Abstract
The spread of resistance to insecticides in disease-carrying mosquitoes poses a threat to the effectiveness of control programmes, which rely largely on insecticide-based interventions. Monitoring mosquito populations is essential, but obtaining phenotypic measurements of resistance is laborious and error-prone. High-throughput genotyping offers the prospect of quick and repeatable estimates of resistance, while also allowing resistance markers to be tracked and studied. To demonstrate the potential of highly-mulitplexed genotypic screening for measuring resistance-association of mutations and tracking their spread, we developed a panel of 28 known or putative resistance markers in the major malaria vector Anopheles gambiae, which we used to screen mosquitoes from a wide swathe of Sub-Saharan Africa (Burkina Faso, Ghana, Democratic Republic of Congo (DRC) and Kenya). We found resistance association in four markers, including a novel mutation in the detoxification gene Gste2 (Gste2-119V). We also identified a duplication in Gste2 combining a resistance-associated mutation with its wild-type counterpart, potentially alleviating the costs of resistance. Finally, we describe the distribution of the multiple origins of kdr resistance, finding unprecedented diversity in the DRC. This panel represents the first step towards a quantitative genotypic model of insecticide resistance that can be used to predict resistance status in An. gambiae.
Subject terms: Ecological genetics, Evolutionary genetics
Introduction
It is estimated that the use of insecticide-treated nets and indoor residual spraying of insecticide has been responsible for 78% of 663 million cases of malaria averted in the period 2000–20151. The documented rise and spread of resistance to currently-used insecticides in the major malaria mosquito vectors therefore presents a worrying trend that may negatively impact malaria control programmes2–4. In particular, the major malaria vector in Sub-Saharan Africa (SSA), Anopheles gambiae, now has widespread resistance to pyrethroids, and is showing pockets of high resistance to carbamates and organophosphates5. To maintain effective malaria control, accurate assessment of the insecticide resistance profile of mosquito populations is essential. Furthermore, as new insecticides are brought to market, it will be crucial to identify and track resistance to these new products in order to react appropriately before the failure of malaria control.
Phenotypic assessment of insecticide resistance requires live mosquitoes of fixed age to be assayed in carefully controlled conditions. The effort and care required to perform these assays make them difficult to perform on a large scale and prone to inconsistency if conditions are not rigorously controlled6. Moreover, phenotyping approaches are only sensitive when resistance has reached appreciable frequency in the population. For these reasons, there is great interest in the prospect of screening mosquitoes for the genetic signatures of insecticide resistance, which would provide a convenient and simple way of assessing resistance from any type of mosquito collection method, including dead mosquitoes routinely collected by monitoring and evaluation programmes. Genetic screening carries the additional benefit of improving our understanding of the origins of resistance mutations, which can inform resistance management policies. For example, by understanding where resistance originates, research can begin to elucidate the environmental and population-level contexts which favour its rise and spread. This has been seen in the evolution of drug resistance in the malaria parasite, which repeatedly appears and spreads from an area in Cambodia7, driving research into understanding the causes of this pattern. Our aim is therefore to develop a highly-multiplexed genotypic panel that can be used in the first instance to quantify the insecticide-resistance association of mutations, and in the second instance to track these mutations across mosquito populations.
There are two main challenges involved in the development of genetic screening assays for insecticide resistance. First, genetic markers of resistance need to be identified and their effect size estimated. Several single nucleotide polymorphism (SNP) mutations associated with insecticide resistance have already been discovered in Anopheles8–15, providing the first opportunities for genetic screening. However, a substantial proportion of the variance in resistance remains unexplained, making it crucial to identify new markers and to quantify their impact.
Second, new approaches are required for large-scale, high-throughput screening of large marker panels, to reduce the costs and time required to screen mosquito populations. The Agena Biosciences iPLEX MassARRAY technology is a platform capable of accurate genotyping combined with high level multiplexing capability. MassARRAY panels have already been developed for a range of infectious disease questions, such as studying resistance to malaria in humans16, mapping genetic diversity in parasites17 and detecting ancestry and inter-specific hybridisation in Anopheles18.
We present the development and application of an iPLEX MassARRAY panel of 28 genotypic markers for screening Anopheles gambiae populations to quantify the effects of known and putative insecticide resistance markers, and to track the origins of spread of knockdown resistance variants (kdr) in the Voltage-gates sodium channel (Vgsc) gene. We applied our panel to samples from four different countries from West (Burkina Faso and Ghana), Central (Democratic Republic of Congo - DRC) and East Africa (Kenya). These samples were assayed for susceptibility to permethrin (all four countries), deltamethrin (Ghana, DRC and Kenya) and DDT (Ghana). This genetic screen:
revealed a heterologous duplication in Gste2 that combines resistance-associated and wild-type alleles,
identified an unprecedented diversity of kdr genetic backgrounds in the DRC,
reported the first evidence of a role for the Gste2-119V mutation in insecticide resistance in An. gambiae.
found evidence that a mutation found alongside kdr reduces insecticide resistance, possibly indicating that this mutation serves to compensate for physiological costs of kdr.
Methods
Sample collection
Samples from Burkina Faso
Anopheles larvae were collected using hand dippers from semi-permanent and temporary water bodies between June and October 2013 at four sites (Bakaridjan: 10.407 N, −4.562 W; Bounouba: 10.357 N, −4.439 W; Naniagara: 10.536 N, −4.669 W and Tiefora: 10.632 N, −4.556 W). Samples were transported to the insectaries in Banfora where they were maintained at a temperature of 27 °C (±2 °C) and a relative humidity of 80% (±10%), and fed with TetraMin Baby®. Adult mosquitoes were tested at a range of permethrin concentrations (5–120 ppm) using an adaptation of the CDC bottle bioassay19 where we exposed the mosquitoes for 60 minutes and recorded mortality after 24 hours. For this study, we selected mosquitoes exposed to 20 ug/ml because this was the concentration that came closest to killing 50% of the mosquitoes (bioassay results at 20 ppm shown in Supplementary Table S1). All mosquitoes were stored individually in perforated PCR tubes and placed into sealed bags with silica gel to avoid the decomposition and ensure DNA preservation. DNA was extracted using the LIVAK method20 and samples were identified to species using SINE PCR21.
