Abstract
Objective
Although several clinical trials have shown that the mid- and long-term safety and efficacy of prasugrel are better than those of clopidogrel after percutaneous coronary intervention (PCI), there are few data regarding the short-term safety.
Methods
In this study, we retrospectively analyzed the short-term (72 hours) PCI-related bleeding complications and mid-term (12 months) efficacy in 250 consecutive coronary artery disease patients who underwent PCI and received aspirin plus prasugrel (prasugrel group; 67.7±10.0 years, 200 men).
Patients
The comparison group consisted of 250 age- and gender-matched patients who received aspirin plus clopidogrel (clopidogrel group: 67.2±11.2 years, 199 men).
Results
The incidence of a composite of PCI-related bleeding complications in the acute phase post-PCI was significantly higher in the prasugrel group than in the clopidogrel group (22.4% vs. 13.2%, p=0.007), although the incidence of non-PCI-related bleeding complications over 12 months was comparable between the 2 groups. The cumulative incidence of major cardiovascular events (MACEs) was comparable between the prasugrel and clopidogrel groups (log-rank test; p=0.561). A multivariate logistic regression analysis of the 250 prasugrel-treated patients showed that acute coronary syndrome tended to be negatively associated with the incidence of PCI-related bleeding complications (p=0.061).
Conclusion
Prasugrel and clopidogrel may have similar efficacy for preventing cardiovascular events as the post-PCI antiplatelet regimen; however, prasugrel should be used cautiously because of the risk of PCI-related bleeding complications.
Keywords: prasugrel, clopidogrel, dual-antiplatelet therapy, percutaneous coronary intervention, bleeding complication
Introduction
Generational advances in percutaneous coronary intervention (PCI) have greatly contributed to reducing the acute phase mortality in patients with ST-elevation myocardial infarction as well as relieving symptoms in those with angina pectoris. Thus, PCI has become an established treatment strategy for coronary artery disease. After coronary stent implantation, dual antiplatelet therapy (a thienopyridine antiplatelet agent plus aspirin) is critically important for the prevention of stent thrombosis, and this therapy is currently recommended for administration over 6 to 12 months after the implantation of a drug-eluting stent (DES) (1, 2). Clopidogrel, a thienopyridine antiplatelet agent and P2Y12 receptor antagonist, has long been used in the dual antiplatelet regimen based on its established safety and efficacy (3). However, clinical events, including myocardial infarction and coronary stent thrombosis, are still observed after PCI in patients treated with clopidogrel. One reason for this is that the pharmacologic response to clopidogrel is affected by CYP2C19 gene polymorphisms, so the inhibition of platelet aggregation is decreased in poor metabolizers (4).
Like clopidogrel, prasugrel is also a prodrug that requires conversion to an active metabolite before binding to the platelet P2Y12 receptor to confer antiplatelet activity (5). This new-generation thienopyridine inhibits adenosine diphosphate-induced platelet aggregation more rapidly, more consistently and to a greater extent than standard or higher doses of clopidogrel (6-8). In addition, prasugrel showed a trend toward fewer ischemic events than clopidogrel, and it had an acceptable safety profile in patients undergoing PCI as well as those with acute coronary syndrome in global as well as Japanese clinical trials (9-11). Since prasugrel is less affected by CYP2C19 gene polymorphisms than clopidogrel, this property may account for the advantageous effects of prasugrel over clopidogrel (11).
Although previous clinical trials on the safety and efficacy of prasugrel assessed the mid-term (6-15 months) outcomes, there are few data regarding the short-term safety. Since prasugrel exerts a stronger inhibitory effect on platelet aggregation than clopidogrel early after administration (11), prasugrel might cause more bleeding complications than clopidogrel in the acute phase post-PCI.
In this study, we retrospectively analyzed the bleeding complications, including PCI-related bleeding, as well as the mid-term (12 months) efficacy in patients with coronary artery disease who underwent PCI. We compared two dual antiplatelet regimens that are used in routine clinical practice: aspirin plus prasugrel versus aspirin plus clopidogrel.
