Abstract
The largest group of deubiquitinases—ubiquitin-specific proteases (UBPs)—perform extensive and significant roles in plants, including the regulation of development and stress responses. A comprehensive analysis of UBP genes has been performed in Arabidopsis thaliana, but no systematic study has been conducted in moso bamboo (Phyllostachys edulis). In this study, the genome-wide identification, classification, gene, protein, promoter region characterization, divergence time, and expression pattern analyses of the UBPs in moso bamboo were conducted. In total, 48 putative UBP genes were identified in moso bamboo, which were divided into 14 distinct subfamilies in accordance with a comparative phylogenetic analysis using 132 full-length protein sequences, including 48, 27, 25, and 32 sequences from moso bamboo, A. thaliana, rice (Oryza sativa), and purple false brome (Brachypodium distachyon), respectively. Analyses of the evolutionary patterns and divergence levels revealed that the PeUBP genes experienced a duplication event approximately 15 million years ago and that the divergence between PeUBP and OsUBP occurred approximately 27 million years ago. Additionally, several PeUBP members were significantly upregulated under abscisic acid, methyl jasmonate, and salicylic acid treatments, indicating their potential roles in abiotic stress responses in plants.
Keywords: moso bamboo, UBP genes, phylogenetic analysis, conserved motif, expression patterns
1. Introduction
Post-translational modifications (PTMs) of target proteins control many cellular processes [1,2], and ubiquitination is involved in many physiological events including DNA repair, cell-cycle control, abiotic or biotic stress tolerance, immune responses, endocytosis, and vesicle trafficking [3,4]. Ubiquitination, the covalent attachment of the small protein modifier ubiquitin (Ub) to a substrate protein is catalyzed by three ordered steps conducted by E1 (Ub activating enzyme), E2 (Ub conjugating enzyme), and E3 (Ub ligase), respectively [5,6,7]. Meanwhile, ubiquitination can be reversed by an opposite process, called deubiquitination, which removes Ub from target proteins and cooperates with Ub to regulate the ubiquitination levels of target proteins [4,8]. To date, deubiquitination has been found to be governed by deubiquitinases/deubiquitinating enzymes (DUBs), which generally affect the activities, stabilities, and fates of the target proteins [9,10].
Among the many types of DUBs, the highly conserved ubiquitin-specific proteases (UBPs) form the largest subfamily in plants. UBP proteins contain an Ub carboxyl-terminal hydrolase (UCH) domain with two similar triads of catalytic residues, each containing two short but highly conserved cysteine (Cys) and histidine (His) boxes, which are key parts of the catalytic sites (Cys in the cysteine box, and His and Asp/Asn in the histidine box) [11,12]. In addition to the conserved UCH domain, UBP proteins also have additional non-UBP protein motifs such as the MYND-type zinc finger domain (ZnF-MYND) which has been reported to be a protein–protein interaction domain in mammalian cells, the zinc finger domain ZnF-UBPs, the meprin and TRAF homology (MATH) domains, the domain present in Ubiquitin-Specific Proteases (DUSPs), and Ubiquitin-associated (UBA) domains [13,14]. There are 27 putative UBP family members in Arabidopsis thaliana classified into 14 sub-groups, and 21 in rice (Oryza sativa) classified into nine sub-groups [12,15]. In plants, UBPs have multiple functions in cell proliferation [13,16], endoreplication [17], root hair elongation [18,19], mitochondria morphogenesis [20], deubiquitination of monoubiquitinated-H2A and -H2B [21,22], pollen development and transmission [22], canavanine resistance [12] and abscisic acid (ABA)-mediated resistance to salt and drought stress [23].
Moso bamboo (Phyllostachys edulis) is a fast-growing non-timber forest product with high ecological, cultural, economic, and social values, and is extensively used as paper, art ware, and food in China and many other countries [24,25]. However, the normal growth and development of moso bamboo are restrained by the progressively deleterious climate and environmental conditions, such as drought, cold, and salt stress [26]. Years ago, a draft genome of moso bamboo was generated [24], which allows research on the gene families present in moso bamboo. Based on A. thaliana, the UBP genes are thought to function throughout plant life. Consequently, identifying their functions in moso bamboo could reveal more roles of UBP genes in plants. In this study, we conducted a systematic, comprehensive analysis of the UBP genes in moso bamboo. We identified 48 putative PeUBP genes and analyzed their phylogenetic relationship, gene structure, protein structure, evolutionary divergence, and expression levels in different tissues. We also examined their expression patterns in response to three plant hormones related to abiotic stress. The results will be beneficial in understanding the roles of UBPs in moso bamboo and other plants.
2. Results
2.1. Identification and Characterization of PeUBP Genes in Moso Bamboo
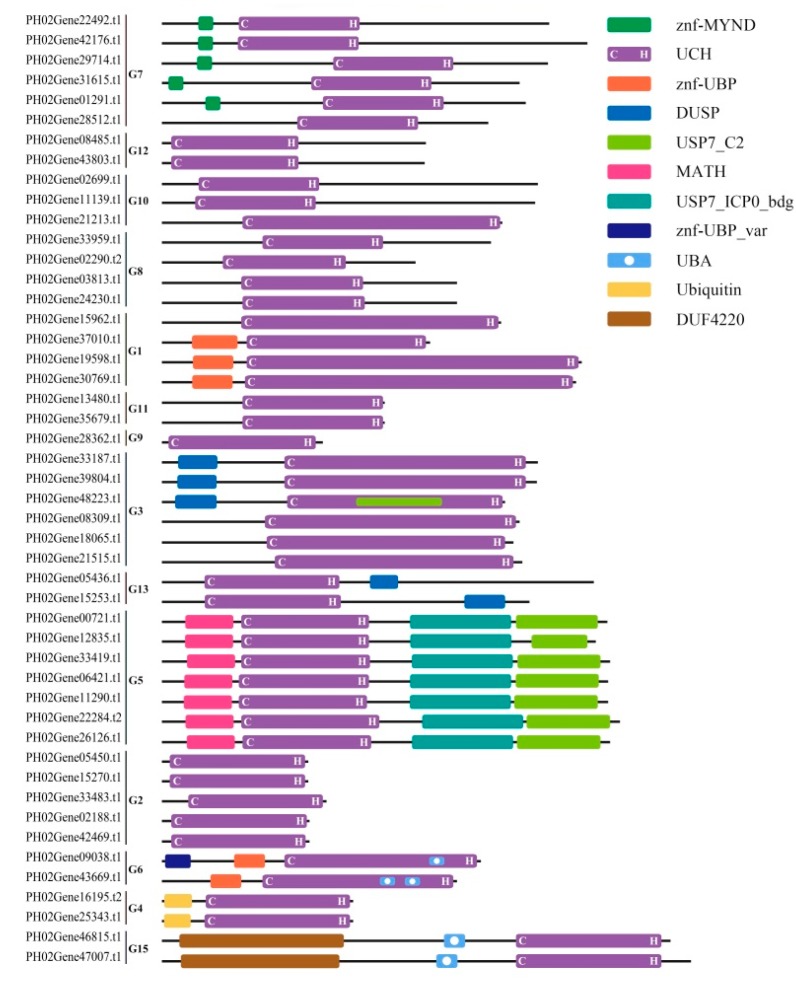
To carry out a genome-wide identification of the UBP gene family in moso bamboo, Arabidopsis UBP protein sequences were used as queries in searches against the protein databases available in the moso bamboo genome bank (ftp://parrot.genomics.cn/gigadb/pub/10.5524/100001_101000/100498/). We identified 67 PeUBP candidate genes, which were applied to confirm the existence of the conserved UCH domain in UBP proteins using the Pfam database (http://pfam.xfam.org) and the NCBI CD-search program (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). After removing sequences that did not satisfy the required conditions, we identified 48 PeUBP genes that exhibited a complete UCH domain with two short but well-conserved motifs specific to UBP proteins (Cys and His) [27]. Like the situation in A. thaliana, the members within each subfamily also had the most comparable non-UBP domains, including ZnF-UBPs, MATHs, UBAs, UBQs, and DUSPs domains. Additionally, a bamboo-specific domain, named DUF4220, did not exist in Arabidopsis (Figure 1).
Figure 1.
48 PeUBP genes can be divided into 14 groups (G1–G13, and G15) based on the predicted amino acid sequence identities and domain structures. The domains are indicated by different colored boxes.
The detailed characteristics of the PeUBPs, such as accession number, location, and physicochemical parameters, are given in Table 1. The lengths of the CDSs ranged from 876 to 3915 bp and encoded sequences ranging from 366 to 1159 aa. The molecular weight (MW) varied from 4.20 to 11.58 kDa, and the theoretical isoelectric point (pI) ranged from 4.85 to 9.34. The transmembrane (TM) regions of PeUBP proteins were predicted using TMHMM Server v2.0. The TM regions only existed in four PeUBP members, of which two (PH02Gene46815.t1 and PH02Gene47007.t1), containing three TMs, belonged to the group (G) 15, and two (PH02Gene01291.t1 and PH02Gene29714.t1), with one TM, belonged to G7 (Figure S1).
Table 1.
