Abstract
Objective
The aim of the analysis is to assess the organisational and economic consequences of adopting an early discharge strategy for the treatment of acute bacterial skin and skin structure infection (ABSSSI) and osteomyelitis within infectious disease departments.
Setting
Infectious disease departments in Greece, Italy and Spain.
Participants
No patients were involved in the analysis performed.
Interventions
An analytic framework was developed to consider two alternative scenarios: standard hospitalisation care or an early discharge strategy for patients hospitalised due to ABSSSI and osteomyelitis, from the perspective of the National Health Services of Greece, Italy and Spain. The variables considered were: the number of annual hospitalisations eligible for early discharge, the antibiotic treatments considered (ie, oral antibiotics and intravenous long-acting antibiotics), diagnosis-related group (DRG) reimbursements, number of days of hospitalisation, incidence and costs of hospital-acquired infections, additional follow-up visits and intravenous administrations. Data were based on published literature and expert opinions.
Primary and secondary outcome measures
Number of days of hospitalisation avoided and direct medical costs avoided.
Results
The total number of days of hospitalisation avoided on a yearly basis would be between 2216 and 5595 in Greece (−8/−21 hospital beds), between 15 848 and 38 444 in Italy (−57/−135 hospital beds) and between 7529 and 23 520 in Spain (−27/−85 hospital beds). From an economic perspective, the impact of the early discharge scenario is a reduction between €45 036 and €149 552 in Greece, a reduction between €182 132 and €437 990 in Italy and a reduction between €292 284 and €884 035 in Spain.
Conclusions
The early discharge strategy presented would have a positive organisational impact on National Health Services, leading to potential savings in beds, and to a reduction of hospital-acquired infections and costs.
Keywords: Hospital acquired infection, Hospital beds, Infectious Diseases, Economic evaluation, Organisation
Strengths and limitations of this study.
The perspective adopted does not consider cost offsets due to hospital bed reductions, and considers only the improvement in efficiency, although it has been acknowledged that reducing hospital beds is per se a budget-saving initiative.
The analysis is focused only on infectious-related pathologies that could best benefit from an early discharge strategy, as suggested by experts, and excludes some infections that could also benefit from early discharge.
The number of days of hospitalisation reduction that could be saved due to the early discharge scenario is based on experts’ opinions, although in line with evidence from literature, and might be considered a limitation of the analysis.
Background
The post-2008 economic crisis, which precipitated a sovereign debt crisis in Europe and squeezed public budgets, added particular urgency to longer term concerns of cost containment. The need to increase efficiency has been reinforced by public budget expenditure reductions and lower rates of growth in healthcare spending, and has been acknowledged across many high-income European Union countries. Among these, Southern Mediterranean states such as Greece, Italy and Spain have been among the most affected by European Commission requests to reduce public spending.1
Efficiency may mean either maximising the benefit from a fixed sum of money or minimising the resources required for a defined benefit. This has important social implications when considering tax-funded National Health Services (NHS): healthcare budgets are limited and spending in one area is unavoidably at the expense of investment in another area, so efficiency can be interpreted as ensuring that the benefits obtained exceed the benefits forgone.2
Where European Union countries have been requested by the ‘Troika’ (European Union, European Central Bank and International Monetary Fund) to increase system efficiency and to reduce costs, one consequence has been a large reduction in the number of hospital beds.3 4 Bed management is an important part of operational planning and control to make efficient use of resources.5 Suboptimal bed management can result in overcrowding, severely impacting hospital departments’ efficiency and creating slow-resolving bed crises. Such crises are frequently precipitated by a surge in acute presentations which a hospital bed management system is incapable of anticipating or responding to.
Achieving a balance between acute and elective hospitalisations and between admissions and discharges can help avoid such crises and reduce length of stay (LoS).6 Reducing LoS, either through the implementation of outpatient programmes or by optimising the duration of either medical or pharmacological therapy, may therefore help increase hospitals’ efficiency and create room to respond to demand pressures.7
Moreover, inpatient LoS has been shown to be one of the determinants of an increased incidence of hospital-acquired infections (HAI).8 HAIs are defined as infections occurring during hospitalisation, which were not present or incubating at the time of admission. Infections that occur more than 48 hours after admission are usually HAIs.
HAIs are known to comprise a large proportion of all adverse events in healthcare, and can cause prolonged LoS and increased healthcare resource consumption.9–12 The WHO and the European Centre for Disease Prevention and Control (ECDC) have repeatedly stressed the relevance and global spread of the phenomenon of HAIs and recognise HAIs as a significant danger to public health.13
Outpatient parenteral antibiotic therapy (OPAT) programmes may help reduce HAIs, by enabling patients to receive intravenous antibiotics after hospital discharge, but they require additional resources and are not available to all patients in Europe.14–18 An earlier switch from intravenous to oral therapy or greater use of long-acting antibiotics (ie, one administration per week) are other strategies which may facilitate early discharge to home or to other facilities, saving days of hospitalisation, possibly reducing HAIs and consequently reducing public healthcare spending.19
The present analysis aims to assess the potential of an ‘early discharge’ strategy to increase the efficiency of hospital bed management, reduce the incidence of HAIs and reduce days in hospital related to the treatment of definite bacterial infections requiring hospitalisation in infectious disease departments. Although it is commonly recognised that an ‘early discharge’ strategy is not applicable to all patients hospitalised in infectious disease departments, it is a possible option for certain patient groups and conditions. Based on clinical expert opinion—taking into account length of pharmacological and medical treatment and course of illness itself—this study focuses on the likely consequences of such a strategy for patients admitted to hospital due to acute bacterial skin and skin structure infection (ABSSSI) and osteomyelitis.
The aim of the present investigation is therefore to provide policymakers and healthcare professionals with estimates of the potential organisational and economic consequences of adopting an early discharge strategy in the setting of infectious disease departments. The results reported may encourage the adoption of more efficient patient pathways for the treatment of ABSSSI and osteomyelitis.
