Abstract
Objectives:
To characterize the radiographic features of maxillary ameloblastoma (AM), odontogenic keratocyst (OKC) and dentigerous cyst (DC) comparatively by using spiral CT and cone beam CT (CBCT).
Methods:
Clinical records, histopathological reports, and nonenhanced spiral CT or CBCT images of 191 consecutive patients with primary maxillary AMs, OKCs, or DCs were retrospectively acquired, and radiographic features were analyzed.
Results:
The study included 118 males and 73 females (age: 5–84 years). 72.0% of AMs and 84.3% of OKCs originated from the posterior maxilla, while 69.6% of DCs occurred in the anterior maxilla. Among 25 AMs, 44.0% were of desmoplastic type, with honey-combed appearance. 84.0% of AMs were circular or oval in shape, 84.0% expanded buccally, and 36.0% invade the nasal floor. Among 89 OKCs of 88 patients, 61.8% were circular or oval, 58.4% expanded buccally, 49.4% were dentigerous, 41.6% nearly filled the maxillary sinus, and 13.5% invaded the nasal floor. 93.7% (74/79) of DCs enveloped a single tooth, and the tooth–cyst relationship was centripetal in 35, eccentric in 30, and circumferential in 9. Moreover, 98.2% (55/56) of the cysts enveloping a supernumerary tooth were DCs, while 80.9% (38/47) of the cysts enveloping the third molar were OKCs.
Conclusions:
Maxillary AMs tend to grow with buccal expansion and invade the nasal floor, and DAs with honey-combed lobularity are common. Maxillary OKCs have variant shapes and tend to invaginate the maxillary sinus. The tooth–cyst relationship of dentigerous OKCs and DCs can be centripetal, eccentric, or circumferential.
Keywords: maxilla; ameloblastoma; odontogenic cyst; tomography, spiral computed; cone-beam computed tomography
Introduction
In the maxillomandibular regions, ameloblastoma (AM), odontogenic keratocyst (OKC), dentigerous cyst (DC), radicular cyst, and nasopalatine duct cyst are the major odontogenic neoplastic lesions.1–3 Radicular cysts are frequently located at the apex of nonvital teeth, and nasopalatine duct cysts are commonly found in the nasopalatine foramen or canal; hence, both can be recognized with relative ease.4 AMs, OKCs and DCs manifest mainly as well-defined radiolucent lesions, with a lower prevalence in the maxilla,3,5–7 however, considerable overlap of the morphological characteristics among them makes further differentiation difficult.5,8–13 Comparative researches focusing on the radiographic features of these three lesions in the mandible are sufficient, however, reports of them in the maxilla are scarcely seen.8,9,14,15
To our knowledge, flimsy of cortical bone in the maxilla and intimacy with the nasal cavities, paranasal sinuses, orbits and vital structures at the skull-base make obvious the different growth patterns of AMs, OKCs and DCs in contrast to those in the mandible.16 Clinically, treatment of maxillary AMs should be more radical, with a longer follow-up period.17–19 However, OKCs and DCs are often treated with curettage and enucleation,20 and sometimes DCs can be treated by marsupialization to allow the tooth to be maintained. Therefore, a definite preoperative diagnosis is necessary for treatment planning.21 Pathologically, biopsies consisting exclusively of partial epithelium may be unable to reflect the true nature of the entire lesion. Further, inflammatory reactions on the cystic wall obviate a definite evaluation of its pathologic nature.
Nowadays, spiral CT or cone beam CT (CBCT) plays an important role in the detection and delineation of maxillary lesions and their impact on adjacent tissues.14,17,19 Further, contrast-enhanced CT or MRI improves the differentiation of these radiolucent lesions. Apajalahti et al20 indicated that contrast-enhanced CT and MRI allowed for better visualization of the mixed cystic and solid content characteristic of non-unicystic AM, or thick rim enhancement of unicystic AM. Konouchi et al22 found that unicystic AM produced relatively thick rim enhancement, whereas OKC demonstrated a thin rim enhancement on contrast-enhanced T1-weighted images. Han et al3 demonstrated that diffusion-weighted imaging and apparent diffusion coefficient determination were helpful for differentiation between unicystic AM and OKC. However, contrast-enhanced CT possesses higher radiation dose and risk of allergy to contrast media; and MRI machines are scarcely equipped in dental hospitals.20 Therefore, the value of non-enhanced CT or CBCT that allows for visualization of fine bony structures shouldn’t be ignored for these benign lesions. In a previous report in Chinese, our own research group analyzed the radiologic features of 22 AMs, 45 OKCs and 20 DCs in the maxilla and obtained some preliminary findings relative to the differential diagnosis among these lesions. However, the sample size seemed a bit small, and the results relative to the site, size and shape of lesions were descriptive.15 The purpose of this study was to characterize the three-dimensional radiographic features of maxillary AMs, OKCs and DCs comparatively by using nonenhanced spiral CT or CBCT imaging, on the basis of a larger case series and further in-depth analysis.
Methods and Materials
Subjects
Spiral CT and CBCT imaging data of patients with benign maxillary lesions from December 2012 through January 2018 were retrospectively acquired from the picture archiving and communication system (PACS, v. 11.0, Carestream Health, Inc. 2008) of Peking University School and Hospital of Stomatology. The study protocol was approved by our institutional review board (PKUSSIRB-201736083).
Inclusion criteria: (i) complete clinical records, (ii) histologically confirmed as AM, OKC, or DC, and (iii) availability of high-quality images without motion artifacts. Exclusion criteria: (i) recurrent lesions in the maxilla and (ii) presence of cleft lip and palate or nevoid basal cell carcinoma syndrome.
In addition, pathological sections of all eligible cases were reviewed by one experienced pathologist to reconfirm the diagnosis. Four variants of AMs were further identified according to the 2005 WHO classification: solid/multicystic ameloblastoma (SMA), unicystic ameloblastoma (UA), desmoplastic ameloblastoma (DA), and peripheral ameloblastome (PA).
In total, 191 patients were recruited, including 25 with AMs, 88 with OKCs, and 78 with DCs. 25 AMs consisted of 6 SMAs, 6 UAs, and 13 DAs. Of all the patients, 134 (21 with AMs, 71 with OKCs and 42 with DCs) had helical CT images (BrightSpeed, GE, USA) and 57 (4 with AMs, 17 with OKCs and 36 with DCs) had CBCT images (Newtom VGi, QR, S.R.L, Verona, Italy). CT images were set at 1.25 mm intervals, and CBCT images were recorded with a voxel size of 300 µm.
All images were displayed on an 18.7-inch monitor with screen resolution set at 1546 × 2048 pixels (Dome E3, NDS Surgical Imaging, San Jose, CA), and were independently viewed by two oral and maxillofacial radiologists (one professor and one resident). They were initially calibrated by examination of 10% of the cases. Images were observed under free angulation of three-dimensional (3D) slices on multiplanar reconstruction (MPR) images. In the event of disagreement in qualitative data, final diagnosis was reached by consensus reading. Quantitative measurements were recorded twice by the same observer at an interval of 2 weeks, and the mean was used for analysis.
