Abstract
Purpose
Retinitis pigmentosa is a family of genetic diseases inducing progressive photoreceptor degeneration. There is no cure for retinitis pigmentosa, but prospective therapeutic strategies are aimed at restoring or substituting retinal input. Yet, it is unclear whether the visual cortex of retinitis pigmentosa patients retains plasticity to react to the restored visual input.
Methods
To investigate short-term visual cortical plasticity in retinitis pigmentosa, we tested the effect of short-term (2 hours) monocular deprivation on sensory ocular dominance (measured with binocular rivalry) in a group of 14 patients diagnosed with retinitis pigmentosa with a central visual field sparing greater than 20° in diameter.
Results
After deprivation most patients showed a perceptual shift in ocular dominance in favor of the deprived eye (P < 0.001), as did control subjects, indicating a level of visual cortical plasticity in the normal range. The deprivation effect correlated negatively with visual acuity (r = −0.63, P = 0.015), and with the amplitude of the central 18° focal electroretinogram (r = −0.68, P = 0.015) of the deprived eye, revealing that in retinitis pigmentosa stronger visual impairment is associated with higher plasticity.
Conclusions
Our results provide a new tool to assess the ability of retinitis pigmentosa patients to adapt to altered visual inputs, and suggest that in retinitis pigmentosa the adult brain has sufficient short-term plasticity to benefit from prospective therapies.
Keywords: retinitis pigmentosa, plasticity, binocular rivalry, psychophysics
Retinitis pigmentosa (RP) is a heterogeneous group of inherited retinal diseases caused by the progressive loss of retinal photoreceptors.1,2 RP affects approximately 1 in 3500 to 4000 people in the United States and Europe,3 and eventually leads to blindness. RP commonly starts with night blindness and peripheral field losses, then progressively affects the central cones, which mediate some of the most specialized visual functions, including visual acuity and fine spatial discrimination.4 There is currently no cure for RP, but much hope is generated by research aimed at reinstating or replacing photoreceptor function.5–16 To this purpose, it is important to evaluate the residual plasticity of the system downstream of photoreceptors in adult age, in order to explore its potential to react to a restored visual input.
Anatomic studies have shown a good preservation of the visual pathways from the retina to the visual cortex,17–20 but much less is known about the visual cortex in RP. In patients with advanced stages of the pathology, abnormal BOLD signals are evoked in the occipital cortex by other nonvisual stimuli (e.g., tactile signals), suggesting a deep reorganization of the circuitry.21–28 However, strong visual stimuli, although not consciously perceived, are still able to drive a BOLD response in late blind RP patients,29 suggesting that visual information can still reach the cortex. Two studies28,29 have compared BOLD responses in the visual cortex before and after retinal prosthetic implants and demonstrated that a degree of visual cortical plasticity is indeed possible in adults who have experienced complete blindness for several years. Evidence for plasticity is also available for adult patients with residual central vision, whose occipital cortex undergoes an apparent reorganization of retinotopic maps; in these patients, the representation of the central visual field spreads over the peripheral region, which is deprived of its normal visual input30,31; there are also structural changes, affecting cortical thickness32; however, the mechanisms underlying this reorganization are debated.33 In addition, these patients show evidence for cross-modal plasticity,28 whereby the deafferented peripheral representation may become responsive to nonvisual signals, and these signals may interact competitively with visual signals from the spared visual field.
While these studies demonstrate that the early visual cortex reorganizes its function and retinotopy after early retinal deficit, they do not indicate whether the reorganization is beneficial. In principle, it could be a maladaptive form of plasticity, which could hinder the possibility to restore visual abilities, for example, with retinal prostheses,34 by reducing the ability of the visual cortex to adjust to short-term visual changes. To address this issue, here we explored the degree of perceptual flexibility after eliciting a form of short-term plasticity of sensory eye dominance in response to a brief monocular visual deprivation. We did so in RP subjects at different stages of the disease and variable degrees of visual deficit, and asked whether short-term plasticity is reduced in RP patients compared to normally sighted controls.
Like in prior studies on short-term monocular deprivation, we quantified sensory eye dominance through measures of binocular rivalry. This phenomenon occurs in the early visual cortex when two dissimilar visual stimuli are simultaneously presented to either eye; despite the constant retinal stimulation, visual perception oscillates between the two monocular images, producing ineluctable perceptual alternations.35–37 Binocular rivalry may be used to measure perceptual ocular dominance, by computing the perceptual dominance time of the stimuli presented to either eye.38–40 Normally, the two eyes are balanced. In the developing brain,41–43 ocular dominance can be plastically changed by monocular deprivation, leading to a weakening of the deprived eye. In adult humans, if one of the two eyes is transiently (2–2.5 hours) deprived of visual information, the opposite occurs: on eye-patch removal the deprived eye dominates visual rivalrous perception for twice as long as the nondeprived eye.44–48 This paradoxical boost of the deprived eye is measurable for up to 90 minutes after re-exposure to binocular vision, and is thought to be mediated by a compensatory upregulation of contrast gain of the deprived eye, reflecting homeostatic plasticity.44–48 In amblyopic patients, the inverse patching effect, when endorsed for several weeks, can last over several months,49 suggesting that it is subtended by a genuine cortical reorganization. Recent studies have found that this form of homeostatic plasticity in normally sighted subjects also occurs for collaborative binocular interactions (after short-term deprivation, the deprived eye dominates in a binocular combination task50–52). Interestingly, evidence indicates that short-term monocular deprivation modulates neural activity as early as the primary visual cortex,47,53,54 accompanied by a decrease of GABA concentration (measured by magnetic resonance spectroscopy) in V1, which correlates with the perceptual boost of the deprived eye.47 These results indicate that short-term monocular deprivation acts by altering the excitation/inhibition balance in the primary visual cortex, one of the key mechanisms underlying visual plasticity.55–57
Methods
RP Patients
A group of 14 Italian RP patients (8 females; mean age, 46 ± 3 years; mean visual acuity, 0.3 ± 0.06 logMAR; mean visual field diameter: deprived eye: 64.6° ± 12.2°, nondeprived eye: 63.7° ± 12.3°) from central and southern Italy, were selected from the database of patients clinically followed up at the Visual Electrophysiology Service of the Institute of Ophthalmology at Università Cattolica del Sacro Cuore, Rome, Italy. Patients had sought consultation because of visual symptoms. All patients had progressive forms of RP based on history, clinical findings, and ERG abnormalities. Table 1 summarizes individual patient baseline features. All clinical measures were obtained in Rome, Italy, at the Visual Electrophysiology Service of the Institute of Ophthalmology at Università Cattolica del Sacro Cuore; the psychophysical visual measures were performed in Pisa, Italy, within 2 months of the clinical assessment.
Table 1. Clinical Data.