Samples from DRC
Samples from the DRC were the same as previously reported22. An. gambiae mosquitoes were collected in March and April 2016 from three rural collection sites (Pambwa, 3.937 N, 20.772 E; Fiwa, 4.318 N, 20.778 E; Bassa, 4.267 N, 21.283 E) in the area surrounding the major town of Gbadolite, near the border with the Central African Republic. Adult mosquito collections were carried out in all three villages using both manual and mechanical (‘Prokopack’23) aspirators and were maintained in a field insectary until egg-laying. Larvae were collected from breeding sites in Fiwa and Pambwa. Larvae collected directly, and those raised from eggs, were reared until the adult stage. All mosquitoes were identified to species group using phenotypic keys24 and insecticide resistance testing was carried out on 3–5 day-old adult An. gambiae s.l. to assess resistance to permethrin (0.75%) and deltamethrin (0.05%) using standard World Health Organization (WHO) protocols25 (overall bioassay results for the population shown in Supplementary Table S2). All mosquitoes were stored on silica gel in 0.2 ml tubes for later DNA analyses. DNA was extracted from individual mosquitoes using Nexttec (Nexttec, Biotechnologie GmbH) extraction plates according to manufacturer’s instructions, and mosquitoes were identified to species using SINE PCR21.
Samples from Ghana
Ghanaian samples were collected from Keta (5.917 N, 0.991 E), an urban community in the Volta Region of Ghana. Keta is located within the coastal savannah agroclimatic zone, with vegetation consisting of shrubs, grasses and a few scattered trees.
An. gambiae s.l. larvae were collected from September to November, 2016, using hand-held ladles. Collections were made from a variety of habitats such as pools, puddles, drainage channels, irrigations and vegetable fields, and transported to the laboratory in partly-filled labelled plastic containers. Larvae were reared in the insectaries at the Animal Science department of the Biotechnology and Nuclear Agricultural Research Institute (BNARI), Accra. Insecticide resistance assays were performed on 3–5 day-old females following standard WHO tube assay protocols (0.05% deltamethrin, 0.75% permethrin or 4% DDT; overall bioassay results for the population shown in Supplementary Table S3).
DNA was extracted from single mosquitoes using the Nexttec 96-well plate DNA Isolation kit. Species identification was performed on the whole-genome-amplified DNA (Section 2.2) using standard species identification PCR26 followed by SINE PCR to further distinguish An. gambiae from An. coluzzii21.
Samples from Kenya
Mosquitoes were sampled from four malaria-endemic regions in Western Kenya (Teso: 0.656 N, 34.353 E, Bondo: −0.098 S, 34.273 E, Rachuonyo: −0.353 S, 34.656 E and Nyando: −0.162 S, 34.921 E). These regions were under distinct vector control interventions, with Teso and Bondo having insecticide treated nets and Nyando and Rachuonyo having both insecticide treated nets and indoor residual spraying. Anopheles larvae were collected from aquatic habitats using the standard dipping method and transferred to plastic tins using wide-mouth pipette for transportation to KEMRI, Kisumu laboratories for rearing. Larvae were reared on a mixture of fish food and brewer’s yeast provided daily. Upon pupation, individuals were transferred to cages to emerge as adults and provided with 10% sucrose in cotton pledgets.
Three-day-old adult females were exposed to either permethrin (0.75%) or deltamethrin (0.05%) using WHO impregnated papers for 1 hour following WHO guidelines27 (overall bioassay results for the population shown in Supplementary Table S4). Mortality was recorded and all mosquitoes were placed in individual tubes and frozen at −20 °C for molecular analysis. DNA was extracted from whole samples using ethanol precipitation28.
A newly-developed melt-curve based species identification assay29 was used because existing techniques are prone to interpretation errors as a result of similar sized PCR bands for An. arabiensis vs An. gambiae on agarose gels. The assay uses SYBR-green with a universal forward primer (5′-ATTGCTACCACCAAAATACATGAAA-3), a reverse primer matching both An. arabiensis and An. gambiae with G-8 extension (5′-GGGGGGGGGAATAATAAGGAACTGCATTTAAT-3′) to slightly increase the amplicon melting temperature, and an An. arabiensis specific reverse primer (5′- GGATGTCTAATAGTCTCAATAGATG -3′).
SNP genotyping
We developed a multiplex panel of 28 SNP markers (Supplementary Data S1) based on the AgamP3 reference genome30 and information on variable sites identified by the An. gambiae 1000 Genomes (Ag1000 G) project31. Primers for these SNPs were developed using the MassARRAY Assay Design software (version 4.0.0.2; Agena Biosciences, Hambrug, Germany).
The SNPs that we chose fell into three categories. First, we included eight SNPs that have previously been associated with insecticide resistance. These include the two kdr mutations (Vgsc-995F and Vgsc-995S), as well as a further Vgsc mutation (Vgsc-1570Y) known to exist on a Vgsc-995F background and conferring increased resistance to pyrethroids9, two mutations associated with resistance to dieldrin (Rdl-296G and Rdl-296S8), a mutation in the gene Gste2 associated with metabolic resistance to DDT (Gste2-114T11) and a mutation associated with resistance to carbamates and organophosphates (Ace1-280S, previously referred to as 119S15). The eighth SNP in this set was a mutation in codon 119 of Gste2 (Gste2-119V), which was included because a mutation in the same codon has been shown to strongly increase resistance to DDT in An. funestus13, raising the prospect that the mutation in An. gambiae may have a similar function.
Second, we included eight non-synonymous SNPs (Vgsc-1868T, Vgsc-1874S, Vgsc-1874L, Vgsc-1853I, Vgsc-1934V, Vgsc-1746S, Vgsc-791M and Vgsc-1597G) that have been found to be strongly associated with the Vgsc-995F mutation31 and are thus regarded as potential candidates influencing resistance either by enhancing the impact of Vgsc-995F or compensating for suspected fitness effects.
