington, D.C.: American Psychiatric Press. [Google Scholar]
- Freedland KE, & Carney RM (2013). Depression as a risk factor for adverse outcomes in coronary heart disease. BMC Med, 11, 131. doi: 10.1186/1741-7015-11-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (1994). Statistical parametric mapping In Thatcher R, Hallett M, Zeffiro T, John E & Huerta M (Eds.), Functional Neuroimaging: Technical Foundations. (pp. 79–93). San Diego: Academic Press. [Google Scholar]
- Friston KJ, Frith C, Liddle P, & Frackowiak R (1991). Comparing functional (PET) images: the assessment of significant change. Journal of Cerebral Blood Flow and Metabolism, 11, 690–699. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, & Neafsey EJ (1994). The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Research, 643, 181–193. [DOI] [PubMed] [Google Scholar]
- Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J, & Santana C (2007). The increasing role of quantification in clinical nuclear cardiology: the Emory approach. Journal of Nuclear Cardiology, 14, 420–432. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, … Post RM (1997). Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop). Journal of Neuropsychiatry & Clinical Neurosciences, 9(1), 55–63. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, & Post RM (1994). Prefrontal cortex dysfunction in clinical depression. Depression, 2, 59–72. [Google Scholar]
- Gianaros PJ, Derbyshire SWG, May JC, Siegle GJ, Gamalo MA, & Jennings JR (2005). Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology, 42, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SWG, & Matthews KA (2007). Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension, 49, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, May JS, Siegle GJ, & Jennings JR (2005). Is there a functional neural correlate of individual differences in cardiovascular reactivity? Psychosomatic Medicine, 67, 31–39. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Jennings JR, Manuck SB, & Hariri AR (2008). Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. The Journal of Neuroscience, 28(4), 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Van der Veen FM, & Jennings JR (2004). Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology, 41, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, … Galluci W (1986). Response to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s Disease. New England Journal of Medicine, 314, 1329–1335. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry, 12, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, … Quyyumi AA (2017). The Mental Stress Ischemia Prognosis Study (MIPS): Objectives, study design, and prevalence of inducible ischemia. Psychosomatic Medicine, 79(3), 311–317. doi: 10.1097/000000000000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, … Quyyumi AA (2017). Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. International Journal of Cardiology, 243, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekens CH (1998). Increasing burden of cardiovascular disease. Current knowledge and future directions for research on risk factors. Circulation, 97, 1095–1102. [DOI] [PubMed] [Google Scholar]
- Hoche F, Guell X, Sherman JC, Vangel MG, & Schmahmann JD (2016). Cerebellar contribution to social cognition. The Cerebellum, 15(6), 732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KL, Su TP, Chen TJ, Chou YH, & Bai YM (2009). Comorbidity of cardiovascular diseases with mood and anxiety disorder: a population based 4-year study. Psychiatry Clin Neurosci, 63(3), 401–409. doi: 10.1111/j.1440-1819.2009.01974.x [DOI] [PubMed] [Google Scholar]
- Jennings JR, Van der Molen MW, & Tanase C (2009). Preparing hearts and minds: Cardiac slowing and a cortical inhibitory network. Psychophysiology, 46, 1170–1178. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, & Mintun MA (1993). Spatial working memory in humans as revealed by PET. Nature, 363, 623–625. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Fiske A, & Gatz M (2009). Major depression and coronary artery disease in the Swedish Twin Registry: phenotypic, genetic, and environmental sources of comorbidity. Archives of General Psychiatry, 66(8), 857–863. doi: 10.1001/archgenpsychiatry.2009.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, & Gardner CO (2000). Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. American Journal of Psychiatry, 157, 1243–1251. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, … Wang PS (2003). The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). Journal of the American Medical Association, 289, 3095–3105. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Archives of General Psychiatry, 62(6), 617–627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, & Nelson CB (1995). Posttraumatic stress disorder in the national comorbidity survey. Archives of General Psychiatry, 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Budhani S, Nakic M, Chen G, Saad ZS, Vythilingam M, … Blair RJ (2006). The role of the amygdala and rostral anterior cingulate in encoding expected outcomes during learning. NeuroImage, 29(4), 1161–1172. doi: 10.1016/j.neuroimage.2005.07.060 [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, & Rolls ET (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72, 341–372. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, & Thayer JF (2009). Neural correlates of heart rate variability during emotion. NeuroImage, 44, 213–222. [DOI] [PubMed] [Google Scholar]
- Lane RD, Waldstein SR, Chesney MA, Jennings JR, Lovallo WR, Kozel PJ, … Cameron OG (2009). The rebirth of neuroscience in psychosomatic medicine, part I: Historical context, methods, and relevant basic science. Psychosomatic Medicine, 71, 117–134. [DOI] [PubMed] [Google Scholar]
