Abstract
Climate variability is highly impacting on mosquito-borne diseases causing malaria and dengue fever across the globe. Seasonal variability change in temperature and rainfall patterns are impacting on human health. Mosquitoes cause diseases like dengue fever, yellow fever, malaria, Chikungunya, West Nile and Japanese encephalitis. According to estimations by health organizations, annually one million human deaths are caused by vector-borne diseases, and dengue fever has increased about 30-fold over the past 50 years. Similarly, over 200 million cases of malaria are being reported annually. Mosquito-borne diseases are sensitive to temperature, humidity and seasonal variability. Both conventional (environmental, chemical, mechanical, biological etc.) and nanotechnology-based (Liposomes, nano-suspensions and polymer-based nanoparticles) approaches are used for the eradication of Malaria and dengue fever. Now green approaches are used to eradicate mosquitoes to save human health without harming the environment. In this review, the impact of climatic conditions on mosquito-borne diseases along with conventional and nanotechnology-based approaches used for controlling malaria and dengue fever have been discussed. Important recommendations have been made for people to stay healthy.
Keywords: climate change, dengue fever, environmental management, eradication of vectors, human health, mosquito-borne diseases, malaria, nanotechnology-based disease control approaches, traditional disease control, public health risk
1. Introduction
Vectors are the organisms responsible for the transmission of communicable diseases from faunas to human beings or among humans. The best-known vector for such diseases is the mosquito. Others may include flies, ticks, sand flies, triatomine bugs, fleas and aquatic snails [1]. The diseases caused by these vectors cause above one million of human deaths yearly. These diseases account for over 17% of the infectious/transmittable diseases [2]. In tropical countries, some vector-borne diseases are still ignored; e.g., lymphatic filariasis, dengue fever, Leishmaniasis, Human African Trypanosomiasis and Schistosomiasis [3]. There are about 3.9 billion people at risk of having dengue fever and 96 million cases of dengue are reported every year in 128 prevalent countries. It follows that almost half of the world’s population is at risk [4]. The prevalence of dengue fever has increased by 30-fold in the past 50 years. In 2017, the estimated malaria cases and corresponding deaths occurred were 219 million and 435,000 respectively, comprising almost 90% of sub-Saharan Africa [5]. In the South-east Asia Region, many of the vector-borne diseases are prevalent, including some mosquito-borne diseases such as malaria, dengue, chikungunya, Japanese encephalitis and lymphatic filariasis, snail-transmitted disease (schistosomiasis) and the sand-fly borne disease (kalaazar). Malaria is prevalent in many tropical and subtropical regions of the world excluding the Maldives, which is officially stated as being malaria-free [6].
As the globe is continuously facing climate change challenges, mosquitoes are benefitting from this competition. As temperatures are getting warmer, the range of disease-carrying mosquitoes is increasing. The increased temperature allows them to breed quicker. Mosquitoes may breed all-year-round in warm areas. Warm temperatures facilitate the increase in hatching and reproduction rates of mosquitoes [7,8]. In South-east Asia, frequent epidemics with hundreds of thousands of reported and unreported cases have arisen during the past decade, resulting in significant numbers of deaths. Countries that are more severely affected such as Pakistan, India, Bangladesh and Sri Lanka can be seen as victims of climate change in terms of mosquito population increases. The risk factors of these vector-borne diseases that may cause corresponding epidemics are poor sanitation, low cleanliness, climate change and the lack of efficient vector control approaches [9,10].
An increase in epidemics of Dengue is due to the absence of active gene therapy or vaccines for its control. The only reliable control approach for these diseases is their prevention and control through source reduction and insecticides. As the mosquitoes are developing resistance against the insecticides, the prime focus of the scientific community is to find new ways of eradicating mosquito populations. The purpose of this study is to assess the existing conventional and novel eradication methods and techniques, which are being used in different countries of the world to eradicate or control vectors and diseases transmitted by such vectors. Moreover, this review identifies missing gaps in the management of vectors, especially malaria and dengue fever, and approaches to manage increases in temperature due to a changing climate.
2. Methodology
Peer-reviewed published research papers and reports along with grey literature were selected from 1981 to 2019 (39 Years). Literature was collected from the world-renowned databases ISI Web of Knowledge (http://isiknowledge.com), Science Direct (http://www.sciencedirect.com), Scopus (https://www.scopus.com) and Google Scholar (https://scholar.google.com.pk). Relevant literature was designated on the basis of inclusion and exclusion criteria; i.e., relevance of literature to the study area on the basis of keywords used. Preference was given to the publication years 1990–2019. The search was curtained by using the following keywords: malaria; dengue fever; Chikungunya; Zika virus; Yellow fever; climate change; health impacts of mosquito-borne diseases; temperature and precipitation effects; conventional control methods for mosquito; and nanotechnology-based methods.
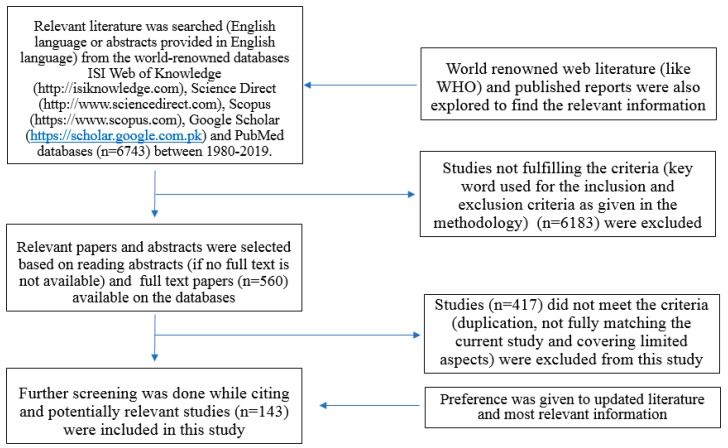
According to the exclusion criterion, literature items, which did not fulfil the above criterion were deleted (Figure 1). Literature was reviewed and properly cited to acknowledge previous studies. The author followed all steps of the methodology proposed by The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Group [11]. PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. PRISMA can be used as a basis for reporting systematic literature reviews.
Figure 1.
PRISMA Diagram showing the literature search strategy.
3. Results
3.1. Climatic Conditions Influence on Mosquito Vectors
3.1.1. Seasonality of Infectious Diseases
A high number of vector-borne diseases like dengue fever and malaria express substantial seasonal patterns around the world. Most of the insects including mosquitoes are poikilothermic ectotherm whose internal temperature is dependent on the ambient temperature of the environment [12]. Researchers described the relationship between water temperature and development rate that is analogous for eggs, larvae and pupae of the insects [13]. An increase in ambient temperature is linked to the increased metabolism of the mosquitoes. The transmission of the mosquito-borne diseases is at a maximum at higher temperature due to an increased rate of pupation, blood meals and a reduced extrinsic incubation period [12,14]. Similarly, optimum rainfall and precipitation is also linked to the growth and development of mosquitoes; and viral replication within the vector [14]. In Peru, a peak of infection with Cyclospora is seen in summer, which subsides in winter. During hot and dry seasons, other infections tend to rise, but decrease at the beginning of the rainy spell in, for example, Africa [15,16].
3.1.2. Vector-Borne Diseases
There are few vector-borne pathogens in the transmission of diseases [17] that may include:
The reproduction and survival rates of vectors in a certain environment.
The level and degree of vector-activity; typically, the rate of biting and the period of year.
Reproduction and development rate of pathogens inside the vector.
The reproduction and survival of pathogens, hosts and vectors is dependent on specific climatic circumstances. Any fluctuation in such conditions may affect the rate of disease transmission. Among these finest environmental conditions for vector-borne diseases, temperature and humidity are the most significant climatic factors while the airstream, daylight duration and elevation of sea level are supplementary vital considerations [18]. Table 1 shows a summary of climatic change impacts to each biotic element of vector-borne illnesses.
Table 1.
Climate change impacts on vector-borne diseases [23].
| Impacts of Temperature on Certain Vectors and Vector-Borne Pathogens | Impacts of Changes in Precipitation on Vector-Borne Pathogens | Impacts of Higher Sea Level on Vector-Borne Pathogens |
|---|---|---|
Vector
|
Vector
|
Vector Reducing or eradicating breeding sites of mosquitoes (e.g., Cs. melanura). The relationship between vector biology and climatic conditions is complex due to the natural capability of vectors to seek out appropriate microclimates for their survival such as hiding places under vegetation, pit toilets during hot or dry weather and tunnels during cold weather [23]. |
| Pathogen |
Pathogen
|
|
Vertebrate host
|
3.1.3. Temperature Sensitivity
Extreme temperatures are mostly fatal for the survival and persistence of pathogens, but any incremental fluctuation in temperature could have varying effects. A slight rise in temperature may become deadly to the disease-causing agent, if a vector is subjected to an environmental condition such as mean temperature, which is approaching the edge of physiological tolerance for the infective agents or pathogens. On the other hand, if a vector lives in an atmosphere of comparatively lower mean temperature, an insignificant rise in temperature can accelerate the growth and replication of pathogens [19,20]. Temperature and development time are inversely correlated, and the optimum growth ranges from 22 to 32 °C [21,22].
The growth rate of a vector may also be modified by temperature due to changing their rates of biting and frequency of interaction with humans. Ultimately, a swing in temperature determines the span of transmission season [23]. Vectors can also adapt to the variations in temperature by shifting geographical distributions. The incidences of malaria in cooler environmental zones of African highlands can be the consequence of shifting habitats of mosquitoes to survive with augmented air temperatures [24]. Additionally, the vectors can also evolve in response to increase in temperatures. Recent evidence suggests that some vectors like the pitcher plant mosquito (Wyeomyia smithii Coquillett) has the ability to genetically adapt with climate change to survive for a longer period of time [8].
3.1.4. Precipitation Sensitivity
Changeability in precipitation has straight consequences on the outbreaks of infectious diseases. Increase in precipitation elevates the incidence of vectors by growing the magnitude of current larval habitation and generating new breeding lands [8]. In addition, this increase in precipitation can also favour the growth in food materials, which ultimately supports a larger population of vertebrate reservoirs. Flooding and decrease in vector population may be triggered by unusually heavy rainfall. On the other hand, flooding may increase the probability of vector human contact. In Brazil, leptospirosis (rodent-borne disease) outbreaks have been reported after extreme flooding [25]. Unexpected droughts may cause rivers to slow in wet tropics, creating additional areas of stagnant water, which is perfect for vector-breeding sites.
3.1.5. Humidity Sensitivity
The spreading of vector-borne illnesses can be greatly affected by humidity as well. The spreading of mosquitoes, which are also reliant on relative humidity, determines the extent of disease transmission [26]. The relative humidity is not only a limiting factor in those areas where it is more than 60%, but also temperature is considered as a main driver [27]. The survival rate of mosquitoes decreases in dry conditions as they can easily desiccate. Water saturation shortage as indicated by relative humidity remains the most critical factor in disease/climate models; e.g., Lyme disease models and dengue fever [23].
3.1.6. Sea Level Sensitivity
At the end of the 21st century, global warming is projected to increase sea levels by 18 to 59 cm through polar ice, melting of glaciers and the thermal expansion of seawater [36]. The increase in sea level related to the change in climate results in the elimination or reduction of breeding sites for coastal wetland mosquitoes. Mammalian and bird hosts that reside in such environmental niches can also be endangered, which support the removal of viruses prevalent in this environment [37]. Otherwise, local invasion of salty water may turn previous freshwater wetlands into coastal ones that help to host vector species evacuated from prior coastal wetland habitats [23].
Vector-borne microbes spend much of their life cycle in cold-blooded arthropods that are subjected to various environmental influences. The transmission of vector-borne diseases affected by climatic factors includes rainfall, temperature, extreme flooding, wind and sea level rise. Flooding can dislocate and lead them to seek out food and shelter [38].