Materials and methods
The perspective assumed in the analysis is that of the NHS of Greece, Italy and Spain.
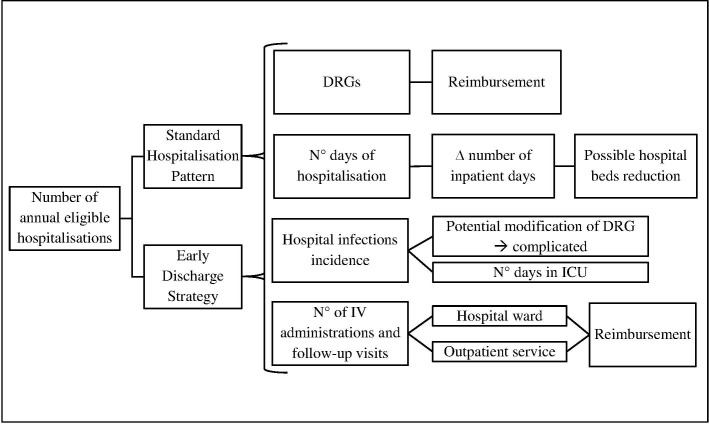
A focus group was held in 2017 to identify the framework of the analysis and the factors correlated with an early discharge strategy for patients admitted to infectious disease wards, involving three directors of infectious disease departments of hospitals located in Greece, Italy and Spain; the director of the pharmacy department of a hospital located in Spain; and three health economists of a university centre for research located in Italy. Following the focus group, a model was implemented to represent the pathway of patients hospitalised due to infection considering two alternative scenarios: a base case scenario in which patients are hospitalised until full recovery, and an alternative (early discharge) scenario in which, after the stabilisation of the infection, patients are discharged to complete their treatment in an outpatient setting. A model schema is reported in figure 1.
Figure 1.
Variables taken into consideration in the analysis. DRG, diagnosis-related group; ICU, intensive care unit; IV, intravenous.
A description of each variable considered in the model is reported below.
Target population: number of annual hospitalisations eligible for early discharge
The target population of the analysis consists of the estimated total annual number of hospitalisations due to infection, in which patients are considered eligible for an early discharge, in each of the national contexts aforementioned. To identify the eligible number of hospitalisations, the total number of annual hospitalisations in each national context due to ABSSSI and osteomyelitis was collected, and then proportion eligible for early discharge was estimated based on data published in the literature, as explained below. The types of infections to be considered in the analysis were selected on the basis of the clinical practice and experience of the three directors of the infectious disease departments involved.
The total number of hospitalisations was estimated starting from the diagnosis-related group (DRG) codes identified by the clinicians involved in the study (for Greece: DRG-KEN Δ12M, Δ12XA, Δ12XB, Δ20M, Δ20X, Δ24X, Δ24M, Δ28MA, Δ28MB, Δ28X for ABSSSI; DRG-KEN M64M, M64X for osteomyelitis; for Italy: DRGs 277 and 278 for ABSSSI; DRG 238 for osteomyelitis; for Spain: DRGs 277 and 278 for ABSSSI; DRGs 238 and 561 for osteomyelitis), and calculating the number of discharges in 2015 for DRGs related to ABSSSI, and osteomyelitis, as published by the Italian Ministry of Health, the Spanish Ministry of Health and ESYNET for Greece (the latter cover only public hospitals, while data relating to Italy and Spain include both public and private hospitals accredited with the NHS).20–22
Starting from these data, the number of hospitalisations eligible for an early discharge strategy was estimated using data published by Eckmann and colleagues,23 who conducted an observational study identifying the percentage of patients infected with ABSSSI eligible for early discharge at 41.1% in Greece, 34.2% in Italy and 38.8% in Spain, based on the analysis of hospital discharge records of 190, 151 and 183 patients, respectively.
Antibiotic treatments
The two possible treatments considered are the standard of care (ie, either oral antibiotics or intravenous short-term multiple dosing) and oral or intravenous long-acting antibiotics. In the early discharge scenario, it is assumed that patients will continue to receive the same antibiotics received in the hospital setting, follow-up visits and, if required, intravenous administrations within outpatient service/clinic. The percentage of patients receiving either short or long-acting antibiotics was estimated for each national context through the expert opinion of the clinicians involved in the analysis, who estimated a proportion of 90% of patients receiving oral antibiotics in Greece, Italy and Spain.
DRG reimbursement and number of days of hospitalisation
As a result of DRG reimbursement, variations or changes in days in hospital will not alter a hospital’s reimbursement unless the threshold of number of days of hospitalisation considered in each DRG is exceeded, in which case a per diem reimbursement was applied to the excess days in the present analysis. However, from an organisational point of view, a decrease in the number of inpatient days creates the opportunity to admit other patients, and over a longer time horizon could permit a reduction in the number of accredited beds at a national level.
The number of days of hospitalisation that might be expected in the early discharge scenario was estimated based on the expert opinion of the three directors of infectious disease departments involved, drawing on their real-world experience of clinical practice. The minimum and maximum LoS considered in the early discharge scenario were: 2–7 days for ABSSSI and 7 days for osteomyelitis. The differential number of inpatient days between the early discharge scenario and the base case scenario was then calculated. When the maximum LoS in the early discharge scenario was higher than the mean LoS of the base case scenario, no difference in days of hospitalisation was assumed. These estimates are conservative when compared with data in the existing literature (available only for ABSSSI among the infections considered), resulting in a mean number of bed-days saved compared with the base case scenario which is less than those estimated in previous literature (Eckmann and colleagues).23
The differential number of inpatient days was then used to calculate the theoretical number of bed-days that could be avoided in each national context. This was also expressed in terms of number of beds, based on the most recent (2015) estimates of bed occupancy rates published by the statistical office of the European Union, of 73.6%, 78.9% and 75.8% in Greece, Italy and Spain, respectively.24 The differential number of inpatient days was divided by the bed occupancy rate multiplied by 365 days, to obtain a number of potential beds that could be made available by implementing this policy in each national context.
