Abstract
Apoptosis is one of the main types of regulated cell death, a complex process that can be triggered by external or internal stimuli, which activate the extrinsic or the intrinsic pathway, respectively. Among various factors involved in apoptosis, several genes and their interactive networks are crucial regulators of the outcomes of each apoptotic phase. Furthermore, mitochondria are key players in determining the way by which cells will react to internal stress stimuli, thus being the main contributor of the intrinsic pathway, in addition to providing energy for the whole process. Other factors that have been reported as important players of this intricate molecular network are miRNAs, which regulate the genes involved in the apoptotic process. Imbalance in any of these mechanisms can lead to the development of several illnesses, hence, an overall understanding of these processes is essential for the comprehension of such situations. Although apoptosis has been widely studied, the current literature lacks an updated and more general overview on this subject. Therefore, here, we review and discuss the mechanisms of apoptosis, highlighting the roles of genes, miRNAs, and mitochondria involved in this type of cell death.
Keywords: regulated cell death, apoptosis, mitochondria, miRNAs, genetics
1. Introduction
The mechanisms underlying cell death and survival have a great impact on maintaining cellular balance, such that their deregulation may lead to the development of various diseases, such as multiple types of cancer and neurodegenerative disorders [1,2]. The classifications about cell death modalities depend mainly on morphological and structural details of individual tissues and cells [3]. Among the different types of cell death, apoptosis stands out as one of the most widely studied in the past years. Still, only a few articles provide a general and descriptive overview of apoptosis.
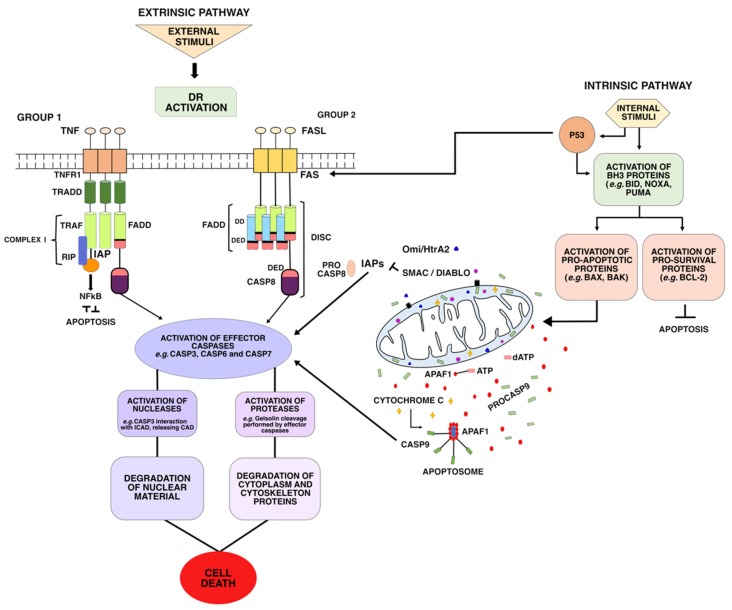
Apoptosis is a normal mechanism that can occur at any stage of the individual’s development or upon cell damage, and is marked by the following characteristics: Protein cleavage (occurring mainly by the activation of cysteine proteases known as caspases), nuclear DNA breakdown, and apoptotic cell recognition by phagocytic cells [4]. These processes are responses to internal (intrinsic or mitochondrial) or external (extrinsic pathway or death receptors, DR) stimuli to the cell, converging in the final stage, known as the apoptotic execution phase (Figure 1).
Figure 1.
General scheme of the process of apoptotic cell death. This phenomenon may occur via the extrinsic or intrinsic pathway, converging in the executing phase.
There are many intricate aspects to both apoptotic pathways and the numerous molecules involved in their mechanisms. The study of the apoptotic pathway has shown to be a great approach in the search for new anticancer therapy, since promising compounds that trigger apoptosis are often non-toxic to healthy cells [5].
In this review, we begin by presenting a general overview of intrinsic and extrinsic pathways and the executioner phase. In more detail, we discuss the roles of major genes involved in apoptosis and highlight the importance of considering epigenetic mechanisms as regulators of the apoptotic machinery.
2. Intrinsic or Mitochondrial Pathway
The intrinsic pathway of apoptosis, also known as the mitochondrial pathway, originates due to internal cellular stress [2]. These internal stimuli, such as DNA damage or endoplasmic reticulum stress, induce the activation of BH3-only (BCL-2 homology 3) proteins, which include BID, BIM, BAD, BIK, BMF, Noxa, PUMA, and HRK. BH3 proteins interact and activate BH3 pro-survival or pro-apoptotic proteins, which may lead to the interruption of apoptotic cell death or to the continuation of this process, respectively [6].
In the first scenario, pro-survival proteins (BCL-2, BCL-X, BCL-w, MCL-1, or BFL-1/A1) prevail, interrupting cell death mechanisms at that given point [7]. In the second scenario, the activation of pro-apoptotic proteins BAX (BCL-2-associated X protein) or BAK (BCL-2 antagonist or killer) lead to mitochondrial outer membrane permeabilization (MOMP), considered a point of no return due to the release of proteins involved in the activation of caspases in the cytoplasm, accompanied by the gradual acidification of the environment around the mitochondria [8,9].
Among proteins released through MOMP, there are cytochrome-c (Cyt-c), involved in the formation of apoptosomes, and the pro-apoptotic proteins Smac/DIABLO and Omi/HtrA2, which interact with inhibitory proteins to activate procaspases such as Procaspase-3 (ProC3) and Procaspase-7 (ProC7) [10]. The release of pro-apoptotic proteins inhibits the negative regulation carried out by inhibitor of apoptosis proteins (IAP) upon procaspases [11].
The release of Cyt-c in the cytoplasm activates the formation of the apoptosome, a cytosolic multiprotein complex that is composed of Cyt-c, apoptotic protease activating factor 1 (Apaf-1), and Procaspase-9 (ProCASP9) [12]. The formation of this complex starts from the association of Cyt-c to the cytoplasmic protein structure composed of Apaf-1 monomers. Then, the release of an ATP molecule and assembly of the heptameric apoptosome takes place. After binding and activation of ProC9, the complete apoptosome is formed.
This activation occurs through binding of ProC9 to the caspase recruitment domain (CARD) of the Apaf-1 adapter protein and, once in its active form (CASP9), it must remain bound to the apoptosome to maintain a substantial catalytic activity [13]. Then, the initiator CASP9 cleaves and activates execution caspases, such as Caspase-3 (CASP3) and Caspase-7 (CASP7), through proteolysis that rearranges critical protein loops in the formation of active sites [14,15]. When activated, execution caspases can cleave and activate other execution caspases in a feedback system during the execution phase [16]. See Execution phase for more detail on caspases.
It is also important to note that, in addition to apoptosome, other protein platforms that trigger specific mechanisms not only in the intrinsic, but also in the extrinsic pathway of apoptosis are currently known and they are all related to diverse initiator caspases [17]. Among these structures, here we highlight PIDDosome, ripoptosome, and FADDosome, of which PIDDosome is involved in the intrinsic pathway. Ripoptosome and FADDosome will be further discussed.
The PIDDosome complex was first described by Tinel and Tschopp [18] as a complex composed by PIDD (p53-induced protein with a death domain) protein, RAIDD (RIP-associated ICH-1/CED-3-homologous protein with a death domain) adaptor, and caspase-2, leading to the activation of the latter. PIDDosome has been suggested to be involved in p53-mediated apoptosis in response to genotoxic stress and DNA damage, but recently it has been associated to other non-apoptotic roles, such as centrosome surveillance during cellular differentiation [19].
3. Extrinsic or Death Receptor Pathway
The extrinsic pathway of apoptosis starts from external stimuli to the cell. It is triggered by the oligomerization of transmembrane proteins from the superfamily of death receptors or when extracellular concentrations of specific factors reach a particular threshold, leading to the transduction of lethal signals or dependence receptors [20].
During the extrinsic pathway, mitochondria amplify caspase activation, but are not considered essential to this type of cell death [20]. Instead, the extrinsic signaling pathway is triggered when DR are activated by receptor binding or aggregation. Such DR belong to the tumor necrosis factor (TNF) receptor superfamily, characterized by cysteine-rich extracellular domains, as well as cytoplasmic death domains [21]. The most well-known members of this superfamily are Fas, TNFR1, DR3, DR4, DR5, and DR6 [22]. Some of these death receptors, such as Fas and DR5, can be activated by different proteins, such as p53 and MYC. The latter interacts and leads to activation of DR5/TRAIL and Fas death receptors, while the former induces DR transcription, in addition to increasing Fas and DR5 levels in the cytosol by non-transcriptional mechanisms [23,24,25].
There are eight types of death receptors (DR1–DR8), which can be divided in two groups according to the adapter protein [26,27]:
-
(i)
The first group includes the receptors Fas (DR2) TRAILR1 (DR4) and TRAILR2 (DR5), which can be activated by Fas ligand (FasL) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), respectively. TRAIL ligands bind to a DR and a TRAIL receptor (TRAIL-R), triggering apoptotic signals and inducing the formation and activation of death-inducing signaling complex (DISC) complex [28]. Once activated, these receptors recruit the death-inducing signaling complex (DISC)—which is composed of FAS-associated via death domain (FADD) and Procaspase-8 (ProC8)—for the transduction of apoptotic signals;
-
(ii)
The second group includes the receptors TNFR1 (DR1), TRAMP (DR3), DR6, and EDAR. These DRs recruit TNF-associated death domain (TRADD) as adapter protein, and bind to the TNF-2,5 receptor-associated factors (TRAF2,5), the receptor-interacting protein kinase (RIP1 or RIPK1), and to cellular inhibitors of apoptosis proteins (cIAP). This forms a signaling complex, called Complex I, for signal transduction of apoptosis and cell survival.
Once DISC is formed, caspase-8 (CASP8) is activated, leading to the activation of other caspases, such as CASP3 [22]. However, the binding of CASP8 to FADD and the further formation of DISC may be prevented if an isoform of one of the DR regulators, called cellular FLICE-like inhibitory protein (cFLIPs), bind to FADD [27]. This is because one of these cFLIP isoforms has a highly similar structure to CASP8, missing only the active catalytic domain. The important regulatory role of cFLIPs is reviewed in Tsuchiya et al. [29].
