Abstract
Background
Hodgkin lymphoma (HL) is one of the most common haematological malignancies in young adults and, with cure rates of 90%, has become curable for the majority of individuals. Positron emission tomography (PET) is an imaging tool used to monitor a tumour’s metabolic activity, stage and progression. Interim PET during chemotherapy has been posited as a prognostic factor in individuals with HL to distinguish between those with a poor prognosis and those with a better prognosis. This distinction is important to inform decision‐making on the clinical pathway of individuals with HL.
Objectives
To determine whether in previously untreated adults with HL receiving first‐line therapy, interim PET scan results can distinguish between those with a poor prognosis and those with a better prognosis, and thereby predict survival outcomes in each group.
Search methods
We searched MEDLINE, Embase, CENTRAL and conference proceedings up until April 2019. We also searched one trial registry (ClinicalTrials.gov).
Selection criteria
We included retrospective and prospective studies evaluating interim PET scans in a minimum of 10 individuals with HL (all stages) undergoing first‐line therapy. Interim PET was defined as conducted during therapy (after one, two, three or four treatment cycles). The minimum follow‐up period was at least 12 months. We excluded studies if the trial design allowed treatment modification based on the interim PET scan results.
Data collection and analysis
We developed a data extraction form according to the Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies (CHARMS). Two teams of two review authors independently screened the studies, extracted data on overall survival (OS), progression‐free survival (PFS) and PET‐associated adverse events (AEs), assessed risk of bias (per outcome) according to the Quality in Prognosis Studies (QUIPS) tool, and assessed the certainty of the evidence (GRADE). We contacted investigators to obtain missing information and data.
Main results
Our literature search yielded 11,277 results. In total, we included 23 studies (99 references) with 7335 newly‐diagnosed individuals with classic HL (all stages).
Participants in 16 studies underwent (interim) PET combined with computed tomography (PET‐CT), compared to PET only in the remaining seven studies. The standard chemotherapy regimen included ABVD (16) studies, compared to BEACOPP or other regimens (seven studies). Most studies (N = 21) conducted interim PET scans after two cycles (PET2) of chemotherapy, although PET1, PET3 and PET4 were also reported in some studies. In the meta‐analyses, we used PET2 data if available as we wanted to ensure homogeneity between studies. In most studies interim PET scan results were evaluated according to the Deauville 5‐point scale (N = 12).
Eight studies were not included in meta‐analyses due to missing information and/or data; results were reported narratively. For the remaining studies, we pooled the unadjusted hazard ratio (HR). The timing of the outcome measurement was after two or three years (the median follow‐up time ranged from 22 to 65 months) in the pooled studies.
Eight studies explored the independent prognostic ability of interim PET by adjusting for other established prognostic factors (e.g. disease stage, B symptoms). We did not pool the results because the multivariable analyses adjusted for a different set of factors in each study.
Overall survival
Twelve (out of 23) studies reported OS. Six of these were assessed as low risk of bias in all of the first four domains of QUIPS (study participation, study attrition, prognostic factor measurement and outcome measurement). The other six studies were assessed as unclear, moderate or high risk of bias in at least one of these four domains. Nine studies were assessed as high risk, and three studies as moderate risk of bias for the domain study confounding. Eight studies were assessed as low risk, and four studies as high risk of bias for the domain statistical analysis and reporting.
We pooled nine studies with 1802 participants. Participants with HL who have a negative interim PET scan result probably have a large advantage in OS compared to those with a positive interim PET scan result (unadjusted HR 5.09, 95% confidence interval (CI) 2.64 to 9.81, I² = 44%, moderate‐certainty evidence). In absolute values, this means that 900 out of 1000 participants with a negative interim PET scan result will probably survive longer than three years compared to 585 (95% CI 356 to 757) out of 1000 participants with a positive result.
Adjusted results from two studies also indicate an independent prognostic value of interim PET scan results (moderate‐certainty evidence).
Progression‐free survival
Twenty‐one studies reported PFS. Eleven out of 21 were assessed as low risk of bias in the first four domains. The remaining were assessed as unclear, moderate or high risk of bias in at least one of the four domains. Eleven studies were assessed as high risk, nine studies as moderate risk and one study as low risk of bias for study confounding. Eight studies were assessed as high risk, three as moderate risk and nine as low risk of bias for statistical analysis and reporting.
We pooled 14 studies with 2079 participants. Participants who have a negative interim PET scan result may have an advantage in PFS compared to those with a positive interim PET scan result, but the evidence is very uncertain (unadjusted HR 4.90, 95% CI 3.47 to 6.90, I² = 45%, very low‐certainty evidence). This means that 850 out of 1000 participants with a negative interim PET scan result may be progression‐free longer than three years compared to 451 (95% CI 326 to 569) out of 1000 participants with a positive result.
Adjusted results (not pooled) from eight studies also indicate that there may be an independent prognostic value of interim PET scan results (low‐certainty evidence).
PET‐associated adverse events
No study measured PET‐associated AEs.
Authors' conclusions
This review provides moderate‐certainty evidence that interim PET scan results predict OS, and very low‐certainty evidence that interim PET scan results predict progression‐free survival in treated individuals with HL. This evidence is primarily based on unadjusted data. More studies are needed to test the adjusted prognostic ability of interim PET against established prognostic factors.
Keywords: Humans, Antineoplastic Combined Chemotherapy Protocols, Antineoplastic Combined Chemotherapy Protocols/therapeutic use, Chemoradiotherapy, Chemoradiotherapy/methods, Disease Progression, Disease‐Free Survival, Hodgkin Disease, Hodgkin Disease/diagnostic imaging, Hodgkin Disease/drug therapy, Positron‐Emission Tomography, Positron‐Emission Tomography/methods, Prognosis, Randomized Controlled Trials as Topic
Imaging with positron emission tomography (PET) during chemotherapy to predict outcome in adults with Hodgkin lymphoma
Review question
This Cochrane Review aimed to find out whether the results of a positron emission tomography (PET) during therapy in people with Hodgkin lymphoma (HL) can help to distinguish between those with a poor prognosis and those with a better prognosis, and predict survival outcomes in each group.
Background
Hodgkin lymphoma is a cancer which affects the lymphoid system of the body. It is considered a relatively rare disease (two to three cases per 100,000 people every year in Western countries), that is most common in young adults in their twenties, but it can also occur in children and elderly people. As treatment options have improved, most people with HL can now be cured. It is important that individuals receive the treatment with the greatest efficacy and least toxicity possible. PET is an imaging tool for assessing the disease stage of an individual, and monitoring tumour activity. It has been suggested that PET performed during therapy (so‐called interim PET, e.g. after two cycles of chemotherapy) can distinguish between people who respond well to therapy and those who do not respond well. The aim of this review was to demonstrate the prognostic ability to distinguish between these groups, and predict survival outcomes in each group, to help clinicians make an informed decision on the treatment pathway to improve long‐term outcomes and safety for people with HL.
Study characteristics
We included 23 studies to explore the association between interim PET scan results after one to four cycles of chemotherapy and survival outcomes in adults with HL (all stages). We contacted 10 authors, and six provided us with relevant information and/or data.
Key results
In 16 included studies, participants received either ABVD chemotherapy or BEACOPP chemotherapy (four studies) only, with or without radiotherapy. In 16 studies, participants underwent an interim PET scan in combination with a computed tomography (CT) (PET‐CT), which have higher accuracy in detecting primary and secondary cancers than a PET scan alone. In the remaining seven studies, PET‐only was conducted. Twenty‐one studies conducted interim PET scans after two cycles (PET2) of chemotherapy.
Eight studies did not report enough data on our outcomes or population of interest, so we reported the results from these studies narratively. We combined individual study results in meta‐analyses to provide robust evidence for our outcomes of interest overall survival and progression‐free survival. No study measured PET‐associated adverse events (harms).
For overall survival, combined results from nine studies (1802 participants) show that there is probably a large advantage in overall survival for people with a negative interim PET scan compared to people with a positive interim PET scan. For progression‐free survival, combined results from 14 studies (2079 participants) show that interim PET‐negative people may have an advantage for progression‐free survival, compared to interim PET‐positive people, but we are uncertain about this result. These are unadjusted results, where interim PET was tested as the only prognostic factor.
Eight studies reported adjusted results, where the independent prognostic ability of interim PET was assessed against other established prognostic factors (e.g. disease stage, B symptoms). We could not combine individual study results because the studies did not include identical sets of prognostic factors. Nevertheless, their results indicate a probable independent prognostic ability of interim PET to predict both outcomes.
Certainty of the evidence
Regarding the unadjusted results, we rated our certainty of the evidence as 'moderate' for overall survival. This means that the true effect is likely to be close to the estimated effect, but there is a possibility that it is substantially different. For progression‐free survival, we rated our certainty of the evidence as 'very low', meaning that we have little confidence in the effect estimate, and that the true effect is likely to be substantially different from the estimated effect.
Regarding the adjusted results, we rated our certainty of the evidence as 'moderate' for overall survival, and 'low' for progression‐free survival.
How up‐to‐date is this review?
We searched data bases up until 2 April 2019, and one trial registry on 25 January 2019.
Summary of findings
Summary of findings for the main comparison.
Comparison of interim PET‐negative and interim PET‐positive individuals with Hodgkin Lymphoma
| Comparison of interim PET‐positive and interim PET‐negative participants with Hodgkin lymphoma | ||||||
| Population: Individuals with Hodgkin lymphoma Setting: Eleven studies recruited participants from a total of 28 haemato‐oncology treatment centres/hospitals in Brazil (N = 1), China (N = 1), Denmark (N = 4), France (N = 4), Italy (N = 3), Poland (N = 11), UK (N = 2) and the USA (N = 2). One study (Straus 2011) included participants from 29 institutions, but did not report the countries. One study (Simon 2016) reported the country (Hungary) but not the number of centres. One multi‐centre study (Hutchings 2014) recruited participants from four countries (USA, Italy, Poland and Denmark). One RCT (Kobe 2018) included participants from 301 hospitals and private practices in Germany, Switzerland, Austria, the Netherlands, and the Czech Republic. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Interim PET‐negative | Risk with Interim PET‐positive | |||||
| Overall survival Follow up: 3 years |
Low | HR 5.09 (2.64 to 9.81) | 1802 (9 studies) | ⊕⊕⊕⊝ MODERATE 2 3 4 | ||
| 900 per 1.000 1 | 585 per 1.0001 (356 to 757) | |||||
| High | ||||||
| 980 per 1.000 1 | 902 per 1.0001 (820 to 948) | |||||
| Progression‐free survival Follow up: 3 years |
Low | HR 4.90 (3.47 to 6.90) | 2079 (14 studies) | ⊕⊝⊝⊝ VERY LOW6 7 8 | ||
| 850 per 1.000 5 | 451 per 1.000 5 (326 to 569) | |||||
| High | ||||||
| 940 per 1.000 5 | 738 per 1.000 5 (653 to 807) | |||||
| Adverse events associated with PET ‐ not reported | No study measured PET‐associated adverse events. | ‐ | ‐ | ‐ | ||
| Overall survival (adjusted effect estimate) | Two studies reported an adjusted effect estimate for overall survival after interim PET2: a hazard ratio of 3.2 (95% CI 1.3 to 8.4, P = 0.02) (Kobe 2018) and 11.51 (95% CI 3.14 to 42.86, P < 0.001) (Simon 2015) indicates the independent prognostic value of interim PET over and above other clinically relevant prognostic factors. | ‐ | 843 (2 studies) | ⊕⊕⊕⊝ MODERATE 9 | ||
| Progression‐free survival (adjusted effect estimate) | Eight studies conducted a multivariable analysis to test the independent prognostic value of interim PET over and above other clinically relevant prognostic factors. Four of these studies reported a hazard ratio as the adjusted effect estimate, of which the value ranges from 2.4 to 36.89, indicating the independent prognostic value of interim PET2.10 | ‐ | 996 (4 studies)10 | ⊕⊕⊝⊝ LOW 11 12 | ||
| *The survival in the PET‐positive group (and its 95% confidence interval) is based on the assumed survival in the PET‐negative group. CI: Confidence interval; HR: Hazard ratio; PET: positron emission tomography | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The assumed event‐free survival in the control group is based on the survival rate of the interim PET‐negative participants at 3 years in the studies included (the lowest survival rate from Cerci 2010 and the highest survival rate from Kobe 2018).
2 Moderate risk of bias in three studies and high risk of bias in six studies for the domain 'study confounding', and high risk of bias in four studies for the domain 'statistical analysis and reporting'. Downgraded by 1 point for risk of bias.
3 For one study we used the reported hazard ratio. For seven studies we had to estimate the hazard ratio and for one study we re‐calculated it (Trivella 2006). Downgraded by 1 point for imprecision.
4 Upgraded by one point due to the large effect showing the large difference between interim PET‐negative and interim PET‐positive participants (HR 5.09, CI 2.64 to 9.81).
5 The assumed event‐free survival in the control group is based on the survival rate of the interim PET‐negative participants at 3 years in the studies included (the lowest survival rate from Rossi 2014 and the highest survival rate from Kobe 2018).
6 Moderate risk of bias in six studies and high risk of bias in eight studies for the domain 'study confounding', and high risk of bias in six studies for the domain 'statistical analysis and reporting'. Downgraded by 1 point for risk of bias.
7The definition of PFS varied across studies, downgraded by 1 point for inconsistency
8 For three studies we used the reported hazard ratio. For ten studies we had to estimate the value, and for one study we had to re‐calculate it (Trivella 2006). Downgraded by 1 point for imprecision.
9 Moderate risk of bias for the domain study confounding in both studies, and high risk of bias for the domain statistical analysis and reporting for one study. Downgraded by 1 point for risk of bias.
10Hutchings 2006; Kobe 2018; Mesguich 2016; Simon 2016.
11 Moderate risk of bias for the domain study confounding in the four studies, and moderate and high risk of bias for the domain statistical analysis and reporting for two studies. Also high risk of bias for the domain study participation in one study. Downgraded by 1 point for risk of bias.
12 Studies included a heterogenous set of covariates in the adjusted analyses. Downgraded by 1 point for inconsistency.
Background
Description of the condition
Hodgkin lymphoma (HL) is a cancer of the lymph nodes and the lymphoid system with possible involvement of other organs such as the liver, lung, bone or bone marrow (Lister 1989). With an annual incidence of approximately two to three per 100,000 inhabitants in Western countries, HL is a comparatively rare disease, but it is one of the most common malignancies in young adults (Howlader 2015). In industrialised countries, the age distribution of HL shows a first peak in the third decade and a second peak after the age of 50 (Thomas 2002).
The World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues distinguishes between two types of HL: classical HL, representing about 95% of all HL; and lymphocyte‐predominant HL, representing about 5% of all HL (Swerdlow 2008). Both types differ in morphology, phenotype and molecular features, and therefore in clinical behaviour and presentation (Re 2005).
The Ann Arbor Classification is used for staging and distinguishes between four different tumour stages. Stages one to three indicate the degree of lymph node and localised extranodal organ involvement, or both, and stage four includes disseminated organ involvement, which can be found in 20% of cases. Factors associated with a poor prognosis include a large mediastinal mass, three or more involved lymph node areas, a high erythrocyte sedimentation rate, extranodal lesions, B symptoms (weight loss > 10%, fever, drenching night sweats) and advanced age, but the factors considered as significant vary slightly between different study groups (German Study Hodgkin Lymphoma Study Group (GHSG); European Organization for Research and Treatment of Cancer (EORTC); National Cancer Institute of Canada (NCIC)). The Cotswold modification of the Ann Arbor Classification also takes into consideration the occurrence of bulky disease (largest tumour diameter greater than 10 cm) (Lister 1989). Hodgkin lymphoma is classified into early favourable, early unfavourable and advanced stage (Engert 2007; Klimm 2005). In Europe, the early favourable‐stage group usually comprises Ann Arbor stages I and II without risk factors. The early unfavourable‐stage group includes individuals with Ann Arbor stages I or II and one or more risk factors. Most individuals with stages IIB, III or IV disease are included in the advanced‐stage risk group (Engert 2003).
With cure rates of up to 90%, HL is one of the most curable cancers worldwide (Engert 2010; Engert 2012; Rancea 2013a; von Tresckow 2012). A combination of adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) is widely accepted as the standard chemotherapy regimen in early‐stage HL (Bröckelmann 2018, Canellos 1992; Engert 2010). Individuals in this stage usually receive a combination of chemotherapy and involved‐field radiation therapy (IF‐RT) (Engert 2010; von Tresckow 2012), whereas those with advanced‐stage disease receive an intensified regimen, such as BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone) (Skoetz 2017a; Borchmann 2011; Engert 2012; Skoetz 2013), or ABVD. A large randomised study showed that two cycles of ABVD followed by 20 Gy of IF‐RT is sufficient for the treatment of early‐favourable HL (Engert 2010), which is implemented into current standard treatment, whereas four cycles of chemotherapy followed by 30 Gy IF‐RT is more suitable for individuals with early‐unfavourable HL. Approximately 10% of people with HL will be refractory to initial treatment or will relapse; this is more common in people with advanced stage or bulky disease. These individuals can be treated with high‐dose chemotherapy and autologous stem cell transplantation (Rancea 2013). Immunotherapy for relapsed HL as another possible approach is under active investigation (Moskowitz 2018).
The current treatment approach for HL aims to maximise progression‐free and OS and to minimise acute and long‐term toxicities like cardiac and pulmonary damage, infertility and secondary cancers. Development of a secondary cancer is one of the major causes of morbidity and mortality once the risk of progression and relapse of HL is over, i.e. from about five years after first‐line treatment onwards. In a large systematic review based on individual patient data in people with HL, Franklin and colleagues demonstrated that treatment de‐intensification by avoiding additional radiotherapy reduces the risk of a secondary cancer (Franklin 2005).
Description of the index (prognostic) factor
A prognostic factor is a characteristic of a patient or the disease (e.g. age, sex, co‐morbidities, disease stage, blood or imaging results) that is likely to predict patient outcomes or health events, often related to OS and disease‐free survival (Moons 2009; Riley 2013). Prognostic information ultimately provides a basis for the determination of treatment and also helps to stratify individuals for treatment according to their risk of future outcomes (Riley 2013). Established prognostic factors in HL include age, gender, B symptoms, Ann Arbor disease stage, bulky disease, albumin level, anaemia and white blood cell count, amongst others (Cuccaro 2014; Josting 2010; Kılıçkap 2013). Particularly male gender, advanced disease stage or age, and a low level of albumin, for example, are associated with worse prognosis and survival outcomes (Cuccaro 2014; Josting 2010).
The prognostic factor to be examined in this review is the tumour's metabolic activity, its stage, and progression as captured by [18F]‐fluorodeoxy‐D‐glucose (FDG)‐positron emission tomography (PET, also called PET scanning), which is an imaging tool. The principle of FDG‐PET is based on a radio‐labelled glucose analogue being a good indicator of the glucose metabolism of a tissue. It comprises two parts: a vector (2‐deoxy‐D‐glucose) taken up by cells with a high metabolic rate, and 18F, a positron‐emitting nuclide, which is detected by scintigraphy. FDG‐PET scanning provides the opportunity to identify the state and degree of progression of FDG‐avid tumours and has therefore become a standard imaging tool for various cancers (Boellaard 2010). Hodgkin lymphoma is a FDG‐avid tumour; in a study of 233 people with HL, 100% were FDG‐avid (Weigler‐Sagie 2010). However, as the field of imaging continuously evolves, it is now widely accepted to use PET in combination with a computed tomography (CT), known as PET‐CT (Barrington 2014). The combination of PET‐CT is argued to provide clearer imaging and a more accurate measurement of nodal size (Cheson 2014). Nevertheless, in the studies included in this review, the use of PET or PET‐CT varied.
Over the last few decades FDG‐PET has been used more and more for staging, prognosis, treatment planning and response evaluation in individuals with HL, and is a widely accepted procedure (Barrington 2017a; Cheson 2014; Fitzgerald 2019; Kobe 2010a; Markova 2009; Meignan 2009; Radford 2015; Specht 2007). FDG‐PET is primarily used for the pretreatment assessment in order to determine the stage of the disease of an individual and thereby to decide on the appropriate treatment regimen (Cheson 2014; Meignan 2009). However, it is now argued that PET should also be conducted during first‐line chemotherapy in individuals with HL, namely interim PET after a few cycles of chemotherapy (Barrington 2017a; Meignan 2009). The result of the interim PET scan (positive or negative) is believed to be a good predictor of outcome, aiding the distinction between individuals with a poor prognosis from those with a better prognosis, while undergoing early treatment (Gallamini 2007; Kobe 2010; Markova 2012). Therapy adaptation based on interim PET results was introduced after detailed exploration of the FDG‐PET procedure (Engert 2012; Kobe 2008a), the idea being to achieve maximum efficacy in terms of OS and progression‐free survival (PFS).We will refer to the prognostic factor henceforth as 'interim PET'.
Why it is important to do this review
There is a need to systematically explore the prognostic ability of the factor (interim PET) in conditions where there is no treatment adaptation. The 'no treatment adaptation' clause is a rather important point in the prognostic exploration as adapting treatment based on interim PET results in daily practice when its prognostic ability is not yet proven is not desired. There is one systematic review on the prognostic value of interim PET without treatment adaptation in individuals with HL (Adams 2015a). However, this review looked at 'treatment failure' as an outcome of the interim PET scan, which is different to the outcomes the current review explored. Moreover, and despite the fact that it is entitled as a review of prognosis studies, the methodology used is akin to diagnostic test evaluation (with calculations of diagnostic odds ratio, specificity and sensitivity), rather than using established prognostic methodology and crucially, the confidence in the calculated estimates was not rated. Moreover, the review included studies published before December 2014 and, therefore, important research published since that time is not included.
One Cochrane Review on the role of PET‐adapted treatment modification for people with HL found some evidence that PFS was decreased in people with early‐stage HL and a negative PET scan receiving only chemotherapy (PET‐adapted therapy) compared to those receiving radiotherapy in addition to chemotherapy (which is the standard therapy regimen) (Sickinger 2015). A similar result was found in another Cochrane Review (Blank 2017). The authors compared the effects of chemotherapy alone versus chemotherapy plus radiotherapy on outcome and safety for adults with early stage HL. They found moderate evidence that when individuals receive the same number of chemotherapy cycles, the addition of radiotherapy can improve PFS. However, both reviews were not able to give definite conclusions on the effect on OS. Another systematic review suggests the change of therapy after interim PET in advanced‐stage individuals only (Amitai 2018). Several randomised controlled trials (RCTs) have recently been published that investigated the consequences of treatment adaptation based on interim PET scan results on outcome and safety for individuals with HL (Andre 2017; Casasnovas 2019; Kobe 2018; Johnson 2016; Radford 2015).
The prognostic role of interim PET in individuals with HL undergoing first‐line chemotherapy is very important and will strongly influence decision‐making particularly regarding the choice of subsequent treatments. Therefore, we have summarised all available data from identified studies and included these in a meta‐analysis when they were sufficiently homogeneous. Our aim was to produce robust evidence based on the improved power that a meta‐analysis provides over the limitations of individual primary studies, and grade the evidence. A reliable answer to the question of the prognostic value of interim PET scan to predict survival outcomes in individuals with HL will strongly influence decision‐making at a crucial point of an individual’s treatment pathway. Moreover, grading the evidence on the prognostic value of interim PET will provide readers with an estimate of how much they can rely on the calculated results.
The aim of this systematic review was to determine whether in previously untreated adults with HL receiving first‐line therapy, interim PET scan results can distinguish between those with a poor prognosis and those with a better prognosis, and whether it can predict survival outcomes in each group. Thereby, we assessed the prognostic value of interim PET scan results. Meta‐analyses and grading of the evidence allow a conclusion of whether interim PET is a prognostic factor. This comprehensive overview will have a great impact on international guidelines and clinical pathways, and will contribute to a high‐grade support in clinical decision‐making for effective, supportive strategies for the individual patient.
Objectives
To determine whether in previously untreated adults with Hodgkin lymphoma (HL) receiving first‐line therapy, interim positron emission tomography (PET) scan results can distinguish between those with a poor prognosis and those with a better prognosis, and thereby predict survival outcomes in each group.
Primary objective
To identify all studies evaluating interim PET scan results as a prognostic factor, describe the characteristics and risk of bias of included studies and meta‐analyse results on the association between PET scan results and overall survival (OS), progression‐free survival (PFS) and PET‐associated adverse events.
PICOTS
We used the PICOTS (population, index, comparator, outcome(s), timing, setting) system to describe the key items for framing this review and its objective and methodology (Table 1) (Debray 2017; Riley 2019).
Table 1. PICOTS system
| Population | Index (prognostic) factor | Comparator | Outcome(s) | Timing | Setting |
|
Interim PET scan results | Not applicable to this review |
The outcome should be measured after a minimum follow‐up of 12 months. |
|
Hospital/treatment centre |
Methods
This is a systematic review of prognostic factor studies.
Criteria for considering studies for this review
Types of studies
We included retrospective and prospective studies evaluating interim PET scan results in a minimum of 10 individuals with Hodgkin lymphoma (HL) undergoing first‐line therapy.
We excluded studies that modified the treatment regimen based on the interim PET scan results in order to draw an unbiased conclusion of the ability of interim PET to predict the outcomes under study.
Participants
We included studies on adults with newly diagnosed classic HL receiving first‐line therapy. If in a study a percentage of the included participants were adolescents but received adult treatment regimen and dosage, and the study considered them as adults, then we also accepted this 'adult' definition.
All participants received an interim PET scan during chemotherapy (e.g. after one, two, three and/or four cycles of chemotherapy), and continued with the planned chemotherapy regimen, without treatment adaptation due to the interim PET scan result.
Index (prognostic) factor
We included studies that assessed interim PET scan results as the index (prognostic) factor to predict survival outcomes. We expected the interim PET scan to be conducted during first‐line treatment of adults with HL, and without interim PET‐guided treatment adaptation, meaning participants should be treated in the same way regardless of the interim PET scan result. We accepted all studies that conducted a PET or PET‐CT (see Background 'Description of index (prognostic) factor').
In the literature, it is generally recommended to use a five‐point scale to assess the grade of uptake and report the PET scan result (Meignan 2009). Generally, scores 1‐3 indicate PET‐negativity, while scores 4‐5 indicate PET‐positivity (Barrington 2014). Most of the included studies used a validated scale, such as the 5‐PS Deauville criteria (Meignan 2009), the Lugano classification (Cheson 2014), the Imaging Subcommittee of International Harmonization Project in Lymphoma criteria (Juweid 2007) or the joint Italian‐Danish study criteria (Gallamini 2007).
Type of outcome measures
Primary outcome
Overall survival (OS), defined as the time to death due to any cause.
We chose OS as our primary outcome because it has the greatest clinical relevance and is most important for individuals with HL. Furthermore, death due to any cause is an objective endpoint not susceptible to bias by the outcome assessor.
Secondary outcomes
Progression‐free survival (PFS), defined as the time to disease progression, relapse, death due to any cause or last follow‐up.
Adverse events (AEs), defined as any event associated with the index factor (e.g. radiation safety).
To report meaningful findings, the required minimum follow‐up period was 12 months for each outcome.
Search methods for identification of studies
Electronic searches
Reporting and therefore retrieval of prognostic factor studies is very poor, as evaluation of guidelines on reporting of prognostic markers in cancer have shown (Altman 2012; Mallett 2010; McShane 2005). Moreover, no specific search filter exists for this new methodological approach, therefore published filters have to be combined for a sensitive search strategy (Geersing 2012). However, as PET scans often are not reported as a prognostic factor, we did not combine our search strategy with a filter for prognosis research. Therefore, the search strategy was not very specific and the results were screened independently and in detail by two teams of two review authors. Furthermore, we did not apply a language restriction in order to reduce the language bias, according to chapter six of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
We searched the following databases.
-
Databases of medical literature
Cochrane Central Register of Controlled Trials (CENTRAL; 2 April 2019, Issue 11) (Appendix 1)
MEDLINE Ovid SP (1946 until 2 April 2019) (Appendix 2)
Embase (1990 until 2 April 2019) (Appendix 2)
Conference proceedings of annual meetings of the following societies for abstracts (2000 to 2019)
American Society of Hematology
European Hematology Association
International Symposium on Hodgkin Lymphoma
We searched ClinicalTrials.gov (on 25 January 2019 using the query PET and Hodgkin lymphoma) to identify clinical trials.
Searching other resources
-
Handsearching of references
We searched the references of all identified studies, relevant review articles and current treatment guidelines for further literature to find other relevant studies and to identify associated articles.
-
Personal contacts
We contacted 10 principal investigators of included studies for further information, of whom six replied and answered our questions for clarification. Two out of these six provided us also with relevant data to conduct our analyses.
Data collection and analysis
Selection of studies
Two teams of two review authors (AA, LE, MHT, NS) independently screened the results of the search strategies to identify eligible studies by reading the titles and abstracts in Covidence (Covidence). In case of disagreements, consensus between the two review authors was reached by discussion of the full‐text publication. When consensus could not be reached, a third review author was consulted for final decision (Higgins 2011).
We documented the study selection process in a flow chart as recommended in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement (Moher 2009), showing the total numbers of retrieved references and the numbers of included and excluded studies (Figure 1).
Figure 1.
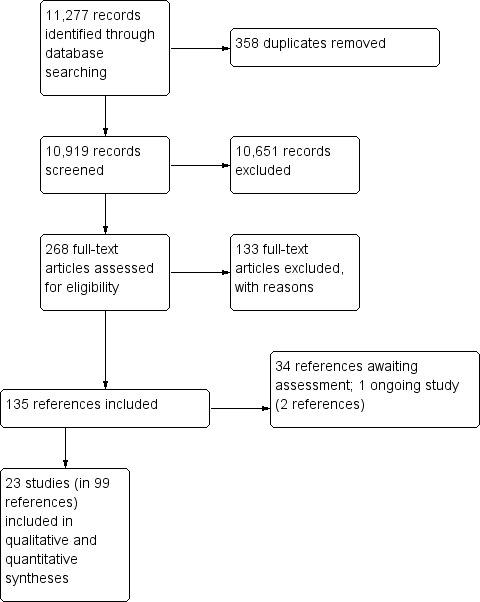
Study flow diagram according to PRISMA
Data extraction and management
We developed a data extraction form specific to studies of prognostic factors based on the Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies (CHARMS) (Moons 2014). The form was piloted using four of the included studies, and then further assessed during several teleconferences between the review authors to discuss required changes. After several amendments of the form, two teams of two review authors (AA, LE, MHT, NS) independently extracted all relevant data from the included studies. After data extraction, we contacted 10 principal investigators of included studies to request additional information.
Our form included the following items (in short).
General information
i.e. Author, title, source, publication date, country, language, duplicate publications
-
Source of data
i.e. Cohort, prospective planned study, randomised study participants, or registry data
-
Participants
Participant eligibility and recruitment method (e.g. consecutive participants, location, number of centres, setting, inclusion and exclusion criteria)
Participant description (e.g. age, gender, stage of disease)
Details of treatments received
Study dates
-
Prognostic factor
Definition and method for measurement of prognostic factor
Timing of prognostic factor measurement (number of chemotherapy cycles before and after measurement of the prognostic factor)
-
Outcomes to be predicted
Definition and method for measurement of outcome
Was the same outcome definition (and method for measurement) used in all individuals?
Was the outcome assessed without knowledge of the prognostic factor (i.e. blinded)?
Time of outcome occurrence or summary of duration of follow‐up
-
Sample size
Number of participants and number of outcomes/events
-
Missing data
Number of participants with any missing value (include predictors and outcomes)
Handling of missing data (e.g. complete‐case analysis, imputation, or other methods)
-
Reported results
Overall survival (OS) (including duration of follow‐up)
Progression‐free survival (PFS) (including duration of follow‐up)
Adverse events (AEs) (including duration of follow‐up)
Risk of bias
For quality assessment (risk of bias) of the prognostic factor studies, we used the Quality in Prognostic Studies (QUIPS) tool (Hayden 2013). Two teams of two review authors (AA, LE, MHT, NS) independently assessed the risk of bias of the included studies according to the domains of the QUIPS tool. We judged each domain by taking into account the criteria listed for each domain in the QUIPS tool (Hayden 2013), and also provided a brief statement supporting our judgement.
After consultation with the original authors of the QUIPS tool, we adapted the scoring, which initially provided three categories for rating the risk of bias of the included studies ('low', 'moderate' and 'high' risk of bias). Due to the inconsistent reporting of the included studies, we added 'unclear' as a further possible rating when information was clearly missing and standard risk of bias rating according to QUIPS was not possible.
We made the following judgements.
Low risk of bias: the relationship between the prognostic factor and outcome is unlikely to be different for participants and eligible non‐participants.
Moderate risk of bias: the relationship between the prognostic factor and outcome may be different for participants and eligible non‐participants.
High risk of bias: the relationship between the prognostic factor and outcome is very likely to be different for participants and eligible non‐participants.
Unclear risk of bias: the study does not provide sufficient information that allows a clear judgement for this domain.
Furthermore, we decided to assess the risk of bias per outcome in each study because not all studies reported all of our outcomes of interest, and even studies reporting at least two of our outcomes showed differences in their outcome reporting.
We judged the following domains and criteria.
-
Study participation
Adequate participation in the study by eligible persons
Description of the source population or population of interest
Description of the baseline study sample
Adequate description of the sampling frame and recruitment
Adequate description of the period and place of recruitment
Adequate description of inclusion and exclusion criteria
-
Study attrition
Adequate response rate for study participants
Description of attempts to collect information on participants who dropped out
Reasons for loss to follow‐up are provided
Adequate description of participants lost to follow‐up
There are no important differences between participants who completed the study and those who did not
-
Prognostic factor measurement
A clear definition or description of the prognostic factor is provided
Method of prognostic factor measurement is adequately valid and reliable
Continuous variables are reported or appropriate cut points are used
The method and setting of measurement of prognostic factor is the same for all study participants
Adequate proportion of the study sample has complete data for the prognostic factor
Appropriate methods of imputation are used for missing prognostic factor data
-
Outcome measurement
A clear definition of the outcome is provided
Method of outcome measurement used is adequately valid and reliable
The method and setting of outcome measurement is the same for all study participants
-
Other prognostic factors (covariates)
Other prognostic factors (covariates) are measured
Clear definitions of the important prognostic factors (covariates) measured are provided
Measurement of all important prognostic factors (covariates) is adequately valid and reliable
The method and setting of prognostic factor measurement are the same for all study participants
Appropriate methods are used if imputation is used for missing data
Important potential prognostic factors (covariates) are accounted for in the study design
Important potential prognostic factors (covariates) are accounted for in the analysis
-
Statistical analysis and reporting
Sufficient presentation of data to assess the adequacy of the analytic strategy
Strategy for model building is appropriate and is based on a conceptual framework or model
The selected statistical model is adequate for the design of the study
There is no selective reporting of results
Reporting deficiencies
Methods and reporting in prognostic research often do not follow current methodological recommendations, limiting retrieval, reliability and applicability of these publications (Bouwmeester 2012; Peat 2014). There is evidence suggesting that prognosis research in cancer is cluttered with false‐positive studies, which would not have been published if the results were negative (Kyzas 2005; Kyzas 2007; Sauerbrei 2005). Moreover, studies evaluating prognostic factors are usually not prospectively registered and no protocol is published (Peat 2014; Riley 2013), resulting in difficulties to identify all studies and to assess potential risks of publication bias. We used sensitive search filters for the disease (HL) and the prognostic factor (interim PET scan results) without any specific filter for research on prognosis in order to increase retrieval.
