Abstract
Trichoderma reesei is a biotechnologically important filamentous fungus with the remarkable ability to secrete large amounts of enzymes, whose production is strongly affected by both the carbon and nitrogen sources. While the carbon metabolism regulators are extensively studied, the regulation of enzyme production by the nitrogen metabolism regulators is still poorly understood. In this study, the GATA transcription factor Are1, which is an orthologue of the Aspergillus global nitrogen regulator AREA, was identified and characterized for its functions in regulation of both protease and cellulase production in T. reesei. Deletion of the are1 gene abolished the capability to secrete proteases, and complementation of the are1 gene rescued the ability to produce proteases. Quantitative RT-PCR analysis revealed that the transcripts of protease genes apw1 and apw2 were also significantly reduced in the Δare1 strain when grown in the medium with peptone as the nitrogen source. In addition, deletion of are1 resulted in decreased cellulase production in the presence of (NH4)2SO4. Consistent with the reduction of cellulase production, the transcription levels of the major cellulase genes, including cbh1, cbh2, egl1, and egl2, were dramatically decreased in Δare1. Sequence analysis showed that all promoter regions of the tested protease and cellulase genes contain the consensus GATA elements. However, the expression levels of the major cellulase transcription activator Xyr1 and the repressor Cre1 had no significant difference between Δare1 and the parental strain QM9414, indicating that the regulatory mechanism deserves further investigation. Taken together, these results demonstrate the important role of Are1 in the regulation of protease and cellulase production in T. reesei, although these processes depend on the kind of nitrogen sources. The findings in this study contribute to the understanding of the regulation network of carbon and nitrogen sources in filamentous fungi.
Keywords: Trichoderma reesei, GATA factors, Are1, Cellulase, Protease
1. Introduction
Filamentous fungi have the excellent capacity to secrete a wide range of enzymes, such as cellulase and protease, involved in the degradation and recycling of complex biopolymers from organisms [1,2]. These hydrolytic enzymes play an important role in nutrition intake for fungi by releasing carbon and nitrogen locked in insoluble macromolecules [3]. Fungi have developed efficient and sensitive genetic regulatory systems, which were highly depended on transcription factors, to enable them to synthesize suitable enzymes for rapidly responding to the fluctuating carbon and nitrogen sources in the environment [4]. So far, many different types of transcription factors, which are involved in regulating the production of secreted enzymes, have been identified in fungi [5,6,7,8,9,10,11].
The filamentous fungus Trichoderma reesei has the capacity to secrete large amounts of cellulolytic enzymes for deconstruction of plant biomass substrates [12]. The expression of cellulase genes is tightly blocked by the carbon catabolite repression (CCR), which was mediated by the repressor Cre1, in the presence of the preferred carbon sources, such as glucose [5]. While, the transcripts of the cellulase genes were activated by the transcription activator Xyr1 in the presence of the non-preferred carbon sources, such as Avicel [9]. However, two recent findings have suggested that among the over 400 CAZy genes in T. reesei, only a few genes appear to be directly regulated by Xyr1 and Cre1 [13,14]. According to these reports, it can be inferred that additional regulatory factors that regulate the expression of cellulase genes should be found in the genome of T. reesei.
Like the CCR that is the important mechanism employed in the utilization of carbon, NMR (nitrogen metabolism repression) is a global regulatory mechanism that modulates the expression of catabolic genes in response to available nitrogen sources in the environment [15]. In filamentous fungi, the global response to limiting nitrogen conditions is mediated by positively acting GATA-type zinc finger proteins, of which AREA from Aspergillus nidulans and NIT2 from Neurospora crassa are the most extensively characterized [16,17]. Another zinc finger protein, AREB, possibly acts as a potential negative regulator of nitrogen catabolism under nitrogen-limiting conditions and may be involved in fungal growth, conidial germination, and asexual development [18]. Particularly, the roles of AREA homologues go beyond the regulation of the pathway involved in nitrogen utilization [19]. AREA homologues are required for the expression of genes involved in the biosynthesis of secondary metabolites, such as aflatoxin in Aspergillus flavus [20], gibberellin in Gibberella fujikuroi [21], and deoxynivalenol in Fusarium graminearum [22,23]. AREA homologues are also involved in chromatin accessibility and are essential for the full virulence of some plant pathogenic fungi [24,25,26,27]. Furthermore, AREA homologues can activate the expression of protease genes in Candida albicans and probably participate in the regulation of cellulase expression in A. nidulans [28,29,30]. Given the importance of T. reesei as a major producer of commercial cellulase product and the absence of research published concerning the function of GATA-type zinc finger regulators in T. reesei, it is of great significance to investigate the roles of AREA/NIT2 orthologues in T. reesei.
Here, the GATA-type transcriptional activator Are1, which regulates the transcription of extracellular enzyme genes, was identified and characterized in T. reesei. Deletion and complementation studies of the are1 gene demonstrated that the are1 is absolutely necessary for activating the expression of extracellular proteases in T. reesei in the presence of the nonpreferred nitrogen sources, such as skim milk. Furthermore, the cellulolytic ability and transcriptional level analysis also revealed that Are1 is involved in the regulation of cellulase expression in the presence of the preferred nitrogen sources, such as ammonium. These findings help to elucidate the regulation network of carbon and nitrogen sources in filamentous fungi.
2. Results
2.1. Generation of the are1, are2, and are3 Gene-Deletion Strains in T. reesei
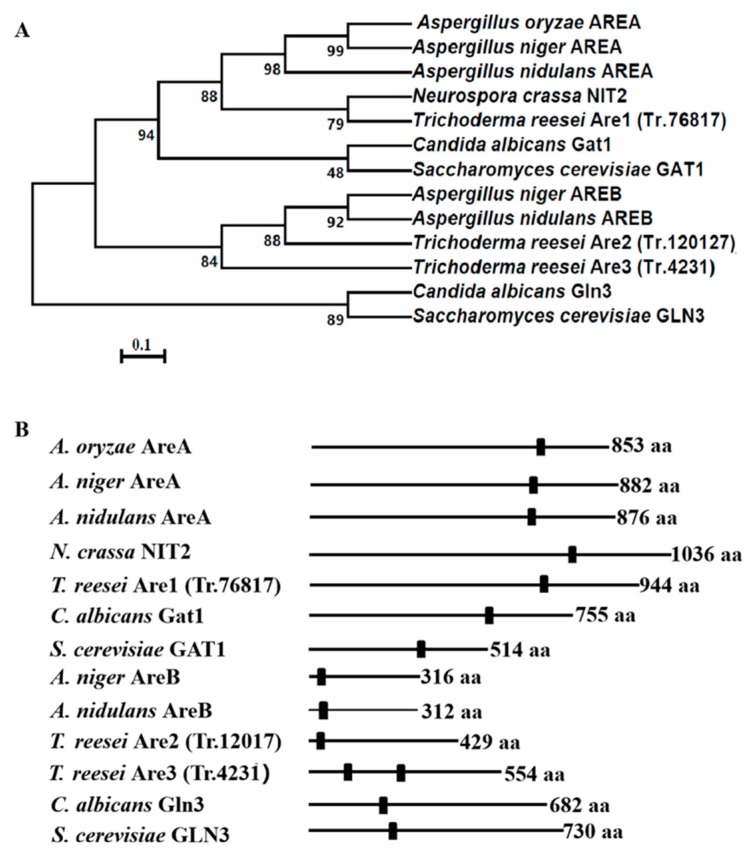
Inspection of the T. reesei genome database (available online: https://genome.jgi.doe.gov/Trire2/Trire2.home.html) with TBLASTX using the classic GATA-type transcription factor, Aspergillus nidulans AREA (GenBank accession no. CAA36731.1), as the query revealed three orthologues, which were named as Are1 (protein ID: tre76817), Are2 (protein ID: Tr_12017), and Are3 (protein ID: Tr_4231), respectively. The Are1-encoding gene are1 contains a predicted 2835-bp open reading frame that is interrupted by two introns and encodes a protein of 944 amino acids, whereas Are2 and Are3 consist of putative 429 and 554 amino acids, respectively. The phylogenetic analysis of AREA/NIT2-related orthologues from different fungal species showed that the T. reesei Are1 is most close to NIT2 of N. crassa, while Are2 and Are3 are most similar to AREB of A. nidulans (Figure 1A). Further dissection of the amino acid sequences of these proteins demonstrated that they all contained the conserved Cys2-Cys2 zinc finger motif (C-X2-C-X17-C-X2-C) (Figure 1B and Figure S1A), indicating the putative conserved role of these orthologues in filamentous fungi. In order to further confirm that the T. reesei Are1 is an orthologue of AreA/NIT2, another phylogenetic analysis was performed focusing on the orthologues of AreA/NIT2 (Figure S1B). The T. reesei Are1, F. fujikuroi AreA, M. grisea NUT1, and N. crassa NIT2 form a well-supported clade, while the other AreA/NIT2 orthologues of filamentous fungi, including A. nidulans AreA, A. niger AreA, A. oryzae AreA, A. parasiticus AreA, P. chrysogenum NRE, and P. roqueforti Nmc fall within another supported clad. In addition, the transcription levels of are1 under various nitrogen sources were investigated. It was found that there was no significant difference (p > 0.05) in the are1 transcript formation between the preferred nitrogen substance (NH4)2SO4 and the non-preferred nitrogen source peptone or no nitrogen source (Figure S2), indicating that the transcription of are1 was possibly nitrogen source-independent.