Detailed information about 48 predicted PeUBP proteins in moso bamboo. The last column lists the number of exons in each gene. CDS, the coding sequence of a gene; MW, molecular weight; pI, protein isoelectric point.
| Gene ID | Location | CDS Length (bp) | Size (aa) | Protein | pl | Exons |
|---|---|---|---|---|---|---|
| MW (Da) | ||||||
| PH02Gene00721.t1 | 16: 30179141-30199136 | 3333 | 1110 | 129890.52 | 5.57 | 32 |
| PH02Gene01291.t1 | 18: 35966647-35977509 | 2721 | 906 | 99762.48 | 6.22 | 12 |
| PH02Gene02188.t1 | 24:40155854-40161467 | 1107 | 368 | 41908.50 | 5.88 | 6 |
| PH02Gene02290.t2 | 23:13745085-13751574 | 1896 | 631 | 69325.70 | 4.85 | 13 |
| PH02Gene02699.t1 | 6:13203375-13209603 | 2823 | 940 | 102597.05 | 9.36 | 11 |
| PH02Gene03813.t1 | 3:84811131-84816494 | 2223 | 740 | 82069.42 | 4.92 | 15 |
| PH02Gene05436.t1 | 15:67480992-67493851 | 3228 | 1075 | 119371.13 | 6.08 | 13 |
| PH02Gene05450.t1 | 15:67882508-67890349 | 1101 | 366 | 42022.55 | 5.86 | 6 |
| PH02Gene06421.t1 | 7:52268122-52288354 | 3354 | 1117 | 131095.16 | 5.62 | 32 |
| PH02Gene08309.t1 | 4:54406565-54413534 | 2673 | 890 | 99416.65 | 5.55 | 12 |
| PH02Gene08485.t1 | 9:53943713-53950726 | 1971 | 656 | 71789.57 | 8.98 | 8 |
| PH02Gene09038.t1 | 16:113303229-113317987 | 2383 | 795 | 88955.33 | 5.27 | 19 |
| PH02Gene11139.t1 | 8:10275805-10281089 | 2805 | 934 | 101955.24 | 9.34 | 11 |
| PH02Gene11290.t1 | 1:21490093-21516076 | 3354 | 1117 | 131044.10 | 5.59 | 32 |
| PH02Gene12835.t1 | 14:60797306-60818319 | 3249 | 1082 | 126562.99 | 5.71 | 30 |
| PH02Gene13480.t1 | 10:1249930-1254111 | 1662 | 553 | 60007.42 | 8.70 | 7 |
| PH02Gene15253.t1 | 21:101647723-101655588 | 2748 | 915 | 101640.25 | 6.34 | 10 |
| PH02Gene15270.t1 | 21:102143034-102149220 | 1101 | 366 | 41960.49 | 6.13 | 6 |
| PH02Gene15962.t1 | 13:51305905-51310945 | 2547 | 848 | 93,234.54 | 4.94 | 4 |
| PH02Gene16195.t2 | 14:31330299-31338713 | 1425 | 474 | 53,211.28 | 5.87 | 17 |
| PH02Gene18065.t1 | 11:42415269-42423636 | 2643 | 880 | 98195.73 | 5.11 | 13 |
| PH02Gene19598.t1 | 3:10292006-10297292 | 3147 | 1048 | 113926.14 | 5.19 | 2 |
| PH02Gene21213.t1 | 3:99405737-99411540 | 2979 | 992 | 109834.65 | 8.62 | 12 |
| PH02Gene21515.t1 | 12:47774473-47795019 | 2694 | 897 | 100426.26 | 5.37 | 12 |
| PH02Gene22284.t2 | 10:28962334-28980176 | 3429 | 1142 | 134013.04 | 5.66 | 31 |
| PH02Gene22492.t1 | 6:34432327-34438439 | 2907 | 968 | 106038.69 | 5.96 | 11 |
| PH02Gene24230.t1 | 2835:25003-30357 | 2217 | 738 | 81849.15 | 5.09 | 15 |
| PH02Gene25343.t1 | 16:69739339-69750041 | 1425 | 474 | 53184.22 | 6.01 | 17 |
| PH02Gene26126.t1 | 4:53402592-53419429 | 3360 | 1119 | 131489.97 | 5.71 | 32 |
| PH02Gene28362.t1 | 23:7647625-7650488 | 1200 | 399 | 45414.32 | 7.91 | 3 |
| PH02Gene28512.t1 | 13:58209169-58217387 | 2439 | 812 | 89586.32 | 6.69 | 10 |
| PH02Gene29714.t1 | 20:29015633-29030162 | 2889 | 962 | 107226.58 | 7.13 | 13 |
| PH02Gene30769.t1 | 18:30083038-30088423 | 3111 | 1036 | 113047.92 | 5.11 | 2 |
| PH02Gene31615.t1 | 17:78162911-78172473 | 2673 | 890 | 98449.22 | 6.41 | 14 |
| PH02Gene33187.t1 | 13:102796122-102807090 | 2808 | 935 | 105880.58 | 5.04 | 13 |
| PH02Gene33419.t1 | 12:32620099-32636788 | 3357 | 1118 | 131431.81 | 5.71 | 32 |
| PH02Gene33483.t1 | 17:40198974-40203749 | 1230 | 409 | 46321.46 | 5.85 | 6 |
| PH02Gene33959.t1 | 5:54814061-54820578 | 2469 | 822 | 92602.24 | 5.49 | 14 |
| PH02Gene35679.t1 | 4:3711703-3715914 | 1662 | 553 | 60216.88 | 8.88 | 7 |
| PH02Gene37010.t1 | 18:22571046-22581812 | 2004 | 667 | 73289.87 | 9.21 | 3 |
| PH02Gene39804.t1 | 5:3099809-3109660 | 2808 | 935 | 105866.37 | 5.02 | 13 |
| PH02Gene42176.t1 | 8:66644923-66652376 | 3180 | 1059 | 115732.16 | 5.90 | 11 |
| PH02Gene42469.t1 | 23:34433041-34449409 | 1107 | 368 | 41893.49 | 5.98 | 6 |
| PH02Gene43669.t1 | 14:97907481-97915260 | 1962 | 733 | 81520.86 | 4.99 | 17 |
| PH02Gene43803.t1 | 7:39435913-39442930 | 1961 | 653 | 71613.43 | 8.84 | 8 |
| PH02Gene46815.t1 | 1:15050591-15059698 | 3810 | 1269 | 144256.52 | 6.22 | 3 |
| PH02Gene47007.t1 | 1:15242214-15246894 | 3693 | 1320 | 151083.45 | 5.89 | 3 |
| PH02Gene48223.t1 | 13:32005753-32013346 | 2565 | 855 | 96768.17 | 5.70 | 12 |
2.2. Phylogenetic Analysis of the PeUBP Genes and Identification of Exon-Intron Structure
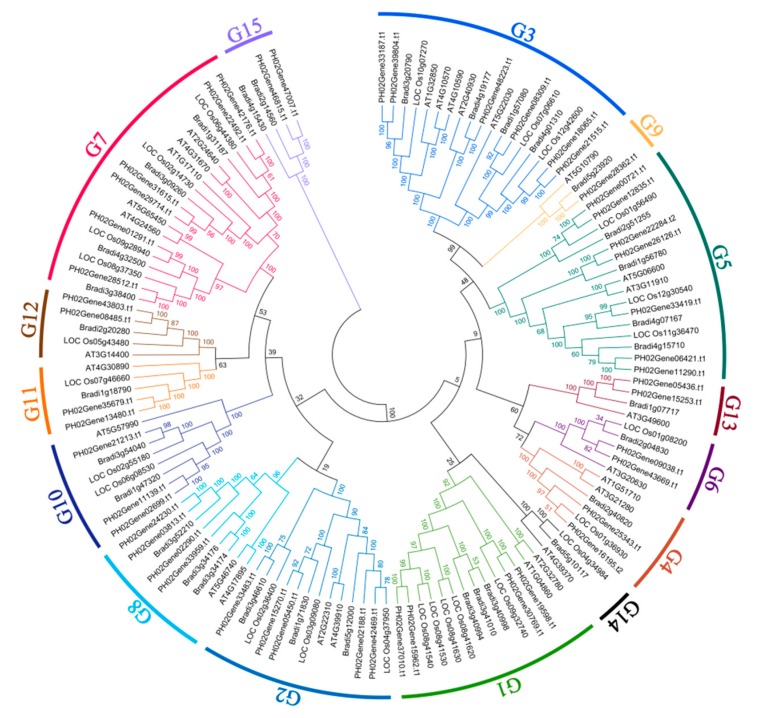
To analyze the phylogenetic relationship of the UBPs among different species and study the potential functions of PeUBPs, a Neighbor-joining phylogenetic tree was constructed based on the alignments of 132 full-length UBP protein sequences from moso bamboo (48), rice (25) [28], A. thaliana (27) [13], and purple false brome (Brachypodium distachyon) (29). The detailed characteristics of the UBP genes from A. thaliana (Dicotyledonous subfamily), rice (Oryza sativa; Poaceae subfamily), and B. distachyon (close relationship with moso bamboo; Monocotyledons) are listed in supplementary Table S1. In the phylogenetic tree, all sequences were classified into 15 groups, and 48 PeUBPs were distributed into 14 (G1–G13, G15), but not G14. G15 formed a branch with four members from moso bamboo (PH02Gene47007.t1 and PH02Gene45815.t1) and B. distachyon (Bradi2g14560 and Bradi4g15430) (Figure 2). In fact, the G15 members had a specific domain (DUF4220) that did not exist in AtUBP and OsUBP proteins. The number of PeUBP members in different groups was uneven. For example, there was none in G14, which had three members, one each from A. thaliana, rice, and B. distachyon; while in other groups, the number of PeUBPs varied, with seven in G5; four in G1, G7, and G8; two in G4, G6, G11, G12, and G14; and one in both G9 and G10.
Figure 2.
Phylogeny of PeUBP proteins from moso bamboo, Arabidopsis thaliana, rice, and Brachypodium distachyon. The tree was generated using the Neighbour-joining method with 1000 replicates. The branches of each group are indicated in a specific color.
