Abstract
Large variations in cancer survival have been recorded between populations, e.g., between countries or between regions in a country. To understand the determinants of cancer survival differentials between populations, researchers have often applied regression analysis. We here propose the use of a non-parametric decomposition method to quantify the exact contribution of specific components to the absolute difference in cancer survival between two populations. Survival differences are here decomposed into the contributions of differences in stage at diagnosis, population age structure, and stage-and-age-specific survival. We demonstrate the method with the example of differences in one-year and five-year breast cancer survival between Denmark’s five regions. Differences in stage at diagnosis explained 45% and 27%, respectively, of the one- and five-year survival differences between Zealand and Central Denmark for patients diagnosed between 2008 and 2010. We find that the introduced decomposition method provides a powerful complementary analysis and has several advantages compared with regression models: No structural or distributional assumptions are required; aggregated data can be used; and the use of absolute differences allows quantification of the survival that could be gained by improving, for example, stage at diagnosis relative to a reference population, thus feeding directly into health policy evaluation.
Keywords: breast cancer, decomposition, stage at diagnosis, Denmark, regions, survival
1. Introduction
Large variations in cancer survival have been reported between populations worldwide, including between European and high-income countries [1,2]. For example, cancer survival is generally lower in Denmark than in a comparable country like Sweden [1,3,4]. Cancer survival differences also occur at sub-national levels, such as lower survival for males than for females in the same country [5,6,7], or differences between regions [8]. These variations in cancer survival suggest that the gap could be lessened if low-survival populations could approximate survival from the high-survival populations by, among other factors, improving national healthcare systems [9] or reducing socioeconomic disparities [10].
Potential explanations of the differences between populations include: more adverse stage at diagnosis [11], greater burden of certain risk factors (e.g., smoking) [12], or biological differences [13]. Studies showed that stage at diagnosis can be a key explanation for cancer differences between countries [11,14,15].
Cancer survival and mortality differences between populations can be studied with descriptive statistics and comparison of survival functions [1,14,15]. These studies show the difference in survival between populations but provide limited information on why these differences occur. For instance, a more adverse stage distribution can be observed in a low-survival population relative to a high-survival population, but this provides no quantification. Regression analyses, such as the Cox proportional hazard model or other forms of generalized linear models, have been used to study the relation between cancer survival or mortality and a set of independent variables (e.g., stage at diagnosis) [11,16,17]. However, regression model assumptions do not always hold (e.g., proportional hazards). Additionally, these models often estimate relative differences only (e.g., hazard ratio, relative risk, or odds ratio), while absolute numbers are sometimes preferable. Absolute differences can be useful for quantifying the differences explained by a specific variable, or the potential gains in survival that could be achieved by modifying that variable.
Non-parametric decomposition methods are valued tools in demography, yet less common in public health [18], and can quantify the exact contribution of specific components, such as ages and causes of death, to a (usually) absolute difference between populations in a given measure [19,20,21,22]. Many decomposition methods require no assumptions (structural or distributional) about the data.
We introduce the Kitagawa decomposition method [22], extend its application to cancer research, and present a novel extension of the method to account for the confounding effect of background population (incl. background survival). We decompose the difference between cancer survival probabilities in two populations by their underlying differences in (1) age composition at diagnosis, (2) stage composition at diagnosis, and (3) age–stage-specific survival. Each of these contributions can relate to different issues in health care. The method is illustrated by decomposing differences in female breast cancer survival between Danish regions. Denmark has five administrative regions: The Capital, Zealand, Southern Denmark, Central Denmark, and Northern Denmark. In 2007, Denmark established a new political and administrative scheme in which the healthcare system is run at three levels: the state, the regions, and the municipalities. The national level regulates and supervises health and elderly care. The regions are responsible for hospitals, general practitioners (GPs), and psychiatric care units. The municipalities mainly oversee primary healthcare services, such as health promotion or extramural rehabilitation [23]. Given the important role of GPs, hospitals and their interactions in prompt diagnosis, and the important role of hospitals in treatment, all falling under the regions’ responsibility, differences in cancer survival between Danish regions are an instructive test case for the method.
2. Methods
The Kitagawa method quantifies the amount of absolute difference in a crude rate that is due to differences in compositions versus differences in component-specific rates between populations, using multiple standardizations. Extensions and similar techniques have been developed [24,25,26,27]. The Kitagawa decomposition can also be applied to probabilities. Indeed, the Kitagawa decomposition can be applied whenever one variable can be expressed as the product of two others, one of which is, generally, a composition. For example, we can express a crude survival probability (S) as the product of some x-composition (e.g., age composition) of a population and the x-specific survival :
| (1) |
To quantify the absolute difference in a crude survival probability between two populations (Y and Z) due to differences in the composition of variable () and differences in x-specific survival probabilities , the Kitagawa formula reads as:
| (2) |
where the bar over the indicator (probability s or composition ) represents the x-specific averages across populations Y and Z (e.g., ) and is the difference between the two populations (e.g., ). If variable is age, then Equation (2) quantifies:
The survival effect: The difference in the crude probability between two populations that is due to the difference in their age-specific probabilities , i.e., the sum of age-specific survival probabilities differences after multiplying for each population by the average age-composition of these two populations (direct standardization).
The age effect (X-effect): The difference in the crude probability between two populations that is due to the difference in their age-specific composition , i.e., the sum of the age composition differences after multiplying for each population by the average age-specific probabilities of these two populations (indirect standardization).
More than one composition effect can be of interest to understand differences in a rate or probability between two populations. When using any two compositions ( and i), the crude survival can be expressed as:
| (3) |
where is the distribution for each and is the composition for each . For example, if is age and is stage, then is the stage-composition at age x. Kitagawa (1955) [22] provided a way to decompose crude rate differences into two or more composition effects (X and I) and one survival effect:
| (4) |
We used the Kitagawa method to decompose differences in crude survival probabilities () between two populations, by an age-at-diagnosis composition effect (X-effect), stage-at-diagnosis composition effect (I-effect), age–stage-specific survival effect (survival effect), and an interaction term between the age and stage compositions, such that:
| (5) |
Confidence intervals (CI) for each contribution and for the total difference were calculated using bootstrapping methods. The original sample was randomly resampled with replacement 1000 times, with the sample size kept constant. The decomposition method was then reapplied to each sample and 95% confidence intervals were calculated based on 2.5% and 97.5% percentiles, as similarly suggested by Wang et al. (2000) [18].