Third, we included twelve SNPs that can differentiate the five haplotype backgrounds of the Vgsc-995F mutation (haplotype backgrounds F1-F5) and the five haplotype backgrounds of the Vgsc-995S mutation (haplotype backgrounds S1-S5) (Anopheles gambiae 1000 Genomes Consortium 2017). These SNPs were identified using the Ag1000G data by searching for SNPs that differentiated each haplotype background from the other haplotypes with the same kdr mutation. Within each of the two kdr mutations (Vgsc-995F and Vgsc-995S), the SNPs always provided perfect separation between the five possible haplotype backgrounds, although a few haplotypes that were not assigned any background in the Ag1000G data could be incorrectly assigned a background based on these SNPs (Supplementary Data S2).
DNA extractions were whole-genome amplified and then genotyped with the Agena Biosciences iPLEX platform as described32, using our panel of 28 SNPs. Genotype calls were obtained from the raw data using the TYPER software (version 4, Agena Biosciences) and raw data as cluster plots were inspected and manually curated to remove ambiguous calls. To assess the quality of the assays and the genotype calling process, we also used our panel to genotype 287 samples previously used in phase 2 of Ag1000G (https://www.malariagen.net/data/ag1000g-phase-2-ar1) and compared the two sets of genotype calls. Raw data and genotype calls are provided in Supplementary Data S3, and genotype calls are also provided along with sample information in Supplementary Data S4. Samples that failed genotyping at more than 50% of loci were excluded from the analysis (this filter removed 39 samples from Ghana and two samples from Kenya).
Deviations from Hardy-Weinberg equilibrium
Tests for deviations from Hardy-Weinberg equilibrium were performed using the HWExact function from the R package HardyWeinberg. These tests were performed for every SNP × location combination in which the SNP was segregating (i.e.: where both alleles were present in the sampling location). This resulted in 72 separate tests, which were adjusted for multiple testing using a Bonferroni correction, with a resulting threshold α value of 0.0007.
Identification of kdr haplotype backgrounds
The process of identifying the kdr haplotype background of a sample based on its genotype was implemented using custom R functions that has been made available as Supplementary Materials and which is described in Supplementary Methods S1.
Statistical analysis of resistance
The two SNPs in kdr (Vgsc-995F and Vgsc-995S) are at different nucleotide positions but affect the same codon and are mutually exclusive. We therefore re-coded these two SNPs as a single variant with three alleles: L (wild-type), F and S. Similarly, we also re-coded the two Rdl SNPs as a single variant with three alleles.
Associations between genotype and insecticide resistance were tested using generalised linear models (GLM) with binomial errors and a logit link function, implemented in R using the package lme433. Genotypes were included as categorical fixed effects. Three candidate resistance markers (Vgsc-1853I, Vgsc-1934V and Vgsc-1874S) were not included in the models due to the scarcity of the mutant allele (n < 5) in the combined data, and the marker for Vgsc-791M was excluded as it was almost perfectly associated with Vgsc-1746S (Supplementary Table S5). For the data from Ghana, where all samples came from the same location, no random effects were included and the analysis was performed using the glm function. For the three other countries, sampling location was included as a random effect using generalised linear mixed models (GLMM) implemented by the function glmer. In one case (permethrin phenotype from DRC) where the mixed models failed to converge, preventing the inclusion of random effects, the modelling was repeated by including location as a fixed effect, using the function glm. In Burkina Faso and DRC, the majority of samples were of the species An. gambiae, with very few An. coluzzii (7 from Burkina Faso, 2 from the DRC), and An. coluzzii samples were thus excluded from the analyses. Similarly, only 6 An. arabiensis samples were found among the samples tested for resistance to deltamethrin in Kenya and were thus excluded. An. arabiensis were better represented in Kenya among the samples tested for resistance against permethrin (18 An. arabiensis samples), and species was thus included as a random factor.
Minimal significant models were obtained by a stepwise backward elimination process using a custom R function provided in the Supplementary Materials and described in Supplementary Methods S2. Briefly, resistance markers were included together into the full model, and the significance of each marker was obtained using the anova function to compare the full model to the model without the marker. Stepwise, the least significant marker was removed until only significant markers were left, providing the minimal model. The P-values reported for the significant markers are the result of the ANOVA comparing the minimal model against the model with the marker removed.
Detection of Gste2 duplication
The Gstue_Dup7 duplication was detected by PCR using primers designed on either side of the duplication breakpoint. The position of the breakpoint was known from previous data34 and primers were designed using NCBI Primer Blast35. Further details on the primers are provided in Supplementary Methods S3.
No human subjects were involved in this research.
Results
High concordance between iPLEX MassARRAY and Ag1000G genotype calls
In total, 29,148 assays were run using the iPLEX MassARRAY panel (1,041 samples × 28 SNPs). Of these, 1,408 (4.8%) failed to produce a genotype call, with none of the individual assays having a failure rate greater than 10% (Supplementary Fig. S1). The failure rate was lowest in An. coluzzii, where 24 out of 7,980 assays failed (0.3%, Supplementary Fig. S2), followed by An. gambiae, where 274 out of 18,788 assays failed (1.46%, Supplementary Fig. S3), and highest in An. arabiensis, where there were 59 failures out of 672 assays (8.8%, Supplementary Fig. S4). The remaining samples were of unknown species as the DNA was not of high enough quality for the species ID assay.
Of the 8,036 individual assays performed on samples from Ag1000G (287 samples × 28 SNPs), only 5 (0.06%) iPLEX calls were divergent from the Ag1000G callset. The rest either gave concordant calls (98.2%) or failed calling by the iPLEX platform (1.7%). The high levels of concordance with the Ag1000G calls indicate that the results of the iPLEX assays can be used with confidence.