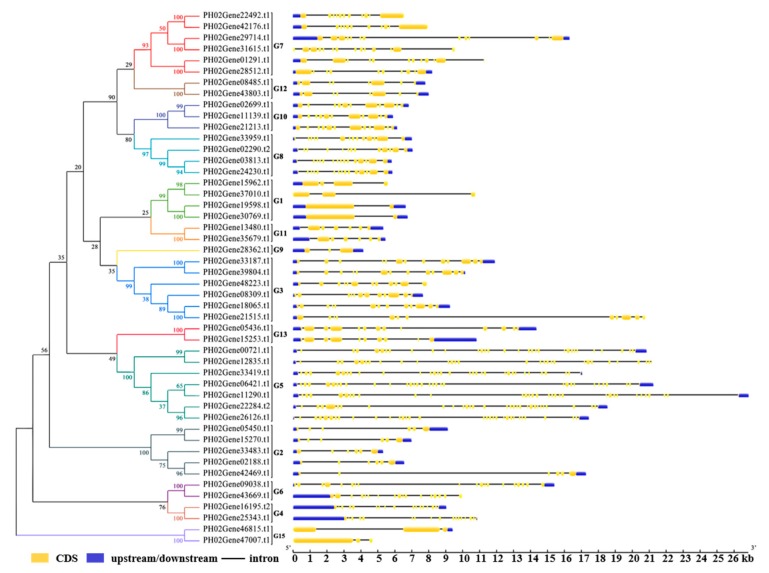
To further analyze the structural diversity of moso bamboo PeUBP genes, a separate phylogenetic tree was constructed only using the full-length UBP protein sequences of moso bamboo. Moso bamboo proteins were also divided into 14 distinct subfamilies, in good agreement with those of the four plant species (Figure 2 and Figure 3). The Gene Structure Display Server (GSDS) online tool was used to identify the exon-intron structure of each predicted PeUBP gene. As shown in Figure 3, the five PeUBP genes (PH02Gene00721.t1, PH02Gene06421.t1, PH02Gene11290.t1, PH02Gene26126.t1, and PH02Gene33419.t1) in G5 contained the largest number of exons (30), and another two genes (PH02Gene12835.t1 and PH02Gene22282.t1) in G5 contained 30 and 31 exons, respectively. In the other 41 PeUBP genes, the exon numbers varied from 2 to 19. Upstream and downstream sequences commonly existed in the PeUBP genomic sequences except for in PH02Gene31615.t1, PH02Gene379010.t1, and PH02Gene47007.t1, which lacked upstream and downstream sequences. Besides, PH02Gene01291.t1, PH02Gene12835.t1, PH02Gene15962.t1, PH02Gene21515.t1, PH02Gene22492.t1, PH02Gene42176.t1, PH02Gene43669.t1, and PH02Gene48223.t1 have only upstream sequences, while PH02Gene46815 has only a downstream sequence. The intron/exon structure of sister gene pairs had both conservative and differential regions. For example, most gene pairs varied greatly in their structural organization and the numbers of intron-exons, except for six gene pairs (PH02Gene02188.t1/PH02Gene42469.t1, PH02Gene03813.t1/PH02Gene379010.t1, PH02Gene05450.t1/PH02Gene15270.t1, PH02Gene08485.t1/PH02Gene43803.t1, PH02Gene13480.t1/PH02Gene35679.t1, and PH02Gene19598.t1/PH02Gene30769.t1), which were shown to have the same intron-exon numbers and intron phase but with variable intron lengths.
Figure 3.
Phylogenetic relationship and gene structures of PeUBPs in moso bamboo. Left: Phylogenetic tree of PeUBPs constructed using the Neighbor-joining method based on the results of a sequence alignment. Bootstrap values from 1000 replicates are indicated at each node. The proteins in the tree were divided into 14 distinct subfamilies, and the branches of different subfamilies are marked using different colors. Right: Exons, introns, and untranslated regions (UTRs) are indicated by yellow rectangles, gray lines, and blue rectangles, respectively.
2.3. Identification of Conserved Sequence Motifs and Determination of Homology Modeling in Moso Bamboo UBP Genes
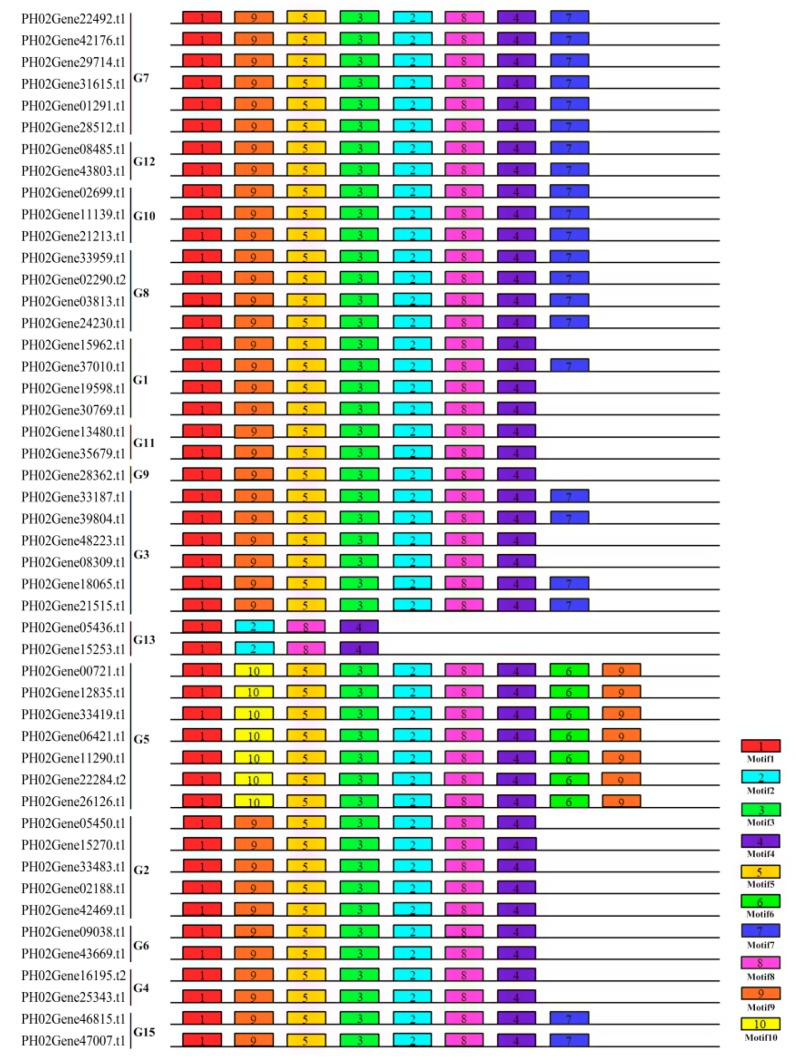
To obtain the compositions and diversification of motifs present in the 48 PeUBP proteins, we searched for the conserved motifs using the MEME program (http://meme-suite.org/tools/meme) (Figure 4). A total of ten distinct motifs were identified, and detailed information on the Logo sequences of these ten motifs are provided in Table S2. Members classified into the same groups had similar or identical conserved motifs, indicating functional similarities among these proteins. All the PeUBP proteins were characterized by Motif 1 in the N-terminal UCH domain, and Motifs 4 and 8 in the C-terminal UCH domain, which represented the Cys and His boxes, respectively. With a few exceptions, the motifs in most of the PeUBP proteins had the same order ranking of 1, 9, 5, 3, 2, 8, and 4. In addition, motifs 6 and 10 were exclusively found in G5.
Figure 4.
Schematic representation of the ten conserved motifs in PeUBPs. Conserved motifs of the PeUBPs were identified using the online MEME program based on 48 full-length aa sequences. The lengths of the motifs are displayed proportionally. The numbers in boxes (1–10) represent the motif numbers.
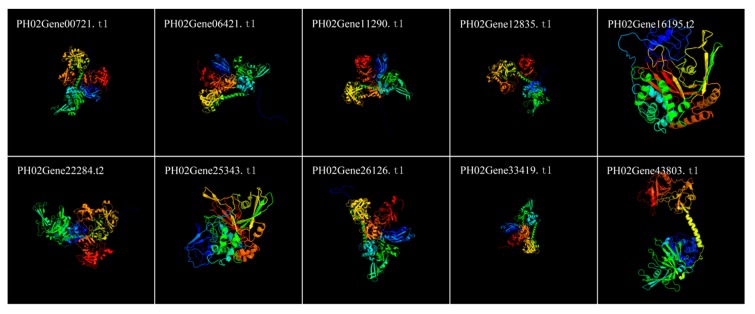
To determine the structures of the PeUBP proteins, we used Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index) to predict the homology modeling, and we aligned the PeUBP protein sequences using the HMM-HMM search in intensive mode [29]. All of the 48 PeUBP proteins could be confidently modeled. As shown in Figure 5, 10 PeUBPs had 100% of their predicted lengths modeled with > 90% confidence (PH02Gene00721.t1, PH02Gene06421.t1, PH02Gene11290.t1, PH02Gene12835.t1, PH02Gene16195.t2, PH02Gene22284.t2, PH02Gene25343.t1, PH02Gene26126.t1, PH02Gene33419.t1, and PH02Gene43803.t1).
Figure 5.
Predicted structures of PeUBP proteins. The structures of 10 PeUBP proteins were predicted with > 90% confidence.
2.4. Evolutionary and Divergence Patterns of the UBP Genes in Moso Bamboo, Rice, and B. distachyon
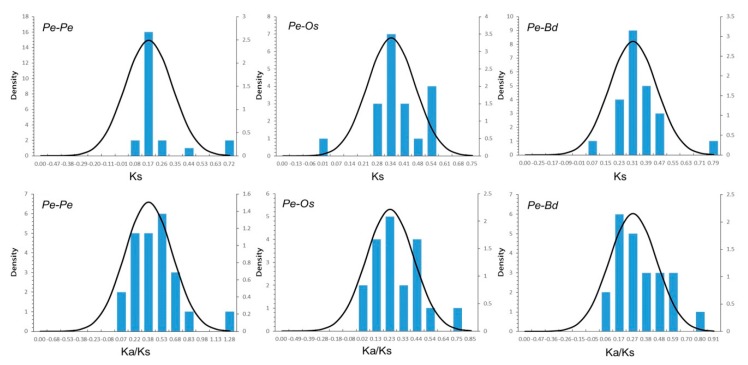
To analyze the UBP’s evolutionary and divergence patterns, homologous pairs among moso bamboo, rice, and B. distachyon were subjected to BLASTn sequence similarity analyses. 21 putative paralogous (Pe-Pe) in moso bamboo, 18 orthologs (Pe-Os) between moso bamboo and rice, and 22 orthologs (Pe-Bd) between moso bamboo and B. distachyon were identified (Table 2). The divergence times of moso bamboo, rice, and B. distachyon were evaluated using the formula T = Ks/2λ, and with the Ks value serving as a proxy for time. The relative Ks values of paralogous pairs (Pe-Pe) averaged ~0.2, indicating that PeUBP genes experienced a large-scale duplication event approximately 15 million years ago (MYA). A previous study reported that bamboo underwent whole-genome duplication 7–12 MYA, indicating that the large-scale duplication of the UBP genes occurred earlier [24]. The Ks value distributions of Pe-Os and Pe-Bd orthologous pairs both peaked at approximately 0.35 (Figure 6), indicating that the divergence time of these genes was 27 MYA. A comparison with a previous study revealed that the divergence times between moso bamboo and rice, and moso bamboo and B. distachyon were 43–57, 42–52 MYA, respectively, indicating that the UBP genes underwent gene evolution prior to the separation of the two progenitor species. In general, Ka/Ks ratio greater than 1, equal to 1, and less than 1 indicate that a gene has experienced positive, neutral, and negative or stabilizing selection, respectively [30,31]. The Ka/Ks ratios of Pe-Pe, Pe-Os, and Pe-Bd genomes were all less than 1, which suggested that the UBP genes between moso bamboo and rice genomes and moso bamboo and Brachypodium genomes, as well as for the paralogous in the moso bamboo genome, have experienced strongly positive purifying selection.
