Abstract
Background
Vancomycin therapeutic drug monitoring (TDM) is based on achieving 24-h area under the concentration–time curve to minimum inhibitory concentration cure breakpoints (AUC24/MIC). Approaches to vancomycin TDM vary, with no head-to-head randomized controlled trial (RCT) comparisons to date.
Objectives
We aimed to compare clinical and pharmacokinetic outcomes between peak–trough-based and trough-only-based vancomycin TDM approaches and to determine the relationship between vancomycin AUC24/MIC and cure rates.
Methods
A multicentered pragmatic parallel-group RCT was conducted in Hamad Medical Corporation hospitals in Qatar. Adult non-dialysis patients initiated on vancomycin were randomized to peak–trough-based or trough-only-based vancomycin TDM. Primary endpoints included vancomycin AUC24/MIC ratio breakpoint for cure and clinical effectiveness (therapeutic cure vs therapeutic failure). Descriptive, inferential, and classification and regression tree (CART) statistical analyses were applied. NONMEM.v.7.3 was used to conduct population pharmacokinetic analyses and AUC24 calculations.
Results
Sixty-five patients were enrolled [trough-only-based-TDM (n = 35) and peak–trough-based-TDM (n = 30)]. Peak–trough-based TDM was significantly associated with higher therapeutic cure rates compared to trough-only-based TDM [76.7% vs 48.6%; p value = 0.02]. No statistically significant differences were observed for all-cause mortality, neutropenia, or nephrotoxicity between the two groups. Compared to trough-only-based TDM, peak–trough-based TDM was associated with less vancomycin total daily doses by 12.05 mg/kg/day (p value = 0.027). CART identified creatinine clearance (CLCR), AUC24/MIC, and TDM approach as significant determinants of therapeutic outcomes. All patients [n = 19,100%] with CLCR ≤ 7.85 L/h, AUC24/MIC ≤ 1256, who received peak–trough-based TDM achieved therapeutic cure. AUC24/MIC > 565 was identified to be correlated with cure in trough-only-based TDM recipients [n = 11,84.6%]. No minimum AUC24/MIC breakpoint was detected by CART in the peak–trough-based group.
Conclusion
Maintenance of target vancomycin exposures and implementation of peak–trough-based vancomycin TDM may improve vancomycin-associated cure rates. Larger scale RCTs are warranted to confirm these findings.
Key points
| Compared to trough-based vancomycin TDM, peak–trough-based vancomycin TDM was associated with a higher clinical success rate and less vancomycin dose requirements. |
| Maintaining AUC24/MIC between 565 and 1256 has been associated with cure. |
Introduction
Therapeutic drug monitoring (TDM) of vancomycin is essential in ensuring the attainment of positive clinical outcomes and minimizing toxicity [1]. Vancomycin clinical pharmacokinetic parameters exhibit large inter-individual variability even with identical dosing regimens [2]. Traditionally, steady-state vancomycin peak (Cmax,ss) and trough (Ctrough,ss) concentrations were measured for vancomycin TDM. In 2009, a paradigm shift in clinical vancomycin dosing and monitoring practices emerged, following the release of a consensus guideline jointly by the American Society of Health-System Pharmacists (ASHP), the Society of Infectious Diseases Pharmacists, and the Infectious Disease Society of America (IDSA) [3]. Based on limited clinical data and animal studies, vancomycin was considered ‘concentration-independent’ and thus Cmax,ss monitoring was no longer recommended. Additionally, a 24-h area under the concentration–time curve (AUC24) to minimum inhibitory concentration (MIC) ratio (AUC24/MIC) of ≥ 400 was defined as the target surrogate to attain clinical effectiveness. These guidelines recommended Ctrough,ss monitoring as a surrogate for achieving AUC24/MIC ≥ 400 with a value of 15–20 mg/L as an acceptable therapeutic range.
Published evidence after 2009 called into question the recommended target ratio (AUC24/MIC ≥ 400) [4], as different AUC/MIC ratios have been found to achieve clinical effectiveness [5–7]. This reported variability in AUC/MIC breakpoints may be attributed to the genetic variability between methicillin-resistant Staphylococcus aureus (MRSA) strains across different geographical areas [8–18], the differences in MRSA site of infection, and the variability of the populations studied in terms of comorbidities and ethnicities [19]. Hence, the generalizability of the published literature remains limited to different disease states, geographical regions, and populations. Furthermore, recent studies questioned the use of Ctrough,ss as an indicator of AUC24/MIC optimal exposure, as discrepancies between optimal AUC24 exposures and the associated trough concentrations have been reported [2, 20–24]. The superior clinical utility of multiple-concentration-based vancomycin dosing approaches compared to trough-only guided dosing has been suggested [21–23]. One of those approaches is peak–trough-based pharmacokinetic dosing [20, 23, 25]. Collectively, these studies raised concerns regarding the optimal vancomycin AUC24/MIC breakpoint for cure, and the best vancomycin TDM approach that would result in the attainment of the optimal AUC24/MIC ratio associated with clinical effectiveness.
The use of vancomycin in the treatment of serious Gram-positive infections has become very challenging in Asia, including the Middle East and North Africa (MENA) region [16, 26, 27]. A meta-analysis of 91 studies exploring the epidemiology of vancomycin intermediate-resistant S.aureus (VISA) strains during 1997 and 2014, reported higher VISA incidence rates in Asia, including MENA, compared to other regions [26]. Given that suboptimal antimicrobial therapy of MRSA contributes to the emergence of resistant strains [27], these findings highlight the potentially high prevalence of inadequate vancomycin treatment in MENA. This can be attributed to non-adherence to clinical practice guidelines or the limited generalizability of vancomycin AUC24/MIC targets reported elsewhere. Genetic and epidemiologic diversity between MRSA clones across various geographical regions and time-points have been reported worldwide, including MENA [12–18]. However, the currently applied vancomycin dosing nomograms in the MENA region are based on published Western vancomycin pharmacokinetic–pharmacodynamic targets, due to the lack of studies reporting MENA-specific targets. Expatriates from different MENA and Asian countries constitute the majority of Qatar’s population, with nationals reported to be < 15% [28, 29]. Therefore, we aimed to explore MENA-specific pharmacokinetic–pharmacodynamic vancomycin targets to understand the reasons for vancomycin treatment failures in the MENA region.
Although the 2009 consensus guidelines recommend trough-only monitoring [3], to the best of our knowledge, no prospective randomized controlled trials (RCTs) have been conducted to compare the clinical and pharmacokinetic outcomes between the traditional peak–trough-based and the trough-only-based vancomycin TDM approaches. Additionally, the vancomycin AUC24/MIC cure breakpoint in MENA-specific populations and bacterial strains has not yet been studied. Therefore, this prospective parallel-group pragmatic multicenter RCT was conducted to compare the clinical and pharmacokinetic outcomes of peak–trough-based and trough-only-based vancomycin TDM approaches and to evaluate the relationship between vancomycin AUC24/MIC ratios and cure in the MENA population.
Methods
Study Design and Setting
A multicenter pragmatic two parallel-group RCT was conducted in three tertiary care hospitals under Hamad Medical Corporation (HMC) in Qatar—Al-Wakrah Hospital (AWH), Al-Khor Hospital (AKH), and Hamad General Hospital (HGH).