In addition, FADD-dependent apoptosis and CASP8 may be indirectly induced by TNFR1, as shown in Figure 1. The TNFR1 receptor recruits the TNFR1-associated death-domain protein, which serves as a platform to recruit different signaling molecules: (i) FADD, which functions as a mediator of the activation of apoptosis through the activation of CASP8, and (ii) TRAF2 (in the TRAF2/cIAP complex) and RIP, eventually leading to the activation of NF-κB and JNK/AP-1 (c-Jun NH2-terminal kinase/Jun proto-oncogene, AP-1 transcription factor subunit), which possess anti-apoptotic activity [21]. As previously mentioned, among the different protein platforms in extrinsic pathway, there are ripoptosome and FADDosome.
Ripoptosome was described by Tenev et al. [30]—and independently by Feoktistova et al. [31]—as composed by RIPK1, FADD, and CASP8 in response to loss of XIAP, cIAP1, and cIAP2 activity induced by genotoxic stress. These IAPs can suppress ripoptosome formation, which can also be negatively regulated by FLIP. This complex is able to convert proinflammatory cytokines into death signals and it may lead to cell death through apoptosis and necroptosis due to RIPK1 function in both types of cell death, which can be modulated by IAPs and cFLIP [30,31].
As described by Henry and Martin [32], FADDosome is a pro-inflammatory complex induced by TRAIL-R stimulation and composed by CASP8, FADD, and RIPK1, in which CASP8 presents non-enzymatic functions in this formation, in addition to CASP8 also being able to lead to NF-kB activation. According to Mohr et al. [33], FADDosome is composed not only by these proteins, but also by CASP10 and TRAF2, and it is mainly induced by DNA damage—protein kinase ATR is essential to FADDosome formation through CASP10 upregulation. In the presence of CASP8 and absence of the other factors in this scenario, FADDosome cannot be assembled and CASP8 mediates cFLIPL cleavage generating an alternative structure called FLIPosome, which leads to NF-κB and TNF-α activation [33]. As highlighted by Mouasni and Tourneur [34], FADDosome seems to be important in inflammatory pathways and tumor growth. Considering that FADDosome has been recently described, much is still to be discovered on this complex.
Nevertheless, induction of apoptosis and activation of NF-κB is considered to be inhibitory to each other: The apoptotic process can be interrupted at different points by the NF-κB pathways, being influenced by the accessibility to IAP proteins or the activation of Akt pathways, and an apoptosis process already in progress may block NF-κB signaling pathway through the cleavage of components by caspases [35,36]. Furthermore, Akt activation prevents Cytochrome C release and directly inhibits proapoptotic signals through the phosphorylation and cytoplasmic sequestration of BAD and the O subfamily of the forkhead box transcription factors (FoxOs) [37,38,39]. FoxOs are involved in a series of cellular functions, such as cell survival and tumor suppression pathways, upregulation of pro-apoptotic molecules, such as Bim and Fas-L, and FoxO’s inhibition leads to decreased expression of TRAIL [40,41,42].
4. Execution Phase
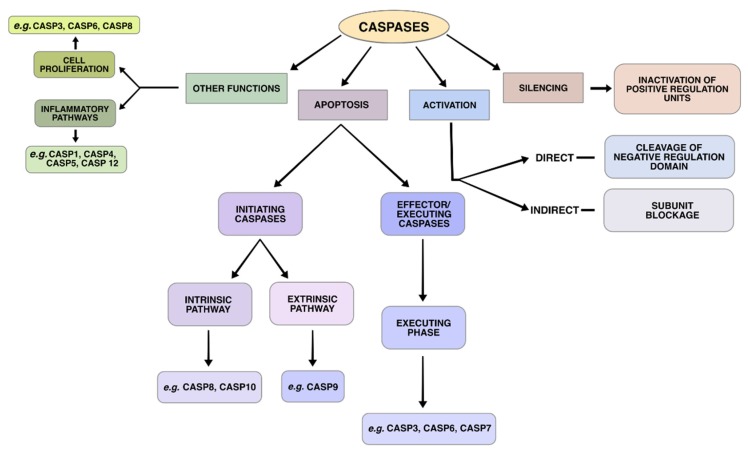
There are many molecules involved in the apoptotic process, among which caspases are one of the most important, and participate in both of the aforementioned pathways. Caspases (cysteine-aspartic proteases) (Figure 2) are endoproteases that hydrolyze peptide bonds in a reaction that depends on catalytic cysteine residues. The above-mentioned series of molecular events lead to the activation of caspases, which then cleave a restricted set of target proteins in the primary sequence, generating either protein inactivation or direct (by cleavage of a negative regulatory domain) or indirect (inactivating a regulatory subunit) activation of proteins [43].
Figure 2.
The main roles played by caspases in different biological pathways, including apoptosis.
There are many types of caspases (Figure 2), namely Caspases -2, -3, -6, -7, -8, -9, -10 and Caspase-activated DNAse (CAD) [44,45]. These proteins are classified as initiators (e.g., CASP2, CASP8, CASP9, and CASP10) or effectors/executioners (e.g., CASP3, CASP6, and CASP7) according to their role in apoptotic pathways [46]. Initiators activate executioners, which in turn activate other executioners in a feedback loop, affecting essential structural proteins and other enzymes, so that key features of apoptotic cell death occur [16].
Caspase classification entails sequence similarity, substrate selection, and catalytic activity. The determination of such features may be assessed though in vivo and in vitro biological assays. Murine caspase knock-out models have been useful for cellular functions carried out by multiple caspases [47]. In some cases, this has also elucidated other roles, such as CASP3, -8, and -9 deficiency leading to embryonic lethality [48,49,50]. The variety of caspases acting in apoptosis has previously suggested possible redundancies. However, proteomic assays have determined more specialized roles [51,52,53]. It is worth mentioning the unique case of initiator CASP10, which is only found in humans and has had its specific biological roles elucidated mainly by in vitro experiments [54].
The activation of the executioner caspases in the intrinsic or extrinsic pathway is considered to be the beginning of apoptotic execution phase, the final part of this cell death process. Executioner caspases activate cytoplasmic endonucleases that degrade nuclear material and proteases that degrade core and cytoskeletal proteins [2].
Among the executioner caspases stands out the role of CASP3, which activates the nuclease CAD through controlled cleavage of the Inhibitor of CAD (ICAD) [55]. CAD is a nuclease that induces DNA breakage within the nuclei, frequently through the p53 protein signaling [56,57]. Another key substract activated by CASP3 is an actin-binding protein named Gelsolin [58]. CASP3 will cleave gelsolin and the cleaved fragments of the protein will cleave actin filaments [2].
This results in cytomorphological changes that include cell shrinkage, chromatin condensation, formation of cytoplasmic blisters and apoptotic bodies, with consequent phagocytosis of bodies by adjacent, neoplastic, or macrophage cells.
5. Genes Involved in the Apoptotic Pathways
Here, we focus on the roles played by nine genes that have essential functions in the intrinsic (APAF1, BCL2, and CASP9), extrinsic (CASP8, FADD, and FAS), both pathways (TP53 and MYC), and in the execution phase (CASP3).
6. Intrinsic Pathway
6.1. APAF1 (Apoptotic Peptidase Activating Factor 1)
The APAF1 gene (Apoptotic peptidase activating factor 1) encodes the Apaf1 protein, which is activated by Cyt-c and regulated by pro-apoptotic molecules (such as Bax and Bid) and anti-apoptotic molecules (such as Bcl-X and Bcl-2). As part of the apoptosome, it is responsible for the activation of CASP9, which leads to CASP3-dependent cell death [59].
Prior to apoptosome formation, Apaf1 is normally present in the cytoplasm in the form of inactive monomers bound to ATP molecules and undergoes conformational changes for binding to Cyt-c [13]. Because it is the intermediate between Cyt-c and ProC9, Apaf1 is considered the main component of apoptosome [12].
Recently, the role of Apaf1 as a major apoptosis regulator was demonstrated in a human embryonic stem cell assay, which concluded that low levels of APAF1 expression were possibly determinant for inhibiting cell death in neuronal development [60]. This is further reinforced by the identification of deleterious variants in the APAF1 gene in human fetuses with neural tube defects, showing the significant impact that mutations in apoptotic genes may have in human development [61].
6.2. BCL2 (B-Cell Lymphoma Protein 2, Apoptosis Regulator)
The BCL2 (B-cell lymphoma protein 2, apoptosis regulator) gene encodes for its homonymous protein, is part of the Bcl-2 (B-cell lymphoma protein 2) family, and acts as pro-survival [7]. This family has at least nine members described, including antiapoptotic members (BCL-2, BCL-XL, MCL-1, BCL-w, BFL-1/A1), pro-apoptotic members (BCL-B, BAX, BAK, and BOK), and four similar proteins (BCL2L13, BCL2L14, BCL2L15, and BID) [62,63]. BCL-B has four BCL-2 Homologous domains (BH), from which pro-apoptotic BH3-only proteins (BAD, BCL-XS, BLK, and BLD) are derived [64]. The balance between pro-apoptotic and anti-apoptotic Bcl-2 proteins influences the sensitivity of cells to apoptotic stimuli [65]. For example, the increase of mitochondrial cAMP leads to the cAMP-dependent protein kinase (PKA)-mediated phosphorylation of BAD, preventing its heterodimerize with Bcl-2 and Bcl-X, thus causing cell survival [66,67]. However, when dephosphorylated, BAD and Bcl-2 are translocated to the mitochondria, and their heterodimerization leads to the release of cytochrome c in the cytoplasm, hence leading to apoptosis [68,69,70].
Considering apoptosis evasion is one of the hallmarks of cancer, it is expected that apoptotic genes are found translocated or mutated in the tumor environment [71]. For the BCL2 gene, this context was crucial to its discovery. First described in follicular B-cell lymphoma, the BCL2 gene is located in the 18q21.3 region and can be linked to a locus in a translocation [72,73] that increases transcription of its protein and causes gain of function [74,75].