Materials and Methods
Study design
This study was a single-center, observational, retrospective, age/gender-matched cohort study. We recruited 250 consecutive patients with coronary artery disease, including both chronic coronary artery disease and acute coronary syndrome, who underwent PCI and received aspirin plus prasugrel after PCI between October 2015 and July 2016 (prasugrel group; 67.7±10.0 years, 200 men). The comparison group consisted of age- and gender-matched coronary artery disease patients (n=250) who underwent PCI and received aspirin plus clopidogrel after PCI between October 2014 and September 2015 (clopidogrel group; 67.2±11.2 years, 199 men). For emergent PCI cases, the loading dose of prasugrel (20 mg) or clopidogrel (300 mg) was administered within 1 hour of the patient leaving the cardiac catheterization laboratory. The maintenance dose of prasugrel (3.75 mg) or clopidogrel (75 mg) was administered once daily, starting on the day after PCI. For elective PCI cases, the maintenance doses of both drugs were started at least 96 hours before PCI without a loading dose. Aspirin 100 mg per day was concomitantly administered during the treatment period. Dual antiplatelet therapy with aspirin plus thienopyridines was continued in principle at least until the time of follow-up coronary angiography, i.e. within 6 months for balloon angioplasty alone or bare metal stent implantation or within 12 months for DES implantation, or until major bleeding complications (defined as below) developed.
We collected data on PCI-related bleeding complications in the acute phase after PCI within 72 hours and outcome data, such as non-PCI-related bleeding events, ischemic events or death, over 12 months after PCI. Patients were excluded if they met any of the following criteria: hemodynamic instability that required circulatory assist with intra-aortic balloon pumping or percutaneous cardiopulmonary support; hemodialysis or hemofiltration; the use of antiplatelet drugs other than thienopyridines and aspirin; the continuous administration of oral acidic non-steroidal anti-inflammatory drugs during the 12-month observation period; and a lack of follow-up data at 12 months after PCI.
In all patients, PCI was performed via a standard radial or femoral approach. Intravenous heparin 100 IU/kg was administered prior to the procedure. The choice of DES, bare-metal stent or balloon angioplasty alone was left to the discretion of the operator. Following the procedures, we used a compression device for hemostatic treatment of the radial access sites or a closure device for treatment of the femoral access sites. If hemostatic treatment failed when attempted via the routine protocol for each device, additional hemostatic treatment was performed using manual compression, based on the discretion of the physician.
We retrospectively collected clinical and laboratory data on these 500 patients. The data were obtained from medical charts during the hospital stay, discharge letters, cardiac catheterization reports at PCI and laboratory data and were confirmed via a clinic medical examination. We collected information on the safety as well as efficacy endpoints.
Endpoints
The safety endpoints included PCI-related bleeding events during the acute phase after PCI. First, we calculated the reduction in the blood hemoglobin from baseline before PCI to the morning after PCI. We then assessed the incidence of the following events: hemoglobin reduction ≥3.0 g/dL, hematoma formation at the puncture sites, additional hemostatic treatment, blood transfusion, and a composite of these 4 events. The incidence of non-PCI-related bleeding events was evaluated up to 12 months after PCI. These events were based upon the thrombolysis in myocardial infarction (TIMI) bleeding criteria and included the following: 1) major bleeding defined as intracranial or clinically significant bleeding with a decrease in hemoglobin of ≥5 g/dL, 2) minor bleeding defined as clinically significant bleeding with a decrease in hemoglobin of 3-5 g/dL, and 3) clinically relevant minimal bleeding with a decrease in hemoglobin of <3 g/dL. We evaluated bleeding from critical sites (e.g. retroperitoneal, intra-pericardial, intra-vitreous/retinal, intra-spinal and intra-articular hemorrhaging), gastrointestinal bleeding accompanied by decreased hemoglobin, gross hematuria not attributed to external factors, epistaxis requiring otolaryngology, gingival bleeding requiring dental treatment and bleeding requiring discontinuation of the study drug at the investigator's discretion.
The efficacy endpoints included the cumulative incidence of the following events during 12 months after PCI: all-cause death; major cardiovascular events (MACEs) including cardiovascular death, non-fatal myocardial infarction and non-fatal ischemic stroke; MACEs plus coronary revascularization; and heart failure requiring hospitalization.
Statistical analyses
Values are expressed as the mean ± standard deviation for continuous variables and the number (percent) of patients for categorical variables. For intergroup comparisons of baseline characteristics and the incidence of events for safety endpoints, we used the unpaired t-test for continuous variables and the chi-square test for categorical variables. The cumulative incidence of events for the efficacy endpoints was assessed by the Kaplan-Meier method, and the groups were compared by the log-rank test. To determine if DES usage affected the efficacy of prasugrel relative to clopidogrel, a Cox proportional hazards regression analysis was performed, and the results were expressed as hazard ratios with 95% confidence intervals. A logistic regression analysis was performed to assess the factors associated with PCI-related bleeding in the prasugrel group. First, we performed a univariate analysis using several factors that might affect the bleeding risk, and then we performed a multivariate analysis using the factors with p<0.3 in the univariate analyses. These results were expressed as the odds ratios with 95% confidence intervals. All values with p<0.05 were considered significant.
