Abstract
Background
Exclusive breastfeeding is recommended for all infants until six months of age due to the many health benefits for both the mother and infant.
Evidence suggests that mothers who are overweight (body mass index (BMI) 25.0 to 29.9 kg/m²) or obese (BMI ≥ 30.0 kg/m²) are less likely to initiate breastfeeding and to breastfeed for a shorter duration. Considering the rising prevalence of overweight and obesity globally and the known benefits of breastfeeding particularly in reducing the long‐term risks of obesity and diabetes for infants, establishing effective ways to support and promote breastfeeding in women who are overweight or obese is paramount in achieving the goal of healthier communities.
Objectives
To assess the effectiveness of interventions to support the initiation or continuation of breastfeeding in women who are overweight or obese.
Search methods
On 23 January 2019 we searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP) and reference lists of retrieved trials.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs that compared interventions to support the initiation and continuation of breastfeeding in women who are overweight or obese. Interventions included social support, education, physical support, or any combination of these. Interventions were compared either with each other or against a control group.
Data collection and analysis
We assessed all potential trials identified from the search strategy. Two review authors extracted data from each included trial and assessed risk of bias. We resolved discrepancies through discussion with the third review author. We assessed the quality of the evidence using the GRADE approach.
Main results
We found no trials comparing one type of support versus another. We included seven RCTs (including one cluster‐RCT) involving 831 women. The number of women in each trial ranged from 36 to 226. The trials were conducted in high‐income countries: USA (5 trials); Denmark (1 trial) and Australia (1 trial), between 2006 and 2015. Three trials only included women who were obese prior to pregnancy and four trials included both women who were overweight and women who were obese. We judged risk of bias in the included trials to be mixed; only one trial was judged to be low risk of bias for random sequence generation, allocation concealment and attrition bias.
Physical breastfeeding support (manual or electric breast pump) versus usual care (no breast pump)
Very low‐certainty evidence from one small trial (39 women) looking at a physical support intervention (manual or electric breast pump) versus usual care (no pump) means it is unclear whether physical support improves exclusive breastfeeding at four to six weeks (risk ratio (RR) 0.55, 95% confidence interval (CI) 0.20 to 1.51) or any breastfeeding at four to six weeks (RR 0.65, 95% CI 0.41 to 1.03). The trial did not report other important outcomes of interest in this review: non‐initiation of breastfeeding, exclusive or any breastfeeding at six months postpartum.
Multiple methods of breastfeeding support versus usual care
Six trials (involving 792 women) used multiple methods of support including education and social support through telephone or face‐to‐face contact. One of these trials also provided physical support through providing a breast pump and a baby sling and one trial provided a small gift to the women at each trial visit. Support in the trials was provided by a professional (four trials) or a peer (two trials). One trial provided group support, with the other five trials supporting women individually. One trial (174 women) did not report on any of our main outcomes of interest.
We are unclear about the effects of the intervention because we identified very low‐certainty evidence for all of the important outcomes in this review: rate of non‐initiation of breastfeeding (average RR 1.03, 95% CI 0.07 to 16.11; 3 trials, 380 women); exclusive breastfeeding at four to six weeks (average RR 1.21, 95% CI 0.83 to 1.77; 4 trials, 445 women); any breastfeeding at four to six weeks (average RR 1.04, 95% CI 0.57 to 1.89; 2 trials, 103 women); rate of exclusive breastfeeding at six months postpartum (RR 7.23, 95% CI 0.38 to 137.08; 1 trial, 120 women); and any breastfeeding at six months postpartum (average RR 1.42, 95% CI 1.08 to 1.87; 2 trials, 223 women).
The included trials under the above comparisons also reported on some of this review's secondary outcomes but very low‐certainty evidence means that we are unclear about the effects of the intervention on those outcomes.
Authors' conclusions
There is insufficient evidence to assess the effectiveness of physical interventions, or multiple methods of support (social, educational or physical) for supporting the initiation or continuation of breastfeeding in women who are overweight or obese. We found no RCTs comparing one type of support to another type of support. All of our GRADE assessments resulted in very low‐certainty evidence, with downgrading decisions based on limitations in trial design (e.g. risk of attrition bias), imprecision, inconsistency. The available trials were mostly of variable quality with small numbers of participants, confounded by poor adherence within both the intervention and control groups.