BCL2 has been well-established as a therapeutic target due to its important roles in apoptosis, and has a number of pharmaceutical initiatives in cancer treatment [76]. Interestingly, a new acquired mutation in BLC2 was recently described inhibiting drug efficacy in chronic lymphocytic leukemia. This single mutation caused a 180-fold reduction in venetoclax binding affinity to BCL2, demonstrating how decisive apoptotic genes are in the context of human disease [77]. In addition, the interactions of Bcl-2 with cellular pathways, such as Akt and RAS, is also important since it can be a mechanism for evasion of apoptosis and chemoresistance in cancer cells [78,79,80].
6.3. CASP9 (Caspase 9)
The CASP9 gene encodes the homonymous protein that acts as the initiating caspase in the intrinsic apoptotic pathway. Its inactive form, ProC9, is composed of three distinct regions: An N-terminal caspase recruitment domain (CARD), a large catalytic subunit, and a small catalytic subunit. Conformational changes lead to the formation of the apoptosome, which seems to serve as a platform for increased concentration and subsequent activation of CASP9 [13]. The active form of this protein must remain attached to the apoptosome to maintain its catalytic activity [81].
The relative concentrations of Apaf-1 and ProC9 appear to influence the proportion of CASP9 homodimers and heterodimers formed in apoptosomes; that is, apoptosomes seem to function as a mediator for the formation of homodimers (induced by Pro-C9 proximity) and heterodimers (resulting from the interaction between the small subunit of ProC9 and the NOD domain of Apaf-1), culminating in the activation of caspases such as CASP3, CASP6, and CASP7 [82,83].
It was demonstrated that high inflammatory responses are associated to increased CASP9 activity and this process may be involved in the progression of inflammatory conditions, such as periodontitis and acute liver disease [84,85]. Furthermore, variants of CASP9 have been associated with cancer [86].
7. Extrinsic Pathway
7.1. CASP8 (Caspase 8)
The CASP8 gene encodes a homonymous protein, which is synthesized as a zymogen and is the first activated caspase in the extrinsic pathway, which occurs after the formation of the DISC complex [87,88,89].
Active CASP8 concentration at this point determines which apoptotic pathway to follow: If CASP8 protein levels are high, it directly cleaves CASP3, which then performs its function; if levels are low, it truncates BID (tBID), which releases Cyt-c from mitochondria, leading to the activation of CASP9, which in turn cleaves CASP3 [90]. Thus, the main function of CASP8 seems to be the activation of executing procaspases, especially ProCASP3. Recently, increased CASP8 plasma concentration was shown to be a candidate biomarker for diabetes mellitus (DM) because it enhances β-cell apoptosis, leading to both circadian rhythm disruption and DM incidence [91]. This discovery reveals that apoptotic activity is related to sleep duration and that CASP8 levels can indicate incidence of an endocrine disease years before onset.
Additionally, CASP8 alterations have also been observed in disease contexts in roles aside from its apoptotic functions. For instance, non-apoptotic functions of CASP8, such as those involved in immune response, are critical in the development of very early onset inflammatory bowel disease through interactions with the immune system [92,93].
7.2. FADD (FAS-Associated Via Death Domain)
The FADD gene (FAS-associated via death domain), also known as MORT1, encodes the FADD protein, which acts as an adapter in the extrinsic pathway. It was discovered and associated with interaction with the Fas receptor by Chinnaiyan et al. [94].
FADD is considered the main adapter for the transmission of DR-mediated apoptotic signals, since the interaction between this adapter and the majority of death receptors has already been described [95]. FADD protein consists of two domains that interact with other domains: Death domain (DD) and death effector domain (DED) [27]. DD/DD interactions lead to recruitment of FADD, initiating the formation of DISC, while DED/DED interactions recruit CASP8 or CASP10 [96].
In addition to its functions in apoptosis, FADD can also induce cell survival, cell cycle progression, and cell proliferation, depending on its phosphorylation and cellular localization [97]. Disrupted FADD phosphorylation has been inversely correlated with tumor aggressiveness in T-cell lymphoblastic lymphomas [98]. Moreover, decreased FADD is associated with cognitive impairment and clinical dementia in the elderly [99]. In addition, a recent review has explored the roles of FADD in the context of cancer and inflammation, highlighting the impact of translational and post-translational modifications in its multiple functions [34].
7.3. FAS (FAS Cell Surface Death Receptor)
The FAS gene (FAS cell surface death receptor), also known as CD95 and APO-1, encodes FAS (or Fas), a type I transmembrane protein located on the cell surface. It is considered a DR due to its main role of inducing cell death when binding to FasL [96]. This binding induces the trimerization of Fas in the cell membrane, which leads to its activation and recruitment of FADD [90].
In addition to its well-defined function in apoptosis, Fas also appears to have non-apoptotic functions: It is involved in the activation of RIPK1, resulting in cellular necrosis; and it may also activate other signaling pathways, resulting in cell differentiation or proliferation [95].
Polymorphisms in FAS gene may decrease the apoptotic potential of the receptor, and have been associated with several illnesses, including cancer and musculoskeletal degenerative disorders [100,101]. Furthermore, there are studies demonstrating the association of FAS polymorphisms with the persistence of HTLV infections and its clinical manifestations, such as Adult T-Cell Leukemia (ATL) and HTLV-1-associated myelopathy (HAM) [102,103,104].
8. Intrinsic and Extrinsic Pathways
8.1. TP53 (Tumor Protein p53)
The TP53 tumor suppressor gene (Tumor protein p53) encodes the p53 protein, a transcription factor that has different functions involving cell and genomic stability, senescence, and cell death. The p53 protein targets more than 900 genes, affecting not only apoptosis, but also metabolism, proliferation, and immune response genes [105].
It has also been demonstrated to induce apoptosis independently of transcription in both pathways. This protein activates the extrinsic pathway by inducing FAS and promoting apoptosis through an element located in intron 1, which binds to FAS promoter regions and reaches maximum transactivation [106]. Its mechanisms through DNA damage may also be implicated in DR5 activation and consequent activation of CASP8 and Poly ADP-ribose polymerase (PARP), with this relationship being a potential therapeutic target in cancer treatment [107]. Recently, a component named Ziyuglycoside I (Ziyu I) was reported to be capable of inducing mitochondrial dependent apoptosis in human WERI-Rb-1 Retinoblastoma cells due to increased expression of p53 [108].
In addition, p53 positively regulates the transcription of the intrinsic pathway factors Noxa, PUMA, and BID that induce apoptosis when highly expressed [109]. Moreover, p53 is necessary for the formation of the apoptosome, by inducing the Apaf1 promoter. It also activates CASP8 and CASP6 in response to cell stress and DNA damage, respectively. P53 localization to the mitochondria also promotes apoptosis by triggering the BAX channel on the mitochondrial surface, responsible for releasing Cyt-c, which activates CASP9 and gives rise to the initiation of apoptosis [110].
8.2. MYC (MYC Proto-Oncogene, bHLH Transcription Factor)
The MYC gene (bHLH transcription factor), also known as c-Myc, acts on the transcriptional regulation of several processes, including cell progression and apoptosis [111] in conjunction with partner protein Max [112,113]. The dual roles played by MYC for being an oncogene and a pro-apoptotic protein are determined by expression levels. For this gene to take part in cell death, the threshold of expression must be much higher than for cell cycle induction, which involves a highly regulated mechanism [114].
Regarding apoptosis, besides its involvement in the extrinsic pathway, MYC is known to drive cell death through p53-mediated apoptosis. MYC activation leads to increased expression of tumor suppressor p14ARF, which in turn blocks p53 inhibitor hMDM2 and ultimately increases p53 expression and the mitochondrial pathway [115]. However, p53-independent MYC-induced apoptosis has been described in certain cell types [116,117]. The main mediator of MYC-induced cell death, however, is reported to be one of its BH3-only targets (BIM) also in a p53-independent mechanism [118].
It seems counter-intuitive that a gene capable of inducing apoptosis at high expression levels may also be related to increased tumorigenesis. However, mechanisms through which MYC triggers apoptosis have been proved to also lead to genomic instability, which explains this paradox [119]. It is important to highlight that MYC is an essential global transcription regulator, capable of controlling up to 15% of genes in the human genome [120,121].
9. Execution Phase
CASP3 (Caspase 3)
The CASP3 gene encodes a homonymous protein that is considered to be the most important effector caspase. During the execution phase of apoptosis, CASP3 activates the CAD endonuclease (Caspase-activated DNAse) by cleaving it from its inhibitor (ICAD). After the release of CAD, it degrades chromosomal DNA, causes chromatin condensation, and induces cytoskeletal reorganization and cell disintegration in apoptotic bodies [2]. It has been suggested that CASP3 has other roles and some that overlap with those of CASP6 and CASP7 [46].
Despite being dependent on chain cleavage and, consequently, initiator proteins, the apoptotic functions of CASP3 have been shown to work in the absence of such activator molecules. This protease may be constitutively activated in the presence of mutation V266E, indicating a possible therapeutic strategy for apoptosis induction [122]. Caspase 3 deregulation studies demonstrate that, in colorectal cancer, CASP3 acts to promote tumor invasiveness and metastasis after exposure to radiation and chemical carcinogens [123], hence it is suggested that targeting CASP3 may increase tumor susceptibility to cancer treatment and inhibit metastasis [124].
10. Mitochondria and Epigenetic Regulation
Mitochondria are cytoplasmic organelles responsible for the generation of cellular energy (ATP) through the process of oxidative phosphorylation (OXPHOS). They have several other functions that are also essential for normal cell functioning, such as control of calcium levels, lipid homeostasis, metabolic cell signaling, and cell death [125]
During OXPHOS, mitochondria generate reactive oxygen species (ROS) through the electron transport chain (ETC). Despite being a natural part of the process, an accumulation of ROS due to a shift in equilibrium causes damage to the mitochondrial DNA (mtDNA) [126]. When a cell is no longer functioning normally, it initiates regulated cell death, most commonly apoptosis. Mitochondria not only have an essential role in intrinsic apoptosis, but also in providing energy for the complete apoptotic process [127].