Results
Baseline characteristics
The baseline characteristics of the prasugrel and clopidogrel groups are shown in Table 1. Although there were no marked differences in most of the clinical, angiographic or procedural characteristics between the two groups, the prevalence of dyslipidemia tended to be higher (70.8% vs. 63.6%, p=0.086), and the syntax score was significantly higher (14.0±9.3 vs. 12.1±8.0, p=0.013) in the prasugrel group than in the clopidogrel group. Furthermore, the DES usage was significantly higher in the prasugrel group than in the clopidogrel group (79.6% vs. 51.2%, p<0.0001).
Table 1.
Baseline Characteristics.
| Prasugrel group (n=250) |
Clopidogrel group (n=250) |
p value | ||
|---|---|---|---|---|
| Age: yrs | 67.7±10.0 | 67.2±11.2 | 0.568 | |
| Male: n (%) | 50 (20.4) | 51 (20.4) | 0.911 | |
| Hypertension: n (%) | 186 (74.4) | 186 (74.4) | 1.000 | |
| Diabetes: n (%) | 106 (42.4) | 93 (37.2) | 0.235 | |
| Dyslipidemia: n (%) | 177 (70.8) | 159 (63.6) | 0.086 | |
| Smoking: n (%) | 140 (56.0) | 148 (59.2) | 0.469 | |
| Body weight<50 kg: n (%) | 25 (10.0) | 22 (8.8) | 0.646 | |
| eGFR: mL/min/1.73m2 | 73.5±23.1 | 73.5±23.1 | 0.428 | |
| Underlying disease: n (%) | 0.207 | |||
| Chronic CAD | 134 (53.6) | 148 (59.2) | ||
| ACS | 116 (46.4) | 102 (40.8) | ||
| Affected vessels: n (%) | 0.230 | |||
| Single vessel disease | 175 (70.0) | 187 (74.8) | ||
| Multi-vessel disease | 75 (30.0) | 63 (25.2) | ||
| Syntax score | 14.0±9.3 | 12.1±8.0 | 0.013 | |
| Approach: n (%) | 0.720 | |||
| Radial | 130 (52.0) | 134 (53.6) | ||
| Femoral | 120 (48.0) | 116 (46.4) | ||
| PCI for LAD: n (%) | 122 (48.8) | 139 (55.6) | 0.128 | |
| DES: n (%) | 199 (79.6) | 128 (51.2) | <0.0001 | |
| Anticoagulants: n (%) | ||||
| Warfarin | 9 (3.6) | 11 (4.4) | 0.648 | |
| DOAC | 9 (3.6) | 13 (5.2) | 0.383 | |
eGFR: estimated glomerular filtration rate, CAD: coronary artery disease, ACS: acute coronary syndrome, PCI: percutaneous coronary intervention, LAD: left anterior descending artery, DES: drug-eluting stent, DOAC: direct oral anticoagulant
*Multi-vessel disease includes two-vessel, three-vessel and left main coronary artery diseases
Study endpoints in the prasugrel and clopidogrel groups
Regarding PCI-related bleeding complications as safety endpoints in the acute phase, the reduction in the blood hemoglobin level, incidence of hemoglobin reduction ≥3.0 g/dL, incidence of puncture site hematoma formation and incidence of blood transfusion were comparable between the two groups. However, the incidence of additional hemostatic treatment (11.6% vs. 6.0%, p=0.027) and the composite of PCI-related bleeding complications (22.4% vs. 13.2%, p=0.007) were significantly higher in the prasugrel group than in the clopidogrel group. The non-PCI-related bleeding complications during the 12 months after PCI were comparable between the two groups (Table 2).
Table 2.