Hospital-acquired infections
The incidence of HAIs is directly correlated with the number of days of hospitalisation, and so a reduction of inpatient days will have a positive impact in terms of reduced numbers of HAIs.8 Due to a lack of data concerning the estimated correlation between patients’ LoS and incidence of HAIs in non-intensive care unit (ICU) wards, we used data published by Wolkewitz and colleagues,25 which reported the incidence of HAIs in ICUs to be 6.75 per 1000 days of hospitalisation. To be consistent with data from the ECDC, which reported the incidence of HAIs in Greece, Italy and Spain to be 3.7%, 3.9% and 5.7%, respectively,26 we adjusted the incidence rate identified by Wolkewitz, following clinicians’ opinion, by a coefficient of 0.63 for Greece, 0.49 for Italy and 1.03 for Spain. The hypothetical number of HAIs per each DRG considered was calculated by dividing the number of inpatient days per DRG by 1000 and then multiplying the result by 4.24 for Greece, by 3.31 for Italy and by 6.95 for Spain, assuming that HAIs are equally spread throughout the LoS.
From an organisational and economic point of view, these policy impacts would also result in a decreased number of admissions to ICUs, and in the initial admission DRG being reclassified as a complicated DRG. The cost related to HAIs was estimated on the basis of available literature for each national context,27 28 and inflated to 2017 values using the annual inflation rates in average consumer prices of Greece, Italy and Spain as reported by the International Monetary Fund.29 Specifically, data for the Italian context were derived from Gianino and colleagues.27 Based on expert opinions, we considered for ABSSSI and osteomyelitis the mean cost of sepsis excluding the cost of urinary tract infections, since the use of catheters in patients affected with ABSSSI and osteomyelitis is less frequent.
The estimated cost to manage an HAI in Italy was set at €4726.27 A cost of €6007 was adopted for the Spanish NHS, as reported by Morano Amado and colleagues.28 Due to lack of published data concerning the cost of HAIs in Greece, the cost range observed in the other two national contexts was used (€4726 and €6007).
Number of intravenous administrations and follow-up visits
Patients receiving oral antibiotics will continue their therapy either in hospital or in a domiciliary setting. Patients treated with intravenous long-acting antibiotics receive up to two weekly administrations. The first administration is performed in hospital in both scenarios, then depending on the LoS, patients discharged from hospital might need to receive a second administration from the hospital’s outpatient service. This would have an impact on costs for the NHS, compared with the base case scenario, in which the DRG reimbursement would cover both administrations.
The same approach is considered for follow-up visits, in terms of additional specialist visits, both for patients receiving oral antibiotics and intravenous long-acting antibiotics.
The economic impact related to specialist visits and the administration of intravenous long-acting antibiotics was estimated using the reimbursement tariffs for specialist visits and intravenous administration at a national level in the contexts investigated.
In detail, the cost of a specialist visit in Greece was set at €10.00, which is the flat reimbursement amount by EOPYY (the National Organisation for the Provision of Health Services) for all visits. The cost of outpatients’ administration was also set at €10.00, using a conservative approach, as no specific reimbursement is considered in Greece.
For Italy, the reimbursement of a specialist visit was derived from the Italian tariff nomenclature, being €20.66 (code 89.7), and the reimbursement for an intravenous administration was set at €9.71.30 Due to the lack of a specific code in the Italian tariff nomenclature for intravenous administrations, we considered the same reimbursement of codes 99.23, 99.25 and 99.29.9, as emerged in an analysis of the tariff nomenclature of Lombardy Region, in which code 99.2A is related to ‘injection or infusion of specific drugs (hypodermic, intramuscular, intravenous),’ whose reimbursement is equal to that of the outpatients’ activities associated with the codes reported on (99.23, 99.25, 99.29.9).
The cost of a specialist visit in the Spanish context was considered equal to the tariff of €17.52 of a ‘consulta sucesiva’ (code A.6.1) and the cost of an outpatient’s administration to be equal to €11.43 as the tariff of a ‘consulta de enfermeria’ (code A.3) as reported by a public insurance scheme accredited with the Spanish social security.31
Sensitivity analysis
The uncertainty surrounding variables such as the number of days of hospitalisation for early discharge strategy was dealt with by considering different scenarios based on the maximum and minimum number of days of hospitalisation before early discharge. A univariate sensitivity analysis was performed to test the impact of variations in national bed occupancy rates (±5% of the baseline rate), HAI incidence (increasing and decreasing by 1 the number of HAIs every 1000 days of hospitalisation), the cost of HAIs (±10%) and the cost of specialist visits and drug administration (+50% and −10%).
Patient and public involvement
No patients were involved in the analysis performed.
Results
Target population and clinical activities
The target population and the activities related to the health services performed in the two scenarios are reported in table 1. In detail, the table shows the total number of hospitalisations and the number of hospitalisations eligible for early discharge in each national context, along with the mean LoS, based on the analyses performed, and the avoided inpatient days due to early discharge for each DRG considered for ABSSSI and osteomyelitis.
Table 1.