3.2. Mosquito-Borne Diseases and Health Impacts
3.2.1. Vector-Host Relationships
It is necessary to understand the relationship between disease transmission and the connection of vectors/mosquitoes in the transmission of pathogens. Mechanical pathogen transmission is mainly accidental. In physically transmitted diseases, a pathogen that causes a disease needs support to move from one host to another. The arthropod obtains the pathogen from one host. The pathogen then grows in the body of the arthropod and is conveyed to another host. Within the invertebrate, the pathogen may or may not reproduce. If the parasite that causes the disease endures the sexual part of its lifecycle in a host, that host is known as the definitive or primary host (as in the case of mosquitoes that cause malaria). In case of malaria, a human is the transitional host in which the asexual phases of the parasite are found [39].
3.2.2. Diseases Transmitted by the Mosquito Vectors
Mosquitoes act as vectors for different diseases, which transfer them from one person to another. Some of these diseases have high mortality and wide distribution. Around 28 viruses, which have a major importance in public health, are transmitted by different mosquitoes. Arboviruses are transmitted by mosquito bites. Yellow fever and Dengue fever are transmitted by Ae. spp., while some kinds of Encephalitis are transmitted by Culex spp. and by Ae. spp. [40,41]. Malaria is a prime disease transmitted by several species of Anopheles, which carry an infective form of a protozoan parasite called Plasmodium. It may also be transmitted by Anophels quadrimaculatus. Anophels hermsi and Anophels freeborni are major vectors, while Anophels Crucians is a minor one. Malaria is one of the serious and lethal diseases around the globe affecting millions of people mostly in tropical and semi-tropical areas.
Five species of malarial parasites (Plasmodium falciparum, Plasmodium malariae, Plasmodium vivax, Plasmodium ovale, Plasmodium knowlesi) are responsible for malaria in humans. Most common signs include severe headache, fever, sweating, vomiting and chills nausea. Parasites first enter the liver and then destroy red blood cells causing anemia and weakness. In complicated cases, there could be kidney and brain damage resulting in renal catastrophe, encephalitis, shock, coma and death [37].
For dengue fever, Ae. aegypti and Ae. albopictus are central vectors in human-to-human transmission. Dengue viruses are endemic in tropical areas while its highly endemic in other areas. Symptoms of dengue fever include skin rash, pain behind the eyes, severe headache, fever as well as muscle and bone pain (thus also named as break bone fever). Recovery form dengue fever is long, but death is seldom. Each of the four strains provides lifelong immunity against the virus, but exposure to the second strain may result in the dengue shock syndrome and dengue haemorrhagic fever, which are much severe than dengue [38].
The Yellow fever virus is transmitted from some forest monkeys to humans by a vector. Ae. aegypti and Ae. albopictus can transmit the virus in urban areas, while Oc. japonicus may also act as potential vector. The symptoms it causes closely resemble those of the dengue fever, which includes fever, backache, headache, jaundice and internal bleeding, which can lead to death [39].
The West Nile Fever is caused by the West Nile virus and transmitted via Culex spp. (mosquito). It is a type of encephalitis and may also be called arboviral encephalitis. The virus is transmitted via the mosquito to their main reservoir host (usually birds). Horses and humans are rather accidental hosts. The symptoms of the West Nile fever include fever, vomiting, headache and swollen lymph nodes. In complicated cases, it may involve the central nerves system [39].
This is caused by the arbovirus, which is spread through Culex tritaeniorhynchus (mosquito). The virus is transferred from pigs or birds, which are their main hosts. Like the West Nile fever, horses and humans are accidental hosts. General symptoms include headache, fever, stupor, disorientation and loss of coordination. It may also lead to paralysis, seizures, coma and death [40].
These viruses may cause encephalitis and are transferred to humans by biting of humans accidently, and thus giving a dead-end host as the vectors cannot take the virus back form the human host. It involves a transmission cycle (similar to arboviruses) from birds or small mammals, which are the primary hosts. Some people are asymptomatic, some may get mild flu-like symptoms like headache and high fever. In some of the cases, people may get encephalitis disturbing the central nervous system, which may result in paralysis, seizures, coma and death [37].
The Alpha virus, which belongs to the family Togaviridae is the causing agent of Chikungunya. It is transmitted by bites of infected mosquitoes to humans. Ae. aegypti and Ae. albopictus are the main agents. Symptoms associated with Chikungunya include headache, nausea, vomiting, skin rash, fever and chills. Some patients may have pain in their joints [40].
3.2.3. Treatment and Vaccines for Mosquito Vectors
According to WHO, no specific treatment is available for dengue fever, but symptomatic treatment and the Dengvaxia® vaccine are used to decrease the severity of the diseases and decrease mortality rate. The Dengvaxia® (CYD-TDV) vaccine was developed in December 2015. In 2016, it was used in areas where dengue fever was highly endemic [40]. During the clinical trials, CYD-TDV was found effective in seropositive individuals. The main method to control dengue is to prevent the vector and to combat mosquitoes. Other vaccines developed by NIAID and Takeda are in the evaluation stage III [42]. Similarly, still no vaccines for Zika and Chikungunya virus have been found [43]. Other vaccines for Chikungunya and Zika are under clinical trials. Similarly, vaccines to provide broad protection against mosquitoes borne diseases like Malaria, Zika and West Nile Fever are under clinical trials by National Institute of Allergy and Infectious Diseases (NIAID) working under NIH [44]. As reported by WHO, yellow fever can be prevented by a single dose of effective vaccines, which are affordable and safe [45]. Effective drugs for malaria are easily available on the market. However, prescription is important before use.
3.3. Conventional Methods Used to Control Mosquito Populations
3.3.1. Overview
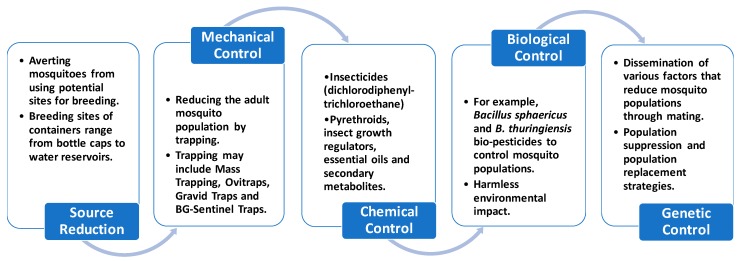
The purpose of monitoring mosquito populations is to lessen the potential for biting nuisance and disease transmission especially important for dengue and malaria. A number of effective methods are used in disease vector control and depend upon the targeted mosquito species. These approaches are classified into five classes: source reduction (environmental control), trapping (mechanical control), insecticides (chemical control), genetic control and biological control. An overview of conventional methods used for the eradication of mosquitoes is given in Figure 2.
Figure 2.
Overview of conventional methods used for the eradication of mosquitoes.
3.3.2. Source Reduction (Environmental Control)
Source reduction methods include averting Aedes spp. (mosquito) from using possible sites for breeding. These sites may consist of a varied range of containers or tanks from bottle covers to water reservoirs (Figure 2). This method is based on eradicating impermanent water tanks and closing permanent water reservoirs. This is frequently the first line of defence against mosquitoes such as A. albopictus, Ae. aegypti and Ae. atropalpus that favour small- and medium-sized artificial water containers and tires placed in or near homes. Source reduction also affects the dispersal of local mosquitoes like Culex spp. in the vicinity by restraining the existing sites for oviposition (process of laying eggs) [46,47,48].
Some breeding areas can be extremely fruitful for certain species. For example, the breeding sites for A. albopictus are catch sinks in northern Italy, ridged extension fountains in USA, and tank basins and tires in South Asia. Operative source reduction, specifically for Aedes spp., needs to be consistent and recurrent; e.g., washing or handling of water containers/vessels for daily use. Success depends largely on owner participation and public awareness. In a study in Brazil, a source lessening drive against Ae. aegypti was started using nylon nets to shelter the most productive sites such as water containers and metal barrels that resulted in a long-lasting drop in female mosquito populations, showing the usefulness of targeting preferable breeding sites. Regrettably, this method does not assist in tracing cryptic productive sites, which are often unseen or inaccessible such as natural reservoirs including leaf litter [49,50].
3.3.3. Trapping (Mechanical Control)
Mass trapping commonly uses odor to attract mosquitoes. For Aedes, trapping methods such as ovitraps or sticky/gravid traps target gravid females. For host-seeking females, the bio-agent-sentinel traps are used. Though the usage of bioagent-sentinel traps is commonly inadequate due to the necessity for electrical supply. Ovitraps uncover the tendency of Aedes mosquitoes to lay their eggs in small containers. An autocidal or larvicide inclusion permits the long-standing use of ovitraps with minor danger of it becoming a major source of adult mosquitoes. Insecticide-treated egg-laying strips are also being used [51,52].
To improve the attractivity of ovitraps, organic infusions (grass, hay and oak) can be applied. Gravid/sticky ovitraps with adhesive surfaces have also been advanced. To improve mosquito collections, researchers developed a bulky autocidal gravid trap with a higher release rate of water vapours and other volatile attractants [53]. As push-pull approaches, combining a repellent with some attractive incentives in tandem, are an effective control method against various mosquitoes. It uses the spatial repellent and contact nuisance of insecticides through insecticide-treated materials or indoor residual spraying. Still, it is important to point out that insecticide-treated materials and indoor residual spraying methods do not target exophylic mosquitoes such as Ae. albopictus [53,54]. There are some other traps like bed net traps, entry and exit traps, sentinel traps, capture-kill traps, host seeking traps, light traps and chemical based traps used for the control of Anophels species. Similarly, CDC traps (CO2 from dry ice) are used to catch 400–500% more mosquitoes [55,56].
3.3.4. Insecticides (Chemical Control)
Chemical control of mosquitoes is undertaken by using natural or synthetic chemicals (insecticides). This allows for instant mosquito reduction control when physical and biological methods are incapable to sustain mosquito numbers below certain thresholds. Larvicides are the compounds proposed to be applied directly to water to control larvae populations. Adulticides along with synergists are used in obscuring and spraying adult mosquitoes to control their numbers.
In the twentieth century, dichlorodiphenyltrichlroethane (commonly known as DDT) was the primary artificial organic insecticide that has been used for active malaria control by decreasing the life span of gravid female mosquitoes. Pyrethroids and insect growth regulators are the effective compounds used worldwide in mosquito control approaches, as adulticides and larvicides along with ovicidal properties possessed by some insect growth regulators such as methoprene pyriproxyfen and diflubenzuron [57].
A new strategy called auto-dissemination consists of manipulating female mosquitoes by contamination with insecticide (pyriproxyfen) through treated nets or diffusion stations designed from modified ovitraps. The method has proved to be effective in inducing high mortality rates of Ae. aegypti and Ae. albopictus at the pupal stage. Furthermore, production and hatchability of eggs were also seen to be affected. A new approach is to synergize the sterile insect technique (SIT) with auto-dissemination to release sterile male mosquitoes treated with pyriproxyfen to infect females during mating [58,59,60]. Common commercially available insecticides currently used are listed in Table 2.
Table 2.