Evaluation of 3D radiographic features
Following 3D radiographic features were analyzed:
Location and size: according to the geographic center, locations of the lesions were classified as (i) intercanine area (ii) left posterior area and (iii) right posterior area. Moreover, the maximum length on axial images set parallel to the plane of occlusion (Figure 1), was measured as an indicator of the lesion size.
Shape: the outline of an entire lesion on axial images set parallel to the occlusal plane was divided into four fundamental types: circular (length: width ratio <1.5, Figure 1), oval (length: width ratio ≥1.5, Figure 2), kidney-shaped (curved oval, Figure 3), and sinus-shaped (occupying the entire sinus, Figure 4). Lesions with scalloped margins were further defined as scalloped-circular (Figure 5), scalloped-oval (Figure 6), and scalloped-kidney (Figure 7). Moreover, certain sinus-shaped lesions bulged buccally and were named as “sinus + circular” (Figure 8).
Internal structures: all lesions were classified as totally radiolucent or mixed density (mixed radiolucent and radiopaque), and locularity was assessed as unilocular, multilocular or honey-combed (consisting of numerous small compartments or loculations, Figure 6).
Buccal and palatal expansion: initially, lesions with buccal and/or palatal expansion were noted and the percentages among different lesions were calculated. Further, a new concept of “Expansion Ratio” (ER) was tentatively applied to evaluate the outline of the bulging part of the leisons. A curved line of normal buccal border was traced in mirror symmetry, and the length of the curve (line A) was measured. The bulging height (line B) was also measured by the line set perpendicular to line A. The ratio of line B/line A was calculated as ER (Figure 8).
Influence on surrounding tissues: the degree of maxillary sinus invagination of the lesions was graded into three levels: no invagination (Level 1), partial invagination (Level 2), and complete filling (Level 3). The influence of a lesion on the nasal floor was scored into three grades: no involvement (Grade I), involvement with intact nasal floor (Grade II), and elevation or discontinuity of the nasal floor (Grade III). Root resorption of the involved teeth was also recorded.
Tooth–cyst relationship: for DCs and “dentigerous” OKCs, the tooth–cyst relationship was classified into three types: centripetal, eccentric, and circumferential. In the centripetal type (classic type), the enveloped tooth was oriented centripetally to the center of the cyst, and the cyst was attached to the region of the cementoenamel junction (CEJ) (Figures 9 and 10). In the eccentric type, long axis of the tooth was oriented laterally to the cyst center, and the cyst was partially attached to the middle or apical region of the root (Figure 11). In the circumferential type, the tooth was adhered to the cyst wall, and the cyst was partially attached to the apex of the root (Figure 12).
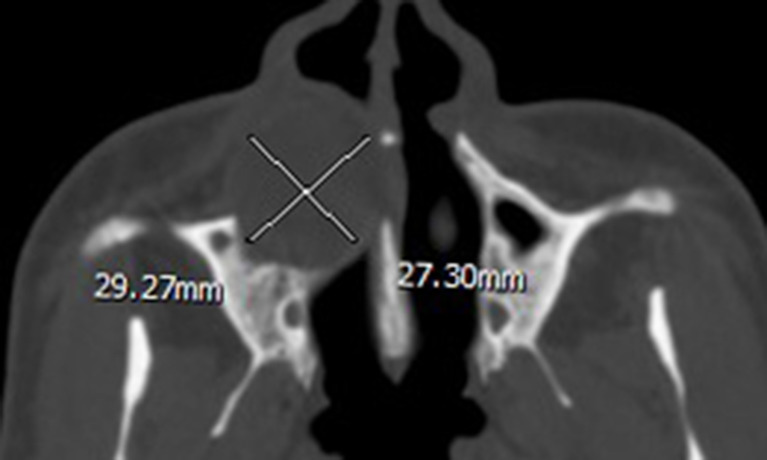
Figure 1.
Axial CT displayed a circular-shaped ameloblastoma occupying the right maxilla with a length of 29.27 mm and a width of 27.30 mm (length/width = 1.07).
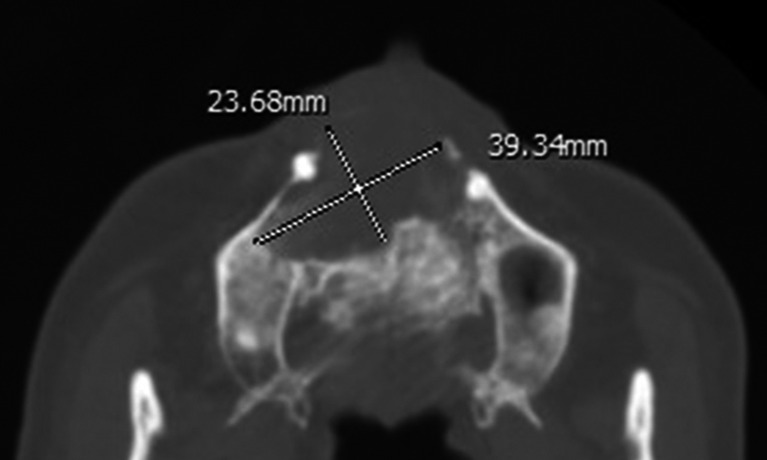
Figure 2.
Axial CT displayed an oval-shaped odontogenic keratocyst occupying the anterior maxilla with a length of 39.34 mm and a width of 23.68 mm (length/width = 1.66).
Figure 3.
Axial CT with bone algorithm showing a kidney-shaped odontogenic keratocyst in the left maxilla.
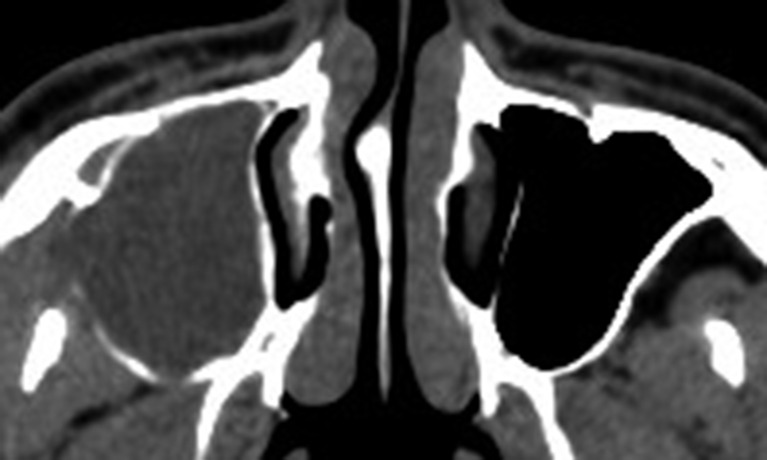
Figure 4.
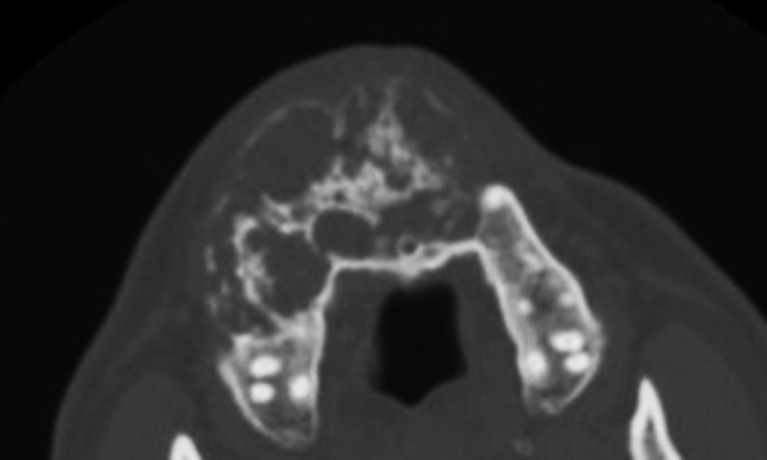
Axial CT showed an odontogenic keratocyst occupying the right maxillary sinus (sinus-shaped).