| Patient | Sex | Age at Visit, y | Onset/Diagnosis, y | Disease Duration, y | fERG Amplitude Right Eye, mV | fERG Amplitude Left Eye, mV | BCVA Right Eye, logMAR | BCVA Left Eye, logMAR | CV V4e Diameter Right Eye, deg | CF V4e Diameter Left Eye, deg | Contrast Sensitivity Right Eye, log | Contrast Sensitivity Left Eye, log |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 45 | 38 | 7 | 0.77 | 0.96 | −0.04 | 0.02 | 22 | 23 | 1.84 | 1.84 |
| 2 | F | 47 | 25 | 22 | 0.1 | 0.18 | 0.68 | 0.58 | 112 | 108 | 1.16 | 0.76 |
| 3 | F | 36 | 18 | 18 | 0.25 | 0.41 | 0.1 | 0.1 | 41 | 38 | 1.80 | 1.80 |
| 4 | F | 26 | 14 | 12 | 0.44 | 0.36 | 0.0 | −0.02 | 70 | 60 | 1.76 | 1.80 |
| 5 | F | 47 | 20 | 27 | N.A. | N.A. | 0.14 | 0.08 | 131 | 115 | 1.56 | 1.48 |
| 6 | M | 30 | 18 | 12 | 0.16 | 0.12 | 0.26 | 0.3 | 88 | 105 | 1.08 | 1.24 |
| 7 | M | 56 | 6 | 50 | 0.06 | 0.54 | 0.46 | 0.36 | 26 | 22 | 1.44 | 1.08 |
| 8 | M | 42 | 27 | 15 | 0.04 | 0.03 | 1.0 | 0.7 | 100 | 90 | 0.80 | 0.80 |
| 9 | M | 52 | 9 | 43 | 0.75 | 0.56 | 0.14 | 0.22 | 140 | 140 | 1.84 | 1.80 |
| 10 | F | 37 | 20 | 17 | 0.27 | 0.56 | 0.44 | 0.44 | 22 | 22 | 0.96 | 0.80 |
| 11 | F | 51 | 16 | 35 | N.A. | N.A. | 0.3 | 0.4 | 20 | 20 | 1.84 | 1.80 |
| 12 | M | 68 | 48 | 20 | 0.3 | 0.26 | 0.2 | 0.3 | 113 | 65 | 1.84 | 1.80 |
| 13 | F | 49 | 15 | 34 | 0.25 | 0.11 | 0.4 | 0.4 | 30 | 30 | 1.44 | 1.08 |
| 14 | M | 63 | 20 | 43 | 0.16 | 0.27 | 0.4 | 0.4 | 22 | 22 | 1.44 | 1.44 |
N.A., not available.
Inclusion Criteria
Patients met the following inclusion criteria: (1) typical RP with a rod-cone pattern of retinal dysfunction, as determined by standard Ganzfeld electroretinography, dark-adapted Tuebingen perimetry, and classic fundus appearance; (2) visual field by Goldmann V/4e >20°; (3) known inheritance pattern and genotype under study; (4) no or minimal ocular media opacities; and (5) no concomitant ocular (e.g., glaucoma, amblyopia) or systemic diseases. Patients with non-Usher syndromic subtypes of RP, Leber’s congenital amaurosis, or early-onset RP with atypical functional patterns were not included. Patients’ genetic data are reported in Table 2.
Table 2. Genetic Data.
| Patient | RP Type Clinical Diagnosis | Mutated Gene | Nucleotide Change | Amino Acid Change | Allele State | Transmission | refSNP |
|---|---|---|---|---|---|---|---|
| 1 | Isolated | ||||||
| 2 | Isolated | ||||||
| 3 | Nonsyndromic Usher 2 | USH2A | c.9815C>T | p.Pro3272Leu | Hom | AR | |
| 4 | Recessive | x | |||||
| 5 | Recessive | EYS | c.5621dup | p.Pro1875Thrfs* | Het | AR | |
| 6 | X-linked | RPGR | c.2311delG | p.G771Rfs*44 | Emiz | X-linked | x |
| 7 | ADRP | PRPF8 | c.5804G>A | p.R1935H | Het | AD | x |
| 8 | Isolated | IMPG2 | c.1100dup | p.Leu367Phefs*12 | Hom | AR | x |
| 9 | Recesive | CNGA1 | c.626_629delAAGA | p.Lys209Argfs55* | Hom | AR | x |
| 10 | X-linked | RPGR | c.A1367A>G | p.Gln456Arg | Het | X-linked | |
| 11 | Isolated | ||||||
| 12 | ADRP | RP9 | c.118C>T | p.(Gln40* | Het | AD | x |
| 13 | Recessive | PDE6B | c.2152G>A | p.(Asp718Asn) | Hom | AR | rs150639487 |
| 14 | Isolated |
AD, autosomal dominant; ADRP, autosomal dominant retinitis; AR, autosomal recessive; Emiz, hemizygous; Het, heterozygous; Hom, homozygous; refSNP, reference single nucleotide polymorphism.
A full general and ophthalmologic examination (including detailed family history, anterior segment biomicroscopy, corrected Snellen visual acuity, direct and indirect ophthalmoscopy, intraocular pressure measurement) was performed on each on several consecutive visits. Ocular motility was assessed by using the cover and uncover far and near tests; all subjects showed normal ocular alignment, except for one (S2), who showed esotropia in the right eye (+15 prism diaopter, near far).
Best corrected visual acuities (BCVAs) were obtained monocularly with a projected Snellen chart. Reported visual acuity in each eye is the average of all available logMAR measurements. Foveal monocular contrast sensitivity was assessed by using Mars Letter Contrast Sensitivity Test.58 Kinetic visual fields were measured to the V4e white test light of the Goldmann perimeter against the standard background of 31.5 apostilbs. To quantify the size of the residual visual field, we reported the diameter of the Goldman V4E visual field. Monocular values for all measurements are reported in Table 1.
Control Subjects
A group of 14 healthy volunteers (1 male) age matched with the RP patient group (mean age, 45 ± 2.5 years) was recruited for a control experiment. The control group of subjects had normal or corrected-to-normal visual acuity and contrast sensitivity. Binocular rivalry was tested in all subjects and a subgroup (N = 9) also performed the monocular deprivation experiment. The group of RP patients performed the binocular rivalry measurements and the monocular deprivation experiment on the same day. The subgroup of control subjects performed the monocular deprivation experiment on a different day, sometime (typically, 1 year) after the initial binocular rivalry measurements. To compare the effect of monocular deprivation between RP patients and normally sighted participants, we also report data from a previous study.48 These data are from a group of 20 adult volunteers (7 males; mean age, 22 ± 3 years), all having normal or corrected-to-normal vision.
Ethical Statement
All patients and typical subjects gave informed consent to participate in the study, which adhered to the tenets of the Declaration of Helsinki and was approved by the Università Cattolica Review Board and by the local ethical committee (Comitato Etico Pediatrico Regionale—Azienda Ospedaliero-Universitaria Meyer—Firenze [FI]), under the protocol “Plasticità del sistema visivo” (March 2011).
Apparatus and Stimuli
Binocular Rivalry
The experiment took place in a dark and quiet room. Visual stimuli were generated by the ViSaGe stimulus generator (Cambridge Research Systems [CRS], Rochester, UK), housed in a PC (Dell, Round Rock, TX, USA) controlled by Matlab programs (The Mathworks Inc., Natick, MA, USA). Visual stimuli were two sinusoidal gratings, oriented either 45° clockwise or counterclockwise (size: 2σ = 2°, spatial frequency: 2 cyc/deg), presented on a uniform background (luminance: 37.4 cd/m2, CIE: 0.442 0.537) in central vision with a central black fixation point and a common squared frame to facilitate dichoptic fusion. Visual stimuli were displayed on a 20-inch Clinton Monoray (Richardson Electronics Ltd., LaFox, IL, USA) monochrome monitor, driven at a resolution of 1024 × 600 pixels, with a refresh rate of 120 Hz. Observers viewed the display at a distance of 57 cm through CRS ferromagnetic shutter goggles that occluded alternately one of the two eyes each frame. Responses were recorded through the computer keyboard.
Flicker Detection Impairment
Flicker detection deficits in the central visual field were assessed via Humphrey Matrix (model 800; Carl Zeiss Meditec, Inc., City, State, Country) using the “macula FDT” protocol. Briefly this test presents a small square grating 2° × 2° of 0.5-cyc/deg spatial frequency flickering at 12 Hz. Targets could appear in 16 possible locations in the central 5° for 300 ms. The subject’s task was to respond within 1.5 seconds from target appearance by means of button key press. The contrast was chosen by following an adaptive procedure (ZEST59) that homed in on threshold; three catch trials per session were included to discourage impulsive key press; fixation stability was monitored according to the Heijl-Krakau blind spot monitoring. All subjects had good monocular fixation and we only observed sporadic errors in fixation (maximum of one fixation error per session). Average detection thresholds across the 16 stimulus locations are reported relative to normative samples and expressed in decibels (dB) (i.e., 20Log10 (THRpatient/THRtypical)).