Endpoint Analysis.
| Prasugrel group (n=250) |
Clopidogrel group (n=250) |
p value | ||
|---|---|---|---|---|
| Safety endpoints | ||||
| PCI-related bleeding | ||||
| Hemoglobin reduction: g/dL | 1.14±1.07 | 1.01±0.99 | 0.266 | |
| Hemoglobin reduction ≥3.0 g/dL: n (%) | 13 (5.2) | 11 (4.4) | 0.676 | |
| Additional hemostasis: n (%) | 29 (11.6) | 15 (6.0) | 0.027 | |
| Hematoma formation: n (%) | 16 (6.4) | 10 (4.0) | 0.243 | |
| Blood transfusion: n (%) | 5 (2.0) | 3 (1.2) | 0.475 | |
| Composite of PCI-related bleeding: n (%) | 56 (22.4) | 33 (13.2) | 0.007 | |
| Non-PCI-related bleeding | ||||
| TIMI major bleeding: n (%) | 1 (0.4) | 1 (0.4) | 1.000 | |
| TIMI minor bleeding: n (%) | 0 (0.0) | 0 (0.0) | 1.000 | |
| Clinically relevant minimal bleeding: n (%) | 6 (2.4) | 3 (1.2) | 0.312 | |
| Efficacy endpoint | ||||
| All-cause death: n (%) | 3 (1.2) | 0 (0.0) | 0.082 | |
| MACE: n (%) | 4 (1.6) | 8 (3.2) | 0.243 | |
| MACE+revascularization: (%) | 12 (4.0) | 26 (10.4) | 0.018 | |
| Heart failure requiring hospitalization: n (%) | 3 (1.2) | 5 (2.0) | 0.402 | |
PCI: percutaneous coronary intervention, TIMI: thrombolysis in myocardial infarction, MACE: major adverse cardiovascular events
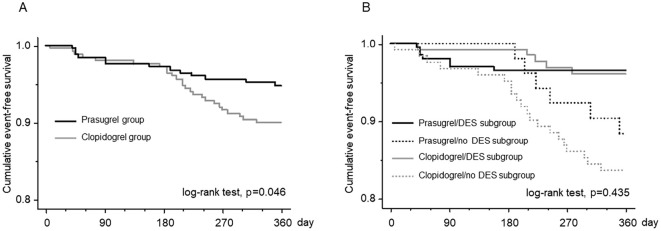
Regarding the efficacy endpoints, all-cause death was seen in 3 patients (1.2%) in the prasugrel group, whereas there were no deaths in the clopidogrel group (p=0.082). The cause of death in the three prasugrel patients was ventricular fibrillation in one and pneumonia in the remaining two. The incidence of MACEs was similar between the two groups. However, the incidence of MACEs plus coronary revascularization was significantly lower in the prasugrel group than in the clopidogrel group (4.0% vs. 10.4%, p=0.018). The incidence of heart failure requiring hospitalization was comparable between the two groups. A Kaplan-Meier survival analysis showed that the cumulative incidence of MACEs was similar between the two groups (log-rank test; p=0.561) (Fig. 1). However, the cumulative incidence of MACE plus coronary revascularization was significantly lower in the prasugrel group than in the clopidogrel group (log-rank test; p=0.046) (Fig. 2A).
Figure 1.
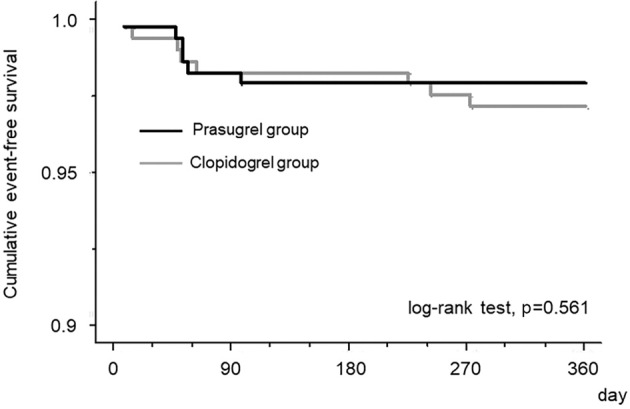
A comparison of the cumulative incidence of major cardiovascular events (MACEs) between the prasugrel and clopidogrel groups by a Kaplan-Meier survival analysis. The incidence was similar between the two groups.
Figure 2.
