Abstract
Objective
Since healthcare providers face an increased risk of hepatitis B virus (HBV) infection because of their work, vaccination plays a critical role in preventing HBV transmission. However, the duration for which acquired HBV surface antibodies (anti-HBs) persist remains unknown. To evaluate the primary immunologic response to HBV vaccination and its persistence in healthy Japanese adolescents.
Methods
In total, 690 young adults underwent HBV vaccination with a three-dose schedule. The primary response was determined by the anti-HBs titers at 1-2 months after the final dosage. Subjects with anti-HBs titers of <10, 10-100, and >100 mIU/mL were classified as “non-responders,” “low-responders,” and “sufficient responders,” respectively. Anti-HB titers were re-measured at 1 or 2 years after vaccination.
Results
First, 95.8% and 72.8% of the subjects had anti-HBs titers of >10 and >100 mIU/mL, respectively, as a primary response. The anti-HBs titers measured at 1 and 2 years after vaccination were significantly correlated with those of the primary response (1 year: r=0.893, p<0.0001; 2 years: r=0.902, p<0.001). Most subjects with a titer of >100 mIU/mL at the primary response maintained an anti-HBs titer of >10 mIU/mL [1 year after vaccination, 208/209 (99.5%); 2 years after vaccination, 72/81 (90.1%)]. However, in subjects with a primary response of 10-100 mIU/mL the anti-HBs titer frequently declined; 17/38 (44.7%) and 9/10 (90.0%) subjects had a titer of <10 mIU/mL at 1 and 2 years, respectively.
Conclusion
The primary response was associated with the anti-HBs titers at 1 and 2 years after vaccination, and the anti-HBs titers of 54.2% of the low responders were not maintained for 2 years, even if they were vaccinated as healthy young adults.
Keywords: HBV vaccination, HBs antibody
Introduction
Hepatitis B virus (HBV) infection causes chronic hepatitis and can result in hepatocellular carcinoma even in the absence of liver cirrhosis (1). Due to the high endemicity of HBV in East Asia, there is a need to focus on the prevention of HBV transmission (2). HBV infection is preventable if a potential host has sufficient HBV surface antibodies (anti-HBs); hence, HBV vaccination is necessary.
Recently, guidelines established by the American College of Physicians and the Centers for Disease Control and Prevention recommend HBV vaccination for subjects at a high risk of HBV transmission (3). In this guideline, healthcare providers are classified as adults at risk because they face an increased risk of HBV infection due to their work (they have a risk of percutaneous or mucosal exposure to blood).
According to the Centers for Disease Control and Prevention (CDC) a post-vaccination anti-HBs titer ≥10 mIU/mL is associated with hepatitis B immunity (4). Immunocompetent persons have long-term protection against HBV and do not require further periodic testing to assess their anti-HBs levels. However, one study reported the development of acute hepatitis B in vaccinated subjects (5). Another study reported that subjects with anti-HBs titers of >10 mIU/mL were subsequently infected with HBV (6). Thus, an insufficient anti-HBs titer may not prevent HBV transmission.
Previous studies have reported that the response to HBV vaccination varies among subjects (7, 8). Because the duration for which anti-HBs titers are sustained in subjects who receive HBV vaccination, the primary response rate of subjects who underwent HBV vaccination and the persistence of the primary response were evaluated in this study.
Materials and Methods
Subjects
Six hundred ninety subjects of 18-25 years of age at Iwate Medical University and Tsukuba University were prospectively enrolled in this study. The age of subjects (rounded down to the year) was recorded. Informed consent was obtained from all subjects, and the Institutional Review Boards of both Iwate Medical University (IRB approval #HG H25-8) and Tsukuba University (IRB approval #230) approved all study procedures.
