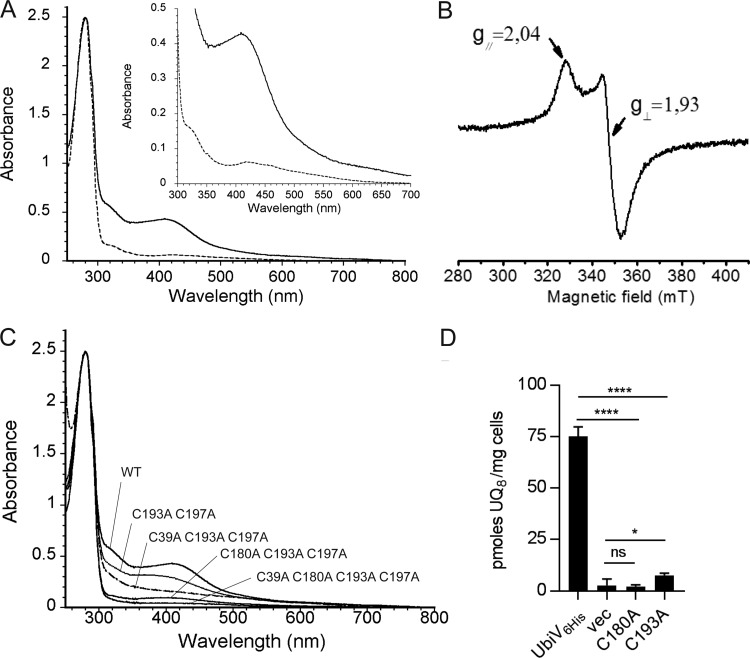
FIG 5.
UbiV binds a [4Fe-4S] cluster. (A) UV-visible absorption spectra of as-purified UbiV (dotted line, 47 μM) and reconstituted holo-UbiV (solid line, 41 μM). The inset is an enlargement of the 300- to 700-nm region. The molar extinction coefficient, ε410nm, was determined to be 10.8 ± 0.4 mM−1 cm−1 for holo-UbiV. (B) X-band EPR spectrum of 785 μM dithionite-reduced holo-UbiV. Recording conditions were the following: temperature, 10K; microwave power, 10 mW; modulation amplitude, 0.6 mT. (C) Comparative UV-visible absorption spectra of WT and different Cys-to-Ala mutants of UbiV after [Fe-S] cluster reconstitution, with the following concentrations: 41 μM WT, 44 μM C193A C197A, 46 μM C39A C193A C197A, 47 μM C180A C193A C197A, and 54 μM C39A C180A C193A C197A. (A to C) Proteins were in 50 mM Tris-HCl, pH 8.5, 25 mM NaCl, 15% glycerol, 1 mM DTT. (D) UQ8 quantification of ΔubiV cells transformed with pBAD-UbiV6His, pBAD-UbiV6His C180A, pBAD-UbiV6His C193A, or empty pBAD and grown overnight in anaerobic SMGN plus 0.02% arabinose. Values are means ± SD (n = 4 to 5). *, P < 0.05; ****, P < 0.0001; both by unpaired Student's t test.