Abstract
Tumor plasticity refers to tumor cell's inherent property of transforming one type of cell to different types of cells. Tumor plasticity is the main cause of tumor relapse, metastasis and drug resistance. Cancer stem cell (CSC) model embodies the trait of tumor plasticity. During carcinoma progression, epithelial-mesenchymal transition (EMT) plays crucial role in the formation of CSCs and vasculogenic mimicry (VM) based on epithelial-mesenchymal plasticity. And the unique tumor microenvironment (TME) not only provides suitable niche for CSCs but promotes the building of CSCs and VM that nourishes tumor tissue together with neoplasm metabolism by affecting tumor plasticity. Therapeutic strategies targeting tumor plasticity are promising ways to treat malignant tumor. In this article, we discuss the recent developments of potential drug targets related to CSCs, EMT, TME, VM, and metabolic pathways and summarize drugs that target these areas in clinical trials.
Keywords: tumor plasticity, cancer stem cells, vasculogenic mimicry, extracellular matrix, tumor microenvironment, targeting
Introduction
The universal methods for cancer treatment include surgery, radiotherapy, and chemotherapy. Chemotherapy is the principle modality for the treatment of malignant tumor, especially tumors in the late stages. Despite significant improvement of cancer chemotherapy in clinical practice, there are still many obstacles that chemotherapeutic drugs must overcome: (1) lack of effective treatments for metastatic tumors; (2) ineffectiveness in killing drug-resistant tumor cells; and (3) lack of new targets based on the characteristics of neoplasm, such as tumor plasticity.
Tumor plasticity prompts tumor cells to differentiate into a variety of cell types to adapt to different environment (1). Emerging evidence suggested that tumor plasticity played critical roles in the emergence of drug resistance and the promotion of tumor growth, invasion and metastasis. Therefore, there is an urgent need to develop new therapeutic agents to target tumor plasticity.
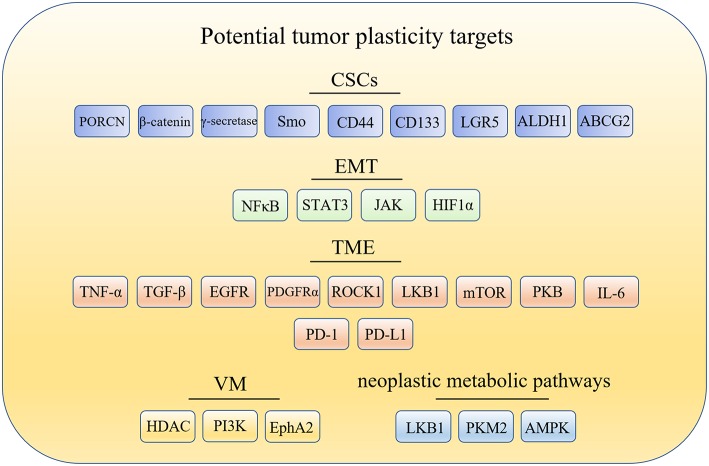
The cancer stem cells (CSCs) model offers one explanation for tumor plasticity. The CSCs model revealed that only a minority of tumorigenic cells contribute to tumor growth and progression. However, there are many other aspects closely related to tumor plasticity. For example: (1) epithelial-mesenchymal transition (EMT), which contributes to the phenotypic plasticity and promotes cancer metastasis; (2) tumor microenvironment (TME), which contains extracellular matrix (ECM) and cells such as fibroblasts, endothelial and immune cells that are the primary source of signals to and from the tumors; (3) vasculogenic mimicry (VM), which is a microcirculation that is independent of angiogenesis in aggressive primary and metastatic tumors and comprised of non-endothelial cell generated by tumor cells and ECM; and (4) neoplastic metabolic pathways, that mainly include glycolysis and oxidative phosphorylation (OXPHOS). Changes of metabolic pathways between glycolysis and OXPHOS in cancer cells is prevalent during tumorigenesis and metastasis. Hence, targeting glycolysis and OXPHOS is essential to wipe out metabolic plasticity in cancer cells. Here, the potential targets related to tumor plasticity was summarized in Figure 1. In this mini review, we summarize the recent advances in anticancer compounds targeting CSCs, ETM, TEM, VM formation, and metabolic pathways, which is associated with tumor plasticity.
Figure 1.
Potential drug targets related to tumor plasticity. CSCs, cancer stem cells; EMT, epithelial-mesenchymal transition; TME, tumor microenvironment; TME, tumor microenvironment; PORCN, porcupine; Smo, smoothened; LGR5, leucine-rich repeat containing G protein-coupled receptor 5; ALDH1, aldehyde dehydrogenase1; ABCG2, breast cancer-resistant protein; NF-κB, nuclear factor-kappa B; JAK/STAT3, the Janus kinase/signal transducer and activator of tran-ions 3; HIF1α, hypoxia-inducible factor 1α; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor-β; EGFR, epidermal growth factor receptor; PDGFRα, platelet derived growth factor receptor alpha; ROCK1, Rho kinase1; LKB1, liver kinase B1; mTOR, mammalian target of rapamycin; PKB/Akt, protein kinase B; IL-6, interleukin-6; PD-1, programmed cell death receptor-1; PD-L1, programmed cell death-ligand 1; HDAC, histone deacetylases inhibitor; PI3K, phosphatidylinositide 3-kinases; MMPs, matrix metalloproteinases; Eck/EphA2, epithelial cell kinase; LKB1, liver kinase B1; PKM2, pyruvate kinase M2; AMPK, AMP-activated protein kinase.
Therapeutic Targeting of CSCs
The concept of CSCs was proposed several decades ago. The existence of CSCs has been confirmed by lineage tracing and cell ablation experiments in tumors (2–6). Similar to normal stem cells, a small subset of CSCs could proliferate and differentiate into a wide variety of cell types to sustain and promote tumor growth. The characteristic of tumor plasticity in CSCs is that CSCs could differentiate in different directions. The CSCs model provides a new explanation for the metastasis and recurrence of malignant tumors. CSCs have also been recognized as a major driver of tumor growth, metastasis and chemotherapeutic resistance. Therefore, CSCs has become crucial targets for cancer treatment. The ways to eliminate CSCs mainly consist of two aspects (7): (1) inhibition of key CSCs signaling pathways, including Wnt pathway, porcupine (PORCN) pathway and Hedgehog (Hh) pathway (8, 9); and (2) ablate CSCs by targeting CSC surface markers, such as CD133, CD44, (leucine-rich repeat containing G protein-coupled receptor 5) LGR5, (aldehyde dehydrogenase1) ALDH1, and breast cancer-resistant protein (BCRP; ABCG2). Table 1 summarizes drugs that target CSCs in recent clinical trials.
Table 1.
Potential drugs targeting CSCs in clinical trials.