Figure 5.
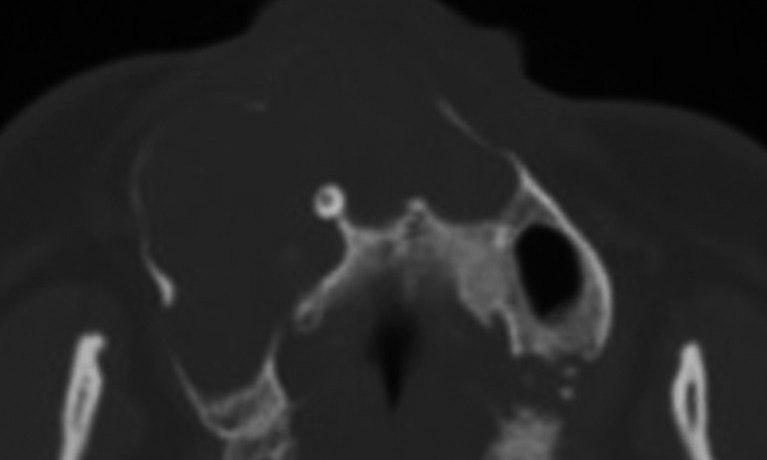
Axial cone-beam CT showing a maxillary ameloblastoma with a lobular-circular shape.
Figure 6.
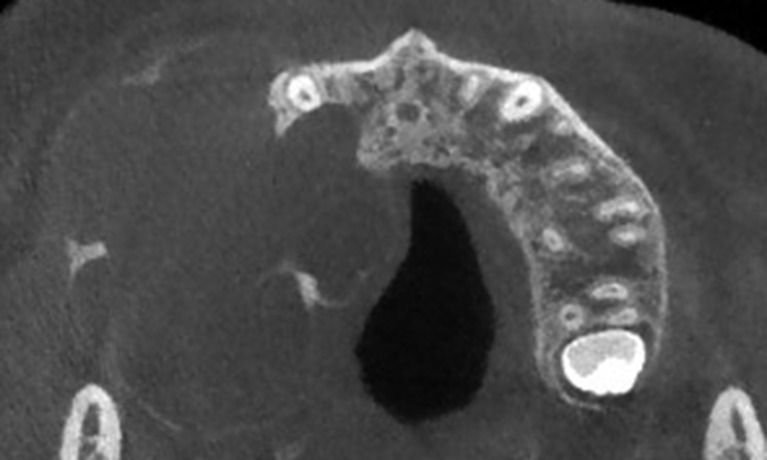
Axial CT showing a maxillary desmoplastic ameloblastoma with a lobular-oval shape. Note the mixed radiolucent and radiopaque appearance, mimicking a fibro-osseous lesion.
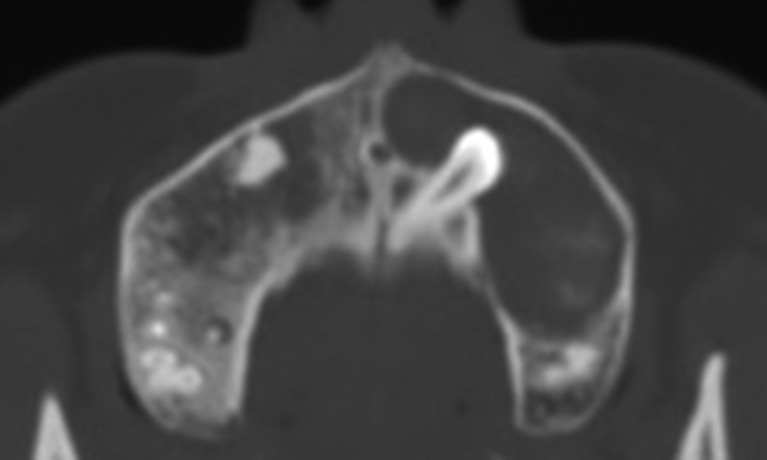
Figure 7.
CT axial image showing a maxillary dentigerous cyst with a lobular-kidney shape. The lesion extends from the right third molar to the left premolar region. Note the scalloped border on the kidney-shaped base.
Figure 8.
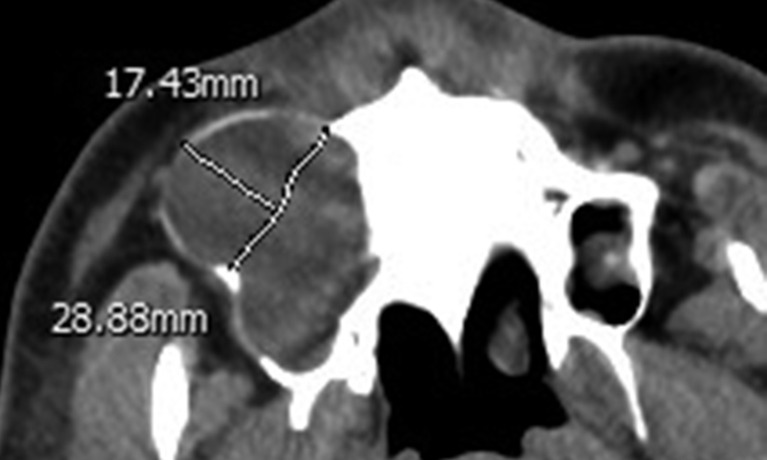
Axial CT showing an odontogenic keratocyst occupying the right maxillary sinus with significant buccal expansion (sinus + circular shaped). The normal border was traced in mirror symmetry, and the length of the curve (line A) was 28.88 mm. The bulging height (line B) was 17.43 mm. Line B/line A = 0.60.
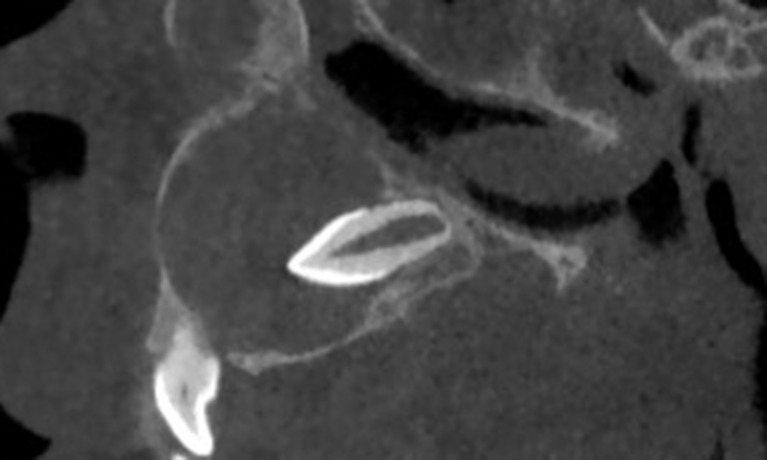
Figure 9.