Due to the expected large effect of hazard ratios (HRs), tests for funnel plot asymmetry could result in publication bias being incorrectly indicated by the test (Macaskill 2010). Therefore, we decided not to evaluate the risk of publication bias by funnel plot asymmetry and describe reporting deficiencies instead.
Data synthesis
We performed analyses according to the recommendations of Cochrane, and the Cochrane Prognosis Methods Group in particular, and used the Cochrane statistical package Review Manager 5 (Deeks 2011; Review Manager 2014). We are aware that since the protocol development, the methodology on assessing studies of prognosis has evolved; hence, some differences between the published protocol and this full review may exist to account for the updated guidance. We have listed these in Differences between protocol and review.
We pooled unadjusted (crude) HRs for OS and PFS by applying meta‐analysis using the RevMan's generic inverse variance methods random‐effects model. Due to reporting inefficiencies and the expected heterogeneity between studies, we only combined studies that were sufficiently similar (e.g. most studies used ABVD as the main therapy regimen, or most studies conducted interim PET after two cycles of chemotherapy). Studies did not always provide an HR and associated standard error (SE), which are the parameters needed for meta‐analysis. Where these values were not available, we estimated them from other available data where possible using an in‐house calculator based on published methods for recovering survival data (Altman 1999; Parmar 1998; Tierney 2007). Recovered data included information and results reported in the text, tables, and Kaplan‐Meier (K‐M) curves. We also contacted 10 principal investigators of included studies to either ask for additional data, or to clarify issues regarding the studies.
As prespecified in the protocol, we would have also pooled adjusted HRs from multivariable analyses of the included studies as adjusted results indicate the independent prognostic value of the main prognostic factor over and above other clinically relevant prognostic factors (Riley 2019). However, pooling of adjusted estimates is recommended only if the same (or very similar) prognostic factors (covariates) are adjusted for in multivariable analyses. Clinically relevant prognostic factors in individuals with HL include, for example, age, gender, disease stage and B symptoms (Cuccaro 2014). Regardless of not pooling the adjusted HR estimates, we still assessed the risk of bias with regard to multivariable analyses for all studies.
Detailed description of the estimation of hazard ratios (HRs) and standard errors (SEs)
We used unadjusted HRs as the effect measure for OS and PFS. In cases where the HR and SE were not reported, we estimated them from available data using an in‐house calculator (Trivella 2006), based on methods reported by Tierney 2007, Altman 1999 and Parmar 1998, or contacted authors to request additional data (Higgins 2011b). Recovered data included sample size, number of events, results such as the logrank P‐value and confidence intervals (CIs), which were reported in the text, tables, and K‐M curves. We kept detailed records of how the HR and SEs were calculated for each outcome in each included study. We identified the following six categories of HR precision.
HR was provided in the study, and the SE was either provided or easily estimated from reported CIs, and/or using the RevMan inbuilt calculator.
HR was provided but on checking while attempting to obtain the SE, there were errors and/or discrepancies with related provided data and we re‐estimated the HR.
HR and SE were not provided but all necessary data for their estimation were available in the study.
HR and SE were not provided. Other necessary data were available but not an exact logrank P value, hence the nearest value was used in the estimation. For example, if they reported P < 0.001, then the nearest exact value was used, in this case P = 0.0009.
HR and SE were not provided. Other necessary data were available but the number of events was estimated from the K‐M curves.
IPD data were available and HR and SE were accurately calculated.
We are aware that categories four and five are likely to over‐ or under‐estimate the HR and associated SE. However, they were the best estimates we could obtain. We consider the remaining categories as precise. We explored the precision of the estimates in a post‐hoc sensitivity analysis where the imprecise studies were temporarily removed to examine the robustness of the pooled result.
Grading the evidence
According to the recommendations of the GRADE working group, we rated and described the confidence in estimates for each outcome by assessing potential risk of bias, inconsistency, imprecision, indirectness and publication bias. We applied an approach that has been proposed for prognosis studies by the GRADE working group, suggesting that the starting point is one of high certainty of the evidence for observational studies (Iorio 2015).
Dealing with missing data
We dealt with missing data as suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We contacted ten principal investigators of included studies to answer our questions regarding the studies and/or to provide us with additional data. Six principal investigators replied and answered our questions, of which two also provided us with additional data necessary to perform our analyses. One investigator kindly provided us with individual participant data for the whole data set. In some studies, the description of the methodology was rather unclear or relevant information was missing. In addition, some studies did not fully report their statistical analyses and data were missing, which complicated a full assessment of the study. We performed sensitivity analysis to assess how sensitive the results were to reasonable changes in the assumptions that were made, and addressed the potential impact of missing data on the findings of this review in the Discussion.
Furthermore, we noticed that most studies applied exclusion criteria on the baseline population (such as unavailability of interim PET or descriptive information) without providing a description of the size of this population and/or reasons for missing information. We treated this as a potential source of selection bias in the domain study participation of the QUIPS tool.
Investigation of heterogeneity
We investigated and discussed clinical and statistical heterogeneity and design aspects of included studies as mentioned in the section 'Data extraction and data management'. We assessed between‐study heterogeneity using the I2 statistic (an I2 greater than 50% = moderate heterogeneity; an I2 greater than 80% = considerable heterogeneity) (Deeks 2011). As most studies of prognosis are observational in nature, we are aware that they are prone to higher and/or inflated heterogeneity. Hence, we also assessed the Tau2 values from the meta‐analyses to be able to make a more robust judgment on the degree of statistical heterogeneity.
As specified in the protocol, we explored potential causes of heterogeneity by subgroup analysis. We considered the following parameters.
Study design (e.g. prospective versus retrospective)
Disease stage (e.g. early versus advanced stages)
Type of chemotherapy (e.g. ABVD versus BEACOPP)
Type of radiotherapy (e.g. involved field versus involved site)
Type of PET measurement (e.g. PET versus PET‐CT) (post‐hoc)
In addition, we conducted a post hoc sensitivity analysis for the timing of the interim PET, as well as the availability/estimation of HR and SE to explore the robustness of the pooled results.
Results
Results of the search
Our literature search in CENTRAL, MEDLINE and Embase (until 2 April 2019, see Appendix 1, Appendix 2 and Appendix 3, respectively) and one trial registry (ClinicalTrials.gov on 25 January 2019), identified 11,277 potentially relevant publications. After removal of 358 duplicates, we screened titles and abstracts of 10,919 references using inclusion and exclusion criteria defined at the protocol stage. These criteria led to the exclusion of 10,651 references, and 268 references were then included for full‐text screening. Before starting full‐text screening, we discussed and determined exclusion reasons. Full‐text screening led to the exclusion of 133 references. Thirty‐four references that were identified are still awaiting assessment (see Studies awaiting classification), and one study is still ongoing (see Ongoing studies). Hence, we finally included 23 studies (from 99 references) in this review. The overall number of publications screened, identified, selected and included in this review is shown in Figure 1
Description of studies
Included studies
See also Characteristics of included studies.
We included 23 studies in this review (Andre 2017; Annunziata 2016; Barnes 2011; Casasnovas 2019; Cerci 2010; Gallamini 2014; Gandikota 2015; Hutchings 2005; Hutchings 2006; Hutchings 2014; Kobe 2018; Markova 2012; Mesguich 2016; Oki 2014; Okosun 2012; Orlacchio 2012; Rossi 2014; Simon 2016; Straus 2011; Touati 2014; Ying 2014; Zaucha 2017; Zinzani 2012), which added up to a total of 99 references when secondary citations were included. To avoid duplication and overlapping of participant data in our analyses, we grouped those publications that assessed the same population (or groups from the same population). In such cases, we chose the publication with the greatest number of participants and/or most information as the primary publication. Duplicate or overlapping study populations were found for eight studies (Andre 2017; Barnes 2011; Gallamini 2014; Kobe 2018; Markova 2012; Simon 2016; Straus 2011; Zinzani 2012). Four studies did not report the duration of follow‐up (Andre 2017; Annunziata 2016; Orlacchio 2012; Straus 2011). The earliest study recruited participants between 1993 and 2004 (Hutchings 2005), and the most recent between 2007 and 2014 (Annunziata 2016).
There was considerable heterogeneity between the included studies, particularly with regard to: stages of disease; treatment regimens; and the timing and criteria for evaluation of the interim PET scans, which are described in detail in the sections below. For meta‐analyses, we only grouped studies that were homogenous enough in order to ensure comparability, and conducted subgroup analyses to explore the potential impact of heterogeneity on our results (see Methods 'Investigation of heterogeneity').
Study design
Of the 23 included studies, seven studies were retrospective single‐centre studies (Annunziata 2016; Markova 2012; Oki 2014; Orlacchio 2012; Rossi 2014; Touati 2014; Ying 2014). Five studies were retrospective multi‐centre studies (ranging between two to 17 centres) (Barnes 2011; Gallamini 2014; Mesguich 2016; Okosun 2012; Zinzani 2012). Two retrospective studies did not report the number of centres from which participants were recruited (Gandikota 2015; Simon 2016). Out of eight studies with a prospective study design, one study was a single‐centre study (Cerci 2010), three were multi‐centre studies (including between four and 11 centres, with Hutchings 2014 not reporting the number of study centres) (Hutchings 2006; Hutchings 2014; Zaucha 2017), and four were clinical trials (Andre 2017; Casasnovas 2019; Kobe 2018; Straus 2011). One study did not report the study design (Hutchings 2005).
For more details see Characteristics of included studies.
Sample size
The smallest study included 23 participants (Okosun 2012) and the largest study included 1945 participants (Kobe 2018).
Location
The included studies were conducted in a variety of countries, including Austria, Belgium, Brazil, Croatia, Czech Republic, Denmark, France, Germany, Hungary, Italy, the Netherlands, Poland, Slovakia, Switzerland, the United Kingdom (UK), the United States of America (USA), and the People's Republic of China. Four studies reported the country but not the study centre (Annunziata 2016; Hutchings 2014; Markova 2012; Simon 2016), and two studies reported neither country nor study centre (Gandikota 2015; Straus 2011).
Participants
This review included a total of 7335 male and female consecutive participants who were newly diagnosed with classic HL and received first‐line therapy. Out of these, a total of 2205 participants were included in meta‐analyses.
Follow‐up
There were differences in the follow‐up time between studies. Three studies did not report follow‐up time (Annunziata 2016; Orlacchio 2012; Straus 2011). Two studies reported follow‐up time per subgroup, i.e. surviving participants only (Kobe 2018; Zaucha 2017). The median follow‐up time for the remaining 18 studies ranged from 23 to 66 months. The total raw range of follow‐up time was between two to 195 months.
Stages of disease
Fifteen studies included all stages of the disease. Four studies included only early stages (Andre 2017; Barnes 2011; Gandikota 2015, Straus 2011) and four studies only advanced stages (Casasnovas 2019; Kobe 2018; Markova 2012; Okosun 2012).
Treatment/therapy
The following chemotherapy regimens were administered.
ABVD (adriamycin/doxorubicin, bleomycin, vinblastine and dacarbazine) in 16 studies (Andre 2017; Annunziata 2016; Barnes 2011; Cerci 2010; Gallamini 2014; Hutchings 2005; Hutchings 2006; Hutchings 2014; Mesguich 2016; Oki 2014; Okosun 2012; Orlacchio 2012; Simon 2016; Touati 2014; Zaucha 2017; Zinzani 2012).
Either ABVD or BEACOPP in one study (Ying 2014).
BEACOPPescalated (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone in escalated doses) in one trial (Casasnovas 2019).
BEACOPPescalated or BEACOPPescalated with rituximab in one trial (Kobe 2018).
BEACOPPescalated or time‐condensed BEACOPP14baseline (BEACOPP in standard, non‐escalated doses repeated on day 15) in one study (Markova 2012).
AVG (doxorubicin, vinblastine and gemcitabine) in one trial (Straus 2011).
ABV/MOPP (adriamycin, bleomycin, vinblastine, mechlorethamine, vincristine, procarbazine and prednisone), ABVD/COPP (ABVD plus cyclophosphamide, vincristine, procarbazine and prednisone), eBEACOPP, or PVAG (prednisone, vinblastine, doxorubicin and gemcitabine) in subgroups of participants in three studies (Hutchings 2005; Hutchings 2006; Touati 2014).
Anthracycline‐based chemotherapy not further specified in one study (Rossi 2014).
The following number of chemotherapy cycles were administered.
Two, three, four, six or eight cycles of chemotherapy alone or combined with radiotherapy in 15 studies (Andre 2017; Annunziata 2016; Barnes 2011; Casasnovas 2019; Cerci 2010; Gallamini 2014; Hutchings 2014; Markova 2012; Mesguich 2016; Orlacchio 2012; Rossi 2014; Simon 2016; Straus 2011; Zaucha 2017; Zinzani 2012). The number of cycles usually depended on the stage of the disease.
Four, six or eight cycles of chemotherapy, depending on the interim PET scan results, in one trial (Kobe 2018). A protocol amendment during the trial introduced a reduction of standard therapy from eight to six cycles.
Six cycles of chemotherapy combined with antiretroviral therapy due to HIV‐positive study population in one study (Okosun 2012).
Six studies did not report the number of cycles (Gandikota 2015; Hutchings 2005; Hutchings 2006; Oki 2014; Touati 2014; Ying 2014).
The following radiotherapy techniques were used either in all or a subgroup of participants.
Involved‐field radiotherapy in eight studies (Barnes 2011; Gallamini 2014; Hutchings 2005; Hutchings 2006; Hutchings 2014; Mesguich 2016; Rossi 2014; Simon 2016), and either involved‐field radiotherapy or extended‐field radiotherapy in one study (Gandikota 2015).
Involved‐node radiotherapy in three studies (Andre 2017; Annunziata 2016; Zaucha 2017).
Involved‐site radiotherapy in two studies (Touati 2014; Zinzani 2012).
Radiotherapy without further specification in five studies (Cerci 2010; Kobe 2018; Markova 2012; Orlacchio 2012; Ying 2014).
No radiotherapy in three studies (Oki 2014; Okosun 2012; Straus 2011).
Stem cell transplantation was conducted in participants who relapsed after first‐line therapy despite treatment escalation or salvage therapy.
Autologous stem cell transplantation in eight studies (Cerci 2010; Gallamini 2014; Hutchings 2014; Mesguich 2016; Touati 2014; Ying 2014; Zaucha 2017).
Autologous and/or allogeneic stem cell transplantation in one study (Zinzani 2012).
Type of stem cell transplantation not specified in four studies (Hutchings 2005; Hutchings 2006; Markova 2012; Orlacchio 2012).
No stem cell transplantation reported in 10 studies (Andre 2017; Annunziata 2016; Barnes 2011; Gandikota 2015; Kobe 2018; Oki 2014; Okosun 2012; Rossi 2014; Simon 2016; Straus 2011).
Index (prognostic) factor
Participants in 16 out of 23 studies underwent PET combined with computed tomography (CT), contrast enhanced CT, or multi detector CT (MDCT), compared to PET‐only for participants in the other studies. Participants in 13 studies underwent PET‐CT (Annunziata 2016; Cerci 2010; Gallamini 2014; Gandikota 2015; Hutchings 2014; Kobe 2018; Mesguich 2016; Okosun 2012; Rossi 2014; Simon 2016; Touati 2014; Ying 2014; Zaucha 2017). Participants in another study underwent either PET or PET‐CT (Barnes 2011); participants in one study underwent PET with contrast‐enhanced CT (Markova 2012); and participants in another study underwent PET/MDCT (Orlacchio 2012). In the remaining seven studies, participants underwent a PET scan only (Andre 2017; Casasnovas 2019; Hutchings 2005; Hutchings 2006; Oki 2014; Straus 2011; Zinzani 2012).
Timing of interim PET
The timing of interim PET imaging varied between studies. In most studies, participants underwent an interim PET scan after two cycles (PET2) of chemotherapy (Andre 2017; Casasnovas 2019; Cerci 2010; Gallamini 2014; Hutchings 2005; Hutchings 2006; Kobe 2018; Mesguich 2016; Oki 2014; Okosun 2012; Orlacchio 2012; Rossi 2014; Simon 2016; Straus 2011; Touati 2014; Zinzani 2012). In another study, participants underwent an interim PET scan after the first cycle (PET1) of chemotherapy only (Annunziata 2016). In one study, participants underwent interim PET scans after the first and second cycle of chemotherapy, but the study protocol was amended after interim analysis to limit PET2 scans to participants with positive results after PET1 (Zaucha 2017). In one multi‐centre study, participants from two centres underwent both PET1 and PET2, whereas participants from the remaining two centres underwent PET2 only if PET1 was positive (Hutchings 2014). Three retrospective studies included participants who underwent interim PET after two to four cycles of chemotherapy (Barnes 2011; Gandikota 2015; Ying 2014), and in another study participants underwent interim PET after four cycles (PET4) of chemotherapy (Markova 2012). For meta‐analyses, we used information at PET2 whenever available in order to ensure homogeneity across studies.
Evaluation of PET scans
In most studies, two nuclear medicine physicians evaluated the PET scans individually, and disagreements in scoring were solved in a consensus meeting (Annunziata 2016; Barnes 2011; Cerci 2010; Hutchings 2005; Hutchings 2006; Hutchings 2014; Mesguich 2016; Orlacchio 2012; Rossi 2014; Ying 2014; Zinzani 2012). Evaluation of PET scans was performed by only one expert in one study (Markova 2012); and by a panel consisting of three to six experts in eight studies (Andre 2017; Casasnovas 2019; Gallamini 2014; Kobe 2018; Oki 2014; Okosun 2012; Straus 2011; Zaucha 2017). Three studies did not report the number or qualification of persons who performed evaluation of PET scans (Gandikota 2015; Simon 2016; Touati 2014). Nine out of 13 multi‐centre studies reported that evaluation of PET scans took place centrally (Andre 2017; Gallamini 2014; Hutchings 2006; Kobe 2018; Mesguich 2016; Okosun 2012; Straus 2011; Zaucha 2017; Zinzani 2012), and two studies did not report how reviewing of PET scans was performed across centres (Barnes 2011; Hutchings 2014).
In 11 studies, outcome assessors were blinded to the outcome (Kobe 2018; Gallamini 2014; Gandikota 2015; Hutchings 2006; Hutchings 2014; Mesguich 2016; Oki 2014; Rossi 2014; Straus 2011; Zaucha 2017; Zinzani 2012). The remaining studies did not report blinding.
Criteria for evaluation
Most studies reported the use of a standardised scale for the evaluation of the PET scans, but the scoring systems and cut‐off points between studies varied.
In 12 studies, the Deauville 5‐point scoring system for evaluation of PET scans was used: in nine studies, Deauville scores 1 ‐ 3 were considered as PET‐negative, and Deauville scores 4 ‐ 5 as PET‐positive (cut‐off ≥4) (Annunziata 2016; Casasnovas 2019; Gallamini 2014; Hutchings 2014; Oki 2014; Okosun 2012; Rossi 2014; Simon 2016; Zaucha 2017); in two studies, both cut‐off points for evaluation of the PET scans were used by scoring each image twice, and comparing performance of interim PET between both scales (Kobe 2018; Mesguich 2016); and in one study, it was reported that the PET scans were re‐interpreted retrospectively using the Deauville criteria, but it was not indicated which cut‐off points were used (Touati 2014).
In one study, the International Harmonization Project criteria were used: a PET scan was considered positive when the residual mass is ≥ 2 cm or, if less than 2 cm, positive if its activity is above that of the surrounding background (Andre 2017). A negative PET scan corresponds to Deauville score 1 (no uptake) and score 2 (uptake ≤ mediastinum).
In two studies, the scoring systems were not specified, but similar scales and cut‐off points as the Deauville scoring system were used: in one study, PET scans were reviewed using a 4‐point scale (Barnes 2011), and in another study using a 5‐point scale (Gandikota 2015).
In three studies, other standardised scales for the evaluation of PET scans were used: one study used the Juweid criteria (Zinzani 2012), and two studies used the International Harmonization Project guidelines (Orlacchio 2012; Straus 2011).
Two studies did not report how PET scans were evaluated (Hutchings 2005; Hutchings 2006); and four studies reported performance of visual evaluation but did not indicate the use of a standardised scoring system (Cerci 2010; Markova 2012; Touati 2014; Ying 2014).
Outcomes
Primary outcome
Overall survival (OS)
Univariable analyses
Twelve out of 23 included studies reported unadjusted results for our primary outcome OS (Barnes 2011; Casasnovas 2019; Cerci 2010; Gallamini 2014; Hutchings 2005; Hutchings 2006; Hutchings 2014; Kobe 2018; Simon 2016; Touati 2014; Zaucha 2017; Zinzani 2012). Of these, nine provided sufficient information and data to be included in meta‐analysis. One study reported an HR that we used (Kobe 2018). Another study reported an HR, but we still re‐calculated it due to discrepancies in values between the graph and table (Simon 2016). For the other seven studies, we estimated the HR using other available data from the publications (Barnes 2011; Cerci 2010; Hutchings 2005; Hutchings 2014; Touati 2014; Zaucha 2017; Zinzani 2012).
Multivariable analyses
Two studies reported adjusted results for OS (Kobe 2018; Simon 2016). Two additional studies planned, but did not conduct the analysis for different reasons (Gallamini 2014; Hutchings 2005).
Secondary outcomes
Progression‐free survival (PFS)
Univariable analyses
Twenty‐one out of 23 studies reported unadjusted results for PFS (Andre 2017; Annunziata 2016; Barnes 2011; Casasnovas 2019; Cerci 2010; Gallamini 2014; Hutchings 2005; Hutchings 2006; Hutchings 2014; Kobe 2018; Markova 2012; Mesguich 2016; Oki 2014; Okosun 2012; Rossi 2014; Simon 2016; Straus 2011; Touati 2014; Ying 2014; Zaucha 2017; Zinzani 2012). Of these, 15 provided sufficient information and data to be included in meta‐analysis. Three studies provided an HR which we used (Annunziata 2016; Kobe 2018; Simon 2016). Another three studies reported an HR, but we still re‐calculated it due to unclear description of the statistical methods used (Hutchings 2006), reporting discrepancies between graphs and tables (Mesguich 2016) or general uncertainties in the reported values (Rossi 2014). For eight studies we estimated the HR using other available data (Barnes 2011; Cerci 2010; Hutchings 2005; Straus 2011; Touati 2014; Ying 2014; Zaucha 2017; Zinzani 2012).
Multivariable analyses
Eight studies reported adjusted results for PFS (Casasnovas 2019; Gallamini 2014; Hutchings 2005; Hutchings 2006; Kobe 2018; Mesguich 2016; Rossi 2014; Simon 2016). Three studies took the importance of adjustment into account, but did not actually conduct a multivariable analysis (Annunziata 2016; Hutchings 2014; Oki 2014).
Definitions of Progression‐free survival (PFS)
The definition of the progression outcome varied between studies. Four studies that reported PFS did not provide a definition (Hutchings 2014; Simon 2016; Straus 2011; Zaucha 2017). One study analysed event‐free survival (Cerci 2010), which was identical with PFS and, therefore, included in the analysis. Table 2 presents an overview of definitions used for progression outcome. Studies with identical definitions were grouped.
Table 2. Definitions of progression outcomes
| Study | Definition of progression outcome |
| Andre 2017 | Progression‐free survival , defined – from the date of random assignment to date of progression – as experiencing relapse after previous complete remission or progression after reaching partial remission (50% decrease and resolution of B symptoms and no new lesions); progressive disease (50% increase from nadir of any previous partial remission lesions or appearance of new lesions) on CT scan measurements during protocol treatment; or death from any cause, whichever occurred first. |
| Casasnovas 2019 | Progression‐free survival defined as the time from randomisation to first progression, relapse, or death from any cause or last follow‐up. |
| Annunziata 2016 | The primary endpoint was PFS, with progression during treatment, lack of complete remission at the end of the first‐line treatment, and relapse counted as adverse events. |
|
Barnes 2011; Ying 2014; Zinzani 2012 |
Progression‐free survival is defined as the time from diagnosis to progression or death from any cause. |
| Kobe 2018 | Progression‐free survival is defined as the time from completion of staging until progression, relapse, or death from any cause, or to the day when information was last received on the patient's disease status. |
| Cerci 2010 | Three‐year event‐free survival was chosen as the endpoint and defined as the time from diagnosis to treatment failure or last follow‐up. Treatment failure was defined as an incomplete response after first‐line treatment, progression during therapy, relapse, or death. |
|
Gallamini 2014; Markova 2012; Mesguich 2016; Oki 2014; |
Progression‐free survival is defined as the time from diagnosis to either disease progression or relapse, or to death as a result of any cause, whichever occurred first. |
| Hutchings 2005; Hutchings 2006 | Progression‐free survival is defined as the time from diagnosis to first evidence of progression or relapse, or to disease‐related death. |
| Okosun 2012 | Progression‐free survival is defined as the time from diagnosis to disease progression or relapse or last follow‐up. |
| Rossi 2014 | Progression‐free survival is defined as the time from the beginning of treatment until progression, relapse, or death from any cause or the date of last follow‐up. Time‐to‐progression (TTP) is defined as the time from the date of the first course of chemotherapy to any treatment failure, including progression, relapse, or death related to lymphoma, or the date of the last follow‐up. |
| Touati 2014 | Progression‐free survival is defined as the time from diagnosis to relapse or death. |
| Hutchings 2014; Simon 2016; Straus 2011; Zaucha 2017 | Definition not reported. |
Adverse events (AEs)
None of the included studies measured PET‐associated AEs.
Conflict of interest
Two studies reported potential conflicts of interest (Andre 2017; Casasnovas 2019). Fourteen studies declared that the investigators had no conflict of interest (Annunziata 2016; Barnes 2011; Hutchings 2006; Hutchings 2014; Kobe 2018; Mesguich 2016; Oki 2014; Okosun 2012; Orlacchio 2012; Rossi 2014; Simon 2016; Straus 2011; Zaucha 2017; Zinzani 2012). Seven studies did not report investigators' disclosures of potential conflicts of interest (Cerci 2010; Gallamini 2014; Gandikota 2015; Hutchings 2005; Markova 2012; Touati 2014; Ying 2014).
Excluded studies
After screening titles and abstracts, we excluded 10651 references that did not match our inclusion criteria. In addition, we excluded a total of 133 references after full‐text screening for the following reasons.
Fifty‐six references had a study design or publication type that did not match our inclusion criteria, i.e. letters and commentaries, case studies with a small sample size or validation studies (Adams 2016; Adams 2017; Adams 2018; Adams 2018a; Adams 2018b; Adams 2019; Afanasyev 2017; Ansell 2016; Barrington 2017; Bar‐Shalom 2003; Basu 2009; Becherer 2002; Bednaruk‐Mlynski 2015; Biggi 2012; Bishop 2015; Bodet‐Milin 2009; Boisson 2007; Borchmann 2016; Bucerius 2006; Cremerius 1999; D'Urso 2018; Dann 2018; deAndres‐Galiana 2015; Diehl 2007; El‐Galaly 2012; Evens 2014; Fanti 2008; Friedberg 2002; Friedberg 2004; Gallamini 2008; Gallamini 2018a; Gallowitsch 2008; Guidez 2016; Hagtvedt 2015; Hartmann 2012; Hartridge‐Lambert 2013; Kobe 2008; Kobe 2014; Lowe 2002; Milgrom 2017; Mocikova 2010; NCT02292979; Pichler 2000; Reinhardt 2005; Rigacci 2002; Rigacci 2017; Rubello 2015; Sakr 2017; Specht 2007; Spinner 2018; Strigari 2016; Tirelli 2015; Xie 2018; Yasgur 2015; Zabrocka 2016; Zaucha 2009).
Thirty‐nine references adapted the treatment based on PET‐results (Albano 2017; Albano 2018; Biggi 2017; Carras 2018; Ciammella 2016; Cuccaro 2016; Damlaj 2017; Damlaj 2019; Danilov 2017; Dann 2009; Dann 2010; Dann 2010a; Dann 2012; Dann 2013; Dann 2016; Dann 2017; Fornecker 2017; Gallamini 2017; Gallamini 2018; Greil 2018; Illidge 2015; Johnson 2015; Johnson 2016; Kamran 2016; Kamran 2018; Moskowitz 2015; NCT00784537; NCT00795613; NCT01358747; NCT01652261; Nguyen 2017; Paolini 2007; Pavlovsky 2019; Simontacchi 2015; Straus 2018; Torizuka 2004; Trotman 2017; Villa 2018; Zinzani 2016).
Eighteen references also included participants with other types of lymphoma and did not report data for HL separately (Awan 2013; Blum 2002; Bodet‐Milin 2008; Cremerius 2001; Filmont 2003; Freudenberg 2004; Fruchart 2006; Goldschmidt 2011; Haioun 2005; Honda 2014; Iagaru 2008; Kostakoglu 2006; Li 2013; Slaby 2002; Tomita 2015; Torizuka 2004; Zinzani 1999; Zinzani 2002).
Ten references included participants who received treatment other than first‐line therapy, i.e. second‐line therapy for relapsed or refractory disease (Bjurberg 2006; Front 1999; Huic 2006; Mocikova 2010; Mocikova 2011; Schot 2007; Sucak 2011; Tseng 2012; Weidmann 1999; Yoshimi 2008).
Eight references reported only end‐of‐chemotherapy PET‐results (Advani 2007; Hueltenschmidt 2001; Hutchings 2007; Jerusalem 2003; Molnar 2010; Naumann 2001; Panizo 2004; Spaepen 2001).
Two were duplicates (Freudenberg 2004; Kobe 2014).
These publications are described in Characteristics of excluded studies.
Risk of bias in included studies
We assessed the risk of bias at outcome level (OS and PFS) for each study using the QUIPS tool. No study reported PET‐associated AE. The detailed assessment can be found in the 'Risk of bias (QUIPS)' section in the Characteristics of included studies.
Risk of bias in studies included in meta‐analyses
The 'Risk of bias' summary (Figure 2) presents the combined judgement made by the review authors in a cross‐tabulation. Studies included in meta‐analysis are highlighted in bold.
Figure 2.
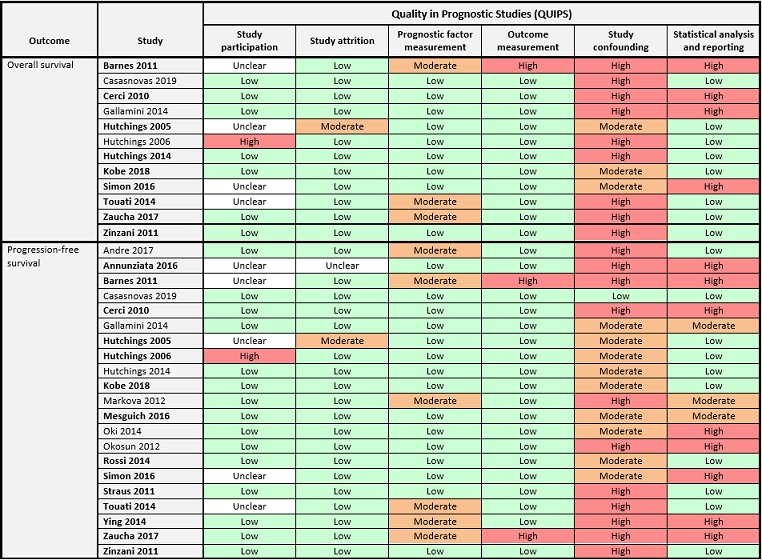
'Risk of bias' assessment according to QUIPS (Quality in Prognostic Studies) by outcome.
Overall survival (OS)
For our primary outcome OS, none of the nine studies included in meta‐analysis were assessed as 'low' in all risk of bias domains. Four studies were assessed as 'unclear' for the domain study participation (Barnes 2011; Hutchings 2005; Simon 2016; Touati 2014), mostly due to a lack of information about the baseline population from which the study sample originated. Most studies had defined exclusion criteria to sample participants from the baseline population (e.g. unavailability of interim PET2) without providing a description of the original population or reasons for missing information. Considering this a potential source of selection bias, we assessed this domain as ‘unclear’ when information about the baseline population was missing. For the domains study attrition, prognostic factor measurement and outcome measurement, risk of bias was assessed as 'low' in most studies. Two studies did not report the use of standardised criteria for prognostic factor measurement, therefore we assessed the risk of bias as 'moderate' (Barnes 2011; Touati 2014). One study was assessed as 'moderate' risk because PET2 availability was dependent on PET1 result (Zaucha 2017). Due to inconsistency in reporting of the timing of the interim PET measurement, the risk of bias for outcome measurement was assessed as 'high' in one study (Barnes 2011), while the remaining studies were all assessed as 'low'. All studies were assessed as 'moderate' or 'high' risk of bias in the domain study confounding, because they either did not adjust for other clinically relevant prognostic factors ('high') or did not provide reasons for their selection of covariates ('moderate'). Six studies provided sufficient information about the methods used for univariable analysis (Hutchings 2005; Hutchings 2014; Kobe 2018; Touati 2014; Zaucha 2017; Zinzani 2012), therefore we assessed the risk of bias for statistical analysis and reporting as 'low'. The same domain was assessed as 'high' in three studies due to discrepancies between text and figures and/or tables (Barnes 2011; Cerci 2010; Simon 2016).
Progression‐free survival (PFS)
For our secondary outcome PFS, none of the 14 studies included in meta‐analysis were assessed as 'low' in all risk of bias domains. Eight studies provided clear descriptions of study characteristics and participants (Cerci 2010; Kobe 2018; Mesguich 2016; Rossi 2014; Straus 2011; Ying 2014; Zaucha 2017; Zinzani 2012), so we assessed the risk of bias as 'low'. Five studies did not report inclusion and/or exclusion criteria (Annunziata 2016; Barnes 2011; Hutchings 2005; Simon 2016; Touati 2014), so we assessed the risk of bias for study participation as 'unclear'. One study reported a high number of participants with unavailable interim PET scans without further information (Hutchings 2006), so we assessed the risk of bias as 'high' in the same domain. Most studies had no loss to follow‐up to report or provided a clear description of how missing data were handled, so we assessed the risk of bias for study attrition as 'low' in the majority of studies. One study was assessed as 'unclear' due to a lack of information regarding loss to follow‐up (Annunziata 2016); another study was assessed as 'moderate' because no explanation was provided as to why some participants were lost to follow‐up (Hutchings 2005). The risk of bias for the domains prognostic factor measurement and outcome measurement was assessed as 'low' in most studies. Three studies did not report the use of standardised criteria for prognostic factor measurement, therefore we assessed the risk of bias as 'moderate' (Barnes 2011; Touati 2014; Ying 2014). A fourth study was assessed as 'moderate' risk because PET2 availability was dependent on PET1 result (Zaucha 2017). Due to lack of outcome definition or inconsistency in the reporting of the timing of the interim PET measurement, the risk of bias for outcome measurement was assessed as 'high' in one study (Barnes 2011). In another study, this domain was also assessed as 'high' because the outcome was not defined (Zaucha 2017). The remaining studies were all assessed as 'low' for the domain outcome measurement. For the domain study confounding, all studies were assessed as 'moderate' or 'high' risk of bias, because they either did not adjust for other clinically relevant prognostic factors ('high') or did not provide reasons for their selection of covariates ('moderate'). Seven studies provided sufficient information about the methods used for univariable analysis (Hutchings 2005; Hutchings 2006; Kobe 2018; Rossi 2014; Straus 2011; Touati 2014; Zinzani 2012), so we assessed the risk of bias for statistical analysis and reporting as 'low'. The same domain was rated 'moderate' in one study because covariates were analysed in separate models (Mesguich 2016). Five studies were assessed as 'high' for this domain because of the poor reporting of results (Annunziata 2016; Barnes 2011; Cerci 2010; Simon 2016; Ying 2014), including discrepancies between text and figures and/or tables in some studies. Another study was also assessed as 'high' because the method of analysis was not sufficiently described (Zaucha 2017).