| Drug | Mechanism of action | Condition or disease | Phase | References |
|---|---|---|---|---|
| WNT-974 | PORCN inhibitors | Colorectal cancer and melanoma | I | (10) |
| ETC-159 | PORCN inhibitors | Advanced solid tumors | I | (11, 12) |
| CGX-1321 | PORCN inhibitors | Refractory solid tumors and advanced gastrointestinal cancers | I | (13) |
| RXC-004 | PORCN inhibitors | Solid tumors | I/II | (14) |
| BC-2059 | β-catenin inhibitors | Desmoid tumors | I | (15) |
| E-7386 | CREB-binding protein (CBP)/β-catenin interaction inhibitors | Solid tumors | I | (16) |
| AL-101 | γ-secretase inhibitors | Adenoid cystic carcinoma | II | (17) |
| Vismodegib | p-glycoprotein inhibitors Breast cancer-resistant protein inhibitors Smo receptor antagonists |
Basal cell carcinoma, other cancers | Launched in 2012 | (18) |
| Sonidegib phosphate | Smo receptor antagonists | Basal cell carcinoma, other cancers | Launched in 2015 | (19) |
| Patidegib | Smo receptor antagonists | Sarcoma, basal cell carcinoma |
III | (20, 21) |
| Taladegib | Smo receptor antagonists | Adenocarcinoma, solid tumors | I/II | (22, 23) |
Therapeutic agents targeting Wnt signaling pathway in clinical trials include porcupine (PORCN) inhibitors, β-catenin inhibitors and antibodies against Wnt signaling molecules (24). Among these, PORCN inhibitors gradually became research focus of antitumor drugs. WNT-974, an orally first-in-class PORCN inhibitor, is a pyridinyl acetamide derivative that target Wnt signaling to inhibit the expression of Wnt related genes and Wnt-dependent LRP6 phosphorylation. WNT-974 showed significant growth inhibitory effect on Wnt-driven neoplasms, such as pancreatic cancer and head and neck squamous cell carcinoma. The pharmacokinetics (PK) and pharmacodynamics (PD) of WNT974 were tested in patients with advanced cancers in phase I clinical trial, and the results showed rapid absorption (median Tmax 1–3 h) and appropriate elimination half-life of 5–8 h. These clinical data demonstrated that WNT-974 possesses favorable safety profile and potential antineoplastic activity in selected populations (25). Currently, WNT-974 is being tested in a phase I study for the treatment of solid tumors including colorectal cancer and melanoma (10). In addition, PORCN inhibitor ETC-159 is in phase I clinical trial for advanced solid tumors, CGX-1321 is in phase I clinical trials for advanced gastrointestinal cancers and RXC-004 is in phase I/II clinical trials for the treatment of solid tumors (11–14). Through the inhibition of β-catenin, Tegavivint (BC-2059), an anthraquinone derivative and E-7386 are both being evaluated in phase I clinical trials to treat symptomatic or progressive unresectable desmoid tumors and solid tumors (15, 16).
The small-molecule inhibitors and macromolecule monoclonal antibodies (mAbs) including γ-secretase inhibitors and mAbs to NOTCH receptors have been tested in clinical trials. A small-molecule inhibitor of γ-secretase, which is a key enzyme in NOTCH signaling pathway, AL-101 with favorable in vitro potency and oxidative metabolic stability, is in phase II clinical development for the treatment of adenoid cystic carcinoma bearing NOTCH activated mutations (17). On the other hand, among the therapeutic molecules targeting Hh pathway, smoothened (Smo) receptor antagonists are the most promising molecules (26). A novel small-molecule inhibitor or antagonist of Smo, Sonidegib phosphate was launched in 2015 for the treatment of advanced basal cell carcinoma (BCC). Sonidegib phosphate exhibited excellent therapeutic effect (roughly 35–60% response rates of patients) in patients with locally advanced, unresectable and metastatic BCC, with high disease control rates and clinical benefit (19, 27). Recent advances in the development of Hh signaling inhibitors include Vismodegib (18), which is launched in 2012 for the treatment of patients with advanced BCC; Patidegib, which is in phase III clinical trial for reducing the incidence of BCC (20, 21) and Taladegib, which is in phase I/II clinical trial) for the treatment of patients with recurrent, advanced solid tumors (22, 23).
Because of the highly plasticity of CSCs in tumors, the identification and eradication of CSCs are difficult. Generally, their identification depends on cell surface markers. CD34, CD44, and CD133 are common examples of CSC-specific surface markers (28). CSC surface markers can mediate adhesion of the cells. A cell surface membrane protein CD133, which was first discovered in hematopoietic stem and progenitor cells, is considered to be one of the common surface markers in multiple stem cells (29). Others like ALDH1 and ABCG2 also play significant roles in the regulation of CSCs (30, 31). Because CSCs drive cancer development, a number of agents targeting the biomarkers of CSCs have been developed (Table 2).
Table 2.
Potential drugs targeting CSC surface marker in clinical trials.
| Drug | Mechanism of action | Condition or disease | Phase | References |
|---|---|---|---|---|
| P5 | Anti-CD49e/CD29 (integrin α5β1) | Non-small cell lung cancer (NSCLC) | III | (32) |
| ALM-201 | Microtubule inhibitors (binds CD44) | Advanced ovarian cancer and other solid tumors | I | (33) |
| RO-5429083 | Anti-CD44 | Acute myeloid leukemia | I | (34) |
| RG-7356 | Anti-CD44 | Acute myeloid leukemia | I | (35) |
| AMC-303 | CD44 Antigen Exon 6 (CD44v6) inhibitors | Advanced or metastatic malignant solid tumors of epithelial origin | I/II | (36) |
| CX-2009 | Tubulin polymerization inhibitors Anti-ALCAM (CD166) |
Solid tumors | I/II | (37) |
| Chrysin | ABCG2 inhibitors | Chronic lymphocytic leukemia (CLL) | II | (38) |
A novel mAb P5, which targets CD49e/CD29, is currently being tested in phase III clinical trials to evaluate its anti-tumor effect, but there are only a few reports about its progress of new clinical trials (32). As a FK506 binding protein like (FKBPL) peptide derivative, ALM-201 can bind to CD44 and inhibit cancer related pathways, such as DLL4/NOTCH signal pathway as well as inhibit cell migration, tubule formation and angiogenesis. ALM-201 showed an excellent safety profile and acceptable PK in patients with advanced solid tumors in a phase I dose-escalation study (39). This candidate is currently in phase I clinical trials for the treatment of patients with advanced ovarian cancer and other solid tumors (33). RO-5429083 and RG-7356 are both humanized monoclonal antibodies against extracellular domain of CD44 which had been used in phase I clinical studies for the treatment of acute myeloid leukemia and solid tumors (34, 35). In addition, AMC-303, a high specific inhibitor of CD44v6, was evaluated as monotherapy to treat patients with advanced epithelial tumors. AMC-303 was proved to be well-tolerated with a favorable PK profile (t1/2 of 4–7 h, CL of 40–60 mL/h/kg) (40). At present, AMC-303 is in phase I/II clinical trials to treat patients with advanced or metastatic malignant solid tumors of epithelial origin (36). A probody drug conjugate CX-2009 against CD166 is in phase I/II clinical development for the treatment of adult patients with metastatic or locally advanced unresectable solid tumors (37). Furthermore, a recent research reported that chrysin, which is an ABCG2 inhibitor, could enhance sorafenib mediated inhibition of cell viability by sustained phosphorylation of ERK1/2 (41). And chrysin is being used in phase II clinical trials to treat CLL (38).
Therapeutic Targeting of EMT
The conversion of cells from epithelial phenotype into mesenchymal phenotype is a critical transformation for embryonic development and during cancer progression. Through EMT process, tumor cells can acquire the ability to disarm anti-tumor defenses in the body, resist apoptosis and antineoplastic drugs, spread through the body and expand the population of tumor cells (42). At the same time, EMT may play an important role in generating CSCs (43). Hence, EMT is an important target for inhibiting tumor metastasis and reducing drug resistance. Various approaches can be used to target the EMT process: (1) targeting the inducing signals in EMT process; (2) reversing EMT to reduce tumor cell aggressiveness; and (3) killing the cells in EMT-like state (44). As one of the key factors of tumor invasion, metastasis and drug resistance, EMT is a promising target for oncotherapy. The following summarized the progress of potential drugs targeting EMT-related signals (Table 3).
Table 3.