In three patients, for whom the Humphreys Matrix was not available for technical reasons, we measured thresholds to discriminate motion direction of a similar drift grating, using a 2AFC instead of a yes/no procedure. We used the CRT monitor (viewed monocularly) and custom-built program running on a MacPro (Apple, Cupertino, CA, USA) with Matlab and with Psychophysics Toolbox.60 Subjects saw a square grating (2° × 2°) of 2 or 4 cyc/deg, with variable contrast, drifting at 8 Hz either leftwards or rightwards. They were asked to report motion direction, and accuracy was plotted as a function of contrast. Data were fitted by a psychometric curve whose median indicates the discrimination threshold. Given the difference in spatial frequency, the sensitivity for the motion discrimination of the drifting grating will be lower than the sensitivity for detecting a flickering grating of the same component contrast. For stimuli of the same spatial frequencies, the two sensitivities are proportional with a factor of square-root 2.61 To compare the two different estimates of sensitivity, threshold values were reported relative to a normative sample (performance of five normally sighted control participants under the same conditions was available in the lab records). The perceptual deficit of RP patients was expressed in decibels as above.
Procedures
Central cone focal ERG (fERG) was recorded from the central 18° region by using a uniform red field superimposed to an equiluminant steady adapting background, used to minimize stray-light modulation.62,63 The stimulus was generated by a circular array of eight red LEDs (λ maximum, 660 nm; mean luminance, 93 cd/m2) presented on the rear of a Ganzfeld bowl (white-adapting background). A diffusing filter in front of the LED array made it appear as a circle of uniform red light. fERGs were recorded in response to the sinusoidal 95% luminance modulation of the central red field. Flicker frequency was 41 Hz. Patients fixated monocularly at a 0.25° central fixation mark, under the constant monitoring of an external observer. Pupils were pharmacologically dilated (1% tropicamide and 2.5% phenylephrine hydrochloride) to a diameter ≥8 mm, and all subjects underwent a preadaptation period of 20 minutes to the stimulus mean illuminance. fERG was recorded by an Ag-AgCl electrode taped on the skin over the lower eyelid. A similar electrode, placed over the eyelid of the contralateral patched eye, was used as reference (interocular recording). fERG signals were amplified (100,000-fold), bandpass filtered between 1 and 100 Hz (6 dB/oct), and averaged (12-bit resolution, 2-kHz sampling rate, 200–600 repetitions in 2–6 blocks). Off-line discrete Fourier analysis quantified the amplitude and phase lag of the response fundamental harmonic (first harmonic) at 41 Hz. Owing to patient unavailability, fERG responses were recorded from 12 patients of the 14 recruited (see Table 1).
Short-Term Monocular Deprivation
Monocular deprivation was performed by using eye patching. The eye patch was custom-made of a translucent plastic material that allowed light to reach the retina (luminance attenuation, 0.07 logUnits) but completely prevented pattern vision, as assessed by the Fourier transform of a natural world image seen through the eye patch. The dominant eye (the eye showing longer perceptual predominance in binocular rivalry) was patched for 2 hours. During the 2 hours of monocular occlusion patients were free to perform normal activities (e.g., walking, going for lunch). The monocular deprivation procedure was repeated twice after a 24-hour interval.
Binocular Rivalry
Each binocular rivalry experimental block lasted 180 seconds. After an acoustic signal (beep), the binocular rivalry stimuli appeared. Subjects reported their perception (clockwise, counterclockwise, or mixed) by continuously pressing with the right hand one of three keys (left, right, and down arrows) of the computer keyboard. At each experimental block, the orientation associated to each eye was randomly varied so that neither subject nor experimenter knew which stimulus was associated with which eye until the end of the session, when it was verified visually.
Because some patients had different visual acuity in the two eyes, balanced perceptual dominance could not be achieved by presenting rivalrous stimuli having the same contrast. To adjust the monocular stimulus contrast to compensate for the patients’ strong ocular dominance, before the first training session, a few 60-second preliminary experimental blocks were performed. At each block, the contrast of stimuli presented to the weaker eye (maximum contrast: 100%) was increased while decreasing the contrast of the stimulus presented to the dominant eye (minimum contrast: 20%), aimed at inducing balanced ocular dominance. Of the 14 patients tested, monocular contrast was adjusted in 7 patients by 41% ± 17% (corresponding to 7.7 ± 3 dB), the remaining 7 patients were presented with orthogonal gratings of equal (50%) contrast. Two binocular rivalry experimental blocks were acquired before short-term monocular deprivation and four blocks after eye-patch removal. All subjects of the control group performed 2 × 180 seconds experimental blocks to estimate binocular rivalry dynamics; a subset of subjects (N = 9) also performed the monocular deprivation experiment.
Analyses
Binocular Rivalry
The perceptual reports recorded through the computer keyboard were analyzed by using Matlab. Mean phase durations for the two orientations and for mixed percepts (the average perceptual duration of each rivalrous stimulus), as well as the total time (T) spent by the observer perceiving the stimulus presented to either eye (deprived and nondeprived), were computed.
Phase duration distributions were fitted by a two-parameter (r, λ) gamma distribution of the form:
| (1) |
where Г is the gamma function, r is the shape parameter, and λ is the scale parameter.
To quantify sensory eye dominance we obtained an index of ocular dominance, ranging from 0 (complete dominance of the nondeprived eye) to 1 (complete dominance of the deprived eye), according to the following equation:
| (2) |
Periods of mixed perception were excluded from the main analyses; the proportion of time spent by the observer perceiving mixed rivalry was analyzed separately.
Statistics
Statistical analyses were performed with SPSS-2.0 (Manufacturer, City, State, Country) and Matlab software. The parameters of the gamma distributions were compared by using a 10,000-repetition bootstrap sign test (two-tailed). Mean phase durations were compared across groups (RP patients and control group) by using an independent samples t-test. Sensory eye dominance values measured before and after short-term deprivation were compared by using a paired-sample t-test (α error fixed at 0.05). Since the assumption of normality was never violated (Shapiro-Wilk normality test, all Ps > 0.05), correlations were computed by using the Pearson’s correlation coefficient (r), statistical significance assessed with a permutation test. To assess the robustness of the correlation, we also computed the Bayes factor (BF): conventionally, a BF lower than 0.3 indicates evidence in favor of the null hypothesis (no correlation), whereas a BF larger than 3 indicates evidence in favor of the alternative hypothesis and therefore a robust correlation between the two variables tested.