In the intrinsic pathway, pro-apoptotic proteins from the Bcl-2 family translocate to the mitochondrial outer membrane and mediate its permeabilization, inducing the release of Cyt-c and IAP inhibitors. This permeabilization is also controlled by certain mitochondrial dynamics, which involve regulation of the organelle’s fission (mediated by Dynamin-related protein 1) [127], fusion of the outer membrane (role played by Optic atrophy 1) [128], lipid content (such as ceramides) [129], and interaction with the endoplasmic reticulum (ER) [130].
In the absence of caspase involvement, mitochondria can undergo apoptosis upon Bax/Bak-induced MOMP, by triggering the release of mtDNA, resulting in IFN expression and a pro-inflammatory type of cell death. Furthermore, the simultaneous activation of caspases inhibits the aforementioned process, meaning mitochondria have a pivotal role in deciding on which pathway it will carry out cell death, a possibly inflammatory or immunologically silent process [131,132]. The extrinsic pathway requires the mitochondria downstream from the activation of death receptor proteins in certain cell types, which interact with this organelle upon the CASP8-mediated activation of Bid, a pro-apoptotic Bcl-2 member [133,134].
Alternatively, apoptosis may be triggered by high levels of calcium ions in the ER. Apoptotic stimuli cause the release of intracellular calcium from the ER and thereby an overload in mitochondria, leading to increased membrane permeability and subsequent flow of pro-apoptotic molecules into the cytoplasm [135].
Considering the number of interacting molecules involved in mitochondrial-mediated apoptosis, it is expected that mutations in mtDNA genes may lead to differences in cell death processes. Additionally, a level of complexity is added once we acknowledge the effects of heteroplasmic variations in mitochondrial genomes. Seeing that the number of mitochondria in a cell may vary widely depending on cell type, proportions of a given mutation are also subject to such fluctuations [136]. This state in which different genotypes of a mutation coexist in the mitochondria of the same cell is called heteroplasmy, while the equality state of the genotypes is called homoplasmy. Studies have shown that different levels of heteroplasmy may have a significant importance in mtDNA and nuclear gene expression, including genes involved directly and indirectly in apoptosis and tumorigenesis [137,138].
Interestingly, much like the nuclear genome (nDNA), mtDNA is also controlled by epigenetic mechanisms. The epigenetic events related to mitochondria are known as mitoepigenetics and are carried out by mtDNA methylation and non-coding RNAs (ncRNAs) [139].
While nDNA methylation is a well-established process, mtDNA methylation is still controversial and poorly understood. However, some studies have demonstrated the presence of nuclei-encoded DNA-methytransferases (DNMTs) inside human mitochondria [140,141]. These enzymes cause DNA modifications by adding a methyl group at the 5′ position of cytosine bases of both nuclear and mitochondrial genomes, resulting in transcriptional regulation [139,142].
Actually, mtDNA methylation levels were related not only to gene expression regulation, but also to mtDNA copy number. Several diseases have been studied from a mitoepigenetic point of view and the evidences suggest that abnormal mtDNA methylation is associated to pathological conditions, particularly to neurodegeneration and aging [143]. Increased DNMTs activity in mitochondria was related to adaption to oxidative stress, suggesting that mtDNA methylation is an apoptotic-related mechanism. In this context, higher levels of mtDNA methylation were found in neuronal mitochondria from patients with amyotrophic lateral sclerosis, indicating that motor neurons apoptosis is driven by DNMT upregulation [139].
Non-coding RNAs are a broad class of regulatory RNAs whose function is not to serve as templates for protein production. Most ncRNAs do not have their roles fully understood, but they were described acting at transcriptional, posttranscriptional, and posttranslational levels, meaning that they can interact with DNA, other classes of RNAs, and proteins. They are classified into two major types according to their length: Long ncRNAs (≥200 nt) and small ncRNAs (<200 nt), which include microRNAs (miRNAs; 17–23 nt), short interfering RNAs (siRNAs; 20–30 nt), and piwi-interacting RNAs (piRNAs; 27–30 nt) [144,145].
Our knowledge about ncRNAs and mitochondria is still very poor, but the evidence suggests that they may have major roles in retrograde and anterograde signals in cellular environment. It is important to note that there are two different contexts inside mitochondria regarding ncRNAs: There are ncRNAs produced by the nuclear genome, which can then be found inside the organelle, and those that are encoded by the mitochondrial genome, which can be found inside and outside the organelle. In general, the first scenario is related to anterograde signals and the latter is associated to the retrograde ones [143,146,147].
So far, there are some long ncRNAs and miRNAs that were described in association with mitochondria in both contexts. Considering the first context, the nuclei-encoded long ncRNA MEG3 was found in mitochondria enhancing apoptosis by reducing the expression of Bcl-2 and ProC9 proteins and promoting the release of Cyt-c to the cytoplasm [148]. Additionally, 15 nuclear-encoded miRNAs were identified in mitochondria of mice, and the functional enrichment suggested their role in cell death (including apoptosis) and cell division [149].
Regarding the second context, studies describing the mtDNA encoding ncRNAs are very sparse. To date, the most studied ncRNAs in this context are ASncmtRNA-1 and ASncmtRNA-2, two types of antisense long ncRNAs derived from mtDNA. They were found inducing apoptosis in several human and mouse tumor cell lines and involved in aging and replicative senescence in normal human cells [150,151]. In 2011, the mitochondrial transcriptome allowed the identification of 31 mitochondrial-encoded small ncRNAs, which presented a large and dynamic range of expression within different cell types, but that still have their biological roles unknown [152].
Overall, very little attention has been devoted to mitochondrial epigenetics, but it is very clear that mitochondria play a critical role in nearly all aspects of apoptosis, ranging from pro-apoptotic protein release, to morphological traits, epigenetic mechanisms, and gene expression regulation, demonstrating the need for a careful analysis of its many layers of complexity.
miRNAs in Apoptosis
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression and many cell processes, including apoptosis. Indeed, it appears that miRNAs have a role in apoptotic pathways mainly in two forms: (i) Acting as anti-apoptotic molecules by targeting pro-apoptotic messenger RNAs (mRNAs) or positive regulators of pro-apoptotic mRNAs, (ii) as pro-apoptotic miRNAs by targeting anti-apoptotic mRNAs or their positive regulators [153]. These roles may be performed upon both the intrinsic and extrinsic machinery of the apoptosis pathway. In fact, many studies have indicated that miRNAs may directly or indirectly influence cancer treatment by regulation of apoptotic cell death through intrinsic or extrinsic pathways.
For instance, miR-491 targets BCL-XL, decreasing cell viability by inducing apoptosis and generating tumor suppression in DLD1 colon cancer cells [128]. miR-133a targets both BCL-XL and MCL-1 and, when upregulated, it has been shown to induce apoptosis and suppress tumorigenesis in osteosarcoma cell lines [154]. Similarly, BCL-XL and epidermal growth factor receptor (EGFR), both tumor suppressors, are also targeted by has-miR-608 [155]. Furthermore, miR-365 acts directly by targeting adaptor protein Src Homology 2 Domain Containing 1 (SHC1) and BAX, promoting tumor cell resistance to gemcitabine and consequent cell proliferation [156].
Moreover, many miRNAs have been reported to influence Bcl-2 expression in the intrinsic pathway. miR-15 and miR-16 negatively regulate Bcl-2; in fact, transfection of both miRNAs in cells lines that express high levels of Bcl-2 expression reduced these levels and resulted in the activation of intrinsic apoptosis pathway [157]. Pro-apoptotic activity of miRNA in response to 5-fluorouracil has been reported in gastric cancer cell lines with down-regulation of the miR-204, which results in ectopic expression of Bcl-2; on the other hand, ectopic expression of miR-204 leads to down-regulation of Bcl-2 and determines colony formation and migration [158].
Similarly, p21-activated kinase 2 (PAK2) and Cyclin D3 (CCND3) were identified as direct targets of miR-4779, a miRNA that inhibits cancer cell growth by inducing apoptosis and cell cycle arrest. Moreover, miR-4779 was downregulated in 9 out of 10 colon cancer tissues, while the expression of PAK2 and CCND3 was significantly higher [159]. miR-378 suppresses intrinsic apoptosis initiation directly through targeting the initiator CASP9; miR-378/378* knockout mice cannot maintain normal muscle weight and display impaired autophagy, accumulation of abnormal mitochondria, and excessive apoptosis in skeletal muscle [160]. The proapoptotic protein BIM, involved in controlling the mitochondrial apoptotic machinery, is regulated by miR-20, miR-92, and miR-302. Therefore, these miRNAs are essential for maintenance of cell survival in stem cells that are primed not only for differentiation but also for cell death [161].
The examples above were mainly focused on intrinsic pathway. However, extrinsic pathway is also regulated by several miRNAs. For instance, overexpression of miR-221 and miR-222 leads to cell sensitivity to TRAIL in resistant tumor cells and induces apoptotic cell death [162]. It has been observed that such miRNAs negatively modulate the expression of proto-oncogenes Kit and p27kipl and reduce resistance to tamoxifen by inducing apoptosis in breast cancer cell lines [163]. In pancreatic cancer resistant to gemcitabine-induced apoptosis, downregulation of FasL and upregulation of its direct regulator miR-21 have been observed [164]. The same has been reported for another FasL regulator, mir-590, in acute myeloid leukemia (AML), promoting cell survival [165].
In osteosarcoma cells, miR-20a has been shown to inhibit Fas expression, promoting an increase of tumor cell survival and enhancing its metastatic capacity [166]. Upregulation of miR-25 also seems to protect tumor cells against TRAIL-induced apoptosis in cholangiocarcinoma [167,168]. Several other miRNAs target proteins in the TRAIL pathway. For instance, it has been predicted that miR-182 and miR-96 target FADD and CASP3, while miR-145 and miR-216 target the receptors of TRAIL, DR4, and DR5 [23]. As for other extrinsic mechanisms, miR-K10a is an oncomiR that increases cell survival and downregulates the expression of apoptotic tumor necrosis factor receptor superfamily member 12A (TWEAK) receptor [169]. In addition, miR-128a targets FADD and it has been observed that transfection of mimic miR-128a has determined Fas resistance by downregulating FADD, while miR-128a antagomir has induced Fas-mediated apoptosis by active FADD expression [170].