Well designed, adequately powered research is needed to answer questions about the social, educational, physical support, or any combination of these interventions that could potentially help mothers who are overweight or obese to achieve optimal breastfeeding outcomes. We need trials that examine interventions designed specifically for women who are overweight or obese, delivered by people with training about how to overcome some of the challenges these women face when establishing and maintaining breastfeeding. Particular attention could be given to the assessment of antenatal interventions aimed at improving breastfeeding initiation in women with a raised BMI, and not just focusing on recruiting women who have an intention to breastfeed. Given that the majority of current trials were undertaken in the USA, further trials in a diverse range of countries and settings are required. Future trials need to give consideration to the theoretical basis of the intervention using established frameworks to enable replicability by others and to better determine the components of effective interventions.
Plain language summary
Interventions to support women who are overweight or obese to start and continue breastfeeding
What is the issue?
Breastfeeding is important for the health of mothers and their infants. Current advice is for exclusive breastfeeding to continue until babies are six months of age. Infants fed with formula milk are at greater risk of infections, asthma and sudden infant death syndrome. Mothers who do not breastfeed are at greater risk of female cancers and type 2 diabetes. Women who are overweight or obese are less likely to start breastfeeding than other women and tend to breastfeed for a shorter length of time. Suggested reasons include physical factors such as larger breasts, which make traditional breastfeeding positions more difficult, and a delay in their milk coming in (normally around 72 hours). This can decrease mothers’ confidence in their milk supply and ability to breastfeed. Cultural factors may also influence women's decision making about starting and continuing breastfeeding, for example, how the woman’s family and friends fed their babies, how confident the mother is in reaching her breastfeeding goals and how the woman views her own body.
Why is this important?
Women who are overweight or obese can experience challenges with breastfeeding that could be overcome with additional encouragement and support. We wanted to find out what types of support are provided and what works best, both before and after birth. Interventions included education, social support and physical methods such as milk expression.
What evidence did we find?
We searched for evidence (January 2019) and identified seven randomised controlled trials (RCTs), involving 831 women (range 36 to 226 women), conducted in high‐income countries (USA, Denmark, Australia) between 2006 and 2015. Three trials only included women who were obese prior to pregnancy and four trials included women who were overweight and women who were obese.
The trials compared different types of breastfeeding support to usual care. There were a limited number of trials for each type of support, and differences in how much support the women received in the support and usual care groups.
One trial (39 women) used a physical support intervention through the loan of an electric or manual breast pump versus usual care (no pump). Very low‐certainty evidence means it is unclear whether physical support improves exclusive breastfeeding at four to six weeks; or any breastfeeding at four to six weeks. The trial did not report other important outcomes of interest: non‐initiation of breastfeeding, and exclusive or any breastfeeding at six months after birth.
Six trials (792 women) used multiple methods of support (including education and social support through telephone or face‐to‐face contact) versus usual care. One trial (174 women) did not report on any of our main outcomes of interest. One of the trials also provided physical support through providing a breast pump and a baby sling, and another provided a small gift to the women at each trial visit. Support in these trials was provided by a professional (four trials) or a peer (two trials), either in a group (one trial) or individually (five trials).
For women receiving an intervention that incorporated multiple methods of support (including social, educational or physical support) versus usual care, we are unclear about the effects of the intervention because we identified very low‐certainty evidence for all of the important outcomes in this review: rate of non‐initiation of breastfeeding; exclusive breastfeeding at four to six weeks; any breastfeeding at four to six weeks; rate of exclusive breastfeeding at six months after birth; and any breastfeeding at six months after birth.
What does this mean?
The effectiveness of interventions for supporting women who are overweight or obese to start and continue breastfeeding remains unclear. The methods used by the available trials varied in quality, with small numbers of participants. No trials compared one type of support to another.
We need high‐quality trials to evaluate whether social, educational, physical support, or any combination of these interventions can give mothers who are overweight or obese the best chance of starting and continuing to breastfeed. The interventions need to be designed specifically for this group of women and delivered by people who understand the challenges these women face when establishing and maintaining breastfeeding.