All 690 subjects were confirmed to be HBV core antibody (anti-HBc)-negative. They received three doses of HBV vaccination (Bimmugen, Kaketsuken, Kumamoto, Japan) by subcutaneous injection according to the following schedule: an initial dose and subsequent doses at 1-2 months and 6-12 months after the initial dose. The serum anti-HB titer was measured at 1-2 months after the final injection; this titer value was defined as the “primary response” to HBV vaccination. The subjects were classified, based on the anti-HBs titer in the primary response, into the following three groups: non-responders (<10 mIU/mL), low responders (10-100 mIU/mL), and sufficient responders (>100 mIU/mL). Forty-three volunteers in non- or low responders received a booster vaccination. Of the original 690 subjects, 280 continued to be part of the study 1 year after its commencement (including 33 who received a booster vaccination), and 101 continued to be part of the study 2 years after its commencement (including 10 who received a booster vaccination) (Fig. 1). To evaluate the decrease in anti-HBs titers over time, we re-measured the serum anti-HBs titers at 1 and 2 years after vaccination in subjects who were not lost to follow-up.
Figure 1.
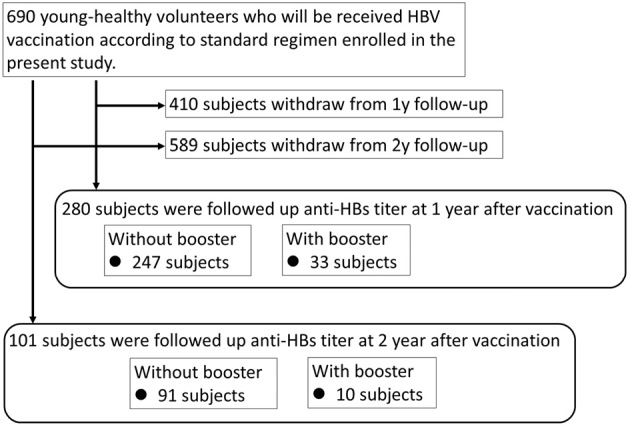
Flow chart of the subjects in the present study.
Laboratory data
Serum anti-HBs titers were measured using a chemiluminescent immunoassay (Architect, Abbott Japan, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using the SPSS software program (version 17.0, IBM, Chicago, USA). The results were expressed as means and standard deviations. Non-parametric tests (Kruskal-Wallis and Friedman tests, followed by Dunn's multiple comparisons) were used to evaluate the statistical significance of the results. The prevalence of subjects with anti-HB titers of <10 mIU/mL at 1 or 2 years after vaccination was analyzed using Fisher's exact test. Two-sided p values of <0.05 were considered to indicate statistical significance. Spearman's correlation test was used to assess the statistical significance of correlations. A linear regression analysis was performed as a multivariate analysis.
Results
Primary responses to HBV vaccination
All subjects were confirmed to have tested negative for the HBs antigen. Prior to HBV vaccination, 3 of 693 subjects (0.43%) were positive for anti-HBc antibodies (Supplementary material). To evaluate the primary response to HBV vaccination, the anti-HB titers of 690 study subjects were measured. No subjects experienced any side-effects associated with the vaccination. The age distribution of the subjects in the present study is presented in Fig. 2A (mean age: 21.6 years; range 19-39 years; standard deviation, 3.0 years). Based on these measurements, the classifications of the subjects were as follows: non-responders, n=29 (4.2%); low responders, n=159 (23.0%); and sufficient responders, n=502 (72.8%). The mean ages of the subjects in each of these groups were as follows: non-responders, 23.5 years; low responders, 22.7 years; and sufficient responders, 21.1 years (Fig. 2B and Table 1). The sufficient responders were significantly younger in comparison to both low responders and non-responders.
Figure 2.