2.1. Sub-Decompositions to Assess the Effect of the Background Population
The background population can inform about the cancer patient’s chance of survival. For example, a low background survival indicates high risk of death irrespective of cancer and can inform on higher competing risks from other causes. The background age composition is also informative, as an older background population is more likely to have older cancer patients. Therefore, we introduce an extension of the Kitagawa decomposition to quantify the contribution of (1) the background survival and (2) the background age composition to the difference in crude cancer survival between two populations. These contributions can approximate the quantity of the survival effect and age effect that is characteristic of the background population in addition to the differences in survival and age-composition of the cancer patients.
2.1.1. Sub-Decomposition of the Survival Effect
Relative survival (), i.e., the survival of cancer patients after adjustment for other causes of death, is often a favored measure over crude survival in cancer research. Relative survival is the ratio of the survival observed among cancer patients to the survival observed in a background population () with similar demographic characteristics (year, sex, age) (), thus controlling for the effect of overall survival.
The survival effect (sE) can be divided into a relative survival effect () and a background survival effect (), such that . Given the formula of , the age–stage-specific survival can be expressed as the product of the relative survival and background survival (). Because can be expressed as a product, the Kitagawa decomposition can be applied to decompose , such that . By replacing in Equation (4) by the previous formulas, we obtain:
| (6) |
| (7) |
2.1.2. Sub-Decomposition of the Age Effect
As with survival, the age-at-diagnosis composition is influenced by the age structure of the background population and by the age structure of the cancer patients. Decomposing differences in a composition is however more complex (see the detailed decomposition in Appendix A). Using compositional data analysis (CoDA) techniques [28,29], we can find the age composition as if the background age composition and the relative age composition were equal between populations Y and Z. The procedure presented in Appendix A is not an exact decomposition but approximates the difference in age composition closely: , where and are the effect of the background age composition and the effect of the relative age composition, respectively.
Confidence intervals for the background effects (age and survival) using the above described method cannot be estimated, as these effects are based on aggregated data only (see Data section below). These contributions should, thus, be seen as an indication of where the differences between populations emerge: From the background structure and survival, or from characteristics of the cancer patients. However, confidence intervals can be estimated for the relative effects (age and survival)
2.2. Decomposition of standardized survival
Standardized survival can also be decomposed. Standardized probabilities were obtained by separating survival from the confounding effects of the age compositions and background survival. The average background survival and age composition of the two regions compared was used as “reference population” for the standardization (see Appendix B for details).
3. Data
3.1. Database
We used data from the Danish Cancer Registry (DCR), in which each tumor is recorded in detail, including histological examination and patient survival. As the Danish five-region classification started in 2007, we used data from 2008 through 2015, thus reflecting the contemporary situation in Denmark. Additionally, the DCR has been using a modernized system since reporting became electronic in January 2008, ensuring more consistent reporting. Over the selected period, the DCR uses the TNM classification to stage cancer and ICD-10 classification of causes of deaths, limiting discontinuities in time series and easing comparisons [30]. Hence, we selected females diagnosed with breast cancer between 2008 and 2010 and calculated the one-year and five-year survival probability. The one-year survival of patient diagnosed between 2011 and 2014 was also studied. Breast cancer was selected because this site had more complete stage-at-diagnosis data than other cancer sites, while being a common cancer.
Within the DCR, 89% of the tumors were morphologically verified, which represents a good validity of the registry [30]. However, information on staging is sometimes missing. The level of completeness depends on the cancer site [31]. For breast cancer diagnosed in Denmark between 2008 and 2010, 5% of the tumors had missing information on tumor size (T), 9% on lymph nodes (N) and 10% on distant metastasis (M).
To obtain information on the background mortality by age, sex and region, we used data from Statistics Denmark [32,33], only available in aggregated form.
3.2. Exclusions
The study was performed on malignant neoplasms stated to be primary only; tumors stated as benign, in-situ, of uncertain behavior or secondary were excluded. Additionally, cancer registered from the death certificate or during autopsy only was excluded as the date of diagnosis is the same as the time of death, thus providing no information on survival. Patients living in Greenland or with unknown (or changed) sex or vital status were also excluded, as were cases where the date of censoring occurred before the date of the diagnosis (e.g., when the patient is reported as being departed from Denmark). If individuals were diagnosed with more than one breast tumor (duplicates), a tumor record was kept for the analysis if the patient had no diagnosis of breast cancer five years prior to the diagnosis of interest (between 2008 and 2010 or between 2011 and 2014). In total, 34,723 tumors were kept for the analysis (Table 1).
Table 1.
Number of diagnoses of primary malignant neoplasms from breast cancer for women diagnosed between 2008 and 2010 and between 2011 and 2014 in Denmark.
| Period | Eligible | Excluded | Included | ||
|---|---|---|---|---|---|
| Duplicates | Death Certificate Only | Other | |||
| 2008–2010 | 16,019 | 90 (0.6%) | 83 (0.4%) | 34 (0.2%) | 15,812 (98.7%) |
| 2011–2014 | 19,176 | 85 (0.4%) | 103 (0.4%) | 77 (0.4%) | 18,911 (98.6%) |
3.3. Staging and Missing Data
We used the TNM data and converted them to prognostic groups using the guide from the American Joint Committee on Cancer (AJCC) 7th edition. In 11% of cases, we were unable to attribute a stage due to at least one missing piece of information on tumor size (T), lymph nodes (N), and/or distant metastasis (M) for women diagnosed with cancer between 2008 and 2010. To avoid bias and information loss, we used multiple imputation to handle missing data. We applied a multiple imputation method using chained equations with the mice R package [34,35] to impute a value to the T, N or M missing data, using 15 imputed datasets and 10 iterations. The procedure is detailed in Appendix C.
The decomposition method presented in Section 2 was calculated for each imputed dataset, including the CI procedure. The CIs shown in the following sections were based on 1000 resamples of each of the 15 imputed datasets, thus accounting for the uncertainty of the multiple imputation procedure.
4. Results
Central Denmark had the highest survival among the regions for both one-year and five-year survival (95.90% and 82.75% respectively, Table 2) and, thus, served as benchmark for the other regions.