Sagittal cone-beam CT showing an anterior maxillary dentigerous cyst. Note the enveloped supernumerary tooth oriented to the epicenter of the cyst (centripetal type).
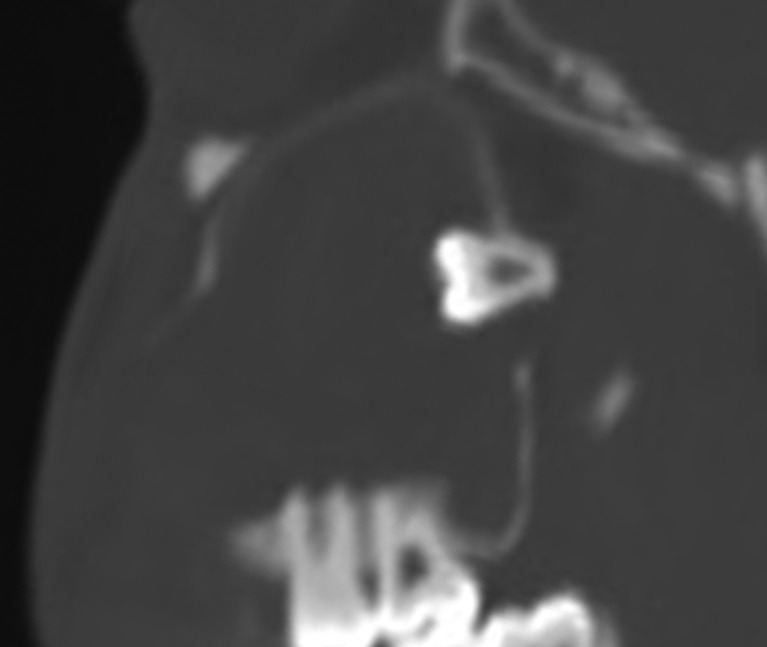
Figure 10.
Sagittal CT showing a maxillary odontogenic keratocyst. Note the superiorly migrated third molar oriented to the epicenter of the cyst (centripetal type).
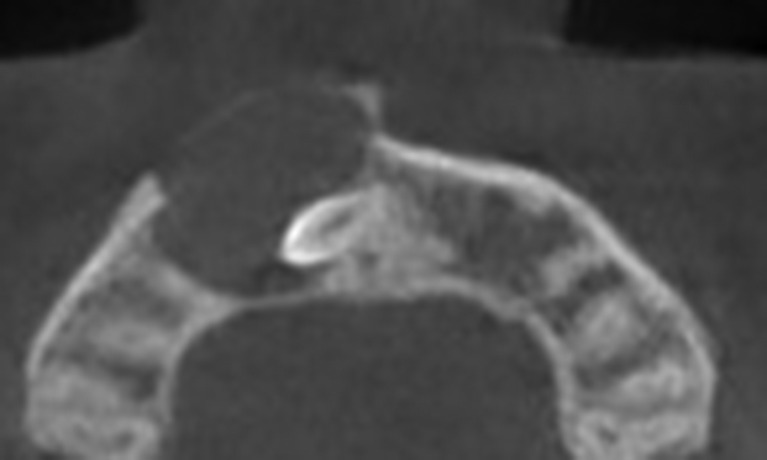
Figure 11.
Axial cone-beam CT showing a maxillary dentigerous cyst. The enveloped supernumerary tooth is oriented laterally to the cyst center (eccentric type).
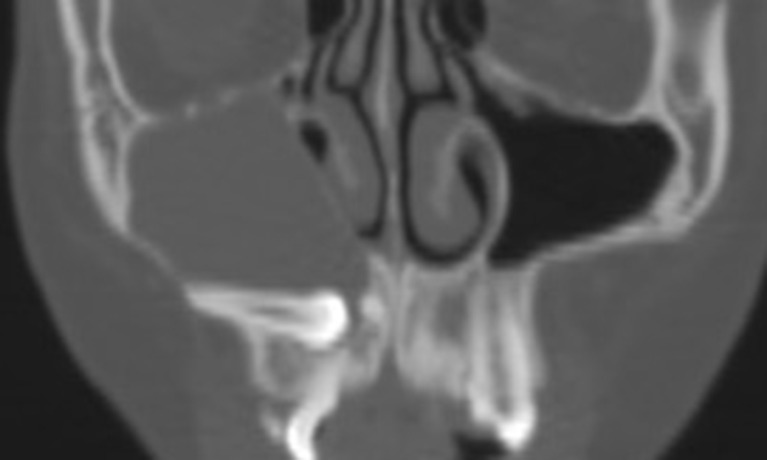
Figure 12.
Coronal CT showing a maxillary dentigerous cyst. The enveloped canine is adhered to the cyst wall, and the cyst is attached cranially to the root apex (circumferential type).
Furthermore, surgical treatment records and follow-up images of all patients were collected and reviewed.
Statistics
Statistical analysis was performed using the SPSS 22.0 (SPSS Inc., Chicago, IL) software program. Descriptive statistics were performed for variables of gender, age, location, size, shape, internal structure, buccal/palatal expansion, influence on surrounding tissue and tooth–cyst relationship. χ2 test and Wilcoxon signed rank sum test was used for analysis among three lesion types. A value of two sided p<0.05 was considered as statistical significance.
Results
Demographic features
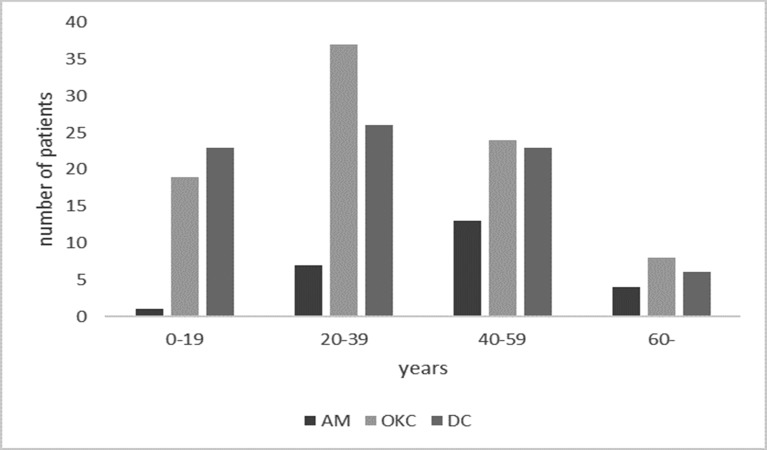
25 patients with single AMs included 16 males and 9 females. 88 OKC patients included 51 males and 37 females, and lesions were single in 87 cases and bilateral in 1. 78 patients with DCs included 51 males and 27 females, and lesions were single in 77 cases and bilateral in 1. No significant differences of male: female ratio were noted among three types (p = 0.598). Age distribution of 191 patients was summarized in Figure 13. The peak age of incidence was between 40 and 59 years for AMs, and between 20 and 39 years for OKCs/DCs, however, no significant differences were found among three types (p = 0.055).
Figure 13.