Risk of bias in studies reported narratively
The risk of bias for all studies reported narratively is included in Figure 2.
Overall survival (OS)
The results for OS from three studies are reported narratively in this review (Casasnovas 2019; Gallamini 2014; Hutchings 2006). For Casasnovas 2019, we assessed the risk of bias as 'low' in five out of six domains of the QUIPS tool. For the domain study confounding, we assessed the risk of bias as 'high' because multivariable analysis for this outcome were not conducted. For Gallamini 2014, we assessed the risk of bias as 'low' in all domains, except for the domains study confounding and statistical analysis and reporting, which were assessed as 'high' risk. The study did not take potential confounders into account and a multivariable analysis was not performed, although it was planned for. For Hutchings 2006, the domain study participation was assessed as 'high' risk because a great number of participants included did not actually undergo an early interim PET. The domain study confounding was also assessed as 'high' risk as no potential confounders were taken into account. All other domains were assessed as 'low' risk of bias.
Progression‐free survival (PFS)
For PFS, the results from seven studies are reported narratively (Andre 2017; Casasnovas 2019; Gallamini 2014; Hutchings 2014; Markova 2012; Oki 2014; Okosun 2012). Out of these, only one study (Casasnovas 2019) was assessed as 'low' risk of bias in all six domains of the QUIPS tool. The other six studies were assessed as 'low' risk of bias for the domain study participation. For the domains study attrition, prognostic factor measurement and outcome measurement, four studies were assessed as a 'low' risk of bias (Gallamini 2014; Hutchings 2014; Oki 2014; Okosun 2012). For the other two studies (Andre 2017; Markova 2012), the domain prognostic factor measurement was assessed as 'moderate' risk because the prognostic factor was measured differently in some participants. For the domain study confounding, three studies were assessed as 'high' risk because they did not take potential confounders into account (Andre 2017; Markova 2012; Okosun 2012). The other three studies were assessed as 'moderate' risk for this domain because potential confounders were taken into account, but the reason behind the choice of these specific confounders was not provided (Gallamini 2014; Hutchings 2014; Oki 2014). Regarding the domain statistical reporting and analysis, two studies were assessed as 'low' risk because they used appropriate methods for the planned analysis (Andre 2017; Hutchings 2014). Another two studies were assessed as 'moderate' risk for this domain mainly because only one confounder was taken into account, without reasons behind this choice (Gallamini 2014; Markova 2012). The remaining two studies were assessed as 'high' risk due to inconsistent conduct and reporting of the analyses (Oki 2014; Okosun 2012).
Other potential sources of bias
Reporting deficiencies and selective reporting
We detected reporting deficiencies in some of the studies, particularly when not all analyses that were planned in the methods were actually conducted. In some cases, this was due to the low number of events (i.e. in PET‐negative participants) that did not allow for further analyses. In other cases, it was unclear why certain analyses were performed and others not. This was particularly the case with regard to multivariable analyses, when studies planned to assess the independent prognostic ability of the interim PET in a prognostic model including other clinically relevant prognostic factors (covariates). Studies either did not perform such an analysis even though they initially planned to, or they did not consider adjustment. None of the studies stated clearly their rationale for the choice of covariates; in some cases, the choice was based on their significance in univariable analysis. For example, in studies that only included two or less covariates in the model in addition to interim PET, the interim PET was always independent in its performance. However, how interim PET possibly performed in comparison to other covariates remains unclear. Hence, it is particularly important to state why certain confounders were taken into account. Thus, we cannot be sure that studies did not only report certain positive ('significant') results, which can be an issue of selective reporting.
In addition, we detected discrepancies in the reporting of results within the texts of some studies, or between text and the corresponding graph(s) (i.e. in the reporting of the HR or number of events). In these cases, we tried to contact the corresponding principal investigator(s) for clarification in order to have a better understanding of the results.
Blinding of prognostic factor assessor
Eleven studies reported that the clinicians evaluating the interim PET scans were blinded to the outcome (Kobe 2018; Gallamini 2014; Gandikota 2015; Hutchings 2006; Hutchings 2014; Mesguich 2016; Oki 2014; Rossi 2014; Straus 2011; Zaucha 2017; Zinzani 2012).
Results of the analyses
Twenty‐three studies evaluated interim PET as a prognostic factor in individuals with HL. Two studies did not report data for our outcomes of interest (Gandikota 2015; Orlacchio 2012) and we have not been able to either obtain or estimate any relevant data. None of the included studies reported PET‐associated AEs. Fifteen studies were included in meta‐analyses. Another six of the included studies in this review reported results for OS and/or PFS, but we were not able to pool results because, despite our approaches for possible estimation of missing data items, there was a lack of accurate information or data to do so (Andre 2017; Casasnovas 2019; Gallamini 2014; Markova 2012; Oki 2014; Okosun 2012). For all studies that were not included in meta‐analyses, we reported the main results narratively in this review.
Overall survival (OS)
Meta‐analysis of unadjusted results
We included nine studies with 1802 participants in meta‐analysis for OS (Barnes 2011; Cerci 2010; Hutchings 2005; Hutchings 2014; Kobe 2018; Simon 2016; Touati 2014; Zaucha 2017; Zinzani 2012). There were 475 interim PET‐positive and 1327 interim PET‐negative participants. Meta‐analysis shows a clear advantage in OS for participants with a negative interim PET scan compared to participants with a positive interim PET scan (HR 5.09, 95% CI 2.64 to 9.81, I2 44%, moderate certainty of evidence) (Analysis 1.1) (Figure 3).
Analysis 1.1.
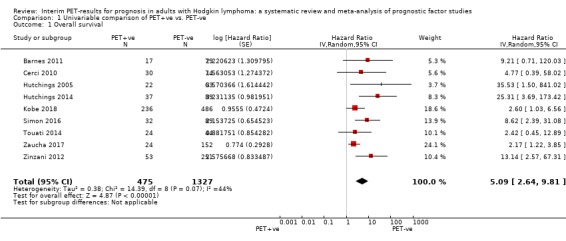
Comparison 1 Univariable comparison of PET+ve vs. PET‐ve, Outcome 1 Overall survival.
Figure 3.
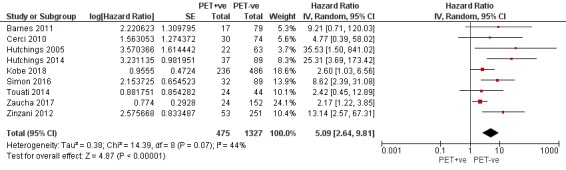
Forest plot of comparison: 1 Univariable comparison of PET+ve vs. PET‐ve, outcome: 1.1 Overall survival
Subgroup analysis
We conducted subgroup analyses to explore the underlying clinical heterogeneity between the studies.
For subgroup analysis by radiotherapy, we found evidence on subgroup difference between the groups (P = 0.05, INRT/ISRT in three studies: N = 548, IFRT in four studies: N = 428, RT not further specified in two studies: N = 826). Results still show an advantage in OS for PET‐negative participants, irrespective of the type of radiotherapy they received (Analysis 2.1).
Analysis 2.1.
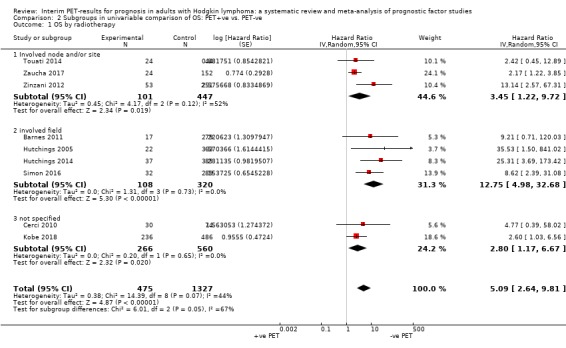
Comparison 2 Subgroups in univariable comparison of OS: PET+ve vs. PET‐ve, Outcome 1 OS by radiotherapy.
For the remaining subgroups, there was no evidence of subgroup differences.
Different study designs (P = 0.28; three prospective studies: N = 406, four retrospective studies: N = 589, one RCT: N = 722) (Analysis 2.2). One study (Hutchings 2005) was not included in this subgroup analysis because they did not explicitly state their study design.
Different chemotherapy regimens (P = 0.33; ABVD in five studies: N = 801, ABVD and other in three studies: N = 279, BEACOPP in one study: N = 722) (Analysis 2.3). Chemotherapy‐regimen in the included studies was mainly ABVD, with differentiating numbers of cycles, with or without radiotherapy (Barnes 2011; Cerci 2010; Hutchings 2014; Simon 2016; Zaucha 2017; Zinzani 2012). In Hutchings 2005, the majority of participants received ABVD, while the remaining received MOPP or MOPP/ABV, or another regimen which was not specified. Some participants also received additional radiotherapy. In Kobe 2018, all participants received eBEACOPP. In Touati 2014, the regimens included ABVD, MOPP/ABV hybrid or BEACOPP. If separate data had been available for each type of chemotherapy, we could have performed more specific subgroup analysis to test for differences between chemotherapies.
PET‐CT versus PET (P = 0.66; PET‐CT in five studies: N = 595, PET only in three studies: N = 1111) (Analysis 2.4). One study (Barnes 2011) was not included in this subgroup analysis because they conducted PET in some participants and PET‐CT in the other participants.
Different stages of disease (P = 0.33; early stages with A or B symptoms in one study: N = 96, all stages in seven studies: N = 984, advanced stages in one study: N = 722) (Analysis 2.5). One study included disease stages IA, IB, IIA and IIB (Barnes 2011) and another study included advanced‐stages only (Kobe 2018). The remaining seven studies included participants representing all disease stages of HL.
Analysis 2.2.
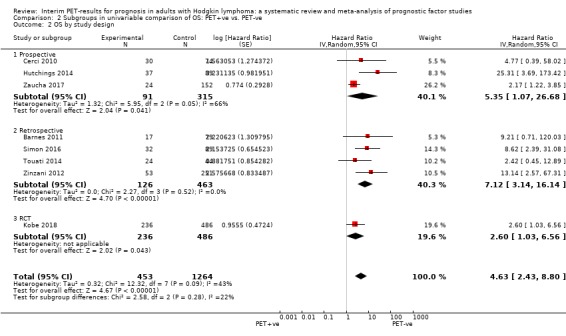
Comparison 2 Subgroups in univariable comparison of OS: PET+ve vs. PET‐ve, Outcome 2 OS by study design.
Analysis 2.3.
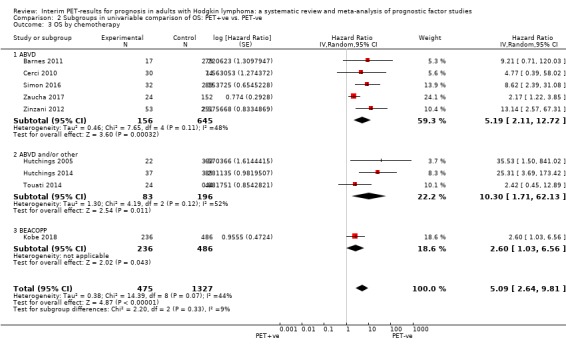
Comparison 2 Subgroups in univariable comparison of OS: PET+ve vs. PET‐ve, Outcome 3 OS by chemotherapy.
Analysis 2.4.
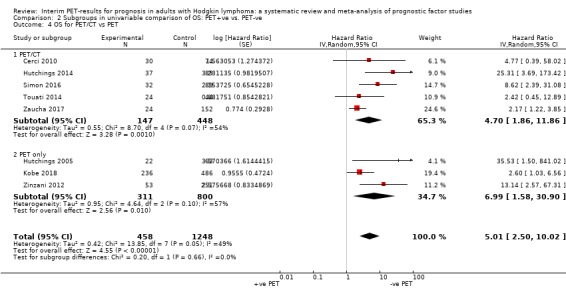
Comparison 2 Subgroups in univariable comparison of OS: PET+ve vs. PET‐ve, Outcome 4 OS for PET/CT vs PET.
Analysis 2.5.
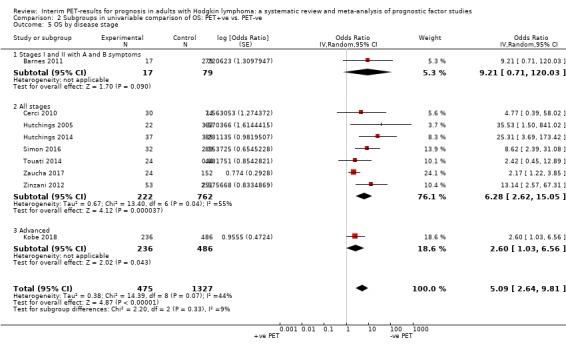
Comparison 2 Subgroups in univariable comparison of OS: PET+ve vs. PET‐ve, Outcome 5 OS by disease stage.
Sensitivity analysis
We conducted sensitivity analyses for the timing of interim PET (removing those that did not conduct a PET2), and the precision of the estimated HR and SE (removing the studies with imprecise HR and SE estimation).
Regarding the timing of the interim PET, interim PET2 was conducted in six studies (N = 1495 participants in total) (Cerci 2010; Kobe 2018; Simon 2016; Touati 2014; Zinzani 2012; Zaucha 2017). In three studies (N = 307 participants in total), interim PET was conducted at other timings: in Barnes 2011, 41 participants received PET2 while the rest of the participants received PET3; in Hutchings 2005, 55 participants received PET2 and 35 participants received PET3; and in Hutchings 2014, PET1 was conducted for all participants (N = 126). Although 89 out of 126 also received a PET2, we used the data for PET1 as the publication provided us with the most information on PET1. At sensitivity analysis, temporarily removing studies that did not perform a PET2 slightly affected the pooled OS (overall: HR 5.09, 95% CI 2.64 to 9.81; sensitivity: HR 3.53, 95% CI 1.97 to 6.32) (Analysis 2.6). It seems that there was an over‐estimation of the HR for the studies that did not perform a PET2. However, the direction of the effect is firm and unchanged. This difference may also be partly explained by the very wide follow‐up ranges within the studies. Hence, following the sensitivity analysis, we consider the overall OS to be robust.
Analysis 2.6.
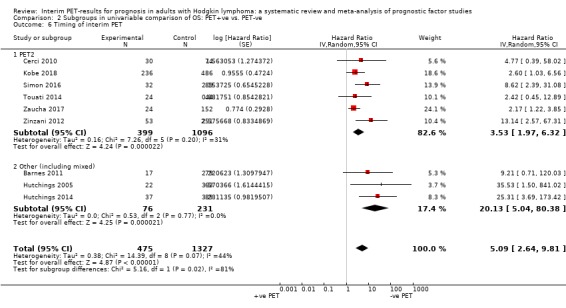
Comparison 2 Subgroups in univariable comparison of OS: PET+ve vs. PET‐ve, Outcome 6 Timing of interim PET.
Regarding the precision of the HR estimation, we were able to either obtain or estimate a precise HR and SE for seven studies (N = 1638 participants in total) (Cerci 2010; Hutchings 2005; Hutchings 2014; Kobe 2018; Simon 2016; Zaucha 2017; Zinzani 2012). For two studies (N = 164 participants in total) (Barnes 2011; Touati 2014), we were only able to provide imprecise estimations of the HR and SE. Temporarily removing the imprecise studies during sensitivity analysis barely affected the pooled results for OS, indicating that the measurements obtained from our imprecise method were quite accurate after all (overall: HR 5.09, 95% CI 2.64 to 9.81; sensitivity: HR 5.70, 95% CI 2.60 to 12.48) (Analysis 2.7). Hence, we concluded that the overall pooled OS is robust.
Analysis 2.7.
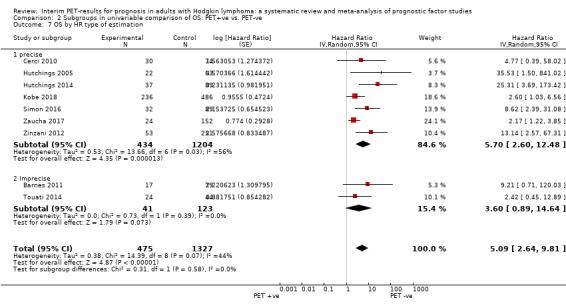
Comparison 2 Subgroups in univariable comparison of OS: PET+ve vs. PET‐ve, Outcome 7 OS by HR type of estimation.
Narrative reporting of results
Univariable analyses
Three studies (Casasnovas 2019; Gallamini 2014; Hutchings 2006) that reported results for OS were not included in meta‐analysis due to lack of adequate data for estimating the HR and associated SE (Table 3).
Table 3. Narrative reporting of results from univariable analysis for OS
| Study | No. of participants + stages | Timing of interim PET scan | Unadjusted results for interim PET scan |
| Casasnovas 2019 | Standard arm N = 413 |
PET2 |
PET2 results (N = 398) Intention‐to‐treat analysis 5‐year OS for entire arm = 95·2% (95% CI 91·1 to 97·4), 13 events Per‐procotol analysis N = 372 participants 5‐year OS for entire arm = 95.6% (95% CI 91.2 to 97.8), 10 events Comment: Separate results for PET2‐negative and PET2‐positive participants in the standard arm were not reported for this outcome. |
| Gallamini 2014 | 260 (stages IIA ‐ IVB) | PET2 | PET‐negative N = 215, 2 deaths, 3‐year OS = 99% PET‐positive N = 45, 6 deaths, 3‐year OS = 87% Comment: Logrank test for difference between groups was not reported and could not be obtained. |
| Hutchings 2006 | 77 (all stages) | PET2 and PET4 |
PET2 results (N = 77) PET‐negative N = 61, no deaths PET‐positive N = 16, 2 deaths Logrank test for difference between groups: P < .01 PET4 results (N = 64) PET‐negative N = 51, no deaths PET‐positive N = 13, 2 deaths Comment: Logrank test for difference between groups after PET4 was not reported and could not be obtained. |
Multivariable analyses
Two studies (Kobe 2018; Simon 2016) reported adjusted effect estimates to test the prognostic ability of PET2 in addition to other prognostic factors. Table 4 displays a list of established prognostic factors (Cuccaro 2014; Josting 2010; Kılıçkap 2013), and shows which were considered as covariates in the final multivariable model. The selection of prognostic factors (covariates) for the final model was either based on the literature (Simon 2016), or on their significance in univariable analysis (Kobe 2018). However, pooling of adjusted data was not possible. In Simon 2016, only the results of those covariates that remained independent prognostic markers in multivariable analysis, namely LMR and PET2‐positivity, were reported. It is unclear whether, or which other covariates were included in the final model. A full list of study‐specific, candidate covariates can be found in the respective table for each study in the Characteristics of included studies.
The statistical methods used were Cox proportional hazards regression model and logistic regression model, which are the appropriate methods for a multivariable analysis.
Table 4. Adjusted results from final multivariable model for OS
| Study | Prognostic factors | Adjusted results for interim PET | |||||||
| Interim PET | Age | Gender | Disease stage | B symptoms | Bulky disease | IPS | Other study‐specific factors | ||
| Kobe 2018 | x | ‐ | ‐ | ‐ | ‐ | ‐ | x | x |
Interim PET‐positivity (DS 4) HR 3.2 (95% CI 1.3 to 8.4), P = 0.02 Comment: Adjusted results indicate an independent prognostic impact of PET2. |
| Simon 2016 | x | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | x |
Interim PET‐positivity HR = 11.51 (95% CI 3.14 to 42.86), P < 0.001 Comment: Adjusted results indicate the independent prognostic impact of PET2. |
|
x = prognostic factor considered for adjustment in the final model ‐ = prognostic factor was not considered in the final model | |||||||||
Progression‐free survival (PFS)
Meta‐analysis of unadjusted results
We included 14 studies with 2079 participants in meta‐analysis for PFS (Annunziata 2016; Barnes 2011; Cerci 2010; Hutchings 2005; Hutchings 2006; Kobe 2018; Mesguich 2016; Rossi 2014; Simon 2016; Straus 2011; Touati 2014; Ying 2014; Zaucha 2017; Zinzani 2012). There were 529 interim PET‐positive and 1550 interim PET‐negative participants. Meta‐analysis shows a clear advantage in PFS for participants with a negative interim PET scan compared to participants with a positive interim PET scan (HR 4.90, 95% CI 3.47, 6.90, I² = 45%, very low certainty of evidence) (Analysis 1.2) (Figure 4).
Analysis 1.2.
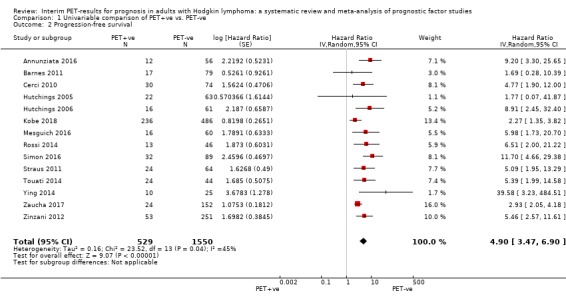
Comparison 1 Univariable comparison of PET+ve vs. PET‐ve, Outcome 2 Progression‐free survival.
Figure 4.
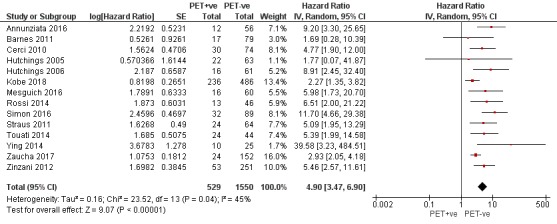
Forest plot of comparison: 1 Univariable comparison of PET+ve vs. PET‐ve, outcome: 1.2 Progression‐free survival
Subgroup analysis
We conducted subgroup analyses to explore the underlying clinical heterogeneity between the studies.
Regarding the disease stage, we detected a significant difference between the groups (P = 0.02, early stages with A or B symptoms in two studies: N = 184, all stages in eleven studies: N = 1173, advanced stages in one study: N = 722). Results still showed an advantage for PFS in PET‐negative participants in any stage of the disease (Analysis 3.4). Twelve studies included all disease stages, while one study included stages IA ‐ IIB (Barnes 2011), and another study included advanced‐stages only (Kobe 2018).
Analysis 3.4.
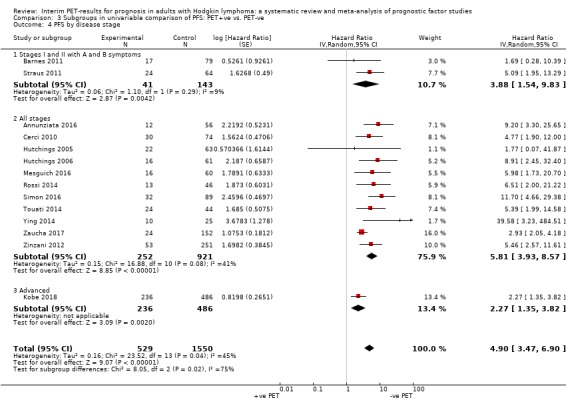
Comparison 3 Subgroups in univariable comparison of PFS: PET+ve vs. PET‐ve, Outcome 4 PFS by disease stage.
For the remaining subgroups, there was no evidence of subgroup differences.
Different study designs (P = 0.29, three prospective studies: N = 357, eight retrospective studies: N = 827, two RCTs: N = 165) (Analysis 3.1). One study (Hutchings 2005) was not included in this subgroup analysis because they did not explicitly state their study design.
Different chemotherapy regimen (P = 0.43; ABVD in seven studies: N = 945, ABVD and other chemotherapy in four studies: N = 265, other chemotherapies in three studies: N = 869) (Analysis 3.2). Chemotherapy‐regimen was ABVD in seven studies, with or without radiotherapy (Annunziata 2016; Barnes 2011; Cerci 2010; Mesguich 2016; Simon 2016; Zaucha 2017; Zinzani 2012). In two studies, participants received either ABVD, ABV/MOPP, ABVD/COPP, BEACOPP esc., PVAG or radiotherapy only (Hutchings 2005; Hutchings 2006). In Touati 2014, the regimens included ABVD, MOPP/ABV hybrid or BEACOPP. In Ying 2014, participants received either ABVD or BEACOPP. In Kobe 2018, all participants received eBEACOPP. In Rossi 2014, all participants received anthracycline‐based chemotherapy, and in Straus 2011, all participants received AVG.
PET versus PET‐CT (P = 0.30; PET‐CT in eight studies: N = 707, PET only in five studies: N = 1276) (Analysis 3.3). One study (Barnes 2011) was not included in this analysis because they conducted PET in some participants and PET‐CT in the other participants.
Different radiotherapy (P = 0.29; INRT/ISRT in five studies: N = 651, IFRT in six studies: N = 514, RT not specified in two studies: N = 826, no RT given in one study: N = 88) (Analysis 3.5).
Analysis 3.1.
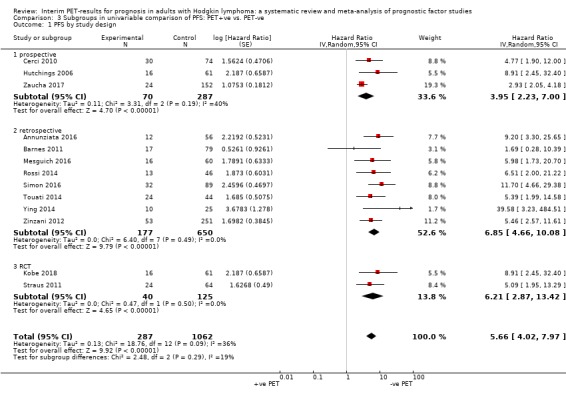
Comparison 3 Subgroups in univariable comparison of PFS: PET+ve vs. PET‐ve, Outcome 1 PFS by study design.
Analysis 3.2.
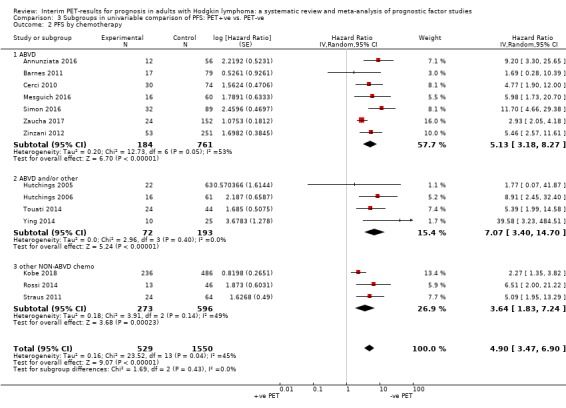
Comparison 3 Subgroups in univariable comparison of PFS: PET+ve vs. PET‐ve, Outcome 2 PFS by chemotherapy.
Analysis 3.3.
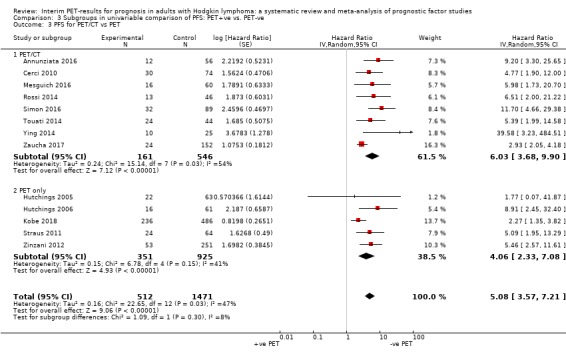
Comparison 3 Subgroups in univariable comparison of PFS: PET+ve vs. PET‐ve, Outcome 3 PFS for PET/CT vs PET.
Analysis 3.5.
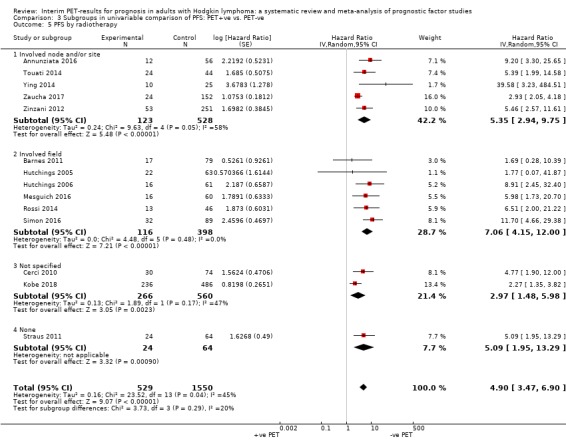
Comparison 3 Subgroups in univariable comparison of PFS: PET+ve vs. PET‐ve, Outcome 5 PFS by radiotherapy.
In addition, we detected variations between the studies with regard to the definition of PFS. However, all trials included in meta‐analysis reported some progression endpoint such as treatment failure, progression or relapse. We have provided the exact reported definitions in Table 2.
Sensitivity analysis
Regarding the timing of interim PET, interim PET was conducted after two cycles of chemotherapy (PET2) in nine studies (N = 1677 participants in total) (Cerci 2010; Hutchings 2006; Kobe 2018; Rossi 2014; Simon 2016; Straus 2011; Touati 2014; Zaucha 2017; Zinzani 2012). In five studies (N = 402 participants in total), interim PET was conducted at other timings: in Annunziata 2016 all participants received PET1; in Barnes 2011 and Hutchings 2005 participants received either PET2 or PET3; and in Hutchings 2006 and Mesguich 2016 participants received either a PET2, PET3 or PET4. At sensitivity analysis, temporarily removing studies that did not perform a PET2 barely affected the results for PFS (overall: HR 4.90, 95% CI 3.47 to 6.90; sensitivity: HR 4.68, 95% CI 3.14 to 6.98) (Analysis 3.6). Hence, the timing of the interim PET measurement (when conducted at a time point other than PET2) did not affect the overall pooled result for PFS.
Analysis 3.6.
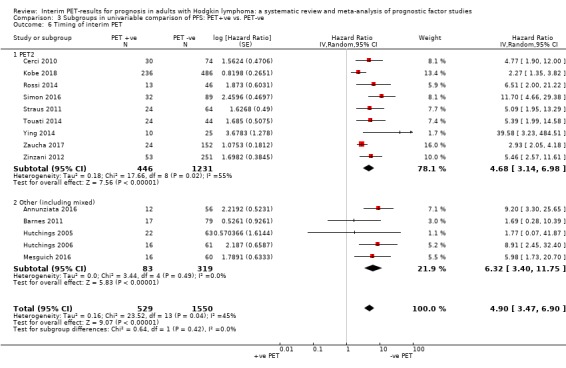
Comparison 3 Subgroups in univariable comparison of PFS: PET+ve vs. PET‐ve, Outcome 6 Timing of interim PET.
Regarding the precision of the HR estimation, we were able to provide a precise estimation of the HR and SE for nine studies (N = 1450 participants in total) (Annunziata 2016; Barnes 2011; Hutchings 2005; Kobe 2018; Rossi 2014; Simon 2016; Straus 2011; Ying 2014; Zaucha 2017). For five studies (N = 629 participants in total) we were only able to provide a slightly imprecise estimation of the HR and SE (Cerci 2010; Hutchings 2006; Mesguich 2016; Touati 2014; Zinzani 2012). However, at sensitivity analysis we found that the imprecise HRs did not significantly affect the pooled results. Temporarily removing the imprecise studies during sensitivity analysis barely affected the pooled results (overall: HR 4.90, 95% CI 3.47 to 6.90; sensitivity: HR 4.69, 95% CI 2.84 to 7.73) (Analysis 3.7). Hence, we concluded that the overall pooled PFS is robust and was not affected by our slightly imprecise method of HR and SE estimation.
Analysis 3.7.
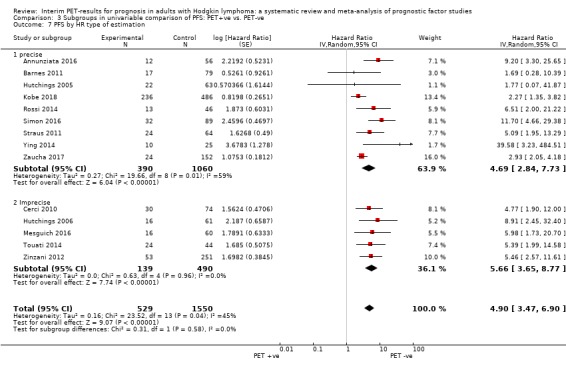
Comparison 3 Subgroups in univariable comparison of PFS: PET+ve vs. PET‐ve, Outcome 7 PFS by HR type of estimation.
Narrative reporting of results
Univariable analyses
Seven studies that reported results for PFS were not included in meta‐analysis (Andre 2017; Casasnovas 2019; Gallamini 2014; Hutchings 2014; Markova 2012; Oki 2014; Okosun 2012). Table 5 presents the results from these studies narratively. We extracted all data that were available and relevant to us (i.e. number of interim PET‐negative and interim PET‐positive participants, number of events and percentages for PFS). Due to strong differences in the reporting between studies, the table presents more information for some studies compared to others.
