Abstract
Objective
Indigo naturalis (IN) is a traditional Chinese medicine that has recently been reported to be effective for ulcerative colitis (UC). The aim of this study was to evaluate the efficacy and safety of IN.
Methods
We performed a retrospective observational study for 14 patients with UC treated with IN from October 2015 to December 2016.
Results
After 8 weeks of oral administration of IN, the partial Mayo score decreased from 4 (2-5) to 1.5 (0-4) [median, interquartile range (IQR), p=0.015]. Among 10 active UC patients, 5 (50%) showed a clinical response, and 4 (40%) achieved clinical remission. Serial changes of endoscopic activity were evaluated in nine patients using the Mayo endoscopic subscore (MES), Rachmilewitz endoscopic index (REI), and UC endoscopy index of severity (UCEIS). The MES decreased from 2 (2-3) to 1 (1-2) [median (IQR), p=0.005], the REI decreased from 7 (5.5-11) to 3 (1-7) [median (IQR), p=0.008], and the UCEIS decreased from 3 (3-4.5) to 1 (0.5-3.5) [median (IQR), p=0.039]. One patient developed acute right-sided colitis with wall thickening and edematous change, and the remaining 13 showed no adverse events.
Conclusion
We conclude that IN is effective for patients with UC as a therapy for inducing remission.
Keywords: ulcerative colitis, indigo naturalis, Qing-Dai, Chinese herbal medicine
Introduction
Ulcerative colitis (UC) is an idiopathic chronic inflammatory disorder of the large intestine characterized by recurrent periods of clinical remission and relapse of the disease. The main goal of medical therapy in UC is to achieve effective and sustained suppression of intestinal inflammation and, as a consequence, to induce and maintain clinical remission. In China, indigo naturalis (IN; also referred to as Qing-Dai) has been traditionally used to treat various inflammatory disorders (1-3), including UC (4, 5). Recently, several reports have suggested the clinical efficacy of IN in intractable Japanese patients with UC (6-8). However, the latest randomized controlled trial evaluating the efficacy of IN for UC in Japan was terminated (8) because of the occurrence of pulmonary arterial hypertension (PAH) (9). The further accumulation of cases is therefore needed in order to clarify the efficacy and adverse events of IN for UC.
We performed a retrospective cross-sectional observational study of 14 Japanese patients with UC treated with IN.
Case Report
Patients
Fourteen Japanese patients with UC who received IN therapy from October 2015 to December 2016 were retrospectively evaluated (Table 1). All patients were recruited at the Division of Gastroenterology, Iwate Medical University Hospital, Morioka, Japan, and diagnosed according to established clinical, endoscopic, radiological, and histological criteria. The median age of the patients was 46 years with a range of 18-69 years. Twelve patients had total colitis, one had left-sided colitis, and the remaining one had proctitis-type. The median duration of UC was 38 months (range, 22-245 months). The partial Mayo score at the start of medication was 4 (range, 1-7).
Table 1.
Patient Characteristics Including Disease Extent and Treatment for Ulcerative Colitis.