Figure 1.
Homologous analysis of the GATA regulators Are1 (Trire2.76817), Are2 (Trire2.120127), and Are3 (Trire2.4231) in T. reesei. (A) Phylogenetic analysis applying the neighbor joining method, including Are1 orthologue sequences from distinct fungi. GenBank accession numbers for the orthologue proteins are as follows: C. albicans (Gat1, AAP50501.1 and Gln3, AORE29836.1); N. crassa (NIT2, P19212.2); A. oryzae (AREA, AAK08066.1); A. niger (AREA, CAA68196.1 and AREB, XP_001399365.2); A. nidulans (AREA, CAA36731.1 and AREB, AAG49353), and S. cerevisiae (GAT1, KZV11580.1 and GLN3, KZV11792.1). The bar marker indicates the genetic distance, which is proportional to the number of amino acid substitutions. (B) Domain architectures in the Are1 orthologues from different fungi. The GATA zinc-finger domain (Pfam: PF00320) is represented by black squares. The length of each protein sequence (in amino acids) is indicated in the right.
To investigate the function of Are1, Are2, and Are3 in T.reesei, the are1, are2, and are3 deletion strains were generated by homologous recombination, respectively (Figures S3A, S4A, and S5A). A total of four putative are1-deletion mutants were constructed and verified by PCR analysis (Figure S3B and Figure S3C). Then, one of these candidates, named as Δare1, was selected for a further Southern blot assay (Figure S3D). The EcoRI/KpnI-digested or EcoRI-digested genomic DNA was hybridized with the are1 probe and showed a 2.7 kb or 5.2 kb fragment for the Δare1 strain, respectively, while a 4.0 kb or 6.5 kb fragment was shown for the parental strain T. reesei QM9414, respectively (Figure S4C). These results suggested that the are1 gene was successfully deleted in T. reesei. Similarly, both of the Δare2 and Δare3 strains were constructed and verified by PCR (Figure S4B, Figure S5B, and Figure S5C). Then, the are1, are2, and are3 gene-deletion strains were used for further investigation.
2.2. Disruption of are1 Abolishes Protease Production in T. reesei
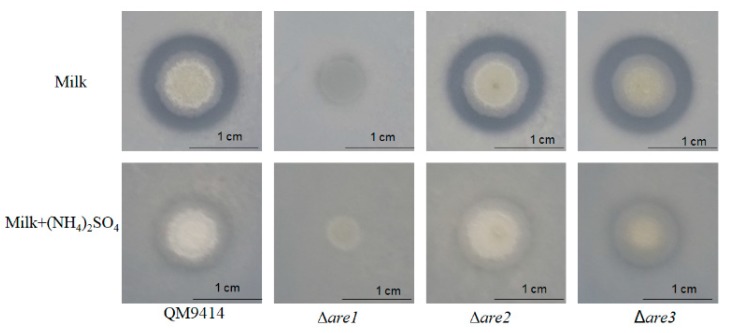
Previous studies showed that the production of extracellular proteases was tightly regulated by AREA/NIT2 in several fungi, such as C. albicans, and P.marneffei [27,28,29,31] and deletion of areA resulted in the disability to produce the extracellular proteases when the deletion strains were grown on the skim milk agar plates [31]. In order to identify whether Are1, Are2, or Are3 is involved in the regulation of the extracellular protease production in T. reesei, the parental strain QM9414 and the deletion strains (Δare1, Δare2, Δare3) were cultured on the skim milk plates. As shown in Figure 2, there was a clear proteolytic halo around the colony of QM9414 with skim milk as the sole nitrogen source, while there was no proteolytic halo around the colony of QM9414 in the presence of (NH4)2SO4, suggesting that the extracellular protease production in T. reesei was repressed by preferred nitrogen sources, such as (NH4)2SO4. Disruption of are2 or are3 still generated the proteolytic halo around the colony on the medium using skim milk as the sole nitrogen source, whereas Δare1 did not grow on this medium, suggesting that Are1 may play a critical role for the uptake of nitrogen on this medium.
Figure 2.
Growth of the are1, are2, and are3 deletion strains on the plates containing 1% skim milk with or without 0.5% (NH4)2SO4 as the nitrogen source. The clear halos surrounding the colonies are due to extracellular protease activity.
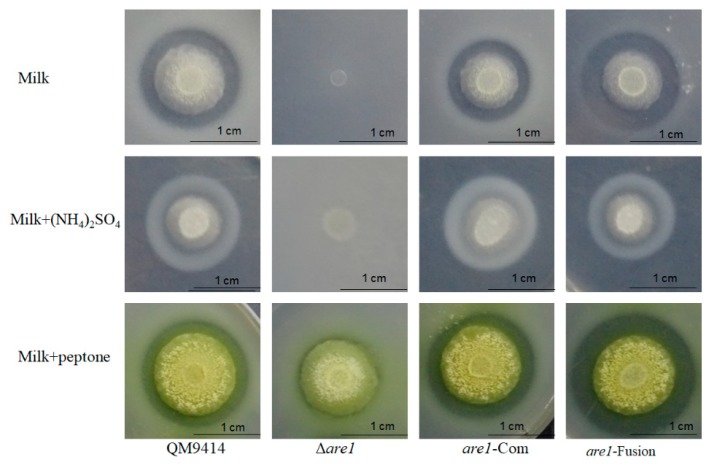
To determine if the re-introduction of Are1 to the T. reesei genome was sufficient to rescue the protease-defective phenotype in Δare1, the are1 complementation (are1-Com) and the PgpdA::are1 fusion (are1-Fusion) strains were constructed and confirmed by PCR analysis (Figure S6). Then, the ability of the are1-Com and the are1-Fusion strains to produce extracellular proteases in the skim milk medium was tested. As shown in Figure 3, there was a clear proteolytic halo around the colony of the are1-Com strain with skim milk as the sole nitrogen source, indicating that complementation of are1 with the are1 gene could restore the production of proteases on the skim milk medium. In addition, it was found that the are1-Fusion strain exhibited a larger hydrolytic halo around the colony than that of QM9414, suggesting that expression of are1 under the control of PgpdA could increase the production of proteases in T. reesei. Considering that Δare1 could not grow on the medium using skim milk as the sole nitrogen source, the Δare1 strain were cultured on the skim milk agar plate plus peptone as the complex nitrogen source to further determine whether the protease-defective phenotype was caused by the impaired growth. As shown in Figure 3, there was no proteolytic halo formed around the colony of Δare1 compared to that of the parental strain QM9414, indicating that the disability of Δare1 to produce proteases was not due to the defective growth, but to the absence of Are1. Taken together, these results demonstrated that Are1 has an essential function in protease production in T. reesei.