Potential drugs targeting EMT-related modulators in clinical trials.
| Drug | Mechanism of action | Condition or disease | Phase | References |
|---|---|---|---|---|
| Denosumab | Receptor activator of NF-κB ligands (RANKL) | Tenosynovial giant cell tumor | Launched in 2013 | (45) |
| TK-006 | Anti-TNFSF11 (RANKL) | Breast cancer-related bone metastases | I | (46) |
| WO-1066 | STAT3 inhibitors, anti-PD-L1, Janus kinase (JAK) inhibitors | Melanoma, brain cancer | I | (47, 48) |
| DSP-0337 | STAT3 inhibitor | Solid tumors | I | (49) |
| Danvatirsen | STAT3 expression inhibitors | Solid tumors | II | (50, 51) |
| OPB-111077 | STAT3 ligands | Solid tumors | II | (52) |
| Napabucasin | STAT3 inhibitors | Colorectal carcinoma, pancreatic cancer | III | (53, 54) |
| PEGPH20 | HIF1α inhibitors | Metastatic breast cancer | I/II | (55, 56) |
| CRLX-101 | HIF1α inhibitors, DNA Topoisomerase I inhibitors | peritoneum cancer | II | (57, 58) |
Modulators of transcription factors, such as nuclear factor-kappa B (NF-κB) and signaling transducer and activator of transcription 3 (STAT3) have made progress in clinical trials (59, 60). Denosumab, which is a macromolecule of humanized mAbs to receptor activator of NF-κB ligand (RANKL), was originally approved to treat and prevent postmenopausal osteoporosis in 2010 (45). Denosumab prevents RANKL binding to RANK, and blocks the development of osteoclasts, leading to restraining the resorption of bone. So far, phase III clinical studies have been ongoing for evaluating its therapeutic effect on metastatic non-small cell lung cancer (NSCLC) together with other chemotherapeutics. TK-006 is another anti-RANKL antibody in early clinical development for the treatment of patients with bone metastases caused by breast cancer through hypodermic injection (46). In addition, WO-1066 is a JAK/STAT3 (the Janus kinase/signal transducer and activator of tran-ions 3) signaling pathway and programmed cell death-ligand 1 (PD-L1) inhibitor, which is derived from the JAK2 inhibitor AG490. In 2019, the compound was granted an orphan drug designation in the U.S. for treating glioblastoma. Currently, the candidate is in phase I clinical trials for patients with melanoma or glioblastoma multiforme with brain metastases (47, 48). DSP-0337, Danvatirsen and OPB-111077, all inhibit STAT3 and are in phase I or II clinical trials to assess their therapeutic efficacy in solid tumors (49–52).
Hypoxia-inducible factor 1α (HIF1α) and β-catenin also regulate the expression of other transcription factors related to EMT (61, 62). PEGPH20 (PEGylated recombinant human hyaluronidase PH20), which enzymatically degrades hyaluronic acid (HA), is currently being evaluated in phase II and III trials. It shows promising efficacy in preclinical and early clinical studies in the treatment of metastatic pancreatic carcinoma and other malignant tumors (55, 56). CRLX-101 was proved to be a potent topoisomerase 1 and HIF1α inhibitor, which is a nanoparticle composed of CPT conjugated to a biocompatible copolymer of cyclodextrin and polyethylene glycol (PEG). Currently, CRLX101 is being evaluated in phase II clinical trials for several tumor types (58).
Therapeutic Targeting of TME
Studies have shown that epigenetic changes of tumor cells caused by TME play a prominent role in tumor progression and invasion (1, 63). Tumor cells usually adapt to the changing external environment through changing the plasticity of tumor cells to meet the demand of tumor development. The research of relationship between TME and tumor plasticity is making progress in recent years (64). TME is composed of a complex mixture of ECM and various cells including cancer associated fibroblasts (CAFs) (65), cancer associated macrophages (CAMs) (66) and endothelial progenitor cells (EPCs) (67). Many components in ECM contribute to tumor growth. TME has become one of the key targets in tumor treatment due to its special pathophysiological characteristics and physicochemical properties (Table 4).
Table 4.
Potential drugs targeting TME in clinical trials.
| Drug | Mechanism of action | Condition or disease | Phase | References |
|---|---|---|---|---|
| Avadomide hydrochloride | TNF-α production inhibitor and cereblon inhibitors | Solid tumors | I/II | (68) |
| NIS-793 | Anti-TGF-β | Solid tumors | I | (69) |
| AVID-200 | TGF-β inhibitors | Solid tumors | I | (70) |
| SAR-439459 | Anti-TGF-β | Solid tumors | I | (71) |
| Fresolimumab | Anti-TGF-β | Lung cancer | I/II | (72) |
| Simotinib hydrochloride | EGFR inhibitors | Lung cancer | I | (73) |
| Amcasertib | PDGFRα inhibitors | Hepatocellular carcinoma, cholangiocarcinoma | II | (74) |
| Olaratumab | Anti-CD140a (PDGFRα) | Soft tissue sarcoma | Launched in 2016 | (75) |
| Cerdulatinib | JAK and Syk kinase inhibitors | Hematologic cancers | II | (76) |
| AZD-8055 | mTORC1/2 inhibitors | Solid tumors | I | (77) |
| BI-860585 | mTORC1/2 inhibitors | Solid tumors | I | (78) |
| DCBCI-0901 | mTORC1/2 inhibitors Phosphatidylinositol 3-Kinase alpha (PI3Kα) inhibitors | Solid tumors | I | (79) |
| LXI-15029 | mTORC1/2 inhibitors | Solid tumors | I | (80) |
| ABI-009 | mTOR inhibitors | Metastatic cancer | II | (81) |
| Sapanisertib | mTORC1/2 inhibitors | Endometrial cancer | II | (82) |
| GSK-690693 | Akt kinases 1 inhibitors | Lymphoma, solid tumors | I | (83) |
| ARQ-751 | pan-Akt inhibitors | Solid tumors | I | (84) |
| TAS-117 | PKB/Akt inhibitors | Solid tumors | II | (85) |
| Ipatasertib | PKB/Akt inhibitors | Prostate cancer | III | (86) |
| Siltuximab | Anti-IL6 | Multiple myeloma | II | (87) |
| Sintilimab | Anti-PD-1 | Lymphoma, Hodgkin's | Launched in 2019 | (88) |
| Avelumab | Anti-PD-L1 | Bladder and kidney cancer | Launched in 2017 | (89) |
Tumor necrosis factor alpha (TNF-α) could promote tumor growth via a PKCa- and AP-1-dependent pathway (90). Avadomide (CC-122) is a small molecule drug that inhibits both TNF-α and cereblon E3 ligase. The first-in-human phase I study, which evaluated the safety and clinical therapeutic effect of avadomide in patients with advanced solid tumors and others, showed acceptable safety and favorable pharmacokinetics (68). Avadomide is currently being evaluated in advanced melanoma in combination with Nivolumab. Transforming growth factor-β (TGF-β) signaling pathway is related to EMT in cancer cells (91). Therapeutic agents modulating the expression of TGF-β that are monoclonal antibodies include: NIS-793 (a humanized anti-TGF-β monoclonal antibody), AVID-200 (a recombinant inhibitor of TGF-β1 and TGF-β3), SAR-439459 (targeting transforming TGF-β) and fresolimumab (a pan-specific human anti-TGF-β monoclonal antibody). Among these therapeutic agents, fresolimumab is able to neutralize all human isoforms of transforming TGF-β and being evaluated in phase I/II trials (72, 92).
Epidermal growth factor receptor (EGFR) regulates ECM and promotes cancer invasion (93). A small EGFR inhibitor Simotinib is used in phase I study to treat NSCLC (73). Platelet derived growth factor receptor alpha (PDGFRα), which contributes to fibroblast reprograming toward CAFs, plays a significant role in colorectal carcinogenesis (94). Amcasertib, a PDGFRα inhibitor and cancer stemness kinase inhibitor, is used to treat hepatocellular carcinoma and cholangiocarcinoma in phase II trials (74). Different from Amcasertib, Lartruvo(R) (olaratumab) is a fully humanized monoclonal antibody to neutralize PDGFRα. It was first launched in the U.S. for front-line treatment with doxorubicin in adults with soft tissue sarcoma in 2016 (75).