Commonly used pesticides on the market against vectors.
| Name | International Union of Pure and Applied Chemistry Name | Molar Mass | Pack Size | Applications | Reference |
|---|---|---|---|---|---|
| Fendona 10% [C22H19Cl2NO3] |
[cyano-(3-phenoxyphenyl) methyl] 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate | 416.300 g/mol | 100, 200 and 1000 mL | It has a broad action spectrum against a large variety of insect pests in households and public facilities. | [61] |
| Lambda Cyhalothrin [C23H19ClF3NO3] |
cyano-(3-phenoxyphenyl) methyl] (1R,3R)-3-[(Z)-2-chloro-3,3,3-trifluoroprop-1-enyl]-2,2 dimethylcyclopropane-1-carboxylate | 449.854 g/mol | 250 and 500 mL | Targets aphids, butterfly larvae, cockroaches, mosquitoes and flies. | [62] |
| Deltamethrin [C22H19Br2NO3] |
Cyano (3-phenoxy-phenyl) methyl; 3-(2,2dibromoethenyl)-2,2-dimethylcyclopropanecarboxylate (CA); [partial diff]-cyano-m-phenoxy benzyl, (1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethyl -cyclopropanol-carboxylate, (S)-[partial diff]-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-carboxylate | 505.240 g/mol | 25 g/L | Control of the malaria vector as a stomach poison for insects. | [63] |
| Fenpropathrin [C22H23NO3] |
2,2,3,3-Tetramethylcyclopropane carboxylic acid cyano(3-phenoxyphenyl) methyl ester | 349.42 g/mol | 1000 L (mini-mum order) |
Controls a variety of pests; specifically, mites present in fruits and vegetables. | [64] |
Plant-based insecticides received much attention, recently, due to their safe, cheap and environmentally friendly nature. Plants have a diversity of phytochemicals (used for defence) that can be applied to lessen insect outbreaks. In chemical ecology, the assessment of how specific chemicals (allelochemicals) are implicated in organism interactions with each other and with their environments is studied. These allelochemicals serve their purpose as defence mechanisms and effect molecular targets such as cellular proteins, enzymes, nervous system signal transduction (synthesis of neurotransmitter, their storage and release as well as receptor binding) and metabolic pathways in herbivores or microbes [65,66].
The secondary metabolites (phenols, alkaloids, slavanoids, sterols, terpenes, carotenoids etc.) of plants protect them against microbial parasites and arthropods; e.g., Chloroxylon swietenia against Anopheles gambiae, Culex quinquefasciatus, Aedes aegypti and Calocedrus decurrens, Juniperus occidentalis against adult Ae. aegypti, and Eucalyptus tereticornis against established Anopheles stephensi.
Plant extract-based larvicides such as essential oils, terpenes and their corresponding elements disturb biochemical processes, particularly effecting the endocrinological balance, neuromuscular fatigue, respiratory capability, neuroexcitation as well as neuro-inhibition, which results in energy depletion, oxygen deprivation, immobility and paralysis that ultimately leads to death (Table 3) [66,67,68,69].
Table 3.
Plant-extracted essential oils and their mechanisms of action (after [65]).
| System | Active Compound | Mechanism | Plant | References |
|---|---|---|---|---|
| Cholinergic system | Essential oils | Acetylecholinestrase (AChE) inhibition | Azadirachtina indica, Lavendula spp. and Mentha spp. | [70] |
| Veratrin | Nerves sodium channels | Schoenocaulon officinale | [71] | |
| Nicotine | Cholinergic acetylcholine nicotinic receptor agonist/antagonist | Nicotiana spp., Haloxylon salicornicum, Delphinium spp. and Stemona japonicum | [72] | |
| GABA system | Thymol and Silphinenes | GABA-gated chloride channel | Thymus vulgaris | [73] |
| Mitochondrial system | Pyrethrin | Potassium ion and sodium exchange disturbance | Crysanthemum cinerariaefolium | [74] |
| Rotenone | Cellular respiration inhibitor (mitochondrial complex I electron transportinhibitor or METI) | Lonchocarpus spp. | [75] | |
| Ryanodine | Calcium channel disturbance | Ryania spp. | [76] | |
| Sabadilla | Alter nerve cell membrane action | Schoenocaulon officinale | [77] | |
| Octopaminergic system | Essential oils | Octopaminergic receptors | Cedrus spp., Pinus spp., Citronella spp. and Eucalyptus spp. | [78,79] |
| Thymol | Block octopamine receptors by functioning through tyramine receptors cascade | Thymus vulgaris | [78,80] | |
| Miscellaneous | Azadirachtin | Anti-mitotic (G2/M Phase) | Azadiractina indica | [71] |
However, it has been proposed that plant extracts, which comprise of several chemicals, are more complex in nature as compared to synthetic insecticides and, therefore, the mixture of physiological and behavioural actions of botanical insecticides prevents the development of resistance.
3.3.5. Biological Control
In recent years, bio-pesticides are receiving increased interest as an effective mosquito control program due to their role in promoting vertebrates’ safety and lowered environmental impact. A number of organisms were discovered as potential bio-pesticides for mosquito control including bacteria, fungi, viruses, protozoa and fish. However, most of these organisms were not considered convenient for the development of an effective mosquito control program due to their limited supply. Some of the spore-forming bacteria such as B. thuringiensis serovar israelensis deBarjac (Bti) and Bacillus sphaericus Neide (Bs) were investigated against operational Ae., Anophels and Cx mosquito populations as they already showed highly toxic effects to such dipteran species. Bti bacteria spores conduct a broader range of activities against Ae. spp., Culex and Anophels Bs is highly specific to certain species and acts only against some Ae. species and majorly effect Culex species [81].
In B. sphaericus (Bs) bacteria, two insecticidal proteins, BinA with 42 kDa and BinB with 51 kDa (known as Binary toxin btx) have shown to be highly toxic for mosquitoes. BinA Recombinant bacteria comprising these toxins separately confirmed that BinA is extremely toxic at high dose without any need of BinB, but BinB alone does not show any toxicity. However, their synergistic effect revealed that their equal concentrations have extreme toxicity to larvae. When the mosquito larvae ingest btx, BinA and BinB solubilize in the midgut, where the proteases process them into 39 kDa and 43 kDa proteins. After processing, btx-active proteins are coupled to receptors present on the brush border membrane of the midgut, where the internalization of active proteins occurs leading to the cell lysis, and ultimately, lysis and inflammation of midgut, which results in the insect’s death. Another mosquitocidal toxin Mtx (100 kDa) appears to be highly toxic in protease negative Bs strains that has been synthesized in low toxicity strains [82,83,84,85,86,87,88].
In Bti bacteria, two insecticidal protein groups Cyt (cytolysins) and Cry (crystal delta-endotoxins) were also shown to be effective for mosquito control [50]. Bti toxins contain four polypeptides: Cry IVA (125 kDa), Cry IVB (135 kDa), Cry IVD (68 kDa) and Cyt A (28 kDa). They are assembled into crystals during sporulation. The genes encoding these Cry toxin proteins are present on the plasmid of the bacterium [89,90]. Cry proteins bind to definite receptors present on the midgut epithelium of the insect, while Cyt proteins do not distinguish specific sites present in the midgut. After ingestion, Bti crystals are processed into smaller fragments by proteases. The activated fragments interrupt the osmotic equilibrium of epithelium cells through making pores into the membrane causing cell death. Most of the mosquitoes die within hours due to a paralyzed gut [91,92,93].
However, there are some resistance problems with these insecticides, because of the insects’ increased evolutionary rate. The factors linked to an increased evolutionary rate are elevated productive rates of species, increased and larger number of progenies, shorter generation times, and higher genetic variability among local species [94].
Recently, genetic engineering techniques are being used for improving bacterial larvicides for mosquito control. The recombinant bacteria of Bti and Bs strains are ten times more effective than wild strains that are also being used for larger scale larvicides production. The recombinant strains comprise all the endotoxins of Bti (Cry4A, Cry4B, Cry11A, and Cyt1A) along with Bs (Binary toxin). The synergy of these toxins will eradicate mosquito populations more effectively [95,96].
3.3.6. Genetic Control
Genetic approaches are attaining greater attention as alternatives to conventional methods for mosquito control. Dissemination of various factors that reduce mosquito damage by mating is defined as genetic control. Such heritable factors are introduced into target species so that the modified mosquito acts as biocontrol agent and their dependence on vertical capacity distinguishes them from biological control methods. Genetic strategies are classified based on their intended outcome into population suppression, population replacement and other key aspects of genetic element persistence [96].
Population suppression programs decrease the number of vectors in the target species, which is comparable to the purpose of insecticide-based strategies. For example, the sterile-male program is intended to suppress the target population when sufficient numbers of sterile males are released. These modified sterile males are disseminated in the environment to mate with unmodified females resulting in the death of the progeny of this mating, and can potentially lead to population eradication [87]. Additionally, population replacement methods diminish the midgut transmission capability of nearly all of the mosquitoes’ present in the target population. For example, a new character (trait) like lower capability to spread a pathogen into the specific mosquito population will be advantageous for humans, while probably being lethal for the mosquito population [97,98].
Persistence or invasiveness is the ability to which the alteration (trait) will be present or disperse in the target group after its release. Concerning self-limiting strategy, the trait has the tendency to vanish from the target mosquito population until replaced by the release of further altered mosquitoes’ periodically. In self-sustaining strategy (gene drive system), use of the proposed alteration can persevere forever and possibly even disperse inside the primary target species or to other species [99].
Regarding the self-limiting strategy, as through the use of sterile male systems, the mutations or inheritable lethal genes are introduced where only a females’ offspring is affected [100]. Sterile insect techniques (SIT), where DNA-damaging agents like γ-radiations are used to introduce lethal mutations and nucleases in the germ line, are expressed to cause chromosomal breaks [101]. Another approach, release of insects carrying a dominant lethal (RIDL) genetic system can be applied to induce toxic gene expression in zygote relative to the father providing more flexibility (to act at a certain time in development), but at the same time make this strategy more vulnerable to the environment [102].
Sterility can also be introduced by diverse strains of Wolbachia bacteria by artificial infection. Wolbachia is maternally transmitted through the mother to her offspring. Female-killing systems can also offer “sterilization”, where released male offspring are homozygous for a female-specific lethal gene that will result in inheritance of the female lethal gene copy; as an effect, offspring (daughters) would die. This approach is known as female-specific RIDL (fsRIDL). It provides mosquito population control much like classical SIT [102,103].
In self-sustaining systems, gene drive is the common phenomenon where all selfish-DNA constituents are introduced that can persist over a target population without giving a suitable benefit to the mosquitoes’ carrying them. Many selfish-DNA elements are known that are being used such as invasive Wolbachia, transposons, synthetic Medea-like elements and the Homing Endonuclease Gene (HEG) [104,105]. The HEG is expressed either as separate genes within introns, as fusions with host proteins or lethal gene where they catalyse the genomic DNA hydrolysis within the cells that produce them.
Artificial Medea-like elements, originating from the naturally present selfish Medea elements of Tribolium castaneum have been created. The method uses reasonably intended synthetic elements that have the ability to replicate the inheritance pattern of Medea deprived of deriving its molecular components [106]. Furthermore, Wolbachia strains are selfish-DNA elements, which can spread by target mosquito species through altering their host’s reproductive biology (generating CI to kill uninfected females).
Gene drive is another technology to propagate a particular gene by altering the probability from the natural to transmit a specific allele in the next generation. This can be performed in different ways to control mosquito species. However, a detailed discussion is beyond the scope of this review.
3.3.7. Integrative Vector Management Approach
This approach is used to achieve globally-set targets to control vector-borne diseases. This includes situation analysis, monitoring and evaluation, surveillance and capacity building. The overall purpose is to apply all strategies to control larvae, adults and bites from mosquitoes. The overall use of biological, chemical and physical methods for the control of mosquitoes is encouraged. Integrated practices are more advisable and a sustainable approach for lowering the mosquito population is advantageous. Appropriate ecofriendly plans and strategies should be adopted [8].
4. Nanotechnology-Based Methods
4.1. Overview
Nanotechnology-based methods have revolutionized a varied area of research fields including entomology. Nanotechnology has a good potential to combat climate change. There is a possibility to develop nanomaterials in the environment naturally. Temperature change can influence the reaction rate and disturb the formation and synthesis of nanoparticles [107]. Nanoparticles show a high antimicrobial activity against various microbes, because of their large surface to volume ratio that is responsible for their high catalytic activity. Nanoparticles have been testified as valuable when compared to chemical insecticides targeting adult mosquitoes and young larval instars. This is likely due to the fact that the overuse of insecticides has led to unfavorable consequences on the environment, plants and human health, along with the insecticide resistance development in targeted populations. Currently, a number of nanomaterials are being used to control mosquito vector-borne diseases.