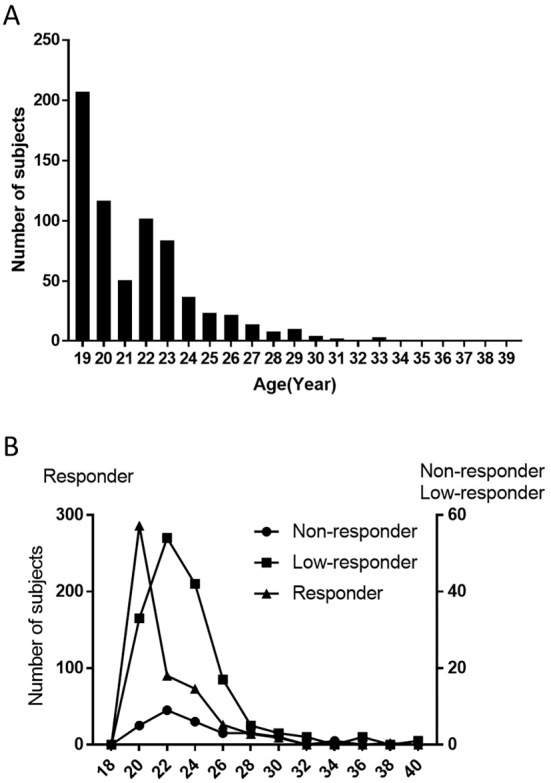
Age distribution of the subjects in the present study. The vertical axis shows the total number of subjects (A) and the subjects classified by the vaccination response (B). The horizontal axis shows the age of the subjects. (B) Circles, squares and triangles indicate non-responders, low-responders and responders, respectively.
Table 1.
Characteristics of Study Subjects, Stratified by Primary Response to HBV Vaccination.
| Non-responders | Low-responders | Responders | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Age, years (mean±SD) |
n | Age, years (mean±SD) |
n | Age, years (mean±SD) |
||||
| Total | 29 | 23.5±0.66 | 159 | 22.7±0.25 | 502 | 21.1±0.12 | *, ** | ||
| Male | 21 | 23.9±0.82 | 101 | 23.2±0.31 | 204 | 22.0±0.24 | *, ** | ||
| Female | 8 | 22.8±1.13 | 58 | 21.7±0.39 | 298 | 20.4±0.11 | ** | ||
*p<0.05, Non-responders vs. Responders; **p<0.05, Low-responders vs. Responders.
HBV: hepatitis B, SD: standard deviation
Spontaneous decrease in anti-HB titers at 1 and 2 years after HBV vaccination
To confirm the spontaneous decreases in anti-HB titers, we re-measured anti-HB titers in 247 and 91 subjects who did not receive a booster HBV vaccination at 1 and 2 years after completing the initial vaccination schedule, respectively (Fig. 3A and B). Overall, the mean anti-HB titers at 1 and 2 years after vaccination were lower than the mean primary response titers (1 year vs. primary response: 339 mIU/mL vs. 1,382 mIU/mL; 2 years vs. primary response: 159 mIU/mL vs. 1,292 mIU/mL). During the observation period, 18 of the 247 (7.3%) and 17 of the 91 (18.7%) subjects showed anti-HB titers of <10 mIU/mL at 1 and 2 years after vaccination, respectively.
Figure 3.
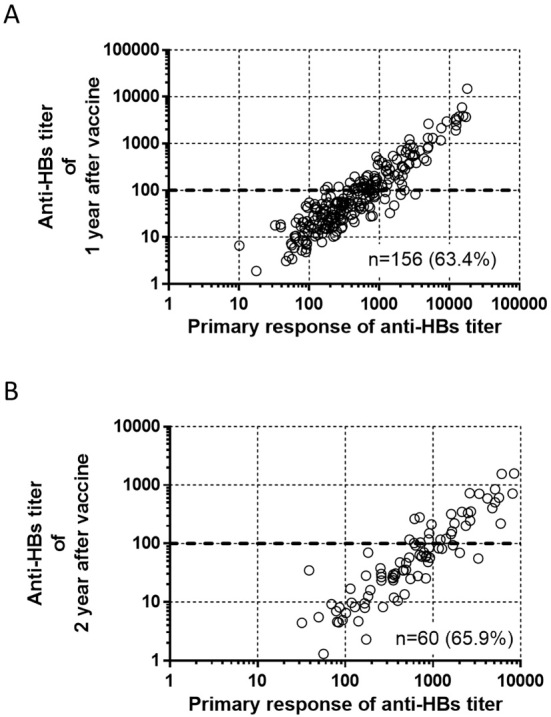
Correlation between the primary response and the anti-HB titer at 1 and 2 years after vaccination. The vertical axis shows the anti-HB titers at (A) 1 year and (B) 2 years after vaccination. The horizontal axis shows the primary response (anti-HB titer) to vaccination. Spearman’s correlation coefficient was used to assess the correlation between the anti-HB titer and the primary response at both time periods; both values were statistically significant: (A) r=0.893, p<0.0001; (B) r=0.902, p<0.001.