| Patient No. |
Sex | Age (yr) | Extent of UC | Duration of UC (mo.) |
PMS at baseline | Current treatment at time of IN initiation |
Past treatments | Intractability† | Daily dose of IN (g) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 23 | Total colitis | 64 | 5 | 5-ASA, PSL, AZA | 5-ASA, ADA, IFX, AZA, PSL, TAC, CAP | Yes | 2.0 |
| 2 | F | 18 | Total colitis | 22 | 4 | 5-ASA | 5-ASA, CAP, IFX, PSL | No | 1.0 |
| 3 | M | 52 | Total colitis | 31 | 5 | 5-ASA, PSL, AZA | 5-ASA, PSL, AZA | Yes | 0.5 |
| 4 | F | 58 | Total colitis | 34 | 1 | 5-ASA, AZA | 5-ASA, PSL, AZA | No | 2.0 |
| 5 | F | 69 | Total colitis | 245 | 6 | 5-ASA | 5-ASA, PSL, AZA | Yes | 1.0 |
| 6 | F | 18 | Left-sided colitis | 29 | 5 | 5-ASA | 5-ASA, PSL | Yes | 0.5 |
| 7 | M | 54 | Total colitis | 28 | 5 | 5-ASA, AZA, IFX | 5-ASA, PSL, AZA, IFX | Yes | 2.0 |
| 8 | M | 69 | Proctitis | 42 | 2 | 5-ASA, AZA | 5-ASA, PSL, AZA, ADA, TAC | Yes | 2.0 |
| 9 | F | 45 | Total colitis | 245 | 2 | 5-ASA, AZA | 5-ASA, PSL, AZA, IFX | Yes | 1.0 |
| 10 | M | 47 | Total colitis | 24 | 3 | 5-ASA, PSL, AZA, IFX | 5-ASA, PSL, AZA, IFX, ADA | Yes | 2.0 |
| 11 | M | 19 | Total colitis | 25 | 1 | none | 5-ASA, PSL, AZA, IFX | Yes | 1.0 |
| 12 | F | 62 | Total colitis | 77 | 4 | 5-ASA, PSL | 5-ASA, PSL, IFX | Yes | 2.0 |
| 13 | M | 20 | Total colitis | 65 | 7 | 5-ASA, AZA | 5-ASA, AZA, IFX | Yes | 2.0 |
| 14 | F | 21 | Total colitis | 46 | 4 | 5-ASA, PSL, TAC | 5-ASA, PSL, AZA, IFX, ADA, TAC | Yes | 1.0 |
†Intractability was defined as steroid dependency or active disease under TNF-a or immunomodulators.
We defined patients who showed an insufficient effect despite proper steroid use, developed recurrence during the reduction in the dose of systemic prednisolone, or who did not respond to immunomodulators (IMs) or anti-tumor necrosis factor (TNF)-α antibodies as having intractable UC. Based on these criteria, 12 patients were regarded as having intractable UC. Thirteen patients were treated with 5-aminosalicylate, 8 with azathioprine, and 5 with prednisolone. All patients purchased IN (Qing-Dai) by themselves from Seishinshoyakudo (Tokyo, Japan). The daily dose of IN was 0.5, 1.0, and 2.0 g in 2, 5, and 7 patients, respectively.
Clinical and endoscopic evaluations
All patients had data available on the white blood cell (WBC) count, serum C-reactive protein (CRP) levels, and partial Mayo score (PMS) before and after eight weeks of IN therapy. Nine patients were evaluated by colonoscopy before and after IN therapy. The endoscopic activity was determined by the Mayo endoscopic subscore (MES), Rachmilewitz endoscopic index (REI), and ulcerative colitis endoscopy index of severity (UCEIS).
Ethical considerations
The study protocol was approved by the ethics committee at Iwate Medical University Hospital, and the study was conducted in accordance with the Declaration of Helsinki (6th revision, 2008).
Statistical analyses
All statistical analyses were performed using the JMPⓇ 13 software program (SAS Institute, Cary, USA). The changes in scores and laboratory results were evaluated by Student's t-test. Clinical characteristics were compared between responders and non-responders. Age and laboratory data were compared with Wilcoxon's test. Frequencies by gender and ongoing medication were compared with the chi-square test. The types of disease extent and daily dose of IN were compared with the Kruskal-Wallis test. P values <0.05 were considered to be statistically significant.
Results
Clinical efficacy of IN
The median WBC (/μL) [interquartile range (IQR)] before and after IN therapy was 6,675 (5,223-8,998) and 6,810 (6,100-11,195), respectively (p=0.72). The median CRP (mg/dL) (IQR) showed a decreasing trend from 0.87 (0.17-1.91) to 0.15 (0.07-0.66) (p=0.079). The median PMS (IQR) significantly decreased from 4 (2-5) to 1.5 (0-4) (p=0.015) (Fig. 1).
Figure 1.