Study Population and Sample Size Calculation
Inclusion criteria included hospitalized adults (≥18 years) with suspected/confirmed staphylococcal or other Gram-positive infection requiring treatment with vancomycin for at least 3 days based on the attending physician’s judgment. Exclusion criteria included renal instability [abrupt absolute increase in serum creatinine (SCr) ≥ 0.5 mg/dL from baseline or a percentage increase in SCr ≥ 50% within 48 h]; end-stage renal disease; transplant; immunosuppression; active malignancy; receiving antineoplastic agents; HIV or absolute neutrophil counts < 1000 cells/mm3; vancomycin allergy; history of recurrent resistant peritonitis; administration of < 4 doses of vancomycin or for < 72 h; vancomycin administration for post-surgical infection prophylaxis; pregnancy; subjects not able to undergo blood sampling per clinician judgment; anuria (urine output < 100 mL/day); symptomatic anemia; and hemoglobin < 8 g/dL.
Sample size was calculated a priori to be 120 subjects (60 subjects per arm) [30]. The primary endpoint used for sample size calculation was clinical effectiveness (therapeutic cure). Based on the meta-analysis of Ye et al., we assumed 85% cure rates in the peak–trough-based vancomycin TDM arm versus 60% cure rates in the control arm [31]. An attrition rate of 20%, a significance level of 5%, and a power of 80% were considered in the a priori power analysis [30]. Given the unexpectedly slow recruitment rate, interim analysis was conducted at 7 months. Statistically significant differences in the primary study outcome of clinical effectiveness were achieved. Thus, the study was ended after the enrollment of 65 subjects since a significant difference was detected in the primary study outcome.
Randomization and Blinding
Participants who provided informed consent and fulfilled the eligibility criteria were randomly assigned to one of the two study groups—(1) peak–trough (intervention) group or (2) trough-only (control) group. An allocation ratio of 1:1 was applied using a computer-generated list of random numbers. Due to the pragmatic nature of this trial, blinding was not possible since the treating clinical team needed to apply the dose change recommendations after justification. Thus, the method used for dose adjustment was revealed as part of justification, when requested by the attending physician.
Study Interventions
All subjects were initiated on vancomycin initial/empiric doses by the attending physician prior to enrollment in the study [3, 32–35]. Initiation or discontinuation of vancomycin treatment was the sole decision of the treating primary team and was not influenced by the study investigators. This trial was pragmatic in nature; thus, subjects were treated as part of routine care. No co-medications, medical procedures, dietary restrictions or restrictions to participation in other concurrent research were applied.
In the two study arms, target Ctrough,ss was as per the recommendations of the HMC institutional guidelines and the clinical practice guidelines: > 10 mg/L for less serious infections such as skin and soft tissue infections (SSTIs); and 15–20 mg/L for complicated infections such as bacteremia, infective endocarditis, osteomyelitis, meningitis, hospital-acquired pneumonia, and serious SSTI (e.g., necrotizing fasciitis) caused by S. aureus [3]. Creatinine clearance (CLCR) was calculated using the Cockcroft–Gault equation [36]. In patients with declined renal function or at risk of nephrotoxicity, the lower end Ctrough,ss targets were used. In the intervention arm, target Cmax,ss was 20–40 mg/L [1], stratified according to the patient’s renal function. In patients with normal CLCR, Cmax,ss ≥ 30–35 mg/L was targeted. Otherwise, a lower Cmax,ss was used, considering clinical judgment and dosing feasibility. This range was used with the objective of sufficient infected tissue penetration while preventing adverse drug reactions (ADRs), by accounting for possible declined renal function and other nephrotoxicity risk factors [1].
Blood Specimen Collection for Initial Vancomycin Concentrations
In both study arms, five initial vancomycin blood samples were collected through venipuncture. Routine vancomycin trough concentrations were collected 30 min before the fourth dose (Ctrough,ss1). For the study purpose, four vancomycin blood samples (10 mL of blood for each) were obtained at 1–2 h post fourth dose infusion (Cmax,ss), 30 min before the fifth dose (Ctrough,ss2), and two concentrations in between the peak and trough concentrations (C1, C2) after the fourth dose (i.e., at steady state). For instance, if the patient was receiving a 12-hourly vancomycin regimen, C1 and C2 were drawn at 4 h and 8 h post fourth dose infusion. On the other hand, if the patient was taking an 8-hourly regimen, C1 and C2 were drawn at 4 h and 6 h post fourth dose infusion.
Biochemistry and Microbiology Specimen Analysis
Vancomycin blood specimens were collected and analyzed at HMC biochemistry laboratories using particle-enhanced turbidimetric inhibition immunoassay (PETINA) [37]. Specimens from HGH and AKH were analyzed using the Architect c16000 (Abbott, USA) analyzer [38], while specimens from AWH were analyzed using the UniCel® DxC 600 (Beckman Coulter, USA) analyzer [39]. To determine vancomycin susceptibilities, microbiology cultures were processed using the broth microdilution test technique (BD Phoenix AP, USA) [40, 41]. Our institution uses the vancomycin MRSA susceptibly breakpoint (≤ 2 mg/L) set out by the Clinical and Laboratory Standards Institute (CLSI). For definitive sensitive cultures, the institutional laboratories reported MICs as ‘< 1 mg/L’ or ‘= 1 mg/L’. A survey of institutional vancomycin MIC of sensitive MRSA isolates collected between April 2015 and January 2016 showed that the MICs for most cultures were reported as 1 mg/L. Thus, we assumed an MIC of 1 mg/L for all specimens in this study.
Trough-Only-Based Vancomycin Dosing Adjustment
In the control arm, only trough concentrations were considered for dosing adjustments. If the target trough was not achieved, a new dose or a new dosing interval was calculated using trough-only linear method equations (Eqs. 1, 2) as below [1, 42, 43], where Ctrough,ss is the target new steady-state trough concentration; Ct is the current trough concentration; Dold is the old dose that resulted in Ct; τold is the old dosing interval that resulted in Ct; and τnew is the new dosing interval. Either the ‘dose-only change’ or the ‘dosing interval-only’ change equation was used based on clinical feasibility and practicality.
Dose-only change:
| 1 |
Dosing interval-only change:
| 2 |
Peak–Trough-Based Vancomycin Dosing Adjustment
Based on both the peak and trough vancomycin concentrations, a patient’s individualized pharmacokinetic parameters were calculated and used in dose adjustment calculations. If either the peak or trough or both concentrations were non-therapeutic, a new vancomycin dosing regimen was calculated and administered. Intravenous (IV) bolus equations (Eqs. 3–7) were used provided that the vancomycin infusion time was short relative to the patient-specific vancomycin half-life [1, 42]. If this assumption was not valid due to augmented renal clearance (ARC) or infusion durations > 1 h, IV intermittent infusion equations (Eqs. 8–13) were used as below [1, 42]; where Ke is the elimination rate constant; k0 is the infusion rate; t1/2 is the half-life; V is the volume of distribution; Cl is the clearance; D is the dose; C1 is the vancomycin concentration at time t1; C2 is the vancomycin concentration at time t2; Cp is the peak concentration; Ct is the trough concentration; Cmax,ss is the target steady-state peak concentration; Ctrough,ss is the target steady-state trough concentration; τ is the dosing interval; and t′ is the infusion duration.