(A) A comparison of the cumulative incidence of MACEs plus coronary revascularization between the prasugrel and clopidogrel groups by a Kaplan-Meier survival analysis. The incidence was significantly lower in the prasugrel group than in the clopidogrel group. (B) A comparison of the cumulative incidence of MACEs plus coronary revascularization among the following four subgroups: prasugrel/DES, prasugrel/no DES, clopidogrel/DES, and clopidogrel/no DES. Although there were no significant differences among the four subgroups, the incidence appeared to be lower in patients with DES usage than in those without it. In the patients with DES usage, the incidence of MACEs plus coronary revascularization was similar between the prasugrel and clopidogrel subgroups; however, in the patients without DES usage, the incidence appeared to be lower in the prasugrel subgroup than in the clopidogrel subgroup. MACEs: major adverse cardiovascular events, DES: drug-eluting stent, PCI: percutaneous coronary intervention
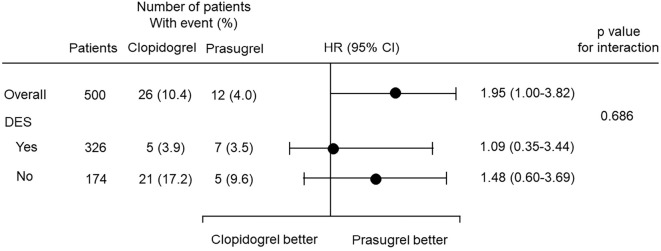
Since the rate of DES usage was significantly higher in the prasugrel group than in the clopidogrel group, this confounding factor might have affected the incidence of MACE plus coronary revascularization. We therefore performed a Kaplan-Meier survival analysis to compare the cumulative incidence of MACEs plus coronary revascularization among the following 4 subgroups: patients in the prasugrel group with DES usage (prasugrel/DES subgroup), those in the prasugrel group without DES usage (prasugrel/no DES subgroup), those in the clopidogrel group with DES usage (clopidogrel/DES subgroup) and those in the prasugrel group without DES usage (clopidogrel/no DES subgroup). Although there were no significant differences among the four subgroups, the incidence of MACEs plus coronary revascularization appeared to be lower in patients with DES usage than in those without it. In the patients with DES usage, the incidence of MACEs plus coronary revascularization appeared to be similar between the prasugrel and clopidogrel subgroups; however, in the patients without DES usage, the incidence appeared to be lower in the prasugrel subgroup than in the clopidogrel subgroup (Fig. 2B). We next performed a Cox proportional hazards regression analysis to compare prasugrel and clopidogrel in the abovementioned subgroups based on the DES usage. This analysis showed that there was no significant interaction between the subgroup factor (DES usage) and the efficacy endpoint (MACEs plus coronary revascularization; Fig. 3).
Figure 3.
A Cox proportional hazards regression analysis comparing the efficacy of prasugrel and clopidogrel in prespecified subgroups based on DES usage. There was no significant interaction between the subgroup factor (DES usage) and the efficacy endpoint (MACEs plus coronary revascularization).
The analysis of the bleeding risk in the prasugrel group
We performed a logistic regression analyses to assess the PCI-related bleeding risk in the 250 patients in the prasugrel group. For this analysis, the dependent variable was a composite of PCI-related bleeding complications that included hemoglobin reduction ≥3.0 g/dL, puncture site hematoma formation, additional hemostatic treatment and the need for blood transfusion. The independent variables included the following factors possibly related to bleeding risk: age, gender, low body weight (<50 kg), hypertension, acute coronary syndrome, estimated glomerular filtration rate (eGFR), DES usage and oral anticoagulant usage. A univariate analysis showed that acute coronary syndrome tended to be associated with a lower incidence of PCI-related bleeding complications (p=0.071). According to a multivariate analysis that included only independent variables with p<0.3 in the univariate analysis (age, eGFR, anticoagulant usage and acute coronary syndrome), acute coronary syndrome tended to be negatively associated with the incidence of PCI-related bleeding complications (p=0.061) (Table 3).
Table 3.
Logistic Regression Analysis for Assessment of PCI-related Bleeding Risk in the Prasugrel Group.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Age | 1.02 (0.99-1.05) | 0.193 | 1.02 (0.99-1.06) | 0.181 | |
| Male gender | 0.71 (0.35-1.43) | 0.334 | |||
| Body weight <50 kg | 0.63 (0.21-1.93) | 0.422 | |||
| Hypertension | 0.73 (0.38-1.42) | 0.356 | |||
| Acute coronary syndrome | 0.57 (0.31-1.05) | 0.071 | 0.54 (0.29-1.03) | 0.061 | |
| eGFR | 0.99 (0.98-1.01) | 0.264 | 1.00 (0.99-1.02) | 0.964 | |
| Femoral approach | 1.46 (0.81-2.66) | 0.212 | 1.61 (0.87-2.98) | 0.132 | |
| DES usage | 1.49 (0.68-3.28) | 0.325 | |||
| Oral anticoagulant usage | 1.82 (0.65-5.09) | 0.254 | 1.64 (0.56-4.59) | 0.482 | |
The dependent variable was a composite of the incidence of hemoglobin reduction ≥3.0 g/dL, puncture site hematoma formation, additioinal hemostatic treatment and blood transfusion.