Mean length of stay and inpatient days per scenario for each national context
| National context | Infection (DRG) | Hospitalisations, n | Base case scenario | Early discharge scenario | ∆ Inpatient days between base case and early discharge scenario (if all LoS were at minimum/ maximum) | |||
| Total | Eligible to early discharge | Mean LoS (days) | Total inpatient days | LoS minimum/ maximum (days) | Total inpatient days (if all LoS were at minimum/ maximum) | |||
| Greece | ABSSSI (DRG-KEN Δ12M) | 11 | 5 | 20.00 | 100 | 2.00/7.00 | 10/35 | −90/−65 |
| ABSSSI (DRG-KEN Δ12XA) | 22 | 9 | 13.00 | 117 | 2.00/7.00 | 18/63 | −99/−54 | |
| ABSSSI (DRG-KEN Δ12XB) | 198 | 81 | 8.00 | 648 | 2.00/7.00 | 162/567 | −486/−81 | |
| ABSSSI (DRG-KEN Δ20M) | 132 | 54 | 16.00 | 864 | 2.00/7.00 | 108/378 | −756/−486 | |
| ABSSSI (DRG-KEN Δ20X) | 418 | 172 | 9.00 | 1548 | 2.00/7.00 | 344/1204 | −1204/−344 | |
| ABSSSI (DRG-KEN Δ24X) | 231 | 95 | 9.00 | 855 | 2.00/7.00 | 190/665 | −665/−190 | |
| ABSSSI (DRG-KEN Δ24M) | 935 | 384 | 4.00 | 1536 | 2.00/4.00 | 768/1536 | −768/0 | |
| ABSSSI (DRG-KEN Δ28MA) | 33 | 14 | 10.00 | 140 | 2.00/7.00 | 28/98 | −112/−42 | |
| ABSSSI (DRG-KEN Δ28MB) | 66 | 27 | 30.00 | 810 | 2.00/7.00 | 54/189 | −756/−621 | |
| ABSSSI (DRG-KEN Δ28X) | 296 | 163 | 4.00 | 652 | 2.00/4.00 | 326/652 | −326/0 | |
| Osteomyelitis (DRG-KEN M64M) | 77 | 32 | 15.00 | 480 | 7.00/7.00 | 224/224 | −256/−256 | |
| Osteomyelitis (DRG-KEN M64X) | 187 | 77 | 8.00 | 616 | 7.00/7.00 | 539/539 | −77/−77 | |
| Total | 2706 | 1113 | 8336 | 2771/6160 | −5595/−2216 | |||
| Italy | ABSSSI (DRG 277) | 5227 | 1788 | 11.00 | 19 710 | 2.00/7.00 | 3576/12 516 | −16 134/−7194 |
| ABSSSI (DRG 278) | 8055 | 2755 | 7.00 | 19 166 | 2.00/7.00 | 5510/19 166 | −13 656/0 | |
| Osteomyelitis (DRG 238) | 2824 | 966 | 16.50 | 15 961 | 7.00/7.00 | 6762/6762 | −9199/−9199 | |
| Total | 16 106 | 5509 | 54 838 | 15 848/38 444 | −38 990/−16 393 | |||
| Spain | ABSSSI (DRG 277) | 4833 | 1875 | 6.82 | 12 788 | 2.00/7.00 | 3750/12 788 | −9038/0 |
| ABSSSI (DRG 278) | 6211 | 2410 | 4.91 | 11 833 | 2.00/7.00 | 4820/11 833 | −7013/0 | |
| Osteomyelitis (DRG 238) | 815 | 316 | 9.44 | 2983 | 7.00/7.00 | 2212/2212 | −771/−771 | |
| Osteomyelitis (DRG 561) | 2792 | 1083 | 13.24 | 14 339 | 7.00/7.00 | 7581/7581 | −6758/−6758 | |
| Total | 14 651 | 5684 | 41 943 | 18 363/34 414 | −23 580/−7529 | |||
DRG 277 Cellulitis age >17 with complications.
DRG 278 Cellulitis age >17 without complications.
DRG 238 Osteomyelitis.
DRG 561 Aftercare, musculoskeletal system and connective tissue without complication or comorbidity (CC)/major complication or comorbidity (MCC).
Δ12Μ Ulcer/cellulite injuries with destructive coexisting conditions—complications.
Δ12XA Ulcer/cellulite-treated ulcers without catastrophic coexisting conditions—complications with skin graft repair or skin flap transfer.
Δ12ΧB Ulcer/cellulite-treated ulcers without catastrophic coexisting conditions—complications without skin graft repair or skin flap transfer.
Δ20M Skin ulcers with disastrous coexisting diseases—complications.
Δ20X Skin ulcers without disastrous coexisting diseases—complications.
Δ24Χ Cellulite without devastating or serious comorbidities—complications.
Δ24Μ Cellulite with devastating or serious comorbidities—complications.
Δ28ΜA Major skin lesions with disastrous (systemic) or serious comorbid conditions—complications.
Δ28ΜB Major skin lesions with catastrophic or serious coexisting conditions—long-term complications.
Μ64Μ Osteomyelitis with severe or serious concomitant diseases—complications.
*M64Χ Osteomyelitis without severe or serious concomitant diseases—complications.
ABSSSI, acute bacterial skin and skin structure infection; DRG, Diagnosis-related group; LoS, length of stay.
The annual hospitalisations eligible for early discharge would be 1113 in Greece, 5509 in Italy and 5684 in Spain. The total number of days of hospitalisation avoided on a yearly basis would be between 2216 and 5595 in Greece, between 16 393 and 38 990 in Italy and between 7529 and 23 580 in Spain.
The consequences of early discharge, related to the number of avoidable hospital beds, avoided HAIs and incremental follow-up visits and outpatients’ intravenous administrations, are reported in table 2.
Table 2.
