Abstract
Huangqi–Danshen decoction (HDD) is a commonly used drug pair for clinical treatment of chronic kidney disease (CKD) in traditional Chinese medicine with good efficacy. However, the potential mechanisms of this action have not been well elucidated. The aim of this study was to explore the metabolic profiling variations in response to HDD treatment in a CKD rat model. CKD rat model was induced by adding 0.75% adenine to the diet for 4 weeks. The rats in the treatment group received HDD extract orally at the dose of 4.7 g/kg/day during the experiment. At the end of the experiment, serum and kidney samples were collected for biochemical and pathological examination. Ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC-QTOF/MS) was used to analyze metabolic profiling variations in the kidney. The results showed that treatment with HDD markedly attenuated kidney injury and improved renal function. A total of 28 metabolites contributing to CKD phenotype were found and identified in the kidney samples. The primary metabolic pathways disordered in the kidney of CKD rats were glycerophospholipid metabolism, glycosylphosphatidylinositol-anchor biosynthesis, and citrate cycle. Partial least squares discriminant analysis (PLS-DA) score plot showed that the three groups of renal samples were obviously divided into three categories, and the metabolic trajectory of the HDD treatment group moved to the control group. (E)-Piperolein A, phosphatidylcholines (PC) (18:1/22:6), phosphatidylinositols (PI) (13:0/18:1), PI (15:0/20:3), phosphatidylserines (PS) (O-20:0/12:0), and triglyceride (TG) (22:4/24:0/O-18:0) represented potential biomarkers of the renoprotective effects of HDD against CKD. In conclusion, HDD has renoprotective effect against adenine-induced CKD, which may be mediated via partially restoration of perturbed metabolism in the kidney.
Keywords: chronic kidney disease, traditional Chinese medicine, Huangqi–Danshen decoction, metabolomics, ultra-high-performance liquid chromatography, mass spectrometry
Introduction
Chronic kidney disease (CKD) arises from many heterogeneous diseases that alter the function and structure of the kidney irreversibly, over months or years (Webster et al., 2017). According to The 2017 Global Burden of Disease (GBD) Study, global absolute CKD prevalence increased by 28.2% from 2007 to 2017 among women and 25.4% among men (James et al., 2018), and the number of global deaths due to CKD will rise from 1.2 million in 2016 to 3.1 million in 2040 (Foreman et al., 2018). The high prevalence and mortality of CKD makes its treatment an urgent task. Normal strategies for the treatment of CKD have emerged over the past years, such as endothelin antagonists, vitamin D receptor agonists, anti-inflammatory agents, bardoxolone, pentoxifylline, etc. Despite promising results in experimental models and small randomized studies, adequately powered randomized trials are required to evaluate the benefits and risks of these therapies in the CKD population (Lambers Heerspink and De Zeeuw, 2013; Pena-Polanco and Fried, 2016). The use of traditional Chinese medicine (TCM) has been a major complementary and alternative branch of CKD therapy (Li and Wang, 2005; Zhong et al., 2013; Zhong et al., 2015). Increasing evidence indicates the beneficial effects of TCM on prevention and treatment of CKD in both clinical CKD patients (Wang et al., 2012; Hsieh et al., 2014; Lin et al., 2015; Zheng et al., 2017) and CKD animal models (Zhao et al., 2015a; Wang et al., 2017b; Dou et al., 2018; Liu et al., 2018; Zhang et al., 2018). Huangqi–Danshen decoction (HDD), a TCM drug pair, is composed of Astragali Radix (Huang-qi) and Salviae Miltiorrhizae Radix et Rhizoma (Dan-shen). According to TCM theory, HDD possesses the efficacies of replenishing Qi and activating blood (Yi-Qi-Huo-Xue), which helps delay the progress of CKD. The previous pharmacological study has showed the renoprotective effect of HDD in adenine-induced CKD rats (Liu et al., 2019). However, the underlying mechanism of HDD on CKD is less known.
Metabolomics has been used to characterize the biochemical metabolites related to pathogenesis of disease that could be used for the diagnosis and monitoring of the disease progression and response to therapeutic interventions (Johnson et al., 2016). In agreement with the holistic thinking of TCM, metabolomics has shown potential in bioactivity evaluation and action mechanism of TCM as well as pharmaceutical research and development (Wang et al., 2017a). Recently, ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC-QTOF/MS)-based metabolomics has been widely applied to evaluate therapeutic effects and potential mechanisms of TCM formulas or herbs in treating CKD (Zhang et al., 2015; Zhao et al., 2015b; Dou et al., 2018; Zhang et al., 2018). Therefore, metabolomics provides an analytical platform in understanding the mechanism of action of Chinese herbal medicine, which will help to promote the modernization of TCM.
In the present study, addition of 0.75% adenine to the diet of rats was used to cause metabolic abnormalities similar to human CKD (Chen et al., 2016). Blood biochemical parameters and kidney pathological changes were used to evaluate the characteristics of CKD and efficacy of HDD treatment. Finally, untargeted metabolomics approach was applied to investigate the metabolic profiling and the response to HDD treatment in adenine-induced CKD rat model.
Materials and Methods
Chemicals and Reagents
Adenine was purchased from Sigma-Aldrich (St. Louis, MO, USA). The primary antibodies used in the present study included fibronectin (FN), type IV collagen (Col-IV) (abcam, Cambridge, MA, USA), and α-smooth muscle actin (α-SMA) (Sigma-Aldrich, St Louis, MO, USA). HDD consists of Astragali Radix [roots of Astragalus membranaceus (Fisch). Bge. var. mongholicus (Bge). Hsiao] and Salviae Miltiorrhizae Radix et Rhizoma (roots and rhizomes of Salvia miltiorrhiza Bge). ( Table 1 ). All the botanical names are recorded and can be validated using http://mpns.kew.org/mpns-portal/?_ga=1.111763972.1427522246.145907734. The HDD water extract was prepared as previously described (Liu et al., 2019). High-performance liquid chromatography–mass spectrometry (HPLC-MS) analysis was conducted to confirm the quality of the HDD extract, as indicated in Supplementary Figure S1 .
Table 1.