Table 2.
Paralogous (Pe-Pe) and orthologous (Pe-Os and Pe-Bd) gene pairs.
| Pe-Pe | Pe-Os | Pe-Bd |
|---|---|---|
| PH02Gene05450.t1/PH02Gene15270.t1 | PH02Gene09038.t1/Os01g08200 | PH02Gene05436.t1/Bradi1g07717 |
| PH02Gene11139.t1/PH02Gene02699.t1 | PH02Gene16195.t2/Os01g36930 | PH02Gene13480.t1/Bradi1g18790 |
| PH02Gene11290.t1/PH02Gene06421.t1 | PH02Gene00721.t1/Os01g56490 | PH02Gene11139.t1/Bradi1g47320 |
| PH02Gene12835.t1/PH02Gene00721.t1 | PH02Gene29714.t1/Os02g14730 | PH02Gene26126.t1/Bradi1g56780 |
| PH02Gene15253.t1/PH02Gene05436.t1 | PH02Gene33483.t1/Os02g36400 | PH02Gene08309.t1/Bradi1g57080 |
| PH02Gene21213.t1/PH02Gene11139.t1 | PH02Gene05450.t1/Os03g09080 | PH02Gene05450.t1/Bradi1g71830 |
| PH02Gene21515.t1/PH02Gene18065.t1 | PH02Gene42469.t1/Os04g37950 | PH02Gene09038.t1/Bradi2g04830 |
| PH02Gene24230.t1/PH02Gene03813.t1 | PH02Gene43803.t1/Os05g43480 | PH02Gene47007.t1/Bradi2g14560 |
| PH02Gene25343.t1/PH02Gene16195.t2 | PH02Gene42176.t1/Os06g44380 | PH02Gene43803.t1/Bradi2g20280 |
| PH02Gene26126.t1/PH02Gene22284.t2 | PH02Gene08309.t1/Os07g06610 | PH02Gene16195.t2/Bradi2g40820 |
| PH02Gene28512.t1/PH02Gene01291.t1 | PH02Gene28512.t1/Os08g37350 | PH02Gene00721.t1/Bradi2g51255 |
| PH02Gene30769.t1/PH02Gene19598.t1 | PH02Gene15962.t1/Os08g41540 | PH02Gene39804.t1/Bradi3g20790 |
| PH02Gene31615.t1/PH02Gene29714.t1 | PH02Gene01291.t1/Os09g28940 | PH02Gene33959.t1/Bradi3g34176 |
| PH02Gene35679.t1/PH02Gene13480.t1 | PH02Gene30769.t1/Os09g32740 | PH02Gene28512.t1/Bradi3g38400 |
| PH02Gene37010.t1/PH02Gene15962.t1 | PH02Gene33187.t1/Os10g07270 | PH02Gene15962.t1/Bradi3g41010 |
| PH02Gene39804.t1/PH02Gene33187.t1 | PH02Gene06421.t1/Os11g36470 | PH02Gene33483.t1/Bradi3g46610 |
| PH02Gene42176.t1/PH02Gene22492.t1 | PH02Gene33419.t1/Os12g30540 | PH02Gene24230.t1/Bradi3g52210 |
| PH02Gene42469.t1/PH02Gene02188.t1 | PH02Gene18065.t1/Os12g42600 | PH02Gene18065.t1/Bradi4g01310 |
| PH02Gene43669.t1/PH02Gene09038.t1 | PH02Gene11290.t1/Bradi4g15710 | |
| PH02Gene43803.t1/PH02Gene08485.t1 | PH02Gene48223.t1/Bradi4g19177 | |
| PH02Gene47007.t1/PH02Gene46815.t1 | PH02Gene42469.t1/Bradi5g12000 | |
| PH02Gene28362.t1/Bradi5g23920 |
Figure 6.
Ks and Ka/Ks value distributions of the PeUBP genes in the genomes of moso bamboo, rice, and Brachypodium distachyon, viewed through the frequency distribution of relative Ks and Ka/Ks modes. Distributions of Ks and Ka/Ks values were obtained from paralogous gene pairs in the moso bamboo genome, orthologous gene pairs between moso bamboo and rice, and orthologous gene-pairs between moso bamboo and B. distachyon.
2.5. PeUBP Expression Levels in Different Tissues
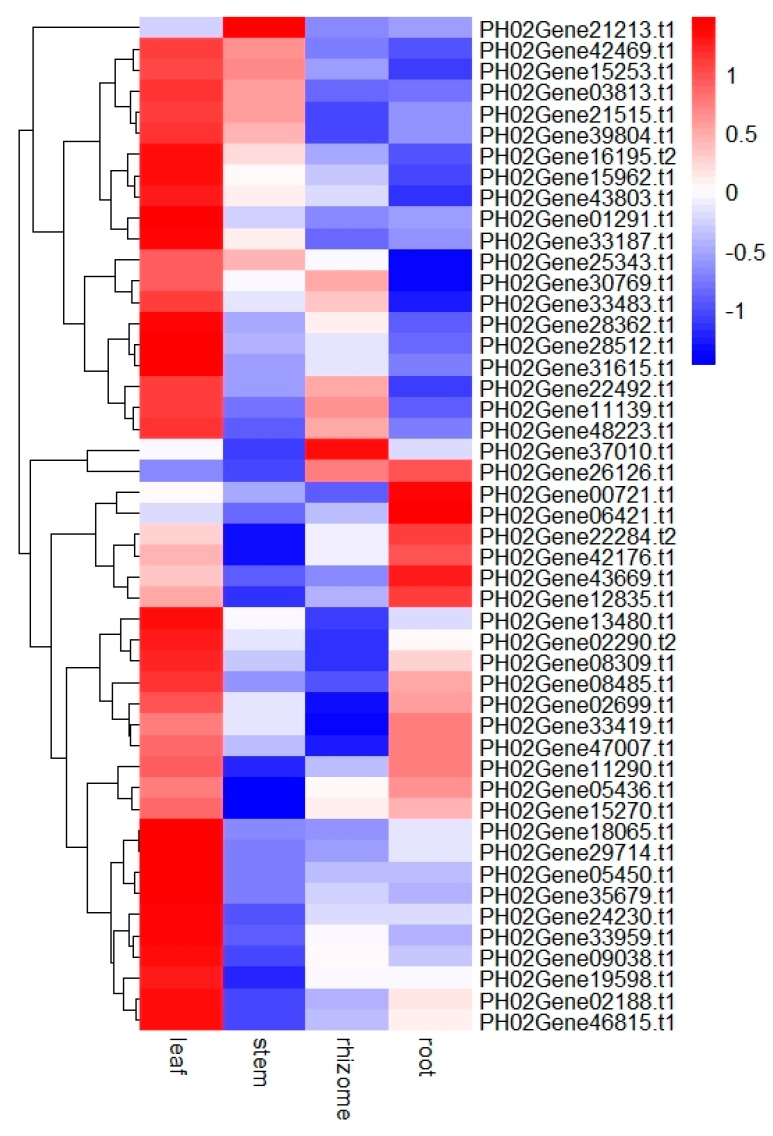
Tissue-specific gene expression provides vital clues for dissecting gene function. To characterize the expression patterns of PeUBP genes, we analyzed their transcription levels in different tissues of moso bamboo, including leaf, stem, rhizome, and root using quantitative real-time PCR (qRT-PCR) (Table S3). As shown in Figure 7, most PeUBP genes (except for PH02Gene00721.t1, PH02Gene06421.t1, PH02Gene21213.t1, PH02Gene26126.t1, and PH02Gene37010.t1) in the leaf, seven genes (PH02Gene03813.t1, PH02Gene15253.t1, PH02Gene21213.t1, PH02Gene21515.t1, PH02Gene25343.t1, PH02Gene39804.t1, and PH02Gene42469.t1) in the stem, seven genes (PH02Gene11139.t1, PH02Gene22492.t1, PH02Gene26126.t1, PH02Gene30769.t1, PH02Gene33483.t1, PH02Gene37010.t1, and PH02Gene48223.t1) in the rhizome, and 14 genes (PH02Gene00721.t1, PH02Gene02699.t1, PH02Gene05436.t1, PH02Gene06421.t1, PH02Gene08485.t1, PH02Gene11290.t1, PH02Gene12835.t1, PH02Gene15270.t1, PH02Gene22284.t1, PH02Gene26126.t1, PH02Gene33419.t1, PH02Gene42176.t1, PH02Gene43669.t1, and PH02Gene47007.t1) in the root showed high expression levels. In particular, for all the PeUBPs, the expression levels were significantly higher in leaf than in other tissues. We also determined that some genes presented a tissue-specific expression profile. For example, PH02Gene05436.t1, PH02Gene11290.t1, PH02Gene15270.t1, PH02Gene33419.t1, and PH02Gene47007.t1 showed high expression levels in leaves and roots, but low levels in stems and rhizomes. PH02Gene03813.t1, PH02Gene15253.t1, PH02Gene21515.t1, PH02Gene39804.t1, and PH02Gene42469.t1 showed high expression levels in the leaf and stem, but low levels in rhizome and root. PH02Gene11139.t1, PH02Gene22492.t1, PH02Gene48223.t1 showed high expression levels in the leaves and rhizomes, but low levels in stems and roots. Only PH02Gene21213.t1 was highly expressed in the stem, and only PH02Gene37010.t1 was highly expressed in the rhizome. In addition, PH02Gene00721.t1 and PH02Gene06421.t1 showed relatively high expression levels in the root compared with the other three tissues. Above all, PeUBP genes exhibited different expression profiles in different tissues, indicating the multiple biological functions of PeUBPs in moso bamboo growth and development.
