Abstract
Objectives
This study assesses U.S. state laws related to prenatal syphilis screening, including whether these laws align with CDC screening recommendations and include legal penalties for failing to screen.
Methods
Statutes and regulations regarding syphilis screening during pregnancy and at delivery effective in 2016 were examined for all 50 U.S. states and the District of Columbia (DC). Targeted search terms were used to identify laws in legal research databases. The timing of the screening mandates for each state law was coded for: (1) first visit, (2) third trimester, and (3) delivery. Descriptive statistics were calculated to examine the number of states with each type of requirement and whether requirements adhered to the CDC STD treatment guidelines.
Results
Only six states (11.8%) do not require prenatal syphilis screening. Of states with screening requirements (n = 45), the majority (84.3%) require testing at first prenatal visit or soon after. 17 states (33.3%) require screening during the third trimester with five requiring screening only if the patient is considered at high risk. 8 (15.7%) states require screening at delivery with five requiring testing only if the woman is at high risk. 14 (27.5%) states include punishments for failing to screen (civil penalties, criminal penalties and license revocation).
Conclusions for Practice
Most states had prenatal syphilis screening requirements; a minority corresponded to or extended CDC recommendations. States vary in when they require testing, who must be tested, and whether a failure to screen could result in a punishment for the provider.
Keywords: Congenital, Syphilis, Pregnancy, Screening, Law, Policy
Introduction
In the United States (U.S.), the number of reported cases of primary and secondary syphilis among women and reported cases of congenital syphilis has increased every year since 2012 (Centers for Disease Control and Prevention 2017b). From 2012 to 2016 the congenital syphilis rate increased from 8.4 to 15.7 cases per 100,000 live births, representing an 87% increase. (Centers for Disease Control and Prevention 2017b). Healthy People 2020 established a target of 9.6 cases of congenital syphilis per 100,000 live births (U.S. Department of Health and Human Services 2010). Congenital syphilis occurs when a pregnant woman with syphilis transmits the infection to her fetus during pregnancy. Congenital syphilis can result in miscarriage, stillbirth, or death of the newborn after delivery. Congenital syphilis can also cause severe birth defects, including bone deformity, severe anemia, and nerve problems, including blindness or deafness (Centers for Disease Control and Prevention 2017a). Historical data indicate that untreated syphilis in pregnant women, if acquired during the 4 years prior to delivery, can lead to infection of the fetus in up to 80% of cases and may result in stillbirth or infant death in up to 40% of cases (Ingraham 1951).
Congenital syphilis prevention relies on screening and treating pregnant women found to have syphilitic infection. By treating woman’s syphilis infection during pregnancy, healthcare providers can effectively decrease morbidity and mortality associated with congenital syphilis (Centers for Disease Control and Prevention 2015a). Moreover, treating a pregnant woman with syphilis reduces her risk of health complications associated with late-stage syphilis. The Centers for Disease Control and Prevention (CDC) recommends syphilis screening for all women at the first prenatal visit. (Centers for Disease Control and Prevention 2015b) For pregnant women who are at high risk of contracting syphilis or live in a community with a high prevalence of syphilis, the CDC recommends repeat testing early in the third trimester (between 28 and 32 weeks) and again at delivery.
The regulation of the medical profession, including screening requirements, is typically a matter of state law under states’ inherent authority in the U.S. legal system. A 2003 study examined the number of syphilis screening tests for pregnant women that each state requires by law (Hollier et al. 2003). However, previous research has not assessed whether state legal requirements were consistent with the CDC guidelines for syphilis screening during pregnancy or if the laws included explicit legal consequences beyond the potential for a civil malpractice claim. These legal consequences include a criminal misdemeanor, a civil penalty, or professional license revocation for providers failing to adhere to these laws. Given that congenital syphilis rates have been increasing in the U.S. and the use of appropriate screening and treatment may prevent congenital syphilis, understanding current state prenatal syphilis testing requirements for pregnant women is important. The purpose of this assessment is to describe U.S. state laws requiring prenatal screening in 2016, including statutes and regulations, with a focus on whether these requirements align with CDC screening recommendations for pregnant women. Additionally, we examined whether the legal requirements included any potential penalties for failing to adhere to these screening requirements.