Age distribution of 191 patients with different diseases. AM, ameloblastoma; DC, dentigerous cyst; OKC, odontogenic keratocyst.
3D radiographic features
Location and size
72.0% (18/25) of AMs and 84.3% (75/89) of OKCs originated from the posterior region. However, 69.6% (55/79) of DCs occurred in the anterior region. (Table 1).
Table 1.
Location distribution of 193 lesions in the maxilla
| Types | Location | p-value | |||
| Anterior | Left-posterior | Right-posterior | |||
| AMa | UA | 2 | 1 | 3 | < 0.001 |
| SMA | - | 4 | 2 | ||
| DA | 5 | 3 | 5 | ||
| OKCa | 14 | 33 | 42 | ||
| DCb | 55 | 9 | 15 | ||
AM, ameloblastoma; DA, desmoplastic ameloblastoma; DC, dentigerous cyst; OKC, odontogenic keratocyst; SMA, solid/multicystic ameloblastoma; UA, unicystic ameloblastoma.
,bSame superscript letters indicate no significant difference in the location distribution
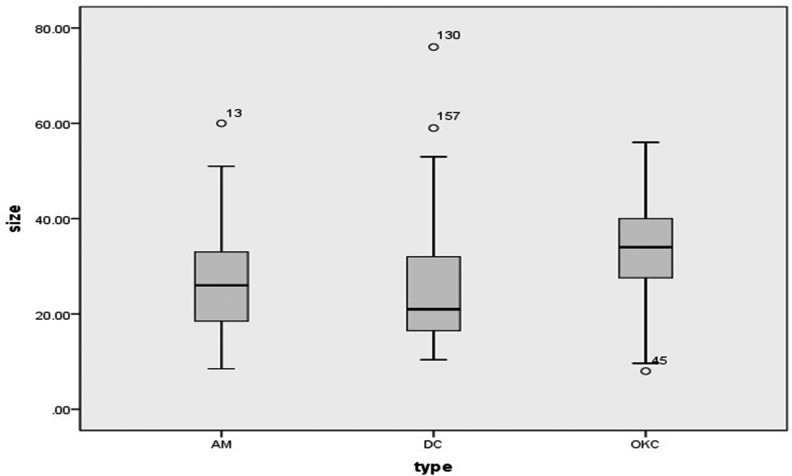
Dimensions of all lesions were showed in box plot diagram (Figure 14) and Wilcoxon signed rank sum test revealed significant differences. Post-hoc tests showed that OKCs (8.0–56.0 mm, median: 34.0 mm) were larger than AMs (8.5–60.0 mm, median: 26.0 mm, p = 0.031) and DCs (10.4–76.0 mm, median: 21.0 mm, p<0.001).
Figure 14.
Box-and-Whisker plot showing the size values of 193 maxillary lesions. The size values of four lesions (numbered 13, 45, 130 and 157) are not included between whiskers. AM, ameloblastoma; DC, dentigerous cyst; OKC, odontogenic keratocyst.
Shape
The shapes of 193 lesions were shown in Table 2. Of note, 84.0% (21/25) of AMs and 97.5% (77/79) of DCs were circular or oval in shape. However, the shape of OKCs varied distinctly, with 61.8% (55/89) being circular or oval. The incidence of circular and oval shapes in OKCs was significantly lower than that in AMs (p = 0.037) and DCs (p<0.001).
Table 2.
Shape distribution of 193 lesions in the maxilla
| Types | Circular | Oval | Kidney | Sinus | Scalloped-circular | Scalloped-oval | Scalloped-kidney | Sinus + circular |
| AM | 20 | 1 | - | - | 1 | 3 | - | - |
| OKC | 48 | 7 | 9 | 11 | 1 | - | 2 | 11 |
| DC | 67 | 10 | - | - | - | - | 2 | - |
AM, ameloblastoma; DC, dentigerous cyst; OKC, odontogenic keratocyst.
Internal structure
Table 3 showed locularity distribution of three types. Among AMs, 13 were honey-combed with mixed density, mimicking a fibro-osseous lesion, including 11 DAs and 2 SMAs. The other AMs (10 unilocular and 2 multilocular) were totally radiolucent. Meanwhile, 84.3% (75/89) of OKCs and all of 79 DCs were completely radiolucent. The remaining 14 OKCs presented with attenuated areas on the radiolucent base.
Table 3.
Locularity distribution of 193 lesions in the maxilla
| Types | Unilocular (%) | Multilocular (%) | Honey-combed (%) | |
| AM | UA | 5 (20.0) | 1 (4.0) | - |
| SMA | 3 (12.0) | 1 (4.0) | 2 (8.0) | |
| DA | 2 (8.0) | - | 11 (44.0) | |
| OKC | 68 (76.4) | 21 (23.6) | - | |
| DC | 77 (97.5) | 2 (2.5) | - | |
AM, ameloblastoma; DA, desmoplastic ameloblastoma; DC, dentigerous cyst; OKC, odontogenic keratocyst; SMA, solid/multicystic ameloblastoma; UA, unicystic ameloblastoma.
Buccal and palatal expansion
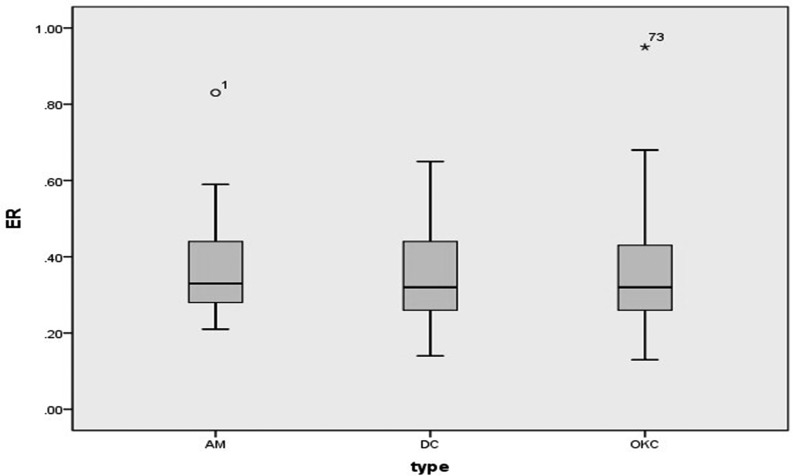
Distribution of buccal and palatal cortical expansion of 193 lesions was summarized in Table 4. χ2 test revealed a significantly lower incidence of buccal expansion in OKCs (58.4%, 52/89), as compared with that in AMs (84.0%, 21/25, p = 0.020) and DCs (78.5%, 62/79, p = 0.005). Besides, palatal expansion was present in 48.0% (12/25) of AMs, 30.3% (27/89) of OKCs and 36.7% (29/79) of DCs, and no significant differences were found among three types. Further, ERs of 135 lesions with buccal expansion were showed in Figure 15. Wilcoxon signed rank sum test revealed no significant differences in the degree of buccal expansion among three types (p = 0.809).
Table 4.