Table 5. Narrative reporting of results from univariable analysis for PFS
| Study | No. of participants analysed | Timing of interim PET scan | Unadjusted results for interim PET |
| Andre 2017 | Favourable: N = 371 standard arm Unfavourable: N = 583 standard arm |
PET2 |
*PET‐negative Favourable group: N = 2 events (both relapses) in the ABVD + INRT arm, ITT 5‐year PFS rate was 99.0% (95% CI 3.8 to 66.1) Unfavourable group: N = 22 events (16 relapses and 6 deaths not related to HL), ITT 5‐year PFS rate was 92.1% (95% CI 88.0 to 94.8) *Results presented here are only for participants without interim PET adaptation (ABVD + INRT arm). Unclear how many of these participants were PET‐positive or PET‐negative. In total (all participants included in the study), there were 465 PET‐negative participants and 361 PET‐positive participants. *PET‐positive N = 41 events (36 relapses and 5 deaths not related to HL) in the ABVD + INRT arm, ITT 5‐year PFS rate was 77.4% (95% CI 70.4 to 82.9) |
| Casasnovas 2019 | Standard arm N = 413 |
PET2 |
PET2 results (N = 398) Intention‐to‐treat analysis PET2‐negative N = 349 participants (88%), 5‐year PFS = 88.4% (95% CI 83.3 to 92) PET2‐positive N = 49 participants (12%), 5‐year PFS = 73.5% (95% CI 58.7 to 83.6) Results for entire standard arm 5‐year PFS = 86.2% (95% CI 81.6 to 89.8) 41 participants relapsed or progressed, 14 deaths Comment: Logrank test for difference between groups in the standard arm after PET2 was not reported and could not be obtained. Per‐protocol analysis N = 372 participants 5‐year PFS = 86.7% (95% CI 81.9 to 90.3) for entire arm |
| Gallamini 2014 | 260 (stages IIA ‐ IV) | PET2 | PET‐negative N = 215, 12 events (progression N = 7, relapse N = 5), 3‐year PFS = 95% PET‐positive N = 45, 33 events (progression N = 27, relapse N = 6), 3‐year PFS = 28% Logrank test for difference between groups: P < 0.0001 |
| Hutchings 2014 | 121 (all stages) | PET1 (N = 121) PET 2 (N = 89) |
PET1 results (N = 126) PET‐negative N = 89, 5 events (relapse), 2‐year PFS = 94.1% PET‐positive N = 37, 22 events (17 primary refractory disease, 5 relapses), 2‐year PFS = 40.8% Log‐rank test for difference between groups: P < 0.01 PET1 vs. PET2 results (N = 89) Participants scanned after PET1 and 2 PET1‐negative 2‐year PFS = 98.3% PET1‐positive 2‐year PFS = 38.5% PET2‐negative 2‐year PFS = 90.2% PET2‐positive 2‐year PFS = 23.1% 14 PET1‐positive converted to a PET2‐negative (6 progressed). All PET1‐negative were also PET2‐negative. |
| Markova 2012 | 69 (advanced stages) | PET4 | PET‐negative N = 51, 2 events (1 relapse and 1 death), % of PFS not reported PET‐positive N = 18, 4 events (progression or relapse), % of PFS not reported Log‐rank test for difference between groups: P = 0.016 |
| Oki 2014 | 229 (all stages) | PET2 |
3‐year PFS rates in PET2‐negative versus PET–positive by disease subgroups Early stage favourable: 100% vs. 100% Early stage unfavourable: 91.5% vs. 56.3% (P < 0.0001) Early stage non‐bulky: 95.9% vs. 76.9% (P = 0.0018) Stage II bulky: 83.3% vs. 20% (P = 0.017) Advanced stage with IPS≤2: 77.0% vs. 30.0% (P < 0.001) Advanced stage with IPS≥3: 71.0% vs. 44.4% (P = 0.155) |
| Okosun 2012 | 23 (stages II ‐ IV) | PET2 or PET3 | PET‐negative: N = 21, no events, 2‐year PFS = 100% PET‐positive: N = 2, 1 event (treatment failure), 2‐year PFS = 50% Log‐rank test for difference between groups: P = 0.0012 |
Multivariable analyses
Eight studies reported adjusted effect estimates for PFS (Casasnovas 2019; Gallamini 2014; Hutchings 2005; Hutchings 2006; Kobe 2018; Mesguich 2016; Rossi 2014; Simon 2016). Table 6 shows which prognostic factors (covariates) were considered in the final multivariable model of the studies. In two studies, only the results of those covariates that remained independent prognostic factors in multivariable analysis were reported (Gallamini 2014; Simon 2016). It is unclear whether, or which other covariates were included in the final multivariable model. The selection of prognostic factors (covariates) for adjustment in the studies was either based on their significance in univariable analysis (Casasnovas 2019; Hutchings 2006; Kobe 2018), or on the literature (established prognostic factors) (Hutchings 2005; Rossi 2014; Simon 2016). In two studies, the rationale for the covariates was not clearly stated (Gallamini 2014; Mesguich 2016).
As there are no final models with an identical set of covariates, pooling of adjusted effect estimates was not feasible. A full list of study‐specific, candidate covariates can be found in the respective table for each study in the Characteristics of included studies.
The statistical methods used were Cox proportional hazards regression model and logistic regression model.
Table 6. Adjusted results from final multivariable model for PFS
| Study | Prognostic factors | Adjusted results for interim PET | |||||||
| Interim PET | Age | Gender | Disease stage | B symptoms | Bulky disease | IPS | Other study‐specific factors | ||
| Casasnovas 2019 | x | ‐ | x | x | x | x | x | x | Multivariable analysis not reported separately for standard treatment group. |
| Gallamini 2014 | x | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | x |
PET2 HR N/A, P < 0.01 (Sig. 0.000), 95% CI 3.136 to 7.917 Comment: Adjusted results indicate the independent prognostic impact of interim PET2. |
| Hutchings 2005 | x | ‐ | ‐ | x | ‐ | ‐ | ‐ | x |
Early interim PET Wald 19.05, HR N/A, P‐value = 0.00007 Comment: Adjusted results indicate the independent prognostic impact of early interim PET. |
| Hutchings 2006 | x | ‐ | ‐ | x | ‐ | ‐ | ‐ | x |
Model 1 (interim PET2 + clinical stage + extranodal disease) PET2 HR = 36.281 (95% CI 7.179 to 183.4), P < .001 Model 2 (interim PET2 + extranodal disease) PET2 HR = 36.887 (95% CI 7.338 to 185.4), P < .001 Comment: Adjusted results indicate the independent prognostic impact of interim PET2. |
| Kobe 2018 | x | ‐ | ‐ | ‐ | ‐ | ‐ | x | x |
Interim PET‐positivity (DS 4) HR 2.4 (95% CI 1.4 to 4.1), P = 0.002 Comment: Adjusted results indicate an independent prognostic impact of PET2. |
| Mesguich 2016 | x | ‐ | ‐ | x | ‐ | x | ‐ | ‐ |
Model 1 (interim PET + disease stage) Positive interim PET HR = 3.73 (95% CI 1.35 to 10.35), P = 0.0112 Model 2 (interim PET + bulky disease) Positive interim PET HR = 3.62 (95% CI 1.30 to 10.05), P = 0.0138 Comment: Adjusted results indicate the independent prognostic impact of interim PET. |
| Rossi 2014 | x | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | x |
SUVmax PET0‐PET2 Relative risk = 7.9 (95% CI 2.9 to 22.9), P = 0.0001 Comment: Adjusted results indicate the independent prognostic impact of SUVmax PET0‐PET2. |
| Simon 2016 | x | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | x |
Interim PET‐positivity HR = 17.74, P < 0.001, 95% CI 6.61 to 47.57 Comment: Adjusted results indicate the independent prognostic impact of PET2. |
|
x = prognostic factor considered for adjustment in the final model ‐ = prognostic factor was not considered in the final model | |||||||||
Adverse events (AEs)
None of the included studies measured PET‐associated AE.
Studies not reporting our outcomes
Two studies (Gandikota 2015; Orlacchio 2012) did not report data for our outcomes of interest, but were still included in this review as they fit our inclusion criteria. Their investigated outcomes were very close to our review outcomes and potentially the authors could have measured them, but did not report them in their publication. However, it has not been possible to obtain the relevant information; therefore, they are reported narratively in this review. Table 7 presents the results from these studies narratively.
Table 7. Narrative reporting of results from studies not reporting our outcomes of interest
| Study | No. of participants | Outcomes/comparison | Results |
| Gandikota 2015 | 77 (stages IIA ‐ IIB) |
|
Analysis of imaging at different time points Baseline imaging
Imaging during and at the end of treatment
Follow‐up imaging
Need for surveillance imaging Quote: “No relapse of cHL was detected at a median follow‐up of 46 months. […] Routine imaging (either CT or PET‐CT) for the early detection of relapse does not appear necessary or justified in these participants.” |
| Orlacchio 2012 | 132 (all stages) | Interim PET2 vs. end PET (three months after the end of chemo‐ and radiotherapy). |
Interim PET results
End PET results Negative interim PET2 group
Positive interim PET2 group
Interim PET vs. end PET Negative interim PET2 group
Positive interim PET2 group
NPV and PPV
|
Discussion
Summary of main results
In this systematic review, we summarised unadjusted data for interim positron emission tomography (PET) scan results as a prognostic factor in individuals with classic Hodgkin lymphoma (HL). The results of an interim PET scan during therapy, e.g. after two cycles of chemotherapy, has been suggested as a good predictor of outcome. Interim PET scan results have also been suggested as an indicator to guide further treatment in order to achieve the best possible outcome in those that have a poor prognosis and those that have a good prognosis, while also minimising adverse events due to the toxicity of the chemotherapy. The results of our review are summarised in the Table 1.
The findings emerging from meta‐analyses are as follows.
Unadjusted results for overall survival (OS) show a large advantage for participants with a negative interim PET scan result compared to participants with a positive interim PET scan result. We rated the certainty of the evidence as 'moderate'.
Unadjusted results for progression‐free survival (PFS) show an advantage for participants with a negative interim PET scan result compared to participants with a positive interim PET scan result, but the evidence is very uncertain. We rated the certainty of the evidence as 'very low'.
The findings of the adjusted results from multivariable analyses, reported narratively in this review, are as follows.
Adjusted results for OS indicate an independent prognostic ability of interim PET beyond other associated factors. We rated our certainty of the evidence as 'moderate'.
Adjusted results for PFS indicate that there may be an independent prognostic ability of interim PET beyond other associated factors. We rated our certainty of the evidence as 'low'.
No study measured adverse events (AEs) associated with PET.
Overall completeness and applicability of the evidence
The evidence in this review mostly applies to adults who were newly diagnosed with classic HL, and who receive a PET scan in combination with CT (PET‐CT) after two cycles of chemotherapy (PET2). The studies included in this review addressed our research question in a total of 7335 male and female participants representing all stages of classic HL (Ann Arbor stages I ‐ IV with A or B symptoms). Nine studies included individuals aged 18 years or older, while the remaining studies also included adolescents and young adults (the youngest being 13 years of age, although most studies started from the age of 16 and onwards). Overall, the findings from this review support the statement that in this group of individuals, interim PET scan results can predict OS and PFS. Most participants in the included studies received ABVD (adriamycin/doxorubicin, bleomycin, vinblastine and dacarbazine) chemotherapy, which is the standard treatment regimen for early‐stage disease (Bröckelmann 2018; Engert 2010). However, as participants can have different therapy regimens, which is decided based on their disease stage and other clinical or individual characteristics, results should always be interpreted with caution for different patient groups, and this naturally restrains the applicability of the evidence for all people with classic HL. Twelve out of 23 studies reported our primary outcome of interest OS, while 21 studies reported PFS. No study reported PET‐associated AE. As the main aim of the review was to identify the prognostic value of interim PET results to predict survival outcomes, it is unlikely that studies on prognosis will measure or report AE.
Heterogeneity between the studies was also found with regard to the evaluation of the interim PET scan, as studies used different criteria for the interpretation of the results. Most studies used the Deauville five‐point scale (DS 1 ‐ 5) for the evaluation of the PET scans. However, different cut‐off values were used for PET‐positivity. Most studies considered scores one to three (DS 1‐3) for PET‐negativity, and scores four to five (DS 4‐5) for PET‐positivity. In some studies, however, DS3 was also considered (or tested) for PET‐positivity. Results from these studies should be interpreted with caution, as using a score of ≥3 can have an important impact on the results and possibly introduce bias. Firstly, using this cut‐off can lead to an increased number of false‐positive results for interim PET (Casasnovas 2019). This can have a relevant impact for the individual, if treatment would be modified based on the interim PET scan results (such as in the studies by Andre 2017; Casasnovas 2019; Kobe 2018). Furthermore, using this cut‐off can lead to an overestimation of the positive outcomes in the interim PET‐positive group. In the study by Kobe 2018, in which cut‐off DS3 and DS4 were tested for PET‐positivity, the results showed no significant difference in DS1‐2 compared to DS3, but a significant difference between DS1‐3 and 4. Thereby, the authors argue for DS4 as the cut‐off value for PET‐positivity, which is interpreted as an [18F]‐fluorodeoxy‐D‐glucose (FDG) uptake higher than in the liver, instead of an uptake higher than in the mediastinum (corresponding DS3) (Kobe 2018). Hence, the implementation of a commonly used cut‐off in clinical practice is important in order to improve interobserver reliability and agreements between central reviewers, and is also highly crucial for the individual (Kobe 2018; Meignan 2009a). In the remaining studies included in this review, either different criteria were used (e.g. International Harmonization Project in Lymphoma criteria (Juweid 2007)), or no specific scale was indicated. However, in most studies, at least two nuclear medicine physicians independently interpreted the interim PET scan results.
One of the greatest issues regarding the prognostic factor studies in this review relates to the difficult reporting of their statistical analyses. Even when the methods of the statistical analyses were appropriate for the study design, the data were insufficiently reported in many of the included studies. We used hazard ratios (HRs) as the effect measure for time‐to‐event data in this review. We were able to pool data from only 15 studies, either because the HR and associated standard error (SE) were not reported, or because we did not have separate data for our participants or outcomes of interest. Out of these 15 studies, six studies reported an HR, but we still re‐calculated the value for four of them for different reasons. For example, values were re‐calculated either when we detected discrepancies between the text and corresponding graph(s) and table(s), or when they were simply not reported, while other relevant data were, helping us to estimate the HR and SE. For the remaining studies, we estimated the HR using other available data where possible (Altman 2012; Parmar 1998; Tierney 2007). For this reason, we contacted 10 principal investigators to clarify our questions and provide us with additional information or data, or both. This step was particularly helpful for deciding which data to pool. Furthermore, the pooled estimates in our analyses are based on crude HRs (no adjustment for covariates), therefore results on the prognostic ability of interim PET are prone to optimism. We prespecified in our protocol that we would only pool adjusted data if analyses were based on an identical set of covariates. Although this was not feasible for our review, we suggest future authors of systematic reviews of prognosis studies to consider pre‐specifying a core set of covariates (established prognostic factors) that are important to the disease under review, that should be investigated in the included studies (Riley 2019). In this way, authors may be able to pool adjusted data, if studies are homogenous enough. In addition, we have moderate between‐study heterogeneity, which is reflected in the I² and wide confidence intervals (CIs). We took these issues around the reporting in the studies into account when we assessed risk of bias and GRADE.
Lastly, although we did not conduct a test for funnel plot asymmetry as this type of test is not necessarily recommended for survival data due to issues of censoring (Debray 2018), we cannot exclude potential publication bias and the presence of small‐study effects in our review (Riley 2019). Firstly, we assume that publication bias may be present in our review as most studies in our analyses have rather small sample sizes, of which all present positive results on the prognostic ability of interim PET scan results. Secondly, most studies included in this review are retrospective studies that have not been pre‐registered, for example, in trial registries. Studies are also not always labelled or indexed as prognosis studies, and search filters for studies on prognosis are still under development, which is the main reason as to why we conducted a broad search with the disease (HL) and prognostic factor (PET) of interest. This led to a high number of search results that had to be screened. Thirdly, we identified a great number of conference abstracts on studies for which we could not find full‐text publications (see Characteristics of studies awaiting classification). Hence, based on these experiences, we cannot preclude that more studies may exist that have either not been published, or not indexed properly.
Certainty of the evidence
Our certainty of the evidence is presented in the Table 1.
Unadjusted results
For our primary outcome OS, we judged the certainty of the evidence as 'moderate'. We included nine studies in the meta‐analysis, of which eight were observational studies and one was a clinical trial. We used the data of participants from the standard arm (no treatment adaptation) of this trial. We judged the certainty of the evidence as 'moderate' due to some methodological issues. We downgraded by one point for risk of bias due to a moderate risk of bias in three studies and a high risk of bias in six studies for the domain study confounding, as well as a high risk of bias in four studies for the domain statistical analysis and reporting. In addition, we downgraded by one point for imprecision because the HR had to be estimated in seven studies, and re‐calculated in one study. Hence, only one out of nine studies reported an HR that we used. Nevertheless, we upgraded by one point for a large effect showing the large difference in the OS between interim PET‐positive and interim PET‐negative participants (HR 5.09, CI 2.64 to 9.81).
For the outcome PFS, we judged the certainty of the evidence as 'very low'. We included 14 studies in the meta‐analysis, of which 12 were observational studies and two were clinical trials (participants from the standard arms). For this outcome, we downgraded by one point for inconsistency because the definition of PFS varied across the studies. We also downgraded by one point for imprecision because the HR had to be estimated in 10 studies and re‐calculated in one study. Hence, we were able to use a reported HR for only three out of 14 studies. In addition, we downgraded by one point for risk of bias, because of moderate risk of bias in six studies and high risk of bias in eight studies for the domain 'study confounding', and high risk of bias in six studies for the domain 'statistical analysis and reporting'.
Adjusted results
For the outcome OS, two studies reported adjusted results from multivariable analyses including established prognostic factors (e.g. International Prognostic Score) in individuals with HL, and the results of both studies indicate the independent prognostic ability of interim PET to predict OS. We judged our certainty in the evidence as 'moderate' for this outcome due to some methodological issues. We downgraded by one point for risk of bias due to a moderate risk of bias for the domain study confounding in both studies, and a high risk of bias for the domain statistical analysis and reporting for one study.
For the outcome PFS, there were eight studies that reported adjusted results (adjusted for e.g. disease stage or B symptoms). All studies found that interim PET scan results have an independent prognostic ability to predict PFS. However, we rated our certainty in the evidence as 'low' for this outcome. We rated down by one point for risk of bias due to moderate risk of bias for the domain study confounding in four studies, and moderate and high risk of bias for the domain statistical analysis and reporting for two studies. For one study, we also rated the domain study confounding as high risk of bias. Furthermore, we rated down by one point for inconsistency because the studies included a heterogenous set of covariates in the multivariable analyses, which made the pooling of adjusted results not feasible.
Potential biases in the review process
To prevent bias in this review, two teams of two review authors independently performed all relevant processes (i.e. screening, data extraction, risk of bias and GRADE assessment). Due to the complexity of assessing bias in prognostic factor studies, as well as assessing the certainty of the evidence from these types of studies, we conducted several teleconferences with different experts in the field of prognosis to discuss our assessments. We consulted Jill Hayden (Hayden 2013) for the 'Risk of bias' assessment, and the GRADE for Prognosis working group for the GRADE assessment. Inparticula, Particularly the methods for grading the evidence from prognosis studies are still under development.
For the 'Risk of bias' assessment, we are aware that adding 'unclear' as a fourth possible rating, thereby setting an example for future authors, can lead to a potential bias in the assessment. However, for our assessment we only used 'unclear' when relevant information was evidently missing, thereby making it difficult to make a fair and transparent judgement for the respective study and domain. We felt that rating a domain as high risk of bias in such cases would be inappropriate. We clearly advise against the use of 'unclear' as a default option and want to recommend future authors of reviews of prognosis studies to use this fourth rating carefully (if the fourth rating will be included in an update of the QUIPS tool).
Our analyses included post‐hoc subgroup analyses on the type of PET measurement (PET versus PET‐CT), as well as post‐hoc sensitivity analyses on the timing of the interim PET and the type of estimation (see Methods) used to estimate missing values. These analyses were necessary due to the heterogeneity between the studies. Results should be interpreted in light of differences that can exist when participants receive a PET‐CT as compared to a PET scan only. Furthermore, the timing of the interim PET is crucial, as PET1 and PET2 may provide different results compared to PET3 and PET4.
Regarding the adjusted results, we refrained from pooling results because, although the studies looked at established prognostic factors, they did not include identical sets of covariates. As the studies are already very heterogeneous, pooling of the adjusted results was not feasible for our review, as the comparison and interpretation of these results may be problematic in this case. To avoid this in the future, we suggest pre‐defining a core set of covariates in order to enable pooling of adjusted results (Riley 2019).
Agreements and disagreements with other studies or reviews
In our review, we included studies that have assessed the prognostic value of interim PET in HL participants without treatment modification. Overall, the findings from this review are in agreement with similar reviews and studies that have investigated the prognostic value of interim PET. Our results are also in agreement with the literature that interim PET can be used for disease and therapy monitoring (Barrington 2017a). Some reviews and studies have investigated this in participants in whom the treatment was changed based on the interim PET scan result, and have come to similar conclusions that interim PET can predict outcome in the different groups (PET‐negative and PET‐positive participants).
We are aware of three systematic reviews (Adams 2015a; Amitai 2018;Sickinger 2015) that have investigated interim PET as a prognostic factor. Adams 2015a included ten studies with limited‐, intermediate‐ and advanced‐stage HL participants in whom the treatment regimen was not modified based on the interim PET scan results. In fact, nine out of these 10 studies are also included in our review. One study was not included in our review because they only included children. The authors of this review concluded that a negative interim PET cannot exclude treatment failure, but that a positive interim PET can identify and predict treatment failure. The authors assessed the quality of the studies with the QUIPS tool (as we did in our review) and judged the overall methodological quality of the included studies as moderate. We have compared their QUIPS assessment with ours for each individual study, and identified that for the domains study participation and study attrition in particular, we found agreements between the authors and our review that there is a low risk of bias in the studies. However, we detected disagreements, particularly with regard to study confounding and statistical analysis, for which in our review we judged the risk of bias as either moderate (for example, when covariates were taken into consideration, but without justification for the chosen factors) or high risk (for example, when multivariable analyses were not conducted, and the reporting of the statistical analyses was poor and, thereby, complicated our own analyses). In Adams 2015a, these domains were judged as low risk of bias in all studies. Disagreement was also found regarding the domain prognostic factor measurement, for which the authors judged the quality as moderate mainly due to the heterogeneity between the studies regarding the use of PET‐CT versus PET only, which is an issue that we have also addressed in our review by subgroup analysis.
Comparison of interim PET with end PET
Nine of the included studies compared the performances of interim PET and end‐of treatment PET (end PET) (Barnes 2011; Hutchings 2006; Hutchings 2014; Markova 2012; Mesguich 2016; Orlacchio 2012; Straus 2011; Ying 2014; Zinzani 2012), as omitting one of the two can have an impact on radiation safety for the patient. However, results between studies are rather contradictory. For example, in Barnes 2011 the authors could not detect a significant difference in OS and PFS between interim PET‐negative and interim PET‐positive participants. In their analyses, interim PET‐positive participants that were negative at end PET had the same good outcomes as participants who were negative both at interim and end PET. In addition, after end PET, the difference between end PET‐positive and end PET‐negative participants was fairly high, with a greater four‐year OS and PFS for end‐PET‐negative participants. In this study, 74 (end PET) out of 79 participants (interim PET) remained PET‐negative, while nine (end PET) out of 17 (interim PET) participants remained PET‐positive. The authors concluded that end‐PET (after six cycles of chemotherapy) predicts outcome, rather than interim PET (after two or four cycles of chemotherapy). In Hutchings 2006, interim PET was conducted after two and four cycles of chemotherapy (total number of cycles was six to eight). Results show that PET2 and PET4 were similarly successful in predicting outcome in participants, but the authors of the study still argue that treatment modifications should be indicated as early as possible (e.g. after PET2) in order to achieve the best possible outcome. In the study by Mesguich 2016, interim PET was also lower in its predictive ability compared to end PET. Out of 60 interim PET‐negative participants, seven converted to a positive end PET. Out of 16 interim PET‐positive participants, seven converted to a negative end PET. In addition, treatment failure was most common in participants with a positive end PET as compared to participants with a positive interim PET. The sensitivity of interim PET was measured as 47% compared to 80% of end PET (Mesguich 2016).
Contrastingly, Orlacchio 2012 detected a very high negative predictive value (NPV) of 98% for interim PET2, with an overall diagnostic accuracy of 86.4%. Out of 104 interim PET‐negative participants, 102 were still negative after end PET. Out of 28 interim PET‐positive participants, however, 16 converted to a negative end PET. A high NPV for interim PET was also found in Hutchings 2005 (interim PET2/3) as interim PET‐negative participants rarely relapsed. In Hutchings 2014, 89 participants had an interim PET1 and PET2, and both show a strong prognostic ability for predicting outcome. In this study, none of the participants in early stages that had a negative interim PET1 progressed or relapsed. Advanced‐stage participants with a negative interim PET1 had a long‐term PFS of more than 90%. The three‐year PFS of interim PET1‐positive participants was 30%. In total, 89 participants had both PET1 and PET2. Out of these, 62 were PET1‐negative, and after treatment, 60 were in complete remission. Twenty‐seven participants were PET1‐positive, of which 15 were in complete remission. To compare, 76 participants were PET2‐negative, of whom 70 were in complete remission. Thirteen participants were PET2‐positive, of which five were in complete remission. The negative predictive value of PET1 was reported as 96.8%, while the positive predictive value was 44.4%. Zinzani 2012 also reported that interim PET after two cycles is highly predictive of OS and PFS. In their study, 92% of the interim PET‐negative participants (n = 251) were in continuous complete remission as compared to 24.5% of the interim PET‐positive participants (n = 53). These conclusions are supported by Ying 2014, although their sample size (n = 35) is too small to provide definite answers. Straus 2011 supported these statements particularly for participants in early stages (as included in their study), as participants with a negative interim PET2 result had a PFS of about 90%, compared to 50% for interim PET‐positive participants, at two years. Markova 2012 reported similar findings for interim PET4, which had a high NPV of 98%. Out of 68 participants in total, 50 had a negative interim PET, but 59 a negative end PET. In other words, nine interim PET‐positive participants were end PET‐negative after chemotherapy. The other nine participants who were interim PET‐positive were also end PET‐positive. At both timings (PET4 and PET6/8) the authors found a significant difference in the survival between PET‐positive and PET‐negative participants. The high NPV of interim PET supports early de‐escalation of chemotherapy, or omitting radiotherapy, in order to reduce the risk of toxicity and adverse events related to the harsh treatment.
Treatment adaptation based on interim PET
Although not an aim of our review, we considered it important to discuss some results from recently published randomised controlled trials (RCTs) in which the interim PET scan result was used to adapt the therapy for individuals with HL in order to improve outcomes (Andre 2017; Casasnovas 2019; Johnson 2016; Kobe 2018), based on the premise that interim PET scan results are indeed prognostic. For example, in the trial by Johnson 2016, the primary aim was to test the omission of bleomycin due to its toxic effects. All participants (N = 1214, advanced stages) started with ABVD chemotherapy. After interim PET2, PET‐positive (DS4‐5) participants (N = 182) were assigned to BEACOPP, and PET‐negative (DS1‐3) participants (N = 935) were randomised to receive either ABVD or AVD. Results show that three‐year PFS was slightly better in the ABVD group compared to the AVD group (85.7% versus 84.4%, respectively). Regarding three‐year OS, the ABVD group reached 97.2% compared to 97.6% in the AVD group. Hence, there were no significant subgroup differences. However, grade 3 and 4 AEs due to the chemotherapy were more common in the ABVD group. In the PET‐positive group, which was escalated to BEACOPP chemotherapy, 3‐year PFS was 67.5% and 3‐year OS was 87.8%.
In another example by Casasnovas 2019, 823 advanced‐stage HL participants were randomly assigned to standard treatment group or PET‐driven treatment group. All participants received two cycles of BEACOPPescalated as the initial therapy and interim PET was conducted thereafter. PET‐positive participants in both groups, as well as PET‐negative participants in the standard group continued with the initial therapy after PET2. PET‐negative participants in the experimental arm, however, were switched to two cycles of ABVD. Results of five‐year PFS show a similar survival of PET‐negative participants in the standard group and experimental group: 88.4% and 89.4%, respectively.
Several systematic reviews were also published that investigated treatment adaptation based on interim PET scan results. Amitai 2018 included 13 studies (of which four were RCTs) that investigated interim PET‐adapted treatment in advanced‐staged HL. Their findings support the statement that PET‐adapted treatment is an appropriate strategy and that it should be considered as standard care for advanced HL (Amitai 2018). This finding is supported by a Phase II RCT (Carras 2018), which assessed interim PET‐response adapted treatment strategy in advanced‐stage HL. The authors concluded that early salvage therapy and high‐dose chemotherapy or autologous stem cell transplant (ASCT) for PET2‐positive participants is safe and can lead to similar positive outcomes as in PET2‐negative participants (Carras 2018). To compare, Sickinger 2015 included studies in which the treatment was also modified, but concluded that PFS was shorter in individuals with early‐stage HL and a negative PET scan receiving chemotherapy only (PET‐adapted therapy) than in those receiving additional RT (standard therapy). This finding was confirmed in another review by Blank 2017, showing improved PFS in early‐stage participants receiving radiotherapy in addition to chemotherapy. However, the overall methodological quality of the included studies in both reviews was judged as moderate (for PFS) to very low (for OS). Constrasting evidence on the clinical and prognostic value of interim PET‐adapted treatment was also found in non‐systematic reviews, which particularly acknowledge the heterogeneity between available studies that makes it difficult to give definite conclusions (Adams 2016a; Berriolo‐Riedinger 2018).
Authors' conclusions
Implications for practice
This review provides moderate‐certainty evidence that interim positron emission tomography (PET) scan results predict overall survival (OS), and very low‐certainty evidence that interim PET scan results predict progression‐free survival (PFS) in individuals with Hodgkin lymphoma (HL) (evidence of the pooled, unadjusted results). The evidence on the ability of interim PET scan results to distinguish between individuals with a poor prognosis and individuals with a good prognosis can aid decision‐making for clinicians and diagnosed individuals, and the evidence may be used in international treatment guidelines for individuals with HL.
Implications for research
Multivariable analyses and prognostic models
Thus far, the prognostic value of interim PET has mostly been assessed in univariable analyses, in which its prognostic ability of determining survival outcomes in individuals with HL has been shown. However, using one single factor is usually not sufficient to give a satisfactory prediction of an outcome, and clinicians, therefore, usually use multiple factors to give an accurate prediction of an individual's disease progression and health outcome (Moons 2009). Hence, it is important to assess the independent prognostic value of the prognostic factor of interest (in this case interim PET) against established prognostic factors such as age, sex, B symptoms or other relevant clinical and individual factors in multivariable analyses as well (Moons 2009; Riley 2019). In such analyses, the independent prognostic ability of a factor, as well as its incremental value on top of other prognostic factors, can be assessed (Moons 2009). In a next step, prognostic models can be built that include multiple prognostic factors that have been proven to be predictive of outcome. Such models are built for risk adaptation and treatment stratification for participants who present those specific factors included in a prediction model for a specific disease, and thereby enables more individualised disease monitoring and treatment guidance. Using a combination of factors, rather than one factor only, allows for a more individual and accurate estimate of the risk of a patient to experience a certain health event (or outcome) within a specific period of time (Moons 2009; Steyerberg 2013).
With regard to our prognostic factor of interest, we could pool adjusted results in meta‐analyses in an update of this review if new studies would adjust for the same set of prognostic factors (covariates). There is a number of different established clinical and individual prognostic factors that can be used to predict survival outcomes in individuals with HL (Cuccaro 2014; Josting 2010; Kılıçkap 2013). In order to enable pooling of adjusted results, future authors of systematic reviews of prognostic factor studies could define a core set of covariates a priori (Riley 2019).
Study design
There is some evidence from retrospective studies that interim PET scan results can predict outcome in individuals during chemotherapy. However, it is commonly agreed that the true prognostic value of this factor can best be assessed in randomised controlled trials (RCTs), in which participants are randomly assigned to a standard or an experimental arm. In the standard arm, participants continue with the planned therapy regimen independent of the interim PET scan result. In the experimental arm, however, different treatments are given according to the interim PET scan result, e.g. de‐escalation of treatment in interim PET‐negative participants. Hence, RCTs are the most suitable study design, with results from experimental arms in which participants receive therapy adaptation based on the interim PET scan result providing the most robust evidence on whether outcome can be approved, while treatment can be safer, by this strategy. Although assessing therapy modification was not an aim of our review, we judged it important to present and discuss some results of published trials that evaluated the impact of PET‐adapted treatment on survival outcomes.
Acknowledgements
This review was published in collaboration with the Cochrane Fast‐Track Service. We particularly thank Helen Wakeford (Managing Editor) and Heather Maxwell (copy‐editor) for their support. The Fast‐Track Service team made comments on the review and managed the editorial process.
We thank Nicola Köhler of the Cochrane Haematological Malignancies (CHM) Editorial Base as well as the editors Dr. Julia Bohlius and PD Dr. Sebastian Theurich and the consumer editor Anne Lyddiatt for commenting on the protocol of this review. We also thank the copy‐editor Heather Maxwell.
We thank Ina Monsef of CHM for developing the search strategy and conducting the search for this review, and Marius Goldkuhle and Vanessa Piechotta for commenting on the first submitted draft of this review.
We thank Jill Hayden for her help with the QUIPS tool, and particularly for agreeing to our adaptation of the tool for the purpose of this review.
We thank Yu‐Tian Xiao for the translation of the Chinese publication (Ying 2014).
We thank all principal investigators who have replied to all of our questions and provided us with more information or data, thereby helping us to include as many studies as possible in our analysis. Hence, we want to thank Prof. Dr. Jan Maciej Zaucha, Prof. Dr. Andrea Gallamini, Prof. Dr. Peter Borchmann and his colleague Helen Görgen, Prof. Dr. Pier Luigi Zinzani, Dr. Martin Hutchings, Prof. Dr. Marc André and his colleague Dr. John Raemaekers.
We thank Kym Snell and Sarah Hodgkinson for commenting on the first submitted draft of this review, as well as Robin Featherstone for commenting on the search strategy.