Figure 3.
The extracellular protease production by the are1 deletion strain Δare1, the are1 complementation strain are1-Com, the PgpdA::are1 fusion stain (are1-Fusion), and the parental strain QM9414. The strains were grown on the plates containing skim milk, skim milk plus (NH4)2SO4, or skim milk plus peptone as the nitrogen source.
2.3. Are1 is a Key Factor in Modulation of Protease Expression in T. reesei
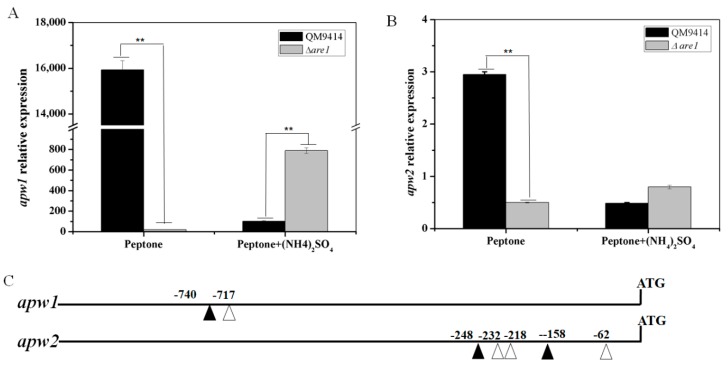
It has been shown that the aspartic proteases APW1 and APW2 may be the major proteases produced by T. reesei when exposed to the non-preferred nitrogen sources, such as BSA [32]. Here, the transcription level of apw1 in the T. reesei QM9414 cultured with peptone as the sole nitrogen source was increased by a fold-change of 156 with a p-value = 1.17 × 10−6 when compared to that with peptone plus (NH4)2SO4 (Figure 4A). In the same conditions, apw2 showed an increase by a fold-change of 5.9 with a p-value = 1.10 × 10−5 (Figure 4B), indicating that the expression of proteases in T. reesei was repressed by ammonium, which was consistent with the results obtained from the skim milk plates. When are1 was deleted, it was found that the transcription levels of apw1 and apw2 were decreased sharply in the Δare1 strain compared to that in QM9414 (a 99.87% decrease with a p-value = 1.16 × 10−6 for apw1 and an 83.51% decrease with a p-value = 1.16 × 10−6 for apw2) using peptone as the sole nitrogen source, demonstrating that Are1 positively regulated the expression of proteases in the non-preferred nitrogen sources, such as peptone (Figure 4). Moreover, the transcription of apw1 was up-regulated in the Δare1 strain when ammonium was added (Figure 4A), suggesting that deletion of are1 could relieve the repression of protease expression by ammonium. It was known that Are1 orthologues recognize the DNA motif, 5′-HGATAR-3′, located in the promoters of AREA-regulated genes [33]. As shown in Figure 4C, the promoter sequences of the protease-genes, apw1 and apw2, contain the conserved motif, indicating that Are1 may participate in the regulation of protease gene expression through directly binding to the protease gene promoters.
Figure 4.
The transcription levels and the putative cis-regulatory elements of the extracellular protease-encoding genes apw1 and apw2 in T. reesei. (A) and (B) RT-qPCR analysis of the transcript abundances of apw1 and apw2 in the T. reesei strains Δare1 and QM9414, respectively. The measured quantity of the qPCR results in each of the samples in (A) and (B) was normalized with the actin gene. Error bars indicate the standard deviation and ** shows a p-value < 0.01. (C) Putative Are1-binding motifs in the promoter regions (within 1000 bp) of apw1 and apw2. White triangle, HGATAR (where H stands for A, T or C, and R stands for A or G); Black triangle, YTATCD (where Y stands for T, G, or A, and D stands for T or C).
2.4. Cellulase Production is Reduced in the Δare1 Strain
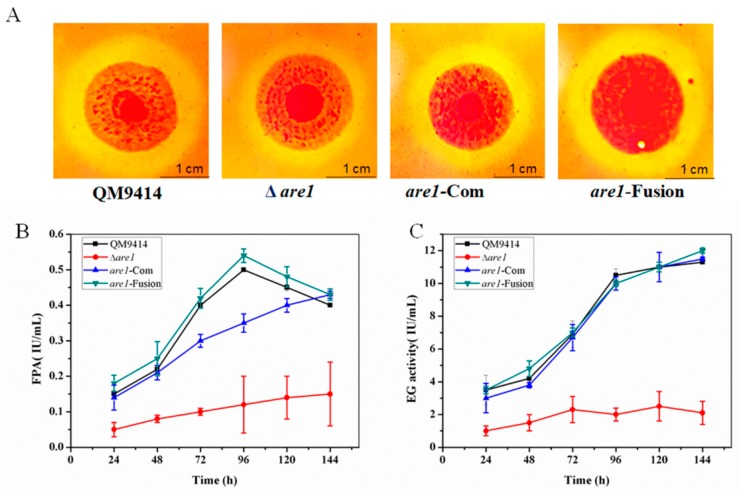
The filamentous fungus T. reesei has the ability to secrete large amounts of cellulolytic enzymes and thus is the main industrial source for cellulase production [12]. So, the effect of deletion of the are1 gene on cellulase production was evaluated. Firstly, the Δare1 strain was grown on the CMC plates containing sodium carboxymethyl cellulose (CMC-Na) as the sole carbon source to test its cellulolytic ability. It was found that the cellulolytic halo around the colony of Δare1 was much smaller than that of the parental strain QM9414, while introduction of a copy of are1 or expression of are1 under the control of PgpdA both restored the cellulolytic defect of Δare1 (Figure 5A), suggesting that Are1 may regulate the production of cellulase in T. reesei. To further examine the influence of are1 deletion on cellulase production, the Δare1, are1-Com, and are1- Fusion strains were cultured in cellulase-inducing medium (CM) at 30 for 7 days. Then, the fermentation supernatants were collected at a specified time interval and the activities of total cellulase (FPA) and endoglucanase (EG) were measured. As shown in Figure 5B,C, both FPA and EG activities were significantly decreased in Δare1 compared to that of the parental strain QM9414. Meanwhile, complementation and expression of are1 under the control of PgpdA completely restored the ability to produce cellulases. Therefore, these results suggested that Are1 also plays a role in the production of cellulases in T. reesei.
Figure 5.
The cellulase production by the are1 deletion strain Δare1, the are1 complementation strain are1-Com, the PgpdA::are1 fusion stain are1-Fusion, and the parental strain QM9414. (A) Detection of the cellulolytic ability of the T. reesei strains on the carboxymethyl cellulose (CMC) agar plates. (B) and (C) The FPA (the activities of total cellulase) activity and the EG (endoglucanase) activity of the T. reesei strains during fermentation for cellulase production, respectively. All the strains were cultured in the cellulase production medium (CPM) for 144 h.