Results
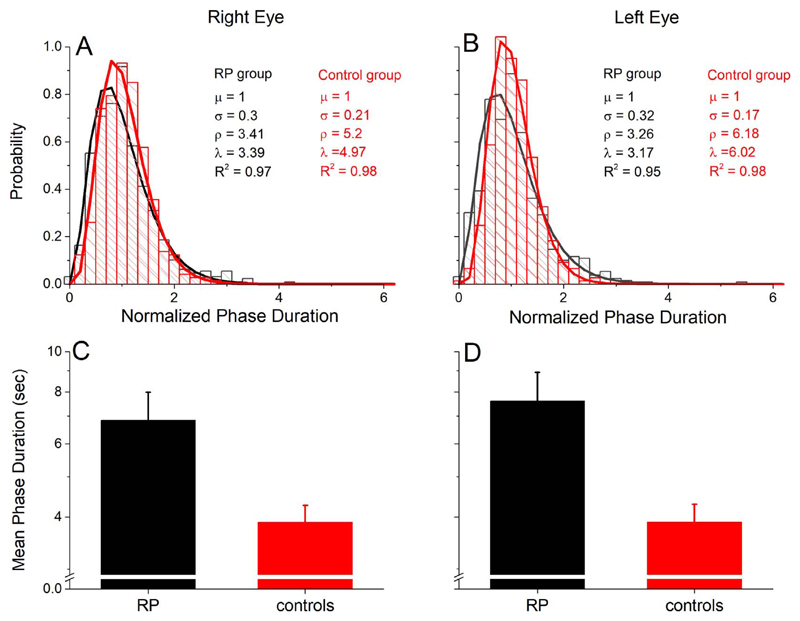
To assess short-term visual plasticity in RP (clinical data in Table 1 and genetic data in Table 2), we measured binocular rivalry in a group of patients diagnosed with RP before and after 2 hours of monocular deprivation. To achieve a balanced perceptual dominance between the eyes before deprivation, in seven patients we adjusted the contrast of the rivalrous stimuli. Overall, all patients showed typical binocular rivalry dynamics, with normalized phase duration distributions well modeled by a gamma distribution (Figs. 1A, 1B), a typical hallmark of binocular rivalry,64 which is not significantly different from the distribution measured in control subjects. The parameters of the gamma distributions of the two eyes (right eye, Fig. 1A; left eye, Fig. 1B) were not significantly different (rate parameter λ, P = 0.68; shape parameter ρ, P = 0.75), indicating that the contrast-balancing procedure used to compensate for differences in monocular vision successfully balanced eye dominance in binocular rivalry. No significant correlations were observed across subjects between the two main parameters characterizing binocular rivalry dynamics, mean phase duration and proportion of mixed percepts (Pearson’s r = −0.38, P = 0.18, BF = 0.48), which only presented a trend for negative correlation, in line with previous studies on normally sighted observers.65 Similarly, no correlation was observed between these parameters and disease-related variables such as BCVA, fERG amplitude, Goldmann visual field area, and flicker detection impairment (all Ps > 0.05, see Supplementary Table S1), indicating that binocular rivalry dynamics were not related to RP severity. However, RP patients do have clearly different (slower) dynamics of binocular rivalry than age-matched controls, as confirmed by a mixed-model ANOVA, with a 2 (EYES, within factor) × 2 (GROUP, between factor) design. This revealed a significant effect of the factor GROUP, with F(1,26) = 6.98, P = 0.014, η2 = 0.21, reflecting that RP patients showed significantly longer mean phase durations than an age-matched control group (Figs. 1C, 1D). Neither the factor EYES nor the EYES*GROUP interaction showed a significant effect (EYES: F(1,26) = 2.03, P = 0.17, η2 = 0.07; EYES*GROUP: F(1,26) = 1.98, P = 0.17, η2 = 0.07), confirming that there was no difference between the two eyes’ mean phase duration within or across groups. Mean phase durations of RP patients and controls were as follows for the right eye: RP patients: 7.61 ± 1.3 seconds, control subjects: 3.89 ± 0.4 seconds, two-tailed, independent samples t-test, t(26) = 2.71, P = 0.012, Cohen’s d = 1.03 (Fig. 1C); and for the left eye: RP patients: 6.84 ± 1.5 seconds, control subjects: 3.88 ± 0.4 seconds, two-tailed, independent samples t-test, t(26) = 2.44, P = 0.022, Cohen’s d = 0.92). Slower binocular rivalry switching rate in RP patients was also confirmed by the comparison of the distributions of phase durations across groups (Figs. 1A, 1B). For both groups the distributions were well fitted by a gamma distribution (function 1); however, the distribution for RP patients was more skewed toward longer phase durations, similarly for both eyes (both the scale, λ, and shape, ρ, parameters differed across groups, P < 10−6). Note that the longer phase duration did not hamper the sharp transition between the left and right eye images: RP patients showed normal-like mixed percepts (i.e., periods of superimposition of the two rivalrous stimuli) both in terms of proportion (independent samples t-test, t(26) = 1.14, P = 0.26) and of duration (independent samples t-test, t(26) = 0.96, P = 0.346).
Figure 1.
Binocular rivalry phase durations in RP patients. Phase duration distributions of the right (A) and left (B) eye are well approximated by a gamma distribution (Equation 1), both for RP patients (black) and for an age-matched sample of normally sighted participants (red), indicating normal binocular rivalry dynamics in RP patients. (C, D) RP patients show longer mean phase durations in binocular rivalry than control subjects both for the right (C) and left (D).
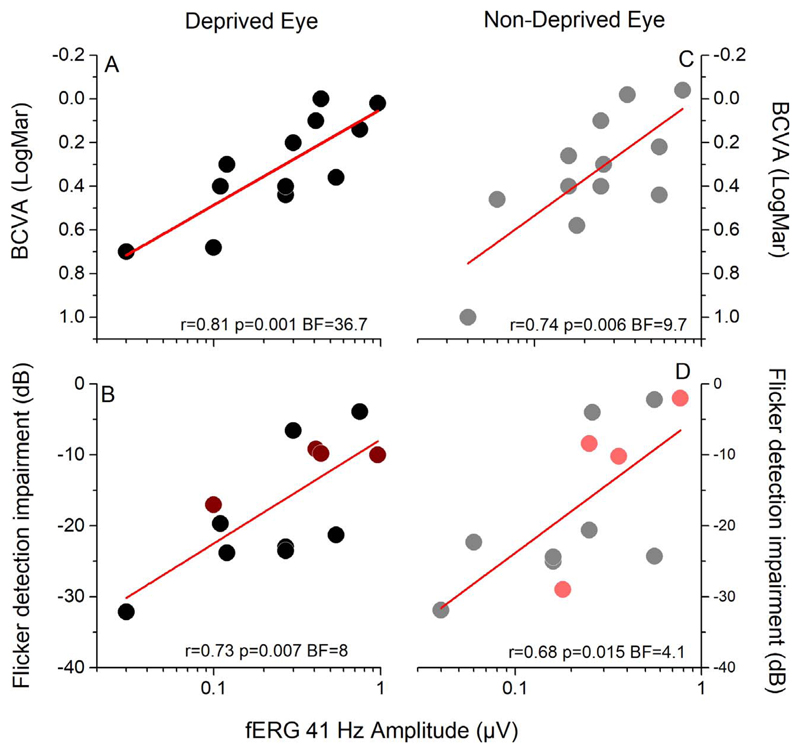
Reliable correlations were found between the amplitude of the central 18° fERG response and both monocular visual acuity (Figs. 2A–C; deprived eye: Pearson’s r = 0.81, P = 0.001, BF = 36.7; nondeprived eye: r = 0.74, P = 0.006, BF = 9.7) and monocular flicker detection impairment (Figs. 2B–D; deprived eye: Pearson’s r = 0.73, P = 0.007, BF = 8; nondeprived eye: r = 0.68, P = 0.015, BF = 4.1). The two sets of sensitivity measures obtained with the Mars Letter test and with the flickering and drifting grating were strongly correlated (deprived eye: r = 0.84, P < 0.0001, BF = 199.6; nondeprived eye: r = 0.87, P < 0.0001, BF = 729.2), validating the Humphrey Matrix method. The good correlation of visual abilities with the fERG reinforces previous evidence66 indicating that the origin of the deficit for these measures is at the retinal level and reinforcing previous evidence that fERG signal is a good predictor of the foveal response, independently of the stimulus size.67
Figure 2.
Correlation between the amplitude of the fERG response and measures of visual functions in RP patients. The amplitude of the fERG correlates across subjects with visual acuity (A–C) and with the impairment in flicker detection (B–D) both for the deprived (A, B) and nondeprived (C, D) eye. Dark and light red symbols in (B) and (D) represent the four patients tested with a different setup probing motion direction discrimination.