Summary of findings
Background
Description of the condition
The World Health Organization (WHO) recommends that infants are exclusively breastfed until six months of age with continued breastfeeding thereafter alongside appropriate complementary foods, due to the many health benefits of breastfeeding for both the mother and infant (WHO 2001). Infants fed with human milk substitutes are at increased risk of infections (Eidelman 2012; Lessen 2015; Salone 2013; Victora 2016), asthma (Eidelman 2012; Lessen 2015; Salone 2013), atopic dermatitis (Eidelman 2012), some childhood leukaemias (Eidelman 2012; Salone 2013), coeliac disease (Eidelman 2012; Lessen 2015) and sudden infant death syndrome (Eidelman 2012; Lessen 2015; Salone 2013; Victora 2016). Long‐term risks to the infant of not receiving breast milk have also been demonstrated such as increased obesity, ischaemic heart disease, and type 1 and type 2 diabetes in later life (Eidelman 2012; Lessen 2015; Salone 2013; Victora 2016). For preterm infants, breastfeeding reduces the risk of developing necrotising enterocolitis (Eidelman 2012; Lessen 2015; Salone 2013; Victora 2016). Mothers who do not breastfeed their infant are at increased risk of breast cancer, ovarian cancer (Eidelman 2012; Lessen 2015; Salone 2013; Victora 2016), type 2 diabetes, postnatal depression (Eidelman 2012; Lessen 2015), and osteoporosis (Lessen 2015). Mother‐infant bonding is also believed to be reduced if the mother does not breastfeed (Lessen 2015). There is much debate around the association between breastfeeding and postnatal weight changes, with some finding no association between breastfeeding and postpartum weight loss (Neville 2014), and others showing less weight loss when not breastfeeding (Lessen 2015).
The internationally recognised definition of being overweight is having a body mass index (BMI) between 25.0 and 29.9 kg/m², and the definition of obesity is a BMI of 30.0 kg/m² or over (WHO 2000). Other definitions also exist for different populations, most notably the WHO definition for Asian populations (WHO 2004). The rate of overweight and obesity across the globe continues to rise, with 34.9% of women currently having a BMI of 25 kg/m² or more and 13.9% a BMI of 30 kg/m² or more (Stevens 2012).
It is well‐established within the literature that women who are overweight or obese have poorer breastfeeding outcomes (Amir 2007; Babendure 2015; Baker 2007; Hauff 2014; Krause 2011; Lepe 2011; Li 2003; Mok 2008; Thompson 2013; Wojcicki 2011). It has been shown that women with a raised BMI are less likely to intend to breastfeed (Krause 2011), and also women who are obese plan to breastfeed for a shorter time period than women with a BMI in the normal range (Amir 2007). In addition, numerous trials have found that compared to women with a BMI in the normal range, women who are overweight or obese are less likely to initiate breastfeeding, initiate breastfeeding later on average, are less likely to breastfeed exclusively and breastfeed for a shorter duration, even when confounders such as age, parity, method of delivery, smoking, delayed lactogenesis and feeding intention are adjusted for (Amir 2007; Hauff 2014; Lepe 2011; Mok 2008; Thompson 2013; Wojcicki 2011). The most recent review suggests that women who have a BMI greater than 30 kg/m² have a 13% decreased rate of breastfeeding initiation and a 20% decreased likelihood of any breastfeeding at six months (Babendure 2015). The risk of early discontinuation of any or full breastfeeding has been shown to increase progressively with increasing BMI (Baker 2007). The link between a high BMI and decreased initiation of breastfeeding has also been shown regardless of gestational weight gain (Li 2003).
Several reasons have been proposed for why women who are overweight or obese are less likely to breastfeed. Factors believed to impact on early breastfeeding success for women who are overweight or obese are anatomical factors and delayed lactogenesis (Babendure 2015). Some women who are obese have larger breasts than women with a BMI in the normal range, which can make traditional breastfeeding positions more difficult (Babendure 2015). Women who are obese have also been shown to experience increased postpartum oedema, which flattens the nipples making it more difficult to latch an infant. The concern that women who are obese may have more mechanical difficulties with breastfeeding is supported by a trial that has shown that prior to discharge from hospital and also at one and three months post‐delivery, more women who are obese than women with a BMI in the normal range report breastfeeding problems such as cracked nipples, which are associated with poor attachment (Mok 2008). Lactogenesis, the onset of copious milk production, is triggered following the removal of the placenta (Babendure 2015). For most women this occurs within 72 hours of birth; however it is suggested that more women with a high BMI have an onset of lactogenesis after 72 hours than women with a BMI in the normal range (Hilson 2004). Even when other confounders are adjusted for, women who were overweight or obese prior to pregnancy have been found to have a reduced prolactin response to suckling at both 48 hours and seven days post‐delivery (Rasmussen 2004). Potential reasons for this delay in lactogenesis in women who are obese are: 1) the increased oedema experienced by these women; 2) an increased likelihood of a prolonged labour and caesarean section; and 3) a less steep decline in insulin concentrations from the end of pregnancy to initiation of lactation (Babendure 2015). It is suggested that insulin is needed for lactogenesis so an insulin imbalance can influence the timing of lactogenesis (Babendure 2015). A delay in lactogenesis can decrease the mother's confidence that her milk is sufficient for her child, leading to early substitution and early cessation of breastfeeding. Women with a raised BMI are more likely to have medical complications such as gestational diabetes, a caesarean section or a preterm birth (Marchi 2015), which have been linked with delayed lactogenesis (Amir 2007), reduced initiation of breastfeeding (Thompson 2013), and increased risk of early termination of full or any breastfeeding (Baker 2007). This may be in part due to pregnancy complications making early separation of the mother and infant more likely. However, even among those with medical conditions that are known to decrease the breastfeeding rate, an association between obesity and reduced breastfeeding continues to exist (Babendure 2015).