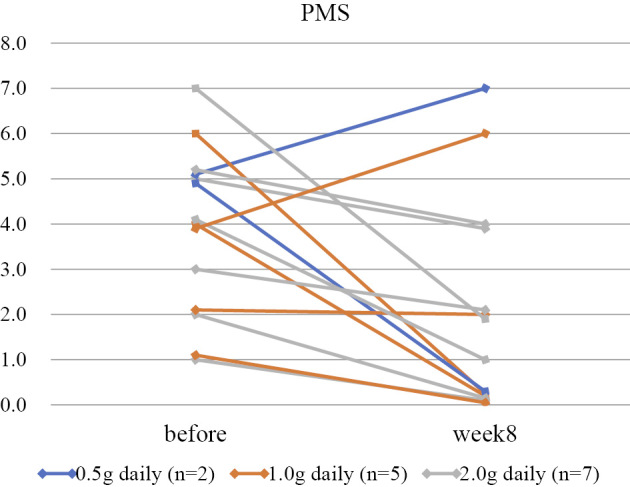
Serial changes in the partial Mayo score before and after indigo naturalis initiation. The partial Mayo score significantly improved from 4 (2-5) to 1.5 (0-4) [median (IQR); t-test, p=0.015].
Ten of the 14 patients had a score of ≥3 for the PMS at the start of IN. When we defined a clinical response as a 2-point decrease in the PMS and clinical remission as a PMS ≤1 with no rectal bleeding, 5 of 10 patients with active disease showed a clinical response, 4 of whom achieved clinical remission after IN therapy (Fig. 2). Table 2 compares the clinical variables between patients with response and those without. As shown in the table, the PMS at baseline and other clinical variables were not markedly different between the two groups. However, there were trends towards higher rates of concomitant use of prednisolone and azathioprine in non-responders than in responders. Although not statistically significant, the response rate was higher in patients treated with 0.5 g or 1.0 g/day of IN than in those treated with 2.0 g/day (60% vs. 40%). When we compared the clinical efficacy between active patients treated with concomitant 5-aminosalicylates (ASA) only and those treated with additional IM or biologics, the decrease in PMS was greater in the former (median: 4.5, IQR: 3.25-5.75) than in the latter (median: 1, IQR: 1-1.5, p=0.004).
Figure 2.
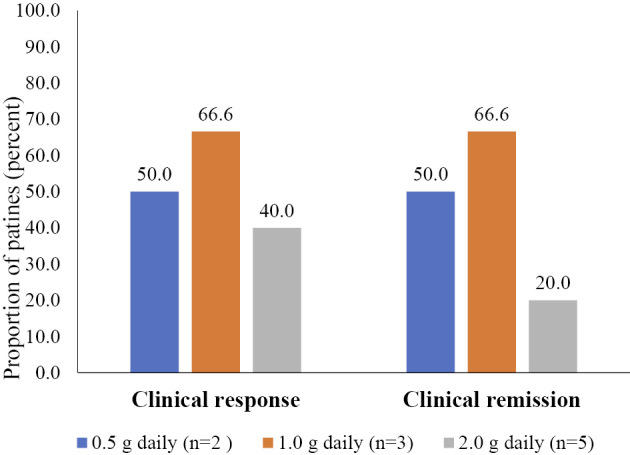
Proportions of patients who achieved a clinical response and clinical remission after eight weeks of indigo naturalis therapy.
Table 2.
Patient Characteristics and Comparison of Responders and Non-responders for IN.