Deviation from Hardy-Weinberg equilibrium implies heterologous duplication of Gste2 in Ghana
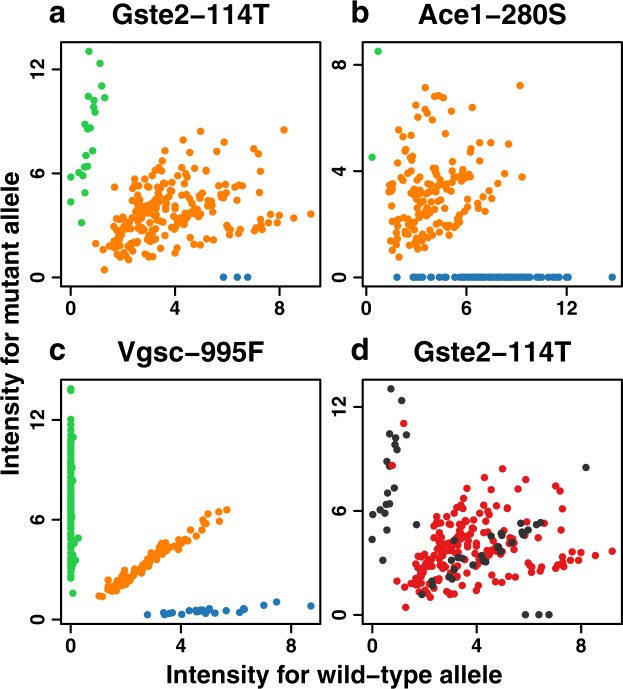
The SNPs Ace1-280S and Gste2-114T showed significant deviation from Hardy-Weinberg expectations in An. coluzzii from Ghana, caused by an excess of heterozygotes at these loci (P < 10-13 in both cases, Table 1). Such heterozygote excess can be caused by the presence of heterologous amplifications, which can create functional heterozygotes by combining wild-type and mutant alleles on a single chromosome. For both Gste2-114 T and Ace1-280 S, the raw intensities for heterozygous samples are split into several clusters rather than the normal single cluster (Fig. 1), which is also consistent with the presence of duplicate copies of one or both alleles. The heterologous Ace1 duplication in Ghana is already well-documented36,37, but no such duplication has yet been reported in Gste2.
Table 1.
Allele frequencies for Gste2-114T and Ace1-280S show large excesses of heterozygotes in Ghana.
| Gste2-114T | Ace1-280S | |||||||
|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | Hardy-Weinberg P |
AA | AG | GG | Hardy-Weinberg P |
|
|
Burkina Faso An. gambiae |
71 | 8 | 1 | 0.26 | 0 | 33 | 56 | 0.04 |
|
DRC An. gambiae |
148 | 0 | 0 | 1 | 0 | 0 | 186 | 1 |
|
Ghana An. coluzzii |
3 | 214 | 21 | 1.6 × 10−41 | 2 | 153 | 83 | 1.4 × 10−14 |
|
Kenya An. gambiae |
139 | 0 | 0 | 1 | 0 | 0 | 155 | 1 |
Gste2-114T: wild-type allele = A, mutant = G. Ace1-280S: wild-type allele = G, mutant = A.
Figure 1.
Raw intensities from the iPLEX MassARRAY assays in Ghana show multiple clusters among heterozygote samples (orange points) for Gste2-114T (a) and Ace1-280S (b). This is typically seen when duplications create ratios of mutant/wild-type alleles that differ from the usual 1/1 in heterozygotes. In comparison, equivalent data for Vgsc-995F (c) shows the normal pattern, with heterozygotes consistently falling in a single cluster along a straight diagonal line. Homozygote wild-type and homozygote mutant samples are shown in blue and green respectively. Colour-coding the Gste2-114T data according to whether samples carry Dup7 (red) or not (black) shows that heterozygotes with no duplication predominantly fall along a straight diagonal line (d). The X and Y axes respectively show intensities for the wild-type and mutant alleles.
To confirm whether the heterozygote excess in Gste2-114T is caused by a duplication, we tested our samples for the presence of the duplication Gstue_Dup7, the only Gste2 duplication known to occur in An. coluzzii from Ghana34. Gstue_Dup7 was present in 185 out of 238 (78%) of samples in our An. coluzzii samples from Ghana, a figure seven times higher than was found in Ag1000G, where 6 out of 55 (11%) An. coluzzii from Ghana had the duplication34.
Gstue_Dup7 was strongly associated with heterozygosity at Gste2-114T (Fig. 1d). Out of 185 Ghanaian An. coluzzii samples that carried Gstue_Dup7, 183 were heterozygote at the 114T locus and 2 were homozygote mutants, while none were homozygote wild-type. In contrast, out of 53 samples that did not carry Gstue_Dup7, 31 were 114T heterozygotes, 19 were homozygote mutants and 3 were homozygote wild-type. Furthermore, the 114T heterozygotes without the duplication did not show the same scatter of raw intensities as the heterozygotes that carried the duplication (Fig. 1d).
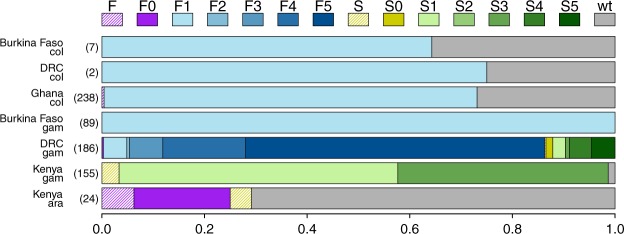
High diversity of kdr haplotypes in the DRC
The Vgsc-995 mutations were at high frequency in all four countries (Fig. 2, Table 2). The wild-type allele was found at 2% frequency in An. gambiae from Kenya, but was completely absent in An. gambiae from Burkina Faso and the DRC. In An. coluzzii, the wild-type codon was found at 27% frequency in Ghana, and at similar frequencies in the few samples from Burkina Faso and the DRC.
Figure 2.
Frequencies of kdr haplotypes in the four countries from the study (total number of individuals, i.e. half the number of haplotypes, shown in brackets). The F1 haplotype is the only one present in Ghana and Burkina Faso, while haplotypes S1 and S3, along with a possible new Vgsc-995F haplotype, are found in Kenya. In the DRC, at least nine out of the ten kdr haplotypes co-exist. F and S indicate samples for which the kdr mutation was known, but the haplotype could not be determined because of failed calls in at least some background markers; F0 and S0 indicate haplotypes where the kdr mutation was known and all of the background markers produced wild-type calls, suggesting a novel haplotype background; wt = wild-type; ara = An. arabiensis; col = An. coluzzii; gam = An. gambiae.