Factors suggested to impact upon the duration of exclusive or any breastfeeding for women who are obese may be physiological, anatomical, psychosocial (Babendure 2015), and cultural (Amir 2007; Mok 2008). Free androgens increase with increasing BMI and are particularly linked to polycystic ovaries, which occurs more often in women who are overweight or obese (Babendure 2015). Mid‐pregnancy androgen levels have been negatively correlated with breastfeeding duration at both three and six months (Carlsen 2010). It is also postulated that women who are overweight or obese may be so due to subclinical hypothyroidism. Thyroid hormones, especially levothyroxine (T4) and liothyronine (T3), are needed for the initiation and maintenance of breastfeeding (Babendure 2015). Animal trials have suggested that obesity in childhood negatively affects the development of breast glandular tissue (Babendure 2015). Anatomically, women who are overweight or obese may therefore have mammary hypoplasia/insufficient glandular tissue (Babendure 2015). Some of the characteristics experienced by women who are overweight or obese are consistent with this, including their reporting of insufficient supply (Mok 2008), describing stopping breastfeeding due to perceived insufficient supply (Guelinckx 2012), and being more likely to try to express in the first two months postpartum but less likely to have successfully expressed than women with a BMI in the normal range (Leonard 2011). Furthermore, no association between BMI and early cessation of breastfeeding has been shown for multiparous women who have successfully breastfed a child previously (Kronborg 2012). This may suggest that the biological factors associated with early cessation of breastfeeding had been overcome in these women or it may have been due to other issues, such as psychological and cultural factors (Kronborg 2012).
Psychosocial factors include confidence to reach breastfeeding goals, feeding practices of friends and family, maternal self‐efficacy and body image (Babendure 2015). Women who are obese have greater body dissatisfaction and lower self‐esteem than women with a BMI in the normal range, both of which could impact upon breastfeeding intentions (Amir 2007). Women who are overweight or obese also usually belong to social classes that traditionally breastfeed less which may lead these women to feel more uncomfortable about breastfeeding in public (Amir 2007). Indeed one French trial found mothers who were obese more often felt uncomfortable about feeding in public or in front of others than women with a weight in the normal range and were less likely to seek breastfeeding support in the first three months post‐delivery (Mok 2008). However, psychosocial factors are not the sole contributor to lower breastfeeding rates in women who are overweight or obese as differences in breastfeeding rates continue to exist after adjusting for socio‐cultural factors (Hauff 2014). Furthermore, research has shown that while socioeconomic status significantly influences long‐term breastfeeding, maternal BMI is consistently a significant predictor of breastfeeding prior to six months (Soltani 2009).
Given that women who are overweight or obese have a lower incidence of breastfeeding initiation and breastfeed for a shorter time period, there is a need for additional encouragement and support for these women, both during pregnancy and in the first year after delivery, to initiate and maintain breastfeeding (Babendure 2015; Hesch Anstey 2011; Krause 2011; Mok 2008). Establishing effective ways to support women who are overweight or obese is of particular importance considering that the proportion of women who are overweight or obese across the globe continues to increase (Heslehurst 2010; Hossain 2007; Stevens 2012).
Description of the intervention
This review evaluates interventions that could potentially increase initiation or duration of breastfeeding in women who are overweight or obese. Various types of interventions exist that can be delivered alone or in combination. This review will include the following intervention types.
Education: this provides women with information about breastfeeding, including physiology, common concerns and their management and an in depth description of the benefits of breastfeeding for mothers and their babies. Education can be in a variety of forms, including verbal and written and can be delivered through different formats; face‐to‐face in an individual or group setting, online or through mobile applications. It is usually provided in the antenatal period, but can also be provided in the postnatal period or both in the antenatal and postnatal periods.