Table 2.
One-year and five-year crude survival probabilities (as percentages) for the five Danish regions, for women diagnosed with breast cancer between 2008 and 2010, ordered by increasing five-year survival.
| Region | One-Year Survival (%) | Five-Year Survival (%) |
|---|---|---|
| Zealand | 94.28 | 78.61 |
| Northern Denmark | 94.24 | 78.86 |
| Capital region | 95.37 | 80.45 |
| Southern Denmark | 95.85 | 82.34 |
| Central Denmark | 95.90 | 82.75 |
4.1. Decomposition of Crude Survival Probabilities (Zealand Versus Central Denmark)
The absolute difference in breast cancer survival between Zealand and Central Denmark was 1.62 percent points for one-year survival and 4.14 for five-year survival, for the period 2008–2010. If Zealand had had the same survival as Central Denmark, the number of breast cancer deaths one year after diagnosis would have been 28.3% lower (corresponding to 40 deaths for the period); five years after diagnosis this number would have been 19.4% (corresponding to 103 deaths for the period).
The survival effect accounted for 31.4% and 43.9% of the difference for the one-year and five-year survival, respectively (Table 3). The survival effect was, however, not significant for the one-year survival. Around 34% of the survival effect can be explained by the background survival components. Central Denmark had better survival from all causes of death combined than Zealand, meaning that cancer patients also benefited from a reduced risk from other causes of death. Female life expectancy for the period 2008–2010 was 81.8 years in Central Denmark and 80.5 in Zealand.
Table 3.
Decomposition of the difference in breast cancer one-year and five-year crude survival. between Zealand and Central Denmark, 2008–2010.
| Components | Contributions | CI (95%) | % | Sub-Components | Contributions | % |
|---|---|---|---|---|---|---|
| One-year survival | ||||||
| Survival | 0.51 | (−0.44, 1.63) | 31.4 | Relative survival | 0.33 | 65.3 |
| Background survival | 0.18 | 34.7 | ||||
| Age | 0.19 | (−0.26, 0.55) | 12.0 | Relative age | 0.25 | 128.9 |
| Background age | −0.06 | −28.9 | ||||
| Stage | 0.73 | (0.14, 1.19) | 44.9 | - | - | - |
| Age–stage interaction | 0.19 | (−0.08, 0.52) | 11.7 | - | - | - |
| Total | 1.62 | (0.54, 2.73) | 100.0 | |||
| Five-year survival | ||||||
| Survival | 1.82 | (0.09, 3.62) | 43.9 | Relative survival | 1.20 | 65.8 |
| Background survival | 0.62 | 34.2 | ||||
| Age | 1.13 | (0.31, 1.92) | 27.4 | Relative age | 1.28 | 112.6 |
| Background age | −0.14 | −12.6 | ||||
| Stage | 1.10 | (0.25, 1.94) | 26.5 | - | - | - |
| Age–stage interaction | 0.09 | (−0.32, 0.50) | 2.2 | - | - | - |
| Total | 4.14 | (1.93, 6.29) | 100.0 | |||
The age effect widened the difference in survival between Zealand and Central Denmark, with a significant contribution for the five-year survival. This difference in the age-at-diagnosis composition of the cancer patients was mainly due the relative age component. The background age component was negative, meaning that Central Denmark had an older background age composition than Zealand. Negative contributions are interpreted as an advantage for Zealand in Table 3.
Zealand also had a more adverse stage-at-diagnosis distribution than Central Denmark, with 44.9% (one-year) and 26.5% (five-year) of the difference in breast cancer survival between the two regions being attributable to the stage effect (both significant, decompositions for other regions in Appendix D).
4.2. Decomposition of Standardized Survival Probabilities
For survival standardized by background survival and age, the differences in the one-year and five-year survival between the Zealand and Central Denmark was 1.24 and 2.39 percent points, respectively, for the period 2008–2010. Table 4 shows the decomposition of the standardized survival by stage-specific survival and stage composition, using the Kitagawa method with one composition effect (Equation (2)). Compared with the contributions of relative survival and stage from the decomposition presented in Table 3, the stage and relative survival contributions remained equal at a two-decimal rounding when decomposing the crude and standardized survival probabilities. However, after standardizing by age, we cannot separate out the interaction effects, which is contained within the stage effect. Standardizing does not affect the absolute contributions of the components, when using the average between populations as reference (Appendix B). After removing the age and background effects, the stage effect is the dominant contributor to the difference in the one-year survival between the two regions, explaining 73.5% of the difference.
Table 4.
Decomposition of the difference in breast cancer one-year and five-year survival standardized (by age and background survival) between Zealand than in Central Denmark, 2008–2010.
| Standardized Survival | Crude Survival (Table 3) | ||||
|---|---|---|---|---|---|
| Components | Contributions | CI (95%) | % | Components | Contributions |
| One-year survival | |||||
| Survival | 0.33 | (0.12, 0.58) | 26.5 | Relative Survival | 0.33 |
| Stage | 0.92 | (0.43, 1.36) | 73.5 | Stage + Interaction | 0.92 |
| Total | 1.24 | (0.97, 1.43) | 100.0 | Sum | 1.24 |
| Five-year survival | |||||
| Survival | 1.20 | (0.84–1.45) | 50.1 | Relative Survival | 1.20 |
| Stage | 1.19 | (0.32–2.05) | 49.9 | Stage + Interaction | 1.19 |
| Total | 2.39 | (2.02–2.62) | 100.0 | Sum | 2.39 |
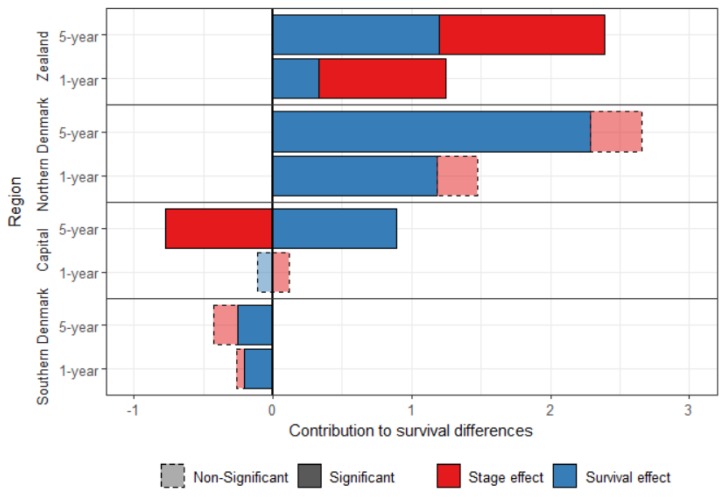
Figure 1 shows the standardized survival decomposition between Central Denmark and the four other regions. After standardization, Southern Denmark has a better survival than Central Denmark, which is mainly attributed to the survival effect. Central Denmark had a better stage-specific survival than the other three regions. The survival effect contributed to 79.8% and 86.0% of the difference in breast cancer one-year and five-year survival, respectively, between Northern Denmark and Central Denmark.