Table 2.
Allele frequencies for the eight SNPs with known associations with insecticide resistance.
| Country | Species | Number of samples |
Vgsc- 995F |
Vgsc- 995S |
Rdl-
296G |
Rdl-
296S |
Ace1-
280S |
Gste2-
114T |
Gste2-
119V |
Vgsc- 1570Y |
|---|---|---|---|---|---|---|---|---|---|---|
| BF | An. coluzzii | 7 | 0.64 | 0 | 0 | 0.43 | 0 | 0.57 | 0 | 0.29 |
| DRC | An. coluzzii | 2 | 0.75 | 0 | 0 | 0.25 | 0 | 0.50 | 0 | 0 |
| Ghana | An. coluzzii | 238 | 0.73 | 0 | 0.54 | 0 | 0.33 | 0.54 | 0 | 0 |
| BF | An. gambiae | 89 | 1 | 0 | 0.35 | 0 | 0.19 | 0.06 | 0.65 | 0.20 |
| DRC | An. gambiae | 186 | 0.86 | 0.14 | 0.05 | 0 | 0 | 0 | 0.35 | 0.01 |
| Kenya | An. gambiae | 155 | 0 | 0.98 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kenya | An. arabiensis | 24 | 0.25 | 0.04 | 0 | 0 | 0 | 0 | 0 | 0 |
We note that the sample size for An. coluzzii in Burkina Faso and the DRC are small and the allele frequencies will thus be imprecise. The full table showing all 28 markers in the panel is provided in Supplementary Data S5.
In the West African populations (Burkina Faso and Ghana), all kdr mutant alleles were Vgsc-995F of the F1 haplotype background (Fig. 2, Supplementary Data S6). In Kenya, all kdr mutants in An. gambiae carried the Vgsc-995S mutant allele from either of two backgrounds (S1 or S3; Supplementary Data S6). In contrast, most kdr haplotypes in Kenyan An. arabiensis were wild-type (34 out of 48), while the rest were predominantly Vgsc-995F (12 out of 48). These Vgsc-995F samples did not carry any of the SNPs associated with the five known haplotype background, possibly indicating that the Vgsc-995F haplotype background in An. arabiensis is distinct from those found to date in An. gambiae. The two Vgsc-995S haplotypes in this population could not be assigned a background due to failure of most of the haplotype background assays.
In the DRC, all five Vgsc-995F haplotype backgrounds were detected, the most frequent background being F5. Similarly, four of the five Vgsc-995S backgrounds were found in the DRC (Fig. 2, Supplementary Data S6), the most frequent background being S5, and haplotype S2 being absent. Previously, eight kdr haplotypes were found in Cameroon, with haplotypes S1 and S3 being absent31.
Association with insecticide resistance
In Burkina Faso, both Gste2 mutations were associated with increased resistance to permethrin, demonstrating for the first time an association between Gste2-119V and insecticide resistance (Table 3). In contrast, the 1874L Vgsc mutation was negatively associated with resistance to permethrin.
Table 3.
Significant associations between genotype and insecticide resistance.
| Country | Insecti- cide |
Codon | P | Intercept genotype |
Effect of het. vs intercept |
Effect of hom. mutant vs intercept |
Effect of hom. mutant vs het. |
|---|---|---|---|---|---|---|---|
| Ghana | Perm | Vgsc-995 | 0.023 | LL | LF: ≥4* ↑ | FF: ≥4* ↑ | 1.58 ↑ |
| Delt | NA | ||||||
| DDT | Rdl-296 | 7.1 × 10−7 | AA | AG: 2.6 ↑ | GG: 2.95 ↑ | 0.35 ↑ | |
| Burkina Faso | Perm | Vgsc-1874L | 0.041 | PP | LP: −1.18 ↓ | LL: −2.4 ↓ | −1.22 ↓ |
| Gste2-114T | 0.022 | II | IT: 3.01 ↑ | TT: ≥4* ↑ | ≥ 4* ↑ | ||
| Gste2-119V | 0.026 | LL | LV: ≥4* ↑ | VV: ≥4* ↑ | 0.35 ↑ | ||
| Kenya | Perm | NA | |||||
| Delt | NA | ||||||
| DRC | Perm | Vgsc-995 | 0.004 | SS | SF: ≥4* ↑ | FF: ≥4* ↑ | 1.1 ↑ |
| Delt | Gste2-119V | 0.0078 | LL | LV: 0.36 ↑ | VV: −1.54 ↓ | −1.91 ↓ | |
| Vgsc-995 | 0.0038 | SS | SF: −0.55 ↓ | FF: 1.5 ↑ | 2.06 ↑ |
Effects are shown as log odds ratio. Direction of effect compared to the reference (intercept) genotype shown by arrows (↑ indicates increase in resistance compared to the intercept; ↓ indicates decrease in resistance). In Ghana, kdr was either wild-type (L) or 995F. In the DRC, kdr was either 995S or 995F.
*Effects ≥4 (corresponding to odds ratio > 50) were usually seen where there were few samples in a category, leading to all samples having the same phenotype and making odds ratios impossible to calculate.
In the DRC, where the wild-type Vgsc-995 codon was absent, the Vgsc-995F mutation was more strongly associated with resistance to both permethrin and deltamethrin than Vgsc-995S, with a slight tendency for heterozygotes to have lower resistance to deltamethrin than Vgsc-995S homozygotes (Table 3). Seemingly contrary to the Burkina Faso data, there was a negative association between the Gste2-119V mutation and resistance to deltamethrin, although heterozygotes showed slightly higher resistance than homozygote wild-types.
In Ghana, no SNPs were associated with resistance to deltamethrin, while the Vgsc-995F mutation was associated with resistance to permethrin and the Rdl-296G mutation was associated with resistance to DDT (Table 3). There were only three homozygous wild-type samples at the Vgsc-995F locus, thus the significant difference is driven by the difference between the homozygote mutant and heterozygotes.
In Kenya, there were no significant associations between any of the SNPs and resistance to either permethrin or deltamethrin, although it is notable that Vgsc-995 was the only resistance candidate SNP found in this population and was nearly fixed in An. gambiae (Table 2 & Supplementary Data S5).
