Abstract
A 62-year-old man presented to our hospital for the further evaluation and treatment of his back pain, general fatigue, and dyspnea, which had developed 4 days after the 29th administration of nivolumab to treat his lung cancer. Based on his clinical history, elevated serum cardiac enzyme values, and cardiac magnetic resonance (CMR) imaging and myocardial biopsy findings, he was diagnosed with myocarditis induced by nivolumab. Corticosteroid therapy improved his condition, and CMR performed on hospital day 11 also showed remarkable improvement. Although nivolumab-induced myocarditis is rare, cardiologists should consider it when encountering patients treated with such a drug for malignant disease.
Keywords: cardiac magnetic resonance imaging, corticosteroid, lung cancer, myocarditis, nivolumab
Introduction
Nivolumab is an immune checkpoint inhibitor (ICI) that has become a new treatment option for malignant tumors. While initially proven effective for melanoma, the diseases for which this agent is administered have been expanding. However, a number of immune-related adverse events (ir-AEs), which are rarely seen in conventional chemotherapy, have been reported with this drug, and as the frequency of its use increases, there are concerns that the number of side effects will also increase.
We experienced a case of myocarditis induced by nivolumab. To our knowledge, only 13 such cases have been reported (1-12). In this case, the onset was delayed until one year after the introduction of nivolumab, and cardiac magnetic resonance imaging (CMR) findings were useful for its differentiation from viral myocarditis. We report this case and review the literature.
Case Report
A 62-year-old man with an unresectable lung adenocarcinoma of clinical stage cT1bN2M0 of the Eighth Lung Cancer Stage Classification (13) diagnosed in August 2013 presented to Saitama Cardiovascular and Respiratory Center complaining of back pain, chest discomfort, general fatigue, and dyspnea in September 2018 and was admitted for a further evaluation. Because of mild fibrosis for which the possibility of interstitial lung diseases on CT could not be ruled out, he had been receiving chemotherapy without radiation therapy since the diagnosis of adenocarcinoma. Since September 2013, he had undergone chemotherapy with cisplatin plus pemetrexed followed by maintenance chemotherapy with pemetrexed and docetaxel. In May 2017, because the contralateral mediastinal lymph node was enlarged, recurrence was diagnosed, and nivolumab was started biweekly. The swollen lymph nodes decreased in size, and no abnormal shadows were noted, so he was considered to be in complete remission.
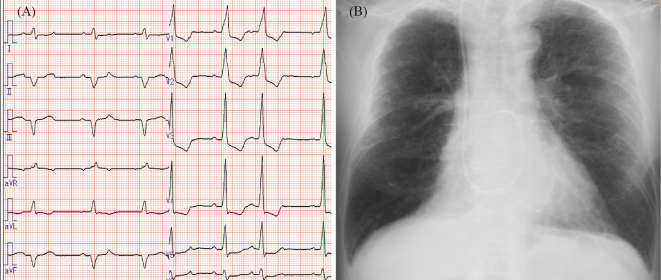
In November 2017, decreased FT3 and FT4 values and an elevated thyroid-stimulating hormone value indicating hypothyroidism were found, but there were no clinical symptoms, and we followed him without treatment. In July 2018, he developed general fatigue, and we considered the symptoms due to hypothyroidism and stopped the nivolumab. By August 2018, his general fatigue had improved, and we re-started the nivolumab. However, in September 2018, 4 days after the 29th administration of nivolumab, he developed back pain, chest discomfort, general fatigue, and dyspnea. His vital signs were as follows: blood pressure of 92/62 mmHg, heart rate of 85 beats/min, and respiratory rate of 20/min. Chest auscultation revealed an irregular heartbeat without murmurs or rales. Pitting edema was found in his lower extremities. The electrocardiogram showed wide QRS waves (Fig. 1A). A transthoracic cardiac ultrasound examination showed an attenuated left ventricular ejection fraction of 45% compared with that of 70% in February 2014 and a decrease in the left ventricular wall motion in the posterior, inferior, and lateral walls. Laboratory data showed white blood cells of 6,200/mm3, hemoglobin of 13.1 g/dL, platelets of 205,000/mm3, serum creatine kinase value of 970 IU/L (normal 56 to 244), and increases in creatine kinase-myocardial band to 78 ng/mL (normal <5 ng/mL) and cardiac troponin T to 4.81 ng/mL (normal <0.1 ng/mL). The C-reactive protein value was also increased to 2.06 mg/dL. Autoantibodies, including antinuclear antibodies, anti-Acl-70 antibodies, anti-ARS antibodies, anti-ds DNA antibodies, anti-RNP antibodies, anti-SS-A/Ro, and anti-SS-B/La antibodies, were all negative. BNP was increased to 466 pg/mL, and chest X-ray showed cardiac enlargement with right-sided pleural effusion (Fig. 1B).