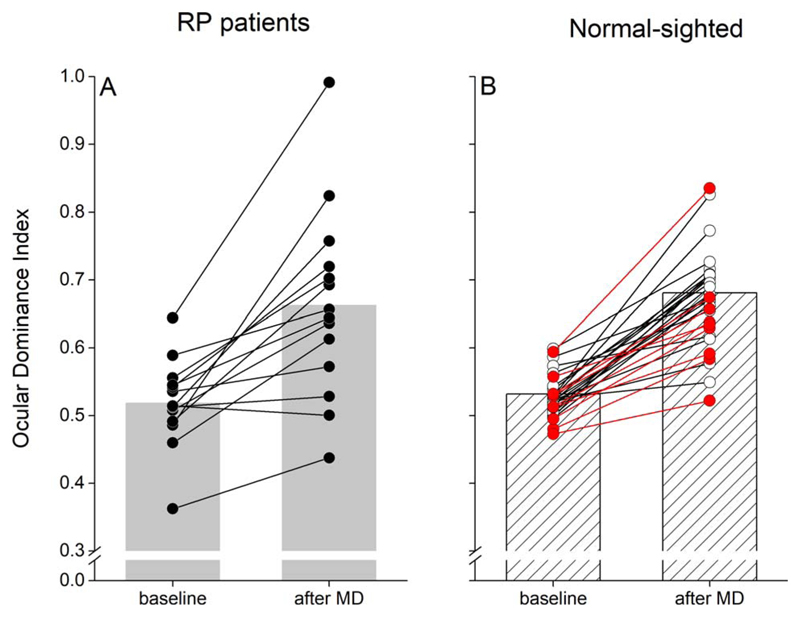
After monocular deprivation, the deprived eye dominance increased significantly (two-tailed paired-samples t-test, α = 0.05, t(13) = 4.9, P = 0.0003, Cohen’s d = 1.2620; Fig. 3A) over the nondeprived eye. Figure 3B compares the effect size of monocular deprivation in RP patients to that measured in young normally sighted subjects48 and in nine age-matched control subjects, showing that in the RP patients the effect is in the typical normal range with perhaps a larger interindividual variability in both pre- and postdeprivation measurements. This was confirmed by a mixed-model ANOVA with the within subjects’ factor TIME (before and after deprivation) and the between subjects’ factor GROUP (RP patients, young controls age-matched controls) that revealed a significant effect of the factor TIME (F[1,40] = 107.1, P < 10−4, η2 = 0.73), but not a significant effect of either the factor GROUP (F[2,40] = 0.56, P = 0.57, η2 = 0.03) or the TIME*GROUP interaction (F[2,40] = 0.35, P = 0.68, η2 = 0.02).
Figure 3.
Effect of monocular deprivation on binocular rivalry. (A) Following 2 hours of monocular deprivation, ocular dominance measured by means of binocular rivalry (ocular dominance index; Equation 2) shifts in favor of the deprived eye (***P < 0.001), indicating normal short-term visual plasticity in RP patients. (B) Effect of monocular deprivation in normally sighted subjects (white symbols: data adapted from Lunghi and Sale48; red symbols: data from nine age-matched control subjects).
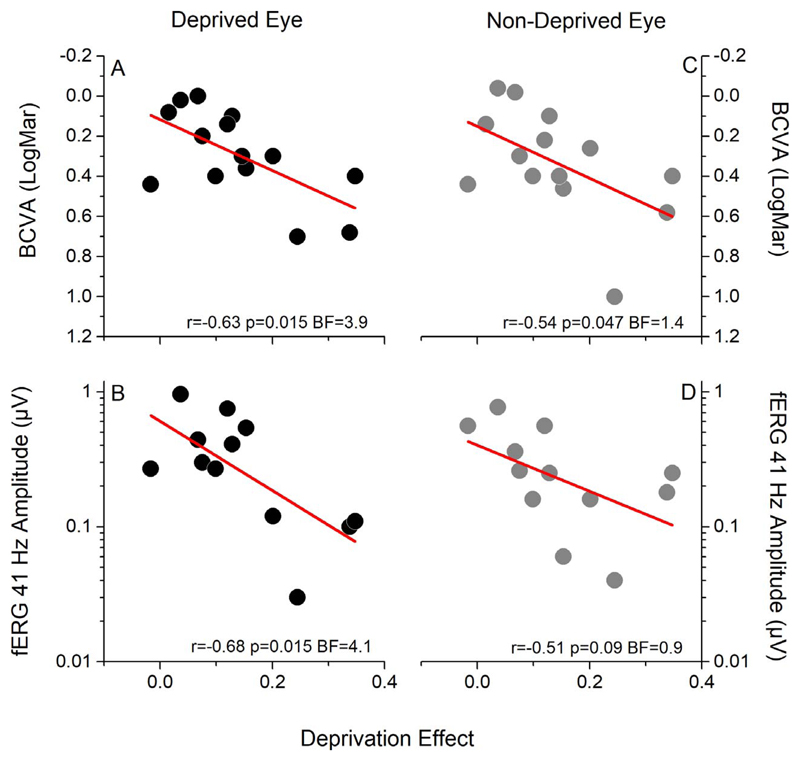
To investigate whether the interindividual variability in the plasticity effect in RP patients could be related to the severity of the pathology, we correlated the effect of monocular deprivation on binocular rivalry (the shift in ocular dominance in favor of the deprived eye) with important behavioral and physiological indexes, such as residual monocular BCVA (measured as logMAR), monocular flicker detection impairment, monocular Goldman visual field size (V4E), and the amplitude of the monocular fERG response. Interestingly, across subjects, the plasticity effect was highly negatively correlated with both BCVA (Fig. 4A; Pearson’s r = −0.63, P = 0.015, BF = 3.9) and fERG amplitude (Fig. 4B; Pearson’s r = −0.68, P = 0.015, BF = 4.1) of the deprived eye. A trend for a negative correlation with nondeprived eye BCVA (Fig. 4C; Pearson’s r = −0.54, P = 0.047, BF = 1.4) and fERG amplitude (Fig. 4D; Pearson’s r = −0.51, P = 0.09, BF = 0.9) was also observed. RP patients retaining a residual normal-like BCVA (logMAR < 0.168) showed reduced visual cortical plasticity in response to short-term monocular deprivation, while plasticity increased with higher visual deficits. No significant correlation was found between the plasticity effect and visual field area of either the deprived (Supplementary Fig. S1A; Pearson’s r = −0.31, P = 0.29, BF = 0.36) or nondeprived (Supplementary Fig. S1C, Pearson’s r =−0.54, P = 0.07, BF = 1.05) eye, or with the flicker detection impairment (Supplementary Fig. S1B; deprived eye: Pearson’s r = 0.1, P = 0.76, BF = 0.2; nondeprived eye: Pearson’s r = 0.14, P = 0.63, BF = 0.2). Nor did we observe significant correlation of the plasticity index with the duration of the disease (Supplementary Fig. S2A; Pearson’s r = 0.26, P = 0.36, BF = 0.31) or its age of onset (Supplementary Fig. S2B; Pearson’s r = −0.09, P = 0.76, BF = 0.21).
Figure 4.
Correlations between the effect of monocular deprivation, visual acuity, and the amplitude of the fERG. Across patients, the effect of monocular deprivation (difference between the ocular dominance index measured before and after deprivation) strongly correlates both with visual acuity (BCVA) and with the amplitude of the fERG of the deprived eye (A, B). A trend for correlation between BCVA and the amplitude of the fERG of the nondeprived eye is also observed (C, D).
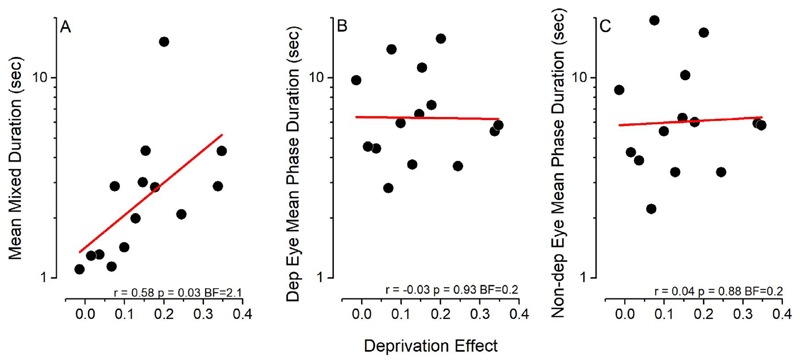
Finally, in order to investigate whether the baseline features of binocular rivalry dynamics could be predictive of the plasticity effect measured after short-term monocular deprivation, we correlated the effect of deprivation with the mean phase duration of coherent and mixed percepts. Interestingly, we observed a correlation trend between the deprivation effect and the average duration of mixed percepts (Fig. 5A; Pearson’s r = 0.58, P = 0.03, BF = 2.1), while the effect of monocular deprivation did not correlate across subjects with binocular rivalry mean phase duration of either the deprived (Fig. 5B; Pearson’s r = −0.03, P = 0.93, BF = 0.2) or the nondeprived (Fig. 5C; Pearson’s r = 0.04, P = 0.88, BF = 0.2) eye.