The herbal composition and proportion of HDD.
| Botanical name | Herbal name | Chinese name | Voucher number | Dosage |
|---|---|---|---|---|
| Astragalus membranaceus (Fisch). Bge. var. mongholicus (Bge). Hsiao | Astragali Radix | Huang-Qi | 2010015Z | 30 g |
| Salvia miltiorrhiza Bge. | Salviae Miltiorrhizae Radix et Rhizoma | Dan-Shen | 2010006Z | 15 g |
Animals
All animal experiments were carried out in accordance with protocols approved by the Ethics Committee of Shenzhen Traditional Chinese Medicine Hospital, Guangzhou University of Chinese Medicine (Shenzhen, China), and all efforts were made to minimize animal suffering. Eight-week-old male Sprague–Dawley (SD) rats were randomly divided into the following three groups: control group (n = 6), CKD group (n = 6), and CKD + HDD group (n = 6). Rats in the CKD and CKD + HDD group were fed a diet containing 0.75% w/w adenine for 4 weeks. CKD + HDD group was administered with HDD extract (4.7 g/kg/day) by gastric irrigation during 4 weeks study period. Control group rats received normal adenine-free feed for 4 weeks. At the end of study, all rats were anesthetized (sodium pentobarbital, 75 mg/kg, intraperitoneal injection), and blood samples were obtained by cardiac puncture. The rats were euthanized by cervical dislocation without regaining consciousness. The kidneys were rapidly harvested and processed for histological examination and metabolomic analysis.
Serum Biochemical Analysis
Serum samples were isolated from blood by centrifugation for 10 min at 1,000 rpm at 4°C. Serum creatinine (Scr), blood urea nitrogen (BUN), and albumin were measured by creatinine serum detection kit, BUN detection kit (StressMarq Biosciences, British Columbia, Canada), and QuantiChrom™ BCG Albumin Assay Kit (BioAssay Systems, Hayward, CA, USA), respectively, following the manufacturer’s instructions.
Histological Analysis
Paraffin-embedded kidneys from rats of three groups were cut into 4-µm sections, dewaxed, and rehydrated. Sections were stained with periodic acid–Schiff (PAS) and Masson’s trichrome stains. The degree of tubular atrophy in PAS staining was determined based on a scale of 0–3 points: 0, normal tubules; 1, rare single atrophic tubule; 2, several clusters of atrophic tubules; and 3, massive atrophy (Lu et al., 2018). Interstitial fibrosis area in Masson staining was measured using Image J software (NIH, Bethesda, MD, USA). Five microscopic fields (200×) of each rat and six rats in each group were performed quantitative analyses in a blinded manner.
Immunohistochemistry
The paraffin-embedded rat kidney slides were dewaxed, rehydrated, and immersed in 3% hydrogen peroxide for 10 min at room temperature to block endogenous peroxidase activity. Then, the slides were blocked with 10% goat serum for 1 h at 37°C and were stained with primary antibodies against fibronectin (FN) (dilution 1:200), type IV collagen (Col-IV) (dilution 1:200), and α-smooth muscle actin (α-SMA) (dilution 1:250) at 4°C overnight followed by SignalStain Boost Detection Reagent (Cell Signaling Technology, Beverly, MA, USA) for 30 min at room temperature. The sections were then treated with SignalStain diaminobenzidine (DAB) substrate (Cell Signaling Technology, Beverly, MA, USA) and counterstained with hematoxylin. The integrated optical density (IOD) values of the positive staining areas were measured by ImagePro Plus 6.0 software (Media Cybernetics, CA, USA). Five microscopic fields (200×) of each rat and three rats in each group were counted in a blinded manner.
Sample Extraction for UHPLC-QTOF/MS Analysis
Renal tissue samples were ground by liquid nitrogen at room temperature, and then 20 mg of the renal tissue was added with 800 μl ice-cold mixture of methanol, acetonitrile, and acetone (1:1:1, v/v). The mixture was thoroughly vortexed for 2 min, incubated at 4°C for 10 min, then centrifuged (14,000 rpm for 10 min at 4°C). The supernatant (600 μl) was transferred and then evaporated to dryness under nitrogen gas at 37°C. The residue was reconstituted with 200 μl 10% acetonitrile and centrifuged (13,000 rpm for 10 min at 4°C) after being vortexed for 60 s. The supernatant was transferred to autosampler vials for further analysis.
Sample Preparation and Extraction of Quality Control Sample
To guarantee the quality of the nontargeted bioanalytical data, quality control (QC) samples were used for method validation. Therefore, 20 mg mixture of renal tissue samples from each group was pooled to obtain a QC sample, and then, 110 μl of the QC sample was added with 10 μl IS (2,4-dichloro-phenylalanine, 0.2 mg/ml). QC samples were extracted using the sample extraction method mentioned above. The QC specimens were inserted between every six experimental samples throughout the whole analysis procedure.
UHPLC-QTOF/MS Analysis
Chromatographic separation was performed on a Dionex UltiMate 3000 UHPLC system (Thermo Scientific, San Jose, CA, USA). UHPLC conditions were as follows: column: Waters Acquity BEH C18 column (2.1 × 100 mm, 1.7 μm, Milford, MA, USA); column temperature: 30°C; mobile phase: 0.1% formic acid water (A) and acetonitrile (B); gradient conditions for positive mode analysis: 0–4 min 10–13% B; 4–6 min 13–50% B; 6–10 min 50% B; 10–14 min 50–85% B; 14–18 min 85% B; 18–20 min 85–100%; 20–25 min 100%; re-equilibrate: 10 min; gradient conditions for negative mode analysis: 0–2 min 15% B; 2–4 min 15–55% B; 4–8 min 55% B; 8–14 min 55–70% B; 14–22 min 70–80% B; 22–24 min 80–100%; 24–28 min 100%; re-equilibrate: 10 min; flowrate: 0.2 ml/min; injection volume: 2 μl.
MS detection was performed on an Orbitrap mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA), which was operated in both positive (ESI+) and negative electrospray ionization interface (ESI−). The MS parameters were as follows: heater temperature, 350°C; sheath gas flowrate, 35 arb; aux gas flowrate, 10 arb; sweep gas flowrate, 0 arb; I spray voltage, 3.2 kV; capillary temperature: 300°C; mass range: m/z 150–1,500.
Data Processing
The acquired raw data were preprocessed by the following procedures: peaks identification, peaks filtration and normalization to total area, and peaks alignment. The three-dimensional data set included sample information, peak intensities, peak retention time (RT), and mass-to-charge ratio (m/z). Then, the three-dimensional data matrices were imported into the SIMCA14.1 (Umetrics, Umeå, Sweden) software for multivariate statistical analysis. The analysis methods including principal components analysis (PCA), partial least-squares discriminant analysis (PLS-DA), and orthogonal partial least-squares discriminant analysis (OPLS-DA) were used for metabolic profile analysis.