It is now well-established that cell death metabolic regulation is a complex and sophisticated mechanism with multiple checkpoints to regulate cell fate in response to multiple stimulus and micro-environment, which can lead to apoptosis, homeostasis, or cell immortality. In this context, these studies highlight the importance of miRNA regulation of different genes involved in metabolic stress and the whole process of apoptosis, as well as miRNA ability to reset the threshold of cell death and attempt to regenerate homeostasis. On the other hand, depending on the target genes, miRNAs can act as anti-apoptotic and generate best conditions to epithelial mesenchymal transition, cell invasion capacity, and subversion of cell cycle arrest. In summary, miRNAs represent a great research field regarding novel therapeutic strategies to management and prevention of various diseases, such as cancer, based on their role in apoptotic pathways.
11. Conclusions
Apoptosis is a regulated cell death mechanism of great importance both in the normal development of the body and in the removal of defective cells. Deregulation of this process can lead to the development of different diseases and it can also influence the treatment of some of these diseases. Therefore, it is necessary to understand the mechanisms by which apoptosis occurs and interacts with other biological systems. By doing so, it is possible to discover biomarkers that may affect the clinical progression of many diseases. To assist in this scenario, here we discussed the apoptotic mechanisms and the major cellular and genetic players involved in them.
As a future perspective, it is important to acknowledge the potential interference of other epigenetic agents, besides miRNA and DNA methylation, that have not yet been described in the context of apoptosis and mitochondrial functions. In addition, it would be interesting to investigate variants in genes involved in apoptotic pathways through a population genetics point of view, given that they may have a differential distribution across worldwide populations and that this could be relevant in the development of various diseases. In summary, in the era of precision medicine, the interactive nature of the many molecules involved in apoptosis should be regarded as a new field for disease management.
Funding
We thank Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Amparo e Desenvolvimento da Pesquisa (FADESP) and Pró-Reitoria de Pesquisa (PROPESP) of Universidade Federal do Pará (UFPA) for the received grants. This work is part of Rede de Pesquisa em Genômica Populacional Humana (Biocomputacional—Protocol no. 3381/2013/CAPES). We highlight that: G.C.C. is supported by CNPq/Brazil (141810/2015-7); A.P.S. is supported by CAPES/Brazil (88882.160155/2017-01); G.F.C. is supported by CAPES/Brazil (88887.162665/2018-00); M.N.S.-d.-S. is supported by CNPq/Brazil (141812/2017-6); Â.R.-d.-S. is supported by CNPq/Produtividade. The funders had no role in the study design or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zhivotovsky B., Orrenius S. Cell death mechanisms: Cross-talk and role in disease. Exp. Cell Res. 2010;316:1374–1383. doi: 10.1016/j.yexcr.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 2.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang D., Kang R., Berghe T.V., Vandenabeele P., Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien M.A., Kirby R. Apoptosis: A review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J. Vet. Emerg. Crit. Care. 2008;18:572–585. doi: 10.1111/j.1476-4431.2008.00363.x. [DOI] [Google Scholar]
- 5.Pfeffer C.M., Singh A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018;19:448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lomonosova E., Chinnadurai G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene. 2008;27:S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M.X., Dewson G. Mitochondria and apoptosis: Emerging concepts. F1000Prime Rep. 2015;7:42. doi: 10.12703/P7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tait S.W.G., Green D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 9.Galluzzi L., Kepp O., Trojel-Hansen C., Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012;111:1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- 10.Cheng T.C., Hong C., Akey I.V., Yuan S., Akey C.W. A near atomic structure of the active human apoptosome. eLife. 2016;5:e17755. doi: 10.7554/eLife.17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan S., Akey C.W. Apoptosome structure, assembly, and procaspase activation. Structure. 2013;21:501–515. doi: 10.1016/j.str.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gortat A., Sancho M., Mondragón L., Messeguer À., Pérez-Payá E., Orzáez M. Apaf1 inhibition promotes cell recovery from apoptosis. Protein Cell. 2015;6:833–843. doi: 10.1007/s13238-015-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bratton S.B., Salvesen G.S. Regulation of the Apaf-1-caspase-9 apoptosome. J. Cell Sci. 2010;123:3209–3214. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y. Caspase activation: Revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Yuan S., Yu X., Asara J.M., Heuser J.E., Ludtke S.J., Akey C.W. The holo-apoptosome: Activation of procaspase-9 and interactions with caspase-3. Structure. 2011;19:1084–1096. doi: 10.1016/j.str.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mace P.D., Riedl S.J. Molecular cell death platforms and assemblies. Curr. Opin. Cell Biol. 2010;22:828–836. doi: 10.1016/j.ceb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinel A., Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- 19.Sladky V., Schuler F., Fava L.L., Villunger A. The resurrection of the PIDDosome - emerging roles in the DNA-damage response and centrosome surveillance. J. Cell Sci. 2017;130:3779–3787. doi: 10.1242/jcs.203448. [DOI] [PubMed] [Google Scholar]
- 20.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., Dawson T.M., Dawson V.L., El-Deiry W.S., Fulda S., et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashkenazi A., Dixit V.M. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 22.Elrod H.A., Sun S.-Y. Modulation of death receptors by cancer therapeutic agents. Cancer Biol. Ther. 2008;7:163–173. doi: 10.4161/cbt.7.2.5335. [DOI] [PubMed] [Google Scholar]
- 23.Haupt S., Berger M., Goldberg Z., Haupt Y. Apoptosis—The p53 network. J. Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 24.Amanullah A., Liebermann D.A., Hoffman B. Deregulated c-Myc prematurely recruits both Type I and II CD95/Fas apoptotic pathways associated with terminal myeloid differentiation. Oncogene. 2002;21:1600–1610. doi: 10.1038/sj.onc.1205231. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Engels I.H., Knee D.A., Nasoff M., Deveraux Q.L., Quon K.C. Synthetic lethal targeting of MYC by activation of the DR5 death receptor pathway. Cancer Cell. 2004;5:501–512. doi: 10.1016/S1535-6108(04)00113-8. [DOI] [PubMed] [Google Scholar]
- 26.Mahmood Z., Shukla Y. Death receptors: Targets for cancer therapy. Exp. Cell Res. 2010;316:887–899. doi: 10.1016/j.yexcr.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Lee E.-W., Seo J., Jeong M., Lee S., Song J. The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep. 2012;45:496–508. doi: 10.5483/BMBRep.2012.45.9.186. [DOI] [PubMed] [Google Scholar]
- 28.Kretz A.-L., Trauzold A., Hillenbrand A., Knippschild U., Henne-Bruns D., von Karstedt S., Lemke J. TRAILblazing Strategies for Cancer Treatment. Cancers. 2019;11:456. doi: 10.3390/cancers11040456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuchiya Y., Nakabayashi O., Nakano H. FLIP the Switch: Regulation of Apoptosis and Necroptosis by cFLIP. Int. J. Mol. Sci. 2015;16:30321–30341. doi: 10.3390/ijms161226232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenev T., Bianchi K., Darding M., Broemer M., Langlais C., Wallberg F., Zachariou A., Lopez J., MacFarlane M., Cain K., et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Feoktistova M., Geserick P., Panayotova-Dimitrova D., Leverkus M. Pick your poison: The Ripoptosome, a cell death platform regulating apoptosis and necroptosis. Cell Cycle. 2012;11:460–467. doi: 10.4161/cc.11.3.19060. [DOI] [PubMed] [Google Scholar]
- 32.Henry C.M., Martin S.J. Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory “FADDosome” Complex upon TRAIL Stimulation. Mol. Cell. 2017;65:715–729.e5. doi: 10.1016/j.molcel.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Mohr A., Deedigan L., Jencz S., Mehrabadi Y., Houlden L., Albarenque S.-M., Zwacka R.M. Caspase-10: A molecular switch from cell-autonomous apoptosis to communal cell death in response to chemotherapeutic drug treatment. Cell Death Differ. 2018;25:340–352. doi: 10.1038/cdd.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouasni S., Tourneur L. FADD at the Crossroads between Cancer and Inflammation. Trends Immunol. 2018;39:1036–1053. doi: 10.1016/j.it.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Wajant H. Death receptors. Essays Biochem. 2003;39:53–71. doi: 10.1042/bse0390053. [DOI] [PubMed] [Google Scholar]
- 36.Dan H.C., Cooper M.J., Cogswell P.C., Duncan J.A., Ting J.P.-Y., Baldwin A.S. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X., Tang N., Hadden T.J., Rishi A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Rytömaa M., Lehmann K., Downward J. Matrix detachment induces caspase-dependent cytochrome c release from mitochondria: Inhibition by PKB/Akt but not Raf signalling. Oncogene. 2000;19:4461–4468. doi: 10.1038/sj.onc.1203805. [DOI] [PubMed] [Google Scholar]