The correlation between the anti-HBs titer at the primary response and the anti-HBs titers at 1 and 2 years after vaccination
We evaluated correlations between the anti-HBs titers at 1 and 2 years after vaccination and the anti-HBs titer at the primary response (Fig. 3). The anti-HBs titers at 1 and 2 years after vaccination were significantly correlated with the anti-HBs titer at the primary response (1 year: r=0.893, p<0.0001; and 2 years: r=0.902, p<0.001). Age, sex, and primary response were included in a multivariate linear regression analysis to identify factors associated with anti-HBs titers at 1 and 2 years after vaccination; the primary response was the only significant factor (Table 2).
Table 2.
Multiple Regression Analysis of Anti-HBs Titers One- and Two- Years Post-vaccination.
| Anti-HBs titer one-year post-vaccination | ||||
|---|---|---|---|---|
| t value | β | p value | 95% confidence interval | |
| Primary response | 22.55 | 0.34 | p<0.05 | 0.31-0.37 |
| Age | 0.37 | n.s. | ||
| Sex | 1.80 | n.s. | ||
| Anti-HBs titer two-years post-vaccination | ||||
| t value | β | p value | 95% confidence interval | |
| Primary response | 14.00 | 0.13 | p<0.05 | 0.12-0.15 |
| Age | -0.14 | n.s. | ||
| Sex | 0.21 | n.s. | ||
n.s.: not significant
A low anti-HB titer in the primary response is a risk factor for negative anti-HBs at 1 and 2 years after HBV vaccination
To ascertain the association between the primary response to vaccination and a negative anti-HBs titer at 1 or 2 years after vaccination, the prevalence of subjects with anti-HBs titers of <10 mIU/mL at 1 or 2 years after vaccination was confirmed among subjects whose anti-HBs titers at the primary response were 10-100 mIU/mL and >100 mIU/mL (Table 3).
Table 3.
Low Anti-HBs Titers at 1 or 2 Years Post-vaccination was Associated with Primary Response.
| <10 mIU/mL | ||
|---|---|---|
| Primary response | 1 year post-vaccination | 2 years post-vaccination |
| 10-100 | 17/38 (44.7%)* | 9/10 (90.0%)* |
| 100< | 1/209 (0.5%) | 8/81 (9.9%) |
*p<0.05, anti-HBs titer of 10-100 mIU/mL vs. anti-HBs titer >100 mIU/mL
The anti-HBs titers of subjects with a titer of >100 mIU/mL at the primary response were maintained: 208/209 (99.5%) and 72/81 (90.1%) showed titers of >10 mIU/mL at 1 and 2 years after vaccination, respectively. In contrast, the anti-HBs titers of subjects with titers of 10-100 mIU/mL at the primary response frequently declined; 17/38 (44.7%) and 9/10 (90.0%) showed titers of <10 mIU/mL at 1 and 2 years, respectively. In total, the anti-HBs titers declined within 2 years after vaccination in 26 of the 48 (54.2%) subjects with titers of 10-100 mIU/mL at the primary response.
The immunological effect of booster vaccination in non-responders and low-responders
To confirm the efficacy of booster HBV vaccination in non-responders and low-responders, we evaluated 33 subjects at 1 year after booster vaccination and 10 subjects at 2 years after booster vaccination. Although the anti-HBs titer increased significantly after booster vaccination, this response was not sustained (Fig. 4).
Figure 4.