PCI: percutaneous coronary intervention, OR: odds ratio, CI: confidence interval, eGFR: estimated glomerular filtration tate, DES: drug-eluting stent
Discussion
In the present study, we compared the safety of prasugrel and clopidogrel based on a retrospective analysis of bleeding complications. The major finding of the present study was that the incidence of additional hemostatic treatment at the puncture site and the composite of PCI-related bleeding complications during the acute phase after PCI were higher in patients treated with prasugrel than in those treated with clopidogrel.
Clopidogrel is converted by the cytochrome P450 (CYP) enzyme system to an active antiplatelet metabolite that binds to the P2Y12 receptor. Since the transformation of clopidogrel into an active compound greatly depends on the CYP enzymes, the CYP2C19 loss-of-function alleles affect the responsiveness of platelets to clopidogrel (12, 13). Like clopidogrel, prasugrel is also an inactive prodrug that requires metabolic processing in vivo to generate an active antiplatelet metabolite that binds to the P2Y12 receptor. However, the enzymatic generation of the active metabolite of prasugrel depends less heavily on the CYP enzyme system than clopidogrel, so prasugrel is less influenced than clopidogrel by CYP2C19 gene polymorphisms. Thus, the more rapid onset, higher potency and lower inter-individual variability of the antiplatelet effects of prasugrel compared with clopidogrel in vivo are due to the more efficient pharmacokinetics of prasugrel (12, 14). These pharmacokinetic characteristics of prasugrel offer an advantage over clopidogrel for the prevention of thrombotic events, although the use of prasugrel may increase the bleeding risk during the acute phase after PCI.
A global clinical trial, the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction (TRITON-TIMI 38 study), showed that there was a lower incidence of ischemic cardiovascular events but a higher incidence of bleeding events with prasugrel than clopidogrel in patients with acute coronary syndrome (9). In contrast, a Japanese trial, Prasugrel Compared with Clopidogrel for Japanese Patients with Acute Coronary Syndrome Undergoing PCI (PRASFIT-ACS) demonstrated that the incidence of bleeding complications was comparable between prasugrel and clopidogrel in acute coronary syndrome patients, although the incidence of ischemic events was lower with prasugrel (10). The Prasugrel for Japanese Patients with Coronary Artery Diseases Undergoing Elective PCI (PRASFIT-Elective) study also showed that prasugrel reduced ischemic events compared with clopidogrel, but bleeding complications were similar in chronic coronary artery disease patients undergoing elective PCI (11). The discrepancy in the incidence of bleeding events between the TRITON-TIMI 38 trial and PRASFIT trial might be due to the difference in the dose of prasugrel (loading dose/maintenance dose: 60/10 mg in the TRITON-TIMI 38 trial and 20/3.75 mg in the PRASFIT trial). Furthermore, both of those trials mainly focused on mid-term (6-15 months) bleeding events, not on those during the acute phase after PCI.
In a post-hoc analyses of the PRASFIT-ACS study, Nishikawa et al. (15) assessed the platelet reactivity, defined as the P2Y12 reaction unit (PRU) (VerifyNowⓇ P2Y12 assay) or the vasodilator-stimulated phosphoprotein phosphorylation reactivity index (VASP-PRI). Their results showed that neither PRU nor VASP-PRI during the acute phase after PCI (5-12 hours) or under steady-state conditions (4 weeks) was associated with either the acute (day 0 to 3) or chronic (day 4 to 12 months) risk of bleeding. In the present study, in which the dose of prasugrel was equivalent to that used in the PRASFIT trial, non-PCI-related mid-term (up to 12 months) bleeding complications, based on the TIMI bleeding criteria, were comparable in the prasugrel and clopidogrel groups, which was similar to the results of the PRASFIT trial. However, PCI-related bleeding complications during the acute phase after PCI were more frequent in the prasugrel group than in the clopidogrel group. This result might be attributable to the stronger antiplatelet action of prasugrel compared with clopidogrel and it also conflicts with the findings of a previous report by Nishikawa et al.(15).