Biomarker Identification and Metabolic Pathway Analysis
OPLS-DA model was used to visualize the metabolic difference between the control group and the model group. Variables with VIP >1 in the OPLS-DA model, variables with p|corr| value >0.58 in S-plot, and variables with cross-validation by jack-knife method were considered as potential biomarkers. The potential biomarkers were identified using Masslynx (Waters, Milford, MA, USA) combined with the METLIN database 1 and Human Metabolome Database (HMDB) 2 . Then, receiver operating characteristic (ROC) curves (Prism 7.00) were applied to analyze data for evaluating the predictive power of the identified biomarkers. Pathway analysis was based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) 3 and MetaboAnalyst 4 .
Statistical Analysis
Unless otherwise stated, data were expressed as mean ± standard error of mean (SEM). The statistical analyses were performed using SPSS statistics software version 16.0 (SPSS Inc., Chicago, IL, USA). Comparison of the same parameter among groups was analyzed by one-way ANOVA followed by post hoc analysis with Tukey test. The value of P < 0.05 was considered statistically significant.
Results
Standardization of HDD Extract
Before drug treatment, HDD was first chemically standardized. An HPLC-MS approach was established to reveal the chemical profile of HDD extract and quantify the main ingredients in the extract. Using the respective individual standard, 11 compounds were identified from HDD extract ( Supplementary Figure S1 ), and the minimum amount in mg/g of dried extract was 0.01659 for protocatechualdehyde, 0.0052 for caffeic acid, 0.0574 for rosmarinic acid, 0.0436 for lithospermic acid, 0.0779 for ononin, 0.0184 for calycosin 7-O-beta-D-glucoside, 0.4503 for salvianolic acid B, 0.0015 for calycosin, 0.0018 for astragaloside IV, 0.0017 for astragaloside III, and 0.0088 for astragaloside II. The chemical analysis of HDD extract here served as quality control for the reproducibility of the below animal study.
HDD Attenuated Adenine-Induced CKD in Rats
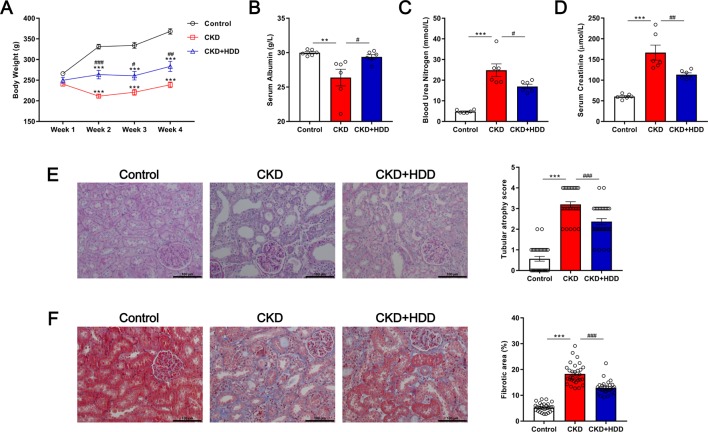
Adenine-induced CKD rats began to lose weight significantly from the second week until the end of the experiment. HDD treatment obviously prevented the decline of body weight in CKD rats ( Figure 1A ). Consistently, we observed that HDD increased serum albumin level, a nutritional indicator, by 11.37% in CKD rats ( Figure 1B ). BUN and Scr levels were measured to evaluate the renal function. As shown by the data, BUN level significantly increased by 5.16-fold in the CKD group compared with the control group and was blocked by 31.65% in the CKD + HDD group ( Figure 1C ). Similarly, Scr level increased by 2.76-fold in CKD rats and was blocked by 32.01% after HDD treatment ( Figure 1D ). In agreement with the improved renal function, massive tubular atrophy in PAS staining and obvious fibrosis in Masson staining in CKD rats were significantly reversed by HDD treatment ( Figures 1E, F ). Moreover, the potential side effects of HDD were estimated by the levels of aspartate transaminase (AST) and alanine transaminase (ALT). There were no significant differences in ALT and AST levels between the three groups ( Supplementary Figure S2 ). These data demonstrated that HDD attenuated adenine-induced CKD in rats without toxic effect on liver function.
Figure 1.
Effects of HDD on adenine-induced CKD. (A) Body weight. (B) Serum albumin. (C) Blood urea nitrogen. (D) Serum creatinine. (E) PAS staining. (F) Masson staining. Data are presented as the means ± SEM, n = 6 rats per group (**P < 0.01, ***P < 0.001 compared with the control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the CKD group).
HDD Inhibited Tubulointerstitial Fibrosis in CKD Rats
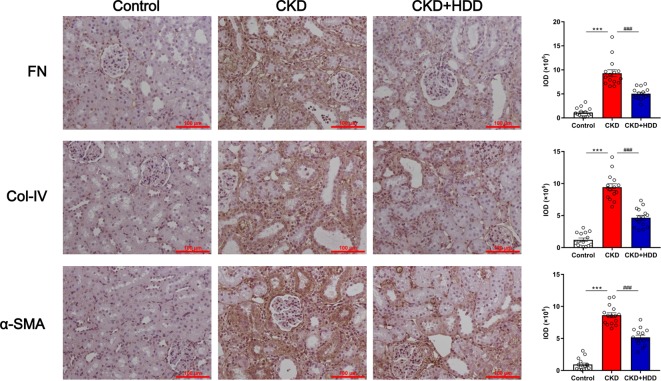
Tubulointerstitial fibrosis (TIF) is the common final pathway for CKD progression to end-stage renal disease (ESRD). Immunohistochemistry staining showed that TIF markers FN, Col-IV, and α-SMA were significantly increased in the kidney of CKD rats and were strikingly inhibited by 45.77, 50.61, and 39.94%, respectively, after HDD administration ( Figure 2 ). These data indicated that HDD inhibited TIF in CKD rats.
Figure 2.
HDD inhibited fibrotic markers expression in CKD rats. Data are presented as the means ± SEM, n = 3 rats per group (***P < 0.001 compared with the control group; ###P < 0.001 compared with the CKD group).