Some signaling pathways are also critical in cancer development. Janus kinase 1 (JAK1)/Rho kinase1 (ROCK1) signaling could promote fibroblast-dependent carcinoma cell invasion (95). Cerdulatinib is a small-molecule anti-cancer drug targeting JAK and syk kinase for the treatment of hematologic cancers (76). Liver kinase B1 (LKB1)/mammalian target of rapamycin (mTOR) signaling axis regulates ECM stiffness and participates in lung adenocarcinoma progression (96). Potential drugs such as AZD-8055, BI-860585, DCBCI-0901, LXI-15029, and ABI-009 are in early clinical stage for various cancers (77–81). Sapanisertib is an orally and highly selective ATP-competitive inhibitor of mTORC1/2 and demonstrates satisfactory anticancer activity. The phase II study of sapanisertib in metastatic castration resistant prostate cancer was not entirely satisfactory likely because of dose reductions secondary to toxicity (82). In addition, abnormal expression of protein kinase B (PKB/Akt) is related to many cancers (97). GSK-690693 (83), ARQ-751 (84), and TAS-117 (85) that can effectively treat solid tumors through inhibiting PKB/Akt are being evaluated in phase I and II clinical studies. Ipatasertib has been combined with other antitumor drugs to treat prostate cancer and breast cancer and is undergoing an investigation in a phase III clinical trial (86).
With the exception of targets above, interleukin-6 (IL-6) showed high expression in prostate cancer (98). Siltuximab, a chimeric monoclonal antibody, was first launched in 2014 to treat HIV-negative and Human Herpes Virus-8 negative multicentric Castleman's disease. Its tight binding to IL-6 inhibits IL-6 bioactivity and thus causes apoptosis of tumor cell. Recently, a phase II clinical trial of siltuximab was conducted for the treatment of multiple myeloma (87). Others like immunity-related programmed cell death receptor-1 (PD-1) and PD-L1 inhibitors show satisfied antitumor effects by restoring antitumor immunity. Sintilimab is a fully human IgG4 mAb, which blocks the interaction of PD-1 with PD-L1 and PL-L2 (88). It was firstly approved in China to treat classical Hodgkin's lymphoma. Avelumab, an anti-PD-L1 antibody, was approved by the FDA in 2019 for first-line treatment of advanced renal cell carcinoma together with axitinib (89).
VM Related Targets and Therapeutic Agents
VM refers to a tumor microcirculation pattern that tumor cells aggregate, migrate and remodel to form a vascular-like structure based on the adhesion of ECM. VM differs from traditional endothelial tumor angiogenesis and plays a crucial role in tumor invasion and spreading. It is worth noting that there is an obvious increase of EMT-related regulators and transcription factors in VM, which indicates the crucial rule of EMT in VM formation (99). VM has been observed in a broad range of tumor types such as prostate cancer, malignant glioma, and melanoma (100). Currently, certain mechanism of VM formation remains matters of frenetic investigation and the mechanism of VM formation mainly include TME, EMT, tumor plasticity, RNA, and other regulators (100). Because VM is important for tumor progression, targeted therapies related to VM could also be a promising antitumor strategy to reducing tumor plasticity.
The major signaling molecules participating in VM formation and promising drugs are summarized in Table 5. Histone deacetylases inhibitor (HDACi) inhibits key molecule MMP-2 in PI3K-MMPs-Ln-5γ2 signaling pathway to block VM formation (112). Panobinostat lactate, which is lunched in 2015, is a first-line HDAC inhibitor applied in combination with bortezomib and dexamethasone to the treatment of multiple myeloma (113). Panobinostat lactate is not only a HDAC inhibitor but also a pan-deacetylase inhibitor. The pharmacokinetics of panobinostat lactate is affected by some factors such as hepatic impairment. HDAC inhibitor romidepsin, which is launched in 2010, could cause cell cycle arrest, differentiation and apoptosis in various cancer cells and is used for the treatment of cutaneous T-cell lymphoma (103). OKI-179 and remetinostat are HDAC inhibitors in early clinical development (101, 102).
Table 5.
Potential drugs targeting VM in clinical trials.
| Drug | Mechanism of action | Condition or disease | Phase | References |
|---|---|---|---|---|
| OKI-179 | HDAC inhibitors | Solid tumor | I | (101) |
| Remetinostat | HDAC inhibitors | Cutaneous T-cell lymphoma | II | (102) |
| Romidepsin | HDAC inhibitors | Cutaneous T-cell lymphoma, peripheral T-cell lymphoma | Launched in 2010 | (103) |
| Panobinostat lactate | HDAC inhibitors | Multiple myeloma | Launched in 2015 | (104) |
| MEN-1611 | PI3K inhibitors | Breast cancer | I | (105) |
| HMPL-689 | PI3Kδ inhibitors | B-cell lymphoma | I | (106) |
| Gedatolisib | PI3K/mTOR inhibitors | Acute myeloid leukemia, solid tumors | II | (107) |
| GDC-0980 | PI3K/mTOR inhibitors | Prostate cancer | II | (108) |
| Buparlisib | PI3K inhibitors | HNSCC | III | (109) |
| Copanlisib hydrochloride | PI3K inhibitors | Lymphoma | Launched in 2017 | (110) |
| siRNA-EphA2-DOPC | EphA2 inhibitors | Solid tumors | I | (111) |
Phosphatidylinositide 3-kinases (PI3K) participate in VM formation by activating matrix metalloproteinases (MMPs) (114). The PI3Kα/δ inhibitor copanlisib hydrochloride was launched in 2017 as a treatment for relapsed follicular lymphoma in patients receiving two or more prior therapy regimens (110). Copanlisib characterizes low risk of PK-related pharmacological interaction due to reduced oxidation metabolism and unchanged excretion of copanlisib. Other PI3K inhibitors in clinical trials include MEN-1611 (phase I for breast cancer), HMPL-689 (phase I for B-cell lymphoma), Gedatolisib (phase II for acute myeloid leukemia and solid tumors), GDC-0980 (phase II for prostate cancer) and Buparlisib (phase III in patients with head and neck squamous cell carcinoma, HNSCC) (105–109).
VE-cadherin mediates the activities of epithelial cell kinase (Eck/EphA2) to affect the formation of VM (115). EphA2 interacts with cell membrane surface ligands by phosphorylation and regulates the extracellular expression of protein kinases ERK and focal adhesion kinase FAK to activate PI3K (116, 117). SiRNA-EphA2-DOPC is a small interfering RNA targeting EphA2 loaded in neutral 1,2-dioleoyl-sn-glycero-3-phosphocholin (DOPC) liposomes (111). SiRNA-EphA2-DOPC reaches to tumor site by interacting with endothelial cells of tumor vasculature. As an EphA2 inhibitor, siRNA-EphA2-DOPC is in early clinical investigations to treat recurrent and advanced solid tumors.
Therapeutic Targeting of Neoplasm Metabolic Pathways
Cancer cells reprogram metabolic pathways by oncogenic mutations, result in enhanced demand of nutrient uptake to supply anabolic metabolism. Not only must energy production and consumption processes in cancer cells be balanced to sustain tumor growth, but also cancer cells have to adapt to the changes in nutrition and oxygen supply caused by their rapid growth. Hence, malignant cells exhibit metabolic flexibility for them to exist and develop. Different from normal cells, cancer cells are more dependent on anaerobic glycolysis even in a sufficient oxygen supply environment, called Warburg effect (118). HIF-1α is crucial for anaerobic glycolysis under oxygen free conditions. Tumor suppressor liver kinase B1 (LKB1) regulates HIF-1α-dependent metabolic reprogramming (119). Recent studies have shown that Pyruvate kinase M2 (PKM2) plays a crucial part in the plasticity of cancer metabolism, and up regulation of PKM2 leads to oxidative metabolism (120). Dimethylaminomicheliolide (DMAMCL), a PKM2 activator, is a prodrug of micheliolide (MCL) that suppresses tumor growth and targets CSCs in the form of guaianolide sesquiterpene lactone. Dimethylaminomicheliolide could inhibit inflammation and tumor growth by releasing MCL into plasma. Early clinical trial using Dimethylaminomicheliolide for patients with solid tumors is being conducted (Table 6) (121).
Table 6.