Figure 7.
Expression profiles of PeUBP genes in different tissues of moso bamboo. Samples were from leaf, stem, rhizome, and root. The heatmap shows the hierarchical clustering of 48 PeUBP genes across the different tissues analyzed. The color scale presented vertically at the right side of the picture represents log10 expression values; blue and red represents low and high levels of transcript abundance. The mean values were obtained from three biological and three technical replicates.
2.6. Expression Profiles of PeUBP Genes under Abscisic Acid (ABA), Methyl Jasmonate (MeJA), and Salicylic Acid (SA) Treatments
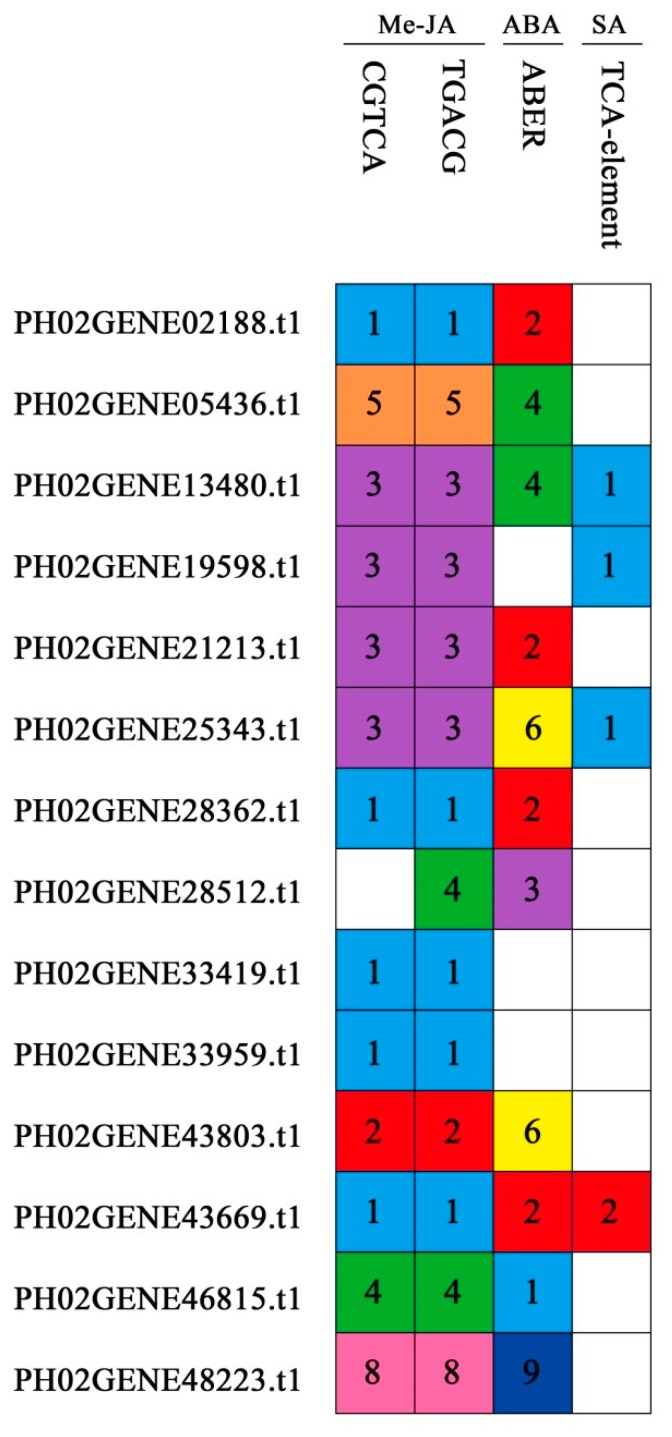
Several AtUBPs have been reported to function in the regulation of ABA (AtUBP24) [23] and MeJA (AtUBP12/13) [32,33,34]; consequently, we hypothesized that the PeUBP genes also had similar functions. We analyzed the promoters of all the PeUBP genes using PlantCARE (http://www. dna.affrc.go.jp/PLACE/) to examine the 2000-bp upstream sequences. Many cis-regulatory elements corresponding to ABA, MeJA, and SA were found, indicating that PeUBPs played roles in plant development and stress responses (Figure 8) [35,36,37,38,39].
Figure 8.
cis-Acting elements related to ABA, MeJA, and SA in the promoter regions of PeUBPs. The colored blocks containing numbers represent the numbers of cis-element in PeUBPs.
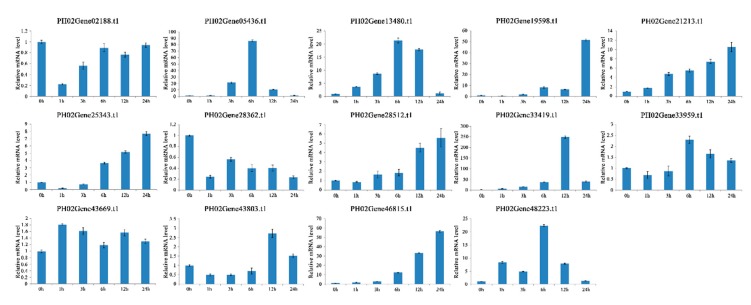
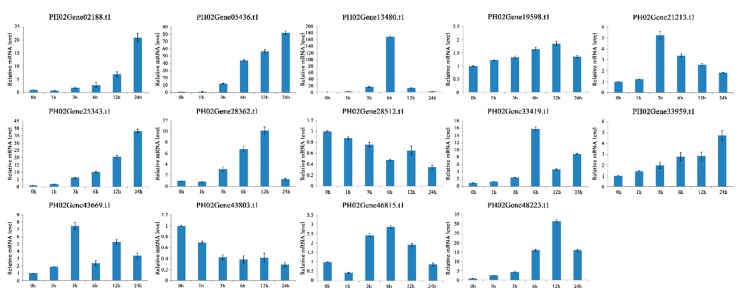
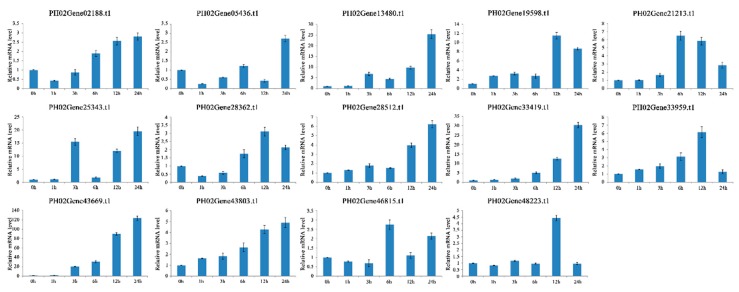
Then, we performed qRT-PCR to evaluate the dynamic expression levels under ABA, MeJA, and SA treatments, with 14 genes as representatives of each subfamily (PH02Gene02188.t1, PH02Gene05436.t1, PH02Gene13480.t1, PH02Gene19598.t1, PH02Gene21213.t1, PH02Gene25343.t1, PH02Gene28362.t1, PH02Gene28512.t1, PH02Gene33419.t1, PH02Gene33959.t1, PH02Gene43669.t1, PH02Gene43803.t1, PH02Gene46815.t1, and PH02Gene48223.t1).
In the MeJA treatment, nine genes (PH02Gene05436.t1, PH02Gene13480.t1, PH02Gene19598.t1, PH02Gene21213.t1, PH02Gene25343.t1, PH02Gene28512.t1, PH02Gene33419.t1, PH02Gene46815.t1, and PH02Gene48223.t1) were distinctly up-regulated. For example, the expression of three genes (PH02Gene05436.t1, PH02Gene 13480.t1, and PH02Gene48223.t1) peaked at 6h, with a gradual decrease at all later time points. The PH02Gene33419.t1 was the most highly expressed (>200-fold that of 0 h) after 12 h of treatment. Five genes (PH02Gene19598.t1, PH02Gene21213.t1, PH02Gene25343.t1, PH02Gene28512.t1, and PH02Gene46815.t1) peaked at 24 h, with a gradual increase over time, except for PH02Gene19598.t1. By contrast, PH02Gene28362.t1 was down-regulated under the MeJA treatment. Additionally, there were five PeUBP genes (PH02Gene02188.t1, PH02Gene28362.t1, PH02Gene33959.t1, PH02Gene43669.t1, and PH02Gene43803.t1) that showed slight (<5-fold that at 0 h) changes in response to MeJA treatment (Figure 9).
Figure 9.
Expression analyses of 14 representative PeUBP genes under MeJA treatment. Sampling occurred 0, 1, 3, 6, 12, and 24 h after treatment, and the relative expression levels were analyzed.
In the ABA treatment, nine genes (PH02Gene02188.t1, PH02Gene05436.t1, PH02Gene13480.t1, PH02Gene21213.t1, PH02Gene25343.t1, PH02Gene28362.t1, PH02Gene33419.t1, PH02Gene43669.t1, and PH02Gene48223.t1) were distinctly up-regulated. For example, two genes (PH02Gene21213.t1 and PH02Gene43669.t1) were gradually up-regulated during the early time points, peaked at 3 h, and decreased during subsequent treatment. Two genes (PH02Gene13480.t1, and PH02Gene33419.t1) were the most highly expressed at 6 h, with a gradual increase during the early time points and a significant decrease during subsequent treatments. Moreover, PH02Gene13480.t1 showed an expression level more than 160-fold higher at 6 h than at 0 h. The expression levels of two genes (PH02Gene 28362.t1 and PH02Gene48223.t1) were highest at 12 h. However, the expression levels of five genes (PH02Gene19598.t1, PH02Gene28512.t1, PH02Gene33959.t1, PH02Gene43803.t1, and PH02Gene46815.t1) changed only slightly (<5 fold that at 0 h) during the 24-h time course (Figure 10).