2.5. Are1 is Involved in Regulation of the Expression of Cellulases in T. reesei
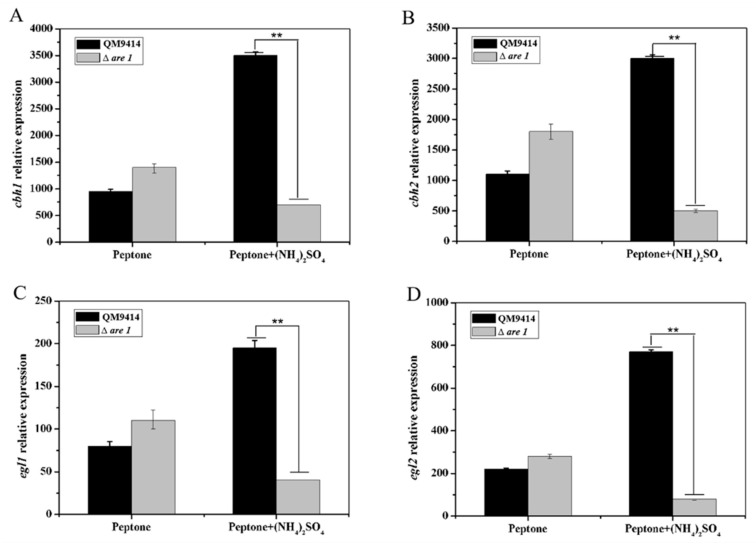
The fact that cellulase production was greatly decreased in the Δare1 strain made us to wonder whether are1 deletion leads to the down-regulated expression of the cellulase genes. Then, the transcription levels of the major cellulase genes cbh1, cbh2, egl1, and egl2 were investigated by RT-qPCR (Figure 6). It was discovered that the transcripts of cbh1, cbh2, egl1, and egl2 were significantly reduced in the Δare1 strain compared with the parental strain QM9414 in the presence of ammonium. However, there was no significant difference (with p > 0.05) in the transcription levels of cellulase genes between Δare1 and QM9414 when cultured in the medium containing the peptone as the sole nitrogen source (Figure 6). These results suggested that are1 possibly positively regulates the expression of cellulase genes in the preferred nitrogen source, such as ammonium.
Figure 6.
The transcription levels of the major cellulase genes cbh1 (A), cbh2 (B), egl1 (C), and egl2 (D) in the T. reesei strains Δare1 and QM9414. The strains were grown in CPM and RT-qPCR analysis was performed to detect the transcript abundance. Error bars indicate the standard deviation and ** shows a p-value < 0.01.
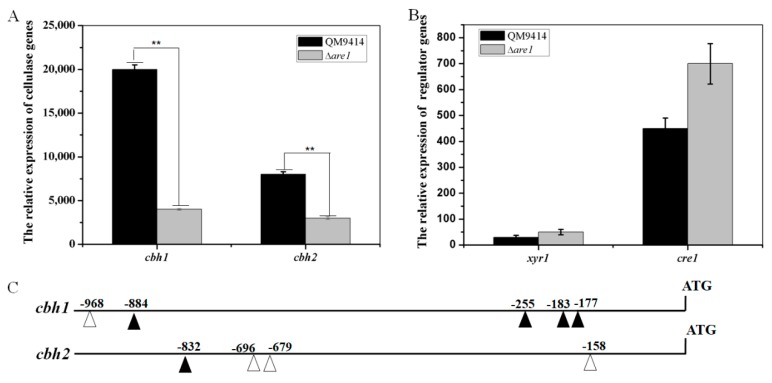
Considering peptone is an organic compound that could be used as both nitrogen and carbon sources and may somehow affect cellulase expression [34], then (NH4)2SO4 was chosen as the sole nitrogen source for cultivation of T. reesei to examine the effect of are1 deletion on cellulase-gene expression. As shown in Figure 7A, the deletion of are1 significantly decreased the expressions of cbh1 and cbh2 in Δare1. This result further demonstrated that Are1 may sense the preferred nitrogen source, such as ammonium, and act as a regulator to modulate cellulase gene expression. It was known that the expression of cellulase genes was positively regulated by the major cellulase regulator, Xyr1, and negatively regulated by the carbon catabolite repressor, Cre1, in T. reesei [5,9]. Here, to investigate whether Are1 modulates cellulase-gene transcription through these regulators, the transcript abundances of xyr1 and cre1 in the Δare1 strain were evaluated. As shown in Figure 7B, there were no remarkable differences (with p > 0.05) in the transcriptional levels of xyr1 and cre1 between Δare1 and the parental strain QM9414. Moreover, it was found that the promoter sequences of cbh1 and cbh2 also contained the Are1-binding motif, 5′-HGATAR-3′ (Figure 7C), indicating that Are1 may act as a regulator through binding to the cellulase gene promoters. Taken together, these results demonstrated that Are1 is also involved in regulation of the expression of cellulases in T. reesei.
Figure 7.
The possible regulation manner of the cellulase gene transcription by Are1 in T. reesei. (A) Validation of the transcription levels of the cellulase genes, cbh1 and cbh2, in the T. reesei strains, which were grown in the MM medium using 10 mM (NH4)2SO4 as the sole nitrogen source. (B) The transcription levels of the major cellulase transcription factors, Xyr1 and Cre1, in the T. reesei strains. RT-qPCR analysis was performed to detect the transcript abundance in (A) and (B). Error bars indicate the standard deviation and ** shows a p-value < 0.01. (C) Putative DNA binding sites of Are1 in promoter regions (within 1000 bp) of cbh1 and cbh2. White triangle, HGATAR (where H stands for A, T, or C and R stands for A or G); Black triangle, YTATCD (where Y stands for T, G, or A, and D stands for T or C).
3. Discussion
Nitrogen metabolite repression mediated by Are1 homologues is widely distributed among filamentous fungi [26,35]. In this study, we identified the nitrogen regulatory factor Are1 from T. reesei and investigated its function in regulation of both protease and cellulase production. The predicted protein Are1 shares extensive regions of homology with the known positive nitrogen regulatory proteins AREA of A. nidulans and NIT2 of N. crassa, which are highly conserved fungal GATA-type zinc finger proteins. Δare1 was unable to grow on medium containing skim milk as the sole nitrogen source, which was similar to the AreA mutant of A. nidulans and the NIT2 mutant of N. crassa [16,17], suggesting that Are1 is required for utilization of the non-preferred nitrogen in T. reesei. However, unlike the AreA and NIT2 mutants, the Δare1 strain showed a severely impaired growth on the medium with ammonium, indicating the different role of are1 on nitrogen utilization from AreA in A. nidulans and NIT2 in N. crassa. It is possible that deletion of are1 could affect the uptake of ammonium in T. reesei. In addition, Δare1 displayed a similar growth to that of the parental strain on the agar plates containing peptone as the nitrogen source. Considering peptone contains free amino acids, it is likely that are1 deletion had no effects on the transportation or metabolism of some amino acids in T. reesei. This is consistent with the results of Bugeja et al. (2012), who reported that AreA is not required for the utilization of some amino acids, such as glutamate and alanine, in P. marneffei [31].