Repeatability and Stability of the UHPLC-QTOF/MS Method
The overlapping total ion current (TIC) chromatograms ( Supplementary Figure S3 ) of QC samples demonstrated the acceptable variations occurred during the large-scale sample analysis. Meantime, six selected extracted ion chromatograms (EICs) in QC samples were used to assess the system repeatability and stability. Taking tissue samples in positive mode as an example, the retention times, peak areas, and mass accuracies of these six selected peaks showed acceptable RSDs. RSDs of these six peaks were 0.00000–2.81406% for retention times, 7.13116–17.37194% for peak areas, and 0.00008–0.07090% for mass accuracies ( Supplementary Tables S1 , S2 ).
Metabolic Profiles of the Control and CKD Rats
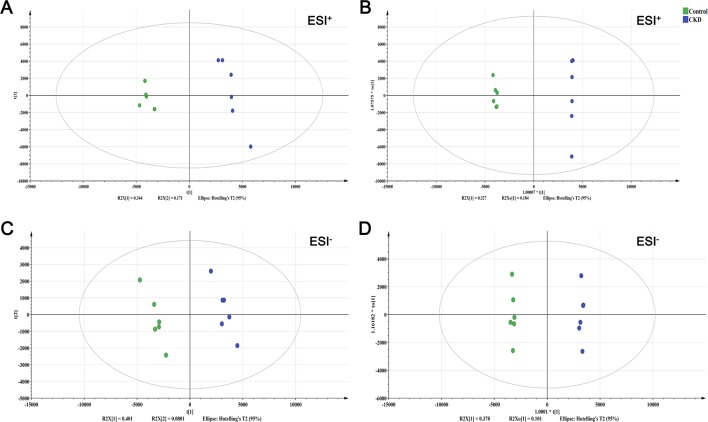
To evaluate the alterations of metabolites in CKD rats, PCA and OPLS-DA were performed using data from the control and CKD groups. PCA score plots showed the obvious separation trend between the control and CKD group ( Figures 3A, C ), indicating that renal metabolic states of CKD rats were significantly changed in relative to control rats. OPLS-DA score plots could also readily be divided into two clusters ( Figures 3B, D ). These results illustrated that the metabolites in the control and CKD groups had been completely separated either the positive or the negative ion mode.
Figure 3.
The score plots from PCA and OPLS-DA model. (A) PCA-X score plot of positive ion mode. (B) OPLS-DA score plot of positive ion mode. (C) PCA-X score plot of negative ion mode. (D) OPLS-DA score plot of negative ion mode. ESI+ means the positive electrospray ionization. ESI− means the negative electrospray ionization. Green circle corresponds to the control group; blue circle corresponds to the CKD group.
Identification of Differential Metabolites in CKD Rats
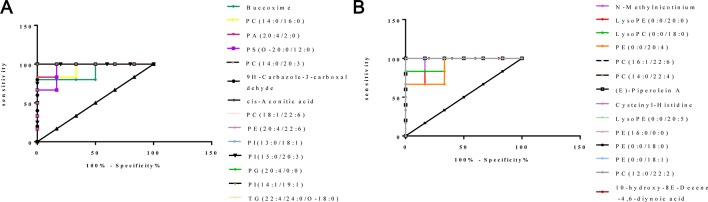
In total, 28 metabolites contributing to CKD phenotype were found and identified in the kidney ( Table 2 ). The ROC curve was performed to evaluate the predictive value of these screened potential biomarkers. The 14 upregulated metabolites provided good diagnostic abilities with average area under the curve (AUC) at 0.9000–1.0000 and p value at 0.0016–0.0163 ( Figure 4A , Supplementary Table S3 ). The 14 downregulated metabolites also provided good diagnostic abilities with AUC at 0.8333–1.0000 and p value at 0.0039–0.0250 ( Figure 4B , Supplementary Table S4 ). The results showed that the identified biomarkers had a powerful diagnostic performance.
Table 2.
Identities of differential metabolites between control and CKD model.
| NO. | Abbreviation | Molecular formula | m/z | Rt (min) | Matched Mass | ppm | VIP | p value | Fold |
|---|---|---|---|---|---|---|---|---|---|
| 1 | N-Methylnicotinium | C11H17N2 | 221.1033 | 2.84 | 221.1031 | 0.9 | 185 | 0.0065 | 0.40 |
| 2 | Buccoxime | C10H17NO | 150.1277 | 27.27 | 150.1283 | -4.0 | 3.18 | 0.0163 | 1.16 |
| 3 | PC (16:1/22:6) | C46H78NO8P | 804.5507 | 20.6 | 804.5538 | -3.9 | 1.32 | 0.0039 | 0.25 |
| 4 | PC (14:0/22:4) | C44H80NO8P | 782.5697 | 27.34 | 782.5694 | 0.4 | 9.91 | 0.0039 | 0.58 |
| 5 | PC (14:0/20:3) | C42H78NO8P | 756.5526 | 27.35 | 756.5538 | -1.6 | 1.93 | 0.0039 | 2.29 |