Figure 10.
Expression analyses of 14 representative PeUBP genes under ABA treatment. Sampling occurred 0, 1, 3, 6, 12, and 24 h after treatment, and the relative expression levels were analyzed. Untreated sample expression levels = 1. X-axes and Y-axes represent time points after ABA treatment and relative gene expression values normalized to reference gene TIP41, respectively. Bars indicate standard deviations from three biological replicates.
In the SA treatment, eight genes (PH02Gene13480.t1, PH02Gene19598.t1, PH02Gene21213.t1, PH02Gene25343.t1, PH02Gene28512.t1, PH02Gene33419.t1, PH02Gene33959.t1, and PH02Gene43669.t1) were distinctly up-regulated. For example, PH02Gene21213.t1 exhibited a gradual increase in expression during the early time points, a peak at 6 h, and a gradual decrease at all later time points. The expression levels of two genes (PH02Gene19598.t1 and PH02Gene33959.t1) were highest at 12 h. Five genes (PH02Gene13480.t1, PH02Gene25343.t1, PH02Gene28512.t1, PH02Gene33419.t1, and PH02Gene43669.t1) were the most highly expressed at 24 h, especially PH02Gene43669.t1, with an expression level over 100-fold higher at 24 h than at 0 h. In addition, the expression levels of six genes (PH02Gene01288.t1, PH02Gene05436.t1, PH02Gene28362.t1, PH02Gene43803.t1, PH02Gene46815.t1, and PH02Gene48223.t1) changed only slightly (<5-fold that at 0 h) over the 24-h time course (Figure 11).
Figure 11.
Expression analyses of 14 representative PeUBP genes under SA treatment. Sampling occurred 0, 1, 3, 6, 12, and 24 h after treatment, and the relative expression levels were analyzed. Untreated sample expression levels = 1. X-axes and Y-axes represent time points after SA treatment and relative gene expression values normalized to reference gene TIP41, respectively. Bars indicate standard deviations from three biological replicates.
In total, four genes (PH02Gene13480.t1, PH02Gene21213.t1, PH02Gene25343.t1, and PH02Gene33419.t1) showed significant changes in response to all three hormone treatments. Additionally, these results were consistent with the putative promoter analysis of PeUBP members that revealed the wide distribution of several ABA-, MeJA-, and SA- responsive cis-elements in these four genes. This implied that a number of PeUBP genes had potential functions in response to ABA, Me-JA, and SA.
Untreated sample expression levels = 1. X-axes and Y-axes represent time points after MeJA treatment and relative gene expression values normalized to reference gene TIP41, respectively. Bars indicate standard deviations from three biological replicates.
3. Discussion
3.1. UBPs in Moso Bamboo
The eukaryotic-specific UBP family, which is one of the largest families of DUBs determined to date, plays an essential role in the processes of plant growth and development [40]. According to a previous study, detailed characteristics and functions of UBP genes have been uncovered in A. thaliana [7]. However, until now, the UBP family members have not been described in moso bamboo. Here, we identified 48 putative UBP genes in moso bamboo using a genome-wide analysis and compared them with 27 AtUBPs, 25 OsUBPs, and 32 BdUBPs. The greater number of PeUBP genes in moso bamboo among these four species was consistent with a genome duplication event occurring in moso bamboo [24,41]. Based on the phylogenetic analysis, the predicted PeUBP gene family was classified into 14 groups (G1–G13, G15). Among them, G1 to G13 shared the same type of domains as those in the corresponding groups of UBP genes in A. thaliana, while PH02Gene47007.t1 and PH02Gene45815.t1 in G15 contained a special DUF4220 domain that only existed in moso bamboo and B. distachyon. In addition, there were no orthologs genes in moso bamboo G14, suggesting a divergence among A. thaliana, rice, B. distachyon, and moso bamboo.
3.2. Divergence of UBPs in Moso Bamboo, Rice, and Brachypodium
Gene duplication events help organisms adapt to variant environments during development and growth, and are also essential for gene evolution and expansion [42,43]. To better explore the macroevolutionary patterns and evolutionary rates in moso bamboo, we analyzed the Ks and Ka models of paralogous genes (Pe-Pe) and orthologous genes (Pe-Os and Pe-Bd) and calculated the Ks and Ka/Ks values for each gene pair. The Ks values indicated that a large-scale duplication event occurred approximately 15 MYA in moso bamboo and that the divergence times for orthologous genes (Pe-Os and Pe-Bd) were both approximate 27 MYA. A previous report showed that a whole-genome duplication event occurred 7–12 MYA in moso bamboo. The divergence time between moso bamboo and rice was 48.6 MYA, while that of moso bamboo and B. distachyon was 46.9 MYA [22]. In addition, the Ka/Ks ratio can be used to determine whether selective pressure acts on the protein-coding gene. Here, the Ka/Ks ratios were all less than 1, which indicated that the PeUBP homologous gene pairs have undergone purification selection during the evolutionary process [30,31].
3.3. The Potential Functions of UBPs in Development and Stress-Responses in Moso Bamboo
The analysis of UBP gene expression profiles in different tissues contributed to understanding gene functions in moso bamboo growth and development. Here, we verified the expression levels of 48 putative PeUBP genes in different tissues (leaf, stem, rhizome, and root). Most PeUBP genes were highly expressed in leaf, suggesting that PeUBPs might be involved in leaf growth and development. However, the high expression level in leaf might result from the faster growth and development, which requires more active metabolism or gene activities. A few PeUBP genes showed tissue-specific expression in moso bamboo. For example, PH02Gene43669.t1—the orthologue of AtUBP14—showed high expression levels in leaf and root, indicating that it plays a role in leaf and root development, similar to AtUBP14, which controls leaf size and root hair elongation [44]. PH02Gene00721.t1, PH02Gene06421.t1, PH02Gene12835.t1, PH02Gene26126.t1, PH02Gene33419.t1, and PH02Gene43669.t1, the orthologues of AtUBP12 and AtUBP13, were highly expressed in the root, indicating that these genes might function in moso bamboo root development, coinciding with the functions of AtUBP12 and AtUBP13 in Arabidopsis root meristem maintenance and development [33,34].
Tissue-specific and stress-responsive gene expression patterns are largely dependent upon cis-elements in the promoter region. Additionally, the cis-regulatory elements are also closely related to multiple stimuli-responsive genes [27,40]. In our study, we analyzed the cis-elements of PeUBPs and found many MeJA-, ABA-, and SA- responsive sequences in PeUBP promoters, indicating the significant roles of PeUBPs in MeJA-, ABA-, and SA- stress responses [45].
The three plant hormones (ABA, MeJA, and SA) have established a good role in plant stress response signaling systems, growth, and development processes. To date, the associations of plant UBP with ABA and MeJA have been uncovered. ABA is produced in dehydrated plant tissues and mature seeds under water deficiency and regulates the expression levels of many genes that take part in drought tolerance [46]. A report [23] showed that AtUBP24 functions upstream of ABI2, and negatively regulates phosphatase activity of PP2C, demonstrating the regulatory role of AtUBP24 in response to ABA in plants. As the homologous gene of AtUBP24 in moso bamboo, the PH02Gene13480.t1 has the same protein structure and conserved domains. Its expression pattern is different from AtUBP24 [23]. However, the PH02Gene13480.t1 showed high ABA sensitivity in our experiments, implying that PH02Gene13480.t1 possibly has a similar role in the ABA-stress response. As a possible air signaling molecule, MeJA plays an important role in regulating communication within and between plants and in regulating plant defense responses, including antioxidant systems [47]. AtUBP12/AtUBP13 positively regulate MYC2 levels in MeJA responses [48] and are involved in plant immunity, circadian clock, and root meristem maintenance [32,34]. Their homologous gene in moso bamboo, PH02Gene33419.t1, had the same conserved UCH and MATH domains and showed high expression levels in the young leaf and root, similarly to AtUBP12/AtUBP13 [48]. In addition, the PH02Gene33419.t1 expression level rises sharply after the MeJA treatment, indicating that PH02Gene33419.t1 may play an important role in MeJA responses, similarly to AtUBP12 and AtUBP13 [48].
4. Materials and Methods
4.1. Identification of Moso Bamboo PeUBP Genes
The UBP protein sequences were originally used as seed sequences to search the Bamboo database (http://parrot.genomics.cn). Then the redundant sequences were removed based on the BLAST results of a ClustalW 2.1 alignment [49], and the putative PeUBP members were confirmed using Pfam (http://pfam.xfam.org/) and the NCBI CD-search program (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) [50,51]. The information on the amino acids, CDS lengths, and physicochemical parameters of the PeUBP genes were obtained from the Bamboo database (http://parrot.genomics.cn). By comparing the CDSs and the corresponding genomic DNA sequences of PeUBP genes, we obtained their exon-intron structures from GSDS (http://gsds.cbi.pku.edu.cn/). Bioinformatic analysis of PeUBP genes was performed using ExPASy (http://www.expasy.ch/tools/pi_tool.html) to determine the number of amino acids in the open reading frame (ORF), molecular weight (MW), isoelectric point (pI), and length of the open reading frame (length) for each gene [45].
4.2. PeUBP Gene Alignments and Phylogenetic Analysis
Four data sets were used for the phylogenetic analysis. In total, 27 Arabidopsis UBP protein sequences were downloaded from TAIR (https://www.arabidopsis.org/), and 25 rice UBP protein sequences and 32 Brachypodium UBP protein sequences were downloaded from the Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) to use as references. A multiple alignment of all the sequences was performed using W algorithm integration [52]. The Neighbor-joining method was used to construct a phylogenetic tree by MEGA 6.0 with bootstrap values calculated using 1000 replicates, and p-distance methods were used with the pairwise deletion option to address gaps in the amino acid sequences [53].