Methods
All 50 U.S. states and the District of Columbia (DC) were included in an assessment of statutes and regulations regarding screening for syphilis during pregnancy and at delivery effective in 2016. Statutes included laws passed by a state legislature, and regulations included those laws that were enacted by a state administrative agency, such as a health department or medical board. Targeted search terms were used to identify state laws in LexisNexis and Westlaw legal databases. The search looked for six key phrases, including “pregnant,” “prenatal,” “blood test,” “serologic,” “STD” (including STI, sexually transmitted disease, and sexually transmitted infection), and “syphilis.” The search results were then cross-referenced with other sources on the subject to ensure that all relevant laws were identified (Hough and Poppe 1998).
Each law identified was coded for its contents to develop several legal measures for each state and DC. First, we categorized whether states had a prenatal syphilis screening law that required a provider to screen for the infection. “Provider” may include any healthcare worker in a position to administer prenatal care including, among others, physicians and physician assistants. We eliminated the prenatal screening requirements that applied only to midwives. Next, the timing of the screening requirements was coded into three different categories: (1) first visit, (2) third trimester, and (3) delivery. The first visit category includes requirements of screening at first visit (or some specified time thereafter), early in pregnancy, or as soon as possible after pregnancy is confirmed. While it would seem that the general theme of these requirements is for the woman to receive testing as early as possible in pregnancy, some laws requiring testing specifically at first visit make clear that the requirement applies to the first time the pregnant patient actually presents to a provider, even if it occurs at delivery. Likewise, if the requirement states that the provider must screen for syphilis as soon as possible after pregnancy confirmation, screening could potentially occur as late as the third trimester or delivery because the pregnancy-confirming exam may happen that late in the pregnancy. The third trimester category covers requirements with language requiring testing during the third trimester, late in pregnancy, or any time between the 28th and 40th week of gestation. Among these requirements, we separately coded requirements that limit the screening requirement to patients considered at high risk for syphilis infection. A state may consider a patient high risk for various reasons, including whether she tested positive for syphilis earlier in her pregnancy, whether she lives in a community with a high syphilis morbidity rate, or whether her sexual behavior indicates an increased risk of infection. The delivery category includes requirements for screening at delivery. Similar to the third trimester category, we also separately coded requirements for screening at delivery only if the patient is deemed high risk. Finally, we coded state laws that punish a provider for failure to adhere to the state’s legal requirements for screening. These laws included three types of punishment: criminal misdemeanor, civil penalty, or professional license revocation. A civil penalty is not a criminal punishment, but rather a fine imposed by a government agency (e.g. state health department) for some misconduct.
To identify recent changes in these requirements, we collected effective dates for each law collected. For laws with effective dates from 2012 to 2016, we coded the law prior to the effective date in order to facilitate comparison with laws effective as of 2016. We also applied our search terms to all states’ laws as of 2012 to identify requirements that were repealed from 2012 to 2015; we identified no repealed requirements.
Based on the coding of the laws, descriptive statistics were calculated using Microsoft Excel to describe the number of states with each type of requirement. The degree to which states adhered to the CDC STD treatment guidelines was also assessed.