Figure 1.
Decomposition of the one-year and five-year standardized survival difference between Central Denmark and the other Danish regions, for women diagnosed with breast cancer between 2008 and 2010.
The stage-effect contributed to the disadvantage of Zealand, but played in favor of the Capital region for the five-year survival. There was no significant stage effect explaining the difference between Central Denmark and the Southern and the Northern regions.
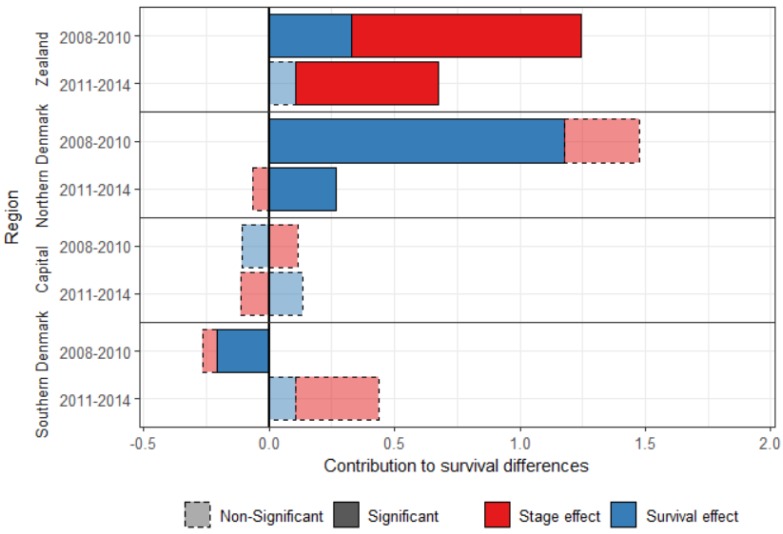
4.3. Diminishing Differences over Time
The five-year survival cannot be calculated for more recent years, but the one-year survival can be calculated for patients diagnosed with breast cancer between 2011 and 2014. Differences between Central Denmark and the other regions have decreased between the periods 2008–2010 and 2011–2014 (Figure 2), showing evidence of progress towards equality. The survival effect was significant for three regions in 2008–2010, but only for Northern Denmark in 2011–2014, for which it decreased four-fold over the study period.
Figure 2.
Decomposition of the one-year standardized survival difference between Central Denmark and the other Danish regions, for women diagnosed with breast cancer between 2008–2010 and between 2011–2014.
Central Denmark had an advantage over Southern Denmark in the most recent period, but it is small (0.44 percent points), and the contributions are not significant.
The stage effect was still significant for Zealand in the most recent period, but smaller, and explains 84.6% of the difference in breast cancer survival with Central Denmark in 2011–2014.
5. Comparison with the Cox Proportional Hazard Model
The Kitagawa decomposition differs from the (commonly-used) Cox proportional hazard (CPH) model, and other types of regression models, in important ways (Table 5).
Table 5.
Summary of the Cox proportional hazard and Kitagawa decomposition models characteristics.
| Cox Proportional Hazard | Kitagawa Decomposition | |
|---|---|---|
| What is measured? | Determinants of survival | Determinants of survival differences |
| Model outputs | Coefficients | Contributions |
| Difference measured | Relative | Absolute |
| Key assumption | Proportional hazards | None |
| Data | Individuals | Individuals and aggregates |
First, the CPH model assesses which variables influence survival. For example, an increase in the age and stage at diagnosis increases the hazard (Table 6) and decreases survival. In contrast, the decomposition method quantifies contributions of specific variables to the difference in survival between two populations.
Table 6.
Results from a Cox proportional hazard model, comparing hazards from Central Denmark and Zealand, 2008–2010.
| Variables | Coefficient | Exp (Coefficient) | CI (95%) |
|---|---|---|---|
| Age at diagnosis | 0.11 | 1.12 | 1.10, 1.14 |
| Stage at diagnosis | 2.24 | 9.38 | 6.40, 13.75 |
| Region Zealand | 1.10 | 2.99 | 1.35, 6.65 |
| Age: stage | −0.02 | 0.98 | 0.98, 0.99 |
| Age: region | −0.01 | 0.99 | 0.98, 1.00 |
| Stage: region | −0.18 | 0.83 | 0.74, 0.94 |
Second, the CPH, and other forms of regression model, estimate coefficients. The coefficients act multiplicatively on the variables’ value and are used to predict survival for an individual with specific characteristics. The decomposition produces variable-specific contributions to the difference, summing up to the total survival difference. The contributions are generally an aggregated value for each variable, without distinction for the value of the variable (e.g., stages 1 to 4).
Third, the CPH estimates relative differences between values of a variable. For example, the ratio of the hazard functions of Zealand/Central Denmark is higher than 1 (Table 6), meaning that people diagnosed with breast cancer in Zealand had a higher hazard than people with similar characteristics in Central Denmark. This approach does not inform, however, on why this difference between region occured. In contrast, the decomposition approach uses absolute differences between two populations. The use of absolute rather than relative differences allows one to quantify the survival that could be gained by improving, for example, stage at diagnosis to the level of a reference region or population: If Zealand had the same stage at diagnosis distribution as Central Denmark in 2008-2010, the five-year crude survival probability (Table 3) would have been 79.80% rather than 78.61%. It also allows one to quantify directly the number of deaths that could be avoided if one of the components were to change. For example, giving Zealand the stage-at-diagnosis distribution of Central Denmark reduces the number of deaths due to breast cancer one year after the diagnosis by 16.0% for patients diagnosed between 2008 and 2010 (23 deaths) and by 5.6% five years after the diagnosis (30 deaths). Most articles tend to report relative measures only, but existing recommendations suggest reporting both relative and absolute measures [36].