Social support: this includes emotional, material or financial, physical, reassurance, praise, networking and meeting with others or the opportunity to discuss and respond to a woman's questions. Support is usually provided in the postnatal period, however initial contact with the woman can be in the antenatal period. Support can be delivered by peers or professional workers. This can include face‐to‐face support or more remote forms of support such as telephone, internet or mobile technologies. It can be provided to women individually or as part of a group and can be reactive, responding to women's requests, or proactive with scheduled visits. The level of support can vary from one‐off support to ongoing support.
Physical support: interventions can include antenatal or postnatal breast expression, provision of breast pumps and hospital practices such as encouragement of skin‐to‐skin contact between mother and infant at delivery.
How the intervention might work
The support a mother receives influences initiation and duration of feeding, as does prenatal education and hospital practices (Lessen 2015; Rollins 2016).
A comprehensive taxonomy for the reporting of specific behaviour‐change techniques incorporated within interventions has been devised by Michie 2013. Within this taxonomy, educational interventions would use behaviour‐change techniques within the 'shape knowledge' cluster, through providing instructions on how to perform the behaviour, such as providing advice on positioning and attachment. Techniques within the 'natural consequences' cluster would also be utilised if information was provided on the health consequences of breastfeeding. Social support falls within the 'social support' cluster of behaviour‐change techniques and could also contain behaviour‐change techniques within the 'reward and treat' cluster if financial incentives or rewards are used. Physical interventions such as antenatal or postnatal breast expression are hypothesised to improve lactogenesis by an early stimulation and hormonal release.
Several reviews have been undertaken on interventions to support breastfeeding. The first has shown that any form of extra support is effective at increasing exclusive breastfeeding and any breastfeeding at both four to six weeks and at six months postpartum in healthy mothers and healthy, term infants (McFadden 2017). In particular, face‐to‐face and proactive support were more likely to be successful, as were interventions in settings with high breastfeeding initiation rates. A second review found that educational and support‐based interventions are effective at increasing exclusive breastfeeding at birth, one month and up to five months of age and at decreasing the rate of no breastfeeding (Haroon 2013). Interventions that included both individual and group counselling were more effective than either an individual or group intervention in isolation. The final review found improved breastfeeding rates for interventions that trained healthcare staff, implemented baby‐friendly support or provided education or support within the health system, the family or in the community (Rollins 2016); a combination of interventions was found to be most effective. None of these reviews have however looked at what interventions are effective for women who are overweight or obese. Due to women with a raised BMI having different breastfeeding expectations and challenges to women with a BMI in the normal range (Mok 2008), and due to the many possible factors noted above that can specifically influence the breastfeeding practices of women who are overweight or obese (Babendure 2015), it is important to establish what interventions are most effective within this group of women.
Why it is important to do this review
The importance of breastfeeding for both the mother and the infant are well known (Eidelman 2012; Lessen 2015; Salone 2013; Victora 2016). It is also well established within the literature that women who are overweight or obese have different breastfeeding expectations, practices and poorer breastfeeding outcomes than women with a BMI in the normal range, including decreased breastfeeding initiation and reduced breastfeeding length for both exclusive and any breastfeeding (Babendure 2015; Hauff 2014). Physical, psychological, socio‐cultural, medical and health services reasons have been proposed for this disparity (Babendure 2015; Lessen 2015), all of which mean that this group of women are in need of extra support both in the antenatal period and post‐delivery to initiate and maintain breastfeeding. It is therefore essential to determine the most beneficial methods of breastfeeding support for women who are overweight or obese. The continuing global trend of increased obesity both in the general and the obstetric populations (Heslehurst 2010; Hossain 2007; Stevens 2012), make this issue particularly important.
Objectives
To assess the effectiveness of interventions to support the initiation or continuation of breastfeeding in women who are overweight or obese.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), cluster‐RCTs and quasi‐RCTs were eligible for inclusion in this review. For trials published in abstract form only, we contacted the trial authors for further details and included the trial if sufficient data were available on the trial quality, intervention and outcomes of interest. Trials using a cross‐over design are not practical for this topic and therefore not eligible for inclusion in this review.
Types of participants
Pregnant or lactating women who were overweight or obese (as defined by trial authors based on pre‐pregnancy or booking pregnancy BMI) and had been recruited into a trial where the intervention was aimed at supporting breastfeeding, either initiation or maintenance. We included all women who were overweight or obese, irrespective of co‐existing medical complications, for example, diabetes, preterm delivery, caesarean section.
Types of interventions
Any intervention specifically aimed at supporting mothers who were overweight or obese to breastfeed that was over and above the care usually provided within that setting. Breastfeeding was classified as the provision of breast milk to the infant either by putting the baby to the breast or by expressing breast milk to give to the infant.