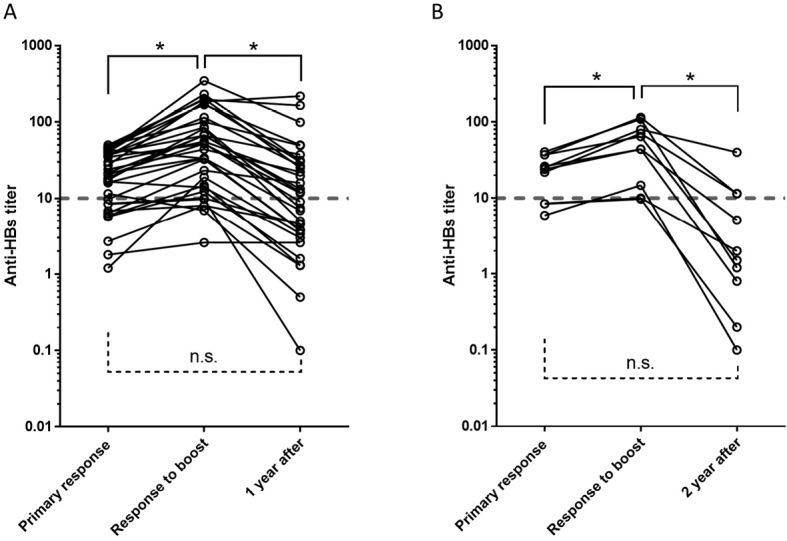
Serial changes in the anti-HB titers of subjects who received a booster vaccination. The vertical axis shows the change in anti-HB titer over time. The horizontal axis shows the indicated time points at which the anti-HB titer was measured at (A) 1 year and (B) 2 years after vaccination. Statistical significance was evaluated using the Friedman test. P values of <0.05 were considered to indicate statistical significance. n.s.: not significant
Discussion
The present study revealed the following four findings: i) 4.2% and 23.0% of the subjects showed anti-HBs titers of <10 mIU/mL and 10-100 mIU/mL, at 1-2 months after primary HBV vaccination, respectively; ii) the primary response predicted the anti-HBs titer at 1 and 2 years after completing the three-dose vaccination schedule; iii) the anti-HBs titers of 54.2% of the primary low responders (anti-HB titers 10-100 mIU/mL) declined within 2 years after vaccination; and iv) a sufficient anti-HBs titer could not be maintained at 1 or 2 years, even after a booster vaccination.
Globally, approximately 240 million people have chronic HBV infection, and >686,000 people die from complications of HBV infection each year (9-11). The increase in the serum anti-HB titer and immune responses with memory B cells after HBV vaccination will generally reduce the risk of HBV transmission and reduce the number of HBV carriers. Thus, universal HBV vaccination protects the general population without post-vaccination measurement of anti-HBs titers. However, subjects who are frequently at risk of exposure to HBV, such as healthcare providers, face an increased risk of HBV infection (3). Thus, follow-up strategies may need to be established for subjects at high risk of HBV transmission. Whether or not the serum anti-HB titer is maintained at a protective level remains to be clarified.
Some reports have demonstrated that insufficient anti-HB titer levels enable HBV transmission (5, 6). Tosti et al. reported that properly vaccinated subjects can develop acute HBV hepatitis (5). Stramer et al. demonstrated that even subjects with anti-HBs of >10 mIU/mL can be infected with HBV (6). Furthermore, Sadlier et al. indicated that hepatitis B vaccine responders, who acquired anti-HBs titers of 35 mIU/mL from genotype A, D HBV vaccines developed chronic hepatitis B infection with genotype F HBV (12). These findings suggest that an anti-HBs titer of >10 mIU/mL is not protective in some cases, especially when the HBV genotype differs from the vaccine-genotypes. Maintaining a sufficient anti-HBs titer for a prolonged period is important for individuals at increased risk of HBV infection, such as healthcare providers. Indeed, the Green Book (published in the UK) recommends that subjects with an anti-HBs titer of <100 mIU/mL should receive booster vaccination to maintain a serum anti-HBs titer that is sufficient to provide protection against HBV transmission (13). In this study, 27.2% of the subjects showed insufficient anti-HB titers (<100 mIU/mL). Importantly, the anti-HBs titers at the primary response were correlated with those at 1 and 2 years after vaccination (Fig. 3). Furthermore, an anti-HBs titer of 10-100 mIU/mL at the primary response was a risk factor for an anti-HBs titer of <10 mIU/mL at 1 and 2 years after vaccination (Table 3). Previous studies have also reported that subjects with a sufficient primary response maintain a high anti-HBs titer long after vaccination (7, 14). Furthermore, other studies have reported that anti-HBs titers chronologically decreased during observation after vaccination (7, 15-17). These results suggest that subjects with a low anti-HBs titer at the primary response should be carefully followed up.