Potential drugs targeting neoplasm metabolic pathways in clinical trials.
In addition to this, oxidative phosphorylation plays an important role in cancer metabolism. Oxidative phosphorylation is mainly regulated by AMP-activated protein kinase (AMPK) (123). As an AMPK activator, acadesine increases the availability of adenosine in tissues under ischemic conditions and shows antitumor activity. Acadesine causes B cells apoptosis selectively in chronic lymphocytic leukemia (CLL) and phase I/II studies are being tested for sieving out the best methods for the treatment of resistant/refractory B-cell chronic lymphocytic leukemia (122). Metabolic plasticity of cancer triggers the adaptive “metabolic switch” needed for cancer development. Mechanism of metabolic switch provides insights into therapies, which could be used to target cancer development.
Conclusions
Tumor plasticity provides new explanation for the mechanisms of drug resistance, metastasis and recurrence of neoplasm. Interfering tumor plasticity is becoming strategies to treat malignant tumors. The drugs in clinical trials that targeting tumor plasticity are still on intense research. However, targeted therapy also has some limitations that most drugs could only be effective on a small part of tumors of genetic transformation and engender drug resistance after a period of time of taking drugs. How to find effective multi-targeted inhibitors or combine with traditional chemotherapeutic drugs and other therapeutics like photodynamic or photothermal therapy become particularly important. The quest for new therapeutic targets toward tumor plasticity continues to be a great impetus to promote cancer treatment.
Author Contributions
DW, X-GY, and L-CZ wrote the draft. Y-JW and Y-YL edited the manuscript. All authors read and approved the final version of manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. (2013) 501:328–37. 10.1038/nature12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. (2003) 100:3983–88. 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. (2007) 445:106–10. 10.1038/nature05372 [DOI] [PubMed] [Google Scholar]
- 4.Dalerba P, Dylla SJ, Park I-K, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. (2007) 104:10158–63. 10.1073/pnas.0703478104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. (2005) 65:9328–37. 10.1158/0008-5472.CAN-05-1343 [DOI] [PubMed] [Google Scholar]
- 6.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. (2007) 445:111–15. 10.1038/nature05384 [DOI] [PubMed] [Google Scholar]
- 7.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. (2017) 23:1124–34. 10.1038/nm.4409 [DOI] [PubMed] [Google Scholar]
- 8.Bariwal J, Kumar V, Dong Y, Mahato RI. Design of Hedgehog pathway inhibitors for cancer treatment. Med Res Rev. (2019) 39:1137–204. 10.1002/med.21555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng X, Ju D. Hedgehog signaling pathway and autophagy in cancer. Int J Mol Sci. (2018) 19:2279. 10.3390/ijms19082279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA. (2013) 110:20224–29. 10.1073/pnas.1314239110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A Study to Evaluate the Safety and Tolerability of ETC-1922159 in Advanced Solid Tumors (NCT02521844) (2015). Available online at: http://clinicaltrials.gov/show/NCT02521844
- 12.Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J, et al. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. (2016) 35:2197–207. 10.1038/onc.2015.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phase I Dose-Escalation Study of CGX-1321 in Subjects With Advanced Gastrointestinal Tumors (NCT03507998) (2018). Available online at: http://clinicaltrials.gov/show/NCT03507998
- 14.Study to Evaluate the Safety and Tolerability of RXC-004 in Advanced Malignancies (NCT03447470) (2018). Available online at: https://clinicaltrials.gov/show/NCT03447470
- 15.Savvidou I, Khong T, Cuddihy A, McLean C, Horrigan S, Spencer A. beta-Catenin inhibitor BC2059 is efficacious as monotherapy or in combination with proteasome inhibitor bortezomib in multiple myeloma. Mol Cancer Ther. (2017) 16:1765–78. 10.1158/1535-7163.MCT-16-0624 [DOI] [PubMed] [Google Scholar]
- 16.A Study of E-7386 in Participants With Advanced Solid Tumor Including Colorectal Cancer (CRC) (NCT03833700) (2019). Available online at: https://clinicaltrials.gov/show/NCT03833700
- 17.Morgan KM, Fischer BS, Lee FY, Shah JJ, Bertino JR, Rosenfeld J, et al. Gamma secretase inhibition by BMS-906024 enhances efficacy of paclitaxel in lung adenocarcinoma. Mol Cancer Ther. (2017) 16:2759–69. 10.1158/1535-7163.MCT-17-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. (2012) 11:437–38. 10.1038/nrd3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burness CB. Sonidegib: first global approval. Drugs. (2015) 75:1559–66. 10.1007/s40265-015-0458-y [DOI] [PubMed] [Google Scholar]
- 20.Campbell VT, Nadesan P, Ali SA, Wang CY, Whetstone H, Poon R, et al. Hedgehog pathway inhibition in chondrosarcoma using the smoothened inhibitor IPI-926 directly inhibits sarcoma cell growth. Mol Cancer Ther. (2014) 13:1259–69. 10.1158/1535-7163.MCT-13-0731 [DOI] [PubMed] [Google Scholar]
- 21.Sasaki K, Gotlib JR, Mesa RA, Newberry KJ, Ravandi F, Cortes JE, et al. Phase II evaluation of IPI-926, an oral Hedgehog inhibitor, in patients with myelofibrosis. Leuk Lymphoma. (2015) 56:2092–97. 10.3109/10428194.2014.984703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin G, Sivaraman A, Lee K. Development of taladegib as a sonic hedgehog signaling pathway inhibitor. Arch Pharmacal Res. (2017) 40:1390–93. 10.1007/s12272-017-0987-x [DOI] [PubMed] [Google Scholar]
- 23.Ueno H, Kondo S, Yoshikawa S, Inoue K, Andre V, Tajimi M, et al. A phase I and pharmacokinetic study of taladegib, a Smoothened inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. (2018) 36:647–56. 10.1007/s10637-017-0544-y [DOI] [PubMed] [Google Scholar]
- 24.Harb J, Lin PJ, Hao J. Recent development of wnt signaling pathway inhibitors for cancer therapeutics. Curr Oncol Rep. (2019) 21:12. 10.1007/s11912-019-0763-9 [DOI] [PubMed] [Google Scholar]
- 25.Janku F, Connolly R, LoRusso P, de Jonge M, Vaishampayan U, Rodon J, et al. Abstract C45: phase I study of WNT974, a first-in-class Porcupine inhibitor, in advanced solid tumors. Mol Cancer Ther. (2015) 14 10.1158/1535-7163.TARG-15-C45 [DOI] [Google Scholar]
- 26.Xin M, Ji X, De La Cruz LK, Thareja S, Wang B. Strategies to target the Hedgehog signaling pathway for cancer therapy. Med Res Rev. (2018) 38:870–913. 10.1002/med.21482 [DOI] [PubMed] [Google Scholar]
- 27.Salati M, Stathis A. Sonidegib Phosphate Smoothened (SMO) receptor antagonist. Drugs Future. (2014) 39:677–84. 10.1358/dof.2014.039.010.2207247 [DOI] [Google Scholar]
- 28.Dianat-Moghadam H, Heydarifard M, Jahanban-Esfahlan R. Cancer stem cells-emanated therapy resistance: implications for liposomal drug delivery systems. J Control Release. (2018) 288:62–83. 10.1016/j.jconrel.2018.08.043 [DOI] [PubMed] [Google Scholar]
- 29.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. (1997) 90:5013–21. [PubMed] [Google Scholar]