Figure 1.
Electrocardiogram and chest X-ray findings on admission. The electrocardiogram showed irregular and wide QRS waves (A). Chest X-ray showed cardiomegaly and right-sided pleural effusion (B).
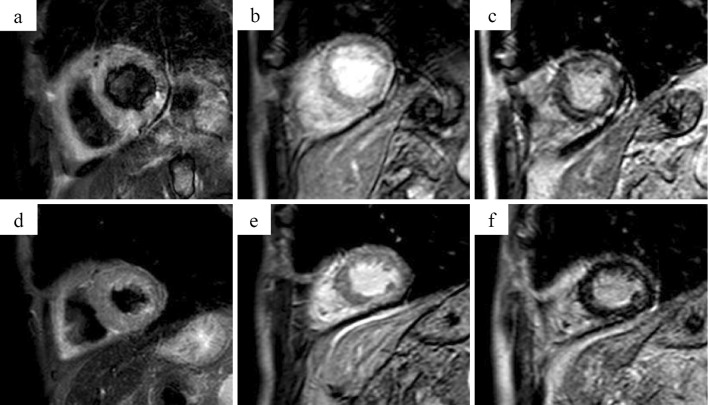
We initially suspected acute myocardial infarction, and a Swan-Ganz catheter was inserted to assist in management in the intensive care unit. The pulmonary artery pressure [systolic/diastolic (mean)] was 28/19 (23) mmHg. Emergency coronary angiography was performed, but no significant stenosis of the coronary arteries was found. We also performed various CMR examinations (Fig. 2). Cine images showed diffuse moderately reduced wall motion abnormality with mild wall hypertrophy, and T2-weighted short-tau inversion recovery (STIR) black-blood (BB) (T2w-STIR-BB) imaging showed diffuse high signal intensity (SI) equal to or greater than the spleen SI. Early gadolinium-enhanced (EGE) imaging showed diffuse hyper-enhancement of the myocardium, and late gadolinium-enhanced imaging showed diffuse patchy enhancement. Given the above findings, we diagnosed him with myocarditis with diffuse myocardial edema.
Figure 2.
Cardiac magnetic resonance imaging. Day 1, T2-weighted short-tau inversion recovery (STIR) black-blood (BB) (T2w-STIR-BB) MRI showed diffuse high signal intensity (SI) equal to or greater than the spleen SI. (a) Day 1, early gadolinium-enhanced (EGE) imaging showed diffuse hyper-enhancement of the myocardium. (b) Day 1, late gadolinium-enhanced (LGE) imaging showed diffuse patchy enhancement. (c) Given the above, myocarditis with diffuse myocardial edema was diagnosed. Day 11, T2w-STIR-BB (d) and EGE (e) images showed improvement of edematous findings, and late gadolinium enhancement (f) of the myocardium was decreased.