Although the anti-HBs titers after booster vaccination were followed up in small number of the subjects in this study, 17 of the 33 subjects who received a booster vaccine showed decreased titers of <10 mIU/mL at 1 year after the initial vaccination (Fig. 4A). Furthermore, 7 of the 10 subjects who received a booster vaccination showed decreased titers of <10 mIU/mL at 2 years after the initial vaccination (Fig. 4B). Although previous reports have described the effectiveness of booster vaccination for maintaining sufficient anti-HBs titers, the subjects included in those studies may not have been non-responders to HBV vaccination (18). In this study, low-responders or non-responders did not maintain sufficient anti-HBs titers after booster vaccination. Based on the results of this study, the anti-HBs titers of low-responders to HBV vaccination should be strictly monitored and regulated to provide effective protection against HBV transmission because their titers rapidly decrease to insufficient levels. Considering that the anti-HBs titers of low-responders and non-responders rapidly declined after booster vaccination, the follow-up schedule to monitor and regulate anti-HBs titers among low-responders and non-responders with a high risk of HBV transmission should be different from the schedule for responders with a high risk of HBV transmission and that for the general population who do not have a high risk of exposure to HBV. The mean age of low-responders and non-responders was significantly higher in comparison to responders (Table 1 and Fig. 2B). Although several factors have been suggested to be associated with the response to HBV vaccination (8, 19-22), age is also reported to be associated with the response to HBV vaccination (23, 24). With the exception of age, none of these factors were evaluated in the present study. Thus, we are not able to evaluate relationship between the response to HBV vaccination and other factors.
The timely prediction of insufficient anti-HBs titers among subjects with a high risk of exposure to HBV improves economic efficiency while screening subjects who require booster vaccination. In this study, the primary response was useful for predicting the anti-HBs titers at 1 and 2 years after vaccination. According to this result, we propose that the follow-up schedule be changed to monitor and regulate anti-HBs titers based on the primary response of each subject.
This study was associated with several limitations. First, the influence of the age-related response to vaccination remains unclear. Because the study subjects were recruited from a university, they were all young. Thus, the vaccination response of older adults and elderly individuals remains unclear; however, previous studies have reported that the response to HBV vaccination tends to decrease at higher ages (23, 24). To resolve this problem, further studies involving older subjects are warranted. Surprisingly, however, even subjects who were vaccinated at a young age showed lower anti-HBs titers at 2 years after vaccination. Second, the number of subjects who withdrew from this observation study was high. Because the subjects are healthy, some subjects refused to undergo additional blood examinations and withdrew from observation study. Third, we were not able to exclude the possibility that the immunity of the subjects was naturally boosted via HBV exposure in the subjects. There was only one subject whose anti-HBs titer at 2 years after the initial vaccination was increased in comparison to the anti-HBs titers produced during the primary response (Fig. 4B). This result suggested that - in some subjects whose anti-HBs titers experienced a natural boost due to HBV exposure - we might have overestimated the duration for which the anti-HBs antibody response was sustained. Since the prevalence of previous HBV infection among our study subjects was low, we considered the possibility that their anti-HBs titers were naturally boosted by HBV exposure to be quite low. Finally, the mechanism underlying the prolonged serum anti-HBs titers remains unclear. Because we focused on the rapid decrease in the anti-HBs titer to <10 mIU/mL, the observation period in this study was only 2 years. To determine how long after HBV vaccination an anti-HBs titer of >10 mIU/mL is maintained, further studies should be performed to prospectively observe the serial changes in anti-HBs titers of subjects who receive HBV vaccination.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported by a grant from the Ministry of Health, Labor and Welfare of Japan for Research on Hepatitis and BSE.