Number of hospitalisations, potential savings of hospital beds, number of hospital-acquired infections, additional visits and intravenous administrations per type of infection, in each national context
| National context | Infection | ∆ Inpatient days between early discharge and base case scenarios | Potential hospital bed saving | Base case scenario—estimated HAIs | Early discharge scenario (if all LoS were at minimum) | Early discharge scenario (if all LoS were at maximum) | ∆ Specialist visits between early discharge and base case scenarios | ∆ Intravenous administrations between early discharge and base case scenarios | ||||||
| If all LoS were at minimum | If all LoS were at maximum | If all LoS were at minimum | If all LoS were at maximum | Estimated HAIs | ∆ HAIs versus base case scenario | Estimated HAIs | ∆ HAIs versus base case scenario | If all LoS were at minimum | If all LoS were at maximum | If all LoS were at minimum | If all LoS were at maximum | |||
| Greece | ABSSSI | −5262 | −1883 | −20 | −7 | 32 | 7 | −25 | 23 | −9 | +1113 | +543 | 0 | 0 |
| Osteomyelitis | −333 | −333 | −1 | −1 | 5 | 3 | −2 | 3 | −2 | +141 | +141 | +11 | +11 | |
| Total | −5595 | −2216 | −21 | −8 | 37 | 10 | −27 | 26 | −11 | +1254 | +684 | +11 | +11 | |
| Italy | ABSSSI | −29 790 | −7194 | −103 | −25 | 128 | 30 | −98 | 104 | −24 | +6331 | +1788 | 0 | 0 |
| Osteomyelitis | −9199 | −9199 | −32 | −32 | 53 | 22 | −31 | 22 | −31 | +1932 | +1932 | +97 | +97 | |
| Total | −38 990 | −16 393 | −135 | −57 | 181 | 52 | −129 | 126 | −55 | +8263 | +3720 | +97 | +97 | |
| Spain | ABSSSI | −16 051 | 0 | −58 | 0 | 171 | 60 | −111 | 171 | 0 | +4285 | 0 | 0 | 0 |
| Osteomyelitis | −7529 | −7529 | −27 | −27 | 121 | 68 | −53 | 68 | −53 | +1399 | +1399 | +140 | +140 | |
| Total | −23 580 | −7529 | −85 | −27 | 292 | 128 | −164 | 239 | −53 | +5684 | +1399 | +140 | +140 | |
ABSSSI, acute bacterial skin and skin structure infection; HAI, hospital-acquired infection; LoS, length of stay.
The potential number of hospital beds saved is between 8 and 21 in Greece, between 57 and 135 in Italy and between 27 and 85 in Spain. The early discharge strategy would lead to a decrease in HAIs between −11 and −27 in Greece, between −55 and −129 in Italy and between −53 and −164 in Spain.
The therapy administered to patients would not change between the base case and early discharge scenarios, but the number of specialist visits and administration of intravenous treatments outside the DRG would increase for those patients discharged earlier than in the base case. This would result in between 684 and 1254 additional follow-up visits and 11 intravenous administrations in Greece, between 3720 and 8263 additional follow-up visits and 97 intravenous administrations in Italy, and between 1399 and 5684 additional follow-up visits and 140 intravenous administrations in Spain.
Economic consequences
From an economic point of view, and adhering to the perspective adopted (ie, NHS), not all the aforementioned differences in variables between the two scenarios lead to economic consequences. For instance, the mean days of hospitalisation does not exceed the inpatient stay’s threshold value for any DRG, and therefore there is no impact on each NHS budget in terms of DRG reimbursement. A lack of economic consequences is also related to the cost of the antibiotic drugs considered (either oral or long-acting, intravenous drugs). The therapy administered to patients, in fact, would not change between the base case and early discharge scenarios, and so the cost of drugs for the NHS would not be affected.
The variables that lead to an impact on NHS’ budgets are the number of HAIs and the number of follow-up visits and intravenous administrations. The costs related to each variable are reported in table 3.
Table 3.
Incremental costs between the base case and early discharge scenarios
| National context | Infection | ∆ Between early discharge and base case scenarios | |||||
| HAI costs (€) | Specialist visits and intravenous administration costs (€) | Total costs (€) | |||||
| If all LoS were at minimum | If all LoS were at maximum | If all LoS were at minimum | If all LoS were at maximum | If all LoS were at minimum | If all LoS were at maximum | ||
| Greece | ABSSSI | −150 186* −118 148† |
−54 067* −42 533† |
+11 130 | +5430 | −139 056* −107 018† |
−48 637* −37 103† |
| Osteomyelitis | −12 015* −9452† |
−12 015* −9452† |
+1519 | +1519 | −10 496* −7933† |
−10 496* −7933† |
|
| Total | −162 201*
−127 599† |
−66 082*
−51 985† |
+12 649 | +6949 | −149 552*
−114 950† |
−59 133*
−45 036† |
|
| Italy | ABSSSI | −463 139 | −113 422 | +130 798 | +36 940 | −332 340 | −76 482 |
| Osteomyelitis | −146 503 | −146 503 | +40 853 | +40 853 | −105 650 | −105 650 | |
| Total | −609 642 | −259 925 | +171 652 | +77 793 | −437 990 | −182 132 | |
| Spain | ABSSSI | −666 824 | 0 | +75 073 | 0 | −591 751 | 0 |
| Osteomyelitis | −318 394 | −318 394 | +26 110 | +26 110 | −292 284 | −292 284 | |
| Total | −985 218 | −318 394 | +101 183 | +26 110 | −884 035 | −292 284 | |
*Result considering the maximum HAI cost.
†Result considering the minimum HAI cost.
ABSSI, acute bacterial skin and skin structure infection; HAI, hospital-acquired infection; LoS, length of stay.
Bringing together the cost reductions from fewer HAIs, and increased costs from additional specialist visits and intravenous administrations, the net impact of the early discharge scenario is somewhere between a reduction of €149 552 and €45 036 in Greece, between a reduction of €182 132 and €437 990 in Italy and between a reduction of €292 284 and €884 035 in Spain, in addition to the reduced numbers of bed-days and beds quantified above.
As the populations across the three countries were not comparable due to the demographic differences, and taking into account that the ‘early discharge’ strategy is applicable to different infectious diseases treated in infectious disease departments, we estimate the economic consequences for a fixed theoretical population of 10 000 and 20 000 patients for each country, as reported in table 4, to aid comparison.
Table 4.