In the present study, we also compared the efficacy of prasugrel and clopidogrel on the incidence of ischemic events using a Kaplan-Meyer survival analysis. Contrary to the results of several previously reported prospective randomized studies (9-11), our retrospective analysis found that the cumulative incidence of MACEs was similar between the two groups. However, the cumulative incidence of MACEs plus coronary revascularization was lower in the prasugrel group than in the clopidogrel group. Although age- and gender-matched patient selection was performed for both groups, the severity of coronary artery disease as demonstrated by the syntax score was higher in the prasugrel group than in the clopidogrel group, possibly leading to the significantly higher rate of DES usage in the prasugrel group. As this background might have affected the results, we performed a Kaplan-Meier survival analysis to compare the four subgroups (prasugrel/DES, prasugrel/no DES, clopidogrel/DES and clopidogrel/no DES). As a result, the incidence of MACEs plus coronary revascularization tended to be lower in the prasugrel/no DES group than in the clopidogrel/no DES group, suggesting some advantage of prasugrel over clopidogrel in cases of PCI without DES implantation. In addition, we performed a Cox proportional hazards regression analysis using the prespecified subgroups based on DES usage. This analysis showed that there were no significant interactions between the subgroup factor (DES usage) and the efficacy endpoint (MACEs plus revascularization). Thus, the lower incidence of MACEs plus coronary revascularization in the prasugrel group appears to be independent of DES usage. Since the very-long-term safety and efficacy of the current DES may be affected by impaired wound healing at the stent site (16-18), PCI without DES implantation should be reevaluated. We believe our results may have some value for the selection of post-PCI antiplatelet regimens in the future. Nevertheless, we cannot conclude an advantage of prasugrel over clopidogrel in terms of its efficacy, since MACEs alone were similar between the prasugrel and clopidogrel groups.
Finally, we evaluated the factors associated with PCI-related bleeding complications in the prasugrel group. Contrary to our expectations, both univariate and multivariate logistic regression analyses indicated that acute coronary syndrome tended to be negatively associated with the incidence of PCI-related bleeding complications. This result might be due to differences in the timing of prasugrel administration between patients with acute coronary syndrome and those with chronic coronary artery disease. The maintenance dose of prasugrel was started at least 96 hours before elective PCI in chronic coronary artery disease patients in order to achieve blood concentrations of the drug that were sufficient to inhibit the platelet function immediately after PCI. In contrast, in acute coronary syndrome patients, prasugrel loading was not started until after the patients had left the cardiac catheterization laboratory, so an effective blood concentration was not achieved immediately after PCI. The subsequently delayed effect of prasugrel in the acute coronary syndrome patients might have reduced PCI-related bleeding complications. Furthermore, other established risk factors for bleeding complications in patients on antiplatelet drugs, such as older age, hypertension, renal dysfunction and a low body weight (19, 20), were not associated with PCI-related bleeding complications.
In the present study, we focused on PCI-related bleeding complications during the acute phase after PCI in patients treated with either prasugrel or clopidogrel. These complications may not be directly life-threatening or associated with the long-term prognosis, but they can impair the quality of life and increase anxiety in both patients and physicians. These complications should therefore be avoided as much as possible. Our study suggests that prasugrel has an advantage over clopidogrel in terms of its efficacy when it is used as the post-PCI antiplatelet regimen, in line with the results of previous reports. However, prasugrel should be used cautiously because of the associated increased risk of short-term PCI-related bleeding complications.
Limitations
This study has several potential limitations. First, it was a single-center, retrospective, observational study in a small number of patients. In addition, the fact that the choice of PCI strategy, such as DES usage, was left to the discretion of the operator may also have been a limitation. Second, the observation periods (based on the date of PCI) differed between the prasugrel and clopidogrel groups. The severity of coronary artery disease was therefore higher in the prasugrel group than in the clopidogrel group, which may have resulted in the usage of DES being higher in the prasugrel group than in the clopidogrel group. Although we adjusted our analyses to account for differences in DES usage, other confounding factors might have affected the results. Indeed, the proportions of patients with diabetes, acute coronary syndrome, multi-vessel disease and syntax score were higher in the prasugrel group than in the clopidogrel group. A specified method, such as a propensity score matching analysis, would have been more appropriate in the present study; however, the sample size was likely too small in our case for stringent propensity score matching. Third, dual anti-platelet therapy was continued in the patients selected for this study in principle at least until the time of follow-up coronary angiography or until major bleeding complications developed. However, we did not include the detailed data concerning the continuation of anti-platelet agents in the analyses. Therefore, precise re-analyses including such data are needed. Fourth, sample size determination was not performed in the present study; thus, a relatively small number of events might have contributed to the lack of significant differences between the two groups. Fifth, the PCI-related bleeding complications that we selected as safety endpoints included a reduction in the blood hemoglobin level, the incidence of hemoglobin reduction ≥3.0 g/dL, the incidence of hematoma formation at the puncture sites, the incidence of additional hemostatic treatment and the incidence of blood transfusion. However, the diagnosis of hematoma and the performance of additional hemostatic treatment were based on each physician's discretion; thus, the incidence of these complications was considerably biased. We should explore more adequate methods of evaluating the PCI-related bleeding complications. Finally, we did not assess CYP2C19 gene polymorphisms, which might affect the pharmacokinetics of clopidogrel and the magnitude of the differences between the prasugrel and clopidogrel groups. In addition to these limitations, there are several other potential weaknesses in the present study. Nevertheless, we believe the results of our study still carry some value as real-world clinical practice data.