IV bolus infusion equations:
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
IV intermittent infusion equations:
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
| 13 |
Post-Dose Adjustment Vancomycin Monitoring
After any dose adjustment, the time to new steady state was calculated and post-dose adjustment peak and trough vancomycin concentrations were measured. If measured vancomycin concentrations were not at target levels, additional dose adjustments were applied as discussed above. Otherwise, vancomycin peak and trough concentration monitoring continued every 24–48 h.
Study Endpoints
Primary Outcome Measures
The primary outcome measures of clinical effectiveness were (1) vancomycin AUC24/MIC cure breakpoint; (2) therapeutic cure (composite endpoint); and (3) therapeutic failure (composite endpoint). Therapeutic cure was defined as clinical cure and/or microbiologic cure [6, 44–46]. Clinical cure was defined as the absence of infection signs/symptoms without the need for additional antibiotic treatment. Microbiologic cure was defined as negative blood cultures at 5 days after vancomycin treatment initiation. Therapeutic failure included at least one of the following [6, 44–46]—clinical failure, microbiologic failure, premature discontinuation due to ADR or all-cause mortality. Clinical failure was defined as insufficient clinical response to initial vancomycin therapy necessitating antibiotic change. Microbiological failure was defined as a positive culture at ≥ 5 days after initiation of vancomycin treatment. Neutropenia was defined as absolute neutrophil counts < 1000/μL [47, 48]. Nephrotoxicity was defined as ‘a minimum of two or three consecutive SCr increases (defined as an increase of 0.5 mg/dL or at least 50% increase from baseline) after several days of vancomycin therapy’ [3]. All-cause mortality was defined as death from any cause during enrollment in the trial.
Secondary Outcome Measures
Secondary outcomes included (1) length of hospital stay (LOS); (2) number of dose adjustments required; (3) cumulative vancomycin doses received; and (4) duration of vancomycin treatment.
Statistical Analysis
Intention-to-treat analysis was applied. Descriptive and inferential statistics were conducted (SPSSv.23; IBM®, Armonk, NY, USA) to compare the differences in clinical outcomes between the intervention (i.e., peak–trough-based vancomycin TDM approach) and the control (i.e., trough-only-based vancomycin TDM approach) arms. Skewness test was applied to ensure normality of data (choice of parametric vs nonparametric tests). For comparison between the groups, Student’s t-test, Mann–Whitney U-test or chi-squared test was used as appropriate. All comparisons were carried out using an a priori significance level of 0.05 (two-sided tests). AUCs were calculated using the nonlinear mixed-effects population pharmacokinetics modeling approach (NONMEM v.7.3, ICON, USA) [49]. Classification and regression tree (CART) analysis was conducted using SPSS v.23 (IBM®; Armonk). AUC24/MIC, vancomycin cumulative doses, treatment duration, infected physiologic compartment, ethnicity, CLCR and TDM approach were tested against clinical effectiveness. To assess the predictive accuracy of the generated models, misclassification risk estimates with standard error were used [50].
Results
Baseline Characteristics of the Study Participants
Sixty-five subjects were enrolled (35 in the trough-only-based vancomycin TDM group and 30 in the peak–trough-based vancomycin TDM group). Baseline characteristics were similar between the study groups and are summarized in Table 1. Central nervous system infections (n = 15, 23.1%), lower respiratory tract infections (n = 16, 24.6%) and sepsis or septic shock (n = 11, 16.9%) were the most frequently occurring infections. Vancomycin was initiated as a definitive treatment in more than half of the cases (n = 35, 53.3%). Of the identified bacteria (n = 35), MRSA (n = 17, 48.6%), MSSA (n = 8, 22.9%), S. epidermidis (n = 5, 14.3%) and Enterococcus faecium (n = 4, 11.4%) constituted the most frequent positive microbiologic cultures. Approximately half of the study participants were critically ill and hospitalized in critical care units (n = 31, 47.7%). Physician-prescribed initial vancomycin dosing regimens were comparable between the study groups.
Table 1.
Baseline characteristics of the study participants
| Variable | Trough-monitoring group | Peak–trough-monitoring group |
|---|---|---|
| (n = 35) | (n = 30) | |
| Age (years), mean ± SD | 41.7 ± 19.56 | 42.4 ± 14.47 |
| BMI (kg/m2), median [IQR] | 26.7 [5.2] | 25.4 [7.8] |
| ABW (kg), median [IQR] | 73.1 [23.6] | 70 [19.3] |
| Height (cm), median [IQR] | 169 [13] | 168 [10.5] |
| Gender, n (%) | ||
| Male | 30 (85.7) | 22 (73.3) |
| Female | 5 (14.3) | 8 (26.7) |
| Ethnicity, n (%) | ||
| MENA | 23 (65.7) | 8 (26.7) |
| Asian (non-MENA) | 11 (31.4) | 20 (66.7) |
| African (non-MENA) | 1 (2.9) | 2 (6.7) |
| Hospitalization ward, n (%) | ||
| Intensive care unitsa | 13 (37.1) | 18 (60) |
| Burns unit | 2 (5.7) | 0 (0) |
| Medical ward | 11 (31.4) | 9 (30) |
| Surgical/orthopedic ward | 9 (25.7) | 3 (10) |
| Diagnosis, n (%) | ||
| CNS infectionb | 5 (15.3) | 10 (33.3) |
| Bacteremia | 4 (11.4) | 2 (6.7) |
| Skin and soft tissue infection | 4 (11.4) | 4 (13.3) |
| Bone and joint infection | 6 (17.1) | 2 (6.7) |
| Sepsis/septic shock | 5 (14.3) | 6 (20) |
| Lower respiratory tract infection | 7 (20) | 5 (16.7) |
| Infective endocarditis | 1 (2.9) | 0 (0) |
| Intra-abdominal infection | 3 (8.6) | 1 (3.3) |
| Infected physiologic compartment, n (%) | ||
| CNS compartmentb | 5 (14.3) | 10 (33.3) |
| Blood compartmentc | 13 (37.1) | 9 (30) |
| Lung compartment | 7 (20) | 5 (16.7) |
| Other tissuesd | 10 (28.6) | 6 (20) |
| Vancomycin treatment type, n (%) | ||