4.2. Nanoliposomes
Liposomes are synthetic nano-assemblies up to few hundred nanometers in diameter comprising one or more outer phospholipid bilayers enclosed by an aqueous core. As a nano-carrier, both hydrophobic and hydrophilic particles can be captured within aqueous core and outer lipophilic layers, respectively. Nanoliposomes are also immunologically compatible due to their targeted uptake by cells through surface adjuvants such as antigens, antibodies and cell-associated specific ligands [108,109]. Nanoliposomes have been already used for malarial treatment as well as for the delivery of malaria vaccine. At present, efficient malarial therapy is restricted due to their deadly side effects and the potential for development of drug resistance. However, the encapsulation of a therapeutic drug (chloroquine) within nanoliposomes can effectively adjust the bioavailability and drug dose within the body that significantly lessen the drug toxic effects, and the risk of drug resistance [110].
Currently, innovative gel-core nanoliposomes that use a composite of polymer and lipid-based carrier systems have been designed and confirmed for the controlled delivery of Pfs25 along with CpGODN (immune-stimulatory adjuvant) as malarial vaccine. In gel-core nanoliposomes, the integration of a polymer into the interior aqueous part of the liposome increases its stability, permitting slower drug release. The slow diffusion through the polymer gel core and phospholipid bilayers actively controls the drug release rate, which enables the capability of nanoliposomes for lasting antigen persistence without the need of boosting [111].
4.3. Nanosuspensions
The biphasic approach containing drug particles of submicron-sized dissolved in an aqueous solution is defined as nano-suspensions, prepared through top-down and bottom-up techniques to lower the size of particles. The purpose of using nano-suspensions is that their lower size increases their surface area, providing higher bioavailability of the drug and lowers its toxicity [112].
The anti-malarial drug Lumefantrine is used to treat multi-drug resistant malaria by inhibiting the ability of P. falciparum to convert heme into toxic hemozoin. The increased accumulation of toxic heme in a parasite causes its death and stops the malarial infection. However, Lumefantrine has lower water solubility that can lead to impaired bioavailability through oral or dietary route [113,114]. Nano-suspensions based drug delivery can be useful to combat malarial infections. Improved antimalarial activity of lumefantrine-based nano-suspensions by reducing its size from 72 μm to 0.251 μm through in-vitro and in-vivo experiments against P. Yoelii nigeriensis and P. falciparum has been observed [115].
The antimalarial action of the dihydroartemisinin (DHA) drug against P. falciparum in in-vitro trials has been demonstrated. The lower concentration of drugs in nanosuspensions have decreased side effects other than antimalarial activity [108].
4.4. Polymer-Based Nanoparticles
Polymer-based nanoparticles are biodegradable and biocompatible solid-colloidal particles ranging from 1 to 1000 nm in size. The second most plentiful polysaccharide is chitin-derived. Chitosan nanoparticles have many advantages owing to their increased stability, biodegradability, biocompatibility, water solubility, controlled drug release, lower toxicity, non-immunogenic and ease-of-fabrication methods, which make them ideal candidates for mosquito control [116,117].
Primaquine (PQ) is a commonly used anti-malarial drug that opposes the relapsing form of malaria by P. vivax and P. ovale plasmodium parasites. However, PQ has some adverse effects due to non-specific targeting, its lower bioavailability and short half-life. Reformulation of PQ into chitosan-based nanoparticles can be a promising approach to overwhelm these side-effects. Scientists tested PQ-loaded solid lipid nanoparticles that showed effective vector reduction with increased half-life, reduced side effects and increased bioavailability compared to the conventional PQ drug [115,116,117,118].
Chitin is a major component of all insects including mosquitoes. It is part of their exoskeleton cuticle and other tissues like foregut, midgut, hindgut and trachea. Chitin biosynthesis is entirely dependent on the chitin synthases enzymes by catalysing the transmission of sugar from triggered sugar donors to definite acceptors present in mosquitoes. CHS-A is a chitin synthesis gene that is present in the cuticle and foregut, hindgut and trachea tissues, while the chitin synthesis gene CHS-B is specifically linked to the midgut. Epithelial cell’s chitin biosynthesis is associated with the peritrophic matrix (PM) [119,120,121].
RNA interference (RNAi) technology is an eco-friendly targeted approach to control mosquito vectors through small interfering RNA (siRNA) activated post-transcriptional gene silencing [114]. Recently, scientists have developed chitosan/dsRNA-based nanoparticles against the African mosquito Anopheles gambiae to silence the chitin synthase genes CHS-A and CHS-B [117].
Mosquito growth and development mostly depends on chitin synthases. Consequently, chitin synthase genes are ideal targets to eradicate mosquito vector populations. Other polymeric nanoparticles such as poly glycolic acid (PGA), poly d, l-lactic acid (PLA), poly d, l, -lactic-co- glycolic acid (PLGA) and poly Ɛ-caprolactone (PCL) are also being used to encapsulate drug active-compounds due to their increased drug stability, control drug release and lower toxicity [122,123].
The primaquine (PQ) drug can be encapsulated in polymeric nanoparticles to control malarial parasite infection [124,125]. Reserachers synthesized nano-chloroquine (Nch) particles from 150–300 nm. Nch particles were tested against liver and spleen resulting in ~29% and ~37% reductions in liver and spleen damage, respectively, compared to conventional chloroquine [126].
4.5. Green-Synthesized Metallic Nanoparticles
The misuse of chemical insecticides is linked to major concerns regarding their toxicity and ecological problems, but green synthesized nanoparticles have reported to be advantageous, because they are eco-friendly and cost-effective compared to conventional methods. For an effective vector control approach, appropriate formulations of botanical-based insecticides need to be improved in terms of their persistence; green synthesized nanoparticles with suitable reducing and capping agents are employed to attain their optimal activity with reduced size and different shape. There are several biophysical techniques such as UV-vis spectroscopy for nano-synthesis monitoring over time, Fourier Transformed Infrared Spectroscopy (FTIR) for functional group studies involved in capping and reduction, Energy Dispersive X-ray (EDX) for elemental composition analysis and X-ray diffraction (XRD) spectroscopy for analysing crystalline structures of nanoparticles [127].
The efficacy of green-synthesized Ag nanoparticles obtained from Dicranopteris linearis, Chenopodium ambrosioides, Aristolochia indica, Gracilaria edulis, Couroupita guianensis and Phyllanthus niruri plant extracts was validated against Anopheline aedine and Culicinae spp. mosquito vectors. The LC50 values of these nano-larvicides was in the range of 1 to 30 μg/mL and the complete eradication of these larval species was recorded within 72 h after first treatment [128,129].
Egg hatchability of Ae. aegypti and Culex Quinquefasciatus was 100% decreased after exposure to green-synthesized Ag nanoparticles (30 μg/mL) from Sargassum muticum. Ag-nanoparticles from D. linearis and S. maritima also reduced the Ae. aegypti egg hatchability after their treatment with 25 μg/mL and 20 μg/mL concentrations, respectively. The Culex Quinquefasciatus eggs were resistant to larvicidal effect of green-synthesized nanoparticles in comparison to Anophels stephensi and Ae. aegypti. Lately, Rubus ellipticus-synthesized Ag nanoparticles showed no hatchability of Ae. aegypti, Anophels stephensi and Culex Quinquefasciatus with single dosage of 75 μg/mL, 90 μg/mL and 60 μg/mL, respectively [130].
Similarly, a number of leaf extract-based Ag nanoparticles have shown their adulticidal activity against Ae. albopictus, Ae. Aegypti, Anophels stephensi and Culex Quinquefasciatus. Unfortunately, no possible toxicity mechanism of ovicidal and adulticidal green-nanoparticles is known, yet [128]. However, it has been assumed that nanoparticle toxicity against mosquito young larvae is because of their ability to penetrate into exoskeleton and their binding to DNA phosphate groups and sulphur-rich proteins. Consequently, this results in DNA alterations, rapid protein degradation and a decrease in membrane permeability, which causes loss in cell function and cell death [129,130,131].
Recently, some studies reported that nanoparticle exposure to Ae. Aegypti and Anophels Stephensi results in adult longevity and female fecundity. Hypnea musciformis-fabricated Ag nanoparticles reduced the longevity of Ae. Aegypti males from 15.64 to 5.32 days and Ae. Aegypti female longevity from 26.87 to 17.34 days. Similarly, exposure of these nanoparticles to Ae. Aegypti results in the reduction of female fecundity from 150 eggs to 98 eggs after 50 ppm dosage.
P. aquilinum-fabricated Ag nanoparticles (50 ppm) reduced the longevity of Anophels Stephensi males and females from 21.44 to 9.90 days and from 37.18 to 20.90 days, respectively. While, post-treatment of 50 ppm nanoparticles reduced the Anophels Stephensi female fecundity from 145 to 66 eggs [132,133]. This method might indicate a way to control mosquito-borne diseases more effectively.
5. Drive to Transform Sick to Healthy Life under Changing Climatic Conditions
Mosquitoes are likely to benefit from climate change [8]. Apart from suitable mosquito breeding sites, temperature, precipitation and absolute humidity of more than 60% can affect parasite transmission in malaria. As the globe is continuously warming, more incidences of dengue and malaria as well as an increase in the distribution of mosquitoes have been related to climate change. The range of disease-vectors may not only increase in latitude but also in altitude. As the mosquito population is increasing globally with time, they will colonize new suitable areas where the temperature is warmer fulfilling their needs.
Due to warmer temperatures, the cell metabolism of mosquitoes will also increase as chemical reactions are increasing. In warm temperatures, mature females feed more frequently and digest blood rapidly, thus producing more progeny. Hard-freeze areas in the winter season have less mosquitoes as they cannot breed during this time and usually eggs are destroyed. However, some species are possibly able to live during the winter season, if the temperature is not too low. At higher temperatures, mosquitoes hatch at a higher rate, subsequently their rate of development and reproduction will rise exponentially [123], and the range of mosquitoes-borne diseases will increase as well [6].
As mosquitoes are poikilothermal, the development of parasites in the mosquito is reliant on the external temperature. For P. vivax and P. falciparum, the nominal temperature at which the development of parasites can be accomplished is 16.5 °C and 14.5 °C, respectively. Higher temperatures accelerate their development, because sporozoites are formed quickly. It follows that the life cycle is completed sooner. Therefore, the likelihood of malarial infection will increase with higher temperatures. There is an inverse relationship between the longevity of mosquitoes and temperature. At higher temperatures, mosquitoes and their parasites die. There is an optimum temperature range for Plasmodium transmission (22–28 °C for P. vivax and A. messeae, and 26–32 °C for P. falciparum and A. gambiae) [134].
The number of females that laid eggs and the number of eggs laid are influenced by both temperature and humidity (Figure 3). A reduction in oviposition rate was detected with increase in temperature, while the intensity of the reduction was affected by humidity. At high temperature (35 °C) and low humidity (relative humidity of 60%), a low oviposition rate (55 ± 4.8 eggs) was observed for adult females. On the other side, at a low temperature (25 °C) and high humidity (relative humidity of 80%), a high oviposition rate (99 ± 3.6 eggs) was noted (Table 4) [135].
Figure 3.
Fertility rates of eggs sustained at different temperatures and humidity values: (a) relative humidity (rh) of 60%; and (b) relative humidity (rh) of 80%; n = number of eggs (adopted from [135]).
Table 4.