Discussion
We have developed a multiplex SNP panel for quick, high-throughput screening of large numbers of An. gambiae s.l. at multiple markers useful for detecting insecticide resistance, testing the importance of putative resistance diagnostic markers and tracking the spread of resistance mutations. We have allied this with a formal analytical approach for analysing phenotypic association of these markers, made available as an R function. By screening 713 mosquitoes from four different countries, we have identified a heterologous duplication in a resistance-conferring SNP, confirmed an association with resistance in a candidate SNP, and found the DRC to be an area in which nearly all evolutionary origins of the kdr resistance mutation co-exist.
The presence of a heterologous duplication in Gste2 in An. gambiae from Ghana was first indicated by a very high excess in Gste2-114T heterozygotes and the characteristic multiple clusters of signal intensities in the raw data, both of which bear a striking resemblance to the pattern seen in Ghanaian Ace1-280S data, known to be caused by a heterologous duplication. We confirmed the role of a duplication by showing that the heterozygosity is associated with the presence of the Gste2 duplication Gstue_Dup734. While the vast majority of Gstue_Dup7 alleles are heterologous, we also found evidence of a rare variant of Gstue_Dup7 in two samples where the duplication was homozygous for the 114T SNP. So far, eleven different duplications in the cluster of genes around Gste2 have been reported in An. gambiae and An. coluzzii from across SSA34, but only one of these, Gstue_Dup1, was shown to be associated with the 114T mutation, being homologous for the mutant allele. The evolution of duplications in Gste2 may therefore mirror what has been found in Ace1, where both homologous and heterologous duplications exist. If the 114T mutation carries a physiological cost in the absence of insecticide pressure, the heterologous duplication discovered here may serve to reduce this cost, as has been found in the case of the Ace1 duplication38.
Gstue_Dup7 was found in 78% of our An. coluzzii samples from Ghana collected in 2015 from Keta (5.9175 N, 0.9916 E), which is seven times higher than was found in samples collected in 2012, albeit from different sites further West in Ghana (Koforidua (6.0945 N, 0.2609 W), Madina (5.6685 N, 0.2193 W), Takoradi (4.9122 N, 1.7740 W), Twifo Praso (5.6086 N, 1.5493 W)34). While we cannot exclude the possibility that this increase reflects differences between sites, the high frequency of this duplication in Keta, combined with the striking difference in frequency between samples collected in 2012 and 2015, strongly suggest that this duplication is rapidly spreading through the An. coluzzii population.
The high diversity of kdr haplotype backgrounds in the DRC points to this area of central Africa as a key to understanding the spread of the most well-characterised insecticide resistance allele in An. gambiae. All five Vgsc-995F backgrounds31 were found in this population, as well as four of the five Vgsc-995S backgrounds, combining haplotypes previously found in Uganda, (S1 and S3), Kenya (S3) and Cameroon (S4 and S5). The only background not confirmed in the DRC was S2, which was previously found in Gabon and Cameroon31. This striking geographical co-existence of kdr haplotype backgrounds could have two explanations. First, most of the main kdr origins could have occured in central Africa, and subsequently spread from there to other countries, with different haplotypes becoming established in different regions. Second, The different kdr haplotypes could have spread from their respective origins and converged in the DRC, where most then persisted without being entirely replaced (although F5 is by far the most prevalent, with a frequency of over 50%). Differentiating between these two hypotheses will require more in-depth analysis of the sequence variability within each haplotype sequence for each background in the DRC and elsewhere.
The Vgsc-995F haplotype found in An. arabiensis from Kenya did not conform to any of the known haplotype backgrounds for this mutation, suggesting that a sixth Vgsc-995F origin exists, which may have originated in East Africa. The Kenyan samples in the present study are from Western Kenya, where Vgsc-995F has previoulsy been reported in both An. arabiensis and A. gambiae39. In contrast, the Kenyan samples included in the Ag1000G data, where the haplotype backgrounds were defined, came from an eastern Kenyan population of An. gambiae where Vgsc-995F was not found. The Kenyan Vgsc-995F background was therefore not present in Ag1000G and could not be included in the definition of haplotype backgrounds. Whether the Vgsc-995F background present in An. gambiae from western Kenya corresponds to one of the five known 995F backgrounds, or to the one found in An. arabiensis from the same area remains to be resolved.
Associations of genetic markers with insecticide resistance revealed several unexpected associations. The Gste2-114T marker, which has been previously associated with resistance to DDT11 was associated with resistance to permethrin in Burkina Faso. To our knowledge, this is the first report of a role for this mutation in permethrin resistance, although one previous study found that it was also associated with resistance to another pyrethroid, deltamethrin40. The evidence is therefore increasing for a role of Gste2-114T in cross-resistance to multiple insecticides. In Ghana, there was no significant association between Gste2-114T and resistance to pyrethroids or DDT, but this is likely due to the very high frequency of heterozygotes, making any association difficult to detect. In the same gene, the Gste2-119V mutation was associated with increased permethrin resistance in Burkina Faso, but with decreased deltamethrin resistance in the DRC. These contrasting effects may reflect different changes in binding affinity to these two insecticides caused by this mutation. While the metabolic properties induced by the Gste2-119V mutation have not been studied, research on the Gste2-119F mutation in An. funestus showed that the mutant form was able to metabolise permethrin, but not deltamethrin13. The negative association with deltamethrin resistance found here is, however, novel, and may be explained by a metabolic cost imposed by the mutation, or by reduced affinity for deltamethrin in the case of the An. gambiae mutation. If the opposing effects on permethrin and deltamethrin resistance detected here are confirmed, it will be important to routinely screen for this mutation when making a choice of insecticides in control campaigns.