| Empiric | 16 (45.7) | 14 (46.7) |
| Definitive | 19 (54.3) | 16 (53.3) |
| Positive microbiologic cultures, n (%) | ||
| MRSA | 8 (42.1) | 9 (56.3) |
| MSSA | 5 (26.3) | 3 (18.6) |
| S. epidermidis | 4 (21.1) | 1 (6.3) |
| S. constellatus | 1 (5.3) | 0 (0) |
| E. faecium | 1 (5.3) | 3 (18.8) |
| Pre-enrollment vancomycin treatment details | ||
| Pre-enrollment days on vancomycin treatment, median [IQR] | 2 [0.5] | 1.5 [1] |
| Dose (mg/dose), median [1QR] | 1000 [0] | 1000 [0] |
| Dose (mg/kg/dose), median [IQR] | 14.3 [5.6] | 14.6 [3.7] |
| Total daily dose (mg/day), median [IQR] | 2000 [1000] | 2000 [125] |
| Total daily dose (mg/kg/day), median [IQR] | 28.6 [16.5] | 29.2 [7.4] |
| Cumulative doses received (mg), median [IQR] | 4000 [1250] | 5000 [2063] |
| Cumulative doses received (mg/kg), median [IQR] | 59.4 [25.04] | 66.8 [29.6] |
| Laboratory parameters | ||
| White blood cells (×109 IU/L), mean ± SD | 13.36 ± 7.9 | 12.8 ± 6.02 |
| Hemoglobin (g/dL), median [IQR] | 11.53 [2.32] | 11.7 [4.15] |
| Neutrophils (×109 IU/L), median [IQR] | 8 [9.8] | 8.2 [7.7] |
| SCr (µmol/L), median [IQR] | 65 [36] | 67 [30] |
| Concomitant antibiotics, n (%) | 18 (51.4) | 22 (55) |
| Beta-lactams | 9 (25.7) | 9 (30) |
| Carbapenems | 10 (15.4) | 11 (16.9) |
| Cephalosporins | 9 (25.7) | 12 (40) |
| Clindamycin | 2 (5.7) | 1 (3.3) |
| Linezolid | 0 (0) | 4 (13.3) |
| Rifampicin | 1 (2.9) | 1 (3.3) |
| Concomitant nephrotoxic agents, n (%) | 12 (34.3) | 12 (40) |
| Amphotericin B | 0 (0) | 2 (6.7) |
| NSAIDs | 8 (22.9) | 10 (33.3) |
| ACEI/ARBs | 4 (11.1) | 1 (3.3) |
| Loop/thiazide diuretics | 4 (11.4) | 6 (20) |
| Acyclovir | 1 (2.9) | 0 (0) |
| Comorbidities, n (%) | ||
| Diabetes mellitus | 6 (17.1) | 8 (26.7) |
| Chronic kidney disease | 1 (2.9) | 2 (6.7) |
| Hypertension | 7 (20) | 11 (36.7) |
| Coronary vascular disease | 2 (5.7) | 4 (13.3) |
| Heart failure | 1 (2.9) | 2 (6.7) |
BMI body mass index, ABW actual body weight, MENA Middle East and North Africa, MRSA methicillin-resistant S. aureus, MSSA methicillin-sensitive S. aureus
aIncludes trauma, medical and surgical intensive care units
bInvolves meningitis, encephalitis and ventriculitis
cIncludes blood, intra-abdominal and cardiac infections
dIncludes skin, soft tissue, bone and joint infections
Clinical Outcomes of Peak–Trough-Based Versus Trough-Only-Based Vancomycin TDM Approaches
Peak–trough-based vancomycin TDM was significantly associated with higher infection cure rates compared to trough-only-based vancomycin TDM [p value = 0.02; Table 2]. Compared to the control group (trough-only-based TDM group), the intervention group (peak–trough-based TDM group) required non-statistically significant shorter duration of vancomycin treatment and hospitalization by 0.5 days and 4.5 days, respectively [p value > 0.05; Table 2]. No statistically significant differences were observed for other safety endpoints between the two monitored groups [p value > 0.05; Table 2].
Table 2.
Clinical outcomes of peak–trough-based versus trough-only-based vancomycin therapeutic drug monitoring approaches
| Variable | Trough-only-monitoring group | Peak–trough-monitoring group | p valuea |
|---|---|---|---|
| (n = 35) | (n = 30) | ||
| Vancomycin treatment efficacy outcomes, n (%) | |||
| Therapeutic cure | 17 (48.6) | 23 (76.7) | 0.020 |
| Therapeutic failure | 18 (51.4) | 7 (23.3) | |
| Vancomycin treatment safety outcomes, n (%) | |||
| Neutropenia | 3 (8.6) | 1 (3.3) | 0.381 |
| Nephrotoxicity | 1 (2.9) | 1 (3.3) | 0.912 |
| All-cause mortality, n (%) | 3 (8.6) | 2 (6.7) | 0.774 |
| Length of hospitalization (days), median [min–max] | 20 [6–117] | 15.5 [4–68.9] | 0.320 |
| Total duration on vancomycin treatment (days), median [min–max] | 7 [1–28] | 6.5 [1–32] | 0.319 |
aChi-squared test or Mann–Whitney U-test
Clinical Pharmacokinetic Outcomes of Peak–Trough-Based Versus Trough-Only-Based Vancomycin TDM Approaches
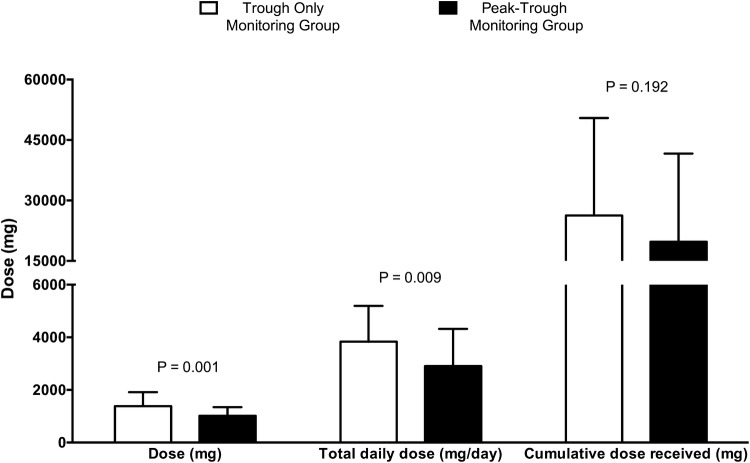
Initial peak and trough vancomycin serum concentrations were not therapeutic in 30.2% (n = 19) and 80% (n = 52) of cases, respectively (Table 3). Individual vancomycin clinical pharmacokinetic parameters were comparable between the study groups (Table 3). Patients enrolled in the peak–trough-based group received TDM earlier than the trough-only-based group by 0.5 days [p value = 0.001]. Vancomycin dosing requirements significantly differed between the two vancomycin TDM approaches; compared to the trough-only-based vancomycin TDM group, the peak–trough-based group required lower average vancomycin single doses and total daily doses by 370 mg/dose and 927 mg/day, respectively [p value < 0.05; Table 3; Fig. 1]. Despite the similar duration on vancomycin treatment between the study groups, the trough-only-based vancomycin TDM recipients received clinically significant higher median cumulative vancomycin doses by 6,522 mg [p value > 0.05; Table 3]. Patients who received trough-only-based vancomycin TDM required more dose adjustments to achieve target serum concentrations compared to the intervention group [p value > 0.05; Table 3]. Furthermore, the trough-only-based monitoring was associated with recommended vancomycin dosing regimens of relatively low dosing frequencies and large single doses, necessitating longer infusion durations that exceeded 1 h (Table 3). The compared TDM approaches resulted in statistically and clinically significant different peak concentrations; peak–trough-based vancomycin dose adjustments compared to trough-only based vancomycin dose adjustments resulted in achievement of target peaks for 94.1% versus 69% of the time, respectively [p value = 0.006; Table 3]. Interestingly, peak–trough-based vancomycin doses resulted in similar rates of therapeutic troughs and AUCs compared to trough-only-based vancomycin doses (Table 3).
Table 3.