Mean number of eggs laid by Aedes aegypti females at different temperature and humidity values (after [135]).
| Temperature | Relative Humidity of 60% | Relative Humidity of 80% | ||
|---|---|---|---|---|
| Eggs (Sample Number) |
Oviposition Variation | Eggs (Sample Number) |
Oviposition Variation | |
| 25 °C | 85.99 ± 3.16 (102) | 4–160 (37.25%) | 99.08 ± 3.56 (92) | 4–155 (55.43%) |
| 30 °C | 82.89 ± 3.33 (111) | 2–143 (37.84%) | 75.75 ± 5.03 (75) | 1–144 (45.33%) |
| 35 °C | 54.53 ± 4.81 (55) | 1–126 (14.55%) | 59.62 ± 3.41 (79) | 2–132 (7.59%) |
| Total | 78.25 ± 2.2 (268) | 79.29 ± 2.53 (246) | ||
A high humidity is suitable even up to saturation (80% or more of relative humidity) at 28 °C. At comparatively lower temperatures (18–20 °C), life expectation may be very substantial even at low humidity and mosquitoes can be kept alive if given water only (without any food) for several weeks. At a temperature of 25 °C and relative humidity of 70% with water provided, low mortality was observed among females earlier the seventh day either fed or not. The duration of life under such conditions without food is about 6 to 8 days. A maximum of 12 days of life expectancy was observed. However, at relatively high temperatures (28 °C) and in the absence of high humidity, a considerable mortality of males was observed even soon after emergence. After the seventh day of emergence, mortality among unfed males and females increased quickly and a high mortality by the tenth day was noted. Few mosquitoes were alive at the fourteenth day at 25 °C even at high humidity and with water given [123,135,136,137].
A temperature below freezing appeared to be fatal for the adults, if continued for 24 h. At freezing temperature, the mosquitoes are completely immobilized. At 4 °C for 1 h, all mosquitoes survived, but extended exposures at 4 °C killed them. However, between 7 and 9 °C, Ae. aegypti mosquitoes were alive for about 82 days. Moreover, half of the females were alive after 30 days. The optimal temperature for mosquitoes is usually around 28 °C. An increase in temperature would be progressively disadvantageous for survival. More than 40 °C is lethal for mosquitoes [138,139].
In order to address climate change, there are two common strategies: mitigation and adaptation. These approaches are interrelated with each other, but the basic difference is that adaptation is linked to the response to the fluctuating climate changing the lifestyle in which humans live as a society, while mitigation means restraining climate change, mainly by lowering greenhouse gas emissions.
Scientists are working to improve the efficacy and availability of alternative energy approaches. To increase domestic energy efficiency, simple measures such as to drive less by biking and use of public transportation would play important roles in reducing climate change worldwide. To limit climate change, our priority should be on changing our own behaviour by replacing to energy sources that release greenhouse gases [140]. Alternative approaches to reduce emissions and lowering temperature can decrease the impacts on human health. Furthermore, adaptation to a changing climate is also important. There is a need for thoughtful planning to minimize hazards.
6. Pakistani Perspective with Reference to Other Countries
Climate change contributes to the spreading of diseases. Conferring to the World Health Organization (WHO), the most fatal vector-borne malarial disease claimed 627,000 lives worldwide in 2012. However, a fast-growing vector-borne disease is dengue; up to 30-fold rise in disease occurrence during the last 50 years [4,5].
Vector-borne diseases add to most health-related complications in developing countries. Since 2005/6, Pakistan is facing steady epidemics of dengue and dengue haemorrhagic fever (DHF). Since 2010, Pakistan has been severely impacted by dengue fever, which led to 16,580 confirmed cases and 257 deaths in Lahore alone. Almost 5,000 confirmed cases and 60 deaths in the other parts of the country have been reported. The entire country is susceptible to dengue through the changeable trends in climate. Pakistan has been categorized in “group three” (Eastern Mediterranean Region) by the World Health Organization together with countries such as Afghanistan, Djibouti and Somalia due to the current alarming situation of vector-borne diseases with an estimated impact of 1.5 million cases annually [7,140]. Worldwide statistical data on dengue cases and their mortality are shown in Table 5 [141].
Table 5.
Statistical data on dengue cases and the corresponding mortality during the period between 2009 and 2017.
| Reports | Saudi Arabia | Sri Lanka | Pakistan | Nepal | Malaysia | India | China | Bangladesh |
|---|---|---|---|---|---|---|---|---|
| Reported cases | 1600 | 35,008 | 1085 | 30 | 41,486 | 17,000 | 322 | 510 |
| Mortality | N/A | 346 | 13 | 0 | 90 | 105 | 0 | 0 |
| Reported cases | 2200 | 34,188 | 11,024 | 917 | 46,171 | 28,000 | 260 | 405 |
| Mortality | N/A | 229 | 40 | 5 | 135 | 112 | 0 | 0 |
| Reported cases | 2400 | 28,473 | 17,057 | 79 | 19,884 | 19,000 | 160 | 1310 |
| Mortality | N/A | 246 | 219 | 0 | 38 | 171 | 0 | 8 |
| Reported cases | 800 | 44,461 | 639 (Karachi only) | 183 | 21,900 | 50,000 | 610 | 750 |
| Mortality | N/A | 220 | 4 | 0 | 37 | 240 | 0 | 1 |
| Reported cases | 4200 | 32,063 | 4388 | 642 | 43,346 | 70,000 | 4779 | 1790 |
| Mortality | N/A | N/A | 32 | 0 | 95 | 140 | 0 | 3 |
| Reported cases | 1950 | 47,502 | 1400 | 355 | 108,698 | 40,571 | 47,056 | 355 |
| Mortality | N/A | N/A | 16 | 0 | 215 | 137 | 6 | 0 |
| Reported cases | 4200 | 29,777 | 3900 | 1500 | 120,836 | 99,913 | 4230 | 3162 |
| Mortality | N/A | N/A | 11 | 0 | 336 | 220 | 0 | N/A |
| Reported cases | N/A | 55,150 | 2500 | 594 till October | 100,028 | 1,29,166 | 2098 | 6010 |
| Mortality | N/A | N/A | 3 | N/A | 231 | 245 | N/A | N/A |
| Reported cases | N/A | 185,688 | N/A | 854 till October | 82,840 | 1,53,635 | 3195 | N/A |
| Mortality | N/A | N/A | N/A | N/A | 171 | 226 | N/A | N/A |
The underprivileged poor in exposed societies existing in distant rural areas with restricted access to health amenities suffer the most. Inappropriate settlements in slums that are without any satisfactory water and sanitation systems are also a challenge. Due to poor living environments, vector-borne diseases flourish. Increasing temperatures and humidity levels are likely to elevate vector-borne disease transmissions such as dengue, malaria, yellow fever and encephalitis. Different studies concluded that an increase in average temperature will enhance the reproduction cycle of the dengue fever virus.
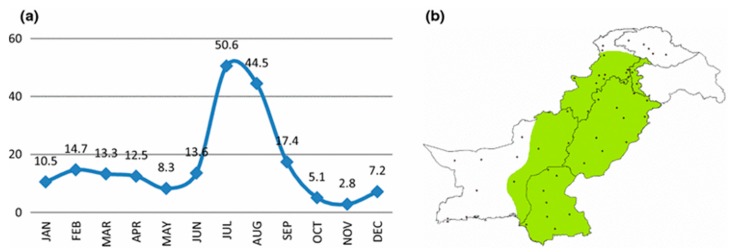
Changing climatic conditions in terms of temperature, rainfall and humidity as well as low levels of immunity among poor populations enhance malaria outbreaks. A temperature range between 20 and 30 °C, a humidity level above 55% and the presence of breeding sites in standing water to rainfall are optimal parameters for development, longevity and transmission of malaria. In Pakistan, vector-borne diseases (predominantly dengue fever and malaria) proliferate after the monsoon. From July to September, more than 80% of total vector-borne diseases are reported every year. The highest numbers of incidences have been reported for the Punjab and followed by Balochistan, Federally Administered Tribal Areas and Khyber Pakhtunkhwa in this order [142]. The monthly rainfall (mm) in Pakistan and locations of meteorological stations are shown in Figure 4. The indicated monsoon zone corresponds to the most affected areas [143].
Figure 4.
(a) Monthly area weighted rainfall (mm) for Pakistan; and (b) locations of meteorological stations that are available for climate data. Note that the green color shows the monsoon zone (adopted from [143]).
7. Conclusions
To fight drug and insecticide resistance of mosquitoes developed through conventional treatment methods to prevent dengue and malaria outbreaks, nano-based technology that is considered to be health- and eco-friendly such as food grade nanoparticles can be used. Food grade nanoparticles have no side effects compared to metallic nanoparticles. They are both eco- and human-friendly. In order to overcome complications, transgenic mosquitoes (genetically modified) and a combination of drugs have been tested. However, these methods are challenging, because mosquito trans-genes are destructed, and multi-drugs can be found in their bodies. Nanomedicines can be anticipated and might aid treatment for malaria and dengue fever. A multi-drug combination employing nanoparticles could be promising. Specific consideration should be given to metallic like Zno nanoparticles, food grade nanoparticles like Curcumin and biodegradable nanoparticles with multi-drug encapsulation to slow down the evolutionary progression. Especially food-based nanoparticles, which have low toxicity compared to metallic drugs, multi-drugs and bio-drugs, might help to eradicate vector-borne diseases in developing countries such as Pakistan in the future. Further investigations are required to test the larvicidal activity of food-based nanoparticles against the larvae of mosquitoes causing malaria and dengue fever.
Outlook
The following recommendations are made to people to stay healthy:
An environmental management approach is very important for the control of breeding sites of mosquitoes especially during and after monsoons. These sites include open drainage, marshes and standing water in pots.
Control of breeding sites as a result of increased rainfall and change in rainfall pattern due to climate change, which increases the vector population like mosquitoes and provide more breeding spaces.
Awareness campaign among the general public at community level and in schools regarding the spread and control of malaria and dengue fever (especially the time period for the diseases).
Personal protective approaches include use of proper clothing, nets, mosquito repellent lotions and other protective measures in homes, businesses, schools and offices.
Allocation of enough funds by the authorities for health departments to control the spread of malaria and dengue fever in the community.
Use of biological, chemical and physical methods to diminish mosquito-related diseases.
Acknowledgments
The authors gratefully acknowledge funding and support received by the Higher Education Commission for the project entitled “Elucidation of Larvicidal Effects of Metallic Nanoparticles against Vectors Causing Malaria and Dengue Fever”. The support received from Muhammad Usman (research associate), who worked with this project for few months, is highly acknowledged.
Author Contributions
T.A. project administration, funding acquisition, conceptualization and writing of the initial draft paper. I.L. writing of section drafts and data collection. M.Z.H. support in writing draft sections and data collection. M.S. writing and editing of the review paper.
Funding
This research was funded by the Higher Education Commission, Government of Pakistan; grant No: 5424/Federal/NRPU/R&D/HEC/2016.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization Dengue Control. [(accessed on 28 March 2019)]; Geneva. Available online: http://www.who.int/denguecontrol/en.
- 2.World Health Organization Neglected Tropical Diseases. [(accessed on 28 March 2019)]; Geneva. Available online: http://www.who.int/neglected_diseases/diseases/en.
- 3.World Health Organization World Health Day—7 April 2014. [(accessed on 28 March 2014)]; Geneva. Available online: http://www.who.int/campaigns/world-health-day/2014/event/en.
- 4.World Health Organization World Malaria Report 2013. [(accessed on 28 March 2019)]; Geneva. Available online: http://www.who.int/malaria/publications/world_malaria_ report_2013/en.