| Parameters at entry | Responders (n=5) | Non-responders (n=5) | p value |
|---|---|---|---|
| Age (years) | |||
| Median (IQR) | 20 (18-66) | 47 (22-53) | 0.68 |
| Sex | |||
| Mele | 1 (20%) | 4 (80%) | 0.06 |
| Female | 4 (80%) | 1 (20%) | |
| Disease extent | |||
| Proctitis | 1 | 0 | |
| Left-sided colitiss | 0 | 0 | 0.42 |
| Total colitiss | 4 | 5 | |
| Baseline PMS (median, IQR) | 5 (4-6.5) | 5 (3.5-5) | 0.44 |
| Laboratory data (median, IQR) | |||
| WBC (/µL) | 6,040 (4,965-11,555) | 6,790 (5,655-12,450) | 0.68 |
| CRP (mg/dL) | 0.8 (0.09-2.1) | 1.23 (0.52-2.33) | 0.53 |
| ESR (mm/hr) | 16 (7-31) | 20 (16-25) | 0.47 |
| Albumin (g/dL) | 3.4 (3.2-3.9) | 3.6 (3.3-3.9) | 0.67 |
| Hemoglobin (g/dL) | 11.5 (10-12.8) | 12.5 (9.8-13.1) | 0.68 |
| Platelet (×1,000/µL) | 297 (234-495) | 494 (359-639) | 0.30 |
| Ongoing treatment | |||
| 5-ASA (oral) | 5 (100%) | 5 (100%) | 1.0 |
| 5-ASA (topical) | 1 (20%) | 1 (20%) | 1.0 |
| Corticosteroid | 1 (20%) | 4 (80%) | 0.06 |
| AZA/6-MP | 1 (20%) | 4 (80%) | 0.06 |
| Tacrolimus | 0 | 1 (20%) | 0.30 |
| Infliximab/adalimumab | 0 | 2 (40%) | 0.11 |
| Probiotics | 2 (40%) | 3 (60%) | 0.53 |
| Daily dose of IN | |||
| 0.5 g | 1 (20%) | 1 (20%) | 0.73 |
| 1.0 g | 2 (40%) | 1 (20%) | |
| 2.0 g | 2 (40%) | 3 (60%) |
ASA: aminosalicylates, AZA: azathioprine, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, IN: indigo natularis, IQR: interquartile range, MP: mercaptopurine, NS: not significant
Endoscopic activity
The median MES (IQR) significantly decreased from 2 (2-3) to 1 (1-2) (p=0.005) (Fig. 3A). The median REI (IQR) decreased from 7 (5.5-11) to 3 (1-7) (p=0.008) (Fig. 3B). The median UCEIS (IQR) also decreased from 3 (3-4.5) to 1 (0.5-3.5) (p=0.039) (Fig. 3C). Fig. 4 shows a representative case of refractory UC successfully treated with IN.
Figure 3.
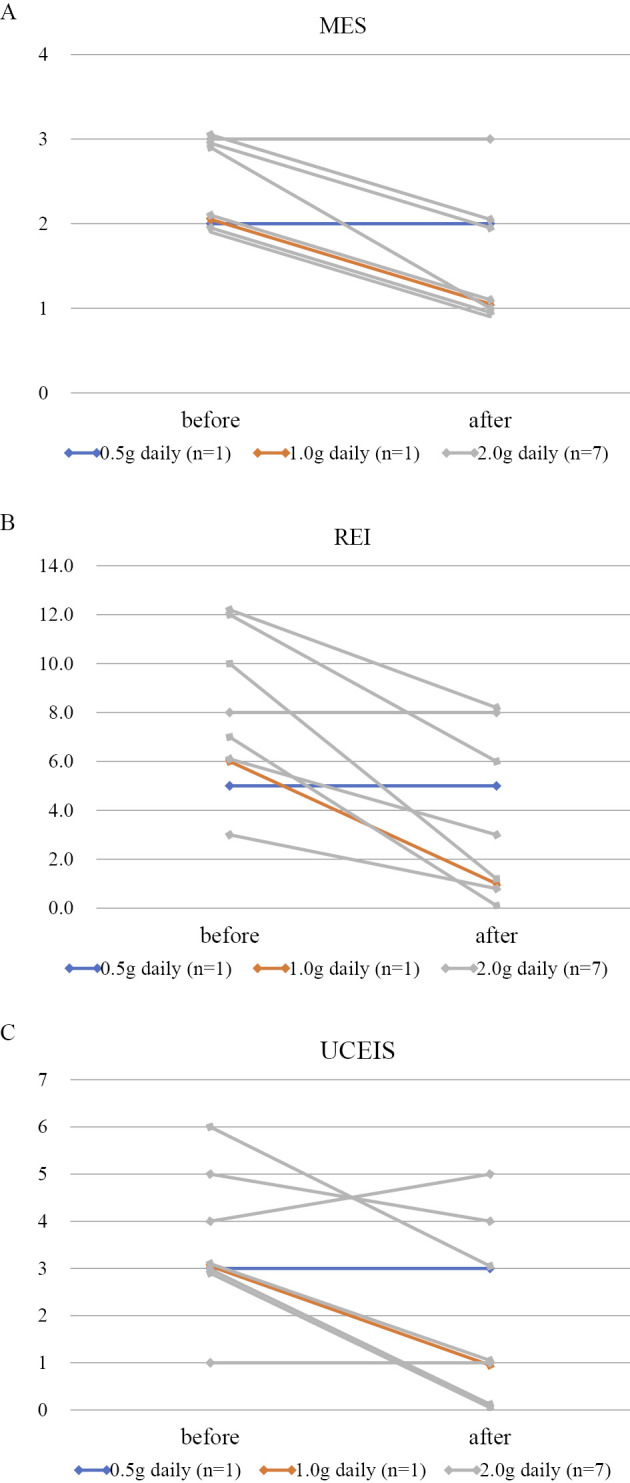
Serial changes in the endoscopic score before and after indigo naturalis initiation. A: The Mayo endoscopic subscore significantly improved from 2 (2-3) to 1 (1-2) [median (IQR); t-test, p=0.005]. B: The Rachmilewitz endoscopic index significantly improved from 7 (5.5-11) to 3 (1-7) [median (IQR); t-test, p=0.008]. C: The ulcerative colitis endoscopic index of severity significantly improved from 3 (3-4.5) to 1 (0.5-3.5) [median (IQR); t-test, p=0.039].