Clinical pharmacokinetic outcomes associated with peak–trough-based versus trough-only-based vancomycin therapeutic drug monitoring approaches
| Variable | Trough-only-monitoring group | Peak–trough-monitoring group | p valuea |
|---|---|---|---|
| (n = 35) | (n = 30) | ||
| Pharmacokinetic parameters at treatment initiation | |||
| Vd (L), mean ± SD | 48.5 ± 10.7 | 51.14 ± 9.96 | 0.311 |
| Ke (h−1), mean ± SD | 0.094 ± 0.05 | 0.089 ± 0.051 | 0.702 |
| Cl (L/h), mean ± SD | 4.15 ± 2.22 | 4.24 ± 2.20 | 0.861 |
| t1/2 (h), median [IQR] | 8.01 [11.12] | 7.23 [9.75] | 0.722 |
| CrCl (L/h), median [IQR] | 6.51 [3.44] | 6.45 [3.12] | 0.374 |
| AUC per initialb dose (mg*h/L), median [IQR] | 227 [195.6] | 228 [273.01] | 0.590 |
| Initialb vancomycin serum concentrations (mg/L), median [IQR] | |||
| Trough-1 | 9 [8.3] | 8.4 [12.9] | 0.732 |
| Peak | 25 [10] | 27.9 [17.8] | 0.863 |
| Random-1 | 18.9 [9.4] | 18 [18.1] | 0.837 |
| Random-2 | 11.9 [8.7] | 11.1 [13.28] | 0.638 |
| Trough-2 | 10.6 [10.5] | 8.9 [15.1] | 0.844 |
| Interpretation of initialb peak vancomycin concentrations, n (%)d | |||
| Therapeutic | 27 (77.1) | 17 (60.7) | 0.158 |
| Non-therapeutic | 8 (22.9) | 11 (39.3) | |
| Interpretation of initialb vancomycin trough concentrations, n (%) | |||
| Therapeutic | 6 (17.1) | 7 (23.3) | 0.534 |
| Non-therapeutic | 29 (82.9) | 23 (76.7) | |
| Minimum number of dose adjustments required to first therapeutic serum concentrations, median [min–max] | 2 [1–5] | 1 [1–3] | 0.105 |
| Overall vancomycin dosing requirements | |||
| Single dose (mg/dose), mean ± SD | 1385.71 ± 530.62 | 1015 ± 332.221 | 0.001 |
| Single dose (mg/kg/dose), mean ± SD | 19.03 ± 7.76 | 14.09 ± 5.68 | 0.005 |
| Total daily dose (mg/day), mean ± SD | 3834.49 ± 1,362.83 | 2907 ± 1,416.08 | 0.009 |
| Total daily dose (mg/kg/day), mean ± SD | 52.83 ± 21.59 | 40.78 ± 21.25 | 0.027 |
| Cumulative doses received (mg), mean ± SD | 26,275 ± 24,190 | 19,753 ± 21,893 | 0.192 |
| Vancomycin dosing interval, n (%) | |||
| Q6 h | 6 (17.1) | 11 (36.7) | 0.091 |
| Q8 h | 16 (45.7) | 12 (40) | |
| Q12 h | 13 (37.1) | 4 (13.4) | |
| Q18 h | 0 (0) | 1 (3.3) | |
| Q24 h | 0 (0) | 1 (3.3) | |
| Q36 h | 0 (0) | 1 (3.3) | |
| Vancomycin infusion duration, n (%) | |||
| Infused over 0.5 h | 1 (2.9) | 0 (0) | 0.297 |
| Infused over 1 h | 19 (54.3) | 22 (73.3) | |
| Infused over 1.5 h | 10 (28.5) | 8 (26.7) | |
| Infused over 2.5 h | 2 (5.7) | 0 (0) | |
| Infused over 3 h | 2 (5.7) | 0 (0) | |
| Infused over 4 h | 1 (2.9) | 0 (0) | |
| AUC per TDM adjusted dose(mg*h/L), median [IQR] | 270 [156.02] | 223 [168.82] | 0.590 |
| AUC24/MIC, median [IQR] | 772 [412.95] | 708 [260.87] | 0.762 |
| Post-dose adjustment peak concentration (mg/L), mean ± SD | 35.94 ± 7.7 | 30.38 ± 5.17 | 0.021 |
| Post-dose adjustment trough concentration (mg/L), mean ± SD | 16.8 ± 3.09 | 15.6 ± 3.49 | 0.596 |
| Interpretation of post-dose adjustmentc peak concentrations, n (%)d | |||
| Therapeutic | 29 (69) | 32 (94.1) | 0.006 |
| Subtherapeutic | 13 (31) | 2 (5.9) | |
| Interpretation of post-dose adjustmentc trough concentrations, n (%) | |||
| Therapeutic | 25 (44.6) | 20 (54.1) | 0.665 |
| Subtherapeutic | 19 (33.9) | 10 (27) | |
| Supratherapeutic | 12 (21.4) | 7 (18.9) | |
aChi-squared test, Mann–Whitney U-test or Student’s t-test
bInitial represents pre-TDM doses and concentrations
c56 dose adjustments were applied in the trough-only arm while 37 dose adjustments were applied in the peak–trough arm
dMissing values
Fig. 1.
Vancomycin dosing requirements of peak–trough-based versus trough-only-based vancomycin therapeutic drug monitoring recipients
Association Between Vancomycin AUC24/MIC and Cure
CART identified CLCR < 7.85 L/h, AUC24/MIC, and the type of vancomycin TDM approach as significant determinants of therapeutic outcomes with 100, 58.4 and 45.8% normalized importance to the model, respectively. Maintaining AUC24/MIC between 565 and 1256 has been associated with cure. All subjects who achieved an AUC24/MIC ≤ 1256 and received peak–trough-based vancomycin TDM achieved clinical success rates [100%, n = 19]. Maintenance of AUC24/MIC > 565 was identified to be correlated with cure in trough-only-based TDM recipients [84.6%, n = 11]. No minimum AUC24/MIC breakpoint was detected by CART in the peak–trough-based group. The predictive performance was high (88.6%) with low misclassification risks (11.4%), suggesting robustness.
Discussion
To our knowledge, this is the first pragmatic head-to-head RCT that (1) prospectively compared two routinely used vancomycin TDM approaches; (2) reported MENA-specific AUC24/MIC targets; and (3) identified a maximum AUC24/MIC threshold for clinical benefit. Studies have suggested that vancomycin TDM was associated with higher clinical success rates and less nephrotoxicity compared to non-TDM groups [31, 51]. To date, studies in this area compared vancomycin TDM recipients with non-TDM recipients, and were mostly based on observational research designs [31]. In addition, no studies reported MENA-specific AUC24/MIC targets. The present pragmatic RCT aimed to address these questions.
Despite similar AUC24 exposures, peak–trough-based TDM was associated with higher cure rates compared to trough-only-based TDM. This unexpected finding can be interpreted in two ways. First, it questions whether AUC24/MIC is the optimal vancomycin pharmacokinetic–pharmacodynamic target. Fukumori’s group reported the area under the trough level as a novel pharmacokinetic–pharmacodynamic parameter that more strongly correlates with vancomycin clinical efficacy compared to AUC24 [52]. Second, it suggests that cure may be more associated with the extent of consistency, sustainability and fluctuations of vancomycin exposure during the course of therapy, rather than a total single exposure estimate. This notion serves as the basis for studies advocating the administration of vancomycin as continuous infusion, rather than intermittent infusion [53–55]. Compared to intermittent infusion, continuous infusion resulted in more consistent and sustained exposure at the infection site, despite similar AUCs [54]. Furthermore, continuous infusion achieved target concentrations faster and was associated with less serum fluctuations [55]. Collectively these studies align with the finding that a minimum cure breakpoint (AUC24/MIC > 565) was only detected in the trough-only TDM arm, while peak–trough-based vancomycin dosing was not associated with a minimum threshold. This suggests that peak–trough-based vancomycin dosing, using the specified peak/trough targets, is associated with more sustained and consistent vancomycin exposure that resulted in achieving the minimum AUC24/MIC threshold for cure at most times, unlike trough-only based dosing. Vancomycin continuous infusion is not feasible for all clinical settings or patient situations. For example, a patient may be on other vancomycin-incompatible intravenous therapy. Indeed, the peak–trough-based approach may serve as a more practical alternative to continuous infusion that needs to be explored in future studies.