| 6 | (E)-Piperolein A | C19H25NO3 | 104.0537 | 1.17 | 104.0539 | 1.9 | 1.09 | 0.0039 | 0.42 |
| 7 | Cysteinyl-Histidine | C9H14N4O3S | 279.0541 | 1.27 | 279.0533 | 2.9 | 1.08 | 0.0062 | 0.27 |
| 8 | 9H-Carbazole-3-carboxaldehyde | C13H9NO | 214.0665 | 1.17 | 214.0668 | 1.4 | 1.18 | 0.0039 | 6.46 |
| 9 | cis-Aconitic acid | C6H6O6 | 173.01 | 2.96 | 173.0092 | 4.6 | 2.03 | 0.0039 | 29.92 |
| 10 | PC (14:0/16:0) | C38H76NO8P | 750.5287 | 27.6 | 750.5291 | 0.5 | 2.07 | 0.0163 | 2.37 |
| 11 | PC (18:1/22:6) | C48H82NO8P | 830.5711 | 27.6 | 830.5705 | 0.7 | 2.67 | 0.0039 | 3.42 |
| 12 | PA (20:4/2:0) | C25H43O7P | 531.2737 | 12.23 | 531.2728 | 1.7 | 1.01 | 0.0065 | 13.54 |
| 13 | LysoPE (0:0/20:5) | C25H42NO7P | 544.2682 | 9.67 | 544.2681 | 0.2 | 1.21 | 0.0039 | 0.04 |
| 14 | LysoPE (0:0/20:0) | C25H52NO7P | 508.3433 | 16.99 | 508.3409 | 4.7 | 3.61 | 0.0163 | 0.54 |
| 15 | PE (20:4/22:6) | C47H74NO8P | 810.5102 | 5028 | 810.5079 | 2.8 | 9.65 | 0.0039 | 7.54 |
| 16 | LysoPC (0:0/18:0) | C26H54NO7P | 568.3604 | 16.99 | 568.362 | 2.8 | 3.55 | 0.0104 | 0.53 |
| 17 | PE (16:0/0:0) | C21H44NO7P | 452.2841 | 12.5 | 452.2783 | 12.8 | 2.87 | 0.0039 | 0.28 |
| 18 | PE (0:0/18:0) | C23H48NO7P | 480.3138 | 16.84 | 480.3096 | 8.8 | 4.13 | 0.0039 | 0.44 |
| 19 | PE (0:0/20:4) | C25H44NO7P | 500.2813 | 10.19 | 500.2783 | 6.0 | 3.47 | 0.0250 | 0.54 |
| 20 | PE (0:0/18:1) | C23H46NO7P | 524.3009 | 17.03 | 524. 2994 | 2.9 | 2.88 | 0.0039 | 0.31 |
| 21 | 10-hydroxy-8E-Decene-4,6-diynoic acid | C10H10O3 | 177.059 | 1.2 | 177.0557 | 18.6 | 1.61 | 0.0039 | 0.07 |
| 22 | PI (13:0/18:1) | C40H75O13P | 793.4846 | 27.6 | 793.4873 | 3.4 | 2.04 | 0.0039 | 3.30 |
| 23 | PI (15:0/20:3) | C44H79O13P | 845.5125 | 27.6 | 845.5186 | 7.2 | 1.89 | 0.0039 | 16.80 |
| 24 | PG (20:4/0:0) | C26H45O9P | 531.2737 | 12.23 | 531.2729 | 1.5 | 1.01 | 0.0065 | 13.54 |
| 25 | PS (O-20:0/12:0) | C38H76NO9P | 766.5232 | 5.36 | 766.524 | 1.0 | 9.08 | 0.0104 | 3.44 |
| 26 | PI (14:1/19:1) | C42H77O13P | 819.4969 | 27.6 | 819.5029 | 7.3 | 1.76 | 0.0039 | 2.34 |
| 27 | TG (22:4/24:0/O-18:0) | C67H124O5 | 335.3083 | 27.17 | 335.3077 | 1.8 | 1.30 | 0.0039 | 10.58 |
| 28 | PC (12:0/22:2) | C42H80NO8P | 758.5695 | 20.43 | 758.5694 | 0.13 | 9.85 | 0.0039 | 0.57 |
LysoPC, lysophosphatidylcholines; LysoPE, lysophosphatidylethanolamine; PA, phosphatidic acids; PC, phosphatidylcholines; PE, phosphatidylethanolamines; PG, phosphoglycerols; PI, phosphatidylinositols; PS, phosphatidylserines; TG, triglyceride.
Figure 4.
ROC curves of 28 potential biomarkers from kidney in CKD rats. (A) ROC analysis of 14 upregulated biomarkers. (B) ROC analysis of 14 downregulated biomarkers.
Metabolic Pathway Analysis
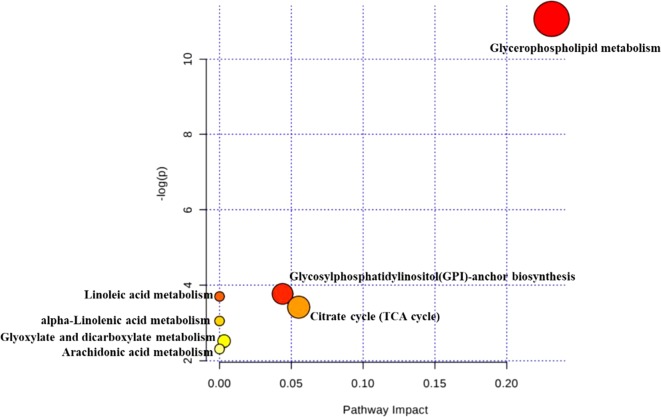
According to MetaboAnalyst database analysis, there were seven main metabolic pathways disordered in CKD rats, which were glycerophospholipid metabolism, glycosylphosphatidylinositol (GPI)-anchor biosynthesis, citrate cycle (TCA cycle), linoleic acid metabolism, alpha-linolenic acid metabolism, glyoxylate and dicarboxylate metabolism, and arachidonic acid metabolism ( Figure 5 ).
Figure 5.
Disordered pathways in the CKD group. The topology map generated from MetaboAnalyst described the impact of 28 metabolites identified between control and CKD groups on metabolic pathway.