- 39.Datta S.R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 40.Modur V., Nagarajan R., Evers B.M., Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J. Biol. Chem. 2002;277:47928–47937. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 41.Stahl M., Dijkers P.F., Kops G.J.P.L., Lens S.M.A., Coffer P.J., Burgering B.M.T., Medema R.H. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 42.Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 43.Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 44.Shalini S., Dorstyn L., Dawar S., Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enari M., Sakahira H., Yokoyama H., Okawa K., Iwamatsu A., Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 46.Parrish A.B., Freel C.D., Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013;5:a008672. doi: 10.1101/cshperspect.a008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng T.S. Learning from Deficiency: Gene Targeting of Caspases. Landes Bioscience; Austin, TX, USA: 2013. [Google Scholar]
- 48.Kuida K., Zheng T.S., Na S., Kuan C., Yang D., Karasuyama H., Rakic P., Flavell R.A. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 49.Varfolomeev E.E., Schuchmann M., Luria V., Chiannilkulchai N., Beckmann J.S., Mett I.L., Rebrikov D., Brodianski V.M., Kemper O.C., Kollet O., et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/S1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 50.Kuida K., Haydar T.F., Kuan C.Y., Gu Y., Taya C., Karasuyama H., Su M.S., Rakic P., Flavell R.A. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/S0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 51.Julien O., Wells J.A. Caspases and their substrates. Cell Death Differ. 2017;24:1380–1389. doi: 10.1038/cdd.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Julien O., Zhuang M., Wiita A.P., O’Donoghue A.J., Knudsen G.M., Craik C.S., Wells J.A. Quantitative MS-based enzymology of caspases reveals distinct protein substrate specificities, hierarchies, and cellular roles. Proc. Natl. Acad. Sci. USA. 2016;113:E2001–E2010. doi: 10.1073/pnas.1524900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tucker M.B., MacKenzie S.H., Maciag J.J., Dirscherl Ackerman H., Swartz P., Yoder J.A., Hamilton P.T., Clay Clark A. Phage display and structural studies reveal plasticity in substrate specificity of caspase-3a from zebrafish. Protein Sci. 2016;25:2076–2088. doi: 10.1002/pro.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wachmann K., Pop C., van Raam B.J., Drag M., Mace P.D., Snipas S.J., Zmasek C., Schwarzenbacher R., Salvesen G.S., Riedl S.J. Activation and Specificity of human Caspase-10. Biochemistry. 2010;49:8307–8315. doi: 10.1021/bi100968m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagata S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018;36:489–517. doi: 10.1146/annurev-immunol-042617-053010. [DOI] [PubMed] [Google Scholar]
- 56.Orth J.D., Loewer A., Lahav G., Mitchison T.J. Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Mol. Biol. Cell. 2012;23:567–576. doi: 10.1091/mbc.e11-09-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larsen B.D., Sørensen C.S. The caspase-activated DNase: Apoptosis and beyond. FEBS J. 2017;284:1160–1170. doi: 10.1111/febs.13970. [DOI] [PubMed] [Google Scholar]
- 58.Kothakota S., Azuma T., Reinhard C., Klippel A., Tang J., Chu K., McGarry T.J., Kirschner M.W., Koths K., Kwiatkowski D.J., et al. Caspase-3-generated fragment of gelsolin: Effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 59.Cecconi F., Gruss P. Human Genome and Diseases: Apaf1 in developmental apoptosis and cancer: How many ways to die? CMLS Cell. Mol. Life Sci. 2001;58:1688–1697. doi: 10.1007/PL00000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karimzadeh S., Hosseinkhani S., Fathi A., Ataei F., Baharvand H. Insufficient Apaf-1 expression in early stages of neural differentiation of human embryonic stem cells might protect them from apoptosis. Eur. J. Cell Biol. 2018;97:126–135. doi: 10.1016/j.ejcb.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Spellicy C.J., Norris J., Bend R., Bupp C., Mester P., Reynolds T., Dean J., Peng Y., Alexov E., Schwartz C.E., et al. Key apoptotic genes APAF1 and CASP9 implicated in recurrent folate-resistant neural tube defects. Eur. J. Hum. Genet. 2018;26:420–427. doi: 10.1038/s41431-017-0025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardwick J.M., Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013;5:a008722. doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chao D.T., Korsmeyer S.J. BCL-2 family: Regulators of cell death. Annu. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 64.Ke N., Godzik A., Reed J.C. Bcl-B, a novel Bcl-2 family member that differentially binds and regulates Bax and Bak. J. Biol. Chem. 2001;276:12481–12484. doi: 10.1074/jbc.C000871200. [DOI] [PubMed] [Google Scholar]
- 65.Siddiqui W.A., Ahad A., Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 66.Lizcano J.M., Morrice N., Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem. J. 2000;349:547–557. doi: 10.1042/bj3490547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harada H., Becknell B., Wilm M., Mann M., Huang L.J., Taylor S.S., Scott J.D., Korsmeyer S.J. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell. 1999;3:413–422. doi: 10.1016/S1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- 68.Martinou J.-C., Youle R.J. Mitochondria in Apoptosis: Bcl-2 family Members and Mitochondrial Dynamics. Dev. Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bergmann A. Survival signaling goes BAD. Dev. Cell. 2002;3:607–608. doi: 10.1016/S1534-5807(02)00328-3. [DOI] [PubMed] [Google Scholar]
- 70.Wang H.G., Pathan N., Ethell I.M., Krajewski S., Yamaguchi Y., Shibasaki F., McKeon F., Bobo T., Franke T.F., Reed J.C. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 71.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Green D.R. Means to an End: Apoptosis and Other Cell Death Mechanisms. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2011. [Google Scholar]
- 73.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C.M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985;229:1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- 75.Tzifi F., Economopoulou C., Gourgiotis D., Ardavanis A., Papageorgiou S., Scorilas A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2012;2012:524308. doi: 10.1155/2012/524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell K.J., Tait S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8:180002. doi: 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blombery P., Anderson M.A., Gong J., Thijssen R., Birkinshaw R.W., Thompson E.R., Teh C.E., Nguyen T., Xu Z., Flensburg C., et al. Acquisition of the Recurrent Gly101Val Mutation in BCL2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia. Cancer Discov. 2019;9:342–353. doi: 10.1158/2159-8290.CD-18-1119. [DOI] [PubMed] [Google Scholar]
- 78.Ma S., Lee T.K., Zheng B.-J., Chan K.W., Guan X.-Y. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 79.Zaanan A., Okamoto K., Kawakami H., Khazaie K., Huang S., Sinicrope F.A. The Mutant KRAS Gene Up-regulates BCL-XL Protein via STAT3 to Confer Apoptosis Resistance That Is Reversed by BIM Protein Induction and BCL-XL Antagonism. J. Biol. Chem. 2015;290:23838–23849. doi: 10.1074/jbc.M115.657833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cox A.D., Der C.J. The dark side of Ras: Regulation of apoptosis. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- 81.Li Y., Zhou M., Hu Q., Bai X., Huang W., Scheres S.H.W., Shi Y. Mechanistic insights into caspase-9 activation by the structure of the apoptosome holoenzyme. Proc. Natl. Acad. Sci. USA. 2017;114:1542–1547. doi: 10.1073/pnas.1620626114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu C.-C., Lee S., Malladi S., Chen M.-D., Mastrandrea N.J., Zhang Z., Bratton S.B. The Apaf-1 apoptosome induces formation of caspase-9 homo- and heterodimers with distinct activities. Nature Commun. 2016;7:13565. doi: 10.1038/ncomms13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shakeri R., Kheirollahi A., Davoodi J. Apaf-1: Regulation and function in cell death. Biochimie. 2017;135:111–125. doi: 10.1016/j.biochi.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Guo R., Lin B., Pan J.F., Liong E.C., Xu A.M., Youdim M., Fung M.L., So K.F., Tipoe G.L. Inhibition of caspase-9 aggravates acute liver injury through suppression of cytoprotective autophagy. Sci. Rep. 2016;6:32447. doi: 10.1038/srep32447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sommer M.E.L., Dalia R.A., Nogueira A.V.B., Cirelli J.A., Vinolo M.A.R., Fachi J.L., Oliveira C.A., Andrade T.A.M., Mendonça F.A.S., Santamaria M., et al. Immune response mediated by Th1 / IL-17 / caspase-9 promotes evolution of periodontal disease. Arch. Oral Biol. 2019;97:77–84. doi: 10.1016/j.archoralbio.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 86.Ercan S., Arinc S., Yilmaz S.G., Altunok C., Yaman F., Isbir T. Investigation of Caspase 9 Gene Polymorphism in Patients With Non-small Cell Lung Cancer. Anticancer Res. 2019;39:2437–2441. doi: 10.21873/anticanres.13361. [DOI] [PubMed] [Google Scholar]
- 87.Bellail A.C., Tse M.C.L., Song J.H., Phuphanich S., Olson J.J., Sun S.Y., Hao C. DR5-mediated DISC controls caspase-8 cleavage and initiation of apoptosis in human glioblastomas. J. Cell. Mol. Med. 2010;14:1303–1317. doi: 10.1111/j.1582-4934.2009.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elrod H.A., Fan S., Muller S., Chen G.Z., Pan L., Tighiouart M., Shin D.M., Khuri F.R., Sun S.-Y. Analysis of death receptor 5 and caspase-8 expression in primary and metastatic head and neck squamous cell carcinoma and their prognostic impact. PLoS ONE. 2010;5:e12178. doi: 10.1371/journal.pone.0012178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Camp N.J., Parry M., Knight S., Abo R., Elliott G., Rigas S.H., Balasubramanian S.P., Reed M.W.R., McBurney H., Latif A., et al. Fine-mapping CASP8 risk variants in breast cancer. Cancer Epidemiol. Biomark. Prev. 2012;21:176–181. doi: 10.1158/1055-9965.EPI-11-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takashina T., Nakayama M. Modifications enhance the apoptosis-inducing activity of FADD. Mol. Cancer Ther. 2007;6:1793–1803. doi: 10.1158/1535-7163.MCT-06-0522. [DOI] [PubMed] [Google Scholar]