Maintaining AUC24/MIC between 565 and 1256 has been associated with cure. This breakpoint is higher than the minimum AUC24/MIC cure breakpoints that ranged from 398 to 451 in seven observational cohort studies [19]. Additionally, this work is the first to identify a maximum AUC24/MIC cure threshold that, if exceeded, no extra clinical benefit is likely as long as the CLCR is < 130 mL/min. This concurs with the emerging concept of ARC (CLCR > 120–150 mL/min), that is associated with decreased vancomycin exposure and negative clinical outcomes [56–60]. Although studies reported that targeting, higher AUC24/MIC ratios was associated with better clinical outcomes [19, 61], the question regarding a maximum AUC24/MIC for clinical benefit remained unanswered in the current literature. It should be carefully noted that pharmacokinetic–pharmacodynamic targets (i.e., AUC24/MIC) serve only for clinical efficacy outcomes while absolute vancomycin exposure measures should be monitored for safety [1, 3, 62, 63].
Emerging evidence suggests the promising clinical usefulness of the peak–trough-based vancomycin TDM approach [22, 64, 65], and questions the clinical benefit of trough-only-based vancomycin dosing [61, 66, 67]. Consistent with other studies, this RCT suggests that peak–trough-based vancomycin dosing may be associated with lower vancomycin exposure, LOS and dose adjustments, which needs to be confirmed in larger scale trials. Finch et al. reported that 2-concentration-based AUC-guided vancomycin dosing resulted in less vancomycin exposure, total daily doses and nephrotoxicity versus trough-only-guided dosing [22]. Similar clinical benefits of vancomycin peak concentration monitoring have been suggested elsewhere [64, 65]. Peak–trough-based TDM allowed significantly better attainment of therapeutic vancomycin concentrations [64]. It has been reported that vancomycin-related ADRs (i.e., nephrotoxicity and neutropenia) may be related to exposure [23, 47, 48, 68–72], with trough concentrations > 15 mg/L having a significantly higher risk [69]. According to a meta-analysis of 17 observational studies, vancomycin dosing that targeted higher trough concentrations (> 15 mg/L) was associated with significantly more nephrotoxicity and no significant improvement in mortality or cure rates [66]. In deep-seated MRSA, trough concentrations > 15 mg/L did not result in shorter LOS, lower mortality rates, or higher treatment success rates versus trough concentrations < 15 mg/L [67]. In fact, vancomycin trough concentrations > 15 mg/L were associated with a higher incidence of nephrotoxicity [67]. A meta-analysis of 14 observational studies showed that vancomycin dosing based on trough concentration targets (15–20 mg/L) was not associated with better clinical outcomes in mortality, bacteremia persistence and treatment failure [61]. Therefore, peak–trough-based vancomycin dosing provides a potential strategy to decrease vancomycin exposure, which will reflect into lower medication utilization, less vancomycin-related ADRs, and decreased emergence of vancomycin-resistant strains. Furthermore, the possibly lower LOS with peak–trough-based dosing would potentially result in a lower incidence of nosocomial infections. Thus, this approach provides a potential strategy to maximize clinical outcomes with vancomycin treatment, as well as decrease the economic burden on healthcare systems.
This study has several strengths. First, the present RCT is of a pragmatic nature. The key feature of pragmatic designs is the ability to assess the effectiveness of an intervention in routine situations to maximize the external validity of the study findings [73]. Due to the limited generalizability of exploratory RCTs to routine clinical practice, the concept of pragmatism has emerged during the past decades [73–75]. Exploratory RCTs are conducted under ideal circumstances in which an intervention is more likely to work, which is not how real-life situations are in clinical settings; thus, they possess limited generalizability and may fail in many routine clinical situations [73]. Therefore, it has been reported that the plethora of exploratory RCTs are of limited use to healthcare policymakers and clinicians [75]. Due to the pragmatic nature of this study, the researchers did not intervene on indication appropriateness and initial dosing of vancomycin; suspected or confirmed Gram-positive infections requiring vancomycin treatment were included, with no restrictions to MRSA like other AUC studies. The study setting included multiple centers and wards in order to be reflective of the variabilities in clinical practice. An important observation is the lack of initial vancomycin target attainment with physician-initiated dosing at most times, which may be due to deviations from the guideline-recommended empiric doses in our clinical setting. The reasons for such non-compliance need to be explored in future audits. In addition, no restrictions on infection type, critical illness state, pharmacotherapeutic or mechanical co-interventions were applied. Thus, the implications of the study findings are of clinical relevance, as it tested effectiveness rather than efficacy alone. Second, the prospective nature of the study allowed accurate vancomycin dosing and blood specimen collection. The accuracy of sampling times and dosing assures the internal validity of clinical pharmacokinetic studies. Unlike most clinical evaluations that estimated the AUC based on estimated renal clearance, which does not accurately predict vancomycin clearance [23], the present work used actual individualized vancomycin clearance to estimate the AUC. Together, these aspects suggest high internal validity of the study, with considerable generalizability.
The present findings need to be interpreted with caution due to some important limitations. This RCT was of limited sample size and was unblinded. The exact MICs were not available for all subjects since many received vancomycin as empiric therapy. For subjects with confirmed sensitive cultures, HMC laboratories reported MIC as 1 mg/L at all instances with values of < 1 mg/L rounded to 1 mg/L. Future larger scale double-blinded pragmatic RCTs are needed to confirm these findings.
Conclusion
In conclusion, this is the first head-to-head pragmatic RCT that compared peak–trough-based versus trough-only-based vancomycin TDM approaches. Compared to trough-only-based vancomycin TDM, peak–trough-based vancomycin TDM strategy was associated with higher cure rates and less vancomycin doses. Furthermore, maintaining an AUC24/MIC between 565 and 1256 was associated with cure. Future larger scale trials are warranted to confirm these study findings.
Ethical Considerations
Informed consent was obtained from all participants included in the study. All procedures performed were in accordance with the 1964 Helsinki declaration and its later amendments and national institutional research committees. This study was approved by HMC Medical Research Center and Qatar University Institutional Review Board as well as the research committees of AWH, AKH and HGH.
Acknowledgements
Open Access funding provided by the Qatar National Library. We would like to thank Dr. Hani Abdelaziz and Dr. Eman El-Mekaty for their efforts and contributions in the design and implementation of this study. We would also like to thank all staff at HMC who helped to conduct the study.
Compliance with Ethical Standards
Funding
This study was funded by Qatar University Internal Research Grant (QUST-CPH-SPR-14/15-12).
Conflicts of interest
All authors declare no conflict of interest.