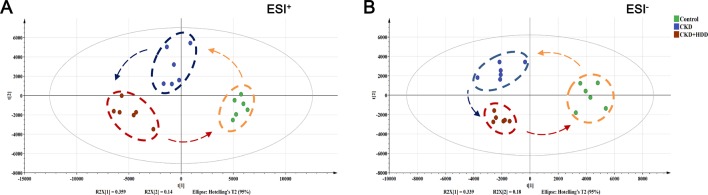
HDD Altered Metabolic Profiles in CKD Rats
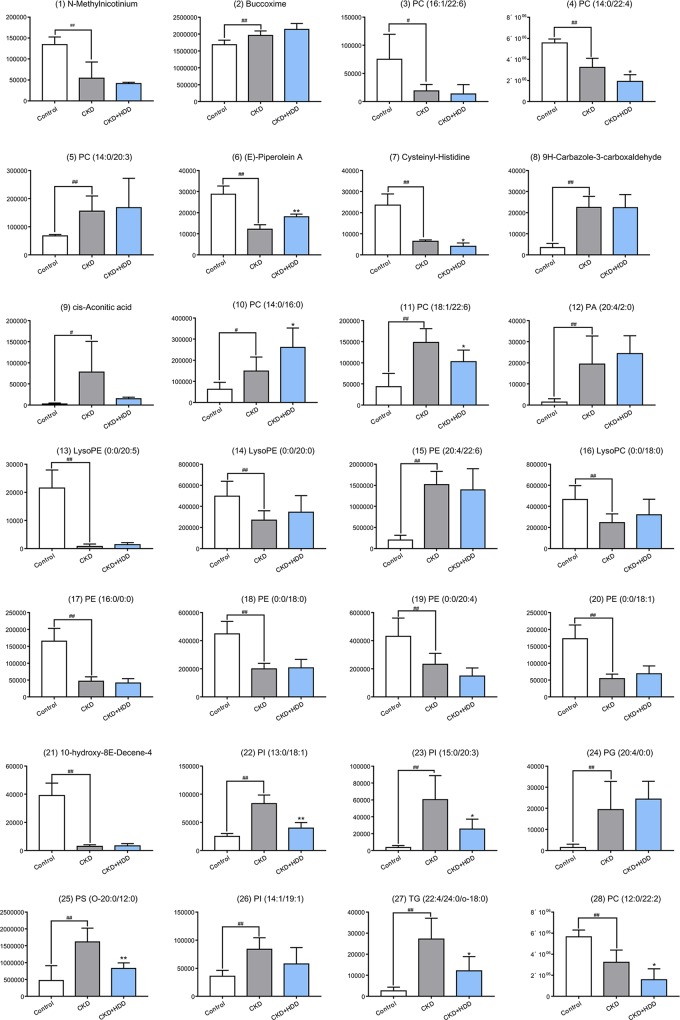
In order to reveal the effect of HDD on metabolic profiles in CKD rats, PLS-DA analysis was performed to obtain the changes in metabolic trajectory. The PLS-DA score plot showed that the three groups of renal samples were obviously divided into three categories, and the metabolic trajectory of the CKD+HDD group deviated from the CKD group and moved to the control group in both positive and negative ion modes ( Figure 6 ), which indicated that renal metabolites were obviously changed and had a tendency to recover after HDD treatment. Figure 7 illustrated the levels of 14 upregulated biomarkers and 14 downregulated biomarkers among the control group and CKD group and CKD + HDD group. After HDD treatment, the levels of (E)-Piperolein A, phosphatidylcholines (PC) (18:1/22:6), phosphatidylinositols (PI) (13:0/18:1), PI (15:0/20:3), phosphatidylserines (PS) (O-20:0/12:0), and triglyceride (TG) (22:4/24:0/O-18:0) were reversed and restored to near control level.
Figure 6.
Effect of HDD on the metabolic profiling by PLS-DA score plots. (A) PLS-DA score plot of positive ion mode. (B) PLS-DA score plot of negative ion mode.
Figure 7.
The relative content of 28 metabolites in the control, CKD, and CKD + HDD group. Data are represented as means ± SD, n ≥ 5 rats per group (*P < 0.05, **P < 0.01 compared with the CKD group; #P < 0.05, ##P < 0.01 compared with the control group).
Discussion
The present study described a renal metabolomic evaluation of CKD rat model induced by adenine and the treatment of HDD based on UHPLC-QTOF/MS metabolomics approach. HDD markedly reduced serum levels of creatinine and BUN, and ameliorated tubular atrophy and interstitial fibrosis in CKD rats. Furthermore, metabolomics analysis indicated that HDD effectively regulated the perturbed metabolism in the kidney of CKD rats.
CKD is a global public health problem, but its pathogenesis, early diagnosis, and treatment are still not satisfactory. Metabolomics is defined as “the quantitative measurement of the dynamic multiparametric metabolic response of living systems to pathophysiological stimuli or genetic modification” (Nicholson et al., 1999; Fiehn, 2002; Nicholson et al., 2002). Metabolites are the final product of cellular metabolism and can be thought of as the ultimate response of the system to heredity and environment. The use of metabolomics methods to study the metabolite composition of the system will provide a powerful tool for understanding the pathological mechanisms of CKD and developing new treatment strategies for treatment (Rhee, 2015; Grams et al., 2018). A series of experimental and clinical studies have been conducted to investigate the metabolic profiles of serum, plasma, urine, or tissue in CKD rodent models or CKD patients and have identified several metabolomics biomarkers and pathways (Zhao, 2013; Hocher and Adamski, 2017; Kalim and Rhee, 2017; Chen et al., 2019). Although TCM has been effective in treating CKD, it lacks the necessary clear molecular mechanisms. Consistent with the overall thinking of TCM, metabolomics has potential for TCM bioactivity and mechanism evaluation (Wang et al., 2017a). Many research groups have used metabolomics to evaluate efficacy and mechanism of TCM in CKD animal models using pure compounds from TCM (Zhao et al., 2014) or TCM extract (Zhang et al., 2016). In the present study, we identified 28 significant changed metabolites in the kidney of CKD rats, most of which were lipids (PCs, PEs, PIs, LysoPCs, or LysoPEs). Behind renopretective effect in CKD, HDD showed a regulatory effect on kidney metabolites, which mainly focused on glycerophospholipid metabolism (PC, PI, and PS). These findings might provide important clues for further mechanism studies.
Glycerophospholipids, glycerol-based phospholipids, are the main component of biological membranes and play a major role in cell signal induction and transport (Hermansson et al., 2011). Our results showed that glycerophospholipid metabolism is the most significantly altered pathway in the kidney of CKD rats and is a major aspect of metabolite regulation in HDD treatment response. Previous studies have shown the presence of glycerophospholipid abnormalities in CKD patients and animal models (Zhao et al., 2015c; Barrios et al., 2016; Ye and Mao, 2016). Nkuipou-Kenfack et al. reported abnormal PC metabolism in mild to advanced CKD patients (Nkuipou-Kenfack et al., 2014). Chen et al. included 180 patients with advanced CKD and found significant increase in the levels of glycerophospholipids which inversely correlated with the level of estimated glomerular filtration rate (eGFR) (Chen et al., 2017). These studies collectively suggest an important role for glycerophospholipids in CKD progression.
According to TCM theory, Qi deficiency and blood stasis (Qi-Xu-Xue-Yu) runs through the CKD process (Liu et al., 2019). HDD is composed of Astragali Radix (Huang-qi) served as replenishing Qi and Salviae Miltiorrhizae Radix et Rhizoma (Dan-shen) served as activating blood, and is one of the most common used drug pair in clinical treatment of CKD. In this study, metabolomics analysis indicated that renal metabolites had a tendency to recover in CKD rats after HDD treatment. Previous studies have reported the effects of Huang-qi or Dan-shen on metabolic pathways in different disease models. Li et al. found that the antifatigue effect of Huang-qi was associated with regulating glycometabolism, lipid metabolism, and energy metabolism (Li et al., 2014). Urinary metabolomics revealed that Huang-qi injections could regulate amino acid metabolism, TCA cycle, fatty acid metabolism, vitamin B6 metabolism, and purine metabolism in cisplatin-induced nephrotoxic rats (Li et al., 2017). Zhang et al. reported that the protective mechanism of Dan-shen in Alzheimer’s disease was related to glutathione metabolism, phenylalanine tyrosine and tryptophan biosynthesis, TCA cycle, glycerophospholipid metabolism, etc. (Zhang et al., 2019). Although the bioactive components of HDD and their interactions were obscure, metabolomics shed light on offering potential biomarkers and metabolic patterns, which will benefit drug efficacy evaluation and potential mechanism research.