- 30.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. (2007) 1:555–67. 10.1016/j.stem.2007.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding X, Wu J, Jiang C. ABCG2: a potential marker of stem cells and novel target in stern cell and cancer therapy. Life Sci. (2010) 86:631–37. 10.1016/j.lfs.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 32.Kim MY, Cho WD, Hong KP, Choi da B, Hong JW, Kim S, et al. Novel monoclonal antibody against beta 1 integrin enhances cisplatin efficacy in human lung adenocarcinoma cells. J Biomed Res. (2016) 30:217–24. 10.7555/JBR.30.2016K0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClements L, Annett S, Yakkundi A, O'Rourke M, Valentine A, Moustafa N, et al. FKBPL and its peptide derivatives inhibit endocrine therapy resistant cancer stem cells and breast cancer metastasis by downregulating DLL4 and Notch4. BMC Cancer. (2019) 19:351. 10.1186/s12885-019-5500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Open-Label Multicenter Dose-Escalation Phase Ia/b Study of RO-5429083 Administered as Intravenous Infusion Alone or in Combination With Cytarabine in Patients With Acute Myelogenous Leukemia (AML) (NCT01641250) (2012). Available online at: https://clinicaltrials.gov/show/NCT01641250
- 35.Vey N, Delaunay J, Martinelli G, Fiedler W, Raffoux E, Prebet T, et al. Phase I clinical study of RG7356, an anti-CD44 humanized antibody, in patients with acute myeloid leukemia. Oncotarget. (2016) 7:32532–42. 10.18632/oncotarget.8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.A Safety and Pharmacokinetic Phase I/Ib Study of AMC-303 in Patients With Solid Tumors (NCT03009214) (2017). Available online at: https://clinicaltrials.gov/show/NCT03009214
- 37.A Trial to Find Safe and Active Doses of an Investigational Drug CX-2009 for Patients With Selected Solid Tumors (NCT03149549) (2017). Available online at: https://clinicaltrials.gov/show/NCT03149549
- 38.Salimi A, Roudkenar MH, Seydi E, Sadeghi L, Mohseni A, Pirahmadi N, et al. Chrysin as an anti-cancer agent exerts selective toxicity by directly inhibiting mitochondrial complex II and v in CLL b-lymphocytes. Cancer Invest. (2017) 35:174–86. 10.1080/07357907.2016.1276187 [DOI] [PubMed] [Google Scholar]
- 39.El-Helali A, Plummer R, Jayson G, Coyle V, Drew Y, Mescallado N, et al. 383PA phase I dose-escalation study of the novel peptide ALM201 in patients (pts) with advanced solid tumours. Ann Oncol. (2017) 28:128 10.1093/annonc/mdx367.01728177460 [DOI] [Google Scholar]
- 40.Calvo E, Aftimos P, Azaro A, de Miguel M, Jungels C, Zeron-Medina Cuairan J, et al. 410O First-in-human, first-in-class study of the CD44v6 inhibitor AMC303 as monotherapy in patients with advanced epithelial tumors. Ann Oncol. (2018) 29:viii134 10.1093/annonc/mdy279.398 [DOI] [Google Scholar]
- 41.Wei C-T, Chen L-C, Hsiang Y-P, Hung Y-J, Chien P-H, Pan H-L, et al. Chrysin-induced ERK1/2 phosphorylation enhances the sensitivity of human hepatocellular carcinoma cells to sorafenib. Anticancer Res. (2019) 39:695–701. 10.21873/anticanres.13165 [DOI] [PubMed] [Google Scholar]
- 42.Marcucci F, Stassi G, De Maria R. Epithelial-mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. (2016) 15:311–25. 10.1038/nrd.2015.13 [DOI] [PubMed] [Google Scholar]
- 43.Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. (2015) 25:675–86. 10.1016/j.tcb.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanneste M, Henry MD. Targeting phenotypic plasticity in prostate cancer. Curr Mol Biol Rep. (2017) 3:183–96. 10.1007/s40610-017-0070-x [DOI] [Google Scholar]
- 45.By:Drooger J, van der Padt A, Sleijfer S, Jager A. Denosumab in breast cancer treatment. Eur J Pharmacol. (2013) 717:12–9. 10.1016/j.ejphar.2013.03.034 [DOI] [PubMed] [Google Scholar]
- 46.Assessment of TK006 in Patients With Breast Cancer-Related Bone Metastases (NCT03239756) (2017). Available online at: https://clinicaltrials.gov/show/NCT03239756
- 47.Geng L, Li X, Zhou X, Fang X, Yuan D, Wang X. WP1066 exhibits antitumor efficacy in nasal-type natural killer/T-cell lymphoma cells through downregulation of the STAT3 signaling pathway. Oncol Rep. (2016) 36:2868–74. 10.3892/or.2016.5091 [DOI] [PubMed] [Google Scholar]
- 48.Tsurumaki H, Katano H, Sato K, Imai R, Niino S, Hirabayashi Y, et al. WP1066, a small molecule inhibitor of the JAK/STAT3 pathway, inhibits ceramide glucosyltransferase activity. Biochem Biophys Res Commun. (2017) 491:265–70. 10.1016/j.bbrc.2017.07.115 [DOI] [PubMed] [Google Scholar]
- 49.A Study of DSP-0337 in Patients With Advanced Solid Tumors to Determine the Safety and the Pharmacokinetic Profile (NCT03416816) (2018). Available online at: https://clinicaltrials.gov/show/NCT03416816
- 50.Neoadjuvant Durvalumab Alone or in Combination With Novel Agents in Resectable Non-small Cell Lung Cancer (NCT03794544) (2019). Available online at: https://clinicaltrials.gov/show/NCT03794544
- 51.Reilley MJ, McCoon P, Cook C, Lyne P, Kurzrock R, Kim Y, et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. J Immunother Cancer. (2018) 6:119. 10.1186/s40425-018-0436-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tolcher A, Flaherty K, Shapiro GI, Berlin J, Witzig T, Habermann T, et al. A first-in-human phase i study of OPB-111077, a small-molecule STAT3 and oxidative phosphorylation inhibitor, in patients with advanced cancers. Oncologist. (2018) 23:658–e72. 10.1634/theoncologist.2017-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loecken H, Clamor C, Mueller K. Napabucasin and related heterocycle-fused naphthoquinones as STAT3 inhibitors with antiproliferative activity against cancer cells. J Nat Prod. (2018) 81:1636–44. 10.1021/acs.jnatprod.8b00247 [DOI] [PubMed] [Google Scholar]
- 54.A Study of Napabucasin (GB-201) in Combination With Paclitaxel and Low-Dose Gemcitabine in Patients With Pancreatic Cancer (NCT03721744) (2018). Available online at: https://clinicaltrials.gov/show/NCT03721744
- 55.Doherty GJ, Tempero M, Corrie PG. HALO-109–301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. (2018) 14:13–22. 10.2217/fon-2017-0338 [DOI] [PubMed] [Google Scholar]
- 56.Wong KM, Horton KJ, Coveler AL, Hingorani SR, Harris WP. Targeting the tumor stroma: the biology and clinical development of pegylated recombinant human hyaluronidase (PEGPH20). Curr Onco Rep. (2017) 19:47. 10.1007/s11912-017-0608-3 [DOI] [PubMed] [Google Scholar]
- 57.Combining CRLX-101 A Nanoparticle Camptothecin With Enzalutamide in People With Progressive Metastatic Castration Resistant Prostate Cancer Following Prior Enzalutamide Treatment (NCT03531827) (2018). Available online at: https://clinicaltrials.gov/show/NCT03531827
- 58.Tian X, Nguyen M, Foote HP, Caster JM, Roche KC, Peters CG, et al. CRLX101, a nanoparticle–drug conjugate containing camptothecin, improves rectal cancer chemoradiotherapy by inhibiting DNA repair and HIF1α. Cancer Res. (2017) 77:112–22. 10.1158/0008-5472.CAN-15-2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erstad DJ, Cusack JC. Targeting the NF-κB path way in cancer therapy. Surg Oncol Clin N Am. (2013) 22:705–46. 10.1016/j.soc.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 60.Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BKH, et al. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors (review). Biochim Biophys Acta. (2014) 1845:136–54. 10.1016/j.bbcan.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 61.Wong CC-L, Zhang H, Gilkes DM, Chen J, Wei H, Chaturvedi P, et al. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis(Article). J Mol Med. (2012) 90:803–15. 10.1007/s00109-011-0855-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. (2008) 68:3645–54. 10.1158/0008-5472.CAN-07-2938 [DOI] [PubMed] [Google Scholar]