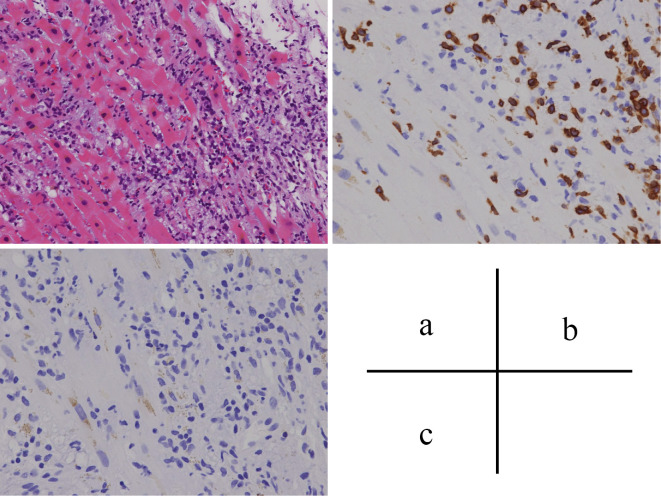
We performed a myocardial biopsy from the endocardial side of the left ventricle. The biopsy revealed fibrosis of the myocardial tissue, infiltration of inflammatory cells, and T cell-dominant lymphocyte infiltration, which were consistent with lymphocytic myocarditis (Fig. 3). Viral PCR testing using myocardial specimens for influenza virus, adenovirus, respiratory syncytial virus, corona virus, parainfluenza virus, bocavirus, echovirus, coxsackie virus echovirus, and enterovirus were all negative. Serum antibodies measured in the convalescent phase against echovirus, coxsackie virus, enterovirus, adenovirus, influenza virus, parainfluenza virus, and respiratory syncytial virus were not increased compared with those measured in the acute phase. We subsequently considered nivolumab-induced myocarditis. We also detected transient complete atrioventricular block during the myocardial biopsy and inserted a temporary pacemaker. Because of these new findings, we diagnosed the patient with nivolumab-induced myocarditis and started administration of methylprednisolone 1 g daily for 3 days.
Figure 3.
Histology of the myocardial biopsy specimen. Myocardial tissue fibrosis, inflammatory cell infiltration (a: Hematoxylin and Eosin staining), and T cell-dominant lymphocyte infiltration (b: immunohistochemical staining for CD3 cells) were found. Infiltration of B cells was minimal (c: immunohistochemical staining for CD20 cells).
From hospital day 4, the internal corticosteroid dose (prednisolone) was reduced to 60 mg daily (1 mg/kg). The patient's condition subsequently improved. His serum creatine kinase value decreased to the normal range within a week after admission. The ejection fraction measured by the transthoracic cardiac ultrasound examination performed on hospital day 9 improved to 55%, and asynergy of the cardiac wall also improved. Electrocardiography performed on hospital day 10 showed narrowing of the QRS. CMR imaging was performed on hospital day 11 and one month following the start of methylprednisolone administration. Cine images showed gradual improvement of the wall motion abnormality with recovery of the left ventricular ejection fraction. T2w-STIR-BB and EGE images showed improvement of edema findings, and late gadolinium enhancement of the myocardium was decreased.
The patient was discharged on hospital day 19. After discharge, the corticosteroid dose was gradually tapered (prednisolone 60 mg daily for 1 week, 50 mg daily for 2 weeks, 40 mg daily for 2 weeks, and 30 mg daily thereafter), and he has been regularly followed up on an outpatient basis. He continues to take prednisolone 30 mg daily, and his myocarditis has not recurred.
Discussion
We herein report a case of nivolumab-induced myocarditis that developed one year after its administration. A cardiac muscle biopsy specimen showed T cell-dominant lymphocyte infiltration, which was successfully improved by corticosteroid therapy.
As an ICI, nivolumab is a novel remedy for malignant tumors that promotes the attack of cancer cells by lymphocytes by suppressing the expression of PD-1 on the cell surface of cancer cells. Nivolumab has transformed the treatment of several cancers by releasing restrained antitumor immune responses (14). Treatment with nivolumab is known to cause several ir-AEs, including thyroid dysfunction, colitis, pneumonitis, and rarely, type 1 diabetes, Guillain-Barré syndrome, and myasthenia gravis. However, the frequency of myocarditis is 0.06% (8), whereas the frequencies of myocarditis due to other ICIs, such as pembrolizumab and atezolizumab, in Japan are reported to be 3 in 4,259 patients (0.07%) (15) and 2 in 2,800 patients (0.07%) (16), respectively. Although its probability of occurrence is not high, myocarditis is an ir-AE that cannot be ignored due to the possibility of a fatal outcome. To our knowledge, only 13 cases of myocarditis induced by nivolumab have been reported (Table). Among these 13 cases, the patients' ages ranged from 49 to 80 years, and 9 men were included. Malignancies for which nivolumab was administered included melanoma in eight cases, lung cancer in four cases, and glioblastoma in one case. Development of nivolumab-induced myocarditis occurred after the initial administration of nivolumab in five cases, after the second administration in three cases, after the third administration in three cases, after the ninth administration in one case, and after the tenth administration in one case. Our patient developed myocarditis one year after the initiation of nivolumab but during the third readministration after a two-month period of drug cessation due to hypothyroidism. As our case indicates, nivolumab-induced myocarditis can develop even one year after its administration. Thus, clinicians should consider nivolumab as a cause of myocarditis even if one year has passed from its introduction.