Fourth, the CPH model makes structural assumptions, primarily the hazards are proportional. With the decomposition model, no distributional or structural assumptions are required: The compositions and component-specific rates or probabilities observed in two populations are directly compared and their effects on survival are quantified.
Finally, the CPH model requires individual data, while the decomposition method can also be used on aggregated data. However, if aggregated data are used, new ways to calculate confidence intervals should be found other than that suggested in the paper.
The CPH and decomposition models serve different purposes and the use of one rather than the other should be determined by the aim of the study. If the aim is to understand the determinants of cancer survival, the CPH model, or other regression models, should be used. However, if the aim is to understand differences in survival between populations or quantify potential gains in survival by modifying one variable, the introduced decomposition method can be preferable. Both methods could also be used to complement each other.
6. Discussion
We presented a non-parametric decomposition method that uncovers the causes of differences in cancer survival probabilities between populations. In the test case of the Danish regions, we found that later stage at diagnosis explained a large share of the difference in breast cancer survival between Zealand and Central Denmark, which tentatively suggests that Zealand could improve cancer survival by diagnosing at an earlier stage, in addition to recent and ongoing improvements.
By the end of 2007, all Danish regions were required to start a breast cancer screening program, the rollout being completed in 2009. However, some differences between regions remained. Zealand recorded more fluctuations in the breast cancer detection rates over time (in contrast to the other regions) with a particularly low detection rate of 0.53% compared with the national average of 0.61% in the fourth screening round (2014–2015) [37]. This could explain, in part, the later stages at diagnosis in Zealand and might be caused by a shortage of experienced radiologist in the region [37].
Lower socioeconomic status has been associated with later cancer stage at diagnosis [38]. Zealand has the highest proportion of residents with low education level among the Danish regions [39], which could also explain its more adverse stage-at-diagnosis distribution. However, Ibfelt et al. (2018) [17] found that even after controlling for differences in socioeconomic status (education and income), the odds ratio of being diagnosed at a later stage remained higher in Zealand than in the Capital region for malignant melanoma. This led the authors to suspect differences in the referral process to specialized care between regions. Other possible explanations include fewer specialized doctors in the outer regions, such as Zealand and Northern Denmark, and other unmeasured social, cultural and behavioral factors [17]. Patient awareness of breast cancer symptoms is high in Denmark, especially in highly educated respondents [40], which suggests that patient delay may be a factor in regions where education is generally lower.
Given the importance of family doctors in the Danish healthcare system, without whose referral one cannot consult a specialist, regional differences in organization, attitudes and number of GPs are likely to lead to some differences in stage distribution. Looking into regional differences in England, Maclean et al. (2015) [41] found that for female breast cancer, being in a practice with short waiting times until referral or detection was associated with a lower proportion of patients diagnosed in stage 3 or 4 rather than stage 1 or 2. Membership of a practice where people thought it less easy to book an appointment was associated with a higher percentage diagnosed later. It would be helpful to know how this translates to the case of Zealand GPs. Presumably, such an effect will depend on the quality, attitudes and organization of family doctors, which may lead to regional differences.
As for stage-specific survival, there are known cases where, while treatment was in principle available, the application of active treatment was different between regions in one country, such as lung cancer in England [42]. However, the Danish Breast Cancer Group (DBCG) established mandatory treatment guidelines, especially regarding the surgical treatment and the (neo)adjuvant treatment [43] (DBCG guidelines, www.DBCG.dk). In principle, patients should get the same treatment across all Denmark’s hospitals. Furthermore, DBCG regularly publish a quality indicator report to identify any variation in the treatment of early breast cancer, showing only small differences [43] (DBCG quality indicator report, www.DBCG.dk). Differences in treatment are, thus, unlikely to cause the differences observed in stage-specific survival between Danish regions (survival effect).
It has also been found that rural dwellers have poorer cancer survival [44]. It is unclear why this would apply only to Northern Denmark, although the vicinity of the capital may make Zealand effectively a little less “rural” than Northern Denmark.
In 2007, many changes occurred regarding breast cancer diagnosis and treatment in Denmark, including the screening program, updates of the national guidelines, and the new regional scheme. These changes might be the cause for the decreasing differences across regions over time, but we cannot assess if or which of these changes are responsible for the convergence, or if it can be the result of previous programs.
More information could be added in the decomposition to further explain the regional differences. If additional data were available, possible extensions could include the compositional difference of socioeconomic status, smoking habits, or medical treatments. The contributions of unspecified components are grouped in the survival-effect, or in the composition effects if an unspecified composition correlates with the age or stage compositions. For example, in Table 3, if the stage at diagnosis was not included in the analysis, the survival effect would be approximated by the sum of the survival and stage effects (e.g., 1.24 for the one-year survival) and the age effect would be the sum of the age and interaction effects (e.g., 0.38 for the one-year survival). This is similar to unmeasured confounders in regression analysis.
7. Conclusions
This paper illustrates the utility of adopting and extending the Kitagawa decomposition to cancer research. The method allows us to understand differences in survival between populations by quantifying the exact contributions of specific variables to this difference. Such quantification can help policy makers and health care professionals improve overall cancer survival, tuning their actions to the dominant contributions. The method presents some advantages compared with other models commonly used in survival analysis, such as the Cox proportional hazard model, when it comes to understanding differences between populations. We argue that decomposition methods are valuable tools and provide a powerful complementary analysis for cancer and public health research.
Acknowledgments
The authors wish to thank the reviewers, Kaare Christensen and James W. Vaupel, for useful comments on earlier versions of this work.
Appendix A
Decomposing the Age Effect
The age composition of cancer patients is likely to be influenced by the age composition of the patient’s source population. For example, Central Denmark has a younger population than Northern Denmark. It is useful in certain cases to assess whether the difference in the age composition at diagnosis of cancer patients has its origin in the composition of the background population, or whether something disease specific is at play. This question can be answered by decomposing differences between two compositions. This, however, presents additional problems: The differences between two compositions always sum to 0. Thus, by decomposing this difference, one component will always be positive and the other component negative, when using the standard Kitagawa decomposition.