Differential costs in each national context, considering a fixed population
| National context | Differential costs if all LoS were at minimum (10 000 patients) (€) |
Differential costs if all LoS were at maximum (10 000 patients) (€) |
Differential costs if all LoS were at minimum (20 000 patients) (€) |
Differential costs if all LoS were at maximum (20 000 patients) (€) |
| Greece | −1 343 680 | −531 291 | −2 687 359 | −1 062 582 |
| −1 032 798 | −404 636 | −2 065 596 | −809 271 | |
| Italy | −795 045 | −330 607 | −1 590 089 | −661 215 |
| Spain | −1 555 305 | −514 223 | −3 110 610 | −1 028 445 |
LoS, length of stay.
Sensitivity analysis results
The results of the sensitivity analysis performed are reported in table 5, in terms of differential costs, differential HAIs and potential hospital beds saved between the standard hospitalisation scenario and the early discharge scenario.
Table 5.
Sensitivity analysis results
| National context | Scenario | If all lengths of stay were at minimum | If all lengths of stay were at maximum | ||||
| ∆ Hospital beds saved | ∆ Number of HAIs | ∆ Costs (€) | ∆ Hospital beds saved | ∆ Number of HAIs | ∆ Costs (€) | ||
| Greece | Base case | −21 | −27 | −149 552* −114 950† |
−8 | −11 | −59 133* −45 036† |
| Bed occupancy rate (+5%) | −20 | −27 | −149 552* −114 950† |
−8 | −11 | −59 133* −45 036† |
|
| Bed occupancy rate (−5%) | −22 | −27 | −149 552* −114 950† |
−9 | −11 | −59 133* −45 036† |
|
| HAI incidence (+1/1000 days) | −21 | −29 | −161 566* −124 402† |
−8 | −13 | −71 148* −54 488† |
|
| HAI incidence (−1/1000 days) | −21 | −18 | −95 485* −72 417† |
−8 | −7 | −35 103* −26 132† |
|
| Cost of HAI (+10%) | −21 | −27 | −165 772* −127 710† |
−8 | −11 | −65 741* −50 234† |
|
| Cost of HAI (−10%) | −21 | −27 | −133 331* −102 190† |
−8 | −11 | −52 525* −39 837† |
|
| Cost of specialist visits and drug administration (+50%) | −21 | −27 | −143 227* −108 626† |
−8 | −11 | −55 658* −41 561† |
|
| Cost of specialist visits and drug administration (−10%) | −21 | −27 | −150 816* −116 215† |
−8 | −11 | −59 828* −45 731† |
|
| Italy | Base case | −135 | −129 | −437 990 | −57 | −55 | −182 132 |
| Bed occupancy rate (+5%) | −129 | −129 | −437 990 | −54 | −55 | −182 132 | |
| Bed occupancy rate (−5%) | −143 | −129 | −437 990 | −60 | −55 | −182 132 | |
| HAI incidence (+1/1000 days) | −135 | −169 | −627 026 | −57 | −71 | −257 746 | |
| HAI incidence (−1/1000 days) | −135 | −89 | −248 954 | −57 | −37 | −97 065 | |
| Cost of HAI (+10%) | −135 | −129 | −498 954 | −57 | −55 | −208 124 | |
| Cost of HAI (−10%) | −135 | −129 | −377 026 | −57 | −55 | −156 139 | |
| Cost of specialist visits and drug administration (+50%) | −135 | −129 | −352 164 | −57 | −55 | −143 235 | |
| Cost of specialist visits and drug administration (−10%) | −135 | −129 | −455 155 | −57 | −55 | −189 911 | |
| Spain | Base case | −85 | −164 | −884 035 | −27 | −53 | −292 284 |
| Bed occupancy rate (+5%) | −81 | −164 | −884 035 | −26 | −53 | −292 284 | |
| Bed occupancy rate (−5%) | −90 | −164 | −884 035 | −29 | −53 | −292 284 | |
| HAI incidence (+1/1000 days) | −85 | −188 | −1 028 214 | −27 | −60 | −334 336 | |
| HAI incidence (−1/1000 days) | −85 | −140 | −739 857 | −27 | −45 | −244 225 | |
| Cost of HAI (+10%) | −85 | −164 | −982 557 | −27 | −53 | −324 123 | |
| Cost of HAI (−10%) | −85 | −164 | −785 514 | −27 | −53 | −260 445 | |
| Cost of specialist visits and drug administration (+50%) | −85 | −164 | −833 444 | −27 | −53 | −279 229 | |
| Cost of specialist visits and drug administration (−10%) | −85 | −164 | −894 154 | −27 | −53 | −294 895 | |
*Result considering the maximum HAI cost.
†Result considering the minimum HAI cost.
HAI, hospital-acquired infection.
Discussion
This analysis has shown how an early discharge strategy for patients admitted due to ABSSSI or osteomyelitis could have a positive organisational impact on NHS. In particular, such a strategy could result in potential beds saved, reduced economic impact and reduced numbers of HAIs.
Even if it were not possible to actually reduce the number of hospital beds in infectious disease departments, higher productivity would be possible, by freeing hospital beds which could be used for further patients to be admitted.
The results reported are dependent on how many days of hospitalisation are assumed in the early discharge scenario; however, sensitivity analyses show that the economic and organisational impact would remain positive for each NHS considered.
The main limitation of this analysis is that the number of days of hospitalisation in the early discharge scenario is based on expert opinions and is not measured in a real-life context. Furthermore, data related to the incidence of HAIs per day of hospitalisation are only available for ICUs, and therefore published data were adapted to the analysis to align with measurements conducted by the ECDC, considering HAIs to be equally spread throughout the LoS. Finally, the hypothetical number of beds saved in each national context might be less in the case of highly decentralised health services, in which hospital treatment for the infections considered may be spread across several hospitals. An additional limitation is that the adopted perspective does not consider cost offsets due to hospital bed reductions, and considers only the improvement in efficiency, although it has been acknowledged that reducing hospital beds is per se a budget-saving initiative. Finally, another limitation is the possible exclusion from the study of some infections that could also benefit from early discharge.