Intriguingly, Rdl-296G was associated with resistance to DDT in Ghana. This mutation is in the target site of cyclodiene insecticides such as dieldrin, which was used in malaria control programmes in Africa during the 1950s and 1960s, but has since been discontinued. Resistance to dieldrin has somehow been maintained in natural mosquito populations41–43, although the selective forces causing its persistence remain a puzzle. One possibility is that cyclodienes continue to be used as insecticides in private agriculture, but another is that the Rdl-296 mutations confer cross-resistance to other insecticides. For example, insertion of the An. gambiae Rdl gene into Xenopus oocytes demonstrated that fipronil, deltamethrin and imidacloprid all inhibited the GABA receptor, of which RDL is a subunit, and that this effect was reduced by the Rdl-296G mutation44. However, the same experiment found no effect of DDT on RDL. Our results may point to an as-yet unrecognised example of cross-resistance between dieldrin and DDT, through mechanisms that remain to be elucidated.
The Vgsc-1874L mutation, exclusively found on the background of the Vgsc-995 F1 haplotype, was negatively associated with resistance to permethrin in Burkina Faso, where the F1 haplotype is fixed in the population. The mutation’s continued presence in the population despite its cost to permethrin resistance might be due to one of two factors. First, the mutation may provide improved resistance to other insecticides. However, since kdr is a target site resistance mechanism, the insecticides that this could apply to are limited to the pyrethroids and DDT. Second, the Vgsc-1874L mutation may serve to compensate the costs of kdr, serving to increase the fitness of the haplotype in the absence of insecticides. This would be a worrying development in the evolution of insecticide resistance, as it would favour the persistence of the kdr mutation during cycles of insecticide rotation in control programmes.
We do not propose that genotypic screening should completely replace phenotypic measurements of resistance in monitoring programmes. Genetic markers cannot yet accurately predict the resistance of a population, although some can be highly diagnostic45, and further studies such as this one are required to address this shortcoming. Furthermore, as and when mosquitoes develop new resistance mutations, phenotypic assays will be required to discover these mutations and quantify their effects. However, once the range and effects of genetic markers are properly understood and can form the basis of predictive models, we hope that it will be possible to scale down the laborious phenotypic assays in favour of large scale genotyping. This will of course require high-throughput, cost-effective genotyping methods. We chose the iPLEX MassARRAY technology in our study due to its ability for multiplexing markers, making it more cost-effective at scale than assays with only one or a few markers. Still greater multiplexing can be achieved by other methods such as amplicon sequencing, which offer a prospective avenue for further development of our panel as new markers are discovered and need to be added.
In conclusion, our results highlight the importance and value of high-throughput genetic screening for the study and monitoring of insecticide resistance in malaria vectors. The three main steps involved in developing new markers of insecticide resistance are 1. discovery of new mutations, 2. confirmation of association with resistance and 3. tracking the mutation across populations (although the order of steps 1 and 2 can be reversed if a forward genetics approach is used to identify mutations responsible for an observed difference in phenotype). Our panel of 28 genetic markers achieved all three of these steps, albeit on different markers. We found evidence for a heterologous duplication in Gste2, we reported the first evidence of resistance association for Gste2-119V, bringing the total number of resistance-associated SNPs in An. gambiae s.l. up to 10, and we investigated the distribution of Vgsc-995 mutations across East, Central and West Africa. Our results also highlight gene and mutations that should be specifically taken into account when investigating resistance to certain insecticides. For example, at least two mutations in Gste2 are involved in resistance to permethrin, while a role for Rdl or its associated markers in DDT resistance would be an interesting topic for further investigation. Work on our genotypic resistance panel should now expand in two directions. First, the panel should be applied to more populations, building a more complete picture of the distribution of these genotypes and their importance in resistance. In particular, more accurate measures of resistance can be obtained by using a range of insecticide concentrations and identifying the mosquitoes killed or surviving at each concentration, thus discerning between slightly resistant and highly resistant phenotypes. Second, more markers should be gradually added to the panel as their importance becomes recognised. Once routine monitoring of important mutations become common-place, our understanding of the genetic underpinnings of insecticide resistance will increase dramatically.
Supplementary information
Acknowledgements
Funding was provided by Award Number R01AI116811 from the National Institute of Allergy and Infectious Diseases (NIAID) and Award MR/P02520X/1 from the Medical Research Council, UK. The work was also supported by the E.C. FP7 Project Grant No: 265660 “AvecNet” and a Wellcome Trust core award to The Wellcome Trust Centre for Human Genetics (090532/Z/09/Z, 203141). K.R. and C.H. are supported by the Wellcome Trust (090770/Z/09/Z, 204911/Z/16/Z).
Author Contributions
E.R.L., K.A.R., E.O., D.P.K., D.W. and M.J.D. designed the study. A.L., J.E., N.G., B.K., J.M. and E.O. collected the samples. K.A.R., A.L., J.E., N.G., B.K., H.N., C.H., E.J.R. and A.E.V.H. performed the lab work. E.R.L. and K.A.R. carried out the analysis. E.R.L., K.A.R., A.L., J.E., N.G., B.K., E.J.R., A.E.V.H., D.W. and M.J.D. wrote the manuscript. All authors read and approved the final manuscript.
Data Availability
All of the raw data for this study are included as Supplementary Materials.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49892-6.