We thank all external peer‐reviewers who read and commented on this review. We thank Robert Wolff, Bob Phillips, Michel Meignan, Tim Illidge and Ulrike Holtkamp who greatly helped to improve this review.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials search strategy
Searches until 07/02/2016
ID Search
#1 MeSH descriptor: [Lymphoma] this term only
#2 MeSH descriptor: [Hodgkin Disease] explode all trees
#3 Germinoblastom*
#4 Reticulolymphosarcom*
#5 Hodgkin*
#6 (malignan* near/2 (lymphogranulom* or granulom*))
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Positron‐Emission Tomography] explode all trees
#9 (pet* or petscan*)
#10 tomograph*
#11 emission*
#12 MeSH descriptor: [Deoxyglucose] explode all trees
#13 MeSH descriptor: [Fluorodeoxyglucose F18] explode all trees
#14 (deoxyglucose* or desoxyglucose* or deoxy‐glucose* or desoxy‐glucose* or deoxy‐d‐glucose* or desoxy‐d‐glucose* or 2deoxyglucose* or 2deoxy‐d‐glucose* or fluorodeoxyglucose* or fluorodesoxyglucose* or fludeoxyglucose* or fluordeoxyglucose* or fluordesoxyglucose* or 18fluorodeoxyglucose* or 18fluorodesoxyglucose* or 18fluordeoxyglucose* or fdg* or 18fdg* or 18f‐dg*)
#15 (fluor* or 2fluor* or fluoro* or fluorodeoxy* or fludeoxy* or fluorine* or 18f* or 18flu*)
#16 glucose*
#17 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 #7 and #17 in trials
Searches from 08/02/2016 ‐ 13/07/2017
ID Search
#1 MeSH descriptor: [Lymphoma] this term only
#2 MeSH descriptor: [Hodgkin Disease] explode all trees
#3 Germinoblastom*
#4 Reticulolymphosarcom*
#5 Hodgkin*
#6 (malignan* near/2 (lymphogranulom* or granulom*))
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Positron‐Emission Tomography] explode all trees
#9 (pet* or petscan*)
#10 tomograph*
#11 emission*
#12 MeSH descriptor: [Deoxyglucose] explode all trees
#13 MeSH descriptor: [Fluorodeoxyglucose F18] explode all trees
#14 (deoxyglucose* or desoxyglucose* or deoxy‐glucose* or desoxy‐glucose* or deoxy‐d‐glucose* or desoxy‐d‐glucose* or 2deoxyglucose* or 2deoxy‐d‐glucose* or fluorodeoxyglucose* or fluorodesoxyglucose* or fludeoxyglucose* or fluordeoxyglucose* or fluordesoxyglucose* or 18fluorodeoxyglucose* or 18fluorodesoxyglucose* or 18fluordeoxyglucose* or fdg* or 18fdg* or 18f‐dg*)
#15 (fluor* or 2fluor* or fluoro* or fluorodeoxy* or fludeoxy* or fluorine* or 18f* or 18flu*)
#16 glucose*
#17 #15 and #16
#18 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #17
#19 #7 and #18 in Trials
#20 #19 Publication Year from 2016 to 2017
Searches from 12/07/2017 ‐ 12/11/2018
Cochrane Central Register of Controlled Trials (Cochrane Library Issue 11, 2018)
ID Search
#1 MeSH descriptor: [Lymphoma] this term only
#2 MeSH descriptor: [Hodgkin Disease] this term only
#3 germinoblastom*
#4 reticulolymphosarcom*
#5 hodgkin*
#6 (malignan* near/2 (lymphogranulom* or granulom*))
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Positron‐Emission Tomography] explode all trees
#9 (pet*)
#10 tomograph*
#11 emission*
#12 MeSH descriptor: [Deoxyglucose] explode all trees
#13 MeSH descriptor: [Fluorodeoxyglucose F18] this term only
#14 (deoxyglucose* or desoxyglucose* or deoxy‐glucose* or desoxy‐glucose* or deoxy‐d‐glucose* or desoxy‐d‐glucose* or 2deoxyglucose* or 2deoxy‐d‐glucose* or fluorodeoxyglucose* or fluorodesoxyglucose* or fludeoxyglucose* or fluordeoxyglucose* or fluordesoxyglucose* or 18fluorodeoxyglucose* or 18fluorodesoxyglucose* or 18fluordeoxyglucose* or fdg* or 18fdg* or 18f‐dg*)
#15 (glucose* and (fluor* or 2fluor* or fludeoxy* or 18f*))
#16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#17 #7and #16 in Trials
key: * : truncation, near/#: adjacent within # number of words
Searches from 12/11/2018 ‐ 02/04/2019
ID Search
#1 MeSH descriptor: [Lymphoma] this term only
#2 MeSH descriptor: [Hodgkin Disease] explode all trees
#3 Germinoblastom*
#4 Reticulolymphosarcom*
#5 Hodgkin*
#6 (malignan* near/2 (lymphogranulom* or granulom*))
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Positron‐Emission Tomography] explode all trees
#9 (pet* or petscan*)
#10 tomograph*
#11 emission*
#12 MeSH descriptor: [Deoxyglucose] explode all trees
#13 MeSH descriptor: [Fluorodeoxyglucose F18] explode all trees
#14 (deoxyglucose* or desoxyglucose* or deoxy‐glucose* or desoxy‐glucose* or deoxy‐d‐glucose* or desoxy‐d‐glucose* or 2deoxyglucose* or 2deoxy‐d‐glucose* or fluorodeoxyglucose* or fluorodesoxyglucose* or fludeoxyglucose* or fluordeoxyglucose* or fluordesoxyglucose* or 18fluorodeoxyglucose* or 18fluorodesoxyglucose* or 18fluordeoxyglucose* or fdg* or 18fdg* or 18f‐dg*)
#15 (fluor* or 2fluor* or fluoro* or fluorodeoxy* or fludeoxy* or fluorine* or 18f* or 18flu*)
#16 glucose*
#17 #15 and #16
#18 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #17
#19 #7 and #18 in Trials
Appendix 2. MEDLINE Ovid search strategy
| # | Searches until 02/02/2016 |
| 1 | *LYMPHOMA/ |
| 2 | exp HODGKIN DISEASE/ |
| 3 | Germinoblastom$.tw,kf,ot. |
| 4 | Reticulolymphosarcom$.tw,kf,ot. |
| 5 | Hodgkin$.tw,kf,ot. |
| 6 | (malignan$ adj2 (lymphogranulom$ or granulom$)).tw,kf,ot. |
| 7 | or/1‐6 |
| 8 | POSITRON‐EMISSION TOMOGRAPHY/ |
| 9 | (pet$ or petscan$).tw,kf,ot. |
| 10 | tomograph$.tw,kf,ot. |
| 11 | emission$.tw,kf,ot. |
| 12 | exp DEOXYGLUCOSE/ |
| 13 | FLUORODEOXYGLUCOSE F18/ |
| 14 | (deoxyglucose$ or desoxyglucose$ or deoxy‐glucose$ or desoxy‐glucose$ or deoxy‐d‐glucose$ or desoxy‐d‐glucose$ or 2deoxyglucose$ or 2deoxy‐d‐glucose$ or fluorodeoxyglucose$ or fluorodesoxyglucose$ or fludeoxyglucose$ or fluordeoxyglucose$ or fluordesoxyglucose$ or 18fluorodeoxyglucose$ or 18fluorodesoxyglucose$ or 18fluordeoxyglucose$ or fdg$ or 18fdg$ or 18f‐dg$).tw. |
| 15 | (fluor$ or 2fluor$ or fluoro$ or fluorodeoxy$ or fludeoxy$ or fluorine$ or 18f$ or 18flu$).tw. |
| 16 | glucose$.tw. |
| 17 | or/8‐16 |
| 18 | 7 and 17 |
| 19 | ANIMALS/ not HUMANS/ |
| 20 | 18 not 19 |
| # | Searches from 03/02/2016 – 12/07/2017 |
| 1 | *LYMPHOMA/ |
| 2 | exp HODGKIN DISEASE/ |
| 3 | Germinoblastom$.tw,kf,ot. |
| 4 | Reticulolymphosarcom$.tw,kf,ot. |
| 5 | Hodgkin$.tw,kf,ot. |
| 6 | (malignan$ adj2 (lymphogranulom$ or granulom$)).tw,kf,ot. |
| 7 | or/1‐6 |
| 8 | exp POSITRON‐EMISSION TOMOGRAPHY/ |
| 9 | (pet$ or petscan$).tw,kf,ot. |
| 10 | tomograph$.tw,kf,ot. |
| 11 | emission$.tw,kf,ot. |
| 12 | exp DEOXYGLUCOSE/ |
| 13 | FLUORODEOXYGLUCOSE F18/ |
| 14 | (deoxyglucose$ or desoxyglucose$ or deoxy‐glucose$ or desoxy‐glucose$ or deoxy‐d‐glucose$ or desoxy‐d‐glucose$ or 2deoxyglucose$ or 2deoxy‐d‐glucose$ or fluorodeoxyglucose$ or fluorodesoxyglucose$ or fludeoxyglucose$ or fluordeoxyglucose$ or fluordesoxyglucose$ or 18fluorodeoxyglucose$ or 18fluorodesoxyglucose$ or 18fluordeoxyglucose$ or fdg$ or 18fdg$ or 18f‐dg$).tw. |
| 15 | (fluor$ or 2fluor$ or fluoro$ or fluorodeoxy$ or fludeoxy$ or fluorine$ or 18f$ or 18flu$).tw. |
| 16 | glucose$.tw. |
| 17 | 15 and 16 |
| 18 | or/8‐14 |
| 19 | 17 or 18 |
| 20 | 7 and 19 |
| 21 | ANIMALS/ not HUMANS/ |
| 22 | 20 not 21 |
| 23 | limit 22 to ed=20160203‐20170712 |
| # | Searches from 12/07/2017 ‐ 12/11/2018 |
| 1 | *LYMPHOMA/ |
| 2 | HODGKIN DISEASE/ |
| 3 | germinoblastom$.tw,kf,ot. |
| 4 | reticulolymphosarcom$.tw,kf,ot. |
| 5 | hodgkin$.tw,kf,ot. |
| 6 | (malignan$ adj2 (lymphogranulom$ or granulom$)).tw,kf,ot. |
| 7 | or/1‐6 |
| 8 | exp POSITRON‐EMISSION TOMOGRAPHY/ |
| 9 | (pet$).tw,kf,ot. |
| 10 | tomograph$.tw,kf,ot. |
| 11 | emission$.tw,kf,ot. |
| 12 | exp DEOXYGLUCOSE/ |
| 13 | FLUORODEOXYGLUCOSE F18/ |
| 14 | (deoxyglucose$ or desoxyglucose$ or deoxy‐glucose$ or desoxy‐glucose$ or deoxy‐d‐glucose$ or desoxy‐d‐glucose$ or 2deoxyglucose$ or 2deoxy‐d‐glucose$ or fluorodeoxyglucose$ or fluorodesoxyglucose$ or fludeoxyglucose$ or fluordeoxyglucose$ or fluordesoxyglucose$ or 18fluorodeoxyglucose$ or 18fluorodesoxyglucose$ or 18fluordeoxyglucose$ or fdg$ or 18fdg$ or 18f‐dg$).tw. |
| 15 | (glucose$ and (fluor$ or 2fluor$ or fludeoxy$ or 18f$)).tw. |
| 16 | or/8‐15 |
| 17 | 7 and 16 |
| 18 | exp ANIMALS/ not HUMANS/ |
| 19 | 17 not 18 |
| 20 | limit 19 to ed=20160203‐20170712 |
| 21 | limit 19 to ed=20170712‐20181112 |
| 22 | ANIMALS/ not HUMANS/ |
| 23 | 21 not 22 |
| 24 | limit 23 to ed=20160203‐20170712 |
| 25 | limit 23 to ed=20170712‐20181112 |
| # | Searches from 12/11/2018 ‐ 02/04/2019 |
| 1 | *LYMPHOMA/ |
| 2 | HODGKIN DISEASE/ |
| 3 | Germinoblastom$.tw,kf,ot. |
| 4 | Reticulolymphosarcom$.tw,kf,ot. |
| 5 | Hodgkin$.tw,kf,ot. |
| 6 | (malignan$ adj2 (lymphogranulom$ or granulom$)).tw,kf,ot. |
| 7 | or/1‐6 |
| 8 | exp POSITRON‐EMISSION TOMOGRAPHY/ |
| 9 | (pet$ or petscan$).tw,kf,ot. |
| 10 | tomograph$.tw,kf,ot. |
| 11 | emission$.tw,kf,ot. |
| 12 | exp DEOXYGLUCOSE/ |
| 13 | Fluorodeoxyglucose F18/ |
| 14 | (deoxyglucose$ or desoxyglucose$ or deoxy‐glucose$ or desoxy‐glucose$ or deoxy‐d‐glucose$ or desoxy‐d‐glucose$ or 2deoxyglucose$ or 2deoxy‐d‐glucose$ or fluorodeoxyglucose$ or fluorodesoxyglucose$ or fludeoxyglucose$ or fluordeoxyglucose$ or fluordesoxyglucose$ or 18fluorodeoxyglucose$ or 18fluorodesoxyglucose$ or 18fluordeoxyglucose$ or fdg$ or 18fdg$ or 18f‐dg$).tw. |
| 15 | (glucose$ and (fluor$ or 2fluor$ or fludeoxy$ or 18f$)).tw. |
| 16 | or/8‐15 |
| 17 | 7 and 16 |
| 18 | exp ANIMALS/ not HUMANS/ |
| 19 | 17 not 18 |
| 20 | limit 19 to ed=20160203‐20170712 |
| 21 | limit 19 to ed=20170712‐20181112 |
| 22 | limit 19 to ed=20181112‐20190402 |
key: exp # /: explode # MeSH subject heading, tw: text word, kf: keyword heading word, ot: original title, ti: title, $: truncation, adj#: adjacent within # number of words
Appendix 3. Embase/Ovid search strategy
| # | Searches |
| 1 | exp CLASSICAL HODGKIN LYMPHOMA/ |
| 2 | *HODGKIN DISEASE/ |
| 3 | germinoblastom*.tw,kw. |
| 4 | reticulolymphosarcom*.tw,kw. |
| 5 | hodgkin*.tw,kw. |
| 6 | (malignan* adj2 (lymphogranulom* or granulom*)).tw,kw. |
| 7 | or/1‐6 |
| 8 | exp POSITRON EMISSION TOMOGRAPHY/ |
| 9 | pet*.tw,kw. |
| 10 | tomograph*.tw,kw. |
| 11 | emission*.tw,kw. |
| 12 | exp DEOXYGLUCOSE/ |
| 13 | FLUORODEOXYGLUCOSE F 18/ |
| 14 | (deoxyglucose* or desoxyglucose* or deoxy‐glucose* or desoxy‐glucose* or deoxy‐d‐glucose* or desoxy‐d‐glucose* or 2deoxyglucose* or 2deoxy‐d‐glucose* or fluorodeoxyglucose* or fluorodesoxyglucose* or fludeoxyglucose* or fluordeoxyglucose* or fluordesoxyglucose* or 18fluorodeoxyglucose* or 18fluorodesoxyglucose* or 18fluordeoxyglucose* or fdg* or 18fdg* or 18f‐dg*).tw. |
| 15 | (glucose* and (fluor* or 2fluor* or fludeoxy* or 18f*)).tw. |
| 16 | or/8‐15 |
| 17 | 7 and 16 |
| 18 | exp ANIMAL/ not HUMAN/ |
| 19 | 17 not 18 |
| 20 | limit 19 to yr="1990 ‐Current" |
| 21 | meta‐analys:.mp. or search:.tw. or review.pt. |
| 22 | (child* or p?ediatric*).ti. |
| 23 | 20 not (21 or 22) |
| 24 | limit 23 to embase |
| 25 | limit 23 to conference abstracts |
| 26 | 24 or 25 |
key: exp # /: explode # EMTREE term, * # /: focus # EMTREE term, /: EMTREE term, tw: text word, kw: keyword, ti: title, mp: multiple purpose, pt: publication type, *: truncation, ?: wildcard
search line #21: Review Embase search filter ‐ best balance of sensitivity and specificity https://hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx
Data and analyses
Comparison 1.
Univariable comparison of PET+ve vs. PET‐ve
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 9 | 1802 | Hazard Ratio (Random, 95% CI) | 5.09 [2.64, 9.81] |
| 2 Progression‐free survival | 14 | 2079 | Hazard Ratio (Random, 95% CI) | 4.90 [3.47, 6.90] |
Comparison 2.
Subgroups in univariable comparison of OS: PET+ve vs. PET‐ve
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 OS by radiotherapy | 9 | 1802 | Hazard Ratio (Random, 95% CI) | 5.09 [2.64, 9.81] |
| 1.1 Involved node and/or site | 3 | 548 | Hazard Ratio (Random, 95% CI) | 3.45 [1.22, 9.72] |
| 1.2 involved field | 4 | 428 | Hazard Ratio (Random, 95% CI) | 12.75 [4.98, 32.68] |
| 1.3 not specified | 2 | 826 | Hazard Ratio (Random, 95% CI) | 2.80 [1.17, 6.67] |
| 2 OS by study design | 8 | 1717 | Hazard Ratio (Random, 95% CI) | 4.63 [2.43, 8.80] |
| 2.1 Prospective | 3 | 406 | Hazard Ratio (Random, 95% CI) | 5.35 [1.07, 26.68] |
| 2.2 Retrospective | 4 | 589 | Hazard Ratio (Random, 95% CI) | 7.12 [3.14, 16.14] |
| 2.3 RCT | 1 | 722 | Hazard Ratio (Random, 95% CI) | 2.60 [1.03, 6.56] |
| 3 OS by chemotherapy | 9 | 1802 | Hazard Ratio (Random, 95% CI) | 5.09 [2.64, 9.81] |
| 3.1 ABVD | 5 | 801 | Hazard Ratio (Random, 95% CI) | 5.19 [2.11, 12.72] |
| 3.2 ABVD and/or other | 3 | 279 | Hazard Ratio (Random, 95% CI) | 10.30 [1.71, 62.13] |
| 3.3 BEACOPP | 1 | 722 | Hazard Ratio (Random, 95% CI) | 2.60 [1.03, 6.56] |
| 4 OS for PET/CT vs PET | 8 | 1706 | Hazard Ratio (Random, 95% CI) | 5.01 [2.50, 10.02] |
| 4.1 PET/CT | 5 | 595 | Hazard Ratio (Random, 95% CI) | 4.70 [1.86, 11.86] |
| 4.2 PET only | 3 | 1111 | Hazard Ratio (Random, 95% CI) | 6.99 [1.58, 30.90] |
| 5 OS by disease stage | 9 | 1802 | Odds Ratio (Random, 95% CI) | 5.09 [2.64, 9.81] |
| 5.1 Stages I and II with A and B symptoms | 1 | 96 | Odds Ratio (Random, 95% CI) | 9.21 [0.71, 120.03] |
| 5.2 All stages | 7 | 984 | Odds Ratio (Random, 95% CI) | 6.28 [2.62, 15.05] |
| 5.3 Advanced | 1 | 722 | Odds Ratio (Random, 95% CI) | 2.60 [1.03, 6.56] |
| 6 Timing of interim PET | 9 | 1802 | Hazard Ratio (Random, 95% CI) | 5.09 [2.64, 9.81] |
| 6.1 PET2 | 6 | 1495 | Hazard Ratio (Random, 95% CI) | 3.53 [1.97, 6.32] |
| 6.2 Other (including mixed) | 3 | 307 | Hazard Ratio (Random, 95% CI) | 20.13 [5.04, 80.38] |
| 7 OS by HR type of estimation | 9 | 1802 | Hazard Ratio (Random, 95% CI) | 5.09 [2.64, 9.81] |
| 7.1 precise | 7 | 1638 | Hazard Ratio (Random, 95% CI) | 5.70 [2.60, 12.48] |
| 7.2 Imprecise | 2 | 164 | Hazard Ratio (Random, 95% CI) | 3.60 [0.89, 14.64] |
Comparison 3.
Subgroups in univariable comparison of PFS: PET+ve vs. PET‐ve
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PFS by study design | 13 | 1349 | Hazard Ratio (Random, 95% CI) | 5.66 [4.02, 7.97] |
| 1.1 prospective | 3 | 357 | Hazard Ratio (Random, 95% CI) | 3.95 [2.23, 7.00] |
| 1.2 retrospective | 8 | 827 | Hazard Ratio (Random, 95% CI) | 6.85 [4.66, 10.08] |
| 1.3 RCT | 2 | 165 | Hazard Ratio (Random, 95% CI) | 6.21 [2.87, 13.42] |
| 2 PFS by chemotherapy | 14 | 2079 | Hazard Ratio (Random, 95% CI) | 4.90 [3.47, 6.90] |
| 2.1 ABVD | 7 | 945 | Hazard Ratio (Random, 95% CI) | 5.13 [3.18, 8.27] |
| 2.2 ABVD and/or other | 4 | 265 | Hazard Ratio (Random, 95% CI) | 7.07 [3.40, 14.70] |
| 2.3 other NON‐ABVD chemo | 3 | 869 | Hazard Ratio (Random, 95% CI) | 3.64 [1.83, 7.24] |
| 3 PFS for PET/CT vs PET | 13 | 1983 | Hazard Ratio (Random, 95% CI) | 5.08 [3.57, 7.21] |
| 3.1 PET/CT | 8 | 707 | Hazard Ratio (Random, 95% CI) | 6.03 [3.68, 9.90] |
| 3.2 PET only | 5 | 1276 | Hazard Ratio (Random, 95% CI) | 4.06 [2.33, 7.08] |
| 4 PFS by disease stage | 14 | 2079 | Hazard Ratio (Random, 95% CI) | 4.90 [3.47, 6.90] |
| 4.1 Stages I and II with A and B symptoms | 2 | 184 | Hazard Ratio (Random, 95% CI) | 3.88 [1.54, 9.83] |
| 4.2 All stages | 11 | 1173 | Hazard Ratio (Random, 95% CI) | 5.81 [3.93, 8.57] |
| 4.3 Advanced | 1 | 722 | Hazard Ratio (Random, 95% CI) | 2.27 [1.35, 3.82] |
| 5 PFS by radiotherapy | 14 | 2079 | Hazard Ratio (Random, 95% CI) | 4.90 [3.47, 6.90] |
| 5.1 Involved node and/or site | 5 | 651 | Hazard Ratio (Random, 95% CI) | 5.35 [2.94, 9.75] |
| 5.2 Involved field | 6 | 514 | Hazard Ratio (Random, 95% CI) | 7.06 [4.15, 12.00] |
| 5.3 Not specified | 2 | 826 | Hazard Ratio (Random, 95% CI) | 2.97 [1.48, 5.98] |
| 5.4 None | 1 | 88 | Hazard Ratio (Random, 95% CI) | 5.09 [1.95, 13.29] |
| 6 Timing of interim PET | 14 | 2079 | Hazard Ratio (Random, 95% CI) | 4.90 [3.47, 6.90] |
| 6.1 PET2 | 9 | 1677 | Hazard Ratio (Random, 95% CI) | 4.68 [3.14, 6.98] |
| 6.2 Other (including mixed) | 5 | 402 | Hazard Ratio (Random, 95% CI) | 6.32 [3.40, 11.75] |
| 7 PFS by HR type of estimation | 14 | 2079 | Hazard Ratio (Random, 95% CI) | 4.90 [3.47, 6.90] |
| 7.1 precise | 9 | 1450 | Hazard Ratio (Random, 95% CI) | 4.69 [2.84, 7.73] |
| 7.2 Imprecise | 5 | 629 | Hazard Ratio (Random, 95% CI) | 5.66 [3.65, 8.77] |
What's new
| Date | Event | Description |
|---|---|---|
| 17 September 2019 | Amended | Acknowledgements updated |
Differences between protocol and review
We included studies that evaluated both adult and adolescent participants (the youngest being 13 years old), as opposed to including adult participants ( ≥ 18 years of age) only as stated in the protocol of this review. Hodgkin lymphoma is a disease with a typical onset in adolescence to mid‐adulthood, with little physiological differences between adolescents and adults. In the studies included in this review, participants under the age of 18 were treated in the same clinic and received the same treatment as participants over ≥ 18 years of age. We believe that the results regarding interim PET are equally relevant to adolescents as they are to adult participants and, therefore, did not see reasons against the inclusion of studies including both younger and older adults. Nevertheless, we did not include studies that evaluated solely paediatric participants. In studies where only paediatric participants are included, it is more likely they will be treated in paediatric clinics and receive a different treatment regimen than adult participants.
We used the Quality in Prognostic Factor Studies (QUIPS) tool to assess the risk of bias of the included studies. However, in consultation with Hayden and colleagues (Hayden 2013), we adapted the QUIPS tool by adding 'unclear (no information)' as a fourth judgement in the tool. In addition, we assessed all six domains (study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, statistical analysis and reporting) per outcome (OS and PFS) in each study. The first three domains ended up always receiving the same judgement as they are indeed to be considered at study level. With regard to the outcomes, however, we identified differences in analysis and reporting within studies.
With regard to data extraction, we developed our own data extraction form specific to prognostic factor studies (particularly those that are included in this review), which includes more items than stipulated in the protocol of this review.
Lastly, we searched Embase as an additional database, as well as one trial registry (ClinicalTrials.gov).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Countries
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
(exclusion criteria reported in Fermé 20071) Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
[1] Fermé C, Eghbali H, Meerwaldt JH, Rieux C, Bosq J, Berger F, et al. Chemotherapy plus involved‐field radiation in early‐stage Hodgkin’s disease. New England Journal of Medicine 2007;357:1916‐1927 |
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
(therapy regimen reported in Raemaekers 20141) |
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
[1] Raemaekers JM, André MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography–negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomised EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32(12):1188‐1194 |
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Countries
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Countries
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country/Countries
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Countries
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
*Potential overlap of Danish participants with those included in Hutchings 2006 Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Countries
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country/Countries
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
*For the whole population (n = 50), but only 35 participants underwent interim PET |
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes | Translated from Chinese to English by Yu‐Tian Xiao. Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
*Data for surviving participants only |
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
| Methods |
Secondary citation(s)
Language of publication
Study design
Study centre(s)
Country
Median follow‐up time (range)
|
|
| Participants |
Number of included participants
Inclusion criteria
Exclusion criteria
Consent
Recruitment period
Age (range, in years)
Ethnic group(s)
Stages of disease
Comorbidities
Therapy regimen
|
|
| Prognostic factor(s) |
Prognostic factor(s)
Definition of prognostic factor(s)
Timing of prognostic factor measurement
Method for measurement (use of specific scale and cut‐off)
Was the same definition and method for measurement used in all participants?
Were prognostic factor(s) assessed blinded for outcome(s), and for each other (if relevant)?
|
|
| Notes |
Conflict of interest
Funding
|
|
ABVD: adriamycin/doxorubicin, bleomycin, vinblastine and dacarbazine; BEACOPP: bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone; ePET: early positron emission tomography; FDG: [18F]‐fluorodeoxy‐D‐glucose; HL: Hodgkin lymphoma; HR: hazard ratio; IF‐RT: involved‐field radiation therapy; ITT: intention‐to‐treat; IQR: interquartile range; NPV: negative predictive value; OS: overall survival; PET: positron emission tomography; PET‐CT: positron emission tomography computed tomography; PFS: progression‐free survival; PPV: positive predictive value.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2016 | Wrong publication type. Letter to the editor. |
| Adams 2017 | Wrong publication type. Letter to the editor. |
| Adams 2018 | Wrong publication type. Letter to the editor. |
| Adams 2018a | Wrong publication type. Letter to the editor. |
| Adams 2018b | Wrong publication type. Letter to the editor. |
| Adams 2019 | Wrong publication type. Letter to the editor. |
| Advani 2007 | Reported only end‐of‐chemotherapy PET scan results. |
| Afanasyev 2017 | Wrong publication type. Protocol. |
| Albano 2017 | PET‐adapted outcomes. |
| Albano 2018 | PET‐adapted outcomes. Treatment was modified according to PET2 results. |
| Altamirano 2008 | Wrong study population. Includes non‐Hodgkin lymphoma patients. |
| Ansell 2016 | Wrong publication type. Article. |
| Awan 2013 | Wrong patient population. Patients with any lymphoma were included; no separate data for Hodgkin lymphoma patients. |
| Bar‐Shalom 2003 | Wrong study design. Comparison FDG PET and 67Ga scintigraphy. |
| Barrington 2011a | Wrong publication type. Meeting abstract. |
| Barrington 2017 | Wrong publication type. Commentary. |
| Basu 2009 | Wrong publication type. Commentary. |
| Becherer 2002 | Wrong study design. End‐of‐chemotherapy PET. Includes non‐Hodgkin lymphoma. |
| Bednaruk‐Mlynski 2015 | Wrong study design. Role of baseline PET/CT. |
| Biggi 2012 | Wrong publication type. Conference abstract. |
| Biggi 2017 | PET‐adapted outcomes. Treatment was modified according to PET2 results. |
| Bishop 2015 | Wrong publication type. Commentary. |
| Bjurberg 2006 | Wrong treatment. Retrospective study of patients with residual tumour or suspected relapse after therapy. |
| Blum 2002 | Wrong patient population. Non‐Hodgkin lymphoma patients |
| Bodet‐Milin 2008 | Wrong patient population. Non‐Hodgkin lymphoma patients. |
| Bodet‐Milin 2009 | Wrong publication type. Article. |
| Boisson 2007 | Wrong publication type. Article. |
| Borchmann 2016 | Wrong study design. Literature review. |
| Bucerius 2006 | Wrong publication type. Conference abstract. |
| Carras 2018 | PET‐adapted outcomes. Treatment was modified according to PET2 results. |
| Ciammella 2016 | PET‐adapted outcomes. |
| Cremerius 1999 | Wrong study design. Retrospective study to validate the clinical value of FDG‐PET for therapy control. |
| Cremerius 2001 | Wrong patient population. Patients with any lymphoma were included; no separate data for Hodgkin lymphoma patients. |
| Cuccaro 2016 | PET‐adapted outcomes. Positive interim PET results led to change in therapy in three patients; data from these patients was not reported separately from the study population in analysis. |
| D'Urso 2018 | Wrong study design. Analysis of metabolic parameters. |
| Damlaj 2017 | PET‐adapted outcomes. Treatment was modified according to PET2 results. |
| Damlaj 2019 | Pet‐adapted outcomes. |
| Danilov 2017 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Dann 2009 | PET‐adapted outcomes. |
| Dann 2010 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Dann 2010a | PET‐adapted outcomes.Treatment was modified according to PET2 results. |
| Dann 2012 | PET‐adapted outcomes.Treatment was modified according to PET2 results. |
| Dann 2013 | PET‐adapted outcomes.Treatment was modified according to PET2 results. |
| Dann 2016 | PET‐adapted outcomes. Treatment was modified according to PET2 results. |
| Dann 2017 | PET‐adapted outcomes. Treatment was modified according to PET2 results. |
| Dann 2018 | Wrong publication type. Response to letter. |
| deAndres‐Galiana 2015 | Wrong study design. Prognostic factor identification study. |
| Diehl 2007 | Wrong study design. The aim was to specify the negative predictive value of PET in patients with residual tumour mass after chemotherapy. |
| El‐Galaly 2012 | Wrong study design. The study evaluated the utility of PET scans for post‐therapy routine surveillance imaging. |
| Evens 2014 | Wrong publication type. Article. |
| Fanti 2008 | Wrong study design. Case study. |
| Filmont 2003 | Wrong patient population. Patients with aggressive lymphoma undergoing salvage therapy. |
| Fornecker 2017 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Freudenberg 2004 | Wrong patient population. Patients with any lymphoma were included; no separate data for Hodgkin lymphoma patients. |
| Friedberg 2002 | Wrong study design. The study intended to compare FDG‐PET to gallium scintigraphy in the staging and follow‐up of newly diagnosed patients with Hodgkin lymphoma. |
| Friedberg 2004 | Wrong study design. The study intended to compare FDG‐PET to gallium scintigraphy in the staging and follow‐up of newly diagnosed patients with Hodgkin lymphoma. |
| Front 1999 | Wrong treatment. The study investigated the utility of gallium scintigraphy performed early during treatment as a means to predict outcome and optimise treatment in Hodgkin lymphoma patients. |
| Fruchart 2006 | Wrong patient population. Patients with B‐cell lymphoma. |
| Gallamini 2008 | Wrong publication type. Article. |
| Gallamini 2017 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Gallamini 2018 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Gallamini 2018a | Wrong publication type. Reply to letter. |
| Gallowitsch 2008 | Wrong publication type. Commentary. |
| Goldschmidt 2011 | Wrong patient population. Relapsed, aggressive non‐Hodgkin lymphoma. |
| Greil 2018 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Guidez 2016 | Wrong publication type. No abstract or full text. |
| Hagtvedt 2015 | Wrong study design. Comparison between FDG‐PET and diffusion‐weighted magnetic resonance imaging for assessment of early treatment response in lymphoma. |
| Haioun 2005 | Wrong patient population. Patients with any lymphoma were included; no separate data for Hodgkin lymphoma patients. |
| Hartmann 2012 | Wrong study design. The study investigated protein expression patterns in different Hodgkin lymphoma subtypes. |
| Hartridge‐Lambert 2013 | Wrong study design. The study evaluated the risk of disease recurrence and the value of radiologic surveillance in patients treated with ABVD alone who achieved a complete remission according to post‐treatment PET. PET was not treated as a prognostic factor. |
| Honda 2014 | Wrong patient population. Letter to the editor, presenting the case of one patient with pulmonary Hodgkin lymphoma. |
| Hueltenschmidt 2001 | Baseline and end‐of‐chemotherapy PET results. |
| Huic 2006 | Wrong treatment. Patients within three months after completion of conventional initial therapy or salvage therapy with high‐dose chemotherapy were included in the study population; no subgroup analysis was reported. |
| Hutchings 2007 | End‐of‐chemotherapy PET. |
| Iagaru 2008 | Wrong patient population. Patients with any lymphoma were included; no separate data for Hodgkin lymphoma patients. |
| Illidge 2015 | PET‐adapted outcomes. Commentary on a research news article about PET‐adapted treatment in Hodgkin lymphoma patients. |
| Jerusalem 2003 | End‐of‐chemotherapy PET. |
| Johnson 2015 | PET‐adapted outcomes. |
| Johnson 2016 | PET‐adapted outcomes. |
| Kamran 2016 | PET‐adapted outcomes. |
| Kamran 2018 | PET‐adapted outcomes. Treatment was modified according to PET2 results. Data was not reported separately for PET‐positive and PET‐negative patients. |
| Kobe 2008 | Wrong study design. The study evaluated the negative predictive value of PET scans in advanced‐stage Hodgkin lymphoma patients. |
| Kobe 2014 | Wrong study design. The study evaluated how computed tomography might help improve the positive predictive value of PET in identifying potential high‐risk patients. |
| Kostakoglu 2006 | Wrong patient population. Patients with either diffuse large cell lymphoma or Hodgkin lymphoma were included; no separate data for Hodgkin lymphoma patients. |
| Li 2013 | Wrong patient population. The study population consisted of patients with mature T‐cell and natural killer cell lymphomas. |
| Lowe 2002 | Wrong study design. Commentary. |
| Milgrom 2017 | Wrong study design. The study population consisted mostly of PET‐positive patients. The study compared data from PET‐positive patients who received salvage chemotherapy or autologous stem cell transplantation with patients who received radiotherapy only. |
| Mocikova 2010 | Wrong study design. The study evaluated the routine use of PET scans in Hodgkin lymphoma patients during follow‐up and in cases of suspected relapse. |
| Mocikova 2011 | Wrong treatment. The study evaluated the prognostic significance of pre‐transplant PET scans after salvage chemotherapy before autologous stem cell transplant in patients with relapsed or refractory Hodgkin lymphoma. |
| Molnar 2010 | End‐of‐chemotherapy PET. |
| Moskowitz 2015 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Naumann 2001 | End‐of‐chemotherapy PET. |
| NCT00784537 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| NCT00795613 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| NCT01358747 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| NCT01652261 | PET‐adapted outcomes. Study closed due to lack of recruitment. |
| NCT02292979 | Wrong study design. |
| Nguyen 2017 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Panizo 2004 | End of chemotherapy PET. |
| Paolini 2007 | PET‐adapted outcomes. |
| Pavlovsky 2019 | PET‐adapted outcomes. |
| Pichler 2000 | Wrong study design. Comparison of FDG‐Hybrid‐PET scans. |
| Reinhardt 2005 | Wrong study design. The study evaluated the accuracy of computed tomography and FDG‐PET for prediction of progression‐free survival of Hodgkin lymphoma and non‐Hodgkin lymphoma patients after completion of therapy. |
| Rigacci 2002 | Wrong study design. Letter. |
| Rigacci 2017 | Wrong study design. Letter. |
| Rubello 2015 | Wrong study design. The study evaluated the variability of FDG liver uptake in patients with Hodgkin lymphoma. |
| Sakr 2017 | Wrong study design. |
| Schot 2007 | Wrong treatment. The study population included patients with recurring lymphoma who were treated with second‐line chemotherapy followed by autologous stem cell transplantation. |
| Simontacchi 2015 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Slaby 2002 | Wrong patient population. Patients with any lymphoma were included; no separate data for Hodgkin lymphoma patients. |
| Spaepen 2001 | Reported only end‐of‐chemotherapy PET scan results. |
| Specht 2007 | Wrong publication type. Article. |
| Spinner 2018 | Wrong publication type. Article. |
| Straus 2018 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Strigari 2016 | Wrong study design. The aim of the study was to present a novel quantitative tool to refine the risk‐class assessment of the Deauville criteria. |
| Sucak 2011 | Wrong treatment. The study population included patients with relapsed or refractory lymphoma post‐autologous stem cell transplantation. |
| Tirelli 2015 | Wrong publication type. Article. |
| Tomita 2015 | Wrong patient population. The study population consisted of patients with peripheral T cell lymphoma. |
| Torizuka 2004 | PET‐adapted outcomes. Includes non‐Hodgkin lymphoma patients. |
| Trotman 2017 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Tseng 2012 | Wrong patient population. The study population included relapsed patients. |
| Villa 2018 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
| Weidmann 1999 | Wrong patient population. Includes relapsed patients. |
| Wilson 2018 | Wrong publication type. Commentary. |
| Xie 2018 | Wrong publication type. Review. |
| Yasgur 2015 | Wrong publication type. Commentary. |
| Yoshimi 2008 | Wrong treatment. The study population included lymphoma patients with a poor prognosis who had received FDG‐PET scans within one month before allogeneic stem cell transplantation. |
| Zabrocka 2016 | Wrong study design. The study evaluated the current usage of PET scans and its clinical usefulness at different points in Hodgkin lymphoma management based on a single‐institution experience. |
| Zaucha 2009 | Wrong publication type. Review. |
| Zinzani 1999 | Wrong patient population. Includes non‐Hodgkin lymphoma patients. |
| Zinzani 2002 | Wrong patient population. Includes non‐Hodgkin lymphoma patients. |
| Zinzani 2016 | PET‐adapted outcomes. Treatment was modified according to interim PET results. |
ABVD: adriamycin/doxorubicin, bleomycin, vinblastine and dacarbazine; FDG: [18F]‐fluorodeoxy‐D‐glucose; PET: positron emission tomography; PET‐CT: positron emission tomography computed tomography.