Table.
Characteristics of Reported Cases of Nivolumab-induced Myocarditis.
| Age/Sex | Primary disease | Duration from initiation of nivolumab to onset of myocarditis | Treatment | Outcome | Reference |
|---|---|---|---|---|---|
| 75/M | Lung cancer | 3 days after the ninth administration | Prednisolone (1 mg/kg/day) | Alive | 1 |
| 64/F | Glioblastoma | 8 days after the second administration | Mycophenolate (1 g twice daily) | Alive | 2 |
| 68/F | Lung cancer | One week after the second administration | Mythylprednisolone (250 mg/day) | Died | 3 |
| 69/M | Melanoma | Two weeks after the third administration | Prednisolone (2 mg/kg/day) | Alive | 4 |
| 72/M | Melanoma | After the tenth administration | Prednisolone (1 mg/kg/day) | Alive | 5 |
| 49/M | Melanoma | Two weeks after the initial administration | Prednisolone (10 mg/day) | Alive | 6 |
| 68/M | Melanoma | Two weeks after the initial administration | Methylprednisolone (1 mg/kg/day) | Alive | 7 |
| 65/F | Melanoma | 12 days after the initial administration | Methylperdnisolone (2 mg/kg/day) | Died | 8 |
| 63/M | Melanoma | 15 days after the initial administration | Methylprednisolone (1,000 mg/day) plus infliximab (5 mg/kg/day) | Died | 8 |
| 80/M | Melanoma | Two weeks after the initial administration | Methylprednisolone (1,000 mg/day) +plus IVIg (400 mg/kg/day) | Alive | 9 |
| 72/M | Melanoma | After the third administration | Prednisolone(1 mg/kg/day) | Alive Effective | 10 |
| 55/M | Lung cancer | 21 days after the second administration | Unknown | Died | 11 |
| 69/F | Lung cancer | One week after the third administration | Methylprednisolone (1,000 mg/day) | Alive | 12 |
| 62/M | Lung cancer | 4 days after the third administration | Methylprednisolone (1,000 mg/day) | Alive | Our case |
M: male, F: female, IVIg: intravenous immunogloburin
Nine of the 13 patients underwent a myocardial biopsy or autopsy (2, 4-6, 8-12). All patients had myocardial tissue fibrosis, inflammatory cell infiltration, and T cell-dominant lymphocyte infiltration, findings that were also found in our patient. From previous reports, the characteristic histological finding of nivolumab-induced myocarditis is T-cell inflammation. Four potential mechanisms of ir-AE have been proposed (17). First, ICIs (monoclonal antibodies) binding directly to cell surface proteins, such as cytotoxic T lymphocyte antigen 4 (CTLA4), which is expressed on normal tissues, can cause T-cell infiltration and injury to tissues due to complement mediation. Second, circulating T cells that recognize antigens expressed by tumor cells can identify either the same tumor antigen or a homologous tissue antigen in healthy tissue. ICI therapy that inhibits programmed cell death protein 1 or CTLA4 can stimulate recognition of or binding of T cells to antigens expressed by non-target organs. Third, there is some evidence to suggest that inhibiting immune checkpoint molecules can increase circulating cytokine levels in affected tissues, thus helping inflammatory molecules infiltrate non-target tissues. Fourth, antibodies administered against ICIs may increase autoantibody levels against target organs or stimulate new autoantibody formation.
CMR was performed in 5 of the 13 cases and showed varied patterns, including diffuse contrast effect, local contrast effect, and normal findings (1, 5-7, 10). The further accumulation of CMR findings of nivolumab-induced myocarditis is needed in order to better determine the characteristic findings of such cases. However, CMR was useful in our case for ruling out viral myocarditis as a cause because viral myocarditis usually affects the epicardium, as reflected by its hematogenous spread (18), whereas our patient showed a diffuse myocardial distribution. CMR may be useful for differentiating viral myocarditis from other disease entities.