References
- 1.Bauer LA. Applied clinical pharmacokinetics. 3. USA: McGraw Hill; 2014. [Google Scholar]
- 2.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58(1):309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 4.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 5.Gawronski KM, Goff DA, Brown J, Khadem TM, Bauer KA. A stewardship program’s retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant staphylococcus aureus bacteremia and osteomyelitis. Clin Ther. 2013;35(6):772–779. doi: 10.1016/j.clinthera.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Brown J, Brown K, Forrest A. Vancomycin AUC24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob Agents Chemother. 2012;56(2):634–638. doi: 10.1128/AAC.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O’Sullivan MV, et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57(4):1654–1663. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HY, Chen CL, Liu SY, Yan YS, Chang CJ, Chiu CH. Impact of molecular epidemiology and reduced susceptibility to glycopeptides and daptomycin on outcomes of patients with methicillin-resistant staphylococcus aureus bacteremia. PLoS One. 2015;10(8):e0136171. doi: 10.1371/journal.pone.0136171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torun MM, Bahar H, Demirci M, Altas K, Bagdatli Y, Kocazeybek B, et al. Two heterogeneously vancomycin-intermediate clinical isolates of methicillin-sensitive and methicillin-resistant staphylococcus aureus in a Turkish university hospital: brief report of a surveillance study. Int J Antimicrob Agents. 2005;26(6):508–510. doi: 10.1016/j.ijantimicag.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51(7):2582–2586. doi: 10.1128/AAC.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson JO, Pearson JC, Christiansen KJ, Coombs GW, Murray RJ. Community-associated versus healthcare-associated methicillin-resistant staphylococcus aureus bacteraemia: a 10-year retrospective review. Eur J Clin Microbiol Infect Dis. 2009;28(4):353–361. doi: 10.1007/s10096-008-0632-1. [DOI] [PubMed] [Google Scholar]
- 12.Deurenberg RH, Stobberingh EE. The evolution of staphylococcus aureus. Infect Genet Evol. 2008;8(6):747–763. doi: 10.1016/j.meegid.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Goudarzi M, Bahramian M, Satarzadeh Tabrizi M, Udo EE, Figueiredo AM, Fazeli M, et al. Genetic diversity of methicillin resistant staphylococcus aureus strains isolated from burn patients in Iran: sT239-SCCmec III/t037 emerges as the major clone. Microb Pathog. 2017;105:1–7. doi: 10.1016/j.micpath.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Goudarzi M, Seyedjavadi SS, Nasiri MJ, Goudarzi H, Sajadi Nia R, Dabiri H. Molecular characteristics of methicillin-resistant staphylococcus aureus (MRSA) strains isolated from patients with bacteremia based on MLST, SCCmec, spa, and agr locus types analysis. Microb Pathog. 2017;104:328–335. doi: 10.1016/j.micpath.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 15.Boswihi SS, Udo EE. Methicillin-resistant staphylococcus aureus: an update on the epidemiology, treatment options and infection control. Curr Med Res Pract. 2018;8(1):18–24. doi: 10.1016/j.cmrp.2018.01.001. [DOI] [Google Scholar]
- 16.Tokajian S. New epidemiology of staphylococcus aureus infections in the Middle East. Clin Microbiol Infect. 2014;20(7):624–628. doi: 10.1111/1469-0691.12691. [DOI] [PubMed] [Google Scholar]
- 17.Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect. 2014;20(7):605–623. doi: 10.1111/1469-0691.12705. [DOI] [PubMed] [Google Scholar]
- 18.Changchien CH, Chen YY, Chen SW, Chen WL, Tsay JG, Chu C. Retrospective study of necrotizing fasciitis and characterization of its associated methicillin-resistant staphylococcus aureus in Taiwan. BMC Infect Dis. 2011;11:297. doi: 10.1186/1471-2334-11-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Men PLH, Zhai S, Zhao R. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. doi: 10.1371/journal.pone.0146224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drennan PG, Begg EJ, Gardiner SJ, Kirkpatrick CMJ, Chambers ST. The dosing and monitoring of vancomycin – what is the best way forward? Int J Antimicrob Agents. 2018. [DOI] [PubMed]
- 21.Neely MN, Kato L, Youn G, Kraler L, Bayard D, van Guilder M, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. doi: 10.1128/AAC.02042-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finch NA, Zasowski EJ, Murray KP, Mynatt RP, Zhao JJ, Yost R, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi: 10.1128/AAC.01293-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–57. doi: 10.1016/j.addr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. Vancomycin: we can’t get there from here. Clin Infect Dis. 2011;52(8):969–974. doi: 10.1093/cid/cir078. [DOI] [PubMed] [Google Scholar]
- 25.Mogle BT, Steele JM, Seabury RW, Dang UJ, Kufel WD. Implementation of a two-point pharmacokinetic AUC-based vancomycin therapeutic drug monitoring approach in patients with methicillin-resistant staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2018;52(6):805–810. doi: 10.1016/j.ijantimicag.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Sun X, Chang W, Dai Y, Ma X. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate staphylococcus aureus isolates. PLoS One. 2015;10(8):e0136082. doi: 10.1371/journal.pone.0136082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahimipour F, Ghazvini K, Youssefi M. Reports of vancomycin-resistant staphylococcus aureus from Middle East countries. Arch Clin Infect Dis. 2018;13(2):e59522. doi: 10.5812/archcid.59522. [DOI] [Google Scholar]
- 28.Qatar population. 2018 [Accessed 24 Feb 2019]. http://worldpopulationreview.com/countries/qatar-population/.
- 29.Quarterly bulletin for population and social statistics. Qatar Ministry of Development and Planning Statistics. 2018 [Accessed 24 Feb 2019]. https://www.mdps.gov.qa/en/statistics1/Pages/LatestStats/20170320.aspx.
- 30.Kane SP. Sample size calculator. ClinCalc: http://clincalc.com/Stats/SampleSize.aspx Updated July 1, 2017 [Accessed 7 October 2015]. http://clincalc.com/Stats/SampleSize.aspx.
- 31.Ye ZK, Tang HL, Zhai SD. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PLoS One. 2013;8(10):e77169. doi: 10.1371/journal.pone.0077169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 33.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 36.Creatinine clearance estimate by Cockcroft-Gault equation. [Accessed 7 October 2015] https://reference.medscape.com/calculator/creatinine-clearance-cockcroft-gault.
- 37.Arneson W, Brickell J. Clinical chemistry: a laboratory perspective. Philadelphia: F. A. Davis Company; 2007. [Google Scholar]
- 38.Architect c16000 USA: Abbott Laboratories 2007. [Accessed 7 October 2015]. http://www.nearmedic.ru/upload/files/Doc_429_207.pdf.
- 39.Synchron systems chemistry information sheet: vancomycin [Package Insert]. Brea, CA:Beckman Coulter, Inc; 2015. [Accessed 3 June 2017]. https://www.beckmancoulter.com/wsrportal/techdocs?docname=/cis/A18566/AN/EN_VANC.pdf.
- 40.Laboratory procedure: BD Pheonix automated microbiology systems [Package insert]. USA: BD Phoenix AP. [Accessed 3 June 2017]. http://legacy.bd.com/ds/technicalCenter/clsi/clsi-Phoenix_GramPositive_V5.15_V4.31.pdf.
- 41.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 42.Shargel L, W-Pong S, Yu A. Applied biopharmaceutics and pharmacokinetics. 6th ed. USA: McGraw-Hill Companies; 2012.