Characteristics of studies awaiting assessment [ordered by study ID]
| Notes | Title: End of treatment but not interim PET scan predicts outcome in non‐bulky limited stage Hodgkin lymphoma. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Interim‐positron emission tomography with [18F]fluorodeoxyglucose (interim‐PET) evaluation in mediastinal lymphoma including Hodgkin lymphoma (HL) and primary mediastinal large B‐cell lymphoma (PMBL). (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Interim FDG PET‐CT in Hodgkin's lymphoma ‐ Does binary response assessment criteria have any prognostic value? (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Interim‐PET in Hodgkin lymphoma: Deauville criteria and metabolic parameters as prognostic factors. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Prognostic value of interim 18FDG‐PET‐CT in patients with Hodgkin's lymphoma using different 5‐point visual scales for interpretation. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Are the Deauville criteria a reliable tool for assessment of interim PET in Hodgkin lymphoma? (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The predictive value of interim PET‐CT in elderly patients with Hodgkin lymphoma. This is an abstract only and a lot of relevant information is missing. A full‐text has not been published yet. It is particularly unclear whether participants have received treatment adaptation based on the interim PET result. Authors need to be contacted for more information. Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Prognostic value of interim 18F‐FDG PET‐CT in mediastinal bulky Hodgkin lymphoma. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Excellent outcome in Hodgkin lymphoma with ABVD and CMT: A single‐centre retrospective analysis. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The complementary prognostic role of baseline and interim PET in predicting treatment outcome in advanced‐stage Hodgkin lymphoma. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Prognostic role of interim 18FDG‐PET in Hodgkin lymphoma: A single‐center experience. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Evaluation of interim 18FDG‐PET in advanced Hodgkin lymphoma (HL) patients (PTS) treated with ChlVPP/ABVVP regimen. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Single institution experience with interim PET evaluation in newly diagnosed CHL receiving ABVD chemotherapy: Need for standardization. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The Deauville criteria and metabolic parameters as prognostic factors in interim PET in Hodgkin lymphoma: A single centre experience. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Prognostic value of 18F‐FDG PET‐CT in Hodgkin lymphoma. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: 'Early FDG‐PET' predicts clinical course of Hodgkin's lymphoma although does not correlate with macrophages infiltration in diagnostic specimens. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Early interim FDG‐PET during intensified BEACOPP therapy for advanced‐stage Hodgkin disease shows a lower positive predictive value than during ABVD. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The importance of PET‐CT as method of evaluation of early response to treatment in HL. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The risk of progression of Hodgkin lymphoma in patients with negative interim PET: A role for the number of tumor‐infiltrating macrophages (CD68+ cell counts) and B symptoms. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: correlation of FDG‐PET results after one cycle and after two cycles of chemotherapy in Hodgkin lymphoma. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Interim FDG PET‐CT to predict progression‐free survival (PFS) better than clinical and baseline metabolic measurements in Hodgkin lymphoma (cHL). (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Significance of early interim PET results in advanced Hodgkin lymphoma treated intensive program EACOPP‐14. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The use of FDG positron emission tomography (FDG‐PET) in patients with Hodgkin lymphoma (HL) in the "real world": A population based study from northern Italy. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Use of 2‐[18F]fluoro‐2‐deoxy‐D‐glucose positron emission tomography in patients with Hodgkin lymphoma in daily practice: a population‐based study from Northern Italy Authors need to be contacted to clarify whether the treatment has been adapted based on the interim PET results. Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The impact of outcome of interim PET‐CT on advanced Hodgkin lymphoma treated with EACOPP‐14. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The value of interim 18F‐FDG PET‐CT in Hodgkin lymphoma. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Interim FDG PET‐CT examinations in advanced stage Hodgkin lymphoma. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Prognostic value of interim vs. end‐of‐treatment PET scan in Hodgkin's lymphoma. (Confernece abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Role of positron emission tomography (PET) after 2 and 4 courses of chemotherapy in patients with Hodgkin's lymphoma: A single center experience. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Bulky disease does not adversely affect overall survival in early stage Hodgkin lymphoma: Role of interim PET and possible omission of radiotherapy in select patients. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: Baseline and dynamic prognostic factors in newly diagnosed classical Hodgkin's lymphoma. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The Deauville criteria and QPET as prognostic factors in interim PET in adult Hodgkin lymphoma: A single centre experience. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: PET‐negative at 2, 3 or 4 cycles of ABVD in Hodgkin's lymphoma is still good. (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
| Notes | Title: The predictive value of interim PET and immunohistochemical markers in Hodgkin lymphoma (HL). (Conference abstract) Aim
Study design
Country/treatment centre(s)
Number of included participants
Inclusion criteria
Exclusion criteria
Treatment
Primary outcome measure(s)
|
ABVD: adriamycin/doxorubicin, bleomycin, vinblastine and dacarbazine; BEACOPP: bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone; EBVD: epirubicin, bleomycin, vinblastine and dacarbazine; FDG: [18F]‐fluorodeoxy‐D‐glucose; HL: Hodgkin lymphoma; IF‐RT: involved‐field radiation therapy; iPET: interim positron emission tomography; MOPP: mustargen, Oncovin, procarbazine and prednisone; NPV: negative predictive value; PET: positron emission tomography; PET‐CT: positron emission tomography computed tomography; RT: radiotherapy.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | HD16 for Early Stages ‐ Treatment optimisation trial in the first‐line treatment of early stage Hodgkin lymphoma; treatment stratification by means of FDG‐PET |
| Starting date | November 2009 |
| Contact information | Prof. Dr. Andreas Engert, University of Cologne, Germany |
| Notes |
Study design
Country/treatment centre
Number of included participants
Inclusion criteria
Exclusion criteria
Arms and interventions
Primary outcome measure(s)
Secondary outcome measure(s)
Estimated study completion date
|
ESR: erythrocyte sedimentation rate; FDG: [18F]‐fluorodeoxy‐D‐glucose; HL: Hodgkin lymphoma; IF‐RT :involved‐field radiation therapy; MDCT: multi detector computed tomography; WHO: World Health Organization
Contributions of authors
Angela Aldin: screening and selection of studies, development of data extraction form, data extraction, 'Risk of bias' assessment, GRADE assessment, data analysis interpretation, 'Summary of findings' tables, writing and drafting of the review, communication with and between authors.
Lisa Umlauff: 'Risk of bias' assessment, characteristics of included and excluded studies (texts and tables), abstract and Plain language summary, proofread and commented on the draft.
Karel Moons: methodological input on reviews of prognosis studies.
Lise J Estcourt: screening and selection of studies, data extraction, risk of bias assessment, clinical and methodological input.
Andreas Engert: medical and content input, particularly on the clinical comparability of studies and subgroup analysis.
Carsten Kobe: nuclear medical input on PET‐CT.
Bastian von Tresckow: clinical input, particularly on the clinical comparability of studies and subgroup analysis.
Gary Collins: methodological input on reviews of prognostic studies.
Madhuri Haque: screening and selection of studies.
Farid Foroutan: input on risk of bias and GRADE assessment of prognostic factor studies.
Nina Kreuzberger: 'Risk of bias' assessment, proofread and commented on the review draft.
Marialena Trivella: screening and selection of studies, data extraction, risk of bias assessment, statistical analysis, proofread and commented on the review draft.
Nicole Skoetz: protocol development, screening and selection of studies, data extraction, risk of bias assessment, GRADE assessment, proofread and commented on the review draft.
Sources of support
Internal sources
-
University Hospital of Cologne, Germany.
Cochrane Haematological Malignancies, Department 1 of Internal Medicine
External sources
-
Federal Ministry of Education and Research, Germany.
Funding Number 01KG1709
Declarations of interest
Angela Aldin: award of the grant by Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Lisa Umlauff: award of the grant by Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Karel Moons: none known.
Lise J Estcourt: award of the grant by Federal Ministry of Education and Research to the University of Oxford to perform this systematic review does not lead to a conflict of interest.
Andreas Engert: none known.
Carsten Kobe: award of the grant by Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Bastian von Tresckow: award of the grant by Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest. Received funds from Novartis Pharma GmbH, Takeda Pharma GmbH and MSD for consultancy and educational presentations, but these were not related to the intervention in this review. No competing interests.
Gary Collins: none known.
Madhuri Haque: award of the grant by Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Farid Foroutan: none known.
Nina Kreuzberger: award of the grant by Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Marialena Trivella: part of the grant from the Federal Ministry of Education and Research to the University Hospital of Cologne, was paid to the University of Oxford for author time spent working on this review. However, the funder played no part in the design and execution of the project and it does not constitute a conflict of interest.
Nicole Skoetz: award of the grant by Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
- Adams HJ, Kwee TC. Interim fluorodeoxyglucose positron emission tomography‐adapted therapy is not an efficient approach to improving outcome in early‐stage Hodgkin lymphoma. Journal of Clinical Oncology 2017;35(24):2850‐1. [DOI] [PubMed] [Google Scholar]; Andre ME, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, et al. Early positron emission tomography response‐adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial (NCT00433433). Journal of Clinical Oncology 2017;35:1786‐94. [DOI] [PubMed] [Google Scholar]; Andre ME, Reman O, Federico M, Brice P, Brusamolino E, Girinski T, et al. First report on the H10 EORTC/GELA/IIL randomized intergroup trial on early FDG‐PET scan guided treatment adaptation versus standard combined modality treatment in patients with supra‐diaphragmatic stage I/II Hodgkin's lymphoma, for the Groupe d'Etude Des Lymphomes De l'Adulte (GELA), European Organisation for the Research and Treatment of Cancer (EORTC) Lymphoma Group and the Intergruppo Italiano Linfomi (IIL). Blood 2009;114(22):97. [Google Scholar]; Andre ME, Reman O, Federico M, Girinski T, Brice P, Brusamolino E, et al. Interim analysis of the randomized EORTC/LYSA/FIL intergroup H10 trial on early PET‐scan driven treatment adaptation in stage I/II Hodgkin lymphoma. Blood 2012;120(21):549. [Google Scholar]; Cottereau AS, Versari A, Loft A, Casanovas O, Bellei M, Ricci R, et al. Prognostic value of baseline metabolic tumor volume in early‐stage Hodgkin lymphoma in the standard arm of the H10 trial. Journal of Nuclear Medicine 2018;131(13):1456‐63. [DOI] [PubMed] [Google Scholar]; Fornecker LM, Lazarovici J, Aurer I, Casasnovas RO, Gac A C, Bonnet C, et al. PET‐based response after 2 cycles of brentuximab vedotin in combination with AVD for first‐line treatment of unfavorable early‐stage Hodgkin lymphoma: First analysis of the primary endpoint of BREACH, a randomized phase II trial of LYSA‐FIL‐EORTC intergroup. Blood 2017;130(Suppl 1):736. [Google Scholar]; Hindie E, Mesguich C, Zanotti‐Fregonara P. On the role of interim fluorine‐18‐labeled fluorodeoxyglucose positron emission tomography in early‐stage favorable Hodgkin lymphoma. Journal of Clinical Oncology 2017;35(24):2851‐2. [DOI] [PubMed] [Google Scholar]; Raemaekers JM, Andre MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32:1188‐94. [DOI] [PubMed] [Google Scholar]
- Annunziata S, Calcagni M, Rufini V, Cuccaro A, Massini G, Bartolomei F, et al. The prognostic role of interim FDG‐PET/CT and CD68+ cells count in the treatment of Hodgkin lymphoma. European Journal of Nuclear Medicine and Molecular Imaging 2014;41(Suppl. 2):S182. [Google Scholar]; Annunziata S, Calcagni ML, Indovina L, Rufini V. Measurement uncertainty and clinical impact of target‐to‐background ratios derived by interim FDG‐PET/CT in Hodgkin Lymphoma: reply to Laffon and Martan. European Journal of Nuclear Medicine and Molecular Imaging 2017;44(12):2140‐1. [DOI] [PubMed] [Google Scholar]; Annunziata S, Cuccaro A, Calcagni M, Indovina L, Hohaus S, Giordano A, et al. Could the ratio between lesion and liver SUVmax (rPET) be a prognostic factor in patients with Hodgkin lymphoma undergoing interim FDG‐PET/CT? A retrospective study. European Journal of Nuclear Medicine and Molecular Imaging. 2015; Vol. 42, issue Suppl. 1:S686. [Google Scholar]; Annunziata S, Cuccaro A, Calcagni ML, Hohaus S, Giordano A, Rufini V. Interim FDG‐PET/CT in Hodgkin lymphoma: the prognostic role of the ratio between target lesion and liver SUVmax (rPET). Annals of Nuclear Medicine 2016;30:588‐92. [DOI] [PubMed] [Google Scholar]; Annunziata S, Cuccaro A, Hohaus S, Calcagni M L, Giordano A, Rufini V. Interim FDG‐PET/CT in patients with Hodgkin lymphoma: The prognostic role of the ratio between target lesion and liver SUVmax (rPET). Journal of Nuclear Medicine 2016;57(Supp 2):651. [DOI] [PubMed] [Google Scholar]; Annunziata S, Cuccaro A, Hohaus S, Giordano A, Rufini V. Semi‐quantitative parameters could improve positive predictive value of interim FDG‐PET/CT in Hodgkin lymphoma. European Journal of Nuclear Medicine and Molecular Imaging 2016;43(Suppl 1):S312. [Google Scholar]; Annunziata S, Cuccaro A, Hohaus S, Rufini V. Interim FDG‐PET/CT in Hodgkin lymphoma: The prognostic value of ratios between target lesion and background SUV. Clinical and Translational Imaging 2017;5(Suppl 1):S44‐5. [Google Scholar]; Annunziata S, Cuccaro A, Rufini V, Calcagni M, Giachelia M, Martini M, et al. The prognostic role of interim FDG‐PET/CT, CD68 1 cells count and levels of plasma thymus and activation‐regulated chemokine in the treatment of Hodgkin lymphoma. Clinical and Translational Imaging 2015;3(Suppl 1):S18‐19. [Google Scholar]; Cuccaro A, Annunziata S, Rufini V, Calcagni M L, Giordano A, Hohaus S. Can the ratio between lesion and liver SUVmax improve outcome prediction in Hodgkin lymphoma with respect to the 5‐point Deauville score?. Haematologica 2015;100(Suppl 3):89. [Google Scholar]
- Barnes JA, LaCasce AS, Zukotynski K, Israel D, Feng Y, Neuberg D, et al. End‐of‐treatment but not interim PET scan predicts outcome in nonbulky limited‐stage Hodgkin's lymphoma. Annals of Oncology 2011;22:910‐5. [DOI] [PubMed] [Google Scholar]; Sher DJ, Mauch PM, Abbeele A, LaCasce AS, Czerminski J, Ng AK. Prognostic significance of mid‐ and post‐ABVD PET imaging in Hodgkin's lymphoma: the importance of involved‐field radiotherapy. Annals of Oncology 2009;20:1848‐53. [DOI] [PubMed] [Google Scholar]
- Casasnovas O, Bouabdallah R, Brice P, Lazarovici J, Ghesquieres H, Stamatoullas A, et al. PET‐adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): a randomised, multicentre, non‐inferiority, phase 3 study. Lancet Oncology 2019;20(2):202‐15. [DOI] [PubMed] [Google Scholar]; Casasnovas O, Brice P, Bouabdallah R, Salles G, Stamatoulas A, Dupuis J, et al. Final analysis of the AHL2011 randomized phase III LYSA study comparing an early pet driven treatment de‐escalation to a not pet‐monitored strategy in patients with advanced stages Hodgkin lymphoma. HemaSphere 2018;2(Suppl. 2):7. [Google Scholar]; Casasnovas O, Brice P, Bouabdallah R, Salles GA, Stamatoulas A, Dupuis J, et al. Randomized phase III study comparing an early PET driven treatment de‐escalation to a not PET‐monitored strategy in patients with advanced stages Hodgkin lymphoma: final analysis of the AHL2011 LYSA study. Journal of Clinical Oncology 2018;36(15):7503. [Google Scholar]; Casasnovas O, Brice P, Bouabdallah R, Salles GA, Stamatoullas A, Dupuis J, et al. Randomized phase III study comparing an early pet driven treatment de‐escalation to a not pet‐monitored strategy in patients with advanced stages hodgkin lymphoma: interim analysis of the AHL2011 LYSA study. Blood 2015;126(23):577. [Google Scholar]; Casasnovas O, Kanoun S, Tal I, Cottereau AS, Edeline V, Brice P, et al. Baseline total metabolic volume (TMTV) to predict the outcome of patients with advanced Hodgkin lymphoma (HL) enrolled in the AHL2011 LYSA trial. Journal of Clinical Oncology 2016;34(15):7509. [Google Scholar]; Casasnovas O, Meignan M, Reman O, Gaillard I, Stamatoullas A, Brice P, et al. AHL 2011: A LYSA randomized phase III study of a treatment driven by early PET response compared to a standard treatment in patients with Ann Arbor stage III‐IV or high‐risk IIB Hodgkin lymphoma. Journal of Clinical Oncology 2013;31(Suppl. 15):8615. [Google Scholar]
- Cerci JJ, Pracchia LF, Linardi CC, Pitella FA, Delbeke D, Izaki M, et al. 18F‐FDG PET after 2 cycles of ABVD predicts event‐free survival in early and advanced Hodgkin lymphoma. Journal of Nuclear Medicine 2010;51:1337‐43. [DOI] [PubMed] [Google Scholar]
- Adams HJ, Kwee TC. Prevention of large‐scale implementation of unnecessary and expensive predictive tests in Hodgkin's lymphoma. Lancet Haematology 2017;4(2):e63‐4. [DOI] [PubMed] [Google Scholar]; Agostinelli C, Gallamini A, Stracqualursi L, Agati P, Tripodo C, Fuligni F, et al. The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin's lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematology 2016;3(10):e467‐79. [DOI] [PubMed] [Google Scholar]; Biggi A, Bergesio F, Bianchi A, Menga M, Chauvie S, Fallanca F, et al. Semi‐quantitative scan assessment improves the accuracy of the deauville 5‐point scale?. Clinical and Translational Imaging 2017;5(Suppl. 1):S43‐4. [Google Scholar]; Biggi A, Gallamini A, Chauvie S, Hutchings M, Kostakoglu L, Gregianin M, et al. International validation study for interim PET in ABVD‐treated, advanced‐stage Hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. Journal of Nuclear Medicine 2013;54:683‐90. [DOI] [PubMed] [Google Scholar]; Biggi A, Kostakoglu L, Barrington S, Chauvie S, Gregianin M, Meignan M, et al. How the threshold of positive results influence progression free survival (PFS) in advanced Hodgkin's lymphoma treated with ABVD: Experience from the International Validation Study. European Journal of Nuclear Medicine and Molecular Imaging 2012;39(Suppl. 2):S222. [Google Scholar]; Gallamini A, Barrington SF, Biggi A, Chauvie S, Kostakoglu L, Gregianin M, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five‐point scale. Haematologica 2014;99:1107‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2‐[18F]fluoro‐2‐deoxy‐D‐glucose positron emission tomography is prognostically superior to international prognostic score in advanced‐stage Hodgkin's lymphoma: a report from a joint Italian‐Danish study. Journal of Clinical Oncology 2007;25:3746‐52. [DOI] [PubMed] [Google Scholar]; Gallamini A, Rigacci L, Merli F, Nassi L, Bosi A, Capodanno I, et al. The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin's disease. Haematologica 2006;91:475‐81. [PubMed] [Google Scholar]; Meignan M. High‐risk interim PET negative patients in Hodgkin's lymphoma. Lancet Haematology 2016;3(10):e449‐50. [DOI] [PubMed] [Google Scholar]; Rigacci L, Puccini B, Zinzani PL, Biggi A, Castagnoli A, Merli F, et al. The prognostic value of positron emission tomography performed after two courses (INTERIM‐PET) of standard therapy on treatment outcome in early stage Hodgkin lymphoma: A multicentric study by the fondazione italiana linfomi (FIL). American Journal of Hematology 2015;90:499‐503. [DOI] [PubMed] [Google Scholar]
- Gandikota N, Hartridge‐Lambert S, Migliacci JC, Yahalom J, Portlock CS, Schoder H. Very low utility of surveillance imaging in early‐stage classic Hodgkin lymphoma treated with a combination of doxorubicin, bleomycin, vinblastine, and dacarbazine and radiation therapy. Cancer 2015;121:1985‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR. Prognostic value of interim FDG‐PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Annals of Oncology 2005;16:1160‐8. [DOI] [PubMed] [Google Scholar]
- Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, et al. FDG‐PET after two cycles of chemotherapy predicts treatment failure and progression‐free survival in Hodgkin lymphoma. Blood 2006;107:52‐9. [DOI] [PubMed] [Google Scholar]
- Hutchings M, Kostakoglu L, Zaucha J M, Malkowski B, Biggi A, Danielewicz I, et al. Early determination of treatment sensitivity in Hodgkin lymphoma: FDG‐PET/CT after one cycle of therapy has a higher negative predictive value than after two cycles of chemotherapy. Annals of Oncology 2011;22(Suppl. 4):138‐9. [Google Scholar]; Hutchings M, Kostakoglu L, Zaucha JM, Malkowski B, Biggi A, Danielewicz I, et al. In vivo treatment sensitivity testing with positron emission tomography/computed tomography after one cycle of chemotherapy for Hodgkin lymphoma. Journal of Clinical Oncology 2014;32:2705‐11. [DOI] [PubMed] [Google Scholar]
- Adams HJ, Kwee TC. Interim FDG‐PET does not predict outcome in advanced‐stage Hodgkin lymphoma patients treated with BEACOPP. British Journal of Haematology 2018;185(4):758‐60. [DOI] [PubMed] [Google Scholar]; Adams HJ, Kwee TC. Interim FDG‐PET/CT in Hodgkin lymphoma: what are we actually looking at?. Acta Oncologica 2018;57(8):1128‐30. [DOI] [PubMed] [Google Scholar]; Borchmann P, Eichenauer DA, Pluetschow A, Haverkamp H, Kreissl S, Fuchs M, et al. Targeted BEACOPP variants in patients with newly diagnosed advanced stage classical Hodgkin lymphoma: Final analysis of a randomized phase II study. Blood 2015;126(23):580. [Google Scholar]; Borchmann P, Engert A. Reply to H.J.A. Adams et al, E.A. Hawkes et al, and C.F. Hess et al. Journal of Clinical Oncology 2017;35(3):375‐6. [DOI] [PubMed] [Google Scholar]; Borchmann P, Goergen H, Kobe C, Eichenauer D, Greil R, Lohri A, et al. EBEACOPP with or without rituximab in interim‐PET‐positive advanced‐stage Hodgkin lymphoma: updated results of the international, randomized phase 3 GHSG HD18 trial. Hematological Oncology 2017;35(Suppl. 2):65. [Google Scholar]; Borchmann P, Goergen H, Kobe C, Fuchs M, Greil R, Zijlstra JM, et al. Treatment reduction in patients with advanced‐stage Hodgkin lymphoma and negative interim pet: final results of the international, randomized phase 3 trial HD18 by the German Hodgkin study group. Haematologica 2017;102(S150):24‐5. [Google Scholar]; Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, et al. Early interim PET in patients with advanced‐stage Hodgkin's lymphoma treated within the phase 3 GHSG HD18 study. Blood 2017;130(Suppl. 1):653. [Google Scholar]; Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, et al. PET‐guided treatment in patients with advanced‐stage Hodgkin's lymphoma (HD18): final results of an open‐label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2018;390(10114):2790‐802. [DOI] [PubMed] [Google Scholar]; Borchmann P, Haverkamp H, Lohri A, Kreissl S, Greil R, Markova J, et al. Addition of rituximab to BEACOPPescalated to improve the outcome of early interim PET positive advanced stage hodgkin lymphoma patients: Second planned interim analysis of the HD18 study. Blood 2014;124(21):500. [Google Scholar]; Borchmann P, Haverkamp H, Lohri A, Mey U, Kreissl S, Greil R, et al. Progression‐free survival of early interim PET‐positive patients with advanced stage Hodgkin's lymphoma treated with BEACOPPescalated alone or in combination with rituximab (HD18): an open‐label, international, randomised phase 3 study by the German Hodgkin Study Group. Lancet Oncology 2017;18(4):454‐63. [DOI] [PubMed] [Google Scholar]; Crump M. Predicting outcomes after a positive interim FDG‐PET scan in advanced Hodgkin's lymphoma. Lancet Oncology 2017;18(4):416‐8. [DOI] [PubMed] [Google Scholar]; Kobe C, Goergen H, Baues C, Kuhnert G, Voltin CA, Zijlstra J, et al. Outcome‐based interpretation of early interim PET in advanced‐stage Hodgkin lymphoma. Blood 2018;132(21):2273‐9. [DOI] [PubMed] [Google Scholar]; Kobe C, Goergen H, Fuchs M, Eich HT, Baues C, Diehl V, et al. Treatment reduction in patients with advanced‐stage Hodgkin lymphoma and negative interim FDG‐PET: final results of the international, randomized, phase 3 HD18 trial by the German Hodgkin Study Group. European Journal of Nuclear Medicine and Molecular Imaging 2017;44(Suppl. 1):S312. [Google Scholar]; Kreissl S, Goergen H, Kobe C, Fuchs M, Greil R, Huttmann A, et al. Treatment reduction in patients with advanced‐stage Hodgkin‐lymphoma and negative interim PET: final results of the international randomized phase 3 trial HD18 by the German Hodgkin Study Goup. Oncology Research and Treatment 2017;40(Suppl. 3):201. [Google Scholar]
- Markova J, Kahraman D, Kobe C, Skopalova M, Mocikova H, Klaskova K, et al. Role of [18F]‐fluoro‐2‐deoxy‐D‐glucose positron emission tomography in early and late therapy assessment of patients with advanced Hodgkin lymphoma treated with bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone. Leukemia & Lymphoma 2012;53:64‐70. [DOI] [PubMed] [Google Scholar]; Markova J, Kobe C, Skopalova M, Dedeckova K, Mocikova H, Klaskova K, et al. Response assessment after 4 cycles of BEACOPP using FDG‐PET in patients with advanced‐stage Hodgkin lymphoma. Annals of Oncology 2011;22(Suppl. 4):160. [Google Scholar]; Markova J, Kobe C, Skopalova M, Klaskova K, Dedeckova K, Plutschow A, et al. FDG‐PET for assessment of early treatment response after four cycles of chemotherapy in patients with advanced‐stage Hodgkin's lymphoma has a high negative predictive value. Annals of Oncology 2009;20:1270‐4. [DOI] [PubMed] [Google Scholar]; Markova J, Kobe C, Skopalova M, Zikavska L, Vernerova Z, Klaskova K, et al. Early and late response assessment with FDG‐PET after BEACOPP‐based chemotherapy in advanced‐stage Hodgkin lymphoma patients has a high negative predictive value. Haematologica. 2009; Vol. 94, issue Suppl. 2:33. [Google Scholar]
- Adams HJ, Kwee TC. Hodgkin lymphoma: is there really a need for interim and end‐of‐treatment FDG‐PET evaluations?. British Journal of Haematology 2018;181(1):122‐3. [DOI] [PubMed] [Google Scholar]; Mesguich C, Cazeau AL, Bouabdallah K, Hindie E. Hodgkin lymphoma: is there really a need for interim and end‐of‐treatment FDG‐PET evaluations? ‐ Response to Adams & Kwee. British Journal of Haematology 2018;181(1):124‐5. [DOI] [PubMed] [Google Scholar]; Mesguich C, Cazeau AL, Bouabdallah K, Soubeyran P, Guyot M, Milpied N, et al. Hodgkin lymphoma: a negative interim‐PET cannot circumvent the need for end‐of‐treatment‐PET evaluation. British Journal of Haematology 2016;175:652‐60. [DOI] [PubMed] [Google Scholar]; Mesguich C, Cazeau AL, Bouabdallah K, Soubeyran P, Guyot M, Milpied N, et al. Hodgkin's lymphoma: Interim FDG‐PET result with a score <= 2 on the 5‐point scale may obviate the need for end‐of‐treatment FDG‐PET evaluation. Journal of Nuclear Medicine 2015;56(Suppl. 3):595. [Google Scholar]
- Oki Y, Chuang H, Chasen B, Jessop A, Pan T, Fanale M, et al. The prognostic value of interim positron emission tomography scan in patients with classical Hodgkin lymphoma. British Journal of Haematology 2014;165:112‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okosun J, Shaw K, Montoto S, Marcus R, Fields P, Virchis A, et al. Interim FDG‐PET scanning in patients with Hodgkin lymphoma and HIV predict response to ABVD chemotherapy. Haematologica 2011;96(Suppl. 2):322‐3. [Google Scholar]; Okosun J, Warbey V, Shaw K, Montoto S, Fields P, Marcus R, et al. Interim fluoro‐2‐deoxy‐D‐glucose‐PET predicts response and progression‐free survival in patients with Hodgkin lymphoma and HIV infection. AIDS 2012;26:861‐5. [DOI] [PubMed] [Google Scholar]
- Orlacchio A, Schillaci O, Gaspari E, Della Gatta F, Danieli R, Bolacchi F, et al. Role of [18F]‐FDG‐PET/MDCT in evaluating early response in patients with Hodgkin's lymphoma. La Radiologia Medica 2012;117:1250‐63. [DOI] [PubMed] [Google Scholar]
- Rossi C, Kanoun S, Berriolo‐Riedinger A, Dygai‐Cochet I, Humbert O, Legouge C, et al. Interim 18F‐FDG PET SUVmax reduction is superior to visual analysis in predicting outcome early in Hodgkin lymphoma patients. Journal of Nuclear Medicine 2014;55:569‐73. [DOI] [PubMed] [Google Scholar]
- Barna S, Miltenyi Z, Simon Z, Jona A, Magyari F, Nagy Z, et al. Prognostical value of Interim and restaging PET/CT on Hodgkin lymphoma with CHEAP (Chemotherapy Effectiveness Assessed by PET/CT) long term observation. European Journal of Nuclear Medicine and Molecular Imaging 2014;41(Suppl. 2):S511. [Google Scholar]; Illes A, Magyari F, Barna S, Simon Z, Payer E, Garai I, et al. Experiences of the first two years with interim PET/CT in Hodgkin lymphoma ‐ the Hungarian CHEAP study. Haematologica 2010;95(Suppl. 4):S45. [Google Scholar]; Miltenyi Z, Barna S, Garai I, Simon Z, Jona A, Magyari F, et al. Prognostic value of interim and restaging PET/CT in Hodgkin lymphoma. Results of the CHEAP (Chemotherapy Effectiveness Assessment by PET/CT) study ‐ long term observation. Neoplasma 2015;62:627‐34. [DOI] [PubMed] [Google Scholar]; Miltenyi Z, Simon Z, Jona A, Magyari F, Barna S, Garai I, et al. Interim PET/CT in Hodgkin lymphoma ‐ final results of the Hungarian CHEAP study (2007‐2011). Haematologica 2013;98(Suppl. 2):42. [Google Scholar]; Simon Z, Barna S, Miltenyi Z, Husi K, Magyari F, Jona A, et al. Combined prognostic value of absolute lymphocyte/monocyte ratio in peripheral blood and interim PET/CT results in Hodgkin lymphoma. International Journal of Hematology 2016;103(1):63‐9. [DOI] [PubMed] [Google Scholar]
- Kostakoglu L, Gandikota N, Hutchings M, Cotter R, Lamonica D, Nanni C, et al. Deauville criteria and post One‐Cycle SUVmax decrease seem to predict progression free survival (PFS) better than metabolic tumor measurements in classical Hodgkin lymphoma (cHL). European Journal of Nuclear Medicine and Molecular Imaging 2012;39(Suppl. 2):S222. [Google Scholar]; Kostakoglu L, Schoder H, Hall N, Straus DJ, Johnson JL, Schwartz L, et al. Interim FDG PET imaging in CALGB 50203 trial of stage I/II non‐bulky Hodgkin lymphoma: Would using combined PET and CT criteria better predict response than each test alone?. Blood 2011;118(21):3644. [Google Scholar]; Kostakoglu L, Schoder H, Johnson JL, Hall NC, Schwartz LH, Straus DJ, et al. Interim [(18)F]fluorodeoxyglucose positron emission tomography imaging in stage I‐II non‐bulky Hodgkin lymphoma: would using combined positron emission tomography and computed tomography criteria better predict response than each test alone?. Leukemia & Lymphoma 2012;53:2143‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]; Straus DJ, Johnson JL, LaCasce AS, Bartlett NL, Kostakoglu L, Hsi ED, et al. Doxorubicin, vinblastine, and gemcitabine (CALGB 50203) for stage I/II nonbulky Hodgkin lymphoma: pretreatment prognostic factors and interim PET. Blood 2011;117:5314‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings M, Kamper P. New clues to the prognostic challenge of Hodgkin Lymphoma. Leukemia & Lymphoma 2015;56(2):277‐8. [DOI] [PubMed] [Google Scholar]; Touati M, Delage‐Corre M, Monteil J, Abraham J, Moreau S, Remenieras L, et al. CD68‐positive tumor‐associated macrophages predict unfavorable treatment outcomes in classical Hodgkin lymphoma in correlation with interim fluorodeoxyglucose‐positron emission tomography assessment. Leukemia and Lymphoma 2015;56:332‐41. [DOI] [PubMed] [Google Scholar]
- Ying Z, Wang X, Song Y, Zheng W, Wang X, Xie Y, et al. Prognostic value of 18F‐FDG PET‐CT in Hodgkin lymphoma. Chinese Journal Of Hematology 2014;35:325‐7. [DOI] [PubMed] [Google Scholar]
- Adams HJ, Kwee TC. The predictive value of interim FDG‐PET in early‐stage Hodgkin lymphoma is not well established. Annals of Oncology 2018;29(2):510‐2. [DOI] [PubMed] [Google Scholar]; Zaucha JM, Chauvie S, Malkowski B, Warszewska A, Biggi A, Kobylecka M, et al. The prognostic role of interim PET after first chemotherapy cycle in ABVD‐treated Hodgkin lymphoma (HL) patients ‐ Polish lymphoma research group (PLRG) observational study. Haematologica 2013;98(Suppl. 2):38. [Google Scholar]; Zaucha JM, Malkowski B, Chauvie S, Subocz E, Tajer J, Kulikowski W, et al. The predictive role of interim PET after the first chemotherapy cycle and sequential evaluation of response to ABVD in Hodgkin lymphoma patients ‐ the Polish Lymphoma Research Group (PLRG) Observational Study. Annals of Oncology 2017;28(12):3051‐7. [DOI] [PubMed] [Google Scholar]; Zaucha JM, Malkowski B, Subocz E, Chauvie S, Tajer J, Kulikowski W, et al. The prognostic role of interim PET after first chemotherapy cycle and PET sequential evaluation of response to ABVD in <hodgkin lymphoma patients ‐ The Polish Lymphoma Research Group (PLRG) observational study. Blood 2015;126(23):3943. [DOI] [PubMed] [Google Scholar]
- Adams HJ, Kwee TC. Does interim 18F‐FDG‐PET response‐adapted therapy really benefit advanced‐stage Hodgkin lymphoma patients?. Nuclear Medicine Communications 2016;37(12):1333‐4. [DOI] [PubMed] [Google Scholar]; Puccini B, Rigacci L, Zinzani PL, Broccoli A, Gallamini A, Merli F, et al. Early positive FDG‐PET scan do not confirm its prognostic impact in localized bulky disease Hodgkin lymphoma patients. Haematologica 2011;96(Suppl. 3):26‐7. [Google Scholar]; Rigacci L, Puccini B, Gallamini A, Merli F, Stelitano C, Balzarotti M, et al. Early FDG‐PET scan confirms its prognostic impact also in localized stage, ABVD treated Hodgkin lymphoma patients. Haematologica 2009;94(Suppl. 2):34. [Google Scholar]; Rigacci L, Puccini B, Zinzani PL, Kovalchuk S, Broccoli A, Evangelista A, et al. Clinical characteristics of patients with negative interim‐PET and positive final PET: Data from the prospective PET‐oriented HD0801 study by Fondazione Italiana linfomi (FIL). Hematological Oncology 2017;35(Suppl. 2):38. [Google Scholar]; Rigacci L, Zinzani PL, Puccini B, Broccoli A, Gallamini A, Merli F, et al. Early FDG‐PET scan confirms its prognostic impact also in localized stage, ABVD treated Hodgkin lymphoma patients. Haematologica 2010;95(Suppl. 2):474. [Google Scholar]; Rigacci L, Zinzani PL, Puccini B, Broccoli A, Gallamini A, Merli F, et al. Early FDG‐PET scan confirms its prognostic impact also in localized stage, ABVD treated Hodgkin lymphoma patients. Haematologica. 2010; Vol. 96, issue Suppl. 4:S13. [Google Scholar]; Rigacci L, Zinzani PL, Puccini B, Broccoli A, Gallamini A, Merli F, et al. Early positive FDG‐PET scan do not confirm its prognostic impact in bulky disease Hodgkin lymphoma patients. Haematologica 2011;96(Suppl. 2):320‐1. [Google Scholar]; Rigacci L, Zinzani PL, Puccini B, Broccoli A, Gallamini A, Merli F, et al. IHP interpretation criteria of interim‐PET scan confirms prognostic impact in early stage Hodgkin lymphoma patients without bulky disease. Blood 2010;116(21):3890. [Google Scholar]; Stefoni V, Broccoli A, Alinari L, Ambrosini V, Derenzini E, Fanti S, et al. Predictive role of early interim FDG‐PET in Hodgkin lymphoma. Blood 2009;114(22):1659. [Google Scholar]; Stefoni V, Pellegrini C, Rigacci L, Broccoli A, Puccini B, Fanti S, et al. Interim PET 2: Is it a real prognostic factor in selecting two different subsets of Hodgkin disease patients?. Haematologica 2011;96(Suppl. 3):123. [Google Scholar]; Zinzani PL, Rigacci L, Stefoni V, Broccoli A, Puccini B, Castagnoli A, et al. Early interim 18F‐FDG PET in Hodgkin's lymphoma: evaluation on 304 patients. European Journal of Nuclear Medicine and Molecular Imaging 2012;39:4‐12. [DOI] [PubMed] [Google Scholar]; Zinzani PL, Tani M, Fanti S, Alinari L, Musuraca G, Marchi E, et al. Early positron emission tomography (PET) restaging: a predictive final response in Hodgkin's disease patients. Annals of Oncology 2006;17:1296‐300. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Adams HJ, Kwee TC. RAPID trial demonstrates low positive predictive value of interim FDG‐PET in early‐stage Hodgkin lymphoma after three cycles of ABVD. Journal of Pediatric Hematology/Oncology 2016;38(2):165. [DOI] [PubMed] [Google Scholar]
- Adams HJ, Kwee TC. Predictive value of interim [18F]fluorodeoxyglucose‐positron emission tomography in advanced‐stage Hodgkin lymphoma is not well established. Journal of Clinical Oncology2017; Vol. 35, issue 3:370‐1. [DOI] [PubMed]
- Adams HJ, Kwee TC. Interim FDG‐PET has no value in selecting patients who require treatment modification in both early‐ and advanced‐stage Hodgkin lymphoma. British Journal of Haematology2018; Vol. 183, issue 1:129‐31. [DOI] [PubMed]
- Adams HJ, Kwee TC. No evidence to promote interim FDG‐PET adapted therapy in the NCCN guidelines for Hodgkin lymphoma. Journal of the National Comprehensive Cancer Network2018; Vol. 16, issue 3:226‐7. [DOI] [PubMed]
- Adams HJ, Kwee TC. Strikingly heterogeneous results among studies on interim fluorodeoxyglucose‐positron emission tomography‐adapted treatment in advanced‐stage Hodgkin lymphoma. Journal of Clinical Oncology2018; Vol. 36, issue 20:2123‐4. [DOI] [PubMed]
- Adams HJ, Kwee TC. Post‐ABVD biopsy results, and not post‐ABVD FDG‐PET results, predict outcome in early‐stage Hodgkin lymphoma. British Journal of Haematology2019; Vol. 184, issue 2:290‐2. [DOI] [PubMed]
- Advani R, Maeda L, Lavori P, Quon A, Hoppe R, Breslin S, et al. Impact of positive positron emission tomography on prediction of freedom from progression after Stanford V chemotherapy in Hodgkin's disease. Journal of Clinical Oncology 2007;25:3902‐7. [DOI] [PubMed] [Google Scholar]
- Afanasyev BV, Moiseev IS, Alekseev SM, Mikhailova NB, Kondakova EV, Ilyin NV, et al. Multicenter prospective escalation‐deescalation PET‐guided clinical study in classical type Hodgkin disease in the north‐west of Russian federation (RNWOHG‐HD1): Rationale and design. Cellular Therapy and Transplantation2017; Vol. 6, issue 4:76‐81.