References
- 1.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Implications of Insecticide Resistance Consortium Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: trends in pyrethroid resistance during a WHO-coordinated multi-country prospective study. Parasites & vectors. 2018;11:550. doi: 10.1186/s13071-018-3101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kafy HT, et al. Impact of insecticide resistance in Anopheles arabiensis on malaria incidence and prevalence in Sudan and the costs of mitigation. Proceedings of the National Academy of Sciences. 2017;114:E11267–E11275. doi: 10.1073/pnas.1713814114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Protopopoff N, et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. The Lancet. 2018;391:1577–1588. doi: 10.1016/S0140-6736(18)30427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends in Parasitology. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Kleinschmidt I, et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. The Lancet infectious diseases. 2018;18:640–649. doi: 10.1016/S1473-3099(18)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miotto O, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nature genetics. 2013;45:648. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du W, et al. Independent mutations in the Rdl locus confer dieldrin resistance to Anopheles gambiae and An. arabiensis. Insect Molecular Biology. 2005;14:179–183. doi: 10.1111/j.1365-2583.2005.00544.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones CM, et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proceedings of the National Academy of Sciences. 2012;109:6614–6619. doi: 10.1073/pnas.1201475109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Torres D, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae ss. Insect molecular biology. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell SN, et al. Metabolic and target-site mechanisms combine to confer strong DDT resistance in Anopheles gambiae. PLoS One. 2014;9:e92662. doi: 10.1371/journal.pone.0092662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranson H, et al. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect molecular biology. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 13.Riveron JM, et al. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biology. 2014;15:R27. doi: 10.1186/gb-2014-15-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weetman D, et al. Candidate-gene based GWAS identifies reproducible DNA markers for metabolic pyrethroid resistance from standing genetic variation in East African Anopheles gambiae. Scientific reports. 2018;8:2920. doi: 10.1038/s41598-018-21265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weill M, et al. Insecticide resistance in mosquito vectors. Nature. 2003;423:136–137. doi: 10.1038/423136b. [DOI] [PubMed] [Google Scholar]
- 16.Network MGE, et al. Reappraisal of known malaria resistance loci in a large multicenter study. Nature genetics. 2014;46:1197–1204. doi: 10.1038/ng.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkman SK, et al. A genome-wide map of diversity in Plasmodium falciparum. Nature genetics. 2007;39:113. doi: 10.1038/ng1930. [DOI] [PubMed] [Google Scholar]
- 18.Norris LC, et al. Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proceedings of the National Academy of Sciences. 2015;112:815–820. doi: 10.1073/pnas.1418892112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brogdon WG, McAllister JC. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. Journal of the American Mosquito Control Association. 1998;14:159–164. [PubMed] [Google Scholar]
- 20.Livak KJ. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107:611–634. doi: 10.1093/genetics/107.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santolamazza F, et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malaria journal. 2008;7:163. doi: 10.1186/1475-2875-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynd A, et al. Insecticide resistance in Anopheles gambiae from the northern Democratic Republic of Congo, with extreme knockdown resistance (kdr) mutation frequencies revealed by a new diagnostic assay. Malaria journal. 2018;17:412. doi: 10.1186/s12936-018-2561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. Journal of medical entomology. 2009;46:1256–1259. doi: 10.1603/033.046.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara. Publications of the South African Institute for Medical Research. 1987;55:1–143. [Google Scholar]
- 25.Geneva: World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. (2016).
- 26.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. The American journal of tropical medicine and hygiene. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 27.Geneva: World Health Organisation. Test procedures for insecticides monitoring in malaria vector mosquitoes. (2013).
- 28.Collins FH, et al. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. The American journal of tropical medicine and hygiene. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- 29.Chabi, J. et al. Rapid high throughput SYBR green assay for identifying the malaria vectors Anopheles arabiensis, Anopheles coluzzii and Anopheles gambiae s.s. Giles. bioRxiv, (2018). [DOI] [PMC free article] [PubMed]
- 30.Sharakhova MV, et al. Update of the Anopheles gambiae PEST genome assembly. Genome biology. 2007;8:R5. doi: 10.1186/gb-2007-8-1-r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anopheles gambiae 1000 Genomes Consortium. Genetic diversity of the African malaria vector Anopheles gambiae. Nature552, 96-100 (2017). [DOI] [PMC free article] [PubMed]
- 32.Fabrigar DJ, Hubbart C, Miles A, Rockett K. High-throughput genotyping of Anopheles mosquitoes using intact legs by Agena Biosciences iPLEX. Molecular ecology resources. 2016;16:480–486. doi: 10.1111/1755-0998.12473. [DOI] [PubMed] [Google Scholar]
- 33.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 34.Lucas, E. R., et al. Whole genome sequencing reveals high complexity of copy number variation at insecticide resistance loci in malaria mosquitoes. Genome Research In press, (2019). [DOI] [PMC free article] [PubMed]
- 35.Ye J, et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assogba BS, et al. The ace-1 locus is amplified in all resistant Anopheles gambiae mosquitoes: fitness consequences of homogeneous and heterogeneous duplications. PLoS Biology. 2016;14:e2000618. doi: 10.1371/journal.pbio.2000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weetman D, et al. Contemporary evolution of resistance at the major insecticide target site gene Ace-1 by mutation and copy number variation in the malaria mosquito Anopheles gambiae. Molecular Ecology. 2015;24:2656–2672. doi: 10.1111/mec.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Assogba BS, et al. An ace-1 gene duplication resorbs the fitness cost associated with resistance in Anopheles gambiae, the main malaria mosquito. Scientific Reports. 2015;5:14529. doi: 10.1038/srep14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochomo E, et al. Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An. arabiensis in western Kenya. Parasites & vectors. 2015;8:616. doi: 10.1186/s13071-015-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opondo KO, et al. Does insecticide resistance contribute to heterogeneities in malaria transmission in The Gambia? Malaria journal. 2016;15:166. doi: 10.1186/s12936-016-1203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asih PBS, et al. Existence of the Rdl mutant alleles among the Anopheles malaria vector in Indonesia. Malaria journal. 2012;11:57. doi: 10.1186/1475-2875-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwiatkowska RM, et al. Dissecting the mechanisms responsible for the multiple insecticide resistance phenotype in Anopheles gambiae ss, M form, from Vallée du Kou, Burkina Faso. Gene. 2013;519:98–106. doi: 10.1016/j.gene.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wondji CS, et al. Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect biochemistry and molecular biology. 2011;41:484–491. doi: 10.1016/j.ibmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor-Wells J, Brooke BD, Bermudez I, Jones AK. The neonicotinoid imidacloprid, and the pyrethroid deltamethrin, are antagonists of the insect Rdl GABA receptor. Journal of neurochemistry. 2015;135:705–713. doi: 10.1111/jnc.13290. [DOI] [PubMed] [Google Scholar]
- 45.Essandoh J, Yawson AE, Weetman D. Acetylcholinesterase (Ace-1) target site mutation 119S is strongly diagnostic of carbamate and organophosphate resistance in Anopheles gambiae ss and Anopheles coluzzii across southern Ghana. Malaria journal. 2013;12:404. doi: 10.1186/1475-2875-12-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the raw data for this study are included as Supplementary Materials.