Distribution of buccal and palatal cortical expansion of 193 lesions in the maxilla
| Types | Buccal expansion | Palatal expansion | ||||
| Presence | Absence | p-value | Presence | Absence | p-value | |
| AMa | 21 | 4 | 0.005 | 12 | 13 | 0.247 |
| OKCb | 52 | 37 | 27 | 62 | ||
| DCa | 62 | 17 | 29 | 50 | ||
AM, ameloblastoma; DC, dentigerous cyst; OKC, odontogenic keratocyst.
,bSame superscript letters indicate no significant differences of buccal expansion.
Figure 15.
Box-and-Whisker plot showing the ER values of 135 maxillary lesions with buccal expansion. The ER values of two lesions (numbered 1 and 73) are not included between whiskers. AM, ameloblastoma; DC, dentigerous cyst; ER, expansion ratio; OKC,odontogenic keratocyst.
(5) Influence on surrounding tissues
In brief, 41.6% (37/89) of OKCs nearly filled the entire maxillary sinus. The incidence of filled maxillary sinuses in OKCs was significantly higher than that in AMs (8.0%, 2/25, p = 0.001) and DCs (10.1%, 8/79, p < 0.001). Meanwhile, 13.5% (12/89) of OKCs invaded the nasal floor (Grade III), and the incidence was significantly lower than that in AMs (9/25, 36.0%, p = 0.023) and DCs (40/79, 50.6%, p < 0.001), as shown in Table 5.
Table 5.
Maxillary sinus invagination, nasal floor invasion among 193 lesions in the maxilla
| Types | Maxillary sinus | Nasal floor | ||||||
| Level 1 | Level 2 | Level 3 | p-value | Grade I | Grade II | Grade III | p-value | |
| AMa | 10 | 13 | 2 | <0.001 | 13 | 3 | 9 | <0.001 |
| OKCb | 11 | 41 | 37 | 52 | 25 | 12 | ||
| DCa | 45 | 26 | 8 | 34 | 5 | 40 | ||
AM, ameloblastoma;
DC, dentigerous cyst; OKC, odontogenic keratocyst.
,bSame superscript letters indicate no significant differences in maxillary sinus invagination and nasal floor invasion.
Moreover, 6 AMs (24.0%, 6/25), 5 OKCs (5.6%, 5/89), and 8 DCs (10.1%, 8/79) presented with root resorption of adjacent teeth.
(6) Tooth–cyst relationship
Among the 25 AMs, only 1 UA enveloped the third molar. Among the 89 OKCs, 44 (49.4%) enveloped a single tooth, including 38 with the third molar, 5 with an anterior tooth and 1 with a supernumerary tooth, and 20 (45.5%, 20/44) of these “dentigerous” OKCs manifested as a typical centripetal type. Among the 79 DCs, 5 originated from two teeth, 55 originated from a supernumerary tooth, 8 from the third molar and 11 from other teeth (four from an incisor, five from a canine, one from a premolar, and one from the first molar). Among the 74 DCs with single tooth contained in this study, the tooth–cyst relationship was centripetal in 35 cases, eccentric in 30 cases, and circumferential in 9 cases. (Table 6).
Table 6.
Distribution of tooth–cyst relationship of OKCs and DCs (%)
| Types | No tooth enveloped | Two teeth enveloped | One tooth enveloped | Total | ||
| Centripetal | Eccentric | Circumferential | ||||
| OKC | 45 (50.6) | - | 20 (22.5) | 11 (12.4) | 13 (14.6) | 89 |
| DC | - | 5 (6.3) | 35 (44.3) | 30 (38.0) | 9 (11.4) | 79 |
DC, dentigerous cyst;OKC, odontogenic keratocyst.
Overall, 98.2% (55/56) of cysts enveloping a supernumerary tooth were DCs, while 80.9% (38/47) of cysts enveloping the third molar were OKCs.
Furthermore, among the 47 lesions enveloping the third molar, distant migration of the wisdom tooth was present in 68.4% (26/38) of OKCs. In the remaining eight OKCs, eight DCs, and one AM, the wisdom tooth was still impacted in the dental arch.
Treatment and follow-up
All patients with OKCs and DCs were treated with curettage. Among the 25 AMs, 9 were treated via curettage and 16 via resection with an adequate margin.
Among the 191 patients, 70 underwent follow-up spiral CT or CBCT scans 3–44 months after surgery. Recurrence was detected in one OKC patient 44 months after curettage, who experienced a second resection uneventfully.
Discussion
AM is the most common benign odontogenic tumor, excluding odontomas,23 and accounts for approximately 11–58% of odontogenic tumors.2,19,24–26 Four distinct growth variants of AM are recognized in the 2005 World Health Organization (WHO) classification for head and neck tumors, however, DA was incorporated into conventional ameloblastoma in the 2017 classification.23 Considering the specific radiographic appearance of DA, the 2005 classification was used in the present study.27,28 AMs have a strong predilection to occur in the mandible than in the maxilla, with a mandible to maxilla ratio of 4:1.23 This can explain the relatively smaller sample size of AMs in this study. Moreover, the male: female ratio of 25 patients with AMs was 1.78:1, and 72.0% of AMs were located in the posterior area. The male and posterior predilection was consistent with descriptions in previous studies.5,15–17,19 Further, 52.0% of AMs were DAs and 84.6% of DAs exhibited honey-combed appearance with mixed density. The incidence of DA was significantly higher than that (29.0%) in a previous study after analysis of 31 maxillary AMs.29 Of the 25 maxillary AMs, 6 (24%) were unicystic, and the percentage was somewhat higher than that in previous reports, in which unicystic AMs represented 5–15% of all intraosseous AMs,20,30 but lower than that in a total analysis of 286 AMs (48% of maxillary cases).29 Meanwhile, the percentage of solid /multicystic type (24%) was significantly lower than 62.5% in a previous report of all intraosseous AMs,20 but was consistent with 23% in maxillary AMs.29
As the third most common cyst of the jaw, OKCs account for approximately 10–20% of odontogenic cysts.4,14,23 In this study, the male:female ratio of 88 OKC patients was 1.38:1, with a slight male preponderance, which was lower than the previously reported ratios.1,11,12,15,31 Most OKCs (84.3%) occurred in the posterior maxilla, implying a posterior predilection, as reported in previous studies.11,12,15,31 Furthermore, 38 OKCs (42.7%, 38/89) enveloped the maxillary third molar, and the ratio was higher than that in the study of Yang (17.9%, 12/67).12
DCs, as the second most common cyst of the jaw, develop around a fully formed crown of an unerupted tooth.23 In this study, the anterior region was the most commonly affected site for DC, as reported in previous investigations.15,32 The proportion of DCs originating from a supernumerary was as high as 69.6%; and the low incidence of DCs originating from canines (6.3%) could be explained by the fact that they were usually treated by marsupialization with the cyst lining unavailable for pathological analysis. Only eight DCs were found to originate from the third molar, which showed a lower incidence as compared with other reports.32
It is generally believed that OKCs have a potentiality to grow through the length of the mandible and demonstrate minimal cortical expansion4,23; AMs and DCs in the mandible differ from OKCs in their tendency to cause cortical expansion. These differences can be probably explained by their pathological nature. DCs are thought to grow solely by osmotic pressure when fluid accumulates in the layers of reduced enamel epithelium or between the epithelium and the crown of the unerupted tooth, nevertheless, the epithelium in OKCs appears to have innate growth potential.4 AMs have aggressive but benign growth characteristics.23 However, the 3D radiographic features of their maxillary entities are scarcely described.12,15,24,25,29 In the present study, 84.0% of AMs and 97.5% of DCs in the maxilla were circular or oval in shape. Nevertheless, OKCs varied greatly in shape, with 61.8% of OKC lesions being circular or oval. In consideration of cortex expansion, most AMs (84.0%) and DCs (78.5%) presented with buccal expansion, while a significant lower percentage (58.4%) of OKCs expanded buccally, which was different from our former result.15 In spite of this, when comparing the ER, which manifested the outline of the bulging part, significant differences were not found among these lesions. Although the exact reason was still unclear, flimsy of the maxillary cortex might play an important role in explaining this finding.16 Therefore, it was concluded that AMs and DCs in the maxilla tended to be circular or oval and grow with buccal expansion, and OKCs assumed variant shapes, with relatively lower incidence of buccal expansion. In any case, the value of buccal expansion was less significant for the differentiation of these maxillary lesions, when compared with their mandibular analogues.