Are1 orthologues have been shown to be transcription activators of the extracellular proteases in some filamentous fungi [26,28,29,31] and it is therefore not surprising that the ability to produce proteases on the skim milk agar plates was abolished in the T. reesei strain Δare1, confirming that Are1 was a key factor to secrete proteases in T. reesei. In addition, the expression of the aspartic protease genes, such as apw1, was induced by peptone and repressed by ammonium in T. reesei, which is consistent with the results reported for other fungi where the expression of proteases was induced by non-preferred nitrogen sources and repressed by the favored nitrogen sources [31,35]. Moreover, the transcription level of apw1 was increased in the presence of ammonium in Δare1, indicating that mutation of Are1 resulted in derepression of protease gene expression, which is similar to that in the AreA mutant of A. nidulans [36].
Evidence is emerging that the cellulase production was reduced in the strain containing the loss function of areA in A. nidulans [30]. In the present study, it was found that the transcripts of the cellulase genes in Δare1 were significantly reduced compared to that in the parental strain QM9414 in the presence of ammonium. However, there was no significant difference in the cellulase-gene transcripts between Δare1 and QM9414 when cultured in the medium containing peptone as the sole nitrogen source. These results suggested that the regulation of cellulase gene expression by Are1 depends on the kind of the nitrogen sources in the environment for T. reesei. Meanwhile, the conserved Are1-binding motif was also found in the promoters of the cellulase genes, cbh1 and cbh2. Although the binding function of Are1 needs to be confirmed experimentally, such as EMSA, the decreased cellulase activities in Δare1 indicated that Are1 is involved in regulation of cellulase expression in T. reesei. The transcript abundances of the major cellulase regulators, Xyr1 and Cre1, in Δare1 were comparable to that of the parental strain QM9414, suggesting that Are1 may act directly on the cellulase gene promoter to regulate the cellulase production, independent of Xyr1 and Cre1.
Carbon catabolite repression (CCR) and nitrogen metabolite repression (NMR), which cooperatively ensure glucose and ammonium are utilized preferentially by preventing the expression of genes required for the metabolism of less preferred carbon and nitrogen sources, are the most important nutrient control laws in the microbial world [37,38]. Protease, as an important participant in the nitrogen source cycle, has been proved to be regulated by nitrogen regulators Are1/AreA/NIT2. Similarly, cellulase, as an important decomposer of lignocellulose (complex carbon source), is also strictly depended on the status of carbon sources. In this study, the expression of both cellulases and proteases for the first time was found to be coupled under the control of the GATA-type transcriptional factor Are1. However, the regulatory mechanisms between the cellulase and protease production were significantly different. When the nitrogen source was sufficient, Are1 could activate the expression of cellulases and inhibit the expression of proteases. When the nitrogen source is insufficient, the expression of proteases is activated and expression of cellulases is inhibited. Taken together, these results demonstrated that Are1 probably acts as a mediator to regulate the expression of cellulases and proteases to facilitate balancing the utilization of carbon and nitrogen sources.
4. Materials and Methods
4.1. Strains and Culture Conditions
The filamentous fungus T. reesei QM9414 (ATCC 26921) was used as the parental strain for construction of all the deletion strains in this study. Relative strains were grown on potato dextrose agar (PDA) plates (200 g/L potato, 20 g/L glucose, 20 g/L agar) at 30 °C for 5–7 days to harvest spores. Then, the spores were collected and then 108 of spores were pre-cultured in 150 mL of minimal medium or induction medium (CPM, Minimal uridine medium with 2% Lactose or Avicel) at 30 °C for 36 h and, subsequently, 1g of mycelia was transferred into 150 mL of CPM for cellulase production [39]. The plasmid T-hph was used as the template to amplify the hygromycin B-resistant gene hph. The pyrithiamine-resistant gene ptrA was amplified from the plasmid T-ptrA. Minimal medium supplemented with 0.3 μg/mL pyrithiamine (Sigma, St. Louis, Missouri, USA) or 300 μg/mL hygromycin B was applied as a selective medium for screening the fungal transformants. The skim-milk agar plates were used to investigate the capacity of T. reesei strains to secrete proteases. The composition of skim-milk medium was MM supplemented with 2% skim milk plus different carbon sources or different nitrogen sources. The CMC plates were used to screen the strains showing cellulase (EG) activity. The CMC medium composition was as follows (g/L): 10 CMC–Na, 1 yeast extract, 5 triton X-100, and 20 agar.
4.2. Molecular Techniques
Primes were designed by using the primer premier 5.00 software (PREMIER Biosoft, Palo Alto, CA, USA). DNA fragments were purified using a Gel Extraction Kit (Omega, Norcross, GA, USA). Oligonucleotides synthesis and DNA sequencing were performed at Sangon Inc. (Shanghai, China). Oligonucleotides used in this study are listed in Table S1 in supplementary materials. Multiple alignments of protein sequences were performed with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/ clustalw2/). Phylogenetic analysis was inferred using the neighbor joining method and the software MEGA4.0. The T. reesei genome database ver2.0 (http://genome.jgi-psf.org/Trire2/Trire2.home.html) was used for DNA and protein predictions in this study.
4.3. Construction of are1, are2, and are3 Genes Deletion Strains
The disruption cassettes for gene disruptions were constructed by the double-joint PCR method previously described [40]. All primers used in this study were listed in Table 1. To construct the Are1 deletion strain, firstly, 1.6 kb- and 1.3 kb- DNA fragments corresponding to the 5′ and 3′ regions of the are1 gene were amplified from the genome of T. reesei using the primer pairs Are1-1618-UF1/Are1-52-UR1 and Are1-232-DF1/Are1-1729-DR1, respectively (Table 1). The hygromycin B resistant gene hph (1.9 kb) was amplified from T-hph with primer pair Hph-F1/Hph-R1 (Table 1). Then, these PCR products were mixed and used as the template to amplify the deletion cassette by PCR using the nest primer pair Are1-1522-UF2/Are1-1426-UR2. A final product (Δare1::hph, 4.4 kb) was obtained and then was transformed into protoplasts of T. reesei using the previously described method (Ghose, 1987). The are1 deletion transformants were identified by PCR using the primer pairs Are1-1618-UF1/Y-hph-168-UR1 and Y-hph-121-DF1/Are1-1729-DR1. Similarly, another deletion cassette, Δare2::prtA (5.4 kb) consisted of three fragments, the 5′ flanking region of are2 gene (1.9 kb), the 3′ flanking region of are2 gene (1.2 kb), and the ptrA gene (2.1 kb). These fragments were generated by the PCR method using the primer pairs Are2-2073-UF1/Are2-173-UR1, Are2-70-DF1/Are2-1537-DR1 and ptrA-F1/ptrA-R1, respectively. Then these PCR products were mixed and were used as the template to amplify the are2-deletion cassette using the Are2-1732-UF2/Are2-1403-UR2 as the primer pair. Δare2 mutants were identified by PCR using primer pairs Are2-2073-UF1/Yan-ptrA-UR1 and Yan-ptrA-DF1/Are2-1537-DR1, which yielded the 2.4 kb- and 1.5 kb- fragments, while there was no fragment for the parental strain. The Δare3::ptrA cassette for are3 deletion was constructed by the same method. Then, the cassette was transformed into the protoplasts of T. reesei. The Δare3 transformants were analyzed by PCR using the primer pairs Are3-2073-UF1/Yan-ptrA-UR1 and Yan-ptrA-DF1/Are1-1949-DR1, which produced the 2.1 kb- and 1.6 kb- fragments in the transformants.
Table 1.