- 63.McCullough KD, Coleman WB. Plasticity of the neoplastic phenotype in vivo is regulated by epigenetic factors. Proc Natl Acad Sci USA. (1998) 95:15333–33. 10.1073/pnas.95.26.15333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhat R, Bissell MJ. Of plasticity and specificity: dialectics of the microenvironment and macroenvironment and the organ phenotype. Wires Dev Biol. (2014) 3:147–63. 10.1002/wdev.130 [DOI] [PubMed] [Google Scholar]
- 65.Chu T, Yang J, Huang T, Liu H. Crosstalk with cancer-associated fibroblasts increases the growth and radiation survival of cervical cancer cells. Radiat Res. (2014) 181:540–47. 10.1667/RR13583.1 [DOI] [PubMed] [Google Scholar]
- 66.Ziebart T, Ziebart J, Gauss L, Pabst A, Ackermann M, Smeets R, et al. Investigation of inhibitory effects on EPC-mediated neovascularization by different bisphosphonates for cancer therapy. Biomed Rep. (2013) 1:719–22. 10.3892/br.2013.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang T, Zhang X, Wang M, Zhang J, Huang F, Cai J, et al. Activation of mesenchymal stem cells by macrophages prompts human gastric cancer growth through NF-κB pathway. PLoS ONE. (2014) 9:e97569. 10.1371/journal.pone.0097569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rasco DW, Papadopoulos KP, Pourdehnad M, Gandhi AK, Hagner P, Li Y, et al. A first-in-human study of novel cereblon modulator avadomide (CC-122) in advanced malignancies. Clin Cancer Res. (2018) 25:90–8. 10.1158/1078-0432.CCR-18-1203 [DOI] [PubMed] [Google Scholar]
- 69.Phase I/Ib Study of NIS-793 in Combination With PDR-001 in Patients With Advanced Malignancies (NCT02947165) (2016). Available online at: https://clinicaltrials.gov/show/NCT02947165
- 70.A Trial of AVID-200 A Transforming Growth Factor Beta (TGFbeta) Inhibitor in Patients Malignancies (NCT03834662) (2019). Available online at: https://clinicaltrials.gov/show/NCT03834662
- 71.A First-in-Human Study of the Safety Pharmacokinetics Pharmacodynamics and Anti-tumor Activity of SAR-439459 Monotherapy and Combination of SAR-439459 and REGN-2810 in Patients With Advanced Solid Tumors (NCT03192345) (2017). Available online at: https://clinicaltrials.gov/show/NCT03192345
- 72.Lacouture ME, Morris JC, Lawrence DP, Tan AR, Olencki TE, Shapiro GI, et al. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor beta by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol Immunother. (2015) 64:437–46. 10.1007/s00262-015-1653-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He L, Li S, Xie F, Cheng Z, Ran L, Liu X, et al. LC-ESI-MS/MS determination of simotinib, a novel epidermal growth factor receptor tyrosine kinase inhibitor: application to a pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci. (2014) 947–8:168–72. 10.1016/j.jchromb.2013.12.021 [DOI] [PubMed] [Google Scholar]
- 74.A Study of BBI-503 in Combination With Selected Anti-cancer Therapeutics in Adult Patients With Advanced Cancer (NCT02483247) (2015). Available online at: https://clinicaltrials.gov/show/NCT02483247
- 75.Deshpande HA, Cecchini M, Ni Choileain S, Jones R. Olaratumab for the treatment of soft tissue sarcoma. Drugs Today. (2017) 53:247–55. 10.1358/dot.2017.53.4.2560077 [DOI] [PubMed] [Google Scholar]
- 76.Coffey GP, Feng J, Betz A, Pandey A, Birrell M, Leeds JM, et al. Cerdulatinib pharmacodynamics and relationships to tumor response following oral dosing in patients with Relapsed/Refractory b-cell malignancies. Clin Cancer Res. (2019) 25:1174–84. 10.1158/1078-0432.CCR-18-1047 [DOI] [PubMed] [Google Scholar]
- 77.Phyu SM, Smith TAD. Combination treatment of cancer cells with pan-Akt and pan-mTOR inhibitors: effects on cell cycle distribution, p-Akt expression level and radiolabelled-choline incorporation. Invest New Drugs. (2019) 37:424–30. 10.1007/s10637-018-0642-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.BI-860585 Dose Escalation Single Agent and in Combination With Exemestane or With Paclitaxel in Patients With Various Advanced and/or Metastatic Solid Tumors (NCT01938846) (2013). Available online at: https://clinicaltrials.gov/show/NCT01938846
- 79.Safety Tolerability and Pharmacokinetics of DCBCI-0901 in Patients With Advanced Solid Tumor (NCT02151357) (2014). Available online at: https://clinicaltrials.gov/show/NCT02151357
- 80.A Study of LXI-15029 in Patients With Advanced Malignant Solid Tumors (NCT03125746) (2017). Available online at: https://clinicaltrials.gov/show/NCT03125746
- 81.Gonzalez-Angulo AM, Meric-Bernstam F, Chawla S, Falchook G, Hong D, Akcakanat A, et al. Weekly nab-rapamycin in patients with advanced nonhematologic malignancies: final results of a phase I trial. Clin Cancer Res. (2013) 19:5474–84. 10.1158/1078-0432.CCR-12-3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Graham L, Banda K, Torres A, Carver BS, Chen Y, Pisano K, et al. A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer. Invest New Drugs. (2018) 36:458–67. 10.1007/s10637-018-0578-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pandey V, Wang BC, Mohan CD, Raquib AR, Rangappa S, Srinivasa V, et al. Discovery of a small-molecule inhibitor of specific serine residue BAD phosphorylation. Proc Natl Acad Sci USA. (2018) 115:E10505–14. 10.1073/pnas.1804897115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Y, Savage RE, Eathiraj S, Meade J, Wick MJ, Hall T, et al. Targeting AKT1-E17K and the PI3K/AKT pathway with an allosteric AKT inhibitor, ARQ 092. PLoS ONE. (2015) 10:e0140479. 10.1371/journal.pone.0140479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mimura N, Hideshima T, Shimomura T, Suzuki R, Ohguchi H, Rizq O, et al. Selective and potent akt inhibition triggers anti-myeloma activities and enhances fatal endoplasmic reticulum stress induced by proteasome inhibition. Cancer Res. (2014) 74:4458–69. 10.1158/0008-5472.CAN-13-3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Bono J, Bracarda S, Chi K, Massard C, Hidalgo D, Sandhu S, et al. Randomized phase III trial of ipatasertib vs. placebo, plus abiraterone and prednisone/prednisolone, in men with asymptomatic or mildly symptomatic previously untreated metastatic castrate-resistant prostate cancer (mCRPC). Ann Oncol. (2017) 28:290 10.1093/annonc/mdx370.051 [DOI] [Google Scholar]
- 87.San-Miguel J, Blade J, Shpilberg O, Grosicki S, Maloisel F, Min CK, et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood. (2014) 123:4136–42. 10.1182/blood-2013-12-546374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoy SM. Sintilimab: first global approval. Drugs. (2019) 79:341–46. 10.1007/s40265-019-1066-z [DOI] [PubMed] [Google Scholar]
- 89.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for advanced renal-cell carcinoma. N Engl J Med. (2019) 380:1103–15. 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arnott CH, Scott KA, Moore RJ, Hewer A, Phillips DH, Parker P, et al. Tumour necrosis factor-alpha mediates tumour promotion via a PKCalpha- and AP-1-dependent pathway. Oncogene. (2002) 21:4728–38. 10.1038/sj.onc.1205588 [DOI] [PubMed] [Google Scholar]