- 5.WHO Malaria vaccine: WHO position paper-January 2016. Vaccine. 2018;36:3576–3577. doi: 10.1016/j.vaccine.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 6.Deichstetter P. The Effect of Climate Change on Mosquito-Borne Diseases. Am. Biol. Teach. 2017;79:169–173. doi: 10.1525/abt.2017.79.3.169. [DOI] [Google Scholar]
- 7.Githeko A.K., Lindsay S.W., Confalonieri U.E., Patz J.A. Climate change and vector-borne diseases: A regional analysis. Bull. World Health OrgAnophels. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- 8.Haider Z., Ahmad F.Z., Mahmood A., Waseem T., Shafiq I., Raza T., Qazi J., Siddique N., Humayun M.A. Dengue fever in Pakistan: A paradigm shift; changing epidemiology and clinical patterns. Perspect. Public Health. 2015;135:294–298. doi: 10.1177/1757913915599019. [DOI] [PubMed] [Google Scholar]
- 9.Yi H., Devkota B.R., Yu J., Oh K., Kim J., Kim H. Effects of global warming on mosquitoes & mosquito-borne diseases and the new strategies for mosquito control. Entomol. Res. 2014;44:215–235. doi: 10.1111/1748-5967.12084. [DOI] [Google Scholar]
- 10.Mohammed A., Chadee D.D. Effects of different temperature regimens on the development of Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes. Acta Trop. 2011;119:38–43. doi: 10.1016/j.actatropica.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Eisen L., Monaghan A.J., Lozano-Fuentes S., Steinhoff D.F., Hayden M.H., Bieringer P.E. The Impact of Temperature on the Bionomics ofAedes (Stegomyia) aegypti, With Special Reference to the Cool Geographic Range Margins. J. Med Èntomol. 2014;51:496–516. doi: 10.1603/ME13214. [DOI] [PubMed] [Google Scholar]
- 13.Hamlet A., Jean K., Perea W., Yactayo S., Biey J., Van Kerkhove M., Ferguson N., Garske T. The seasonal influence of climate and environment on yellow fever transmission across Africa. PLoS Negl. Trop. Dis. 2018;12:e0006284. doi: 10.1371/journal.pntd.0006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madico G., McDonald J., Gilman R.H., Cabrera L., Sterling C.R. Epidemiology and Treatment of Cyclospora cayetanensis Infection in Peruvian Children. Clin. Infect. Dis. 1997;24:977–981. doi: 10.1093/clinids/24.5.977. [DOI] [PubMed] [Google Scholar]
- 15.Moore P.S. Meningococcal Meningitis in Sub-Saharan Africa: A Model for the Epidemic Process. Clin. Infect. Dis. 1992;14:515–525. doi: 10.1093/clinids/14.2.515. [DOI] [PubMed] [Google Scholar]
- 16.Magori K., Drake J.M. The Population Dynamics of Vector-borne Diseases. Nat. Educ. Knowl. 2013;4:14. [Google Scholar]
- 17.Kovats R.S., Campbell-Lendrum D.H., McMichael A.J., Woodward A., Cox J.S. Early effects of climate change: Do they include changes in vector-borne disease? Philos. Trans. R. Soc. Lond. Ser. B. Biol. Sci. 2001;356:1057–1068. doi: 10.1098/rstb.2001.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley D.J. Human tropical diseases in a changing environment. Ciba Found. Symp. 1993;175:146–162. [PubMed] [Google Scholar]
- 19.Lindsay S.W., Birley M.H. Climate change and malaria transmission. Ann. Trop. Med. Parasitol. 1996;90:573–588. doi: 10.1080/00034983.1996.11813087. [DOI] [PubMed] [Google Scholar]
- 20.Beserra E.B., Fernandes C.R.M., Silva S.A.D.O., Da Silva L.A., Santos J.W. Efeitos da temperatura no ciclo de vida, exigências térmicas e estimativas do número de gerações anuais de Aedes aegypti (Diptera, Culicidae) Iheringia. Série Zool. 2009;99:142–148. doi: 10.1590/S0073-47212009000200004. [DOI] [Google Scholar]
- 21.Marinho R.A., Beserra E.B., Bezerra-Gusmão M.A., Porto V.D., Olinda R.A., dos Santos C.A. Effects of temperature on the life cycle, expansion, and dispersion of Aedes aegypti (Diptera: Culicidae) in three cities in Paraiba, Brazil. J. Vector Ecol. 2016;41:1–10. doi: 10.1111/jvec.12187. [DOI] [PubMed] [Google Scholar]
- 22.Gubler D.J., Reiter P., Ebi K.L., Yap W., Nasci R., Patz J.A. Climate variability and change in the United States: Potential impacts on vector- and rodent-borne diseases. Environ. Health Perspect. 2001;109:223–233. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox J., Craig M., Sueur D.L., Sharp B. Mapping Climate Vulnerability and Poverty in Africa: Report to the Department for International Development. ILRI; Nairobi, Kenya: 1999. Mapping Malaria Risk in the Highlands of Africa; p. 96. [Google Scholar]
- 24.Ko A.I., Galvão Reis M., Ribeiro Dourado C.M., Johnson W.D., Jr., Riley L.W. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–825. doi: 10.1016/S0140-6736(98)11019-X. [DOI] [PubMed] [Google Scholar]
- 25.Bai L., Morton L.C., Liu Q. Climate change and mosquito-borne diseases in China: A review. Glob. Health. 2013;9:10. doi: 10.1186/1744-8603-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang G.J., Gao Q., Zhou S.S., Malone J.B., McCarroll J.C., Tanner M., Vounatsou P., Bergquist R., Utzinger J., Zhou X.N. Mapping and predicting malaria transmission in the People’s Republic of China, using integrated biology-driven and statistical models. Geospat. Health. 2010;5:11–22. doi: 10.4081/gh.2010.183. [DOI] [PubMed] [Google Scholar]
- 27.Focks D.A., Daniels E., Haile D.G., Keesling J.E. A Simulation Model of the Epidemiology of Urban Dengue Fever: Literature Analysis, Model Development, Preliminary Validation, and Samples of Simulation Results. Am. J. Trop. Med. Hyg. 1995;53:489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- 28.Reeves W.C., Hardy J.L., Reisen W.K., Milby M.M. Potential Effect of Global Warming on Mosquito-Borne Arboviruses. J. Med Èntomol. 1994;31:323–332. doi: 10.1093/jmedent/31.3.323. [DOI] [PubMed] [Google Scholar]
- 29.Reiter P. Weather, Vector Biology, and Arboviral Recrudescence. In: Monath T.P., editor. The Arboviruses: Epidemiology and Ecology. CRC Press; Boca Raton, FL, USA: 1988. pp. 245–255. [Google Scholar]
- 30.Reiter P. Global-warming and vector-borne disease in temperate regions and at high altitude. Lancet. 1998;351:839–840. doi: 10.1016/S0140-6736(05)78979-0. [DOI] [PubMed] [Google Scholar]
- 31.Reisen W.K. Effect of Temperature on Culex tarsalis (Diptera: Culicidae) from the Coachella and San Joaquin Valleys of California. J. Med Èntomol. 1995;32:636–645. doi: 10.1093/jmedent/32.5.636. [DOI] [PubMed] [Google Scholar]
- 32.Kramer L.D., Hardy J.L., Presser S.B. Effect of Temperature of Extrinsic Incubation on the Vector Competence of Culex tarsalis for Western Equine Encephalomyelitis Virus. Am. J. Trop. Med. Hyg. 1983;32:1130–1139. doi: 10.4269/ajtmh.1983.32.1130. [DOI] [PubMed] [Google Scholar]
- 33.Day J.F., Curtis G.A. Influence of Rainfall on Culex nigripalpus (Diptera: Culicidae) Blood-Feeding Behavior in Indian River County, Florida. Ann. Èntomol. Soc. Am. 1989;82:32–37. doi: 10.1093/aesa/82.1.32. [DOI] [Google Scholar]
- 34.Hardy J.L., Meyer R.P., Presser S.B., Milby M.M. Temporal Variations in the Susceptibility of a Semi-Isolated Population of Culex tarsalis to Peroral Infection with Western Equine Encephalomyelitis and St. Louis Encephalitis Viruses. Am. J. Trop. Med. Hyg. 1990;42:500–511. doi: 10.4269/ajtmh.1990.42.500. [DOI] [PubMed] [Google Scholar]
- 35.Lansdowne C., Hacker C.S. The Effect of Fluctuating Temperature and Humidity on the Adult Life Table Characteristics of Five Strains of Aedes Aegypti. J. Med Èntomol. 1975;11:723–733. doi: 10.1093/jmedent/11.6.723. [DOI] [PubMed] [Google Scholar]
- 36.United Nations (UN) Intergovernmental Panel on Climate Change (IPCC) Fourth Assessment Report Climate Change 2007. Intergovernmental Panel on Climate Change; Geneva, Switzerland: 2007. [Google Scholar]
- 37.Gratz N.G. Emerging and resurging vector-borne diseases. Annu. Rev. Èntomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- 38.Halstead S.B. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 39.Scholte E.J., Schaffner F. Waiting for the Tiger: Establishment and Spread of the Ae. albopictus Mosquito in Europe. In: Takken W., Knols B.G.J., editors. Emerging Pests and Vector-borne Disease in Europe. Wageningen Academic Publishers; Wageningen, The Netherlands: 2007. pp. 241–460. [Google Scholar]
- 40.Guerra C.A., Gikandi P.W., Tatem A.J., Noor A.M., Smith D.L., I Hay S., Snow R.W. The Limits and Intensity of Plasmodium falciparum Transmission: Implications for Malaria Control and Elimination Worldwide. PLoS Med. 2008;5:e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO Dengue and Severe Dengue. [(accessed on 2 July 2019)]; 2019 Report. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- 42.Kantor I.N. Dengue, Zika, Chikungunya y el Desarrollo de Vacunas. Volume 78. MEDICINA; Buenos Aires, Argentina: 2018. pp. 23–28. [PubMed] [Google Scholar]
- 43.Paixão E.S., Teixeira M.G., Rodrigues L.C. Zika, chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Health. 2018;3:000530. doi: 10.1136/bmjgh-2017-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NIH, NIH Begins Study of Vaccine to Protect Against Mosquito-Borne Diseases. [(accessed on 18 August 2019)];2017 Available online: https://www.nih.gov/news-events/news-releases/nih-begins-study-vaccine-protect-against-mosquito-borne-diseases.
- 45.WHO Yellow Fever. [(accessed on 18 August 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/yellow-fever.