Oligonucleotides used in this study.
| Primers | Sequences (5′-3′) | Employment |
|---|---|---|
| ARE1-1618-UF1 | AAGCACTGGTTGTTGGTTGG | mutant construction |
| ARE1-52-UR1 | TGCTCCTTCAATATCAGTTAAGGTCGAAGAAGGCTAATGGGGGAGAA | mutant construction |
| ARE1-232-DF1 | AAATTCCGTCACCAGCCCTGGGTTGTGATACCTGGGTCTTTTGTGTG | mutant construction |
| ARE1-1729-DR1 | TCTGGTTCCGATAGCCGA | mutant construction |
| ARE1-1522-UF2 | CCCCTGTCCGTTAGCAGTTCAT | mutant construction |
| ARE1-1426-UR2 | AGCATTTGGCATTTGCGAGAGA | mutant construction |
| Hph-F1 | CGACGTTAACTGATATTGAA | mutant construction |
| Hph-R1 | CAACCCAGGGCTGGTGACGG | mutant construction |
| ARE2 -2073-UF1 | AGGGGCACTGGCAAATAT | mutant construction |
| ARE2 -173-UR1 | GAAGCATAAAGTGTAAAGCCTGGGGGCCCGTTTTAGGCTTAGAG | mutant construction |
| ARE2-70-DF1 | ATACAAACAAAGATGCAAGAGCGGCGTGGCAACGATGGATGT | mutant construction |
| ARE2-1537-DR1 | ACCCTGAATGGTGTCCCTC | mutant construction |
| PtrA-F1 | CCCCAGGCTTTACACTTTAT | mutant construction |
| ptrA-R1 | CCGCTCTTGCATCTTTGTT | mutant construction |
| ARE3-1676-UF1 | AGGGAGGCTGTCGGAGTG | mutant construction |
| ARE3-36-UR1 | GAAGCATAAAGTGTAAAGCCTGGGGGGGCTTTTGTTGATGTTTCTC | mutant construction |
| ARE3-457-DF1 | ATACAAACAAAGATGCAAGAGCGGCCCTGTCTATTCCTGAGTGT | mutant construction |
| ARE3-1949DR1 | GGTATGAGTCGCAGGGAG | mutant construction |
| ARE3-1585-UF2 | CGAACGAAGGTGGTGGAGAT | mutant construction |
| ARE3-1415-DR2 | CCGCTTGTATGCTGGGGACT | mutant construction |
| Y-hph-121-DF1 | ACTGAGGAATCCGCTCTTGG | mutant construction |
| Y-hph-168-UR1 | ACTGCTTACAAGTGGGCTGA | mutant construction |
| Y-ptrA-UR1 | CATTGTCGGTTGGTTTGG | mutant construction |
| Y-ptrA-DF1 | ATGTAACGGTGGGGCATT | mutant construction |
| ARE1-1064-UF | TTATCGGTCAGTCTCCATCTTCA | mutant construction |
| ARE1-535-DR | TCCATTTTCCCAACATACTCCA | mutant construction |
| PgpdA-S | AGACCTAATACAGCCCCTAC | mutant construction |
| PgpdA-A | AACAGCTCCTCGCCCTTGCTCACCATATGTCTGCTCAAGCGGGGTAG | mutant construction |
| ARE1-3-F | GGCATGGACGAGCTGTACAAGGCAGCTGTCGGACCGCTT | mutant construction |
| ARE1-923-DR1 | ATGAGCCCAAGAAAGACTGAAGAG | mutant construction |
| ARE1-340-DR2 | CAGGCAGAGCAGAAAGAAATAAAAG | mutant construction |
| ARE1-1072-R | GTAGGGGCTTCTGTTCCA | mutant construction |
| ARE1-384DF | GAAGAACAGCCTCGGTGCG | probe |
| ARE1-1206DR | CTGCCTATCCCCAAGCGTC | probe |
| real-APW1-F1 | AGGCACGGACAGAACGGCAGCT | RT-qPCR for apw1 |
| real-APW1-R1 | CGTTGGCGTAGTAGGCATCG | RT-qPCR for apw1 |
| real-APW2-F1 | GCGATGTCTACCACGATATTGTCTC | RT-qPCR for apw2 |
| real-APW2-R1 | TCAAGGCTGCCGACGATGTT | RT-qPCR for apw2 |
| real-cbh1-F1 | CCGAGCTTGGTAGTTACTCTG | RT-qPCR for cbh1 |
| real-cbh1-R1 | GGTAGCCTTCTTGAACTGAGT | RT-qPCR for cbh1 |
| real-cbh2-F1 | CTGGTCCAACGCCTTCTTCA | RT-qPCR for cbh2 |
| real-cbh2-R1 | GACCCAGACAAACGAATCCAG | RT-qPCR for cbh2 |
| real-egl1-F1 | CTCAGATGGACGAGAACGGG | RT-qPCR for egl1 |
| real-egl1-R1 | CTGGTGGCTAGTGTTGAGGG | RT-qPCR for egl1 |
| real-egl2-F1 | AACAAGTCCGTGGCTCCATT | RT-qPCR for egl2 |
| real-egl2-R1 | TCCGCTCCAACCAATACCTC | RT-qPCR for egl2 |
| real-actin-F1 | CCCAAGTCCAACCGTGAGA | RT-qPCR for actin |
| real-actin-R1 | CAATGGCGTGAGGAAGAGC | RT-qPCR for actin |
4.4. Complementation of are1 in the Δare1 Strain
To construct the complementation strain, a PCR-amplified product (5.0 kb) containing the entire Are1 encoding gene was amplified by primer pair Are1-1064-UF/Are1-535-DR. To constitutively express the are1 gene, the are1 encoding region (4.1 kb) and 3′flanking region (1.5 kb) were amplified by PCR with primer pairs Are1-1-F/Are1-923 DR1 and Are1-1618-UF1/Are1-52-UR1 and fused with the constitutive A. nidulans gpdA promoter (1.0 kb). The promoter was obtained from the plasmid pAB4-1 with primer pair gpdA-S/gpdA-A (Table 1). Then, these DNA fragments were fused by double-joint PCR with the nested primer pair Are1-1522-UF2/Are1-340-DR2 (Table 1), resulting in a 6.2 kb product. The complementation cassette and constitutive expression cassette were transformed into the protoplasts of Δare1 strain. Candidates were selected on regeneration medium containing 2 g/L NaNO3. Transformants with the successful complementation and constitutive expression of are1 were confirmed by PCR using primer pair ARE1-1-F/ARE1-1072-R, which expected a 1.0 kb product in the mutant.
4.5. RNA Extraction and RT-qPCR Analysis
For RNA extraction, 108 spores were inoculated in minimal medium with 1% glucose and cultured for 36 h at 30 °C. Then, the mycelia were harvested and transferred into the induction medium (with peptone) supplemented with or without (NH4)2SO4. Considering the peptone used as nitrogen and carbon sources, the mycelia also transferred into the induction medium (without peptone) containing the (NH4)2SO4 as the sole nitrogen source. Then, the mycelia were harvested after 10 h of cultivation and then total RNA were isolated using the RNAiso™ reagent. The integrity of the extracted RNA was evaluated by agarose gel electrophoresis and then the total RNA was treated with DNAaseI. cDNA was synthesized from total RNA using a PrimeScript RT reagent kit, following the manufacturer’s description. The transcript levels of the apw1, apw2, cbh1, cbh2, egl1, and egl2 were detected using an RT-PCR Kit with actin gene as a control. All of these primers used for PCR are listed in Table 1. The amplification efficiency of each gene was determined, which were between 93% and 102%. RT-qPCR analysis was performed on a LightCycler 480 System (Roche Diagnostics, Germany). The procedure of RT-qPCR was as follows: Initial denaturation of 1 min at 95 °C, followed by 40 cycles of 5 s at 95 °C and 20 s at 60 °C. Melting curve analysis from 65 to 95 °C was performed to confirm the specifics of the applications. Light-Cycler480 software 1.5.0 was used for the Ct value calculation. Data analysis was performed using the relative quantitation/comparative CT (ΔΔCT) method and were normalized to an endogenous control (actin). Three biological replicates were performed for each analysis and the results are shown as the mean and SD from the replicates. Statistical analysis was performed using the Student’s t-test analysis.