- Albano D, Patti C, Lagalla R, Midiri M, Galia M. Whole‐body MRI, FDG‐PET/CT, and bone marrow biopsy, for the assessment of bone marrow involvement in patients with newly diagnosed lymphoma. Journal of Magnetic Resonance Imaging 2017;45(4):1082‐9. [DOI] [PubMed] [Google Scholar]
- Albano D, Patti C, Matranga D, Lagalla R, Midiri M, Galia M. Whole‐body diffusion‐weighted MR and FDG‐PET/CT in Hodgkin Lymphoma: Predictive role before treatment and early assessment after two courses of ABVD. European Journal of Radiology 2018;103:90‐8. [DOI] [PubMed] [Google Scholar]
- Altamirano J, Esparza JR, Garza Salazar J, Calvo PS, Vera SR, Chalapud Revelo JR, et al. Staging, response to therapy, and restaging of lymphomas with18F‐FDG PET. Archives of Medical Research 2008;39(1):69‐77. [DOI] [PubMed] [Google Scholar]
- Ansell SM. Hodgkin lymphoma: 2016 update on diagnosis, risk‐stratification, and management. American Journal of Hematology 2016;91(4):434‐42. [DOI] [PubMed] [Google Scholar]
- Awan UE, Siddiqui N, SaadUllah M, Bashir H, Farooqui ZS, Muzaffar N, et al. FDG‐PET scan in assessing lymphomas and the application of Deauville Criteria. Journal of the Pakistan Medical Association 2013;63(6):725‐30. [PubMed] [Google Scholar]
- Barrington SF, O'Doherty MJ, Pike L, Franceschetto A, Cucca M, Brun E, et al. Standardised PET‐CT reporting for an international multicentre trial in lymphoma (RATHL). European Journal of Nuclear Medicine and Molecular Imaging 2011;38 (SUPPL. 2):S115. [Google Scholar]
- Barrington SF, Johnson PW. <18F‐FDG PET/CT in lymphoma: Has imaging‐directed personalized medicine become a reality?. Journal of Nuclear Medicine 2017;58(10):1539‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐Shalom R, Yefremov N, Haim N, Dann EJ, Epelbaum R, Keidar Z, et al. Camera‐based FDG pet and 67Ga SPECT in evaluation of lymphoma: Comparative study. Radiology 2003;227(2):353‐60. [DOI] [PubMed] [Google Scholar]
- Basu S. Early FDG‐PET response‐adapted risk stratification and further therapeutic decision‐making in lymphoma: Will this replace the established prognostic indices and be the standard‐of‐care in clinical management?. European Journal of Nuclear Medicine and Molecular Imaging 2009;36(12):2089‐90. [DOI] [PubMed] [Google Scholar]
- Becherer A, Mitterbauer M, Jaeger U, Kalhs P, Greinix HT, Karanikas G, et al. Positron emission tomography with 2‐fluoro‐D‐2‐deoxyglucose (FDG‐PET) predicts relapse of malignant lymphoma after high‐dose therapy with stem cell transplantation. Leukemia 2002;16(2):260‐7. [DOI] [PubMed] [Google Scholar]
- Bednaruk‐Mlynski E, Pienkowska J, Skorzak A, Malkowski B, Kulikowski W, Subocz E, et al. Comparison of positron emission tomography/computed tomography with classical contrast‐enhanced computed tomography in the initial staging of Hodgkin lymphoma. Leukemia and Lymphoma 2015;56(2):377‐82. [DOI] [PubMed] [Google Scholar]
- Biggi A, Kostakoglu L, Barrington S, Chauvie S, Gregianin M, Meignan M, et al. How the threshold of positive results influence progression free survival (PFS) in advanced Hodgkin's lymphoma treated with ABVD: Experience from the International Validation Study. European Journal of Nuclear Medicine and Molecular Imaging 2012;39(2):S222. [Google Scholar]
- Biggi A, Bergesio F, Menga M, Bianchi A, Fallanca F, Chauvie S, et al. Diagnostic accuracy of FDG PET/CT at the of treatment of Hodgkin lymphoma in the HD0607 trial. Clinical and Translational Imaging 2017;5:S44. [Google Scholar]
- Bishop G. PET‐directed therapy for Hodgkin's lymphoma. New England Journal of Medicine 2015;373(4):392. [DOI] [PubMed] [Google Scholar]
- Bjurberg M, Gustavsson A, Ohlsson T, Brun E. FDG‐PET in the detection of residual disease and relapse in patients with Hodgkin's lymphoma. Experience from a Swedish centre. Acta Oncologica 2006;45(6):743‐9. [DOI] [PubMed] [Google Scholar]
- Blum R, Prince HM, Hicks RJ, Patrikeos A, Seymour J. Discordant response to chemotherapy detected by PET scanning: Unveiling of a second primary cancer. American Journal of Clinical Oncology: Cancer Clinical Trials 2002;25(4):368‐70. [DOI] [PubMed] [Google Scholar]
- Bodet‐Milin C, Kraeber‐Bodere F, Moreau P, Campion L, Dupas B, Gouill S. Investigation of FDG‐PET/CT imaging to guide biopsies in the detection of histological transformation of indolent lymphoma. Haematologica 2008;93(3):471‐2. [DOI] [PubMed] [Google Scholar]
- Bodet‐Milin C, Salaun PY, Crespin C, Vuillez JP, Kraeber‐Bodere F. FDG‐PET scanning in managing patients with lymphoma. Medecine Nucleaire 2009;33(8):486‐90. [Google Scholar]
- Boisson N, Cachin F, Kelly A, Freitas D, Isnardi V, Mestas D, et al. PET‐CT in the Hodgkin's disease. Medecine Nucleaire 2007;31(10):562‐7. [Google Scholar]
- Borchmann S, Tresckow B, Engert A. Current developments in the treatment of early‐stage classical Hodgkin lymphoma. Current Opinion in Oncology 2016;28(5):377‐83. [DOI] [PubMed] [Google Scholar]
- Bucerius J, Herkel C, Joe AY, Altehoefer C, Finke J, Moser E, et al. <sup>18</sup>F‐FDG PET and conventional imaging for assessment of Hodgkin's disease and non Hodgkin's lymphoma: An analysis of 193 patient studies. Nuklearmedizin 2006;45(3):105‐10. [PubMed] [Google Scholar]
- Carras S, Dubois B, Senecal D, Jais JP, Peoc'h M, Quittet P, et al. Interim PET response‐adapted strategy in untreated advanced stage Hodgkin lymphoma: Results of GOELAMS LH 2007 phase 2 multicentric trial. Clinical Lymphoma, Myeloma & Leukemia 2018;18(3):191‐8. [DOI] [PubMed] [Google Scholar]
- Ciammella P, Filippi AR, Simontacchi G, Buglione M, Botto B, Mangoni M, et al. Post‐ABVD/pre‐radiotherapy 18F‐FDG‐PET provides additional prognostic information for early‐stage Hodgkin lymphoma: A retrospective analysis on 165 patients. British Journal of Radiology 2016;89(1061):20150983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremerius U, Fabry U, Kroll U, Zimny M, Neuerburg J, Osieka R, et al. [Clinical value of FDG PET for therapy monitoring of malignant lymphoma‐‐results of a retrospective study in 72 patients]. Clinic for Nuclear Medicine (Stuttgart) 1999;38(1):24‐30. [PubMed] [Google Scholar]
- Cremerius U, Fabry U, Neuerburg J, Zimny M, Bares R, Osieka R, et al. Prognostic significance of positron emission tomography using fluorine‐18‐fluorodeoxyglucose in patients treated for malignant lymphoma. Clinic for Nuclear Medicine (Stuttgart) 2001;40(1):23‐30. [PubMed] [Google Scholar]
- Cuccaro A, Annunziata S, Cupelli E, Martini M, Calcagni ML, Rufini V, et al. CD68+ cell count, early evaluation with PET and plasma TARC levels predict response in Hodgkin lymphoma. Cancer Medicine 2016;5(3):398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Urso D, Stefano A, Romano A, Russo G, Cosentino S, Fallanca F, et al. Analysis of metabolic parameters coming from basal and interim PET in Hodgkin lymphoma. Current Medical Imaging Reviews 2018;14(4):533‐44. [Google Scholar]
- Damlaj M, Al‐Zahrani M, Syed G, Gmati G, Pasha T, Abuelgasim K, et al. Escalation from ABVD following positive interim functional imaging improves progression free survival but not overall survival in advanced classical Hodgkin lymphoma‐a real world analysis. Blood 2017;130(Suppl. 1):2799. 29089309 [Google Scholar]
- Damlaj M, Al‐Zahrani M, Syed G, Gmati G, Alahmari B, Pasha T, et al. Interim functional imaging is an independent predictor of progression‐free survival in advanced classical Hodgkin lymphoma ‐ A real‐world analysis. Clinical Lymphoma, Myeloma and Leukemia 2019;19(1):e71‐9. [DOI] [PubMed] [Google Scholar]
- Danilov AV, Li H, Press OW, Shapira I, Swinnen LJ, Noy A, et al. Feasibility of interim positron emission tomography (PET)‐adapted therapy in HIV‐positive patients with advanced Hodgkin lymphoma (HL): a sub‐analysis of SWOG S0816 Phase 2 trial. Leukemia & Lymphoma 2017;58(2):461‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann EJ, Bar‐Shalom R, Tamir A, Ben‐Shachar M, Avivi I, Zuckerman T, et al. For standard and high‐risk patients with Hodgkin lymphoma six cycles of tailored BEACOPP, based on interim scintigraphy, are effective and female fertility is preserved. Blood 2009;114(22):1552. [Google Scholar]
- Dann EJ, Bar‐Shalom R, Tamir A, Epelbaum R, Avivi I, Ben‐Shachar M, et al. A functional dynamic scoring model to elucidate the significance of post‐induction interim fluorine‐18‐fluorodeoxyglucose positron emission tomography findings in patients with Hodgkin's lymphoma. Haematologica 2010;95(7):1198‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann EJ, Bairey O, Bar‐Shalom R, Izak M, Korenberg A, Akria L, et al. Tailored therapy in Hodgkin lymphoma, based on predefined risk factors and early interim PET/CT, can lead to modification and safe reduction in therapy: Results of 134 patients on the Israel National Hodgkin Study. Blood 2010;116(21):2809. [Google Scholar]
- Dann EJ, Bairey O, Bar‐Shalom R, Mashiach T, Izak M, Korenberg A, et al. Early Hodgkin lymphoma therapy, based on predefined risk factors and early interim PET/CT. Israeli H2 protocol: Preliminary report. Haematologica 2012;97(Suppl. 1):85. [Google Scholar]
- Dann EJ, Bairey O, Bar‐Shalom R, Izak M, Korenberg A, Akria L, et al. Tailored therapy in Hodgkin lymphoma, based on predefined risk factors and early interim PET/CT, Israeli H2 protocol: Preliminary report on 317 patients. Haematologica 2013;98(Suppl. 2):37. [Google Scholar]
- Dann EJ, Bairey O, Bar‐Shalom R, Mashiach T, Barzilai E, Korenberg A, et al. Adjustment of therapy for Hodgkin lymphoma based on interim PET is beneficial and radiotherapy may be substituted with chemotherapy in patients with negative interim study: Final results of H2 trial. European Haematology Association Open Access Library. Unknown: https://library.ehaweb.org/eha/2016/21st/135140/eldad.dann.adjustment.of.therapy.for.hodgkin.lymphoma.based.on.interim.pet.is.html, 2016, issue S107:4‐5.
- Dann EJ, Bairey O, Bar‐Shalom R, Mashiach T, Barzilai E, Kornberg A, et al. Modification of initial therapy in early and advanced Hodgkin lymphoma, based on interim PET/CT is beneficial: a prospective multicentre trial of 355 patients. British Journal of Haematology 2017;178:709‐718. [DOI] [PubMed] [Google Scholar]
- Dann EJ, Paltiel O. Interim FDG‐PET has no value in selecting patients who require treatment modification in both early‐ and advanced‐stage Hodgkin lymphoma: Response to Adams and Kwee. British Journal of Haematology 2018;183(1):131‐3. [DOI] [PubMed] [Google Scholar]
- deAndres‐Galiana EJ, Fernandez‐Martinez JL, Luaces O, Coz JJ, Fernandez R, Solano J, et al. On the prediction of Hodgkin lymphoma treatment response. Clinical and Translational Oncology 2015;17(8):612‐9. [DOI] [PubMed] [Google Scholar]
- Diehl V, Kobe C, Haverkamp H, Dietlein M, Engert A. FDG‐PET for assessment of residual tissue after completion of chemotherapy in Hodgkin lymphoma report on the 2nd interim analysis of the PET investigation in the trial HD15 of the GHSG. Blood. The American Society of Hematology. 2007, issue 11. [Google Scholar]
- El‐Galaly TC, Mylam KJ, Brown P, Specht L, Christiansen I, Munksgaard L, et al. Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica 2012;97(6):931‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evens AM, Kostakoglu L. The role of FDG‐PET in defining prognosis of Hodgkin lymphoma for early‐stage disease. Blood 2014;124(23):3356‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti S, Castellucci P, Stefoni V, Nanni C, Tani M, Rubello D, et al. Early relapse in a patient with Hodgkin's disease and negative interim FDG‐PET. Annals of Nuclear Medicine 2008;22(5):429‐32. [DOI] [PubMed] [Google Scholar]
- Filmont J E, Czernin J, Yap C, Silverman DH, Quon A, Phelps ME, et al. Value of F‐18 fluorodeoxyglucose positron emission tomography for predicting the clinical outcome of patients with aggressive lymphoma prior to and after autologous stem‐cell transplantation. Chest 2003;124(2):608‐13. [DOI] [PubMed] [Google Scholar]
- Fornecker LM, Lazarovici J, Aurer I, Casasnovas RO, Gac AC, Bonnet C, et al. Pet‐based response after 2 cycles of brentuximab vedotin in combination with avd for first‐line treatment of unfavorable early‐stage Hodgkin Lymphoma: first analysis of the primary endpoint of breach, a randomized phase II trial of LYSA‐FIL‐EORTC intergroup. Blood 2017;130(Suppl. 1):736. [Google Scholar]
- Freudenberg LS, Antoch G, Schutt P, Beyer T, Jentzen W, Muller SP, et al. FDG‐PET/CT in re‐staging of patients with lymphoma. European Journal of Nuclear Medicine and Molecular Imaging 2004;31(3):325‐9. [DOI] [PubMed] [Google Scholar]
- Friedberg JW, Fischman A, Neuberg D, Kim H, Takvorian T, Mauch PM, et al. FDG‐PET is superior to gallium scintigraphy in the staging and follow‐up of patients with de novo Hodgkin's disease: a prospective, blinded comparison. Leukemia & Lymphoma2004; Vol. 45, issue 1:85‐92. [DOI] [PubMed]
- Friedberg JW, Fischman A, Neuberg D, Kim H, Takvorian T, Ng AK, et al. FDG‐PET is superior to gallium scintigraphy in staging and more sensitive in the follow‐up of patients with de novo Hodgkin lymphoma: a blinded comparison. Leukemia and Lymphoma 2004;45(1):85‐92. [DOI] [PubMed] [Google Scholar]
- Front D, Bar‐Shalom R, Mor M, Haim N, Epelbaum R, Frenkel A, et al. Hodgkin disease: prediction of outcome with 67Ga scintigraphy after one cycle of chemotherapy. Radiology 1999;210(2):487‐91. [DOI] [PubMed] [Google Scholar]
- Fruchart C, Reman O, Stang N, Musafiri D, Cheze S, Macro M, et al. Prognostic value of early 18 fluorodeoxyglucose positron emission tomography and gallium‐67 scintigraphy in aggressive lymphoma: A prospective comparative study. Leukemia and Lymphoma 2006;47(12):2547‐57. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Hutchings M, Avigdor A, Polliack A. Early interim PET scan in Hodgkin lymphoma: Where do we stand?. Leukemia and Lymphoma 2008;49(4):659‐62. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Tarella C, Viviani S, Rossi A, Patti C, Mulé A, et al. Early chemotherapy intensification with escalated BEACOPP in advanced‐stage Hodgkin lymphoma with a positive interim PET‐CT after 2 ABVD cycles: Long‐term results of the GITIL/FIL HD 0607 trial. Journal of Clinical Oncology 2018;36(5):454‐462. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Tarella C, Viviani S, Rossi A, Patti C, Mule A, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced‐stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: Long‐term results of the GITIL/FIL HD 0607 trial. Journal of Clinical Oncology 2018;36(5):454‐62. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Viviani S, Pavoni C, Rambaldi A. Reply to H.J.A. Adams et al and C. Mesguich et al. Journal of Clinical Oncology 2018;36(20):2127‐8. [DOI] [PubMed] [Google Scholar]
- Gallowitsch HJ, Igerc I, Kohlfurst S, Lind P. The incremental value of F‐18 FDG PET and PET/CT in malignant lymphoma. Imaging Decisions MRI 2008;12(4):2‐6. [Google Scholar]
- Goldschmidt N, Or O, Klein M, Savitsky B, Paltiel O. The role of routine imaging procedures in the detection of relapse of patients with Hodgkin lymphoma and aggressive non‐Hodgkin lymphoma. Annals of Hematology 2011;90(2):165‐71. [DOI] [PubMed] [Google Scholar]
- Greil R. Treatment optimisation trial in the first‐line treatment of advanced stage Hodgkin lymphoma; comparison of 4‐6 cycles of escalated BEACOPP with 4‐6 cycles of BrECADD. Memo ‐ Magazine of European Medical Oncology. 2018;11(1):1‐28. [Google Scholar]
- Guidez S, Delwail V, Brice P. PET‐scan in management of Hodgkin's lymphoma. Hematologie 2016;22(6):389‐91. [Google Scholar]
- Hagtvedt T, Seierstad T, Lund KV, Løndalen AM, Bogsrud TV, Smith HJ, et al. Diffusion‐weighted MRI compared to FDG PET/CT for assessment of early treatment response in lymphoma. Acta Radiologica 2015;56(2):152‐8. [DOI] [PubMed] [Google Scholar]
- Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro‐2‐deoxy‐D‐glucose positron emission tomography (FDG‐PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood 2005;106(4):1376‐81. [DOI] [PubMed] [Google Scholar]
- Hartmann S, Agostinelli C, Diener J, Doring C, Fanti S, Zinzani PL, et al. GLUT1 expression patterns in different Hodgkin lymphoma subtypes and progressively transformed germinal centers. BMC Cancer 2012;12:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartridge‐Lambert SK, Schoder H, Lim RC, Maragulia JC, Portlock CS. ABVD alone and a PET scan complete remission negates the need for radiologic surveillance in early‐stage, nonbulky Hodgkin lymphoma. Cancer 2013;119(6):1203‐9. [DOI] [PubMed] [Google Scholar]
- Honda A, Nakamura F, Nannya Y, Shintani Y, Fukayama M, Ichikawa M, et al. Pulmonary lymphocyte‐rich classical Hodgkin lymphoma with early response to ABVD therapy. Annals of Hematology 2014;93(6):1073‐4. [DOI] [PubMed] [Google Scholar]
- Hueltenschmidt B, Sautter‐Bihl ML, Lang O, Maul FD, Fischer J, Mergenthaler HG, et al. Whole body positron emission tomography in the treatment of Hodgkin disease. Cancer 2001;91(2):302‐10. [PubMed] [Google Scholar]
- Huic D, Mutvar A, Radman I, Grosev D, Labar B, Zuvic M, et al. The value of F‐18 FDG triple‐head coincidence PET in the posttreatment evaluation of patients with lymphoma. Clinical Nuclear Medicine 2006;31(5):275‐8. [DOI] [PubMed] [Google Scholar]
- Hutchings M, Loft A, Hansen M, Berthelsen AK, Specht L. Clinical impact of FDG‐PET/CT in the planning of radiotherapy for early‐stage Hodgkin lymphoma. European Journal of Haematology 2007;78(3):206‐12. [DOI] [PubMed] [Google Scholar]
- Iagaru A, Wang Y, Mari C, Quon A, Goris ML, Horning S, et al. (18)F‐FDG‐PET/CT evaluation of response to treatment in lymphoma: when is the optimal time for the first re‐evaluation scan?. Hellenic Society of Nuclear Medicine 2008;11(3):153‐6. [PubMed] [Google Scholar]
- Illidge T. Personalised approach to treating early Hodgkin's lymphoma. BMJ 2015;350:h2927. [DOI] [PubMed] [Google Scholar]
- Jerusalem G, Beguin Y, Fassotte MF, Belhocine T, Hustinx R, Rigo P, et al. Early detection of relapse by whole‐body positron emission tomography in the follow‐up of patients with Hodgkin's disease. Annals of Oncology 2003;14(1):123‐30. [DOI] [PubMed] [Google Scholar]
- Johnson PW, Federico M, Fossa A, O'Doherty M, Roberts T, Stevens L, et al. Response‐adapted therapy based on interim FDG‐PET scans in advanced Hodgkin lymphoma: first analysis of the safety of deescalation and efficacy of escalation in the international RATHL study (CRUK/07/033). Clinical Advances in Hematology and Oncology 2015;13(8 Suppl. 9):6‐7. 26430869 [Google Scholar]
- Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A, et al. Adapted treatment guided by interim PET‐CT scan in advanced Hodgkin's lymphoma. New England Journal of Medicine 2016;374(25):2419‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran SC, Jacene HA, Chen YH, Mauch PM, Ng AK. Clinical outcome of patients with early stage favorable Hodgkin lymphoma treated with ABVDX2 cycles followed by PET/CT restaging and 20 Gy of involved‐site radiotherapy. Haematologica 2016;101(Suppl. 5):14. [DOI] [PubMed] [Google Scholar]
- Kamran SC, Jacene HA, Chen YH, Mauch PM, Ng AK. Clinical outcome of patients with early stage favorable Hodgkin lymphoma treated with ABVD x two cycles followed by FDG‐PET/CT restaging and 20 Gy of involved‐site radiotherapy. Leukemia and Lymphoma 2018;59(6):1384‐90. [DOI] [PubMed] [Google Scholar]
- Kobe C, Dietlein M, Franklin J, Markova J, Lohri A, Amthauer H, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first‐line chemotherapy in advanced‐stage Hodgkin lymphoma. Blood 2008;112(10):3989‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe C, Kuhnert G, Kahraman D, Haverkamp H, Eich HT, Franke M, et al. Assessment of tumor size reduction improves outcome prediction of positron emission tomography/computed tomography after chemotherapy in advanced‐stage Hodgkin lymphoma. Journal of Clinical Oncology 2014;32(17):1776‐81. [DOI] [PubMed] [Google Scholar]
- Kostakoglu L, Goldsmith SJ, Leonard JP, Christos P, Furman RR, Atasever T, et al. FDG‐PET after 1 cycle of therapy predicts outcome in diffuse large cell lymphoma and classic Hodgkin disease. Cancer 2006;107:2678‐87. [DOI] [PubMed] [Google Scholar]
- Li YJ, Li ZM, Xia XY, Huang HQ, Xia ZJ, Lin TY, et al. Prognostic value of interim and posttherapy 18F‐FDG PET/CT in patients with mature T‐cell and natural killer cell lymphomas. Journal of Nuclear Medicine 2013;54(4):507‐15. [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Wiseman GA. Assessment of Lymphoma Therapy Using (18)F‐FDG PET. Journal of Nuclear Medicine 2002;43(8):1028‐30. [PubMed] [Google Scholar]
- Milgrom SA, Pinnix CC, Chuang H, Oki Y, Akhtari M, Mawlawi O, et al. Early‐stage Hodgkin lymphoma outcomes after combined modality therapy according to the post‐chemotherapy 5‐point score: can residual pet‐positive disease be cured with radiotherapy alone?. British Journal of Haematology 2017;179(3):488‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocikova H, Obrtlikova P, Vackova B, Trneny M. Positron emission tomography at the end of first‐line therapy and during follow‐up in patients with Hodgkin lymphoma: a retrospective study. Annals of Oncology 2010;21(6):1222‐7. [DOI] [PubMed] [Google Scholar]
- Mocikova H, Pytlik R, Markova J, Steinerova K, Kral Z, Belada D, et al. Pre‐transplant positron emission tomography in patients with relapsed Hodgkin lymphoma. Leukemia & Lymphoma 2011;52(9):1668‐74. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Simon Z, Borbenyi Z, Deak B, Galuska L, Keresztes K, et al. Prognostic value of FDG‐PET in Hodgkin lymphoma for posttreatment evaluation. Long term follow‐up results. Neoplasma 2010;57(4):349‐54. [PubMed] [Google Scholar]
- Moskowitz CH. Early FDG‐PET adapted treatment improves the outcome of early FDG‐PET‐positive patients with stages I/II hodgkin lymphoma (HL): Final results of the randomized intergroup EORTC/LYSA/FIL H10 trial. Clinical advances in Hematology & Oncology: H&O 2015;13(8 Supplement 9):16‐7. [Google Scholar]
- Naumann R, Vaic A, Beuthien‐Baumann B, Bredow J, Kropp J, Kittner T, et al. Prognostic value of positron emission tomography in the evaluation of post‐treatment residual mass in patients with Hodgkin's disease and non‐Hodgkin's lymphoma. British Journal of Haematology 2001;115(4):793‐800. [DOI] [PubMed] [Google Scholar]
- NCT00784537. High‐dose chemotherapy and stem cell transplantation, in patients PET‐2 positive, after 2 courses of ABVD and comparison of RT versus no RT in PET‐2 negative patients. clinicaltrials.gov/show/nct00784537 (first received 4 November 2008).
- NCT00795613. Positron Emission Tomography (PET)‐adapted chemotherapy In advanced Hodgkin Lymphoma (HL). clinicaltrials.gov/show/nct00795613 (first received 21 November 2008).
- NCT01358747. Study of a treatment driven by early PET response to a treatment not monitored by early PET in patients with AA stage 3‐4 or 2B HL. clinicaltrials.gov/show/nct01358747 (first received 24 May 2011).
- NCT01652261. Very early FDG‐PET/CT‐response adapted therapy for advanced Hodgkin Lymphoma (H11). clinicaltrials.gov/show/nct01652261 (first received 30 July 2012).
- NCT02292979. Brentuximab vedotin associated with chemotherapy in untreated patients with Hodgkin Lymphoma. clinicaltrials.gov/show/nct02292979 (first received 18 November 2014).