Interventions included social, educational, physical support, or any combination of these. Interventions were compared either with each other or against a control group that received standard care for that setting. This led to consideration of five separate comparisons.
Social support only versus usual care
Educational support only versus usual care
Physical support only versus usual care
Multiple methods of breastfeeding support versus usual care
One or multiple forms of breastfeeding support versus another form of breastfeeding support
Antenatal, postnatal or combined antenatal and postnatal interventions were eligible for inclusion so long as they were designed to improve breastfeeding rates among women who were overweight or obese.
We included interventions delivered at the level of the individual, in groups or a combination of these; we included interventions provided by either peer or professional workers and in hospital or community settings.
Types of outcome measures
Primary outcomes
Non‐initiation of breastfeeding ‐ where initiation is defined as the baby being put to the breast or being given any of the mother's breast milk within 48 hours of delivery (NHS England 2014)
Exclusive breastfeeding at four to six weeks ‐ as defined by trial authors
Any breastfeeding at four to six weeks
Exclusive breastfeeding at six months ‐ as defined by trial authors
Any breastfeeding at six months
Secondary outcomes
Breastfeeding intention
Excusive breastfeeding at one week, two weeks, two, three, four months ‐ as defined by trial authors
Any breastfeeding at two weeks, two, three, four, nine, 12 months
Duration of exclusive breastfeeding ‐ as defined by trial authors
Duration of any breastfeeding
Maternal postpartum weight retention at two, three, four, six, nine and 12 months
Maternal postpartum BMI at two, three, four, six, nine and 12 months
All‐cause infant or neonatal morbidity ‐ as reported by trial authors, for example, neonatal hypoglycaemia, low weight gain, infections
All‐cause infant or neonatal mortality
Infant weight gain at two, three, four, six, nine and 12 months
Maternal satisfaction with care
Maternal satisfaction with feeding method
Maternal nipple health ‐ as defined by trial authors, for example, cracked nipples, sore nipples
Cost‐effectiveness of the intervention
Search methods for identification of studies
The following methods section of this protocol is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (23 January 2019)
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (23 January 2019) for unpublished, planned and ongoing trial reports using the search methods described in Appendix 1.
Searching other resources
We searched the reference lists of retrieved trials for further eligible trials.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential trials identified through the search strategy. We resolved any disagreements through discussion and consultation with the third review author.
We created a trial flow diagram to map out the number of records identified, included and excluded (Figure 1).
1.
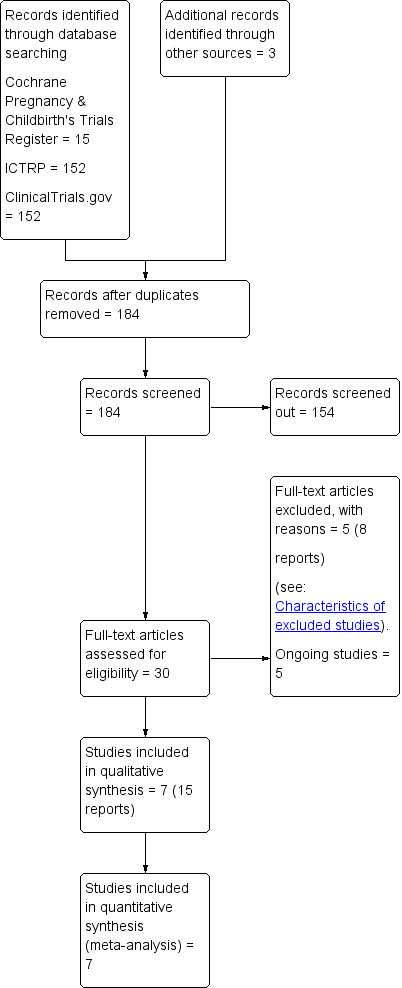
Study flow diagram
Data extraction and management
We designed a form to extract data. For eligible trials, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion with all review authors. We entered data into Review Manager 5 software and checked for accuracy (Review Manager 2014). When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreement by discussion or by involving a third assessor. In addition, for the included cluster‐randomised trial we assessed risk of 1) recruitment bias; 2) baseline imbalance; 3) loss of clusters; 4) incorrect analysis; and 5) comparability with individually randomised trials as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.3.2) (Higgins 2011).
1. Random sequence generation (checking for possible selection bias)
We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias)
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
3.1. Blinding of participants and personnel (checking for possible performance bias)
For this type of intervention, blinding women and clinical staff is generally not feasible, although it may be possible to blind outcome assessors.