Figure 5.
Correlation between the effect of monocular deprivation and binocular rivalry parameters. The effect of monocular deprivation correlates across subjects with the average duration of mixed percepts (A), but not with the mean phase duration of either the deprived (B) or nondeprived (C) eye measured before deprivation.
Discussion
We assessed short-term visual plasticity in RP patients by testing the effect of 2 hours of monocular deprivation on sensory eye dominance measured by means of binocular rivalry. We found that RP patients show a normal plastic response to short-term monocular deprivation: after deprivation, ocular dominance by the deprived eye significantly increased, reflecting homeostatic plasticity. This result demonstrates that even after many years of abnormal visual experience the central visual field representation in early visual cortex (possibly V129) retains a normal capability to plastically adapt to visual change—despite the potential recruitment of early visual areas by other senses.69,70 We also found that RP patients show longer mean dominance durations in binocular rivalry, indicating slower switching rate. Binocular rivalry switching rate has been related to visual cortical inhibition, with subjects with slower switching rate having higher GABA concentration in the primary visual cortex.71 Our result might therefore indicate an abnormal excitation/inhibition balance in RP patients’ visual cortex, even though we did not find a correlation across subjects between binocular rivalry mean phase duration and RP-related physiological measures. Interestingly, it has been shown that binocular rivalry mean phase durations are modulated by luminance level: under scotopic viewing conditions, slower alternation rates are observed.72 This suggests that the longer mean phase durations observed in RP patients might be also related to the altered capability of photoreceptors to respond to light. The slower switching rate observed in RP patients might hence reflect the longer visual processing times associated with the disease.25,73 A further suggestion that mean phase duration and plasticity effect leverage on a different neurobiological basis, respectively subcortical and cortical, comes from the lack of correlation between the two variables (Pearson’s r = 0.01, P = 0.97, BF = 0.2). A potential abnormal excitation/inhibition balance in the visual cortex of RP patients is also suggested by the correlation trend between the mixed percepts duration and the plasticity effect, as mixed percepts have been recently linked to altered GABAergic inhibition in other pathologies.74
Interestingly, the plasticity effect (perceptual boost of the deprived eye measured with binocular rivalry) showed a strong negative correlation across subjects both with BCVA and with an objective physiological measure of central retinal function (the central 18° fERG amplitude) of the deprived eye. In particular, patients retaining normal-like acuities (logMAR < 0.1) showed lower plastic changes in ocular dominance following deprivation, which increased in subjects with lower acuities. BCVA is related to central cones and is one of the most resistant visual functions to survive disease progression in RP,75–77 decaying after most ERG and visual field parameters.78 A similar negative trend is observed for the relationship between flicker detection and plasticity effect, but the correlation is nonsignificant, presumably reflecting the multi-factorial nature of flicker detection (combining the contributions of multiple retinal and cortical mechanisms—more than visual field amplitude and BVCA) compared to objective measures like fERG.
The effect of short-term monocular deprivation may be dubbed “plasticity” or “adaptation.” The border between the two is fuzzy: although adaptation is generally considered to have more transient effects, there are cases where its consequences last for days.79 We prefer the term “plasticity” given recent work from our laboratory showing that the short-term monocular deprivation in adult amblyopes can produce changes of visual function that remain stable for over 1 year.49
Importantly, in our sample of RP patients, we observed a specific pattern of correlations between the effect of short-term monocular deprivation and disease-related variables. We found a negative correlation between the plasticity effect and both visual acuity and the amplitude of the fERG, suggesting increased plasticity when the foveal signals are hampered by the disease, specifically so for the deprived eye. This result is in line with a recent study49 showing that short-term monocular deprivation boosts neural responses of the deprived eye in the early visual cortex. It is tempting to speculate that this phenomenon may correlate with a readiness to switch from visually guided to non–visually guided behavior, “opening” the visual cortex to nonvisual signals, a phenomenon known as cross-modal plasticity.69,70 At more initial stages of the disease, when retinal function and visual acuity are relatively preserved, there might be a temporary reduction of plasticity, which might reflect a form of cortical modulation aimed at optimized filtering of the altered retinal input. This may have the important functional consequence of providing stable processing despite degenerating visual input. Interestingly, at this stage, the retinotopy associated with the central visual field recruits a larger cortical territory, expanding to the representation of peripheral visual field that is deafferented.30 The larger neuronal territory associated with central vision may mediate the initial cortical gating, stabilizing the circuitry that supports central vision and thus increasing the threshold necessary to endorse visual plasticity. At later stages of the disease, when the incoming visual signal becomes too poor and provides only coarse visual information, the plasticity threshold might be reduced to allow the colonization of the visual cortex by other sensory modalities69,70 observed in both early and late blind subjects.21–28 All these latter studies, however, show that rewiring is more likely in associative cortex than in early visual cortex, while many studies demonstrate that the intracortical circuitry of primary visual areas remains stable even after many year of total blindness.80 This suggests taking with some caution the interpretation of increased plasticity being related to cross-modal recruiting of the early visual cortex. Yet, the fact that we observed a greater plastic response to a phenomenon shown to specifically involve V154 suggests that in the later stage of the disease also the V1 region subserving macular vision might have the capability to rewire. In line with this hypothesis, two recent studies of RP patients, analyzed before and after training with an artificial prosthesis,28,29 demonstrate that very little cortical rearrangement takes place after a few months of the prosthetic implants. However, after approximately 1 year, the colonized V1 response of tactile signal is reduced in one subject28 and the aberrant V1 response to flashes of light is increased in three other subjects.29 Given that binocular rivalry can be modulated by tactile signals81–84 and can reveal the small changes of tactile modulation of V1 responses after short-term monocular deprivation in adult humans,85 it would be useful to test directly the hypothesis of cross-modal recruitment in RP subjects in future studies.
Independently of these speculations, our paradigm reveals a progression of visual cortical plasticity in RP and may thus provide a benchmark, noninvasive testing tool to assess a patient’s ability to respond to altered visual inputs,29 and in particular his/her likelihood to benefit from prospective therapeutic strategies.
Supplementary Material
Acknowledgments
Supported by Fondazione Roma under the Grants for Biomedical Research: Retinitis Pigmentosa (RP)-Call for proposals 2013 “Cortical Plasticity in Retinitis Pigmentosa: an Integrated Study from Animal Models to Humans,” the European projects ERA-NET Neuro-DREAM, ECSPLAIN (European Research Council under the Seventh Framework Programme, FPT/2007-2013, Grant agreement No. 338866) and PUPILTRAITS (European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme, Grant agreement No. 801715), and the Italian Ministry of University and Research under the project “PRIN 2015,” Grant agreement No. RBFR1332DJ.