Results
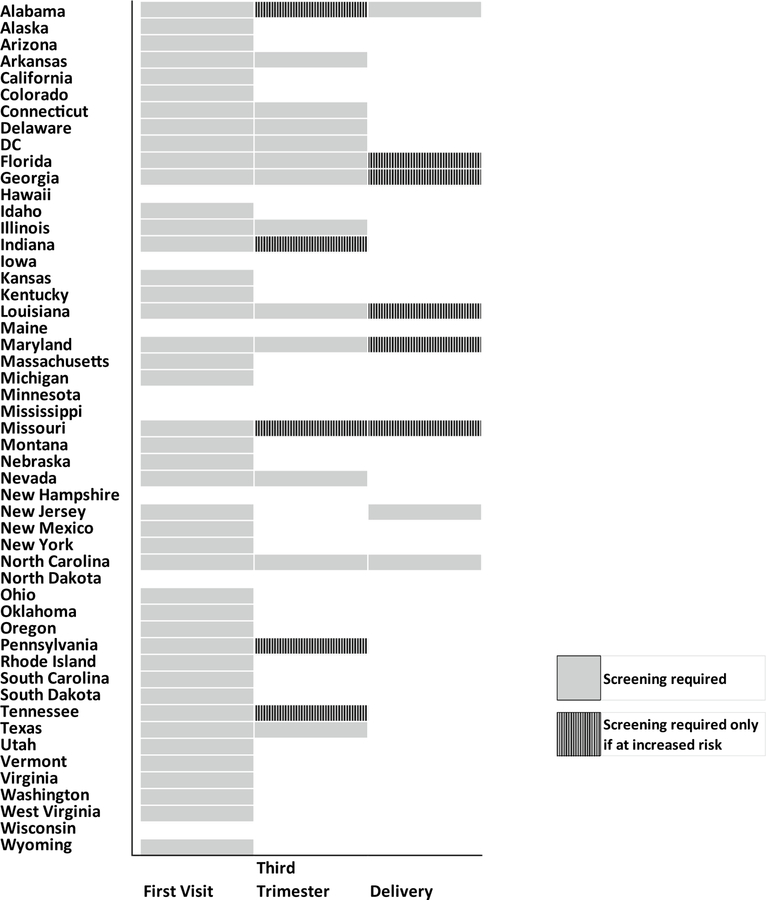
Among the 50 U.S. states and DC, only 6 (11.8%) states—Iowa, Minnesota, Mississippi, New Hampshire, North Dakota, and Wisconsin—have no prenatal syphilis screening requirements for providers other than midwives (Fig. 1). Of the 45 states that do have prenatal syphilis screening requirements, the time for required testing differs among states. 2 (3.9%) states, Maine and Hawaii, require testing at some point during gestation or delivery, but do not specify exactly when that testing must occur. The other states with a requirement fall into the category of requiring testing at the first prenatal visit (84.3%, n = 43). Among the 43 states in this category, states phrase this screening requirement in different ways. Most states require testing at the first visit with a provider or within a specified time period immediately after the first visit, e.g. “10 days” (78.4%, n = 40). Among the states in this category that do not specifically link testing to the first visit, Vermont requires testing early in pregnancy (“[a healthcare provider] attending a pregnant woman shall take samples of blood of such woman, if possible, prior to the third month of gestation…”, 18 V.S.A. § 1102), and 2 (3.9%) states—Kentucky and West Virginia—require testing as soon as possible after pregnancy has been confirmed. In contrast to the first category of states (requiring screening at first visit), a smaller proportion of U.S. states (33.3%, n = 17) have third trimester screening requirements. Among these 17 states in this third trimester screening category, 12 (70.6%) require third trimester screening with all pregnancies, and 5 (29.4%) require testing during the third trimester only if the patient is considered at high risk for syphilis infection. 8 (15.7%) U.S. states require screening at delivery. Among these 8 states, 3 require testing at delivery for all women and 5 states only require screening at delivery if the patient is deemed high risk. 7 (13.7%) of U.S. states require screening at first visit, during the third trimester, and at delivery.
Fig. 1.
Legal requirements for syphilis screening among pregnant women by state. Figure describes which states have laws requiring prenatal syphilis screening at the first visit, during the third trimester, and at delivery, and whether these screenings are conditioned on the patient being at increased risk. *If a state requires screening of all women at a particular time and requires screening of women at increased risk at that same time, then the state was coded using the category of broader applicability—all women. **Maine and Hawaii require prenatal screening, but do not specify an exact time for the test to be administered
While the majority of states have syphilis screening requirements, 14 (32.6%) states specify penalties for failing to administer a prenatal syphilis test as specified by law (Fig. 2). Failure to adhere to a state’s screening procedures may result in a criminal misdemeanor for health providers in 12 (23.5%) states. Hawaii may impose a civil penalty on those providers who do not follow the state mandated screening requirements. In Virginia, a provider may face professional license (physician, nurse, etc.) revocation if he or she fails to adhere to the state’s prenatal syphilis screening requirements.
Fig. 2.