- 91.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS, Wang Y-L. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial–mesenchymal transition. Mol Biol Cell. (2012) 23:781–91. 10.1091/mbc.e11-06-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE. (2014) 9:e90353 10.1371/journal.pone.0090353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grasset EM, Bertero T, Bozec A. Matrix stiffening and EGFR cooperate to promote the collective invasion of cancer cells. Cancer Res. (2018) 78:5229–42. 10.1158/0008-5472.CAN-18-0601 [DOI] [PubMed] [Google Scholar]
- 94.Saplacan RMM, Balacescu L, Gherman C, Chira RI, Craiu A, Mircea PA, et al. The role of PDGFs and PDGFRs in colorectal cancer. Mediat Inflamm. (2017) 2017:1–9. 10.1155/2017/4708076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Albrengues J, Bourget I, Pons C, Butet V, Hofman P, Tartare-Deckert S, et al. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. (2014) 7:1664–78. 10.1016/j.celrep.2014.04.036 [DOI] [PubMed] [Google Scholar]
- 96.Gao Y, Xiao Q, Ma HM, Li L, Liu J, Feng Y, et al. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc Natl Acad Sci USA. (2010) 107:18892–97. 10.1073/pnas.1004952107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Q, Zhang X-F, Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. (2011) 20:538–49. 10.1016/j.ccr.2011.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu SH, Maynard JP, Vaghasia AM, Marzo AMD, Drake CG, Sfanos KS. A role for paracrine interleukin-6 signaling in the tumor microenvironment in prostate tumor growth. Prostate. (2019) 79:215–22. 10.1002/pros.23726 [DOI] [PubMed] [Google Scholar]
- 99.Liu Q, Qiao L, Liang N, Xie J, Zhang J, Deng G, et al. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. J Cell Mol Med. (2016) 20:1761–69. 10.1111/jcmm.12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu D, et al. Advanced research on vasculogenic mimicry in cancer. J Cell Mol Med. (2015) 19:315–26. 10.1111/jcmm.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.A Study of OKI-179 in Patients With Solid Tumors (NCT03931681) (2019). Available online at: https://clinicaltrials.gov/show/NCT03931681
- 102.Efficacy Safety and Tolerability Study of SHAPE in IA IB or IIA Cutaneous T-Cell Lymphoma (NCT02213861) (2014). Available online at: https://clinicaltrials.gov/show/NCT02213861
- 103.O'Connor OA, Oezcan M, Jacobsen ED, Roncero JM, Trotman J, Demeter J, et al. Randomized phase III study of alisertib or investigator's choice (selected single agent) in patients with relapsed or refractory peripheral t-cell lymphoma. J Clin Oncol. (2019) 37:613–23. 10.1200/JCO.18.00899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Surati M, Valla K, Shah KS, Panjic EH, Lonial S. Panobinostat for the treatment of multiple myeloma. Expert Opin Orphan D. (2015) 229–38. 10.1517/21678707.2015.999665 [DOI] [Google Scholar]
- 105.Blagden S, Olmin A, Josephs D, Stavraka C, Zivi A, Pinato DJ, et al. First-in-human study of CH5132799, an oral class I PI3K inhibitor, studying toxicity, pharmacokinetics, and pharmacodynamics, in patients with metastatic cancer. Clin Cancer Res. (2015) 21:660–60. 10.1158/1078-0432.CCR-14-3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trial to Evaluate the Safety and Pharmacokinetics of HMPL-689 in Patients With Lymphomas (NCT03786926) (2018). Available online at: https://clinicaltrials.gov/show/NCT03786926
- 107.Gazi M, Moharram SA, Marhaell A, Kazi JU. The dual specificity PI3K/mTOR inhibitor PKI-587 displays efficacy against T-cell acute lymphoblastic leukemia (T-ALL). Cancer Lett. (2017) 392:9–16. 10.1016/j.canlet.2017.01.035 [DOI] [PubMed] [Google Scholar]
- 108.Makker V, Recio FO, Ma L, Matulonis UA, Lauchle JO, Parmar H, et al. A multicenter, single-arm, open-label, phase 2 study of apitolisib (GDC-0980) for the treatment of recurrent or persistent endometrial carcinoma (MAGGIE study). Cancer. (2016) 122:3519–28. 10.1002/cncr.30286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Gooijer MC, Zhang P, Buil LCM, Citirikkaya CH, Thota N, Beijnen JH, et al. Buparlisib is a brain penetrable pan-PI3K inhibitor. Sci Rep. (2018) 8:10784–92. 10.1038/s41598-018-29062-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Markham A. Copanlisib: first global approval. Drugs. (2017) 77:2057–62. 10.1007/s40265-017-0838-6 [DOI] [PubMed] [Google Scholar]
- 111.Shen H, Rodriguez-Aguayo C, Xu R, Gonzalez-Villasana V, Mai J, Huang Y, et al. Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clin Cancer Res. (2013) 19:1806–15. 10.1158/1078-0432.CCR-12-2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu X, Wang JH, Li S, Li LL, Huang M, Zhang YH, et al. Histone deacetylase 3 expression correlates with vasculogenic mimicry through the phosphoinositide3-kinase/ERK-MMP-laminin5gamma2 signaling pathway. Cancer Sci. (2015) 106:857–66. 10.1111/cas.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Veggel M, Westerman E, Hamberg P. Clinical pharmacokinetics and pharmacodynamics of panobinostat. Clin Pharmacokinet. (2018) 57:21–9. 10.1007/s40262-017-0565-x [DOI] [PubMed] [Google Scholar]
- 114.Hess AR, Seftor EA, Seftor REB, Hendrix MJC. Phosphoinositide 3-kinase regulates membrane Type 1-matrix metalloproteinase (MMP) and MMP-2 activity during melanoma cell vasculogenic mimicry. Cancer Res. (2003) 63:4757–62. Available online at: https://cancerres.aacrjournals.org/content/63/16/4757 [PubMed] [Google Scholar]
- 115.Hess AR, Seftor EA, Gardner LMG, Carles-Kinch K, Schneider GB, Seftor REB, et al. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2). Cancer Res. (2001) 61:3250–55. Available online at: https://cancerres.aacrjournals.org/content/61/8/3250 [PubMed] [Google Scholar]
- 116.Hess AR, Hendrix MJ. Focal adhesion kinase signaling and the aggressive melanoma phenotype. Cell Cycle. (2006) 478–80. 10.4161/cc.5.5.2518 [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N, et al. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. (2017) 31–44. 10.1016/j.canlet.2017.02.035 [DOI] [PubMed] [Google Scholar]
- 118.Warburg O. On the origin of cancer cells. Science. (1956) 123:309–14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 119.Faubert B, Vincent EE, Griss T, Samborska B, Izreig S, Svensson RU, et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1α. Proc Natl Acad Sci USA. (2014) 111:2554–59. 10.1073/pnas.1312570111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wong N, De Melo J, Tang D. PKM2, a central point of regulation in cancer metabolism. Int J Cell Biol. (2013) 2013:1–11. 10.1155/2013/242513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu N, Hua ZY, Ba G, Zhang SM, Liu ZH, Thiele CJ, et al. The anti-tumor growth effect of a novel agent DMAMCL in rhabdomyosarcoma in vitro and in vivo. J Exp Clin Cancer Res. (2019) 38:118. 10.1186/s13046-019-1107-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Du L, Yang F, Fang H, Sun H, Chen Y, Xu Y, et al. AICAr suppresses cell proliferation by inducing NTP and dNTP pool imbalances in acute lymphoblastic leukemia cells. FASEB J. (2019) 33:4525–37. 10.1096/fj.201801559RR [DOI] [PubMed] [Google Scholar]
- 123.Yu L, Lu M, Jia D, Ma J, Ben-Jacob E, Levine H, et al. Modeling the genetic regulation of cancer metabolism: Interplay between glycolysis and oxidative phosphorylation. Cancer Res. (2017) 1564–74. 10.1158/0008-5472.CAN-16-2074 [DOI] [PMC free article] [PubMed] [Google Scholar]