Footnotes
Disclosure: C. Lunghi, None; L. Galli-Resta, None; P. Binda, None; G.M. Cicchini, None; G. Placidi, None; B. Falsini, None; M.C. Morrone, None
References
- 1.Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1:40. doi: 10.1186/1750-1172-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verbakel SK, van Huet RAC, Boon CJF, et al. Non-syndromic retinitis pigmentosa. Prog Retin Eye Res. 2018;66:157–186. doi: 10.1016/j.preteyeres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Fahim AT, Daiger SP, Weleber RG. Nonsyndromic Retinitis Pigmentosa Overview. Seattle, WA: University of Washington, Seattle; 1993. [Google Scholar]
- 4.Berson EL. Long-term visual prognoses in patients with retinitis pigmentosa: the Ludwig von Sallmann lecture. Exp Eye Res. 2007;85:7–14. doi: 10.1016/j.exer.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rachitskaya AV, Yuan A. Argus II retinal prosthesis system: an update. Ophthalmic Genet. 2016;37:260–266. doi: 10.3109/13816810.2015.1130152. [DOI] [PubMed] [Google Scholar]
- 6.Luo YH-L, da Cruz L. The Argust® II retinal prosthesis system. Prog Retin Eye Res. 2016;50:89–107. doi: 10.1016/j.preteyeres.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Petrs-Silva H, Linden R. Advances in gene therapy technologies to treat retinitis pigmentosa. Clin Ophthalmol. 2013;8:127–136. doi: 10.2147/OPTH.S38041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busskamp V, Picaud S, Sahel JA, Roska B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012;19:169–175. doi: 10.1038/gt.2011.155. [DOI] [PubMed] [Google Scholar]
- 9.Humayun MS, Dorn JD, da Cruz L, et al. Interim results from the International Trial of Second Sight’s Visual Prosthesis. Ophthalmology. 2012;119:779–788. doi: 10.1016/j.ophtha.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stingl K, Bartz-Schmidt KU, Besch D, et al. Subretinal visual implant alpha IMS—clinical trial interim report. Vision Res. 2015;111:149–160. doi: 10.1016/j.visres.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Stingl K, Bartz-Schmidt KU, Besch D, et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc R Soc B Biol Sci. 2013;280 doi: 10.1098/rspb.2013.0077. 20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow AY. Retinal prostheses development in retinitis pigmentosa patients—progress and comparison. Asia Pac J Ophthalmol (Phila) 2013;2:253–268. doi: 10.1097/APO.0b013e3182a0b4fe. [DOI] [PubMed] [Google Scholar]
- 13.Chow AY, Bittner AK, Pardue MT. The artificial silicon retina in retinitis pigmentosa patients [an American Ophthalmological Association thesis] Trans Am Ophthalmol Soc. 2010;108:120–154. [PMC free article] [PubMed] [Google Scholar]
- 14.Chow AY, Chow VY, Packo KH, Pollack JS, Peyman GA, Schuchard R. The artificial silicon retina microchip for the treatment of visionloss from retinitis pigmentosa. Arch Ophthalmol. 2004;122:460–469. doi: 10.1001/archopht.122.4.460. [DOI] [PubMed] [Google Scholar]
- 15.Ghezzi D. Retinal prostheses: progress toward the next generation implants. Front Neurosci. 2015;9:290. doi: 10.3389/fnins.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis PM, Ackland HM, Lowery AJ, Rosenfeld JV. Restoration of vision in blind individuals using bionic devices: a review with a focus on cortical visual prostheses. Brain Res. 2015;1595:51–73. doi: 10.1016/j.brainres.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 18.Sauvé Y, Girman SV, Wang S, Lawrence JM, Lund RD. Progressive visual sensitivity loss in the Royal College of Surgeons rat: perimetric study in the superior colliculus. Neuroscience. 2001;103:51–63. doi: 10.1016/s0306-4522(00)00557-1. [DOI] [PubMed] [Google Scholar]
- 19.Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205. doi: 10.1016/s1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- 20.Schoth F, Burgel U, Dorsch R, Reinges MHT, Krings T. Diffusion tensor imaging in acquired blind humans. Neurosci Lett. 2006;398:178–182. doi: 10.1016/j.neulet.2005.12.088. [DOI] [PubMed] [Google Scholar]
- 21.Berkowitz BA, Bissig D, Roberts R. MRI of rod cell compartment-specific function in disease and treatment in vivo. Prog Retin Eye Res. 2016;51:90–106. doi: 10.1016/j.preteyeres.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gothe J, Brandt SA, Irlbacher K, Röricht S, Sabel BA, Meyer B-U. Changes in visual cortex excitability in blind subjects as demonstrated by transcranial magnetic stimulation. Brain. 2002;125:479–490. doi: 10.1093/brain/awf045. [DOI] [PubMed] [Google Scholar]
- 23.Kitajima M, Korogi Y, Hirai T, et al. MR changes in the calcarine area resulting from retinal degeneration. AJNR Am J Neuroradiol. 1997;18:1291–1295. [PMC free article] [PubMed] [Google Scholar]
- 24.Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholl HPN, Kremers J. Alterations of L- and M-cone driven ERGs in cone and cone-rod dystrophies. Vision Res. 2003;43:2333–2344. doi: 10.1016/s0042-6989(03)00411-5. [DOI] [PubMed] [Google Scholar]
- 26.Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 27.Sadato N, Pascual-Leone A, Grafman J, et al. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham SI, Weiland JD, Bao P, Lopez-Jaime GR, Tjan BS. Correlation of vision loss with tactile-evoked V1 responses in retinitis pigmentosa. Vision Res. 2015;111:197–207. doi: 10.1016/j.visres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castaldi E, Cicchini GM, Cinelli L, Biagi L, Rizzo S, Morrone MC. Visual BOLD response in late blind subjects with argus II retinal prosthesis. PLoS Biol. 2016;14:e1002569. doi: 10.1371/journal.pbio.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira S, Pereira AC, Quendera B, Reis A, Silva ED, Castelo-Branco M. Primary visual cortical remapping in patients with inherited peripheral retinal degeneration. NeuroImage Clin. 2017;13:428–438. doi: 10.1016/j.nicl.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabbah N, Sanda N, Authié CN, et al. Reorganization of early visual cortex functional connectivity following selective peripheral and central visual loss. Sci Rep. 2017;7 doi: 10.1038/srep43223. 43223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanda N, Cerliani L, Authié CN, et al. Visual brain plasticity induced by central and peripheral visual field loss. Brain Struct Funct. 2018;223:3473–3485. doi: 10.1007/s00429-018-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wandell BA, Smirnakis SM. Plasticity and stability of visual field maps in adult primary visual cortex. Nat Rev Neurosci. 2009;10:873–884. doi: 10.1038/nrn2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray MM, Matusz PJ, Amedi A. Neuroplasticity: unexpected consequences of early blindness. Curr Biol. 2015;25:R998–R1001. doi: 10.1016/j.cub.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 35.Alais D, Blake R. Binocular Rivalry. Cambridge, Massachusetts; London, England: MIT Press; 2005. [Google Scholar]
- 36.Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 37.Levelt WJ. On Binocular Rivalry. Soesterberg, The Netherlands: Institution for Perception; 1965. [Google Scholar]
- 38.Ooi TL, He ZJ. Sensory eye dominance. Optometry. 2001;72:168–178. [PubMed] [Google Scholar]
- 39.Dieter KC, Sy JL, Blake R. Individual differences in sensory eye dominance reflected in the dynamics of binocular rivalry. Vision Res. 2017;141:40–50. doi: 10.1016/j.visres.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handa T, Mukuno K, Uozato H, Niida T, Shoji N, Shimizu K. Effects of dominant and nondominant eyes in binocular rivalry. Optom Vis Sci. 2004;81:377–383. doi: 10.1097/01.opx.0000135085.54136.65. [DOI] [PubMed] [Google Scholar]
- 41.Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- 42.Blakemore C, Garey LJ, Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey’s visual cortex. J Physiol. 1978;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 44.Binda P, Lunghi C. Short-term monocular deprivation enhances physiological pupillary oscillations. Neural Plast. 2017;2017 doi: 10.1155/2017/6724631. 6724631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lunghi C, Burr DC, Morrone MC. Long-term effects of monocular deprivation revealed with binocular rivalry gratings modulated in luminance and in color. J Vis. 2013;13(6):1. doi: 10.1167/13.6.1. [DOI] [PubMed] [Google Scholar]