- 43.Bauer LA. Evaluation of a simplified method to adjust vancomycin trough concentrations. Pharmacotherapy. 2005;25(10):1503. [Google Scholar]
- 44.Walraven CJ, North MS, Marr-Lyon L, Deming P, Sakoulas G, Mercier RC. Site of infection rather than vancomycin MIC predicts vancomycin treatment failure in methicillin-resistant staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2011;66(10):2386–2392. doi: 10.1093/jac/dkr301. [DOI] [PubMed] [Google Scholar]
- 45.Clemens EC, Chan JD, Lynch JB, Dellit TH. Relationships between vancomycin minimum inhibitory concentration, dosing strategies, and outcomes in methicillin-resistant staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2011;71(4):408–414. doi: 10.1016/j.diagmicrobio.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Castillo JS, Leal AL, Cortes JA, Alvarez CA, Sanchez R, Buitrago G, et al. Mortality among critically ill patients with methicillin-resistant staphylococcus aureus bacteremia: a multicenter cohort study in Colombia. Rev Panam Salud Publica. 2012;32(5):343–350. doi: 10.1590/S1020-49892012001100004. [DOI] [PubMed] [Google Scholar]
- 47.Pai MP, Mercier RC, Koster SA. Epidemiology of vancomycin-induced neutropenia in patients receiving home intravenous infusion therapy. Ann Pharmacother. 2006;40(2):224–228. doi: 10.1345/aph.1G436. [DOI] [PubMed] [Google Scholar]
- 48.Morris A, Ward C. High incidence of vancomycin-associated leucopenia and neutropenia in a cardiothoracic surgical unit. J Infect. 1991;22(3):217–223. doi: 10.1016/S0163-4453(05)80002-7. [DOI] [PubMed] [Google Scholar]
- 49.Bonate P. Pharmacokinetic-pharmacodynamic modeling and simulation. 2. USA: Springer; 2014. [DOI] [PubMed] [Google Scholar]
- 50.Sutton CD. Classification and regression trees bagging and boosting. Handbook of statistics. 24. ebook: Elsevier; 2005.
- 51.Welty TE, Copa AK. Impact of vancomycin therapeutic drug monitoring on patient care. Ann Pharmacother. 1994;28(12):1335–1339. doi: 10.1177/106002809402801201. [DOI] [PubMed] [Google Scholar]
- 52.Fukumori S, Tsuji Y, Mizoguchi A, Kasai H, Ishibashi T, Iwamura N, et al. Association of the clinical efficacy of vancomycin with the novel pharmacokinetic parameter area under the trough level (AUTL) in elderly patients with hospital-acquired pneumonia. J Clin Pharm Ther. 2016;41(4):399–402. doi: 10.1111/jcpt.12399. [DOI] [PubMed] [Google Scholar]
- 53.Waineo MF, Kuhn TC, Brown DL. The pharmacokinetic/pharmacodynamic rationale for administering vancomycin via continuous infusion. J Clin Pharm Ther. 2015;40(3):259–265. doi: 10.1111/jcpt.12270. [DOI] [PubMed] [Google Scholar]
- 54.Byl B, Jacobs F, Wallemacq P, Rossi C, de Francquen P, Cappello M, et al. Vancomycin penetration of uninfected pleural fluid exudate after continuous or intermittent infusion. Antimicrob Agents Chemother. 2003;47(6):2015–2017. doi: 10.1128/AAC.47.6.2015-2017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vuagnat A, Stern R, Lotthe A, Schuhmacher H, Duong M, Hoffmeyer P, et al. High dose vancomycin for osteomyelitis: continuous vs. intermittent infusion. J Clin Pharm Ther. 2004;29(4):351–357. doi: 10.1111/j.1365-2710.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 56.Hobbs AL, Shea KM, Roberts KM, Daley MJ. Implications of augmented renal clearance on drug dosing in critically ill patients: a focus on antibiotics. Pharmacotherapy. 2015;35(11):1063–1075. doi: 10.1002/phar.1653. [DOI] [PubMed] [Google Scholar]
- 57.Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med. 2014;42(3):520–527. doi: 10.1097/CCM.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 58.Fuster-Lluch O, Geronimo-Pardo M, Peyro-Garcia R, Lizan-Garcia M. Glomerular hyperfiltration and albuminuria in critically ill patients. Anaesth Intensive Care. 2008;36(5):674–680. doi: 10.1177/0310057X0803600507. [DOI] [PubMed] [Google Scholar]
- 59.Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care. 2013;28(5):695–700. doi: 10.1016/j.jcrc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39(5):420–423. doi: 10.1016/j.ijantimicag.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Prybylski JP. Vancomycin trough concentration as a predictor of clinical outcomes in patients with staphylococcus aureus bacteremia: a meta-analysis of observational studies. Pharmacotherapy. 2015;35(10):889–898. doi: 10.1002/phar.1638. [DOI] [PubMed] [Google Scholar]
- 62.Chavada R, Ghosh N, Sandaradura I, Maley M, Van Hal SJ. Establishment of an AUC0-24 threshold for nephrotoxicity is a step towards individualized vancomycin dosing for methicillin-resistant staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2017;61(5):e02535-16. doi: 10.1128/AAC.02535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zasowski EJ, Murray KP, Trinh TD, Finch NA, Pogue JM, Mynatt RP, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2018;62(1):e01684-17. doi: 10.1128/AAC.01684-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong J, Krop LC, Johns T, Pai MP. Individualized vancomycin dosing in obese patients: a two-sample measurement approach improves target attainment. Pharmacotherapy. 2015;35(5):455–463. doi: 10.1002/phar.1588. [DOI] [PubMed] [Google Scholar]
- 65.Iwamoto T, Kagawa Y, Kojima M. Clinical efficacy of therapeutic drug monitoring in patients receiving vancomycin. Biol Pharm Bull. 2003;26(6):876–879. doi: 10.1248/bpb.26.876. [DOI] [PubMed] [Google Scholar]
- 66.Meng L, Fang Y, Chen Y, Zhu H, Long R. High versus low vancomycin serum trough regimen for gram-positive infections: a meta-analysis. J Chemother. 2015;27(4):213–220. doi: 10.1179/1973947814Y.0000000182. [DOI] [PubMed] [Google Scholar]
- 67.Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9–14. doi: 10.1517/14740330903413514. [DOI] [PubMed] [Google Scholar]
- 68.Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52(4):1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother. 2011;45(5):629–638. doi: 10.1345/aph.1P583. [DOI] [PubMed] [Google Scholar]
- 71.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255. doi: 10.1007/s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]
- 72.Segarra-Newnham M, Tagoff SS. Probable vancomycin-induced neutropenia. Ann Pharmacother. 2004;38(11):1855–1859. doi: 10.1345/aph.1E187. [DOI] [PubMed] [Google Scholar]
- 73.Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13(2):217–224. doi: 10.31887/DCNS.2011.13.2/npatsopoulos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams HC, Burden-Teh E, Nunn AJ. What is a pragmatic clinical trial? J Invest Dermatol. 2015;135(6):1–3. doi: 10.1038/jid.2015.134. [DOI] [PubMed] [Google Scholar]
- 75.Zwarenstein M, Oxman A. Why are so few randomized trials useful, and what can we do about it? J Clin Epidemiol. 2006;59(11):1125–1126. doi: 10.1016/j.jclinepi.2006.05.010. [DOI] [PubMed] [Google Scholar]