- Nguyen VT, Pophali PA, Tsai J P, Jagadeesh D, Dean RM, Pohlman B, et al. Early stage, bulky Hodgkin lymphoma patients have a favorable outcome when treated with or without consolidative radiotherapy: potential role of PET scan in treatment planning. British Journal of Haematology 2017;179(4):674‐6. [DOI] [PubMed] [Google Scholar]
- Panizo C, Perez‐Salazar M, Bendandi M, Rodriguez‐Calvillo M, Boan JF, Garcia‐Velloso MJ, et al. Positron emission tomography using 18F‐fluorodeoxyglucose for the evaluation of residual Hodgkin's disease mediastinal masses. Leukemia and Lymphoma 2004;45(9):1829‐33. [DOI] [PubMed] [Google Scholar]
- Paolini R, Rampin L, Rodella E, Ramazzina E, Banti E, Al‐Nahhas A, et al. The prognostic value of 18F‐FDG PET‐CT in the management of Hodgkin's lymphoma: Preliminary results of a prospective study. Nuclear Medicine Review 2007;10(2):87‐90. [PubMed] [Google Scholar]
- Pavlovsky A, Fernandez I, Kurgansky N, Prates V, Zoppegno L, Negri P, et al. PET‐adapted therapy after three cycles of ABVD for all stages of Hodgkin lymphoma: results of the GATLA LH‐05 trial. British Journal of Haematology 2019;185(5):865‐73. [DOI] [PubMed] [Google Scholar]
- Pichler R, Maschek W, Hatzl‐Griesenhofer M, Huber H, Wimmer G, Wahl G, et al. Clinical value of FDG hybrid‐PET in staging and restaging of malignant lymphoma ‐ compared with conventional diagnostic methods. NuklearMedizin 2000;39(6):166‐73. [PubMed] [Google Scholar]
- Reinhardt MJ, Herkel C, Altehoefer C, Finke J, Moser E. Computed tomography and 18F‐FDG positron emission tomography for therapy control of Hodgkin's and non‐Hodgkin's lymphoma patients: when do we really need FDG‐PET?. Annals of Oncology 2005;16(9):1524‐9. [DOI] [PubMed] [Google Scholar]
- Rigacci L, Castagnoli A, Carpaneto A, Carrai V, Vaggelli L, Matteini M. Can (18)F‐FDG PET after first cycle chemotherapy predict the efficacy of therapy in Hodgkin's disease?. Haematologica 2002;87(5):ELT24. [PubMed] [Google Scholar]
- Rigacci L, Puccini B, Zinzani P, Kovalchuk S, Broccoli A, Evangelista A, et al. Clinical characteristics of patients with negative interim‐pet and positive final PET: data from the prospective PET‐oriented HD0801 study by Fondazione Italiana linfomi (FIL). Hematological Oncology 2017;35(Suppl. 2):38. [Google Scholar]
- Rubello D, Gordien P, Morliere C, Guyot M, Bordenave L, Colletti PM, et al. Variability of hepatic 18F‐FDG uptake at interim PET in patients with Hodgkin lymphoma. Clinical Nuclear Medicine 2015;40:e405‐10. [DOI] [PubMed] [Google Scholar]
- Sakr R, Massoud M, Kerbage F, Rached L, Zeghondy J, Akoury E, et al. Real‐life Experience for Integration of PET‐CT in the Treatment of Hodgkin Lymphoma in Lebanon. Clinical Lymphoma, Myeloma & Leukemia 2017;17S:S92‐5. [DOI] [PubMed] [Google Scholar]
- Schot BW, Zijlstra JM, Sluiter WJ, Imhoff GW, Pruim J, Vaalburg W, et al. Early FDG‐PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood 2007;109(2):486‐91. [DOI] [PubMed] [Google Scholar]
- Simontacchi G, Filippi AR, Ciammella P, Buglione M, Saieva C, Magrini SM, et al. Interim PET after two ABVD cycles in early‐stage Hodgkin lymphoma: Outcomes following the continuation of chemotherapy plus radiotherapy. International Journal of Radiation Oncology Biology Physics 2015;92:1077‐83. [DOI] [PubMed] [Google Scholar]
- Slaby J, Belohlavek O, Taborska K, Prochazka M, Trneny M, Klener P. [Predictive features of positron emission tomography after two cycles of induction therapy in malignant lymphoma]. Casopis Lékaru Ceských [Journal of Czech Physicians] 2002;141(10):312‐5. [PubMed] [Google Scholar]
- Spaepen K, Stroobants S, Dupont P, Thomas J, Vandenberghe P, Balzarini J, et al. Can positron emission tomography with [(18)F]‐fluorodeoxyglucose after first‐line treatment distinguish Hodgkin's disease patients who need additional therapy from others in whom additional therapy would mean avoidable toxicity?. British Journal of Haematology 2001;115:272‐8. [DOI] [PubMed] [Google Scholar]
- Specht L. FDG‐PET scan and treatment planning for early stage Hodgkin lymphoma. Radiotherapy and Oncology 2007;85(2):176‐7. [DOI] [PubMed] [Google Scholar]
- Spinner MA, Advani RH. Risk‐adapted therapy for advanced‐stage Hodgkin lymphoma. Hematology 2018;1:200‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DJ, Jung SH, Pitcher B, Kostakoglu L, Grecula JC, Hsi ED, et al. CALGB 50604: risk‐adapted treatment of nonbulky early‐stage Hodgkin lymphoma based on interim PET. Blood 2018;132(10):1013‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigari L, Attili A, Duggento A, Chiaravalloti A, Schillaci O, Guerrisi MG. Quantitative analysis of basal and interim PET/CT images for predicting tumor recurrence in patients with Hodgkin's lymphoma. Nuclear Medicine Communications 2016;37:16‐22. [DOI] [PubMed] [Google Scholar]
- Sucak GT, Ozkurt ZN, Suyani E, Yasar DG, Akdemir OU, Aki Z, et al. Early post‐transplantation positron emission tomography in patients with Hodgkin lymphoma is an independent prognostic factor with an impact on overall survival. Annals of Hematology 2011;90(11):1329‐36. [DOI] [PubMed] [Google Scholar]
- Tirelli U, Spina M. PET‐adapted salvage therapy in Hodgkin's lymphoma. Lancet Oncology 2015;16(3):239‐40. [DOI] [PubMed] [Google Scholar]
- Tomita N, Hattori Y, Fujisawa S, Hashimoto C, Taguchi J, Takasaki H, et al. Post‐therapy 18F‐fluorodeoxyglucose positron emission tomography for predicting outcome in patients with peripheral T cell lymphoma. Annals of Hematology 2015;94(3):431‐6. [DOI] [PubMed] [Google Scholar]
- Torizuka T, Nakamura F, Kanno T, Futatsubashi M, Yoshikawa E, Okada H, et al. Early therapy monitoring with FDG‐PET in aggressive non‐Hodgkin's lymphoma and Hodgkin's lymphoma. European Journal of Nuclear Medicine and Molecular Imaging 2004;31(1):22‐8. [DOI] [PubMed] [Google Scholar]
- Trotman J, Fossa A, Federico M, Stevens L, Kirkwood A, Clifton‐Hadley L, et al. Response‐adjusted therapy for advanced Hodgkin lymphoma (RATHL) trial: longer follow up confirms efficacy of de‐escalation after a negative interim PET scan (CRUK/07/033). Hematological Oncology 2017;35:65‐7. [Google Scholar]
- Tseng D, Rachakonda LP, Su Z, Advani R, Horning S, Hoppe RT, et al. Interim‐treatment quantitative PET parameters predict progression and death among patients with Hodgkin's disease. Radiation Oncology 2012;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa D, Sehn LH, Aquino‐Parsons C, Tonseth P, Scott D W, Gerrie AS, et al. Interim PET‐directed therapy in limited stage Hodgkin lymphoma initially treated with ABVD. Haematologica 2018;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann E, Baican B, Hertel A, Baum RP, Chow KU, Knupp B, et al. Positron emission tomography (PET) for staging and evaluation of response to treatment in patients with Hodgkin's disease. Leukemia and Lymphoma 1999;34(5‐6):545‐51. [DOI] [PubMed] [Google Scholar]
- Wilson D, Benard F, Gascoyne RD, Slack GW, Farinha P, Morris J, et al. Interim PET‐directed therapy in limited‐stage Hodgkin lymphoma initially treated with ABVD. Haematologica 2018;103(12):e590‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Jiang X F, Zhao W L, Wang L. Prognostic evaluation of different PET/CT reading methods in Hodgkin lymphoma and diffused large B‐cell lymphoma. Journal of Shanghai Jiaotong University 2018;38(8):954‐9. [Google Scholar]
- Yasgur BS. Interim PET results guide ongoing therapy in Hodgkin lymphoma. Oncology Report 2015;11(8):available at: https://www.mdedge.com/hematologynews/nhlhub/article/101136/indolent‐lymphoma/interim‐pet‐results‐guide‐ongoing‐therapy. [Google Scholar]
- Yoshimi A, Izutsu K, Takahashi M, Kako S, Oshima K, Kanda Y, et al. Conventional allogeneic hematopoietic stem cell transplantation for lymphoma may overcome the poor prognosis associated with a positive FDG‐PET scan before transplantation. American Journal of Hematology 2008;83(6):477‐81. [DOI] [PubMed] [Google Scholar]
- Zabrocka E, Sierko E, Wojtukiewicz MZ. Positron emission tomography scanning in the management of Hodgkin lymphoma patients: a single‐institution experience. Advances in Clinical and Experimental Medicine 2016;25(6):1185‐92. [DOI] [PubMed] [Google Scholar]
- Zaucha J, Danielewicz I, Malkowski B, Zaucha R, Lesniewski‐Kmak K. The role of PET for interim response assessment in patients with Hodgkin's lymphoma. Wspolczesna Onkologia 2009;13(4):161‐6. [Google Scholar]
- Zinzani PL, Magagnoli M, Chierichetti F, Zompatori M, Garraffa G, Bendandi M, et al. The role of positron emission tomography (PET) in the management of lymphoma patients. Annals of Oncology 1999;10(10):1181‐4. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Chierichetti F, Zompatori M, Tani M, Stefoni V, Garraffa G, et al. Advantages of positron emission tomography (PET) with respect to computed tomography in the follow‐up of lymphoma patients with abdominal presentation. Leukemia and Lymphoma 2002;43(6):1239‐43. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Broccoli A, Gioia DM, Castagnoli A, Ciccone G, Evangelista A, et al. Interim positron emission tomography response‐adapted therapy in advanced‐stage hodgkin lymphoma: final results of the phase II part of the HD0801 study. Journal of Clinical Oncology2016; Vol. 34, issue 12:1376‐85. [DOI] [PubMed]
References to studies awaiting assessment
- Abramson JS, Barnes JA, LaCasce AS, Zukotynski K, Israel D, Feng Y, et al. End of treatment but not interim PET scan predicts outcome in non‐bulky limited stage Hodgkin lymphoma. Haematologica 2010;95(Suppl. 4):S16. [DOI] [PubMed] [Google Scholar]
- Algrin C, Berenger N, Chevret S, Vercellino L, Bazelaire C, Brice P, et al. Interim‐positron emission tomography with [18F]fluorodeoxyglucose (interim‐PET) evaluation in mediastinal lymphoma including Hodgkin lymphoma (HL) and primary mediastinal large B‐cell lymphoma (PMBL). Blood 2010;116(21):2860. [Google Scholar]
- Arce‐Calisaya P, Scarsbrook A, Thygesen H, Chowdhury F, Patel C. Interim FDG PET/CT in Hodgkin's lymphoma ‐ Does binary response assessment criteria have any prognostic value?. European Journal of Nuclear Medicine and Molecular Imaging 2013;40(Suppl. 2):S476. [Google Scholar]
- Baratto L, Guerra L, Elisei F, Crivellaro C, Ponti E, Bolis S, et al. Interim‐PET in Hodgkin lymphoma: Deauville criteria and metabolic parameters as prognostic factors. Clinical and Translational Imaging 2015;3(Suppl. 1):S16‐7. [Google Scholar]
- Barna S, Fedinecz N, Magyari F, Varga J, Illes A, Garai I. Prognostic value of interim 18FDG‐PET/CT in patients with Hodgkin's lymphoma using different 5‐point visual scales for interpretation. European Journal of Nuclear Medicine and Molecular Imaging 2011;38(Suppl. 2):S377. [Google Scholar]
- Barrington SF, Kostakoglu L, Hutchings M, Meignan M, Biggi A, Gregianin M, et al. Are the Deauville criteria a reliable tool for assessment of interim PET in Hodgkin lymphoma?. European Journal of Nuclear Medicine and Molecular Imaging. 2011; Vol. 38, issue Suppl. 2:S164. [Google Scholar]
- Bentur OS, Eldad DJ, Paran E, Lavie D, Nachmias B, Dally N, et al. The predictive value of interim PET‐CT in elderly patients with Hodgkin lymphoma. Haematologica. Conference: 22th Congress of the European Hematology Association. Spain. 2017; Vol. 102:460‐1. [Google Scholar]
- Berenger N, Vercellino L, Algrin C, Groheux D, Hindie E, Lussato D, et al. Prognostic value of interim 18F‐FDG PET/CT in mediastinal bulky Hodgkin lymphoma. European Journal of Nuclear Medicine and Molecular Imaging 2010;37(Suppl. 2):S436. [Google Scholar]
- Bhatwadekar S, Deshpande S, Khadse S, Shah B, Desai D, Kachchhi U, et al. Excellent outcome in Hodgkin lymphoma with ABVD and CMT: A single‐centre retrospective analysis. Hematological Oncology 2017;35(Suppl. 2):316‐7. [Google Scholar]
- Cimino G, Zaucha JM, Cirillo S, Saviolo C, Hutchings M, El‐Galaly TC, et al. The complementary prognostic role of baseline and interim PET in predicting treatment outcome in advanced‐stage Hodgkin lymphoma. Blood 2014;124(21):4405. [Google Scholar]
- Cocorocchio E, Vanazzi A, Botteri E, Alietti A, Negri M, Bassi S, et al. Prognostic role of interim 18FDG‐PET in Hodgkin lymphoma: A single‐center experience. Journal of Clinical Oncology 2009;27(15 suppl. 1):e19520. [Google Scholar]
- Cocorocchio E, Botteri E, Gigli F, Bassi S, Bertazzoni P, Sammassimo S, et al. Evaluation of interim 18FDG‐PET in advanced Hodgkin lymphoma (HL) patients (PTS) treated with ChlVPP/ABVVP regimen. Annals of Oncology. 2011; Vol. 22, issue Suppl. 4:216. [Google Scholar]
- Copeland A, Fanale M, Chuang H, Macapinlac H, Faria SC, Siegmund B, et al. Single institution experience with interim PET evaluation in newly diagnosed CHL receiving ABVD chemotherapy: Need for standardization. Haematologica 2010;95(Suppl. 4):S50. [Google Scholar]
- Cuzzocrea M, Guerra L, Elisei F, Crivellaro C, Ponti E, Bolis S, et al. The Deauville criteria and metabolic parameters as prognostic factors in interim PET in Hodgkin lymphoma: A single centre experience. European Journal of Nuclear Medicine and Molecular Imaging 2015;42(1 suppl. 1):S152‐3. [Google Scholar]
- Rueda B, Costilla L, Catalina S, Grasa J, Rubio D, Giraldo P. Prognostic value of 18F‐FDG PET/CT in Hodgkin lymphoma. Haematologica 2013;98(Suppl. 1):572‐3. [Google Scholar]
- Fabbri A, Rigacci L, Lazzi S, Lollo S, Pietrini A, Puccini B, et al. 'Early FDG‐PET' predicts clinical course of Hodgkin's lymphoma although does not correlate with macrophages infiltration in diagnostic specimens. Haematologica 2011;96(Suppl. 2):321‐2. [Google Scholar]
- Fiore F, Viviani S, Luminari S, Levis A, Raimondo F, Merli F, et al. Early interim FDG‐PET during intensified BEACOPP therapy for advanced‐stage Hodgkin disease shows a lower positive predictive value than during ABVD. Haematologica 2010;95(Suppl. 4):S19. [Google Scholar]
- Gallegos C, Rueda B, Grasa JM, Banso A, Giraldo P. The importance of PET/CT as method of evaluation of early response to treatment in HL. Haematologica 2012;97(Suppl. 1):566. [Google Scholar]
- Hohaus S, Cuccaro A, Annunziata S, Martini M, D'Alo F, Calcagni ML, et al. The risk of progression of Hodgkin lymphoma in patients with negative interim PET: A role for the number of tumor‐infiltrating macrophages (CD68+ cell counts) and B symptoms. Haematologica 2015;100(Suppl. 3):7. 25552678 [Google Scholar]
- Hutchings M, Kostakoglu L, Loft A, Coleman M, Specht L. Correlation of FDG‐PET results after one cycle and after two cycles of chemotherapy in Hodgkin lymphoma. Journal of Clinical Oncology 2010;28(15):8061. [Google Scholar]
- Knight‐Greenfield A, Marshall RA, Hutchings M, Doucette J, Stern J, Coleman M, et al. Interim FDG PET/CT to predict progression‐free survival (PFS) better than clinical and baseline metabolic measurements in Hodgkin lymphoma (cHL). Journal of Clinical Oncology 2013;31(15):8555. [Google Scholar]
- Leontjeva A, Demina E, Ryabukhina J, Tumyan G, Trofimova O, Sotnikov V, et al. Significance of early interim PET results in advanced Hodgkin lymphoma treated intensive program EACOPP‐14. British Journal of Haematology 2016;173(Suppl. 1):107. [Google Scholar]
- Luminari S, Cesaretti M, Tomasello C, Guida A, Bagni B, Merli F, et al. The use of FDG positron emission tomography (FDG‐PET) in patients with Hodgkin lymphoma (HL) in the "real world": A population based study from northern Italy. Haematologica 2010;95(Suppl. 4):S42‐3. [Google Scholar]
- Luminari S, Cesaretti M, Tomasello C, Guida A, Bagni B, Merli F, et al. Use of 2‐[18F]fluoro‐2‐deoxy‐D‐glucose positron emission tomography in patients with Hodgkin lymphoma in daily practice: a population‐based study from Northern Italy. Leukemia and Lymphoma 2011;52(9):1689‐96. [DOI] [PubMed] [Google Scholar]
- Medvedovskaya E, Leontjeva A, Demina E, Ryabukhina J, Tumyan G, Kokosadze N, et al. The impact of outcome of interim PET/CT on advanced Hodgkin lymphoma treated with EACOPP‐14. Haematologica 2016;101(Suppl. 5):26. 26546504 [Google Scholar]
- Molnar Z, Deak B, Kajary K, Lengyel Z, Molnar P, Rosta A, et al. The value of interim 18F‐FDG PET/CT in Hodgkin lymphoma. European Journal of Nuclear Medicine and Molecular Imaging 2011;38(Suppl. 2):S165. [Google Scholar]
- Molnar Z, Deak B, Kajary K, Lengyel Z, Molnar P, Rosta A, et al. Interim FDG PET/CT examinations in advanced stage Hodgkin lymphoma. Nuclear Medicine Review 2011;14(Suppl. A):A4. [Google Scholar]
- Moreira C, Fidalgo P, Queiroz L, Domingues N, Moreira I, Oliveira I, et al. Prognostic value of interim vs. end‐of‐treatment PET scan in Hodgkin's lymphoma. Hematological Oncology 2013;31(Suppl. 1):257. [Google Scholar]
- Perrone T, Gaudio F, Giordano A, Risi C, Spina A, Curci P, et al. Role of positron emission tomography (PET) after 2 and 4 courses of chemotherapy in patients with Hodgkin's lymphoma: A single center experience. Haematologica 2009;94(Suppl. 4):216. [Google Scholar]
- Pophali PA, Rybicki LA, Fenner KB, Jagadeesh D, Dean RM, Pohlman B, et al. Bulky disease does not adversely affect overall survival in early stage Hodgkin lymphoma: Role of interim PET and possible omission of radiotherapy in select patients. Blood 2014;124:21. [Google Scholar]
- Rusconi C, Ravelli E, Gabutti C, Zilioli V, Meli E, Nichelatti M, et al. Baseline and dynamic prognostic factors in newly diagnosed classical Hodgkin's lymphoma. Haematologica 2010;95(Suppl. 4):S43. [Google Scholar]
- Spallino M, Guerra L, Cuzzocrea M, Elisei F, Crivellaro C, Ponti E, et al. The Deauville criteria and QPET as prognostic factors in interim PET in adult Hodgkin lymphoma: A single centre experience. Clinical and Translational Imaging 2017;5(Suppl. 1):S43. [Google Scholar]
- Yaghmour G, Farhat M, Valdivieso BS, Janakiraman N. PET‐negative at 2, 3 or 4 cycles of ABVD in Hodgkin's lymphoma is still good. Journal of General Internal Medicine 2012;27(Suppl. 2):S258‐9. [Google Scholar]
- Zanoni L, Agostinelli C, Gallamini A, Rigacci L, Sista M, Piccaluga P, et al. The predictive value of interim PET and immunohistochemical markers in Hodgkin lymphoma (HL). European Journal of Nuclear Medicine and Molecular Imaging 2011;38(Suppl. 2):S165. [Google Scholar]
References to ongoing studies
- Kobe C, Dietlein M, Kuhnert G, Holstein A, Kahraman D, Haverkamp H, et al. Recruitment and PET interpretation in the HD16 trial for early stage Hodgkin lymphoma. Treatment stratification by FDG‐PET. NuklearMedizin. 2012; Vol. 50. Jahrestagung der Deutschen Gesellschaft für Nuklearmedizin. Bremen, Germany, issue 51 (2):A39‐40. [Google Scholar]; NCT00736320. HD16 for early stages ‐ treatment optimization trial in the first‐line treatment of early stage Hodgkin lymphoma; treatment stratification by means of FDG‐PET. clinicaltrials.gov/ct2/show/nct00736320 (first received 15 August 2008).
Additional references
- Adams HJ, Nievelstein RA, Kwee TC. Prognostic value of interim FDG‐PET in Hodgkin lymphoma: systematic review and meta‐analysis. British Journal of Haematology 2015;170(3):356‐66. [DOI] [PubMed] [Google Scholar]
- Adams HJ, Kwee TC. Controversies on the prognostic value of interim FDG‐PET in advanced‐stage Hodgkin lymphoma. European Journal of Haematology 2016;97(6):491‐8. [DOI] [PubMed] [Google Scholar]
- Altman DG. Practical Statistics for Medical Research. 1. London: Chapman & Hall, 1991. [Google Scholar]
- Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLOS Medicine 2012;9(5):e1001216. [PUBMED: 22675273] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai I, Gurion R, Vidal L, Dann EJ, Raanani P, Gafter‐Gvili A. PET‐adapted therapy for advanced Hodgkin lymphoma – systematic review. Acta Oncologica 2018;57(6):765‐72. [DOI] [PubMed] [Google Scholar]
- Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. Journal of Clinical Oncology 2014;32(27):3048‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non‐Hodgkin lymphomas. European Journal of Nuclear Medicine and Molecular Imaging 2017;44(1):97‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berriolo‐Riedinger A, Becker S, Casasnovas O, Vander Borght T, Edeline V. Role of FDG PET‐CT in the treatment management of Hodgkin lymphoma. Cancer Radiotherapy 2018;22(5):393‐400. [DOI] [PubMed] [Google Scholar]
- Blank O, Tresckow B, Monsef I, Specht L, Engert A, Skoetz N. Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early stage Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD007110.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. European Journal of Nuclear Medicine and Molecular Imaging 2010;37(1):181‐200. [PUBMED: 19915839] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchmann P, Haverkamp H, Diehl V, Cerny T, Markova J, Ho AD, et al. Eight cycles of escalated‐dose BEACOPP compared with four cycles of escalated‐dose BEACOPP followed by four cycles of baseline‐dose BEACOPP with or without radiotherapy in patients with advanced‐stage Hodgkin's lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. Journal of Clinical Oncology 2011;29(32):4234‐42. [PUBMED: 21990399] [DOI] [PubMed] [Google Scholar]
- Bouwmeester W, Zuithoff NP, Mallett S, Geerlings MI, Vergouwe Y, Steyerberg EW, et al. Reporting and methods in clinical prediction research: a systematic review. PLOS Medicine 2012;9(5):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröckelmann PJ, Eichenauer DA, Jakob T, Follmann M, Engert A, Skoetz N. Clinical practice guideline: Hodgkin lymphoma in adults—diagnosis, treatment, and follow‐up. Deutsches Ärzteblatt International 2018;115:535‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. New England Journal of Medicine 1992;327(21):1478‐84. [PUBMED: 1383821] [DOI] [PubMed] [Google Scholar]
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. Journal of Clinical Oncology 2014;32(27):3059‐68. [PUBMED: 25113753] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veritas Health Innovation. Covidence. Version accessed 4 March 2019. Melbourne, Australia.: Veritas Health Innovation.
- Cuccaro A, Bartolomei F, Cupelli E, Galli E, Giachelia M, Hohaus S. Prognostic factors in hodgkin lymphoma. Mediterranean Journal of Hematology and Infectious Diseases 2014;6(1):e2014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debray T, Damen J, Snell K, Ensor J, Hooft L, Reitsma J, et al. A guide to systematic review and meta‐analysis of prediction model performance. BMJ 2017;356:i6460. [DOI] [PubMed] [Google Scholar]
- Debray T, Moons K, Riley R. Detecting small‐study effects and funnel plot asymmetry in meta‐analysis of survival data: A comparison of new and existing tests. Research Synthesis Methods 2018;9(1):41‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, et al. Involved‐field radiotherapy is equally effective and less toxic compared with extended‐field radiotherapy after four cycles of chemotherapy in patients with early‐stage unfavorable Hodgkin's lymphoma: results of the HD8 trial of the German Hodgkin's Lymphoma Study Group. Journal of Clinical Oncology 2003;21(19):3601‐8. [DOI] [PubMed] [Google Scholar]
- Engert A, Franklin J, Eich HT, Brillant C, Sehlen S, Cartoni C, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended‐field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. Journal of Clinical Oncology 2007;25(23):3495‐502. [DOI] [PubMed] [Google Scholar]
- Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, et al. Reduced treatment intensity in patients with early‐stage Hodgkin's lymphoma. New England Journal of Medicine 2010;363(7):640‐52. [PUBMED: 20818855] [DOI] [PubMed] [Google Scholar]
- Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced‐intensity chemotherapy and PET‐guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open‐label, phase 3 non‐inferiority trial. Lancet 2012;379(9828):1791‐9. [PUBMED: 22480758] [DOI] [PubMed] [Google Scholar]
- Fitzgerald TJ. Optimisation of adaptive therapy for advanced Hodgkin lymphoma. Lancet Oncology 2019;20(2):167‐8. [DOI] [PubMed] [Google Scholar]
- Franklin JG, Paus MD, Pluetschow A, Specht L. Chemotherapy, radiotherapy and combined modality for Hodgkin's disease, with emphasis on second cancer risk. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003187.pub2; PUBMED: 16235316] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2‐[18F]fluoro‐2‐deoxy‐D‐glucose positron emission tomography is prognostically superior to international prognostic score in advanced‐stage Hodgkin's lymphoma: a report from a joint Italian‐Danish study. Journal of Clinical Oncology 2007;25(24):3746‐52. [PUBMED: 17646666] [DOI] [PubMed] [Google Scholar]
- Geersing GJ, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLOS One 2012;7(7):e32844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JA, Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Annals of Internal Medicine 2013;158(4):280‐6. [PUBMED: 23420236] [DOI] [PubMed] [Google Scholar]
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975‐2010. National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2010/ based on November 2012 SEER data submission, posted to the SEER web site, April 2013 (accessed 23 June 2015).
- Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:1‐8. [DOI] [PubMed] [Google Scholar]
- Josting A. Prognostic factors in Hodgkin lymphoma. Expert Review of Hematology 2010;3(5):583‐92. [DOI] [PubMed] [Google Scholar]
- Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. Journal of Clinical Oncology 2007;25:571‐8. [DOI] [PubMed] [Google Scholar]
- Klimm B, Engert A, Diehl V. First‐line treatment of Hodgkin's lymphoma. Current Hematology Reports 2005;4(1):15‐22. [PubMed] [Google Scholar]
- Kobe C, Dietlein M, Franklin J, Markova J, Lohri A, Amthauer H, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first‐line chemotherapy in advanced‐stage Hodgkin lymphoma. Blood 2008;112(10):3989‐94. [PUBMED: 18757777] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe C, Dietlein M, Fuchs M. Interpretation and validation of interim positron emission tomography in Hodgkin lymphoma. Leukemia and Lymphoma2010; Vol. 51, issue 3:552‐3. [PUBMED: 20141443] [DOI] [PubMed]
- Kobe C, Dietlein M, Kriz J, Furth C, Fuchs M, Borchmann P, et al. The role of PET in Hodgkin's lymphoma and its impact on radiation oncology. Expert Review of Anticancer Therapy 2010;10(9):1419‐28. [PUBMED: 20836677] [DOI] [PubMed] [Google Scholar]
- Kyzas PA, Loizou KT, Ioannidis JP. Selective reporting biases in cancer prognostic factor studies. Journal of the National Cancer Institute 2005;97(14):1043‐55. [PUBMED: 16030302] [DOI] [PubMed] [Google Scholar]
- Kyzas PA, Denaxa‐Kyza D, Ioannidis JP. Quality of reporting of cancer prognostic marker studies: association with reported prognostic effect. Journal of the National Cancer Institute 2007;99(3):236‐43. [PUBMED: 17284718] [DOI] [PubMed] [Google Scholar]
- Kılıçkap S, Barışta I, Ulger S, Celik I, Selek U, Yıldız F, et al. Clinical Features and Prognostic Factors of Hodgkin's Lymphoma: A Single Center Experience. Balkan Medical Journal 2013;30(2):178‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. Journal of Clinical Oncology 1989;7(11):1630‐6. [DOI] [PubMed] [Google Scholar]
- Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Chapter 10 Analysing and Presenting Results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 1.0. The Cochrane Collaboration, 2010. Available from: http://srdta.cochrane.org/. [Google Scholar]
- Mallett S, Timmer A, Sauerbrei W, Altman DG. Reporting of prognostic studies of tumour markers: a review of published articles in relation to REMARK guidelines. British Journal of Cancer 2010;102(1):173‐80. [PUBMED: 19997101] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markova J, Kobe C, Skopalova M, Klaskova K, Dedeckova K, Plutschow A, et al. FDG‐PET for assessment of early treatment response after four cycles of chemotherapy in patients with advanced‐stage Hodgkin's lymphoma has a high negative predictive value. Annals of Oncology 2009;20(7):1270‐4. [PUBMED: 19228806] [DOI] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumour MARKer prognostic studies (REMARK). British Journal of Cancer 2005;93(4):387‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim‐PET‐Scan in Lymphoma. Leukemia and Lymphoma 2009;50(8):1257‐60. [DOI] [PubMed] [Google Scholar]
- Meignan M, Itti E, Bardet S, Lumbroso J, Edeline V, Olivier P, et al. Development and application of a real‐time on‐line blinded independent central review of interim PET scans to determine treatment allocation in lymphoma trials. Journal of Clinical Oncology 2009;27(16):2739‐41. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
- Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how?. BMJ 2009;338:b375. [DOI] [PubMed] [Google Scholar]
- Moons KG, Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Medicine 2014;11:e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, et al. Five‐year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood Journal 2018;132(25):2639. [DOI] [PubMed] [Google Scholar]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
- Peat G, Riley RD, Croft P, Morley KI, Kyzas PA, Moons KG, et al. Improving the transparency of prognosis research: the role of reporting, data sharing, registration, and protocols. PLOS Medicine 2014;11(7):e1001671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET‐directed therapy for early‐stage Hodgkin's lymphoma. New England Journal of Medicine 2015;372(1):1598‐607. [DOI] [PubMed] [Google Scholar]
- Rancea M, Monsef I, Tresckow B, Engert A, Skoetz N. High‐dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009411.pub2; PUBMED: 23784872] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancea M, Engert A, Tresckow B, Halbsguth T, Behringer K, Skoetz N. Hodgkin's lymphoma in adults: diagnosis, treatment and follow‐up. Deutsches Arzteblatt International 2013;110(11):177‐83, 183e1‐3. [PUBMED: 23555321] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re D, Thomas RK, Behringer K, Diehl V. From Hodgkin disease to Hodgkin lymphoma: biologic insights and therapeutic potential. Blood 2005;105(12):4553‐60. [PUBMED: 15728122] [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Riley RD, Hayden JA, Steyerberg EW, Moons KG, Abrams K, Kyzas PA, et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLOS Medicine 2013;10(2):e1001380. [PUBMED: 23393429] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RD, Moons KG, Snell KE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta‐analysis of prognostic factor studies. BMJ 2019;364:k4597. [DOI] [PubMed] [Google Scholar]
- Sauerbrei W. Prognostic factors. Confusion caused by bad quality design, analysis and reporting of many studies. Advances in Oto‐Rhino‐Laryngology 2005;62:184‐200. [PUBMED: 15608428] [DOI] [PubMed] [Google Scholar]
- Sickinger MT, Tresckow B, Kobe C, Engert A, Borchmann P, Skoetz N. Positron emission tomography‐adapted therapy for first‐line treatment in individuals with Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010533.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoetz N, Trelle S, Rancea M, Haverkamp H, Diehl V, Engert A, et al. Effect of initial treatment strategy on survival of patients with advanced‐stage Hodgkin's lymphoma: a systematic review and network meta‐analysis. Lancet. Oncology 2013;14(10):943‐52. [PUBMED: 23948348] [DOI] [PubMed] [Google Scholar]
- Skoetz N, Will A, Monsef I, Brillant C, Engert A, Tresckow B. Comparison of first‐line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD007941.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg EW, Moons KG, Windt DA, Hayden JA, Perel P, Schroter S, et al. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLOS Medicine 2013;10(2):e1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Fourth. Vol. 2, Lyon: WHO Press, 1211 Geneva 27, Switzerland, 2008. [ISBN‐13: 9789283224310] [Google Scholar]
- Thomas RK, Re D, Zander T, Wolf J, Diehl V. Epidemiology and etiology of Hodgkin's lymphoma. Annals of Oncology 2002;13:147‐52. [PUBMED: 12401681] [DOI] [PubMed] [Google Scholar]
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivella M. Systematic Reviews of Prognostic Factor Studies (Section: Estimating the Hazard Ratio) [DPhil]. University of Oxford (Altman DG, thesis advisor) June 10th 2006:219.
- Tresckow B, Plutschow A, Fuchs M, Klimm B, Markova J, Lohri A, et al. Dose‐intensification in early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin study group HD14 trial. Journal of Clinical Oncology 2012;30(9):907‐13. [PUBMED: 22271480] [DOI] [PubMed] [Google Scholar]
- Weiler‐Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. (18)F‐FDG avidity in lymphoma readdressed: a study of 766 patients. Journal of Nuclear Medicine 2010;51(1):25‐30. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Skoetz N, Collins G, Moons K, Estcourt LJ, Engert A, Kobe C, et al. Interim PET for prognosis in adults with Hodgkin lymphoma: a prognostic factor exemplar review. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD012643] [DOI] [Google Scholar]