- Lane RD, Waldstein SR, Critchley HD, Derbyshire SWG, Drossman DA, Wager TD, … Cameron OG (2009). The rebirth of neuroscience in psychosomatic medicine, part II: Clinical applications and implications for research. Psychosomatic Medicine, 71, 135–151. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, & Dow RS (1989). Reappraising the cerebellum: What does the hindbrain contribute to the forebrain? Behavioral Neuroscience, 103(5), 998–1008. [DOI] [PubMed] [Google Scholar]
- Lichtman JH, Bigger JT Jr., Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, … Froelicher ES (2008). Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation, 118(17), 1768–1775. doi: 10.1161/circulationaha.108.190769 [DOI] [PubMed] [Google Scholar]
- Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, … American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing. (2014). Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation, 129(12), 1350–1369. doi: 10.1161/CIR.0000000000000019 [DOI] [PubMed] [Google Scholar]
- Luo Q, Mitchell D, Jones M, Mondillo K, Vythilingam M, & Blair RJ (2007). Common regions of dorsal anterior cingulate and prefrontal-parietal cortices provide attentional control of distracters varying in emotionality and visibility. NeuroImage, 38(3), 631–639. doi: 10.1016/j.neuroimage.2007.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Blair KS, Vythilingam M, Busis S, & Blair RJ (2007). Response options and expectations of reward in decision-making: the differential roles of dorsal and rostral anterior cingulate cortex. NeuroImage, 35(2), 979–988. doi: 10.1016/j.neuroimage.2006.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, … Fox PT (1997). Cingulate function in depression: a potential predictor of treatment response. Neuroreport, 8, 1057–1061. [DOI] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature, 420, 70–73. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, & Quirk GJ (2006). Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol, 73(1), 61–71. [DOI] [PubMed] [Google Scholar]
- Otte C, Marmar CR, Pipkin SS, Moos R, Browner WS, & Whooley MA (2004). Depression and 24-hour urinary cortisol in medical outpatients with coronary heart disease: The Heart and Soul Study. Biological Psychiatry, 56, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, & Raichle ME (1991). Localization of a human system for sustained attention by positron emission tomography. Nature, 349, 61–64. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, & Raichle ME (1990). The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences USA, 87, 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun MA, & Raichle ME (1988). Positron emission tomographic studies of the cortical anatomy of single word processing. Nature, 331, 585–589. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, & Kaufmann C (2007). Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research 1141, 178–187. [DOI] [PubMed] [Google Scholar]
- Price JL, & Drevets WC (2012). Neural circuits underlying the pathophysiology ofmood disorders. Trends in Cognitive Science, 16, 61–71. [DOI] [PubMed] [Google Scholar]
- Quirk GJ (2002). Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learning & Memory, 9, 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, & Gonzalez-Lima F (2006). Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry, 60(4), 337–343. [DOI] [PubMed] [Google Scholar]
- Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, & Sheps DS (2006). Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. Journal of the American College of Cardiology, 47(5), 987–991. [DOI] [PubMed] [Google Scholar]
- Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, & Quyyumi AA (2013). Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. Journal of the American Heart Association, 2, e000321. doi: 10.1161/JAHA.113.000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, … Chen K (1997). Neuroanatomical correlates of externally and internally generated human emotion. The American Journal of Psychiatry, 154(7), 918–925. [DOI] [PubMed] [Google Scholar]
- Rosen SD, Paulesu E, Nihoyannopoulos P, Tousoulis D, Frackowiak RSJ, Frith CD, … Camici PG (1996). Silent ischemia as a central problem: Regional brain activation compared in silent and painful myocardial ischemia. Annals of Internal Medicine, 124(11), 939–949. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Stein MB, Afifi TO, Fleet C, & Asmundson GJ (2007). Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med, 69(3), 242–248. [DOI] [PubMed] [Google Scholar]
- Schutter DJLG, & van Honk J (2009). The cerebellum in emotion regulation: A repetitive transcranial magnetic stimulation study. The Cerebellum, 8(1), 28–34. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barcha DM, Price JL, Rundleb MM, Vaishnavib SN, Snyderb AZ, … Raichle ME (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheps DS, McMahon RP, Becker L, Carney RM, Freedlan KE, Cohen JD, … Kaufmann PG (2002). Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia Study. Circulation, 105, 1780–1784. [DOI] [PubMed] [Google Scholar]
- Soufer R (2004). Neurocardiac interaction during stress-induced myocardial ischemia: How does the brain cope? Circulation, 110, 1710–1713. [DOI] [PubMed] [Google Scholar]