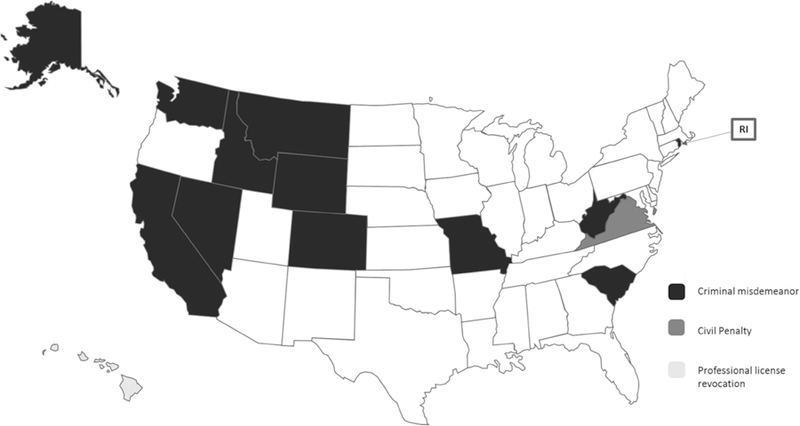
State may impose penalty on providers for failure to adhere to prenatal screening laws. Figure describes which states explicitly penalize providers for failing to perform a screening that is required by law, and whether these penalties are in the form of a criminal misdemeanor, a civil penalty, or professional license revocation
Since 2012, 4 (7.8%) states—Arkansas, Georgia, Louisiana, and Texas—have changed their legal requirements in a way that is relevant to CDC’s screening recommendations. Texas replaced a requirement that all women be screened at delivery with a requirement that all women be screened in the third trimester. Louisiana and Arkansas added a screening requirement for all women in the third trimester. Georgia and Louisiana both added a screening requirement at delivery for women at high risk of infection.
Discussion
A previous analysis of these legal requirements in 2003 found that most states have at least one relevant law. Our findings from 2016 are consistent with this research and suggest that the majority of states still have at least some form of prenatal syphilis screening requirement. Additionally, our study indicates that over three-quarters of states (84%) have statutes or regulations requiring screening for syphilis at the first visit or early in pregnancy. One-third of states (33.3%) have statutes or regulations addressing third trimester screening, but fewer states (15.7%) have statutes or regulations addressing screening at delivery. Every state that requires testing at third trimester or delivery also requires testing at the first visit.
CDC recommends screening at the first prenatal visit for all pregnant women and recommends repeat screening early in the third trimester and again at delivery for women at increased risk of infection. Only seven states have statutes or regulations that correspond to or extend the full CDC guidelines for prenatal syphilis screening, addressing screening at each timeframe including the first prenatal visit, during the third trimester, and at delivery. Missouri has language that mirrors the CDC recommendations for prenatal testing (screening required at first visit for all women, and repeat screening during the third trimester and at delivery required only if the patient is high risk). Florida, Georgia, Louisiana, and Maryland only require screening at delivery if the patient is considered high risk, but they require screening at the third trimester for all women, regardless of risk. Conversely, Alabama requires screening at the third trimester only if the patient is deemed high risk, but requires screening for all women at delivery. North Carolina requires providers to screen all women, regardless of risk factors, at all three stages: first visit, third trimester, and delivery.
Additionally, we found that a substantial minority of states (32.6%) have laws that permit punishment of providers for failure to perform syphilis screenings for pregnant patients as required by their state laws. The types of punishment include criminal misdemeanor, civil penalty, and professional license revocation. Such penalty laws may encourage providers to adhere to the testing requirements out of fear of consequences. Alternatively, providers might still follow the screening requirements even without an enforcement mechanism because such a requirement would be indicative of a provider’s standard of care in the event of a civil malpractice lawsuit. Importantly, women who do not receive prenatal screening to prevent congenital syphilis cases often may lack resources that are required to access legal counsel necessary to bring such a civil malpractice case. For instance, bringing a civil lawsuit can be very expensive for the plaintiff and includes the risk of losing the lawsuit along with the costs of litigation. A plaintiff must also be aware of their right to bring the lawsuit, and know how to take the actions necessary to secure legal counsel. Therefore, how perceptions of medical malpractice liability affects practice for prenatal syphilis screening is unclear. Future research could use our legal results to investigate provider perception of these policies and any associations with provider practice.