4.3. Gene Structure Analysis
The intron-exon structures of each gene were mapped to their corresponding genomic sequences. The intron-exon structures were determined by comparing CDSs with their corresponding genomic DNA sequences, and schematics were generated using the GSDS v2.0 (http://gsds.cbi.pku.edu.cn/) [54].
4.4. Protein Structure Analyses
We used the Phyre2 website (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index) to predict protein homology models, and applied Hidden Markov Models (HMM) to the alignment of the UBP protein sequences using the HMM–HMM search in intensive mode [55]. Conserved motifs present in the PeUBP proteins were identified with the online MEME tool (http://meme-suite.org/tools/meme), with the following parameter settings: maximum number of motifs—10, and maximum width—50.
4.5. Calculation of Ka/Ks Values
Ka/Ks values mean the ratio of the number of nonsynonymous substitutions per nonsynonymous site (Ka) to the number of synonymous substitutions per synonymous site (Ks). Ka and Ks were calculated using KaKs Calculator 2.0 with the NY model [56] based on the pairwise alignment of paralogous and orthologous pairs between moso bamboo, and both rice and Brachypodium. Clustal W 2.1 was used to study the gene duplication events. The divergence time (T) was calculated according to T = Ks/2 λ (λ = 6.5 × 10−9) by converting the date of duplication events for moso bamboo, rice, and Brachypodium [24].
4.6. Putative Promoter Region Analysis of PeUBP Genes
The 2000-bp upstream sequences of the PeUBP genes were chosen to identify the cis-elements in the putative promoter regions. We used the PlantCARE (http://www.dna.affrc.go.jp/PLACE/) to predict the putative cis-regulatory elements present in the promoter sequences [57,58]. Then, we screened out cis-elements that responded to ABA, MeJA, and SA stress.
4.7. Plant Material and Growth Conditions
THE 90-d-old moso bamboo seeds (from Gongcheng Yao Autonomous County, Guangxi Zhuang Autonomous Region, China) were germinated and grown in an artificial growth chamber under long daylight conditions (16-h light/8-h dark) maintained at 28 °C and 80% relative humidity. These seedlings were used in experiments to analyze gene expression levels in response to three stress treatments when they were 3 months of age. To study the expression patterns of PeUBP genes under stress-related hormone treatments, the young leaves were sprayed individually with 20 μM ABA, 100 μM MeJA, or 1 mM SA. The young leaves sprayed with distilled water were used as negative controls. The leaves of the hormone-treated plants were collected at 0, 3, 6, 12, and 24 h and all the samples were rapidly frozen in liquid nitrogen and stored at −80 °C prior to RNA extraction.
4.8. PeUBP Expression Levels in Different Tissues
The samples (leaf, root, stem, and rhizome) were collected from a 90-d-old moso bamboo. Leaf, root, stem, and rhizome refer to the young leaves, the first three tender stems, stems with roots in the last two segments, and roots growing in the soil, respectively. Then, we determined a comprehensive expression profile for each PeUBP genes, using the Tonoplast intrinsic protein 41 (TIP41) gene as an internal standard [59,60]. The heatmap of PeUBP gene expression was constructed using R studio for four moso bamboo tissues (leaf, root, stem, and rhizome).
4.9. RNA Isolation and qRT-PCR
The qRT-PCR was performed using 48 genes based on their similarity levels to the reference genes in the phylogenetic tree. The primers of qRT-PCR of the 48 PeUBP genes are listed in Table S4. Total RNA was isolated from samples of each tissue using an RNAprep Pure Plant Kit (TransGen Biotech, Beijing, China) according to the user manual. Total RNAs were used for complementary cDNA synthesis using SuperScript III transcriptase (Invitrogen, Carlsbad, American) in accordance with the manufacturer’s instructions [61]. The qRT-PCR analysis was performed on a Bio-Rad CFX96 using the Light Cycler 480 SYBR Green Master Mix (TaKaRa, Dalian, China). The PCR reaction conditions were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, and 60 °C for 30 s. The expression values of the individual genes were normalized using the expression level of TIP41 as an internal standard [60]. The mean expression values and SE values were calculated from the results of three independent experiments.
5. Conclusions
In this study, 48 UBP family genes in moso bamboo were identified and characterized by the systematic analyses of a phylogenetic tree, protein structure, gene structure, structural domains, and divergence time, which indicated a complex evolutionary history for this family in moso bamboo. The expression profiles of UBP genes indicated that these genes play pivotal roles in stress responses, as well as growth and development. This is a comprehensive study of moso bamboo UBP genes that may aid in the selection of appropriate candidate genes for further cloning and functional analyses in moso bamboo.
Acknowledgments
We thank Lianfeng Gu, from the Fujian Agriculture and Forestry University, for kindly providing the seeds of moso bamboo; We thank Kaihua Jia and Jianfeng Mao, from the College of Biological Sciences and Technology, Beijing Forestry University, for providing technical support. We also thank Lesley Benyon, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Abbreviations
| UBPs | Ubiquitin-specific proteases |
| DUBs | Deubiquitinases/Deubiquitinating enzymes |
| ABA | Abscisic acid |
| MeJA | Methyl jasmonate |
| SA | Salicylic acid |
| E1 | Ubiquitin activating enzyme |
| E2 | Ubiquitin conjugating enzyme |
| E3 | Ubiquitin ligase= |
| UCH | Carboxyl-terminal hydrolase |
| Cys | Cysteine |
| His | Histidine |
| DUSP | Domain present in UBP |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/17/4309/s1.
Author Contributions
Conceptualization, R.W.; Formal analysis, R.W.; Investigation, C.L., and L.D.; Methodology, Y.S.; W.Z.; and S.C. Project administration, C.L., and L.D.; Software, R.W.; Y.S. and Q.Z.; Writing—original draft, R.W.
Funding
This study was financially supported by the National Key Research and Development Program of China (Grant No. 2018 YFD0600101), the Fundamental Research Funds for the Central Universities [Grant No. BLX201418], and the National Natural Science Foundation of China [Grant No. 31501168].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Clague M., Coulson J., Urbe S. Cellular functions of the DUBs. J. Cell Sci. 2012;125:277–286. doi: 10.1242/jcs.090985. [DOI] [PubMed] [Google Scholar]
- 2.Isono E., Nagel M. Deubiquitylating enzymes and their emerging role in plant biology. Front. Plant Sci. 2014;5:56. doi: 10.3389/fpls.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickart C. Back to the future with ubiquitin. Cell. 2004;116:181–190. doi: 10.1016/S0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- 4.Vierstra R. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009;10:385–397. doi: 10.1038/nrm2688. [DOI] [PubMed] [Google Scholar]
- 5.Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1999;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 6.Atanassov S., Koutelou E., Dent Y. The role of deubiquitinating enzymes in chromatin regulation. FEBS Lett. 2011;585:2016–2023. doi: 10.1016/j.febslet.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H., Zhao J., Cai J., Patil S. Ubiquitin-specific proteases function in plant development and stress responses. Plant Mol. Biol. 2017;94:565–576. doi: 10.1007/s11103-017-0633-5. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson K. Ubiquitination and deubiquitination: Targeting of proteins for degradation by the proteasome. Semin Cell Dev. Biol. 2000;11:141. doi: 10.1006/scdb.2000.0164. [DOI] [PubMed] [Google Scholar]
- 9.Katz E., Isasa M., Crosas B. A new map to understand deubiquitination. Biochem. Soc. Trans. 2010;38:21–28. doi: 10.1042/BST0380021. [DOI] [PubMed] [Google Scholar]
- 10.Neutzner M., Neutzner A. Enzymes of ubiquitination and deubiquitination. Essays Biochem. 2012;52:37–50. doi: 10.1042/bse0520037. [DOI] [PubMed] [Google Scholar]
- 11.Varshavsky A. The ubiquitin system. Annu. Rev. Biochem. 1998;22:383–387. doi: 10.1016/S0968-0004(97)01122-5. [DOI] [Google Scholar]
- 12.Yan N., Doelling J., Falbel T., Durski A., Vierstra R. The ubiquitin-specific proteases family from Arabidopsis. AtUBP1 and 2 are required for the resistance to the amino acid analog canavanine. Plant Physiol. 2000;124:1828–1843. doi: 10.1104/pp.124.4.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Wang F., Zhang H., He H., Ma L., Deng X. Functional characterization of the Arabidopsis ubiquitin-specific protease gene family reveals specific role and redundancy of individual members in development. Plant J. 2008;55:844–856. doi: 10.1111/j.1365-313X.2008.03557.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson K. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997;11:1245–1256. doi: 10.1096/fasebj.11.14.9409543. [DOI] [PubMed] [Google Scholar]
- 15.Moon Y., Hong J., Cho Y., Yang S., An G., Kim W. Structure and expression of OsUBP6, an ubiquitin-specific protease 6 homolog in rice (Oryza sativa L.) Mol. Cells. 2009;28:463–472. doi: 10.1007/s10059-009-0138-4. [DOI] [PubMed] [Google Scholar]