- Soufer R, Bremner JD, Arrighi JA, Cohen I, Zaret BL, Burg MM, & Goldman-Rakic P (1998). Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proceedings of the National Academy of Sciences of the United States of America, 95, 6454–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufer R, Jain H, & Yoon AJ (2009). Heart-brain interactions in mental stress-induced myocardial ischemia. Current Cardiology Reports, 11, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg DE, & Jarvik ME (1976). Memory functions in depression. Archives of General Psychiatry, 33, 219–224. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, … Vaccarino V (2018). Sex differences in hemodynamic and microvascular mechanisms of myocardial ischemia induced by mental stress. Arteriosclerosis, Thrombosis, and Vascular Biology, 38(2), 473–480. doi: doi: 10.1161/ATVBAHA.117.309535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, & Tournoux J (1988). Co-Planar Atlas of the Human Brain. New York: Thieme Medical Publishers; Tanaka M, Yoshida M, Emoto H, & Ishii H (2000). Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies European Journal of Pharmacology, 405, 397–406. [DOI] [PubMed] [Google Scholar]
- Tanida M, Sakatani K, Takano R, & Tagai K (2004). Relation between asymmetry of prefrontal cortex activities and the autonomic nervous system during a mental arithmetic task: Near infrared spectroscopy study. Neuroscience Letters, 369, 69–74. [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 33, 81–88. [DOI] [PubMed] [Google Scholar]
- Traskman L, Tybring G, Asberg M, Bertilsson L, Lantto O, & Schalling D (1980). Cortisol in the CSF of depressed and suicidal patients. Archives of General Psychiatry, 37, 761–767. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, & Bremner JD (2013). Traumatic stress is heartbreaking. Biol Psychiatry, 74(11), 790–792. doi: 10.1016/j.biopsych.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, & Bremner JD (2014). Psychiatric and behavioral aspects of cardiovascular disease In Bonow RO, Mann DL, Zipes OP & Libby P (Eds.), Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. (9th ed., Vol. 2). Philadelphia, PA: Saunders. [Google Scholar]
- Vaccarino V, & Bremner JD (2015). Posttraumatic Stress Disorder and Risk of Cardiovascular Disease In Alvarenga M & Byrne D (Eds.), Handbook of Psychocardiology. Singapore: Springer. [Google Scholar]
- Vaccarino V, & Bremner JD (2017). Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neuroscience & Biobehavioral Reviews, 74((Pt B)), 297–309. doi: 10.1016/j.neubiorev.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, …. Bremner JD (2013). Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. Journal of the American College of Cardiology, 62(11), 97–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, … Raggi P (2014). Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosomatic Medicine, 76(3), 171–180. doi: 10.1161/JAHA.116.003630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, … Raggi P (2018). Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: Sex differences and mechanisms. Circulation, 137, 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Votaw J, Faber T, Veledar E, Murrah NV, Jones LR, … Bremner JD (2009). Major depression and coronary flow reserve detected by positron emission tomography. Archives of Internal Medicine, 169, 1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, … Quyyumi AA (2016). Sex differences in mental stress-Induced myocardial ischemia in patients with coronary heart disease. Journal of the American Heart Association, 5(9), pii: e003630. doi: doi: 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Train KF, Garcia EV, Maddahi J, Areeda J, Cooke CD, Kiat H, … Matzer L (1994). Multicenter trial validation for quantitative analysis of same-day rest-stress technetium-99m-sestamibi myocardial tomograms. Journal of Nuclear Medicine, 35, 609–618. [PubMed] [Google Scholar]
- Vogt BA, Finch DM, & Olson CR (1992). Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex, 2, 435–443. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, … Bremner JD (2004). Hippocampal volume, memory and cortisol status in major depressive disorder: Effects of treatment. Biological Psychiatry, 56, 101–112. [DOI] [PubMed] [Google Scholar]
- Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, … Vaccarino V (2014). Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. Plos One, 9, e102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, … Vaccarino V (2014). Meta-analysis of mental sress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. American Journal of Cardiology, 114(2), 187–192. doi: 10.1016/j.amjcard.2014.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, & Rauch SL (1998). The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry, 44, 1219–1228. [DOI] [PubMed] [Google Scholar]
- Willner P, Scheel-Krüger J, & Belzungc C (2013). The neurobiology of depression and antidepressant action. Neuroscience & Biobehavioral Reviews, 37(10), 2331–2371. [DOI] [PubMed] [Google Scholar]
- Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, … Champion HC (2005). Neurohumoral features of myocardial stunning due to sudden emotional stress. New England Journal of Medicine, 352(6), 539–548. [DOI] [PubMed] [Google Scholar]
- Yehuda R (2002). Post-traumatic stress disorder. New England Journal of Medicine, 346, 108–114. [DOI] [PubMed] [Google Scholar]
- Yehuda R (2006). Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Annals of the New York Academy of Sciences, 1071, 137–166. [DOI] [PubMed] [Google Scholar]
- Young EA, Haskett RF, & Grunhaus L (1994). Increased circadian activation of the hypothalamic pituitary adrenal axis in depressed patients in the evening. Archives of General Psychiatry, 51, 701–707. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Neggers SFW, Kahn RS, & Vink M (2013). Expectations and violations: delineating the neural network of proactive inhibitory control. Human Brain Mapping, 34, 2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]