Conclusion
Although prasugrel and clopidogrel may have similar efficacy for preventing cardiovascular events as post-PCI antiplatelet regimens, prasugrel should be used cautiously in this setting because of the risk of short-term PCI-related bleeding complications.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Ayumi Matsunuma, Clinical Research Coordinator in the Department of Cardiovascular Medicine, Dokkyo Medical University, for her efforts in data acquisition and technical support. We also thank Kiyosoh Yamagata for his assistance with the statistical analyses.
References
- 1.2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124: e574-e651, 2011. [DOI] [PubMed] [Google Scholar]
- 2.2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 35: 2541-2619, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Sadanandan S, Singh IM. Clopidogrel: the data, the experience, and the controversies. Am J Cardiovasc Drugs 12: 361-374, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Mao L, Jian C, Changzhi L, et al. . Cytochrome CYP2C19 polymorphism and risk of adverse clinical events in clopidogrel-treated patients: a meta-analysis based on 23,035 subjects. Arch Cardiovasc Dis 106: 517-527, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Niitsu Y, Jakubowski JA, Sugidachi A, Asai F. Pharmacology of CS-747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin Thromb Hemost 31: 184-194, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Brandt JT, Payne CD, Wiviott SD, et al. . A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. Am Heart J 153: e9-e16, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Jernberg T, Payne CD, Winters KJ, et al. . Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 27: 1166-1173, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Wiviott SD, Trenk D, Frelinger AL, et al. . Prasugrel compared to high loading and maintenance dose clopidogrel in patients with planned percutaneous coronary intervention: the PRINCIPLE-TIMI 44 trial. Circulation 116: 2923-2932, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, Braunwald E, McCabe CH, et al. . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357: 2001-2015, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Saito S, Isshiki T, Kimura T, et al. . Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J 78: 1684-1692, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Isshiki T, Kimura T, Ogawa H, et al. . Prasugrel, a third-generation P2Y12 receptor antagonist, in patients with coronary artery disease undergoing elective percutaneous coronary intervention. Circ J 78: 2926-2934, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol 50: 126-142, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Jiang M, You JH. Review of pharmacoeconomic evaluation of genotype-guided antiplatelet therapy. Expert Opin Pharmacother 16: 771-779, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Schrör K, Siller-Matula JM, Huber K. Pharmacokinetic basis of the antiplatelet action of prasugrel. Fundam Clin Pharmacol 26: 39-46, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa M, Isshiki T, Kimura T, et al. . No association between on-treatment platelet reactivity and bleeding events following percutaneous coronary intervention and antiplatelet therapy: a post hoc analysis. Thromb Res 136: 947-954, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Sata M, Hikichi Y, et al. . Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation: impact on restenosis. Circulation 115: 553-561, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular inflammation and repair: implication for reendothelialization, restenosis, and stent thrombosis. JACC Cardiovasc Interv 4: 1057-1166, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakuma M, Nasuno T, Abe S, et al. . Mobilization of progenitor cells and assessment of vessel healing after second generation drug-eluting stenting by optical coherence tomography. Int J Cardiol Heart Vasc 18: 17-24, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biondi-Zoccai G, Abbate A, D'Ascenzo F, Lotrionte M, Modena MG. Prasugrel during primary percutaneous coronary intervention: evidence from clinical data. Curr Vasc Pharmacol 10: 454-457, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Roe MT, Goodman SG, Ohman EM, et al. . Elderly patients with acute coronary syndromes managed without revascularization: insights into the safety of long-term dual antiplatelet therapy with reduced-dose prasugrel versus standard-dose clopidogrel. Circulation 128: 823-833, 2013. [DOI] [PubMed] [Google Scholar]