3.2. Blinding of outcome assessment (checking for possible detection bias)
We described for each included trial the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
In trials examining breastfeeding support, women may be followed up over many months. We therefore used a cut‐off of 20% missing data to assess a trial as low risk of bias either at six months post‐delivery or at trial end if the trial finished prior to six months to coincide with the primary outcomes. We described for each included trial, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups; maximum of 20% missing data);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
5. Selective reporting (checking for reporting bias)
We described for each included trial how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the trial’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the trial’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; trial fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
6. Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included trial any important concerns we have about other possible sources of bias.
We assessed whether each trial was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
7. Overall risk of bias
We made explicit judgements about whether trials were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). With reference to 1 to 6 above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. For the purpose of this review, we defined 'high quality' as a trial having adequate sequence generation, allocation concealment and an attrition rate of less than 20%. We explored the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Assessing the quality of the body of evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE Handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons (Schünemann 2013).
Non‐initiation of breastfeeding
Exclusive breastfeeding at four to six weeks
Any breastfeeding at four to six weeks
Exclusive breastfeeding at six months
Any breastfeeding at six months
We used the GRADEpro Guideline Development Tool (GRADEpro GDT), to import data from Review Manager 5.3 (Review Manager 2014), in order to create 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (trial limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We included cluster‐randomised trials in the analyses along with individually randomised trials. We adjusted their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.3.4), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a trial of a similar population (Higgins 2011). We reported where we used ICCs from other sources and conducted sensitivity analyses to investigate the effect of variation in the ICC. We synthesised relevant information identified from both cluster‐randomised trials and individually randomised trials. We considered it reasonable to combine the results from both if there was little heterogeneity between the trial designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
We acknowledged heterogeneity in the randomisation unit and performed a sensitivity analysis to investigate the effects of the randomisation unit.
Multiple‐armed trials
We included multi‐armed trials and attempted to overcome potential unit of analysis errors by combining groups to create a single pair‐wise comparison or by selecting one pair of interventions and excluding the others as described in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.5; Higgins 2011).
Dealing with missing data
For included trials, we noted levels of attrition. We explored the impact of including trials with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomised to each group in the analyses and all participants were analysed in the group to which they had been allocated regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003), and Chi² statistics (Deeks 2017). We regarded heterogeneity as substantial if an I² statistic value was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more trials, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it (Sterne 2017).
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (Review Manager 2014). We anticipated some heterogeneity between trials in terms of the intervention and trial populations, we therefore used random‐effects meta‐analysis for combining data. The random‐effects analyses results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I² statistics. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not combine trials.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses for the review's primary outcomes.
BMI category (overweight and obese versus obese)
Intervention provider (professional versus partner/family member/peer support)
Type of intervention delivery (face‐to‐face versus remote support; group versus individual)
Timing of intervention (antenatal and postnatal versus postnatal alone)
Setting of the intervention (Baby‐Friendly Initiative accredited institution versus non Baby‐Friendly Initiative accredited institution)
Intensity of intervention (number of scheduled contacts)
Socioeconomic status of the population (mixed versus low (> 75% of participants from low‐income backgrounds))
Gestational age at birth of infant (term infants only versus preterm and term infants)
Mode of delivery (vaginal delivery (normal or assisted) versus caesarean section)
Background breastfeeding initiation rates (high (≥ 80%) and medium (60% to < 80%) versus low (< 60%))
Co‐morbidities (without complications versus with co‐morbidities, such as gestational diabetes mellitus, pre‐existing diabetes and preterm birth)
We have insufficient data to perform meaningful subgroup analyses at this time. However, data stratified by subgroups are presented in Comparisons 3 to 12 for the above planned subgroups (apart from 9 and 10) for information only, and to inform future updates.
In future updates, where appropriate, we will assess subgroup differences by interaction tests available within Review Manager 5 software (Review Manager 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² statistic value.
Sensitivity analysis
There were insufficient trials to carry out our planned sensitivity analysis for risk of bias because we classified only one included trial as high quality. For the purpose of this review, 'high quality' was defined as a trial having adequate sequence generation, allocation concealment and an attrition rate of less than 20%. In future updates we will carry out planned sensitivity analysis based on the quality of the included trials to identify the impact of the methodological quality on the overall results for our primary outcomes.
For the included cluster‐RCTs, we also used sensitivity analysis to investigate the effect of variation in the ICC and to investigate the effect of the unit of randomisation.
We restricted sensitivity analyses to the primary outcomes.