Conclusion
In this study, UHPLC-QTOF/MS-based nontargeted metabolomic approach was applied to investigate the protective effects of HDD against adenine-induced CKD in rats. Twenty-eight metabolites contributing to CKD phenotype were identified in the kidney. The primary metabolic pathways disordered in the kidney of CKD rats were glycerophospholipid metabolism, GPI-anchor biosynthesis, and TCA cycle. Treatments with HDD attenuated kidney injury, improved renal function, and partially reversed abnormalities of renal metabolome.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Ethics Committee of Shenzhen Traditional Chinese Medicine Hospital, Guangzhou University of Chinese Medicine.
Author Contributions
XL and SL conceived and designed the experiments. LZ and JC performed herbal preparation. BZ, SH, and FW carried out animal experiment and conducted the pathological analysis. JL, YZ, and LZ contributed to data collection and manuscript review. XL, BZ, and JC performed the experiments, analyzed the data, prepared figures, and wrote the manuscript. All authors have read and approved the manuscript.
Funding
This study was supported by Shenzhen Science and Technology Plan Project (JCYJ20170307154652899 and ZDSYS201606081515458), Natural Science Foundation of China (81603437 and 81804052), Natural Science Foundation of Guangdong Province (2018A030313305), and Shenzhen Municipal Health Commission (SZLY2018005).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00992/full#supplementary-material
References
- Barrios C., Spector T. D., Menni C. (2016). Blood, urine and faecal metabolite profiles in the study of adult renal disease. Arch. Biochem. Biophys. 589, 81–92. 10.1016/j.abb.2015.10.006 [DOI] [PubMed] [Google Scholar]
- Chen D. Q., Cao G., Chen H., Argyopoulos C. P., Yu H., Su W., et al. (2019). Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat. Commun. 10, 1476. 10.1038/s41467-019-09329-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Cao G., Chen D. Q., Wang M., Vaziri N. D., Zhang Z. H., et al. (2016). Metabolomics insights into activated redox signaling and lipid metabolism dysfunction in chronic kidney disease progression. Redox Biol. 10, 168–178. 10.1016/j.redox.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chen L., Liu D., Chen D. Q., Vaziri N. D., Yu X. Y., et al. (2017). Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. J. Proteome Res. 16, 1566–1578. 10.1021/acs.jproteome.6b00956 [DOI] [PubMed] [Google Scholar]
- Dou F., Miao H., Wang J. W., Chen L., Wang M., Chen H., et al. (2018). An integrated lipidomics and phenotype study reveals protective effect and biochemical mechanism of traditionally used Alisma orientale Juzepzuk in chronic kidney disease. Front. Pharmacol. 9, 53. 10.3389/fphar.2018.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn O. (2002). Metabolomics—the link between genotypes and phenotypes. Plant Mol. Biol. 48, 155–171. 10.1023/A:1013713905833 [DOI] [PubMed] [Google Scholar]
- Foreman K. J., Marquez N., Dolgert A., Fukutaki K., Fullman N., Mcgaughey M., et al. (2018). Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 392, 2052–2090. 10.1016/S0140-6736(18)31694-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grams M. E., Shafi T., Rhee E. P. (2018). Metabolomics research in chronic kidney disease. J. Am. Soc. Nephrol. 29, 1588–1590. 10.1681/ASN.2018030256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansson M., Hokynar K., Somerharju P. (2011). Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res. 50, 240–257. 10.1016/j.plipres.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Hocher B., Adamski J. (2017). Metabolomics for clinical use and research in chronic kidney disease. Nat. Rev. Nephrol. 13, 269–284. 10.1038/nrneph.2017.30 [DOI] [PubMed] [Google Scholar]
- Hsieh C. F., Huang S. L., Chen C. L., Chen W. T., Chang H. C., Yang C. C. (2014). Non-aristolochic acid prescribed Chinese herbal medicines and the risk of mortality in patients with chronic kidney disease: results from a population-based follow-up study. BMJ Open 4, e004033. 10.1136/bmjopen-2013-004033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Abate D., Abate K. H., Abay S. M., Abbafati C., Abbasi N., et al. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 392, 1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. H., Ivanisevic J., Siuzdak G. (2016). Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 17, 451–459. 10.1038/nrm.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalim S., Rhee E. P. (2017). An overview of renal metabolomics. Kidney Int. 91, 61–69. 10.1016/j.kint.2016.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers Heerspink H. J., De Zeeuw D. (2013). Novel drugs and intervention strategies for the treatment of chronic kidney disease. Br. J. Clin. Pharmacol. 76, 536–550. 10.1111/bcp.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Song H. T., Wang X. X., Wan Y. Y., Ding X. S., Liu S. J., et al. (2017). Urinary metabolomics reveals the therapeutic effect of HuangQi injections in cisplatin-induced nephrotoxic rats. Sci. Rep. 7, 3619. 10.1038/s41598-017-03249-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang H. (2005). Chinese herbal medicine in the treatment of chronic kidney disease. Adv. Chronic Kidney Dis. 12, 276–281. 10.1016/j.ackd.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Li Z. Y., He P., Sun H. F., Qin X. M., Du G. H. (2014). (1)H NMR based metabolomic study of the antifatigue effect of Astragali Radix. Mol. Biosyst. 10, 3022–3030. 10.1039/C4MB00370E [DOI] [PubMed] [Google Scholar]
- Lin M. Y., Chiu Y. W., Chang J. S., Lin H. L., Lee C. T., Chiu G. F., et al. (2015). Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney Int. 88, 1365–1373. 10.1038/ki.2015.226 [DOI] [PubMed] [Google Scholar]