There is a rich literature on how to treat compositional data [28,29], labeled compositional data analysis (CoDA). In CoDA, a perturbation (noted or ) amounts to multiplying (or dividing) one composition by another and then “closing” the result, i.e., scaling the newly obtained compositions so that their sum is the same as for the original compositions, usually 1 or 100. With this procedure, it is possible to perturb one composition by another to obtain a third composition:
| (A1) |
where is the relative age composition and is the background age composition. By using the perturbation procedure, it is possible to standardize an age composition by its background age composition and relative age composition:
| (A2) |
By replacing in Equation (4) by and , we obtain Equations (A3) and (A4), which approximate the effect of the background age composition and the relative age composition to the difference in survival between two populations:
| (A3) |
| (A4) |
The notations and refer to the effect of the background age composition and the effect of the relative age composition, respectively. As mentioned in the main text, this procedure does not produce an exact decomposition, but an approximation : We tend to lose a limited amount of information by passing from an Aitchison space (compositional data) to Euclidean space. Given that the decomposition is exact within the Aitchison space, we think this procedure is justified.
Appendix B
Standardization and Decomposition
When standardizing the survival probability by age and background survival, the standardization should be done at the most disaggregated level:
| (A5) |
The standardization of population Y by background survival and age composition is written as:
| (A6) |
where is the standard background survival and is the standard age composition, within each stage i.
Appendix C
Multiple Imputations
We imputed a value directly for the T, N and M variables rather than on the grouped stages (1 to 4), as in some cases, information was available for one or two of these variables, providing important information on staging. For instance, if only the information on lymph nodes is available for a patient, with a value N2 (with N2 being defined as: “Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted; or in clinically-detected ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases” [45], p. 360.), then the stage can only be 3 or 4. The imputed value on M will determine if the stage is either 3 or 4.
We used an ordinal logistic regression to impute values to the T, N and M variables, using the vital status one year after diagnosis, age at diagnosis, region of residence and the available T, N and/or M variables as independent variables, with an interaction term between age and vital status. We used 15 imputed datasets and 10 iterations.
After multiple imputations, the parameters of interests are generally calculated on each imputed dataset and the average of the estimates is used [34]. However, given the equivalence necessary for the decomposition, as shown in Equations (1) and (3) in the main text, using the average age and/or stage composition and the average age–stage survival across the 15 datasets does not guarantee that these equivalences are respected. Thus, we used the imputed dataset that minimized the root mean square error with the mean.
Appendix D
Results for All Regions
Table A1.
One-year crude survival decomposition between Central Denmark and three other Danish regions, females diagnosed with breast cancer between 2008 and 2010.
| Components | Contributions | CI | % | Sub-Components | Contributions | % |
|---|---|---|---|---|---|---|
| Northern Denmark | ||||||
| Survival | 1.28 | (0.12, 2.51) | 77.0 | Relative survival | 1.17 | 91.9 |
| Background survival | 0.11 | 8.1 | ||||
| Age | 0.08 | (−0.43, 0.54) | 5.0 | Relative age | −0.05 | −57.8 |
| Background age | 0.14 | 157.8 | ||||
| Stage | 0.05 | (−0.66, 0.65) | 2.9 | - | - | - |
| Age–stage interaction | 0.25 | (−0.08, 0.67) | 15.1 | - | - | - |
| Total | 1.66 | (0.32, 2.81) | 100.0 | |||
| Southern Denmark | ||||||
| Survival | −0.16 | (−1.10, 0.68) | −363.6 | Relative survival | −0.20 | 126.0 |
| Background survival | 0.04 | −26.0 | ||||
| Age | 0.27 | (−0.03, 0.65) | 596.5 | Relative age | 0.18 | 67.5 |
| Background age | 0.09 | 32.5 | ||||
| Stage | −0.08 | (−0.45, 0.44) | −150.0 | - | - | - |
| Age–stage interaction | 0.01 | (−0.28, 0.25) | 17.1 | - | - | - |
| Total | 0.04 | (−0.90, 1.02) | 100.0 | |||
| Capital | ||||||
| Survival | 0.02 | (−0.85, 0.82) | 4.5 | Relative survival | −0.12 | −482.9 |
| Background survival | 0.14 | 582.9 | ||||
| Age | 0.38 | (0.10, 0.73) | 73.1 | Relative age | 0.25 | 65.3 |
| Background age | 0.13 | 34.7 | ||||
| Stage | −0.08 | (−0.56, 0.36) | −17.9 | - | - | - |
| Age–stage interaction | 0.21 | (−0.06, 0.43) | 40.3 | - | - | - |
| Total | 0.53 | (−0.33, 1.36) | 100.0 | |||
Table A2.
Five-year crude survival decomposition between Central Denmark and three other Danish regions, females diagnosed with breast cancer between 2008 and 2010.
| Components. | Contributions | CI | % | Sub-Components | Contributions | % |
|---|---|---|---|---|---|---|
| Northern Denmark | ||||||
| Survival | 2.62 | (0.87, 4.72) | 67.4 | Relative survival | 2.29 | 87.2 |
| Background survival | 0.34 | 12.8 | ||||
| Age | 0.90 | (−0.22, 1.69) | 23.1 | Relative age | 0.37 | 41.1 |
| Background age | 0.53 | 58.9 | ||||
| Stage | 0.32 | (−0.77, 1.23) | 8.2 | - | - | - |
| Age–stage interaction | 0.05 | (−0.31, 0.66) | 1.3 | - | - | - |
| Total | 3.89 | (1.62, 6.36) | 100.0 | |||
| Southern Denmark | ||||||
| Survival | −0.10 | (−1.56, 1.52) | −24.8 | Relative survival | −0.25 | 247.2 |
| Background survival | 0.15 | −147.2 | ||||
| Age | 0.69 | (−0.04, 1.31) | 166.9 | Relative age | 0.34 | 49.9 |
| Background age | 0.35 | 50.1 | ||||
| Stage | −0.40 | (−1.08, 0.30) | −96.9 | - | - | - |
| Age–stage interaction | 0.22 | (−0.08, 0.60) | 54.9 | - | - | - |
| Total | 0.41 | (−1.29, 2.31) | 100.0 | |||
| Capital | ||||||
| Survival | 1.44 | (−0.02, 2.91) | 62.5 | Relative survival | 0.99 | 64.1 |
| Background survival | 0.52 | 35.9 | ||||
| Age | 1.64 | (0.95, 2.28) | 71.3 | Relative age | 1.15 | 70.0 |
| Background age | 0.49 | 30.0 | ||||
| Stage | −1.09 | (−1.87, −0.41) | −47.2 | - | - | - |
| Age–stage interaction | 0.31 | (0.04, 0.65) | 13.4 | - | - | - |
| Total | 2.30 | (0.60, 3.97) | 100.0 | |||
Author Contributions
The six authors have contributed in the following way to the manuscript: Conception and design of the study: M.-P.B.-B., M.J.W., R.L.-J. and J.O. Access and interpretation of data: M.-P.B.-B., M.J.W., R.L.-J., N.V.H. Analysis and production of results: M.-P.B.-B. and M.J.W. Interpretation of results: M.-P.B.-B., M.J.W., J.O. and H.M.N. Drafting or revision of the manuscript: M.-P.B.-B., M.J.W., J.O., N.V.H., H.M.N. and R.L.-J. Approval of the final version: M.-P.B.-B., M.J.W., J.O., N.V.H., H.M.N. and R.L.-J.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
No conflict of interests have been declared by the authors.