To our knowledge, the analysis presented is the first one to consider the impact of an early discharge strategy assuming the perspective of NHS in Italy and Greece. Durojaiye and colleagues assessed the costs for the NHS in the UK of an OPAT service activated in Sheffield, comparing the cost of treating 3812 patient episodes in 10 years through OPAT service with the theoretical cost of treating them as inpatients.32 The results showed savings as a consequence of the activation of the OPAT service between £32.9 million and £6.2 million. González-Ramallo and colleagues33 assessed direct medical cost associated with a hospital at home OPAT in a multicentre retrospective evaluation in Spain. The results show an 80% decrease of costs through OPAT compared with inpatient management. Previous analyses have assumed the point of view of hospitals.23 34 Eckmann and colleagues performed a retrospective observational analysis of the medical charts of patients affected with complicated skin and soft tissue infection, in which they considered various treatment scenarios including the use of early discharge. This strategy would have reduced the mean LoS by 7.5 days in Greece, 7.0 days in Italy and 6.7 days in Spain. Our analysis showed a theoretical number of inpatient days saved for ABSSSI of up to 5.2 in Greece, up to 6.56 in Italy and up to 3.75 in Spain; these are lower than the estimates of Eckmann and colleagues, suggesting that our assumptions and methods are conservative. The potential costs avoided considered by Eckmann were from the hospital point of view, in terms of the ‘hotel’ component of hospital costs, as estimated by the WHO.
Palmieri and colleagues34 assessed the economic impact of an early discharge strategy for patients hospitalised due to ABSSSI in Italy. That analysis considered a home care service after patient discharge for a period of 8.6 days, a scenario which is not considered in the present analysis, considering that patients would be autonomous in continuing their antibiotic therapy, and should attend follow-up visits for intravenous antibiotic administration. Palmieri and colleagues estimated a lower cost for early discharge assuming a hospital point of view. In the Discussion section, the authors also consider the likely impact on the Regional Health Service of Lazio Region (Italy), estimating an increase in costs due to the home care assistance required for discharged patients: this perspective was considered in our analysis, which also reports the costs of outpatient care.
Conclusions
In conclusion, an early discharge strategy for patients hospitalised due to ABSSSI and osteomyelitis would have a positive impact on the NHS in Greece, Italy and Spain in terms of reduced HAIs, possible hospital bed saving or increased productivity and reduced direct health costs.
Supplementary Material
Footnotes
Contributors: UR participated in the design of the study, performed the economic analysis and drafted the manuscript. MB and DC participated in the design of the study, in the performance of the economic analysis, in the interpretation of the results, and critically revised the manuscript. SG, SMG, GR and SM provided their expert opinion for the study design and for the clinical variables considered in the analysis, participated in the interpretation of the results and critically revised the manuscript. VP participated in the performance of the economic analysis, in the interpretation of the results, and critically revised the manuscript. AV and AG provided their expert opinion for the economic analysis performed, participated in the interpretation of the results and critically revised the manuscript. MS participated in the interpretation of the results and critically revised the manuscript. All authors read and approved the final manuscript.
Funding: This analysis was funded by an unconditional grant from Angelini.
Competing interests: UR, MB, DC, VP and AV declares grants from Angelini during the conduct of the study; SG declares personal fees from Angelini during the conduct of the study, and grants form Astellas Pharma and Pfizer. SM and SMG declare personal fees from Angelini during the conduct of the study. GR declares personal fees from Angelini during the conduct of the study, and personal fees from Gilead, MSD and ViiV. MS is employed by Angelini. AG declares grants from Angelini and is partly supported by the NIHR Biomedical Research Centre, Oxford.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article.
References
- 1. Wenzl M, Naci H, Mossialos E. Health policy in times of austerity-A conceptual framework for evaluating effects of policy on efficiency and equity illustrated with examples from Europe since 2008. Health Policy 2017;121:947–54. 10.1016/j.healthpol.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 2. Ottolini FL, Buggio L, Somigliana E, et al. . The complex interface between economy and healthcare: an introductory overview for clinicians. Eur J Intern Med 2016;36:1–6. 10.1016/j.ejim.2016.07.030 [DOI] [PubMed] [Google Scholar]
- 3. Karanikolos M, Mladovsky P, Cylus J, et al. . Financial crisis, austerity, and health in Europe. Lancet 2013;381:1323–31. 10.1016/S0140-6736(13)60102-6 [DOI] [PubMed] [Google Scholar]
- 4. McKee M, Karanikolos M, Belcher P, et al. . Austerity: a failed experiment on the people of Europe. Clin Med 2012;12:346–50. 10.7861/clinmedicine.12-4-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Proudlove NC, Gordon K, Boaden R. Can good bed management solve the overcrowding in accident and emergency departments? Emerg Med J 2003;20:149–55. 10.1136/emj.20.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scott IA. Public hospital bed crisis: too few or too misused? Aust Health Rev 2010;34:317–24. 10.1071/AH09821 [DOI] [PubMed] [Google Scholar]
- 7. Gray A, Dryden M, Charos A. Antibiotic management and early discharge from Hospital: an economic analysis. J Antimicrob Chemother 2012;67:2297–302. 10.1093/jac/dks194 [DOI] [PubMed] [Google Scholar]
- 8. Wolkewitz M, Zortel M, Palomar-Martinez M, et al. . Landmark prediction of nosocomial infection risk to disentangle short- and long-stay patients. J Hosp Infect 2017;96:81–4. 10.1016/j.jhin.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization Report on the burden of endemic health care-associated infection worldwide. Geneva, Switzerland: World Health organization, 2011. Available: http://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf;jsessionid=ABAE37F5457971EA33C42DCF8E7EFFD5?sequence=1 [Accessed Nov 2017].