Supplementary Material
Characteristics of subjects with positive anti HBc antibody
Acknowledgement
We thank Koko Motodate for providing excellent secretarial support.
References
- 1.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 49: S45-S55, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Locarnini S, Hatzakis A, Chen DS, Lok A. Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs. J Hepatol 62: S76-S86, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Abara WE, Qaseem A, Schillie S, et al. . Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med 167: 794-804, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Schillie S, Murphy TV, Sawyer M, et al. . CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 62: 1-19, 2013. [PubMed] [Google Scholar]
- 5.Tosti ME, Alfonsi V, Lacorte E, et al. . Acute hepatitis B after the implementation of universal vaccination in Italy: results from 22 years of surveillance (1993-2014). Clin Infect Dis 62: 1412-1418, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Stramer SL, Wend U, Candotti D, et al. . Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med 364: 236-247, 2011. [DOI] [PubMed] [Google Scholar]
- 7.McMahon BJ, Dentinger CM, Bruden D, et al. . Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis 200: 1390-1396, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Sakai A, Noguchi E, Fukushima T, et al. . Identification of amino acids in antigen-binding site of class II HLA proteins independently associated with hepatitis B vaccine response. Vaccine 35: 703-710, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 30: 2212-2219, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Mortality GBD, Causes of Death C Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385: 117-171, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 11: 97-107, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Sadlier C, Madden K, O'Gorman S, Crowley B, Bergin C. Development of chronic hepatitis B infection in a hepatitis B vaccine responder. Int J STD AIDS 28: 526-528, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Hepatitis B: the green book, chapter 18 [Intrernet]. [cited 2018 Jan. 12]. Available from: https://www.gov.uk/government/publications/hepatitis-b-the-green-book-chapter-18
- 14.Yoshioka N, Deguchi M, Hagiya H, et al. . Durability of immunity by hepatitis B vaccine in Japanese health care workers depends on primary response titers and durations. Plos One 12: e0187661, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Der Meeren O, Behre U, Crasta P. Immunity to hepatitis B persists in adolescents 15-16 years of age vaccinated in infancy with three doses of hepatitis B vaccine. Vaccine 34: 2745-2749, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Behre U, Bleckmann G, Crasta PD, et al. . Long-term anti-HBs antibody persistence and immune memory in children and adolescents who received routine childhood hepatitis B vaccination. Hum Vaccin Immunother 8: 813-818, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Whittle H, Jaffar S, Wansbrough M, et al. . Observational study of vaccine efficacy 14 years after trial of hepatitis B vaccination in Gambian children. BMJ 325: 569, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan PKS, Ngai KLK, Lao TT, et al. . Response to booster doses of hepatitis B vaccine among young adults who had received neonatal vaccination. Plos One 9, 2014(in English). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rave S, Heijtink RA, Bakker-Bendik M, Boot J, Schalm SW. Immunogenicity of standard and low dose vaccination using yeast-derived recombinant hepatitis B surface antigen in elderly volunteers. Vaccine 12: 532-534, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Denis F, Mounier M, Hessel L, et al. . Hepatitis-B vaccination in the elderly. J Infect Dis 149: 1019, 1984. [DOI] [PubMed] [Google Scholar]
- 21.Egea E, Iglesias A, Salazar M, et al. . The cellular basis for lack of antibody response to hepatitis B vaccine in humans. J Exp Med 173: 531-538, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godkin A, Davenport M, Hill AV. Molecular analysis of HLA class II associations with hepatitis B virus clearance and vaccine nonresponsiveness. Hepatology 41: 1383-1390, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis 35: 1368-1375, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Mast EE, Weinbaum CM, Fiore AE, et al. . A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 55: 1-33, quiz CE31-34, 2006. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of subjects with positive anti HBc antibody