References
- 1.Coleman M.P., Forman D., Bryant H., Butler J., Rachet B., Maringe C., Nur U., Tracey E., Coory M., Hatcher J., et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Angelis R., Sant M., Coleman M.P., Francisci S., Baili P., Pierannunzio D., Trama A., Visser O., Brenner H., Ardanaz E., et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 3.Engeland A., Haldorsen T., Dickman P.W., Hakulinen T., Möller T.R., Storm H.H., Tulinius H., Engeland T.H.A. Relative Survival of Cancer Patients: A Comparison between Denmark and the other Nordic Countries. Acta Oncol. 1998;37:49–59. doi: 10.1080/028418698423177. [DOI] [PubMed] [Google Scholar]
- 4.Tryggvadottir L., Gislum M., Bray F., Klint Å., Hakulinen T., Storm H.H., Engholm G. Trends in the survival of patients diagnosed with breast cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49:624–631. doi: 10.3109/02841860903575323. [DOI] [PubMed] [Google Scholar]
- 5.Cook M.B., McGlynn K.A., Devesa S.S., Freedman N.D., Anderson W.F. Sex disparities in cancer mortality and survival. Cancer Epidemiol. Biomark. Prev. 2011;20:1629–1637. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberaigner W., Siebert U. Do women with cancer have better survival as compared to men after adjusting for staging distribution? Eur. J. Public Health. 2010;21:387–391. doi: 10.1093/eurpub/ckq099. [DOI] [PubMed] [Google Scholar]
- 7.Micheli A., Ciampichini R., Oberaigner W., Ciccolallo L., De Vries E., Izarzugaza I., Zambon P., Gatta G., De Angelis R. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur. J. Cancer. 2009;45:1017–1027. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Rachet B., Maringe C., Nur U., Quaresma M., Shah A., Woods L.M., Ellis L., Walters S., Forman D., Steward J., et al. Population-based cancer survival trends in England and Wales up to 2007: An assessment of the NHS cancer plan for England. Lancet Oncol. 2009;10:351–369. doi: 10.1016/S1470-2045(09)70028-2. [DOI] [PubMed] [Google Scholar]
- 9.Gatta G., Trama A., Capocaccia R. Variations in cancer survival and patterns of care across Europe: roles of wealth and health-care organization. J. Natl. Cancer Inst. Monogr. 2013;2013:79–87. doi: 10.1093/jncimonographs/lgt004. [DOI] [PubMed] [Google Scholar]
- 10.Byers T.E., Wolf H.J., Bauer K.R., Bolick-Aldrich S., Chen V.W., Finch J.L., Fulton J.P., Schymura M.J., Shen T., Van Heest S., et al. The impact of socioeconomic status on survival after cancer in the United States: Findings from the national program of cancer registries patterns of care study. Cancer. 2008;113:582–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 11.Sant M., Allemani C., Capocaccia R., Hakulinen T., Aareleid T., Coebergh J.W., Coleman M.P., Grosclaude P., Martínez C., Bell J., et al. Stage at diagnosis is a key explanation of differences in breast cancer survival across Europe. Int. J. Cancer. 2003;106:416–422. doi: 10.1002/ijc.11226. [DOI] [PubMed] [Google Scholar]
- 12.Islami F., Torre L.A., Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl. Lung Cancer Res. 2015;4:327–338. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorak M.T., Karpuzoglu E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012;3:268. doi: 10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walters S., Maringe C., Butler J., Rachet B., Barrett-Lee P., Bergh J., Boyages J., Christiansen P., Lee M., Wärnberg F., et al. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000–2007: A population-based study. Br. J. Cancer. 2013;108:1195–1208. doi: 10.1038/bjc.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walters S., Maringe C., Coleman M.P., Peake M.D., Butler J., Young N., Bergström S., Hanna L., Jakobsen E., Kölbeck K., et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: A population-based study, 2004–2007. Thorax. 2013;68:551–564. doi: 10.1136/thoraxjnl-2012-202297. [DOI] [PubMed] [Google Scholar]
- 16.Licaj I., Braaten T., Langhammer A., Le Marchand L., Hansen M.S., Gram I.T. Sex differences in risk of smoking-associated lung cancer: Results from a cohort of 600,000 norwegians. Am. J. Epidemiol. 2017;187:971–981. doi: 10.1093/aje/kwx339. [DOI] [PubMed] [Google Scholar]
- 17.Ibfelt E.H., Steding-Jessen M., Dalton S.O., Lundstrøm S.L., Osler M., Holmich L.R. Influence of socioeconomic factors and region of residence on cancer stage of malignant melanoma: A Danish nationwide population-based study. Clin. Epidemiol. 2018;10:799–807. doi: 10.2147/CLEP.S160357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Rahman A., Siegal H.A., Fisher J.H. Standardization and decomposition of rates: Useful analytic techniques for behavior and health studies. Behav. Res. Methods Instrum. Comput. 2000;32:357–366. doi: 10.3758/BF03207806. [DOI] [PubMed] [Google Scholar]
- 19.Beltrán-Sánchez H., Preston S.H., Canudas-Romo V. An integrated approach to cause-of-death analysis: Cause-deleted life tables and decompositions of life expectancy. Demogr. Res. 2008;19:1323. doi: 10.4054/DemRes.2008.19.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreev E., Shkolnikov V., Begun A.Z. Algorithm for decomposition of differences between aggregate demographic measures and its application to life expectancies, healthy life expectancies, parity-progression ratios and total fertility rates. Demogr. Res. 2002;7:499–522. doi: 10.4054/DemRes.2002.7.14. [DOI] [Google Scholar]