- 10. Burke JP. Infection control - a problem for patient safety. N Engl J Med 2003;348:651–6. 10.1056/NEJMhpr020557 [DOI] [PubMed] [Google Scholar]
- 11. Rutberg H, Borgstedt Risberg M, Sjödahl R, et al. . Characterisations of adverse events detected in a university hospital: a 4-year study using the global trigger tool method. BMJ Open 2014;4:e004879 10.1136/bmjopen-2014-004879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graves N, Weinhold D, Tong E, et al. . Effect of healthcare-acquired infection on length of hospital stay and cost. Infect Control Hosp Epidemiol 2007;28:280–92. 10.1086/512642 [DOI] [PubMed] [Google Scholar]
- 13. Weist K, Högberg LD, Diaz Högberg L. Ecdc publishes 2015 surveillance data on antimicrobial resistance and antimicrobial consumption in Europe. Euro Surveill 2016;21 10.2807/1560-7917.ES.2016.21.46.30399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapman ALN, Dixon S, Andrews D, et al. . Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother 2009;64:1316–24. 10.1093/jac/dkp343 [DOI] [PubMed] [Google Scholar]
- 15. Matthews PC, Conlon CP, Berendt AR, et al. . Outpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother 2007;60:356–62. 10.1093/jac/dkm210 [DOI] [PubMed] [Google Scholar]
- 16. Tice AD, Rehm SJ, Dalovisio JR, et al. . Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004;38:1651–71. 10.1086/420939 [DOI] [PubMed] [Google Scholar]
- 17. Vargas-Palacios A, Meads DM, Twiddy M, et al. . Cost-Effectiveness of outpatient parenteral antibiotic therapy: a simulation modelling approach. J Antimicrob Chemother 2017;72:2392–400. 10.1093/jac/dkx123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smismans A, Vantrappen A, Verbiest F, et al. . OPAT: proof of concept in a peripheral Belgian hospital after review of the literature. Acta Clin Belg 2018;73:257–67. 10.1080/17843286.2018.1424503 [DOI] [PubMed] [Google Scholar]
- 19. Russo A, Concia E, Cristini F, et al. . Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect 2016;22(Suppl 2):S27–36. 10.1016/S1198-743X(16)30095-7 [DOI] [PubMed] [Google Scholar]
- 20. Italian Ministry of Health Rapporto annuale sull’attivit di ricovero ospedaliero – Settembre, 2016. Available: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2548_allegato.pdf [Accessed Nov 2017].
- 21. Ministerio de Sanidad, Servicios Sociales e Igualdad Portal Estadístico. Available: https://www.msssi.gob.es/estadEstudios/estadisticas/inforRecopilaciones/anaDesarrolloGDR.htm [Accessed Nov 2017].
- 22. Greek Ministry of health. Available: http://www.moh.gov.gr/articles/bihealth/stoixeia-noshleytikhs-kinhshs/3865-stoixeia-noshleythentwn-sta-nosokomeia-toy-esy-etoys-2015?dl=1 [Accessed Nov 2017].
- 23. Eckmann C, Lawson W, Nathwani D, et al. . Antibiotic treatment patterns across Europe in patients with complicated skin and soft-tissue infections due to meticillin-resistant Staphylococcus aureus: a plea for implementation of early switch and early discharge criteria. Int J Antimicrob Agents 2014;44:56–64. 10.1016/j.ijantimicag.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 24. EUROSTAT Curative care bed occupancy rate, last update: 14-06-2017. Available: http://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do [Accessed Nov 2017].
- 25. Wolkewitz M, Cooper BS, Palomar-Martinez M, et al. . Multilevel competing risk models to evaluate the risk of nosocomial infection. Crit Care 2014;18 10.1186/cc13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. European Centre for Disease Prevention and Control Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. 2011-2012. Stockholm: ECDC, 2013. Available: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf [Accessed Nov 2017].
- 27. Gianino MM, Vallino A, Minniti D, et al. . A model for calculating costs of hospital-acquired infections: an Italian experience. J Health Organ Manag 2007;21:39–53. 10.1108/14777260710732259 [DOI] [PubMed] [Google Scholar]
- 28. Morano Amado LE, Del Campo Pérez V, López Miragaya I, et al. . [Nosocomial bacteremia in the adult patient. Study of associated costs]. Rev Clin Esp 2002;202:476–84. [DOI] [PubMed] [Google Scholar]
- 29. International Monetary Fund World economic outlook database, 2017. Available: http://www.imf.org/external/pubs/ft/weo/2017/01/weodata/weorept.aspx?sy=2000&ey=2018&scsm=1&ssd=1&sort=country&ds=%2C&br=1&pr1.x=16&pr1.y=11&c=174%2C184%2C136&s=PCPI%2CPCPIPCH&grp=0&a= [Accessed Nov 2017].
- 30. Italian Ministry of Health Nomenclatore dell'assistenza specialistica ambulatoriale. Available: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=1767&area=programmazioneSanitariaLea&menu=lea [Accessed Nov 2017].
- 31. Tariffs for outpatients activities , 2017. Available: https://www.fraternidad.com/sites/default/files/descargas-fm/Tarifas%20Sanitarias%202017.pdf [Accessed Nov 2017].
- 32. Durojaiye OC, Bell H, Andrews D, et al. . Clinical efficacy, cost analysis and patient acceptability of outpatient parenteral antibiotic therapy (OPAT): a decade of Sheffield (UK) OPAT service. Int J Antimicrob Agents 2018;51:26–32. 10.1016/j.ijantimicag.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 33. González-Ramallo VJ, Mirón-Rubio M, Mujal A, et al. . Costs of outpatient parenteral antimicrobial therapy (OPAT) administered by hospital at home units in Spain. Int J Antimicrob Agents 2017;50:114–8. 10.1016/j.ijantimicag.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 34. Palmieri F, Alberici F, Deales A, et al. . Early discharge of infectious disease patients: an opportunity or extra cost for the Italian healthcare system? Infez Med 2013;21:270–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.