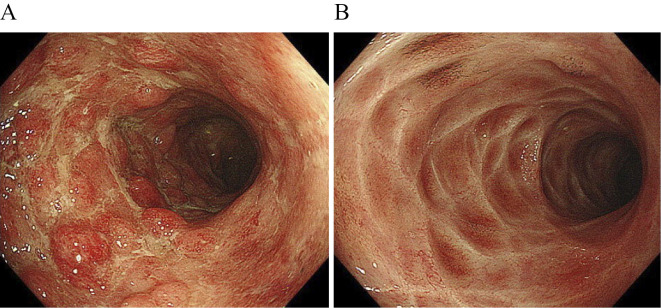
Figure 4.
Clinical endoscopic examples before and after indigo naturalis therapy (most effective case). A: Before indigo naturalis therapy: Partial Mayo score of 3. B: After indigo naturalis therapy: Partial Mayo score of 0.
Safety
One patient developed acute right-sided colitis with wall thickening and edematous change after she increased her dose of IN by herself (10), resulting in the patient requiring hospitalization. The remaining 13 patients showed no adverse events as reported in previous studies (headache, nausea, liver dysfunction, PAH) (3, 9, 11).
Discussion
In the present study, we investigated the clinical efficacy and adverse events of IN in patients with UC. The results showed that IN significantly reduced both the PMS and endoscopic scores, and IN was effective in half of patients with active UC.
Since 2007, Xilei-san, a major ingredient in IN, has been reported to be effective in patients with refractory UC of proctitis type (12-14). Other recent studies have reported the strong efficacy of IN in Japanese patients with UC (6-8). Among these studies, the only prospective multicenter randomized, double-blind, placebo-controlled study found 8 weeks of IN to be effective in inducing a clinical response in 75% (48/64) of patients (8). In that study, the clinical response rate was high in IN groups of all dosages (69.6% for IN 0.5 g/day, 75% for IN 1.0 g/day, 81% for IN 2.0 g/day). However, the clinical remission rate was unfavorable (39%), being 26% for the subgroup receiving IN 0.5 g/day, 55% for that receiving IN 1.0 g/day, and 38%, for that receiving IN 2.0 g/day.
In our present study, clinical response rate at week 8 was 50%, and the clinical remission rate at week 8 was 40%. The remission rate at week 8 in our study (40%) was similar to that in the above prospective study (39%), while the response rate in our study (50%) was lower than in the prospective study (75%). However, most of our study patients were taking prednisolone (5/10 cases) and/or IM (8/10 cases), which might have been associated with their poor response to IN therapy. The significant difference in the reduction in the PMS between patients treated with 5-ASA only and those treated with IM or biologics in our study may support this speculation. Alternatively, the lack of dose dependency in the efficacy of IN for the treatment of UC as shown in our patients and in a prospective study by Naganuma et al. (8) suggests that IN is not synergistic with the medications widely accepted for the treatment of UC. A recent report describing a novel modulation in interleukin (IL)-22 induced by IN in rodent experimental colitis may further support our speculation (15).