- 46.Chebabi Abramides G., Roiz D., Guitart R., Quintana S., Guerrero I., Gimenez N. Effectiveness of a multiple intervention strategy for the control of the tiger mosquito (Ae. albopictus) in Spain. Trans. R. Soc. Trop. Med. Hyg. 2011;105:281–288. doi: 10.1016/j.trstmh.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Fonseca D.M., Unlu I., Crepeau T., Farajollahi A., Healy S.P., Bartlett-Healy K., Strickman D., Gaugler R., Hamilton G., Kline D., et al. Area-wide management of Ae. albopictus. Part 2, Gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest Manag Sci. 2013;69:1351–1361. doi: 10.1002/ps.3511. [DOI] [PubMed] [Google Scholar]
- 48.Dowling Z., Armbruster P., LaDeau S.L., Decotiis M., Mottley J., Leisnham P.T. Linking Mosquito Infestation to Resident Socioeconomic Status, Knowledge, and Source Reduction Practices in Suburban Washington, DC. EcoHealth. 2013;10:36–47. doi: 10.1007/s10393-013-0818-6. [DOI] [PubMed] [Google Scholar]
- 49.Petrić D., Bellini R., Scholte E.-J., Rakotoarivony L.M., Schaffner F. Monitoring population and environmental parameters of invasive mosquito species in Europe. Parasites Vectors. 2014;7:187. doi: 10.1186/1756-3305-7-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacups S.P., Rapley L.P., Johnson P.H., Benjamin S., Ritchie S.A. Bacillus thuringiensis var. israelensis misting for control of Aedes in cryptic ground containers in North Queensland, Australia. Am. J. Trop. Med. Hyg. 2013;88:490–496. doi: 10.4269/ajtmh.12-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kline D.L. Semiochemicals, traps/targets and mass trapping technology for mosquito management. J. Am. Mosq. Control. Assoc. 2007;23:241–251. doi: 10.2987/8756-971X(2007)23[241:STAMTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.Mackay A.J., Amador M., Barrera R. An improved autocidal gravid ovitrap for the control and surveillance of Aedes aegypti. Parasites Vectors. 2013;6:225. doi: 10.1186/1756-3305-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook S.M., Khan Z.R., Pickett J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Èntomol. 2007;52:375–400. doi: 10.1146/annurev.ento.52.110405.091407. [DOI] [PubMed] [Google Scholar]
- 54.Paz-Soldan V.A., Plasai V., Morrison A.C., Rios-Lopez E.J., Guedez-Gonzales S., Grieco J.P., Mundal K., Chareonviriyaphap T., Achee N.L. Initial assessment of the acceptability of a push-pull Ae. aegypti control strategy in Iquitos, Peru and Kanchanaburi, Thailand. Am. J. Trop. Med. Hyg. 2011;84:208–217. doi: 10.4269/ajtmh.2011.09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lima J.B.P., Rosa-Freitas M.G., Rodovalho C.M., Santos F., Lourenço-De-Oliveira R. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches? - A Review. Memórias Do Inst. Oswaldo Cruz. 2014;109:685–705. doi: 10.1590/0074-0276140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salazar F.V., Achee N.L., Grieco J.P., Prabaripai A., Ojo T.A., Eisen L., Dureza C., Polsomboon S., Chareonviriyaphap T. Effect of Ae. aegypti exposure to spatial repellent chemicals on BG-Sentinel (TM) trap catches. Parasit. Vect. 2013;6:145. doi: 10.1186/1756-3305-6-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suman D.S., Wang Y., Bilgrami A.L., Gaugler R. Ovicidal activity of three insect growth regulators against Aedes and Culex mosquitoes. Acta Trop. 2013;128:103–109. doi: 10.1016/j.actatropica.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 58.Mulla M.S. The future of insect growth regulators in vector control. J. Am. Mosq. Control. Assoc. 1995;11:269–273. [PubMed] [Google Scholar]
- 59.Ocampo C.B., Julieth M.N., Carabali M., Alexander N., Osorio L. Reduction in dengue cases observed during mass control of Aedes (Stegomyia) in street catch basins in an endemic urban area in Colombia. Acta Trop. 2014;132:15–22. doi: 10.1016/j.actatropica.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.PubChem U.S National library of medicine (NIH), National Center for Biotechnology Information. [(accessed on 29 August 2019)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fendona#section=Depositor-Supplied-Synonyms.
- 61.PubChem U.S National library of medicine (NIH), National Center for Biotechnology Information. [(accessed on 29 August 2019)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/lambda-Cyhalothrin.
- 62.PubChem U.S National library of medicine (NIH), National Center for Biotechnology Information. [(accessed on 29 August 2019)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/deltamethrin.
- 63.PubChem U.S National library of medicine (NIH), National Center for Biotechnology Information. [(accessed on 29 August 2019)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fenpropathrin.
- 64.Regnault-Roger C. The potential of botanical essential oils for insect pest control. Integr. Pest Manag. Rev. 1997;2:25–34. doi: 10.1023/A:1018472227889. [DOI] [Google Scholar]
- 65.Roman P. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crop. Prod. 2015;76:174–187. [Google Scholar]
- 66.Dias C.N., Moraes D.F. Essential oils and their compounds as Ae. aegypti L. (Diptera: Culicidae) larvicides: Review. Parasitol. Res. 2014;113:565–592. doi: 10.1007/s00436-013-3687-6. [DOI] [PubMed] [Google Scholar]
- 67.Maheswaran R., Ignacimuthu S. A novel herbal formulation against dengue vector mosquitoes Ae. aegypti and Ae. albopictus. Parasitol. Res. 2012;110:1801–1813. doi: 10.1007/s00436-011-2702-z. [DOI] [PubMed] [Google Scholar]
- 68.Mikami A., Yamashita N. The inhibitory effects of a neem formulation on emergence of Oc. japonicus and Culex pipiens pallens. Med. Entomol. Zool. 2004;55:239–242. doi: 10.7601/mez.55.239. [DOI] [Google Scholar]
- 69.Felipe C.F.B., Fonsêca K.S., Barbosa A.L.R., Bezerra J.N.S., Neto M.A., França Fonteles M.M., Barros Viana G.S. Alterations in behavior and memory induced by the essential oil of Zingiber officinale Roscoe (ginger) in mice are cholinergic-dependent. J. Med. Plants Res. 2008;2:163–170. [Google Scholar]
- 70.Rameshwar S.R. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010;29:913–920. [Google Scholar]
- 71.Federici B.A., Park H.W., Bideshi D.K., Wirth M.C., Johnson J.J., Sakano Y., Tang M. Developing recombinant bacteria for control of mosquito larvae. J. Am. Mosq. Control. Assoc. 2007;23:164–175. doi: 10.2987/8756-971X(2007)23[164:DRBFCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 72.Bloomquist J.R., Boina D.R., Chow E., Carlier P.R., Reina M., González-Coloma A. Mode of action of the plant-derived silphinenes on insect and mammalian GABAA receptor/chloride channel complex. Pestic. Biochem. Physiol. 2008;91:17–23. doi: 10.1016/j.pestbp.2007.12.002. [DOI] [Google Scholar]
- 73.Casida J.E. Pyrethrum, the Natural Insecticide. Academic Press; New York, NY, USA: 1973. [Google Scholar]
- 74.Khambay B.P., Batty D., Jewess P.J., Bateman G.L., Hollomon D.W. Mode of action and pesticidal activity of the natural product dunnione and of some analogues. Pest Manag. Sci. 2003;59:174–182. doi: 10.1002/ps.632. [DOI] [PubMed] [Google Scholar]
- 75.Copping L.G., Menn J.J. Biopesticides: A review of their action, applications and efficacy. Pest Manag. Sci. 2000;56:651–676. doi: 10.1002/1526-4998(200008)56:8<651::AID-PS201>3.0.CO;2-U. [DOI] [Google Scholar]
- 76.Bloomquist J.R. Ion Channels as Targets for Insecticides. Annu. Rev. Èntomol. 1996;41:163–190. doi: 10.1146/annurev.en.41.010196.001115. [DOI] [PubMed] [Google Scholar]
- 77.Enan E.E. Molecular and pharmacological analysis of an octopamine receptor from american cockroach and fruit fly in response to plant essential oils. Arch. Insect Biochem. Physiol. 2005;59:161–171. doi: 10.1002/arch.20076. [DOI] [PubMed] [Google Scholar]
- 78.Kostyukovsky M., Rafaeli A., Gileadi C., Demchenko N., Shaaya E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002;58:1101–1106. doi: 10.1002/ps.548. [DOI] [PubMed] [Google Scholar]
- 79.Enan E.E. Molecular response of Drosophila melanogaster tyramine receptor cascade to plant essential oils. Insect Biochem. Mol. Biol. 2005;35:309–321. doi: 10.1016/j.ibmb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 80.Subbiah P. Current trends in the control of mosquito vectors by means of biological larvicides. J. Biofertil. Biopestici. 2012;3 doi: 10.4172/2155-6202.1000125. [DOI] [Google Scholar]
- 81.Mahmood F. Laboratory bioassay to compare susceptibilities of Ae. aegypti and Anophels albimanus to Bacillus thuringiensis var. israelensis as affected by their feeding rates. J. Am. Mosq. Control Assoc. 1998;14:69–71. [PubMed] [Google Scholar]
- 82.Singh G.J., Gill S.S. An electron microscope study of the toxic action of Bacillus sphaericus in Culex quinquefasciatus larvae. J. Invertebr. Pathol. 1998;52:237–247. doi: 10.1016/0022-2011(88)90131-0. [DOI] [PubMed] [Google Scholar]
- 83.Poopathi S., Mani T.R., Rao D.R., Baskaran G., Kabilan L. Susceptibilty levels of resistance of Culex quinquefasciatus to the insecticidal toxin of Bacillus sphaericus (strain 2362) Environ. Ecol. 2000;18:703–710. [Google Scholar]
- 84.Poopathi S., Mani T.R., Raghunatha R.D., Baskaran G., Lalitha K. Effect of Bacillus sphaericus and Bacillus thuringiensis yare israelensis on the ultrastructural changes in the midgut of Culex quinquefasciatus Say (Diptera: Culicidae) J. Ent. Res. 1999;23:347–357. [Google Scholar]
- 85.Charles J.F., Silva-Filha M.H., Nielsen-LeRoux C., Humphreys M.J., Berry C. Binding of the 51- and 42-kDa individual components from the Bacillus sphaericus crystal toxin to mosquito larval midgut membranes from Culex and Anophels sp. (Diptera: Culicidae) FEMS Microbiol. Lett. 1997;156:153–159. doi: 10.1016/S0378-1097(97)00419-9. [DOI] [PubMed] [Google Scholar]
- 86.Silva-Filha M.H., Nielsen-LeRoux C., Charles J.F. Identification of the receptor for Bacillus sphaericus crystal toxin in the brush border membrane of the mosquito Culex pipiens (Diptera: Culicidae) Insect Biochem. Mol. Biol. 1999;29:711–721. doi: 10.1016/S0965-1748(99)00047-8. [DOI] [PubMed] [Google Scholar]
- 87.Baumann P., A Clark M., Baumann L., Broadwell A.H. Bacillus sphaericus as a mosquito pathogen: Properties of the organism and its toxins. Microbiol. Rev. 1991;55:425–436. doi: 10.1128/mr.55.3.425-436.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ignoffo C.M., Couch T.L., Garcia C., Kroha M.J. Relative activity of Bacillus thuringiensis var. kurstaki and B. thuringiensis var. israelensis against larvae of Ae. aegypti, Culex quinquefasciatus, Trichoplusia ni, Heliothis zea, and Heliothis virescens. J. Econ. Entomol. 1981;74:218–222. doi: 10.1093/jee/74.2.218. [DOI] [PubMed] [Google Scholar]
- 89.Höfte H., Whiteley H.R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 1989;53:242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Delecluse A., Rosso M.L., Ragni A. Cloning and expression of a novel toxin gene from Bacillus thuringiensis sub sp. jegathesan encoding a highly mosquitocidal protein. Appl. Environ. Microbiol. 1995;61:4230–4235. doi: 10.1128/aem.61.12.4230-4235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frutos R., Rang C., Royer M. Managing Insect Resistance to Plants ProducingBacillus thuringiensisToxins. Crit. Rev. Biotechnol. 1999;19:227–276. doi: 10.1080/0738-859991229251. [DOI] [Google Scholar]
- 92.Pimentel D., Burgess M. Effects of Single Versus Combinations of Insecticides on the Development of Resistance. Environ. Èntomol. 1985;14:582–589. doi: 10.1093/ee/14.5.582. [DOI] [Google Scholar]
- 93.McGaughey W.H., Gould F., Gelernter W. Bt resistance management. Nat. Biotechnol. 1998;16:144–146. doi: 10.1038/nbt0298-144. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y.B., Tabashnik B.E. Experimental evidence that refuges delay insect adaptation to Bacillus thuringiensis. Proc. R. Soc. B Biol. Sci. 1997;264:605–610. doi: 10.1098/rspb.1997.0086. [DOI] [Google Scholar]