Considering the mechanism underlying the side effects of ICIs as described above, steroids are often administered to counteract serious ir-AEs (16). In our patient, in addition to heart failure, complete atrioventricular block developed, and it was necessary to administer a steroid. Among the 13 cases reported so far, none of the patients underwent observation without treatment. The recently published clinical practice guidelines state that all ICI patients complicated with myocarditis should be treated with corticosteroids regardless, of the grade of cardiac toxicities (19). The therapeutic options in the 13 reported cases include prednisolone in 5, methylprednisolone in 4, mycophenolate mofetil in 1, methylprednisolone and infliximab in 1, methylprednisolone and intravenous anti-thymus immunoglobulin in 1, and no mention of therapy in 1 case. Corticosteroid therapy seems effective in most cases, but deaths in patients treated with steroid therapy have also been reported (3). One patient with recurrence of myocarditis after nivolumab readministration eventually died; we therefore consider that nivolumab should not be readministered in such cases (5).
In conclusion, we experienced a case of nivolumab-induced myocarditis successfully treated with steroid therapy. We evaluated the CMR findings in the acute phase and after discharge. Although reports of nivolumab-induced myocarditis are limited, myocarditis is a life-threatening adverse event, and clinicians should consider it in the differential diagnosis when it is encountered in patients treated with nivolumab.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank our colleagues at the Department of Cardiology, Saitama Cardiovascular and Respiratory Center for their detailed comments
References
- 1.Semper H, Muehlberg F, Schulz-Menger J, Allewelt M, Grohe C. Drug-induced myocarditis after nivolumab treatment in a patient with PDL1- negative squamous cell carcinoma of the lung. Lung Cancer 99: 117-119, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Tay RY, Blackley E, McLean C, et al. . Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer 117: 921-924, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson R, Delaune J, Szady A, Markham M. Suspected autoimmune myocarditis and cardiac conduction abnormalities with nivolumab therapy for non-small cell lung cancer. BMJ Case Rep 2016: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tadokoro T, Keshino E, Makiyama A, et al. . Acute lymphocytic myocarditis with anti-PD-1 antibody nivolumab. Circ Heart Fail 9: 2016. [DOI] [PubMed] [Google Scholar]
- 5.Tajmir-Riahi A, Bergmann T, Schmid M, Agaimy A, Schuler G, Heinzerling L. Life-threatening autoimmune cardiomyopathy reproducibly induced in a patient by checkpoint inhibitor therapy. J Immunother 41: 35-38, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Norwood TG, Westbrook BC, Johnson DB, et al. . Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer 5: 91, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tawbi HA, Forsyth PA, Algazi A, et al. . Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 379: 722-730, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson DB, Balko JM, Compton ML, et al. . Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375: 1749-1755, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura T, Fukushima S, Miyashita A, et al. . Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci 107: 1055-1058, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinzerling L, Ott PA, Hodi FS, et al. . Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 4: 50, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matson DR, Accola MA, Rehrauer WM, Corliss RF. Fatal myocarditis following treatment with the PD-1 inhibitor nivolumab. J Forensic Sci 63: 954-957, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Fukasawa Y, Sasaki K, Natsume M, et al. . Nivolumab-induced myocarditis concomitant with myasthenia gravis. Case Rep Oncol 10: 809-812, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest 151: 193-203, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Wolchok JD. PD-1 Blockers. Cell 162: 937, 2015. [DOI] [PubMed] [Google Scholar]
- 15. KEYTRUDAⓇ Interview Form ver9 [Internet]. [cited 2019 Jan. 29]. Available from: http://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/4291435A1(in Japanese)
- 16. Chugai Pharmaceutical. TECENTRIQⓇ Intravenous Infusion 1200 mg - Unresectable, advanced or recurrent non-small cell lung cancer - Report of adverse drug reactions collected in early post-marketing phase vigilance, 2019 January.
- 17.Varricchi G, Galdiero MR, Marone G, et al. . Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2: e000247, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahrholdt H, Goedecke C, Wagner A, et al. . Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 109: 1250-1258, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Brahmer JR, Lacchetti C, Schneider BJ, et al. . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36: 1714-1768, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]