- 91.Svensson T., Svensson A.K., Kitlinski M., Almgren P., Engström G., Nilsson J., Orho-Melander M., Nilsson P.M., Melander O. Plasma Concentration of Caspase-8 Is Associated With Short Sleep Duration and the Risk of Incident Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2018;103:1592–1600. doi: 10.1210/jc.2017-02374. [DOI] [PubMed] [Google Scholar]
- 92.Lehle A.S., Farin H.F., Marquardt B., Michels B.E., Magg T., Li Y., Liu Y., Ghalandary M., Lammens K., Hollizeck S., et al. Intestinal Inflammation and Dysregulated Immunity in Patients With Inherited Caspase-8 Deficiency. Gastroenterology. 2019;156:275–278. doi: 10.1053/j.gastro.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 93.Jäger R., Zwacka R.M. The enigmatic roles of caspases in tumor development. Cancers. 2010;2:1952–1979. doi: 10.3390/cancers2041952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chinnaiyan A.M., O’Rourke K., Tewari M., Dixit V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 95.Kaufmann T., Strasser A., Jost P.J. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu Q., Fu T.-M., Cruz A.C., Sengupta P., Thomas S.K., Wang S., Siegel R.M., Wu H., Chou J.J. Structural Basis and Functional Role of Intramembrane Trimerization of the Fas/CD95 Death Receptor. Mol. Cell. 2016;61:602–613. doi: 10.1016/j.molcel.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tourneur L., Chiocchia G. FADD: A regulator of life and death. Trends Immunol. 2010;31:260–269. doi: 10.1016/j.it.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 98.Marín-Rubio J.L., de Arriba M.C., Cobos-Fernández M.A., González-Sánchez L., Ors I., Sastre I., Fernández-Piqueras J., Villa-Morales M. Deregulated FADD expression and phosphorylation in T-cell lymphoblastic lymphoma. Oncotarget. 2016;7:61485–61499. doi: 10.18632/oncotarget.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramos-Miguel A., García-Sevilla J.A., Barr A.M., Bayer T.A., Falkai P., Leurgans S.E., Schneider J.A., Bennett D.A., Honer W.G., García-Fuster M.J. Decreased cortical FADD protein is associated with clinical dementia and cognitive decline in an elderly community sample. Mol. Neurodegener. 2017;12:26. doi: 10.1186/s13024-017-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edathara P.M., Gorre M., Kagita S., Vuree S., Cingeetham A., Nanchari S.R., Meka P.B., Annamaneni S., Digumarthi R.R., Satti V. Association of promoter polymorphisms of Fas-FasL genes with development of Chronic Myeloid Leukemia. Tumor Biol. 2016;37:5475–5484. doi: 10.1007/s13277-015-4295-0. [DOI] [PubMed] [Google Scholar]
- 101.Huang D., Xiao J., Deng X., Ma K., Liang H., Shi D., Wu F., Shao Z. Association between Fas/FasL gene polymorphism and musculoskeletal degenerative diseases: A meta-analysis. BMC Musculoskel. Disord. 2018;19:137. doi: 10.1186/s12891-018-2057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vallinoto A.C.R., Santana B.B., dos Santos E.L., do Espírito Santo R.R., Hermes R.B., Sousa R.C.M., Cayres-Vallinoto I., Machado L.F.A., Ishak M.O.G., Ishak R. FAS-670A/G single nucleotide polymorphism may be associated with human T lymphotropic virus-1 infection and clinical evolution to TSP/HAM. Virus Res. 2012;163:178–182. doi: 10.1016/j.virusres.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 103.Vallinoto A.C.R., Santana B.B., Queiroz M.A.F., da Silva A.N.M.R., Cayres-Vallinoto I.M.V., da Costa C.A., de Sousa M.S., Ishak R. Family Aggregation of HTLV-1 Infection Associated with FAS-670A/G Polymorphism: A Case Report. Front. Microbiol. 2018;8:2685. doi: 10.3389/fmicb.2017.02685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Menezes S.M., Leal F.E., Dierckx T., Khouri R., Decanine D., Silva-Santos G., Schnitman S.V., Kruschewsky R., López G., Alvarez C., et al. A Fashi Lymphoproliferative Phenotype Reveals Non-Apoptotic Fas Signaling in HTLV-1-Associated Neuroinflammation. Front. Immunol. 2017;8:97. doi: 10.3389/fimmu.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nguyen T.-A.T., Grimm S.A., Bushel P.R., Li J., Li Y., Bennett B.D., Lavender C.A., Ward J.M., Fargo D.C., Anderson C.W., et al. Revealing a human p53 universe. Nucleic Acids Res. 2018;46:8153–8167. doi: 10.1093/nar/gky720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Müller M., Wilder S., Bannasch D., Israeli D., Lehlbach K., Li-Weber M., Friedman S.L., Galle P.R., Stremmel W., Oren M., et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J. Exp. Med. 1998;188:2033–2045. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Willms A., Schittek H., Rahn S., Sosna J., Mert U., Adam D., Trauzold A. Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells. PLoS ONE. 2019;14:e0214847. doi: 10.1371/journal.pone.0214847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu X., Wang K., Yao Y., Zhang K., Zhou F., Zhu L. Triggering p53 activation is essential in ziyuglycoside I-induced human retinoblastoma WERI-Rb-1 cell apoptosis. J. Biochem. Mol. Toxicol. 2018;32 doi: 10.1002/jbt.22001. [DOI] [PubMed] [Google Scholar]
- 109.Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 110.Basu A., Haldar S. The relationship between BcI2, Bax and p53: Consequences for cell cycle progression and cell death. Mol. Hum. Reprod. 1998;4:1099–1109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- 111.Chen B.-J., Wu Y.-L., Tanaka Y., Zhang W. Small Molecules Targeting c-Myc Oncogene: Promising Anti-Cancer Therapeutics. Int. J. Biol. Sci. 2014;10:1084–1096. doi: 10.7150/ijbs.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blackwood E.M., Eisenman R.N. Max: A helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251:1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 113.Lüscher B. Function and regulation of the transcription factors of the Myc/Max/Mad network. Gene. 2001;277:1–14. doi: 10.1016/S0378-1119(01)00697-7. [DOI] [PubMed] [Google Scholar]
- 114.Murphy D.J., Junttila M.R., Pouyet L., Karnezis A., Shchors K., Bui D.A., Brown-Swigart L., Johnson L., Evan G.I. Distinct thresholds govern Myc’s biological output in vivo. Cancer Cell. 2008;14:447–457. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zindy F., Eischen C.M., Randle D.H., Kamijo T., Cleveland J.L., Sherr C.J., Roussel M.F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trudel M., Lanoix J., Barisoni L., Blouin M.-J., Desforges M., L’Italien C., D’Agati V. C-MYC–induced Apoptosis in Polycystic Kidney Disease Is Bcl-2 and p53 Independent. J. Exp. Med. 1997;186:1873–1884. doi: 10.1084/jem.186.11.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boone D.N., Qi Y., Li Z., Hann S.R. Egr1 mediates p53-independent c-Myc-induced apoptosis via a noncanonical ARF-dependent transcriptional mechanism. Proc. Natl. Acad. Sci. USA. 2011;108:632–637. doi: 10.1073/pnas.1008848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muthalagu N., Junttila M.R., Wiese K.E., Wolf E., Morton J., Bauer B., Evan G.I., Eilers M., Murphy D.J. BIM is the primary mediator of MYC-induced apoptosis in multiple solid tissues. Cell Rep. 2014;8:1347–1353. doi: 10.1016/j.celrep.2014.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cartwright I.M., Liu X., Zhou M., Li F., Li C.-Y. Essential roles of Caspase-3 in facilitating Myc-induced genetic instability and carcinogenesis. Elife. 2017;6:e26371. doi: 10.7554/eLife.26371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Knoepfler P.S. Myc goes global: New tricks for an old oncogene. Cancer Res. 2007;67:5061–5063. doi: 10.1158/0008-5472.CAN-07-0426. [DOI] [PubMed] [Google Scholar]
- 121.Winkle M., van den Berg A., Tayari M., Sietzema J., Terpstra M., Kortman G., de Jong D., Visser L., Diepstra A., Kok K., et al. Long noncoding RNAs as a novel component of the Myc transcriptional network. FASEB J. 2015;29:2338–2346. doi: 10.1096/fj.14-263889. [DOI] [PubMed] [Google Scholar]
- 122.Walters J., Pop C., Scott F.L., Drag M., Swartz P., Mattos C., Salvesen G.S., Clark A.C. A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem. J. 2009;424:335–345. doi: 10.1042/BJ20090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu X., He Y., Li F., Huang Q., Kato T.A., Hall R.P., Li C.-Y. Caspase-3 Promotes Genetic Instability and Carcinogenesis. Mol. Cell. 2015;58:284–296. doi: 10.1016/j.molcel.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou M., Liu X., Li Z., Huang Q., Li F., Li C.-Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells: Metastasis of colon cancer cells. Int. J. Cancer. 2018;143:921–930. doi: 10.1002/ijc.31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Know L. Life—The Epic Story of Our Mitochondria: How the Original Probiotic Dictates Your Health, Illness, Ageing, and Even Life Itself. FriesenPress; Victoria, BC, Canada: 2014. [Google Scholar]
- 126.Karbowski M., Lee Y.-J., Gaume B., Jeong S.-Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C.L., Youle R.J. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frezza C., Cipolat S., Martins de Brito O., Micaroni M., Beznoussenko G.V., Rudka T., Bartoli D., Polishuck R.S., Danial N.N., De Strooper B., et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 128.Nakano H., Miyazawa T., Kinoshita K., Yamada Y., Yoshida T. Functional screening identifies a microRNA, miR-491 that induces apoptosis by targeting Bcl-X(L) in colorectal cancer cells. Int. J. Cancer. 2010;127:1072–1080. doi: 10.1002/ijc.25143. [DOI] [PubMed] [Google Scholar]
- 129.Mullen T.D., Obeid L.M. Ceramide and apoptosis: Exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med. Chem. 2012;12:340–363. doi: 10.2174/187152012800228661. [DOI] [PubMed] [Google Scholar]
- 130.Rowland A.A., Voeltz G.K. Endoplasmic reticulum–mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rongvaux A., Jackson R., Harman C.C.D., Li T., West A.P., de Zoete M.R., Wu Y., Yordy B., Lakhani S.A., Kuan C.-Y., et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peña-Blanco A., García-Sáez A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018;285:416–431. doi: 10.1111/febs.14186. [DOI] [PubMed] [Google Scholar]