- 21.Arriaga E.E. Measuring and explaining the change in life expectancies. Demography. 1984;21:83–96. doi: 10.2307/2061029. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa E.M. Components of a difference between two rates. J. Am. Stat. Assoc. 1955;50:1168–1194. [Google Scholar]
- 23.Ministry of Health . Healthcare in Denmark: An Overview. Ministry of Health; Copenhagen, Denmark: 2017. [Google Scholar]
- 24.Das Gupta P. A general method of decomposing a difference between two rates into several components. Demography. 1978;15:99–112. doi: 10.2307/2060493. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y.J., Strobino D.M. Decomposition of the difference between two rates with hierarchical factors. Demography. 1984;21:361–372. doi: 10.2307/2061165. [DOI] [PubMed] [Google Scholar]
- 26.Vaupel J.W., Canudas-Romo V. Decomposing demographic change into direct vs. compositional components. Demogr. Res. 2002;7:1–14. doi: 10.4054/DemRes.2002.7.1. [DOI] [Google Scholar]
- 27.Chevan A., Sutherland M. Revisiting das gupta: refinement and extension of standardization and decomposition. Demography. 2009;46:429–449. doi: 10.1353/dem.0.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aitchison J. The Statistical Analysis of Compositional Data. Chapman & Hall, Ltd.; London, UK: 1986. [Google Scholar]
- 29.Pawlowsky-Glahn V., Buccianti A., editors. Compositional Data Analysis: Theory and Applications. John Wiley & Sons; Chichester: West Sussex, United Kingdom: 2011. [Google Scholar]
- 30.Gjerstorff M.L. The Danish cancer registry. Scand. J. Public Health. 2011;39(Suppl. 7):42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 31.Søgaard M., Olsen M. Quality of cancer registry data: Completeness of TNM staging and potential implications. Clin. Epidemiol. 2012;4(Suppl. 2):1–3. doi: 10.2147/CLEP.S33873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Statistics Denmark FOD207: Deaths by Municipality Sex and Age. [(accessed on 15 June 2018)]; Available online: http://www.statbank.dk/statbank5a/selectvarval/define.asp?PLanguage=1&subword=tabsel&MainTable=FOD207&PXSId=146254&tablestyle=&ST=SD&buttons=0.
- 33.Statistics Denmark FOLK1A: Population at the First Day of the Quarter by Region, Sex, Age and Marital Status. [(accessed on 15 June 2018)]; Available online: http://www.statbank.dk/statbank5a/selectvarval/define.asp?PLanguage=1&subword=tabsel&MainTable=FOLK1A&PXSId=199113&tablestyle=&ST=SD&buttons=0.
- 34.Azur M.J., Stuart E.A., Frangakis C., Leaf P.J. Multiple imputation by chained equations: What is it and how does it work? Int. J. Methods Psychiatr. Res. 2011;20:40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Buuren S., Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 36.King N.B., Harper S., Young M.E. Use of relative and absolute effect measures in reporting health inequalities: Structured review. BMJ. 2012;345:e5774. doi: 10.1136/bmj.e5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynge E., Bak M., Von Euler-Chelpin M., Kroman N., Lernevall A., Mogensen N.B., Schwartz W., Wronecki A.J., Vejborg I. Outcome of breast cancer screening in Denmark. BMC Cancer. 2017;17:897. doi: 10.1186/s12885-017-3929-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyratzopoulos G., Abel G.A., Brown C.H., Rous B.A., Vernon S.A., Roland M., Greenberg D.C. Socio-demographic inequalities in stage of cancer diagnosis: Evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann. Oncol. 2013;24:843–850. doi: 10.1093/annonc/mds526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henriksen D.P., Rasmussen L., Hansen M.R., Hallas J., Pottegård A. Comparison of the five Danish regions regarding demographic characteristics, healthcare utilization, and medication use—a descriptive cross-sectional study. PLoS ONE. 2015;10:e0140197. doi: 10.1371/journal.pone.0140197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hvidberg L., Lagerlund M., Pedersen A.F., Hajdarevic S., Tishelman C., Vedsted P. Awareness of cancer symptoms and anticipated patient interval for healthcare seeking. A comparative study of Denmark and Sweden. Acta Oncol. 2016;55:917–924. doi: 10.3109/0284186X.2015.1134808. [DOI] [PubMed] [Google Scholar]
- 41.MacLean R., Jeffreys M., Ives A., Jones T., Verne J., Ben-Shlomo Y. Primary care characteristics and stage of cancer at diagnosis using data from the national cancer registration service, quality outcomes framework and general practice information. BMC Cancer. 2015;15:500. doi: 10.1186/s12885-015-1497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Møller H., Coupland V.H., Tataru D., Peake M.D., Mellemgaard A., Round T., Baldwin D.R., Callister M.E.J., Jakobsen E., Vedsted P., et al. Geographical variations in the use of cancer treatments are associated with survival of lung cancer patients. Thorax. 2018;73:530–537. doi: 10.1136/thoraxjnl-2017-210710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danish Breast Cancer Cooperative Group (DBCC) [(accessed on 1 May 2019)]; Available online: http://www.dbcg.dk/
- 44.Carriere R., Adam R., Fielding S., Barlas R., Ong Y., Murchie P. Rural dwellers are less likely to survive cancer—An international review and meta-analysis. Health Place. 2018;53:219–227. doi: 10.1016/j.healthplace.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Edge S.B., Byrd D.R., Compton C.C., Fritz A.G., Greene F.L., Trotti A., editors. AJCC Cancer Staging Manual. 7th ed. Springer; New York, NY, USA: 2010. [Google Scholar]