- 46.Lunghi C, Burr DC, Morrone C. Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Curr Biol. 2011;21:R538–R539. doi: 10.1016/j.cub.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Lunghi C, Emir UE, Morrone MC, Bridge H. Short-term monocular deprivation alters GABA in the adult human visual cortex. Curr Biol. 2015;25:1496–1501. doi: 10.1016/j.cub.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lunghi C, Sale A. A cycling lane for brain rewiring. Curr Biol. 2015;25:R1122–R1123. doi: 10.1016/j.cub.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lunghi C, Sframeli AT, Lepri A, et al. A new counterintuitive training for adult amblyopia. Ann Clin Transl Neurol. 2019;6:274–284. doi: 10.1002/acn3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J, Clavagnier S, Hess RF. Short-term monocular deprivation strengthens the patched eye’s contribution to binocular combination. J Vis. 2013;13(5):12. doi: 10.1167/13.5.12. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J, Reynaud A, Hess RF. Real-time modulation of perceptual eye dominance in humans. Proc Biol Sci. 2014;281 doi: 10.1098/rspb.2014.1717. 20141717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou J, Baker DH, Simard M, Saint-Amour D, Hess RF. Short-term monocular patching boosts the patched eye’s response in visual cortex. Restor Neurol Neurosci. 2015;33:381–387. doi: 10.3233/RNN-140472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lunghi C, Berchicci M, Morrone MC, Di Russo F. Short-term monocular deprivation alters early components of visual evoked potentials. J Physiol. 2015;593:4361–4372. doi: 10.1113/JP270950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Binda P, Kurzawski JW, Lunghi C, Biagi L, Tosetti M, Morrone MC. Response to short-term deprivation of the human adult visual cortex measured with 7T BOLD. Elife. 2018;7:e40014. doi: 10.7554/eLife.40014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heimel JA, van Versendaal D, Levelt CN. The role of GABAergic inhibition in ocular dominance plasticity. Neural Plast. 2012;2011 doi: 10.1155/2011/391763. 391763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 57.Baroncelli L, Maffei L, Sale A. New perspectives in amblyopia therapy on adults: a critical role for the excitatory/inhibitory balance. Front Cell Neurosci. 2011;5:25. doi: 10.3389/fncel.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arditi A. Improving the design of the letter contrast sensitivity test. Invest Ophthalmol Vis Sci. 2005;46:2225–2229. doi: 10.1167/iovs.04-1198. [DOI] [PubMed] [Google Scholar]
- 59.King-Smith PE, Grigsby SS, Vingrys AJ, Benes SC, Supowit A. Efficient and unbiased modifications of the QUEST threshold method: theory, simulations, experimental evaluation and practical implementation. Vision Res. 1994;34:885–912. doi: 10.1016/0042-6989(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 60.Brainard DH. The Psychopysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 61.Levinson E, Sekuler R. The independence of channels in human vision selective for direction of movement. J Physiol. 1975;250:347–366. doi: 10.1113/jphysiol.1975.sp011058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falsini B, Iarossi G, Fadda A, et al. The fundamental and second harmonic of the photopic flicker electroretinogram: temporal frequency-dependent abnormalities in retinitis pigmentosa. Clin Neurophysiol. 1999;110:1554–1562. doi: 10.1016/s1388-2457(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 63.Iarossi G, Falsini B, Piccardi M. Regional cone dysfunction in retinitis pigmentosa evaluated by flicker ERGs: relationship with perimetric sensitivity losses. Invest Ophthalmol Vis Sci. 2003;44:866–874. doi: 10.1167/iovs.01-1256. [DOI] [PubMed] [Google Scholar]
- 64.Levelt WJ. The alternation process in binocular rivalry. Br J Psychol. 1966;57:225–238. [Google Scholar]
- 65.Antinori A, Smilliec LD, Carter OL. Personality measures link slower binocular rivalry switch rates to higher levels of self-discipline. Front Psychol. 2017;7:2008. doi: 10.3389/fpsyg.2016.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westheimer G. The grain of visual space. Cold Spring Harb Symp Quant Biol. 1990;55:759–763. doi: 10.1101/sqb.1990.055.01.071. [DOI] [PubMed] [Google Scholar]
- 67.Galli-Resta L, Piccardi M, Ziccardi L, et al. Early detection of central visual function decline in cone-rod dystrophy by the use of macular focal cone electroretinogram. Invest Opthalmol Vis Sci. 2013;54:6560–6569. doi: 10.1167/iovs.13-12676. [DOI] [PubMed] [Google Scholar]
- 68.ICD-10 World Health Organization. The ICD-10 classification of mental and behavioural disorders. Int Classif. 1992;10:1–267. [Google Scholar]
- 69.Kupers R, Ptito M. Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neurosci Biobehav Rev. 2014;41:36–52. doi: 10.1016/j.neubiorev.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Cohen LG, Weeks RA, Sadato N, Celnik P, Ishii K, Hallett M. Period of susceptibility for cross-modal plasticity in the blind. Ann Neurol. 1999;45:451–460. doi: 10.1002/1531-8249(199904)45:4<451::aid-ana6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 71.van Loon AM, Knapen T, Scholte HS, John-Saaltink E, Donner TH, Lamme VA. GABA shapes the dynamics of bistable perception. Curr Biol. 2013;23:823–827. doi: 10.1016/j.cub.2013.03.067. [DOI] [PubMed] [Google Scholar]
- 72.O’Shea RP, Blake R, Wolfe JM. Binocular rivalry and fusion under scotopic luminances. Perception. 1994;23:771–784. doi: 10.1068/p230771. [DOI] [PubMed] [Google Scholar]
- 73.Kiser AK, Mladenovich D, Eshraghi F, Bourdeau D, Dagnelie G. Reliability and consistency of dark-adapted psychophysical measures in advanced eye disease. Invest Ophthalmol Vis Sci. 2006;47:444–452. doi: 10.1167/iovs.04-1146. [DOI] [PubMed] [Google Scholar]
- 74.Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic action in the autistic brain. Curr Biol. 2016;26:80–85. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 75.Holopigian K, Greenstein V, Seiple W, Carr RE. Rates of change differ among measures of visual function in patients with retinitis pigmentosa. Ophthalmology. 1996;103:398–405. doi: 10.1016/s0161-6420(96)30679-9. [DOI] [PubMed] [Google Scholar]
- 76.Birch DG, Anderson JL, Fish GE. Yearly rates of rod and cone functional loss in retinitis pigmentosa and cone-rod dystrophy. Ophthalmology. 1999;106:258–268. doi: 10.1016/S0161-6420(99)90064-7. [DOI] [PubMed] [Google Scholar]
- 77.Madreperla SA, Palmer RW, Massof RW, Finkelstein D. Visual acuity loss in retinitis pigmentosa: relationship to visual field loss. Arch Ophthalmol. 1990;108:358–361. doi: 10.1001/archopht.1990.01070050056030. [DOI] [PubMed] [Google Scholar]
- 78.Galli-Resta L, Placidi G, Campagna F, et al. Central retina functional damage in usher syndrome type 2: 22 years of focal macular ERG analysis in a patient population from central and southern Italy. Invest Opthalmol Vis Sci. 2018;59:3827–3835. doi: 10.1167/iovs.17-23703. [DOI] [PubMed] [Google Scholar]
- 79.McCollough C. Color adaptation of edge-detectors in the human visual system. Science. 1965;149:1115–1116. doi: 10.1126/science.149.3688.1115. [DOI] [PubMed] [Google Scholar]
- 80.Bock AS, Binda P, Benson NC, Bridge H, Watkins KE, Fine I. Resting-state retinotopic organization in the absence of retinal input and visual experience. J Neurosci. 2015;35:12366–12382. doi: 10.1523/JNEUROSCI.4715-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lunghi C, Binda P, Morrone MC. Touch disambiguates rivalrous perception at early stages of visual analysis. Curr Biol. 2010;20:R143–R144. doi: 10.1016/j.cub.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 82.Lunghi C, Morrone MC. Early interaction between vision and touch during binocular rivalry. Multisens Res. 2013;26:291–306. doi: 10.1163/22134808-00002411. [DOI] [PubMed] [Google Scholar]
- 83.Lunghi C, Alais D. Touch interacts with vision during binocular rivalry with a tight orientation tuning. PLoS One. 2013;8:e58754. doi: 10.1371/journal.pone.0058754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lunghi C, Alais D. Congruent tactile stimulation reduces the strength of visual suppression during binocular rivalry. Sci Rep. 2015;5 doi: 10.1038/srep09413. 9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lo Verde L, Morrone MC, Lunghi C. Early cross-modal plasticity in adults. J Cogn Neurosci. 2017;29:520–529. doi: 10.1162/jocn_a_01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.