- 133.Kantari C., Walczak H. Caspase-8 and bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 134.Huang K., Zhang J., O’Neill K.L., Gurumurthy C.B., Quadros R.M., Tu Y., Luo X. Cleavage by Caspase 8 and Mitochondrial Membrane Association Activate the BH3-only Protein Bid during TRAIL-induced Apoptosis. J. Biol. Chem. 2016;291:11843–11851. doi: 10.1074/jbc.M115.711051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Snustad D.P., Simmons M.J. Fundamentos de Genética. Volume 4 Guanabara Koogan; Rio de Janeiro, Brazil: 2008. [Google Scholar]
- 137.Park J.S., Sharma L.K., Li H., Xiang R., Holstein D., Wu J., Lechleiter J., Naylor S.L., Deng J.J., Lu J., et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum. Mol. Genet. 2009;18:1578–1589. doi: 10.1093/hmg/ddp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Picard M., Zhang J., Hancock S., Derbeneva O., Golhar R., Golik P., O’Hearn S., Levy S., Potluri P., Lvova M., et al. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl. Acad. Sci. USA. 2014;111:E4033–E4042. doi: 10.1073/pnas.1414028111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iacobazzi V., Castegna A., Infantino V., Andria G. Mitochondrial DNA methylation as a next-generation biomarker and diagnostic tool. Mol. Genet. Metab. 2013;110:25–34. doi: 10.1016/j.ymgme.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 140.Shock L.S., Thakkar P.V., Peterson E.J., Moran R.G., Taylor S.M. DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proc. Natl. Acad. Sci. USA. 2011;108:3630–3635. doi: 10.1073/pnas.1012311108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chestnut B.A., Chang Q., Price A., Lesuisse C., Wong M., Martin L.J. Epigenetic Regulation of Motor Neuron Cell Death through DNA Methylation. J. Neurosci. 2011;31:16619–16636. doi: 10.1523/JNEUROSCI.1639-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Biswas S., Rao C.M. Epigenetic tools (The Writers, The Readers and The Erasers) and their implications in cancer therapy. Eur. J. Pharmacol. 2018;837:8–24. doi: 10.1016/j.ejphar.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 143.D’Aquila P., Bellizzi D., Passarino G. Mitochondria in health, aging and diseases: The epigenetic perspective. Biogerontology. 2015;16:569–585. doi: 10.1007/s10522-015-9562-3. [DOI] [PubMed] [Google Scholar]
- 144.Maia B.M., Rocha R.M., Calin G.A. Clinical significance of the interaction between non-coding RNAs and the epigenetics machinery: Challenges and opportunities in oncology. Epigenetics. 2014;9:75–80. doi: 10.4161/epi.26488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Han B., Yao W., Oh Y.-T., Tong J.-S., Li S., Deng J., Yue P., Khuri F.R., Sun S.-Y. The novel proteasome inhibitor carfilzomib activates and enhances extrinsic apoptosis involving stabilization of death receptor 5. Oncotarget. 2015;6:17532–17542. doi: 10.18632/oncotarget.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mercer T.R., Neph S., Dinger M.E., Crawford J., Smith M.A., Shearwood A.-M.J., Haugen E., Bracken C.P., Rackham O., Stamatoyannopoulos J.A., et al. The human mitochondrial transcriptome. Cell. 2011;146:645–658. doi: 10.1016/j.cell.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lambertini L., Byun H.-M. Mitochondrial Epigenetics and Environmental Exposure. Curr. Environ. Health Rep. 2016;3:214–224. doi: 10.1007/s40572-016-0103-2. [DOI] [PubMed] [Google Scholar]
- 148.Wang M., Huang T., Luo G., Huang C., Xiao X.-Y., Wang L., Jiang G.-S., Zeng F.-Q. Long non-coding RNA MEG3 induces renal cell carcinoma cells apoptosis by activating the mitochondrial pathway. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015;35:541–545. doi: 10.1007/s11596-015-1467-5. [DOI] [PubMed] [Google Scholar]
- 149.Kren B.T., Wong P.Y.-P., Sarver A., Zhang X., Zeng Y., Steer C.J. MicroRNAs identified in highly purified liver-derived mitochondria may play a role in apoptosis. RNA Biol. 2009;6:65–72. doi: 10.4161/rna.6.1.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Borgna V., Villegas J., Burzio V.A., Belmar S., Araya M., Jeldes E., Lobos-González L., Silva V., Villota C., Oliveira-Cruz L., et al. Mitochondrial ASncmtRNA-1 and ASncmtRNA-2 as potent targets to inhibit tumor growth and metastasis in the RenCa murine renal adenocarcinoma model. Oncotarget. 2017;8:43692–43708. doi: 10.18632/oncotarget.18460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bianchessi V., Badi I., Bertolotti M., Nigro P., D’Alessandra Y., Capogrossi M.C., Zanobini M., Pompilio G., Raucci A., Lauri A. The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in Endothelial Cells. J. Mol. Cell. Cardiol. 2015;81:62–70. doi: 10.1016/j.yjmcc.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 152.Zhao Y., Sun L., Wang R.R., Hu J.-F., Cui J. The effects of mitochondria-associated long noncoding RNAs in cancer mitochondria: New players in an old arena. Crit. Rev. Oncol. Hematol. 2018;131:76–82. doi: 10.1016/j.critrevonc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 153.Lima R.T., Busacca S., Almeida G.M., Gaudino G., Fennell D.A., Vasconcelos M.H. MicroRNA regulation of core apoptosis pathways in cancer. Eur. J. Cancer. 2011;47:163–174. doi: 10.1016/j.ejca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 154.Ji F., Zhang H., Wang Y., Li M., Xu W., Kang Y., Wang Z., Wang Z., Cheng P., Tong D., et al. MicroRNA-133a, downregulated in osteosarcoma, suppresses proliferation and promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone. 2013;56:220–226. doi: 10.1016/j.bone.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y., Schiff D., Park D., Abounader R. MicroRNA-608 and microRNA-34a regulate chordoma malignancy by targeting EGFR, Bcl-xL and MET. PLoS ONE. 2014;9:e91546. doi: 10.1371/journal.pone.0091546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hamada S., Masamune A., Miura S., Satoh K., Shimosegawa T. MiR-365 induces gemcitabine resistance in pancreatic cancer cells by targeting the adaptor protein SHC1 and pro-apoptotic regulator BAX. Cell. Signal. 2014;26:179–185. doi: 10.1016/j.cellsig.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 157.Zhou M., Liu Z., Zhao Y., Ding Y., Liu H., Xi Y., Xiong W., Li G., Lu J., Fodstad O., et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J. Biol. Chem. 2010;285:21496–21507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cimmino A., Calin G.A., Fabbri M., Iorio M.V., Ferracin M., Shimizu M., Wojcik S.E., Aqeilan R.I., Zupo S., Dono M., et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koo K.H., Kwon H. MicroRNA miR-4779 suppresses tumor growth by inducing apoptosis and cell cycle arrest through direct targeting of PAK2 and CCND3. Cell Death Dis. 2018;9:77. doi: 10.1038/s41419-017-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li Y., Jiang J., Liu W., Wang H., Zhao L., Liu S., Li P., Zhang S., Sun C., Wu Y., et al. microRNA-378 promotes autophagy and inhibits apoptosis in skeletal muscle. PNAS. 2018;115:E10849–E10858. doi: 10.1073/pnas.1803377115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pernaute B., Spruce T., Smith K.M., Sánchez-Nieto J.M., Manzanares M., Cobb B., Rodríguez T.A. MicroRNAs control the apoptotic threshold in primed pluripotent stem cells through regulation of BIM. Genes Dev. 2014;28:1873–1878. doi: 10.1101/gad.245621.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miller T.E., Ghoshal K., Ramaswamy B., Roy S., Datta J., Shapiro C.L., Jacob S., Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Garofalo M., Quintavalle C., Di Leva G., Zanca C., Romano G., Taccioli C., Liu C.G., Croce C.M., Condorelli G. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene. 2008;27:3845–3855. doi: 10.1038/onc.2008.6. [DOI] [PubMed] [Google Scholar]
- 164.Wang P., Zhuang L., Zhang J., Fan J., Luo J., Chen H., Wang K., Liu L., Chen Z., Meng Z. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FasL. Mol. Oncol. 2013;7:334–345. doi: 10.1016/j.molonc.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shaffiey F., Cross E., Sathyanarayana P. Mir-590 Is a Novel STAT5 Regulated Oncogenic miRNA and Targets FasL In Acute Myeloid Leukemia. Blood. 2013;122:3811. [Google Scholar]
- 166.Huang G., Nishimoto K., Zhou Z., Hughes D., Kleinerman E.S. miR-20a encoded by the miR-17-92 cluster increases the metastatic potential of osteosarcoma cells by regulating Fas expression. Cancer Res. 2012;72:908–916. doi: 10.1158/0008-5472.CAN-11-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ovcharenko D., Kelnar K., Johnson C., Leng N., Brown D. Genome-scale microRNA and small interfering RNA screens identify small RNA modulators of TRAIL-induced apoptosis pathway. Cancer Res. 2007;67:10782–10788. doi: 10.1158/0008-5472.CAN-07-1484. [DOI] [PubMed] [Google Scholar]
- 168.Slattery M.L., Mullany L.E., Sakoda L.C., Wolff R.K., Samowitz W.S., Herrick J.S. Dysregulated genes and miRNAs in the apoptosis pathway in colorectal cancer patients. Apoptosis. 2018;23:237–250. doi: 10.1007/s10495-018-1451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abend J.R., Uldrick T., Ziegelbauer J.M. Regulation of tumor necrosis factor-like weak inducer of apoptosis receptor protein (TWEAKR) expression by Kaposi’s sarcoma-associated herpesvirus microRNA prevents TWEAK-induced apoptosis and inflammatory cytokine expression. J. Virol. 2010;84:12139–12151. doi: 10.1128/JVI.00884-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yamada N., Noguchi S., Kumazaki M., Shinohara H., Miki K., Naoe T., Akao Y. Epigenetic regulation of microRNA-128a expression contributes to the apoptosis-resistance of human T-cell leukaemia jurkat cells by modulating expression of fas-associated protein with death domain (FADD) Biochim. Biophys. Acta. 2014;1843:590–602. doi: 10.1016/j.bbamcr.2013.11.022. [DOI] [PubMed] [Google Scholar]