4.6. Detection of Cellulase Activity
The filter paper activity (FPA) was measured using Whatman No. 1 filter paper as a substrate, as described by Ghose (Ghose, 1987) [41]. The reaction mixtures containing 50 mg of Whatman No. 1 filter paper, 1.5 mL of 50 mM citrate buffer (pH 4.8), and 500 μL of the suitably diluted enzyme fractions were incubated at 50 °C for 60 min. EG activity was assayed with CMC–Na as a substrate. The enzyme reactions were performed in 2 mL of 1% CMC–Na (pH 4.8) at 50 °C for 30 min. The amount of reducing sugar released was determined using the DNS method. One unit of enzyme activity was defined as the amount of enzyme releasing 1 μmol of glucose per minute. Three biological triplicates were designed in all experiments.
5. Conclusions
In this study, the GATA-type transcription factor Are1 in T. reesei was identified and characterized for its function in the regulation of both protease and cellulase production. The T. reesei Δare1 was unable to produce extracellular proteases on the skim milk agar plates and showed a significant decrease in cellulase activity on the CMC agar plate. Further transcriptional analysis revealed that the regulation of protease and cellulase gene expression by Are1 highly depended on the kind of nitrogen sources. Sequence analysis showed that all promoter regions of the tested protease and cellulase genes contain the Are1-binding motif. These findings suggested that Are1 plays an important role both in the regulation of protease and cellulase production and may function in the manner of acting directly on the promoters of the target genes. To our knowledge, this is the first report of the function of Are1 in T. reesei, which provides new insights into the role of the GATA-type transcription factors in fungi.
Acknowledgments
We would like to thank the researchers and their hard work that were able to make this article possible.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/17/4100/s1.
Author Contributions
Y.C.Q. and Y.H.Z. designed the experiments; Y.C.Q., Y.S., L.X.Z., N.N.S., and Y.F.S. performed experiments; Y.C.Q., Y.B.Q., and Y.H.Z. analyzed the data; Y.C.Q. and Y.H.Z. wrote the paper.
Funding
This research was supported by the grants from the National Key R&D Program of China (No. 2018YFA0900503), the Shandong Key Research and Development Program (No. 2017GSF21111), the Fundamental Research Fund of Shandong University (No. 2018JC020) and the Postdoctoral Science Foundation of China (No. 2019M652372).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Musser R., Farmer E., Pieffer M., Felton G. Caterpiller salivary gland ablation technique for the classification of role of liable enzyme glucose oxidase. J. Chem. Ecol. 2004;28:1691–1696. doi: 10.1023/A:1019985417720. [DOI] [Google Scholar]
- 2.Saleemi M.K., Khan M.Z., Khan A., Mehmood M.A., Farooq M., Hameed S., Hassan Z.U., Javed M.R., Javed I. Molecular identification of black Aspergilli isolated from poultry feeds by sequencing of its region. Pak. Vet. J. 2012;32:171–174. [Google Scholar]
- 3.Zia M.A., Riaz A., Rasul S., Abbas R.Z. Evaluation of antimicrobial activity of glucose oxidase from Aspergillus niger EBL-A and Penicillium notatum. Braz. Arch. Biol. Technol. 2013;56:956–961. doi: 10.1590/S1516-89132013005000010. [DOI] [Google Scholar]
- 4.Fernandez J., Wright J.D., Hartline D., Quispe C.F., Madayiputhiya N., Wilson R.A. Principles of carbon catabolite repression in the rice blast fungus: Tps1, Nmr1-3, and a MATE-family pump regulate glucose metabolism during infection. PLoS Genet. 2012;8:e1002673. doi: 10.1371/journal.pgen.1002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilmen M., Thrane C., Penttila M. The glucose repressor gene cre1 of Trichoderma: Isolation and expression of a full-length and a truncated mutant form. Mol. Gen. Genet. 1996;251:451–460. doi: 10.1007/BF02172374. [DOI] [PubMed] [Google Scholar]
- 6.Saloheimo A., Aro N., Ilmen M., Penttila M. Isolation of the ace1 gene encoding a Cys(2)-His(2) transcription factor involved in regulation of activity of the cellulase promoter cbh1 of Trichoderma reesei. J. Biol. Chem. 2000;275:5817–5825. doi: 10.1074/jbc.275.8.5817. [DOI] [PubMed] [Google Scholar]
- 7.Aro N., Pakula T., Penttilä M. Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol. Rev. 2005;29:719–739. doi: 10.1016/j.femsre.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Peij N.V., Gielkens M., Vries R.D., Visser J., Graaff L.D. The transcriptional activator XlnR regulates both xylanolytic and endoglucanase gene expression in Aspergillus niger. Appl. Environ. Microbiol. 1998;64:3615–3619. doi: 10.1128/aem.64.10.3615-3619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stricker A.R., Grosstessner-Hain K., Wurleitner E., Mach R.L. Xyr1 (xylanase regulator 1) regulates both the hydrolytic enzyme system and d-xylose metabolism in Hypocrea jecorina. Eukaryot. Cell. 2006;5:2128–2137. doi: 10.1128/EC.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Znameroski E.A., Coradetti S.T., Roche C.M., Tsai J.C., Iavarone A.T., Cate J.H.D. Induction of lignocellulose-degrading enzymes in Neurospora crassa by cellodextrins. Proc. Natl. Acad. Sci. USA. 2012;109:6012–6017. doi: 10.1073/pnas.1118440109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y., Zheng F., Wang L., Zhao G., Chen G., Zhang W., Liu W. Rce1, a novel transcriptional repressor, regulates cellulase gene expression by antagonizing the transactivator Xyr1 in Trichoderma reesei. Mol. Microbiol. 2017;105:65–83. doi: 10.1111/mmi.13685. [DOI] [PubMed] [Google Scholar]
- 12.Gao J., Qian Y.C., Wang Y.F., Qu Y.B., Zhong Y.H. Production of the versatile cellulase for cellulose bioconversion and cellulase inducer synthesis by genetic improvement of Trichoderma reesei. Biotechnol. Biofuels. 2017;15:272. doi: 10.1186/s13068-017-0963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dos Santos Castro L., de Paula R.G., Antonieto A.C., Persinoti G.F., Silva-Rocha R., Silva R.N. Understanding the role of the master regulator XYR1 in Trichoderma reesei by global transcriptional analysis. Front. Microbiol. 2016;7:175. doi: 10.3389/fmicb.2016.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonieto A.C., Dos Santos Castro L., Silva-Rocha R., Persinoti G.F., Silva R.N. Defining the genome-wide role of CRE1 during carbon catabolite repression in Trichoderma reesei using RNA-Seq analysis. Fungal Genet. Biol. 2014;73:93–103. doi: 10.1016/j.fgb.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Arst J.H.N., Cove D.G. Nitrogen metabolite repression in Aspergillus nidulans. Melee. Gen. Genet. 1973;126:111–141. doi: 10.1007/BF00330988. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y.H., Marzluf G.A. nit-2, the major nitrogen regulatory gene of Neurospora crassa, encodes a protein with a putative zinc finger DNA-binding domain. Mol. Cell. Biol. 1990;10:1056–1065. doi: 10.1128/MCB.10.3.