- Liu X., Chen J., Liu X., Wang D., Zheng P., Qi A., et al. (2018). Jian-Pi-Yi-Shen Formula ameliorates chronic kidney disease: involvement of mitochondrial quality control network. BMC Complement. Altern. Med. 18, 340. 10.1186/s12906-018-2395-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Huang S., Wang F., Zheng L., Lu J., Chen J., et al. (2019). Huangqi–Danshen decoction ameliorates adenine-induced chronic kidney disease by modulating mitochondrial dynamics. Evid. Based Complement. Alternat. Med. 2019, 9574045. 10.1155/2019/9574045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Liu X., Liao Y., Wang D., Chen J., Li S. (2018). Jian-Pi-Yi-Shen Formula regulates inflammatory cytokines production in 5/6 nephrectomized rats via suppression of NF-kappaB activation. Evid. Based Complement. Alternat. Med. 2018, 7203547. 10.1155/2018/7203547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., Connelly J., Lindon J. C., Holmes E. (2002). Metabonomics: a platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 1, 153–161. 10.1038/nrd728 [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Lindon J. C., Holmes E. (1999). ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29, 1181–1189. 10.1080/004982599238047 [DOI] [PubMed] [Google Scholar]
- Nkuipou-Kenfack E., Duranton F., Gayrard N., Argiles A., Lundin U., Weinberger K. M., et al. (2014). Assessment of metabolomic and proteomic biomarkers in detection and prognosis of progression of renal function in chronic kidney disease. PLoS One 9, e96955. 10.1371/journal.pone.0096955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Polanco J. E., Fried L. F. (2016). Established and emerging strategies in the treatment of chronic kidney disease. Semin. Nephrol. 36, 331–342. 10.1016/j.semnephrol.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Rhee E. P. (2015). Metabolomics and renal disease. Curr. Opin. Nephrol. Hypertens. 24, 371–379. 10.1097/MNH.0000000000000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Chen L., Liu D., Chen H., Tang D. D., Zhao Y. Y. (2017. a). Metabolomics highlights pharmacological bioactivity and biochemical mechanism of traditional Chinese medicine. Chem. Biol. Interact. 273, 133–141. 10.1016/j.cbi.2017.06.011 [DOI] [PubMed] [Google Scholar]
- Wang X., Yu S., Jia Q., Chen L., Zhong J., Pan Y., et al. (2017. b). NiaoDuQing granules relieve chronic kidney disease symptoms by decreasing renal fibrosis and anemia. Oncotarget 8, 55920–55937. 10.18632/oncotarget.18473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. J., He L. Q., Sun W., Lu Y., Wang X. Q., Zhang P. Q., et al. (2012). Optimized project of traditional Chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. J. Ethnopharmacol. 139, 757–764. 10.1016/j.jep.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Webster A. C., Nagler E. V., Morton R. L., Masson P. (2017). Chronic kidney disease. Lancet 389, 1238–1252. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- Ye L., Mao W. (2016). Metabonomic biomarkers for risk factors of chronic kidney disease. Int. Urol. Nephrol. 48, 547–552. 10.1007/s11255-016-1239-6 [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu Y., Liu M., Liu B., Li N., Dong X., et al. (2019). UHPLC-QTOF/MS-based metabolomics investigation for the protective mechanism of Danshen in Alzheimer’s disease cell model induced by Abeta1-42. Metabolomics 15, 13. 10.1007/s11306-019-1473-x [DOI] [PubMed] [Google Scholar]
- Zhang Z. H., Li M. H., Liu D., Chen H., Chen D. Q., Tan N. H., et al. (2018). Rhubarb protect against tubulointerstitial fibrosis by inhibiting TGF-beta/Smad pathway and improving abnormal metabolome in chronic kidney disease. Front. Pharmacol. 9, 1029. 10.3389/fphar.2018.01029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. H., Vaziri N. D., Wei F., Cheng X. L., Bai X., Zhao Y. Y. (2016). An integrated lipidomics and metabolomics reveal nephroprotective effect and biochemical mechanism of Rheum officinale in chronic renal failure. Sci. Rep. 6, 22151. 10.1038/srep22151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. H., Wei F., Vaziri N. D., Cheng X. L., Bai X., Lin R. C., et al. (2015). Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Sci. Rep. 5, 14472. 10.1038/srep14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Qian D., Shang E. X., Jiang S., Guo J., Liu P., et al. (2015. a). Comparative pharmacokinetics of the main compounds of Shanzhuyu extract after oral administration in normal and chronic kidney disease rats. J. Ethnopharmacol. 173, 280–286. 10.1016/j.jep.2015.07.037 [DOI] [PubMed] [Google Scholar]
- Zhao T., Zhang H., Zhang X., Zhao T., Lan H. Y., Liang Q., et al. (2015. b). Metabolomic and lipidomic study of the protective effect of Chaihuang–Yishen formula on rats with diabetic nephropathy. J. Ethnopharmacol. 166, 31–41. 10.1016/j.jep.2015.02.019 [DOI] [PubMed] [Google Scholar]
- Zhao Y. Y. (2013). Metabolomics in chronic kidney disease. Clin. Chim. Acta 422, 59–69. 10.1016/j.cca.2013.03.033 [DOI] [PubMed] [Google Scholar]
- Zhao Y. Y., Chen H., Tian T., Chen D. Q., Bai X., Wei F. (2014). A pharmaco-metabonomic study on chronic kidney disease and therapeutic effect of ergone by UPLC-QTOF/HDMS. PLoS One 9, e115467. 10.1371/journal.pone.0115467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. Y., Vaziri N. D., Lin R. C. (2015. c). Lipidomics: new insight into kidney disease. Adv. Clin. Chem. 68, 153–175. 10.1016/bs.acc.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Cai G. Y., He L. Q., Lin H. L., Cheng X. H., Wang N. S., et al. (2017). Efficacy and safety of Niaoduqing particles for delaying moderate-to-severe renal dysfunction: a randomized, double-blind, placebo-controlled, multicenter clinical study. Chin. Med. J. (Engl.) 130, 2402–2409. 10.4103/0366-6999.216407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Deng Y., Chen Y., Chuang P. Y., Cijiang He J. (2013). Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int. 84, 1108–1118. 10.1038/ki.2013.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Menon M. C., Deng Y., Chen Y., He J. C. (2015). Recent advances in traditional Chinese medicine for kidney disease. Am. J. Kidney Dis. 66, 513–522 10.1053/j.ajkd.2015.04.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.