As for the locularity distribution, 40.0% of AMs, 76.4% of OKCs and 97.5% of DCs were unilocular. Differential diagnosis among the unilocular AMs, OKCs and DCs should be based on several other characteristics. Noticeably, it can be presumably stated that cysts enveloping a supernumerary tooth were mostly DCs, while cystic lesions enveloping the third molar were generally OKCs. This finding was scarcely described in previous reports.9,15
In the present study, invasion of maxillary sinus and nasal floor was analyzed for all three lesion types. 41.6% (37/89) of OKCs showed complete maxillary sinus invasion, and in contrast, merely 8.0% (2/25) AMs and 10.1% (8/79) DCs filled the sinuses, implying that OKCs differed from AMs and DCs in a tendency of filling the maxillary sinus, as implied in previous studies.15,31 On the other hand, the incidence of nasal floor invasion in OKCs was 13.5% (12/89), which was significantly lower than that in AMs (36.0%) and DCs (50.6%). These findings were scarcely described previously, which further verified the presumption that OKCs manifested as higher potentiality of sinus filling, whereas DCs and AMs showed higher incidence of nasal floor invasion.15 When comparing these maxillary lesions, the value of maxillary sinus and nasal floor invasion should not be ignored, especially for those with larger sizes.
Radiographically, DCs usually present as a well-defined unilocular radiolucency with a sclerotic border. The cyst wall is typically attached to the CEJ, and the crown of the enveloped tooth can be oriented centripetal to or diverge from the center of the cyst.4,33 It was reported that the relationship between DCs and the crown of the impacted tooth might vary as: (i) central, symmetric enveloping of the crown of an unerupted tooth; (ii) lateral, arising from the side of a crown; and (iii) circumferential, extension of the lesion below the CEJ.33,34 These variations were also verified in the present study.15 Moreover, 49.4% of OKCs were “dentigerous,” and 22.5% of OKCs manifested as a typical centripetal type. Given that DCs are usually enucleated whereas OKCs are either marsupialized or removed with wide margin,4 differential diagnosis between DCs and “dentigerous” OKCs is imperative and can be based upon aforementioned radiographic appearances.
Our study had several limitations. Due to the retrospective nature, as well as the relatively low incidences of these histopathologically proven lesions in the maxilla, patients examined by spiral CT and by CBCT were both recruited into this study. The greater contrast resolution of spiral CT and the generally greater spatial resolution of CBCT could have resulted in a bias of the outcomes. For instance, the evaluation of attachment points in “dentigerous” lesions using spiral CT was occasionally challenging. Further, the Housefield unit was not comparatively measured given that some patients were examined by CBCT.
In summary, both AMs and OKCs have a predilection to involve the posterior maxilla, while DCs enveloping a supernumerary tooth are commonly seen in the anterior maxilla. AMs in the maxilla tend to be circular or oval and grow with buccal expansion, and DAs with honey-combed appearance are commonly seen. With variant shapes, maxillary OKCs tend to invaginate the sinus, while have a low incidence of nasal floor invasion. Certain OKCs manifest as dentigerous lesions. The tooth–cyst relationship of “dentigerous” OKCs and DCs can be centripetal, eccentric, or circumferential. Three-dimensional images provided by nonenhanced spiral CT and CBCT are highly valuable for diagnosis and differentiation of benign radiolucent lesions in the maxilla.
Contributor Information
Yuan Meng, Email: kqldg@bjmu.edu.cn.
Deng-Gao Liu, Email: 1515519934@qq.com.
REFERENCES
- 1.Kilinc A, Saruhan N, Gundogdu B, Yalcin E, Ertas U, Urvasizoglu G. Benign tumors and tumor-like lesions of the oral cavity and jaws: an analysis of 709 cases. Niger J Clin Pract 2017; 20: 1448–54. doi: 10.4103/1119-3077.187309 [DOI] [PubMed] [Google Scholar]
- 2.Johnson NR, Gannon OM, Savage NW, Batstone MD. Frequency of odontogenic cysts and tumors: a systematic review. J Investig Clin Dent 2014; 5: 9–14. doi: 10.1111/jicd.12044 [DOI] [PubMed] [Google Scholar]
- 3.Han Y, Fan X, Su L, Wang Z. Diffusion-weighted MR imaging of unicystic odontogenic tumors for differentiation of unicystic ameloblastomas from keratocystic odontogenic tumors. Korean J Radiol 2018; 19: 79–84. doi: 10.3348/kjr.2018.19.1.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White SC, Pharoah MJ. Oral radiology principles and interpretation. 7th ed Missouri: The British Institute of Radiology.; 2014. [Google Scholar]
- 5.Li J, Wu T, Yu SF, Yu GY. Unicystic ameloblastoma a clinicopathologic study of 33 Chinese patients. Am J Surg Pathol 2000; 24: 1385–92. [DOI] [PubMed] [Google Scholar]
- 6.Nunez-Urrutia S, Figueiredo R, Gay-Escoda C. Retrospective clinicopathological study of 418 odontogenic cysts. Med Oral 2009; 15: e767–73. doi: 10.4317/medoral.15.e767 [DOI] [PubMed] [Google Scholar]
- 7.Dunsche A, Babendererde O, Luttges J, Springer ING. Dentigerous cyst versus unicystic ameloblastoma - differential diagnosis in routine histology. J Oral Pathol Med 2003; 32: 486–91. doi: 10.1034/j.1600-0714.2003.00118.x [DOI] [PubMed] [Google Scholar]
- 8.MacDonald-Jankowski DS, Yeung R, Lee KM, Li TK. Ameloblastoma in the Hong Kong Chinese. Part 2: systematic review and radiological presentation. Dentomaxillofac Radiol 2004; 33: 141–51. doi: 10.1259/dmfr/28001874 [DOI] [PubMed] [Google Scholar]
- 9.Barrett AW, Sneddon KJ, Tighe JV, Gulati A, Newman L, Collyer J, Norris PM, et al. Dentigerous cyst and ameloblastoma of the jaws. Int J Surg Pathol 2017; 25: 141–7. doi: 10.1177/1066896916666319 [DOI] [PubMed] [Google Scholar]
- 10.Araujo JP, Lemos CA, Miniello TG, Alves FA. The relevance of clinical and radiographic features of jaw lesions: a prospective study. Braz Oral Res 2016; 30: e96. 10.1590/1807-3107BOR-2016.vol30.0096 [DOI] [PubMed] [Google Scholar]