Only one patient developed adverse events (acute right-sided colitis) (10), and no relapse of this event has been noted since the patient discontinued IN therapy. Similar cases of IN-associated right-sided colitis with wall thickening and edematous change have also been described (7, 16). Although the precise mechanism has not yet been clarified, some ischemic event might have contributed to the pathogenesis (16, 17).
Several limitations associated with the present study warrant mention. First, this was a cross-sectional observational study, and its retrospective nature might have induced some selection biases. Second, the daily dose of IN was not unified among patients. Third, the timing of the endoscopic examination was not scheduled and varied to some degree among cases. Fourth, we failed to evaluate the efficacy of IN by colonoscopy in five patients. Finally, the study was performed at a single center and involved a limited number of patients.
In conclusion, we herein report the practical efficacy of IN for patients with intractable UC. We confirmed that IN improved both the clinical and endoscopic activity. We also found that the clinical response rate to IN was not as high in our patients as has been reported previously. Since the safety profile of IN remains to be elucidated, further studies will be necessary before IN can be recommended as a treatment choice for UC.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Yuan G, Ke Q, Su X, Yang J, Xu X. Qing Dai, a traditional Chinese medicine for the treatment of chronic hemorrhagic radiation proctitis. Chinese-German J Clin Oncol 8: 114-116, 2009. [Google Scholar]
- 2.Stasiak N, Kukula-Koch W, Glowniak K. Modern industrial and pharmacological applications of indigo dye and its derivatives-a review. Acta Pol Pharm 71: 215-221, 2014. [PubMed] [Google Scholar]
- 3.Zhou D, Chen W, Li X, et al. Evidence-based practice guideline of Chinese herbal medicine for psoriasis vulgaris (Bai Bi). Eur J Integr Med 6: 135-146, 2014. [Google Scholar]
- 4.Jiang XL, Cui HF. An analysis of 10218 ulcerative colitis cases in China. World J Gastroenterol 8: 158-161, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouyang Q, Hu PJ, Qian JM, Zheng JJ, Hu RW. Consensus on the management of inflammatory bowel disease in China in 2007. J Dig Dis 9: 52-62, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki H, Kaneko T, Mizokami Y, et al. Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis. World J Gastroenterol 19: 2718-2722, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugimoto S, Naganuma M, Kiyohara H, et al. Clinical efficacy and safety of oral Qing-Dai in patients with ulcerative colitis: a single-center open-label prospective study. Digestion 93: 193-201, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Naganuma M, Sugimoto S, Mitsuyama K, et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154: 935-947, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Nishio M, Hirooka K, Doi Y. Chinese herbal drug natural indigo may cause pulmonary artery hypertension. Eur Heart J 37: 1992, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Yanai S, Nakamura S, Matsumoto T. Indigo naturalis-induced colitis. Dig Endosc 2018(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 11.Ferber KH. Toxicology of indigo. A review. J Environ Pathol Toxicol Oncol 7: 73-83, 1987. [PubMed] [Google Scholar]
- 12.Fukunaga K, Hida N, Ohnishi K, et al. A suppository Chinese medicine (xilei-san) for refractory ulcerative proctitis: a pilot clinical trial. Digestion 75: 146-147, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Fukunaga K, Ohda Y, Hida N, et al. Placebo controlled evaluation of Xilei San, a herbal preparation in patients with intractable ulcerative proctitis. J Gastroenterol Hepatol 27: 1808-1815, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, Li Y, Xu F, Chu Y, Zhao W. Comparison of Xilei-san, a Chinese herbal medicine, and dexamethasone in mild/moderate ulcerative proctitis: a double-blind randomized clinical trial. J Altern Complement Med 19: 838-842, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Kawai S, Iijima H, Shinzaki S, et al. Indigo Naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J Gastroenterol 52: 904-919, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Kondo S, Araki T, Okita Y, et al. Colitis with wall thickening and edematous changes during oral administration of the powdered form of Qing-dai in patients with ulcerative colitis: a report of two cases. Clin J Gastroenterol 11: 268-272, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Suo BJ, Zhou LY, Ding SG, et al. [The endoscopic and clinical features of Indigo Naturalis-associated ischemic lesions of colonic mucosa]. Zhonghua Nei Ke Za Zhi 50: 646-649, 2011(in Chinese, Abstract in English). [PubMed] [Google Scholar]