This analysis has some limitations. The precise language in each prenatal screening requirement varies from state to state. For example, some states require the first prenatal test to occur specifically at the patient’s first visit whereas others require providers to test a patient as soon as possible after pregnancy confirmation. The patient’s first visit, however, could theoretically occur during the third trimester or even at delivery. Additionally, some states might consider the entire state low morbidity and limit their law to only focus on first visit screening. In jurisdictions or within subpopulations where access to prenatal care is suboptimal, our exclusion of midwives from this analysis might result in excluding a source of prenatal care for some women. This analysis was not able to assess the reasons why state laws for prenatal syphilis screening vary. For example, we do not know if state budgets or other state-level factors or characteristics may influence decisions to require screening at more than one prenatal visit. Also unclear is how the presence or absence of state regulations, with or without penalties, affect clinical practice. Similarly, while many states require screenings at various times based on the woman being at “high risk,” this term is rarely defined in law, nor is it defined by CDC or other organizations. Accordingly, screening requirements that depend on the individual risk of the patient may be subject to interpretation and inconsistently performed between providers.
Furthermore, prenatal syphilis screening requirements are triggered by a healthcare examination. Therefore, for women who lack adequate prenatal care, these requirements will not assure timely screening. A substantial proportion of women at risk of congenital syphilis do not access prenatal care: women did not receive prenatal care in 21.8% of congenital syphilis cases from 2008 to 2014 (Bowen et al. 2015). Similarly, many states contain a provision in their prenatal screening requirements that allows women to object to the testing. Therefore, if a patient refuses syphilis testing at pregnancy or delivery, a provider will be exempted from any screening requirement and subsequently may not face a penalty for failure to adhere to the state screening requirements. We do not have information on how many women across these states utilize their power to object, but it could influence the syphilis screening rates.
In conclusion, the majority of states have prenatal screening requirements for syphilis; however, few had requirements that followed or extended the CDC screening guidelines for syphilis screening during pregnancy. Variation exists across states in when these tests must be performed, under what conditions they must be performed, and whether the state explicitly provides a punishment for failure follow the testing requirements. As rates of congenital syphilis have increased in recent years, few states have made substantive changes to their requirements. Furthermore, relatively few states have requirements addressing screening during the third trimester or at delivery. Future research could examine the relationship between these requirements, providers’ syphilis screening practices of pregnant women, and the potential impact of these requirements on congenital syphilis prevention.
Significance Statement.
Congenital syphilis (C.S.) has been increasing in the United States since 2012. The Centers for Disease Control and Prevention recommends syphilis screening at the first prenatal visit for all women and early in the third trimester and at delivery for women at high risk of infection. Most states require that pregnant women be screened for syphilis at various times during pregnancy. Given that C.S. rates have been increasing in the U.S. and the use of appropriate screening and treatment may prevent C.S., an updated understanding of recent state laws requiring prenatal syphilis testing may be important for C.S. prevention efforts.
Acknowledgments
Funding This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC.
Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflict of interest The authors report no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10995-018-2592-0) contains supplementary material, which is available to authorized users.
References
- Bowen V, Su J, Torrone E, Kidd S, & Weinstock H (2015). Incidence of congenital syphilis—United States, 2012–2014. Morbidity and Mortality Weekly Report, 64(44), 1241–1245. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2015a). 2015 STD treatment guidelines for congenital syphilis Accessed August 31, 2017, from http://www.cdc.gov/std/tg2015/congenital.htm.
- Centers for Disease Control and Prevention. (2015b). 2015 STD treatment guidelines for syphilis during pregnancy Accessed August 31, 2017, from http://www.cdc.gov/std/tg2015/syphilis-pregnancy.htm.
- Centers for Disease Control and Prevention. (2017a). Congenital syphilis—CDC fact sheet Accessed August 31, 2017, from http://www.cdc.gov/std/syphilis/stdfact-congenital-syphilis.htm.
- Centers for Disease Control and Prevention. (2017b). Sexually transmitted disease surveillance 2016
- Hollier LM, Hill J, Sheffield JS, & Wendel GD (2003). State laws regarding prenatal syphilis screening in the United States. American Journal of Obstetrics & Gynecology, 189, 1178–1183. [DOI] [PubMed] [Google Scholar]
- Hough MK, & Poppe JA (1998). Sexually transmitted diseases: A policymaker’s guide and summary of state laws Denver, CO: National Conference of State Legislatures. [Google Scholar]
- Ingraham NR (1951). The value of penicillin alone in the prevention and treatment of congenital syphilis. Acta Dermato-Venereologica, 31(24), 60–88. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2010). Healthy people 2020 topics & objectives: Sexually transmitted diseases Accessed August 31, 2017, from https://www.healthypeople.gov/2020/topics-objectives/topic/sexually-transmitted-diseases/objectives.