- 11.Boffano P, Ruga E, Gallesio C. Keratocystic odontogenic tumor (odontogenic keratocyst): preliminary retrospective review of epidemiologic, clinical, and radiologic features of 261 lesions from University of Turin. J Oral Maxillofac Surg 2010; 68: 2994–9. doi: 10.1016/j.joms.2010.05.068 [DOI] [PubMed] [Google Scholar]
- 12.Yang S-I, Park Y-I, Choi S-Y, Kim J-W, Kim C-S. A retrospective study of 220 cases of keratocystic odontogenic tumor (KCOT) in 181 patients. Asian J Oral Maxillofac Surg 2011; 23: 117–21. doi: 10.1016/j.ajoms.2011.03.002 [DOI] [Google Scholar]
- 13.Lawal AO, Adisa AO, Olajide MA. Cystic ameloblastoma: a Clinico-pathologic review. Ann Ib Postgrad Med 2014; 12: 49–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Kocak-Berberoglu H, Cakarer S, Brkic A, Gurkan-Koseoglu B, Altug-Aydil B, Keskin C. Three-dimensional cone-beam computed tomography for diagnosis of keratocystic odontogenic tumours; evaluation of four cases. Med Oral 2012: e1000–5. doi: 10.4317/medoral.17629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng Y, Zhang YQ, Ye X, Zhao YN, Chen Y, Liu DG. Imaging analysis of ameloblastoma, odontogenic keratocyst and dentigerous cyst in the maxilla using spiral CT and cone beam CT. Chin J Stomatol 2018; 10: 659–64. doi: https://doi.org/DOI:10.3760/cma.i.issn.1002-0098.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Zwahlen R, Grätz KW. Maxillary ameloblastomas: a review of the literature and of a 15-year database. J Craniomaxillofac Surg 2002; 30: 273–9. doi: 10.1016/S1010-5182(02)90317-3 [DOI] [PubMed] [Google Scholar]
- 17.Parmar S, Al-Qamachi L, Aga H. Ameloblastomas of the mandible and maxilla. Curr Opin Otolaryngol Head Neck Surg 2016; 24: 148–54. doi: 10.1097/MOO.0000000000000238 [DOI] [PubMed] [Google Scholar]
- 18.Philipsen HP, Reichart PA. Unicystic ameloblastoma. A review of 193 cases from the literature. Oral Oncology 1998; 34: 317–25. doi: 10.1016/S1368-8375(98)00012-8 [DOI] [PubMed] [Google Scholar]
- 19.Milman T, Ying G-S, Pan W, LiVolsi V. Ameloblastoma: 25 Year Experience at a Single Institution. Head and Neck Pathol 2016; 10: 513–20. doi: 10.1007/s12105-016-0734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apajalahti S, Kelppe J, Kontio R, Hagström J. Imaging characteristics of ameloblastomas and diagnostic value of computed tomography and magnetic resonance imaging in a series of 26 patients. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 120: e118–30. doi: 10.1016/j.oooo.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Rastogi V, Pandilwar PK, Maitra S. Ameloblastoma: an evidence based study. J Maxillofac Oral Surg 2010; 9: 173–7. doi: 10.1007/s12663-010-0060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konouchi H, Asaumi J-ichi, Yanagi Y, Hisatomi M, Kawai N, Matsuzaki H, et al. Usefulness of contrast enhanced-MRI in the diagnosis of unicystic ameloblastoma. Oral Oncol 2006; 42: 481–6. doi: 10.1016/j.oraloncology.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 23.Takata T, Slootweg PJ. Odontogenic and maxillofacial bone tumors : El-Naggar AE, Chan JKC, Grandis JR, Takata T, Slootweg PJ, WHO classification of head and neck tumors (4th ed). Lyon, France: The British Institute of Radiology.; 2017. 203–60. [Google Scholar]
- 24.Fregnani ER, da Cruz Perez DE, de Almeida OP, Kowalski LP, Soares FA, de Abreu Alves F. Clinicopathological study and treatment outcomes of 121 cases of ameloblastomas. Int J Oral Maxillofac Surg 2010; 39: 145–9. doi: 10.1016/j.ijom.2009.11.022 [DOI] [PubMed] [Google Scholar]
- 25.Adeline VL, Dimba EAO, Wakoli KA, Njiru AK, Awange DO, Onyango JF, et al. Clinicopathologic features of ameloblastoma in Kenya: a 10-year audit. J Craniofac Surg 2008; 19: 1589–93. doi: 10.1097/SCS.0b013e31818c0504 [DOI] [PubMed] [Google Scholar]
- 26.Carvalho K, Dhupar A, Spadigam A, Syed S. Ameloblastoma: a 16-year clinicopathological study on Goan population. Indian J Pathol Microbiol 2017; 60: 157–60. doi: 10.4103/0377-4929.208374 [DOI] [PubMed] [Google Scholar]
- 27.Anand R, Sarode GS, Sarode SC, Reddy M, Unadkat HV, Mushtaq S, et al. Clinicopathological characteristics of desmoplastic ameloblastoma: a systematic review. J Invest Clin Dent 2018; 9: e12282. 10.1111/jicd.12282 [DOI] [PubMed] [Google Scholar]
- 28.Sun Z-J, Wu Y-R, Cheng N, Zwahlen RA, Zhao Y-F. Desmoplastic ameloblastoma - A review. Oral Oncol 2009; 45: 752–9. doi: 10.1016/j.oraloncology.2009.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darshani Gunawardhana KSN, Jayasooriya PR, Rambukewela IK, Tilakaratne WM. A clinico-pathological comparison between mandibular and maxillary ameloblastomas in Sri Lanka. J Oral Pathol Med 2010; 39: 236–41. doi: 10.1111/j.1600-0714.2009.00850.x [DOI] [PubMed] [Google Scholar]
- 30.Singh A, Shaikh S, Samadi FM, Shrivastava S, Verma R. Maxillary unicystic ameloblastoma: a review of the literature. Natl J Maxillofac Surg 2011; 2: 163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apajalahti S, Hagström J, Lindqvist C, Suomalainen A. Computerized tomography findings and recurrence of keratocystic odontogenic tumor of the mandible and maxillofacial region in a series of 46 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 111: e29–37. doi: 10.1016/j.tripleo.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 32.Lo Muzio L, Mascitti M, Santarelli A, Rubini C, Bambini F, Procaccini M, et al. Cystic lesions of the jaws: a retrospective clinicopathologic study of 2030 cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2017; 124: 128–38. doi: 10.1016/j.oooo.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 33.Mortazavi H, Baharvand M. Jaw lesions associated with impacted tooth: a radiographic diagnostic guide. Imaging Sci Dent 2016; 46: 147–57. doi: 10.5624/isd.2016.46.3.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scarfe WC, Toghyani S, Azevedo B. Imaging of benign odontogenic lesions. Radiol Clin North Am 2018; 56: 45–62. doi: 10.1016/j.rcl.2017.08.004 [DOI] [PubMed] [Google Scholar]