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudla B., Caddick M.X., Langdon T., Martinez-Rossil N.M., Bennett C.F., Sibley S., Davies R.W., Arst J.H.N. The regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans. EMBO J. 1990;9:1355–1364. doi: 10.1002/j.1460-2075.1990.tb08250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong K.H., Hynes M.J., Todd R.B., Davis M.A. Deletion and overexpression of the Aspergillus Nidulans GATA factor AreB reveals unexpected pleiotropy. Microbiology. 2009;155:3868–3880. doi: 10.1099/mic.0.031252-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim H., Woloshuk C.P. Role of AREA, a regulator of nitrogen metabolism, during colonization of maize kernels and fumonisin biosynthesis in Fusarium verticillioides. Fungal Genet. Biol. 2008;45:947–953. doi: 10.1016/j.fgb.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich K.C., Cotty P.J. Variability in nitrogen regulation of aflatoxin production by Aspergillus flavus strains. Appl. Microbiol. Biotechnol. 2002;60:174–178. doi: 10.1007/s00253-002-1094-5. [DOI] [PubMed] [Google Scholar]
- 21.Martina M. AREA directly mediates nitrogen regulation of gibberellin biosynthesis in Gibberella fujikuroi, but its activity is not affected by NMR. Mol. Microbiol. 2003;47:975–991. doi: 10.1046/j.1365-2958.2003.03326.x. [DOI] [PubMed] [Google Scholar]
- 22.Gardiner D.M., Kazan K., Manners J.M. Novel Genes of Fusarium graminearum That Negatively Regulate Deoxynivalenol Production and Virulence. Mol. Plant Microbe Interact. 2009;22:1588–1600. doi: 10.1094/MPMI-22-12-1588. [DOI] [PubMed] [Google Scholar]
- 23.Min K., Shin Y., Son H., Lee J., Kim J.C., Choi G.J., Lee Y.W. Functional analyses of the nitrogen regulatory gene areA in Gibberella zeae. FEMS Microbiol. Lett. 2012;334:66–73. doi: 10.1111/j.1574-6968.2012.02620.x. [DOI] [PubMed] [Google Scholar]
- 24.Muro-Pastor M., Gonzalez R., Strauss J., Narendja F., Scazzocchio C. The GATA factor AreA is essential for chromatin remodelling in an eucaryotic bidirectional promoter. EMBO J. 1999;18:1584–1597. doi: 10.1093/emboj/18.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger H., Basheer A., Bock S., Reyes-Dominguez Y., Dalik T., Altmann F., Strauss J. Dissecting individual steps of nitrogen transcription factor cooperation in the Aspergillus nidulans nitrate cluster. Mol. Microbiol. 2008. 69:1385–1398. doi: 10.1111/j.1365-2958.2008.06359.x. [DOI] [PubMed] [Google Scholar]
- 26.Bi F., Ment D., Luria N., Meng X., Prusky D. Mutation of AREA affects growth, sporulation, nitrogen regulation, and pathogenicity in Colletotrichum gloeosporioides. Fungal Genet. Biol. 2017. 99:29–39. doi: 10.1016/j.fgb.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Todd R.B., Fraser J.A., Wong K.H., Davis M.A., Hynes M.J. Nuclear accumulation of the GATA factor AreA in response to complete nitrogen starvation by regulation of nuclear export. Eukaryot. Cell. 2005;4:1646–1653. doi: 10.1128/EC.4.10.1646-1653.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dabas N., Morschhauser J.A. transcription factor regulatory cascade controls secreted aspartic protease expression in Candida albicans. Mol. Microbiol. 2008;69:586–602. doi: 10.1111/j.1365-2958.2008.06297.x. [DOI] [PubMed] [Google Scholar]
- 29.Morschhäuser J. Nitrogen regulation of morphogenesis and protease secretion in Candida albicans. Int. J. Med. Microbiol. 2011;301:390–394. doi: 10.1016/j.ijmm.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Lockington R.A., Rodbourn L., Barnett S., Carter C.J., Kelly J.M. Regulation by carbon and nitrogen sources of a family of cellulases in Aspergillus nidulans. Fungal Genet. Biol. 2002;37:190–196. doi: 10.1016/S1087-1845(02)00504-2. [DOI] [PubMed] [Google Scholar]
- 31.Bugeja H.E., Hynes M.J., Andrianopoulos A. AreA controls nitrogen source utilisation during both growth programs of the dimorphic fungus Penicillium marneffei. Fungal Biol. 2012;116:145–154. doi: 10.1016/j.funbio.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhang G., Zhu Y., Wei D., Wang W. Enhanced production of heterologous proteins by the filamentous fungus Trichoderma reesei via disruption of the alkaline serine protease SPW combined with a pH control strategy. Plasmid. 2014;71:16–22. doi: 10.1016/j.plasmid.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Ravagnani H., Gorfinkiel L., Langdon T., Diallinas G., Adjadj E., Demais S., Gorton D., Arst J.H.N., Scazzocchio C. Subtle hydrophobic interactions between the seventh residue of the zinc finger loop and the first base of an HGATAR sequence determine promoter-specific recognition by the Aspergillus nidulans GATA factor AreA. EMBO J. 1997;16:3974–3986. doi: 10.1093/emboj/16.13.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jun H., Bing Y., Keying Z., Xuemei D., Daiwen C. Strain improvement of Trichoderma reesei Rut C-30 for increased cellulase production. Indian J. Microbiol. 2009;49:188–195. doi: 10.1007/s12088-009-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellier A.L., Laugé R., Veneault-Fourrey C., Langin T. CLNR1 the AREA/NIT2-like global nitrogen regulator of the plant fungal pathogen Colletotrichum lindemuthianum is required for the infection cycle. Mol. Microbiol. 2003;48:639–655. doi: 10.1046/j.1365-2958.2003.03451.x. [DOI] [PubMed] [Google Scholar]
- 36.Pateman J.A., Dunn E., Kinghorn J.R., Forbes E.C. The transport of ammonium and methylammonium in wild type and mutant cells of Aspergillus nidulans. Mol. Gen. Genet. 1974;133:225–236. doi: 10.1007/BF00267672. [DOI] [PubMed] [Google Scholar]
- 37.Yamada T., Makimura K., Abe S. Isolation, characterization, and disruption of dnr1, the areA/nit-2-like nitrogen regulatory gene of the zoophilic dermatophyte, Microsporum canis. Med. Mycol. 2006;44:243–252. doi: 10.1080/13693780500410909. [DOI] [PubMed] [Google Scholar]
- 38.DeBusk R.M., Ogilvie S. Regulation of amino acid utilization in Neurospora crassa: Effect of nmr-l and ms-5 mutations. J. Bucteriol. 1984;160:656–661. doi: 10.1128/jb.160.2.656-661.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian Y., Zhong L., Hou Y., Qu Y., Zhong Y. Characterization and strain improvement of a Hypercellulytic variant, Trichoderma reesei SN1, by genetic engineering for optimized cellulase production in biomass conversion improvement. Front. Microbiol. 2016;7:1349. doi: 10.3389/fmicb.2016.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J.H., Hamari Z., Han K.H., Seo J.A., Reyes-Dominguez Y., Scazzocchio C. Double-joint PCR: A PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal. Genet. Biol. 2004;41:973–981. doi: 10.1016/j.fgb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Ghose T.K. Measurement of Cellulase Activities. Pure Appl. Chem. 1987;59:257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.