- 95.Wirth M.C., Zaritsky A., Ben-Dov E., Manasherob R., Khasdan V., Boussiba S., Walton W.E. Cross-resistance spectra of Culex quinquefasciatus resistant to mosquitocidal toxins of Bacillus thuringiensis towards recombinant Escherichia coli expressing genes from B. thuringiensis subsp. israelensis. Environ. Microbiol. 2007;9:1393–1401. doi: 10.1111/j.1462-2920.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- 96.Alphey L. Genetic control of mosquitoes. Annu. Rev. Entomol. 2014;59:205–224. doi: 10.1146/annurev-ento-011613-162002. [DOI] [PubMed] [Google Scholar]
- 97.Alphey L., Benedict M., Bellini R., Clark G.G., Dame D.A., Service M.W., Dobson S.L. Sterile-Insect Methods for Control of Mosquito-Borne Diseases: An Analysis. Vector Borne Zoonotic Dis. 2010;10:295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lambrechts L., Koella J.C., Boëte C. Can transgenic mosquitoes afford the fitness cost? Trends Parasitol. 2008;24:4–7. doi: 10.1016/j.pt.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 99.FAO/IAEA Status and Risk Assessment of the Use of Transgenic Arthropods in Plant Protection; Proceedings of the a Technical Meeting Organized by the Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture and the Secretariat of the International Plant Protection Convention; Rome, Italy. 8–12 April 2002. [Google Scholar]
- 100.Benedict M. The first releases of transgenic mosquitoes: An argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. doi: 10.1016/S1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 101.Dame D., Curtis C., Benedict M., Robinson A., Knols B. Historical applications of induced sterilization in field populations of mosquitoes. Malar. J. 2009;8:S2. doi: 10.1186/1475-2875-8-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Windbichler N., Papathanos P.A., Crisanti A. Targeting the X chromosome during spermatogenesis induces Y chromosome transmission ratio distortion and early dominant embryo lethality in Anophels gambiae. PLoS Genet. 2008;4:e1000291. doi: 10.1371/journal.pgen.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Werren J.H., Baldo L., Clark M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Genet. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 104.Burt A., Trivers R. Genes in Conflict: The Biology of Selfish Genetic Elements. Belknap Press/Harvard University Press; Cambridge, MA, USA: 2006. p. 610. [Google Scholar]
- 105.Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. R. Soc. B Biol. Sci. 2003;270:921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen C.H., Huang H., Ward C.M., Su J.T., Schaeffer L.V., Guo M., Hay B.A. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- 107.Lee S.W., Chang S.H., Lai Y.S., Lin C.C., Tsai C.M., Lee Y.C., Chen J.C., Huang C.L. Effect of Temperature on the Growth of Silver Nanoparticles Using Plasmon-Mediated Method under the Irradiation of Green LEDs. Materials. 2014;7:7781–7798. doi: 10.3390/ma7127781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Santos-Magalhães N.S., Mosqueira V.C.F. Nanotechnology applied to the treatment of malaria. Adv. Drug Deliv. Rev. 2010;62:560–575. doi: 10.1016/j.addr.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 109.Lasic D.D. Novel applications of liposomes. Trends Biotechnol. 1998;16:307–321. doi: 10.1016/S0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 110.Peeters P.A.M., Huiskamp C.W.E.M., Eling W.M.C., Crommelin D.J.A. Chloroquine containing liposomes in the chemotherapy of murine malaria. Parasitology. 1989;98:381–386. doi: 10.1017/S003118200006145X. [DOI] [PubMed] [Google Scholar]
- 111.Agrawal A.K., Singhal A., Gupta C. Functional drug targeting to erythrocytes in vivo using antibody bearing liposomes as drug vehicles. Biochem. Biophys. Res. Commun. 1987;148:357–361. doi: 10.1016/0006-291X(87)91118-1. [DOI] [PubMed] [Google Scholar]
- 112.Wu Y., Yang W., Wang C., Hu J., Fu S. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int. J. Pharm. 2005;295:235–245. doi: 10.1016/j.ijpharm.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 113.Nakache E., Poulain N., Candau F., Orecchioni A., Irache J. Biopolymer and Polymer Nanoparticles and Their Biomedical Applications. In: Nalwa H.S., editor. Handbook of Nanostructured Materials and Nanotechnology. Academic Press; New York, NY, USA: 2000. [Google Scholar]
- 114.Baird J.K., Hoffman S.L. Primaquine therapy for malaria. Clin. Infect. Dis. 2000;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 115.Omwoyo W.N., Ogutu B., Oloo F., Swai H., Kalombo L., Melariri P., Mahanga G.M., Gathirwa J.W. Preparation, characterization, and optimization of primaquine-loaded solid lipid nanoparticles. Int. J. Nanomed. 2014;9:3865–3874. doi: 10.2147/IJN.S62630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baird J., Rieckmann K.H. Can primaquine therapy for vivax malaria be improved? Trends Parasitol. 2003;19:115–120. doi: 10.1016/S1471-4922(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 117.Dennis E., Peoples V.A., Johnson F., Bibbs R.K., Topps D., Bopda-Waffo A., Coats M.T. Utilizing Nanotechnology to Combat Malaria. J. Infect. Dis. Ther. 2015;3:4. doi: 10.4172/2332-0877.1000229. [DOI] [Google Scholar]
- 118.Ahmed T.A., Aljaeid B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016;10:483–507. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kilama W., Ntoumi F. Malaria: A research agenda for the eradication era. Lancet. 2009;374:1480–1482. doi: 10.1016/S0140-6736(09)61884-5. [DOI] [PubMed] [Google Scholar]
- 120.Schwendener R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines. 2014;2:159–182. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sen G.L. A brief history of RNAi: The silence of the genes. FASEB J. 2006;20:1293–1299. doi: 10.1096/fj.06-6014rev. [DOI] [PubMed] [Google Scholar]
- 122.Airs P.M., Bartholomay L.C. RNA Interference for Mosquito and Mosquito-Borne Disease Control. Insects. 2017;8:4. doi: 10.3390/insects8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang X., Zhang J., Zhu K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anophels gambiae) Insect Mol. Biol. 2010;19:683–693. doi: 10.1111/j.1365-2583.2010.01029.x. [DOI] [PubMed] [Google Scholar]
- 124.Bennet D., Kim S. Application of Nanotechnology in Drug Delivery. IntechOpen; London, UK: 2014. Polymer Nanoparticles for Smart Drug Delivery. [Google Scholar]
- 125.Moon J.J., Suh H., Polhemus M.E., Ockenhouse C.F., Yadava A., Irvine D.J. Antigen-Displaying Lipid-Enveloped PLGA Nanoparticles as Delivery Agents for a Plasmodium vivax Malaria Vaccine. PLoS ONE. 2012;7:e31472. doi: 10.1371/journal.pone.0031472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tripathy S., Das S., Chakraborty S.P., Sahu S.K., Pramanik P., Roy S. Synthesis, characterization of chitosan--tripolyphosphate conjugated chloroquine nanoparticle and it’s in vivo anti-malarial efficacy against rodent parasite: A dose and duration dependent approach. Int. J. Pharm. 2012;434:292–305. doi: 10.1016/j.ijpharm.2012.05.064. [DOI] [PubMed] [Google Scholar]
- 127.Tripathy S., Das S., Dash S.K., Chattopadhyay S., Roy S. The impact of nanochloroquine on restoration of hepatic and splenic. J. Nanopart. 2013;9:106152. doi: 10.1155/2013/106152. [DOI] [Google Scholar]
- 128.Benelli G., Maggi F., Pavela R., Murugan K., Govindarajan M., Vaseeharan B., Petrelli R., Cappellacci L., Kumar S., Hofer A., et al. Mosquito control with green nanopesticides: Towards the One Health approach? A review of non-target effects. Environ. Sci. Pollut. Res. 2017;25:10184–10206. doi: 10.1007/s11356-017-9752-4. [DOI] [PubMed] [Google Scholar]
- 129.Benelli G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: A review. Parasitol. Res. 2016;115:23–34. doi: 10.1007/s00436-015-4800-9. [DOI] [PubMed] [Google Scholar]
- 130.Azarudeen R.M.S.T., Govindarajan M., Amsath A., Muthukumaran U., Benelli G. Single-step biofabrication of silver nanocristals using Naregamia alata: A cost effective and eco-friendly control tool in the fight against Malaria, Zika virus and St Louis encephalitis mosquito vectors. J. Clust. Sci. 2017;28:179–203. doi: 10.1007/s10876-016-1067-y. [DOI] [Google Scholar]
- 131.Benelli G. Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer—a brief review. Enzym. Microb. Technol. 2016;95:58–68. doi: 10.1016/j.enzmictec.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 132.Panneerselvam C., Murugan K., Roni M., Aziz A.T., Suresh U., Rajaganesh R., Madhiyazhagan P., Subramaniam J., Dinesh D., Nicoletti M., et al. Fern-synthesize nanoparticles in the fight against malaria: LC/MS analysis of Pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitol. Res. 2016;115:997–1013. doi: 10.1007/s00436-015-4828-x. [DOI] [PubMed] [Google Scholar]
- 133.Alto B.W., Juliano S.A. Precipitation and temperature effects of populations of Ae. albopictus (Diptera: Culicidae): Implications for range expansion. J. Med. Entomol. 2001;38:646–656. doi: 10.1603/0022-2585-38.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Roni M., Murugan K., Panneerselvam C., Subramaniam J., Nicoletti M., Madhiyazhagan P., Dinesh D., Suresh U., Khater H.F., Wei H., et al. Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Ae. aegypti and Plutella xylostella. Ecotoxicol. Environ. Saf. 2015;121:31–38. doi: 10.1016/j.ecoenv.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 135.Costa E.A.P.A., Santos E.M., Correia J.C., Albuquerque C.M.R. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae) Rev. Bras. Entomol. 2010;54:488–493. doi: 10.1590/S0085-56262010000300021. [DOI] [Google Scholar]
- 136.Becker N. Influence of climate change on mosquito development and mosquito-borne diseases in Europe. Parasitol. Res. 2008;103:19–28. doi: 10.1007/s00436-008-1210-2. [DOI] [PubMed] [Google Scholar]
- 137.Canyon D.V., Hii J.L.K., Muller R. Adaptation of Aedes aegypti (Diptera: Culicidae) oviposition behaviour in response to humidity and diet. J. Insect Physiol. 1999;45:959–964. doi: 10.1016/S0022-1910(99)00085-2. [DOI] [PubMed] [Google Scholar]
- 138.Reiter P., Amador M.A., Anderson R.A., Clark G.G. Short report: Dispersal of Ae. aegypti in an urban area after blood feeding as demonstrated by rubidium-marked eggs. Am. J. Trop. Med. Hyg. 1995;52:177–179. doi: 10.4269/ajtmh.1995.52.177. [DOI] [PubMed] [Google Scholar]
- 139.Mogi M., Miyagi I., Abadi K., Syafruddin Inter- and intraspecific variation in resistance to desiccation by adult Aedes (Stegomyia) spp. (Diptera: Culicidae) from Indonesia. Med. Vet. Entomol. 1996;33:53–57. doi: 10.1093/jmedent/33.1.53. [DOI] [PubMed] [Google Scholar]
- 140.Ahmed T., Scholz M., Al-Faraj F., Niaz W. Water-Related Impacts of Climate Change on Agriculture and Subsequently on Public Health: A Review for Generalists with Particular Reference to Pakistan. Int. J. Environ. Res. Public Health. 2016;13:1051. doi: 10.3390/ijerph13111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kreft S., Eckstein D., Melchior I. Global Climate Risk Index 2017 Who Suffers Most from Extreme Weather Events? Weather-related Loss Events in 2015 and 1996 to 2015, 2017. Germanwatch e.V.; Bonn, Germany: 2014. [Google Scholar]
- 142.Jahan F. Short report: Dengue Fever (DF) in Pakistan. Asia Pac. Fam. Med. 2011;10:1. doi: 10.1186/1447-056X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kazmi D.H., Li J., Ruan C., Zhao S., Li Y., Sen Z. A statistical downscaling model for summer rainfall over Pakistan. Clim. Dyn. 2016;47:2653–2666. doi: 10.1007/s00382-016-2990-1. [DOI] [Google Scholar]