- 16.Du L., Li N., Chen L., Xu Y., Li Y., Zhang Y., Li C., Li Y. The ubiquitin receptor DA1 regulates seed and organ size by modulating the stability of the ubiquitin-specific protease UBP15/SOD2 in Arabidopsis. Plant Cell. 2014;26:665–677. doi: 10.1105/tpc.114.122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., Jin W., Li N., Zhang W., Liu C., Li C., Li Y. Ubiquiitin-specific protease 14 interacts with ultiraviolet-B insensitive 4 to regulate endoreduplication and cell and organ growth in Arabidopsis. Plant Cell. 2016;28:1200–1214. doi: 10.1105/tpc.16.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doelling J., Yan N., Kurepa J., Walker J., Vierstra R. The ubiquitin-specific protease UBP14 is essential for early embryo development in Arabidopsis thaliana. Plant J. 2001;27:393–405. doi: 10.1046/j.1365-313X.2001.01106.x. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Zheng L., Corke F., Smith C., Bevan M. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev. 2008;22:1331–1336. doi: 10.1101/gad.463608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan F., Wu M., Hu W., Liu R., Yan H., Xiang Y. Genome-wide identification and expression analyses of the bZIP transcription factor genes in moso bamboo (Phyllostachys edulis) Int. J. Mol. Sci. 2019;20:2203. doi: 10.3390/ijms20092203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton A., Stes E., Cybulski N., Van Bel M., Iñigo S., Durand A.N., Timmerman E., Heyman J., Pauwels L., De Veylder L., et al. It’s time for some “site”-seeing: novel tools to monitor the ubiquitin landscape in Arabidopsis thaliana. Plant Cell. 2016;28:6–16. doi: 10.1105/tpc.15.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassrallah A., Rougée M., Bourbousse C., Drevensek S., Fonseca S., Iniesto E., Ait-Mohamed O., Deton-Cabanillas A.F., Zabulon G., Ahmed I., et al. DET1-mediated degradation of a SAGA-like deubiquitination module controls H2Bub homeostasis. Elife. 2018;7:1–19. doi: 10.7554/eLife.37892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J., Zhou H., Zhang M., Gao Y., Li L., Gao Y., Li M., Yang Y., Guo Y., Li X. Ubiquitin-specific protease 24 negatively regulates abscisic acid signaling in Arabidopsis thaliana. Plant Cell Environ. 2016;39:427–440. doi: 10.1111/pce.12628. [DOI] [PubMed] [Google Scholar]
- 24.Peng Z., Lu Y., Li L., Zhao Q., Feng Q., Gao Z., Lu H., Hu T., Yao N., Liu K. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla) Nat. Genet. 2013;45:456–461. doi: 10.1038/ng.2569. [DOI] [PubMed] [Google Scholar]
- 25.Wu H., Lv H., Li L., Liu J., Mu S., Li X., Gao J. Genome-wide analysis of the AP2/ERF transcription factors family and the expression patterns of DREB genes in moso bamboo (Phyllostachys edulis) PLoS ONE. 2015;10:e0126657. doi: 10.1371/journal.pone.0126657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gui Y., Zhou Y., Wang Y., Wang S., Wang S., Hu Y., Bo S., Chen H., Zhou C., Ma N., et al. Insights into the bamboo genome: Syntenic relationships to rice and sorghum. Chin. Bull. Bot. 2010;52:1008–1015. doi: 10.1111/j.1744-7909.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 27.Amerik A., Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta. 2004;1695:189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Wang D., Song W., Wei S., Zheng Y., Chen Z., Han J., Zhang H., Luo J., Qin Y., Xu Z., et al. Characterization of the ubiquitin C-terminal hydrolase and ubiquiitin-specific protease families in rice (Oryza sativa) Front Plant Sci. 2018;15:1636. doi: 10.3389/fpls.2018.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soding J. Protein homology detection by HMM-HMM comparison. Bioinformatics. 2005;21:951–960. doi: 10.1093/bioinformatics/bti125. [DOI] [PubMed] [Google Scholar]
- 30.Cannon B., Mitra A., Baumgarten A., Young D., May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4:10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiu S., Karlowski W., Pan R., Tzeng Y., Mayer K., Li W. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;16:1220–1234. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An Z., Liu Y., Ou Y., Li J., Zhang B., Sun D., Sun Y., Tang W. Regulation of the stability of RGF1 receptor by the ubiquitin-specific proteases UBP12/UBP13 is critical for root meristem maintenance. Proc. Natl. Acad. Sci. USA. 2018;115:1123–1128. doi: 10.1073/pnas.1714177115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui X., Lu F., Li Y., Xue Y., Kang Y., Zhang S., Qiu Q., Cui X., Zheng S., Liu B., et al. Ubiquitin-specific proteases UBP12 and UBP13 act in circadian clock and photoperiodic flowering regulation in Arabidopsis. Plant Physiol. 2013;162:897–906. doi: 10.1104/pp.112.213009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derkacheva M., Liu S., Figueiredo D., Gentry M., Mozgova I., Nanni P., Tang M., Mannervik M., Köhler C., Hennig L. H2A deubiquitinases UBP12/13 are part of the Arabidopsis polycomb group protein system. Nat. Plants. 2016;2:16126. doi: 10.1038/nplants.2016.126. [DOI] [PubMed] [Google Scholar]
- 35.Chico J., Fernandez-Barbero G., Chini A., Fernandez-Calvo P., Diez-Diaz M., Solano R. Repression of jasmonate-dependent defenses by shade involves differential regulation of protein stability of MYC transcription factors and their JAZ repressors in Arabidopsis. Plant Cell. 2014;26:1967–1980. doi: 10.1105/tpc.114.125047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii H., Chinnusamy V., Rodrigues A., Rubio S., Antoni R., Park S., Cutler S., Sheen J., Rodriguez P., Zhu J. In vitro reconstitution of an abscisic acid signaling pathway. Nature. 2009;462:660–664. doi: 10.1038/nature08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan M., Mehar F., Per S., Anjum N., Khan N. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015;6:642. doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le D., Nishiyama R., Watanabe Y., Vankova R., Tanaka M., Seki M., Yamaguchi-Shinozaki K., Shinozaki K., Tran L. Identification and expression analysis of cytokinin metabolic genes in soybean under normal and drought conditions in relation to cytokinin levels. PLoS ONE. 2012;7:e42411. doi: 10.1371/journal.pone.0042411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walther D., Brunnemann R., Selbig J. The regulatory code for transcriptional response diversity and its relation to genome structural properties in A. thaliana. PLoS Genet. 2007;3:e11. doi: 10.1371/journal.pgen.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eduardo M., Sara F. Plant deubiquitinases and their role in the control of gene expression through modification of histones. Front Plant Sci. 2014;8:2274. doi: 10.3389/fpls.2017.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Opanowicz M., Vain P., Draper J., Parker D., Doonan H. Brachypodium distachyon: Making hay with a wild grass. Trends Plant Sci. 2008;13:172–177. doi: 10.1016/j.tplants.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Bowers E., Chapman A., Rong J., Paterson H. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 2003;422:433–438. doi: 10.1038/nature01521. [DOI] [PubMed] [Google Scholar]
- 43.Gu Z., Steinmetz L., Gu X., Li W. Role of duplicate genes in genetic robustness against null mutations. Nature. 2003;421:63–66. doi: 10.1038/nature01198. [DOI] [PubMed] [Google Scholar]
- 44.Li W.F., Perry P.J., Prafulla N.N., Schmidt W. Ubiquitin-specific protease 14 547 (UBP14) is involved in root responses to phosphate deficiency in Arabidopsis. Mol. Plant. 2010;3:212–223. doi: 10.1093/mp/ssp086. [DOI] [PubMed] [Google Scholar]
- 45.Liu H., Wu M., Li F., Gao Y., Chen F., Xiang Y. TCP transcription factors in moso bamboo (Phyllostachys edulis): Genome-wide identification and expression analysis. Front Plant Sci. 2018;9:1263. doi: 10.3389/fpls.2018.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchishinozaki K., Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005;10:88–94. doi: 10.1016/j.tplants.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Reyes-Díaz M., Tomas L., Liliana C., Adriano N.-N., Jorge R., Laura J., Miren A., Alejandra R.-F. Methyl jasmonate: An alternative for improving the quality and health properties of fresh fruits. Molecules. 2016;21:567. doi: 10.3390/molecules21060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong J., Jung C., Seo J., Kim J., Chua N. The deubiquitinating enzymes UBP12 and UBP13 positively regulate MYC2 levels in jasmonate responses. Plant Cell. 2017;29:1406–1424. doi: 10.1105/tpc.17.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson J., Gibson T., Plewniak F., Jeanmougin F., Higgins D. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finn R.D., Mistry J., Schuster-Böckler B., Griffiths-Jones S., Hollich V., Lassmann T., Moxon S., Marshall M., Khanna A., Durbin R., et al. Pfam: Clans, web tools and services. Nucleic Acids Res. 2006;34:D247–D251. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finn R.D., Bateman A., Clements J., Coggill P., Eberhardt R.Y., Eddy S.R., Heger A., Hetherington K., Holm L., Mistry J., et al. Pfam: The protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao H., Lou Y., Sun H., Li L., Wang L., Dong L., Gao Z. Transcriptome and comparative gene expression analysis of Phyllostachys edulis in response to high light. BMC Plant Biol. 2016;16:34. doi: 10.1186/s12870-016-0720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou D., Cheng Z., Xie L., Li X., Li J., Mu S., Gao J. The R2R3 MYB gene family in Phyllostachys edulis: Genome-wide analysis and identification of stress or development-related R2R3MYBs. Front. Plant Sci. 2018;9:738. doi: 10.3389/fpls.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J. The Phyre2 web portal for protein modeling, prediction, and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D., Zhang Y., Zhang Z., Zhu J., Yu J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinf. 2010;8:77–80. doi: 10.1016/S1672-0229(10)60008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higo K., Ugawa Y., Iwamoto M., Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lescot M., Déhais P., Thijs G., Marchal K., Moreau Y., Van D., Rouze P., Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He Y., Mao S., Gao Y., Wu D., Cui Y., Li J., Qian W. Genome-wide identification and expression analysis of WRKY transcription factors under multiple stresses in Brassica napus. PLoS ONE. 2016;11:e0157558. doi: 10.1371/journal.pone.0157558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu M., Li Y., Chen D., Liu H., Zhu D., Xiang Y. Genome-wide identification and expression analysis of the IQD gene family in moso bamboo (Phyllostachys edulis) Sci. Rep. 2016;6:24520. doi: 10.1038/srep24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan C., Ma J., Guo Q., Li X., Wang H., Lu M. Selection of reference genes for quantitative real-time PCR in bamboo (Phyllostachys edulis) PLoS ONE. 2013;8:e56573. doi: 10.1371/journal.pone.0056573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.