Results
Description of studies
Results of the search
The search of Cochrane Pregnancy and Childbirths' Trials Register retrieved 15 trial reports, we found 304 records in ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP), and three further potential reports from other sources (see Figure 1).
Included studies
In total, we included seven trials from 15 reports (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a; Rasmussen 2011b; Reifsnider 2018; Stuebe 2016). It should be noted that one publication contained two separate trials, one trial called Bassett Improving Breastfeeding Study (BIBS) and the other BIBS 2. We have separated these two trials, with BIBS identified as (Rasmussen 2011a), and BIBS2 as (Rasmussen 2011b). There are also five potential ongoing trials (NCT01668316; NCT02260518; NCT02520167; NCT02534051; NCT02756169).
Design
There were six parallel‐RCTs and one cluster‐RCT (Stuebe 2016).
Sample sizes
Trials recruited 831 women. Sample sizes ranged from a minimum of 36 women (Martin 2015), to a maximum of 226 women (Carlsen 2013). Four of the trials (Carlsen 2013; Chapman 2013; Reifsnider 2018; Stuebe 2016), had a sample size of 100 or more women.
Setting
All trials were conducted in high‐income countries. Five trials were conducted in the USA (Chapman 2013; Rasmussen 2011a; Rasmussen 2011b; Reifsnider 2018; Stuebe 2016), and one trial each in Denmark (Carlsen 2013), and Australia (Martin 2015). Three trials (Chapman 2013; Rasmussen 2011a; Rasmussen 2011b), were conducted between 2006 and 2009. The other four trials were conducted between 2010 and 2015 (Carlsen 2013; Martin 2015; Reifsnider 2018; Stuebe 2016).
Only one trial reported being undertaken in a Baby Friendly Initiative (BFI)‐accredited institution (Chapman 2013), two trials reported not having BFI accreditation (Carlsen 2013; Stuebe 2016), with the other four trials not mentioning their accreditation status. Four of the trials that were run at units with no accreditation or of unknown accreditation status specifically mentioned that the unit(s) running the trial promoted and supported breastfeeding (Carlsen 2013; Rasmussen 2011a; Rasmussen 2011b; Stuebe 2016).
None of the trials reported background rates of 'ever breastfed' within their institution. We therefore used background rates of 'ever breastfed' published in either the WHO Global Data in Infant and Young Child Feeding (www.who.int/nutrition/databases/infantfeeding/countries/en/; accessed November 2017), or from the published supplementary material in Victora 2016. For Denmark, we took background rates from the Organisation for Economic Co‐operation and Development (OECD) Family database (www.oecd.org/general/searchresults/?q=breastfeed&cx=012432601748511391518:xzeadub0b0a&cof=FORID:11&ie=UTF‐8; accessed January 2019). The five trials undertaken in the USA (Chapman 2013; Rasmussen 2011a; Rasmussen 2011b; Reifsnider 2018; Stuebe 2016) were undertaken in four separate states all of which had medium background breastfeeding rates (60% to 80% of women ever breastfeeding). The background rates of 'ever breastfed' in Australia (Martin 2015), and Denmark (Carlsen 2013), were high (80% to 100% of women ever breastfeeding).
Participants
All trials recruited women with a raised pre‐pregnancy BMI. Three trials (Carlsen 2013; Rasmussen 2011a; Rasmussen 2011b), exclusively recruited women who were obese, while the other four trials recruited both women who were overweight and those who were obese.
One trial recruited women in the postnatal period, within 48 hours of delivery (Carlsen 2013). All other trials recruited women in the antenatal period, with Martin 2015 recruiting women under 26 weeks' gestation, Stuebe 2016 between 22 and 37 weeks' gestation, Rasmussen 2011a and Rasmussen 2011b at 35 or less weeks' gestation, Chapman 2013 at 36 or less weeks' gestation and Reifsnider 2018 during the third trimester. Six trials reported only including women expecting or giving birth to a singleton infant (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a; Rasmussen 2011b; Reifsnider 2018).
Two trials only recruited women who could read/write/speak English (Martin 2015; Stuebe 2016), and one trial women who were English or Spanish speaking (Chapman 2013). Chapman 2013 and Reifsnider 2018 only recruited women from a low‐income background, with two other trials recruiting more than 50% of women from low‐income backgrounds (Rasmussen 2011a; Rasmussen 2011b).
Other inclusion criteria for the trials included no history of previous breast surgery (Carlsen 2013; Rasmussen 2011a; Rasmussen 2011b), not intending to move out of the area during the trial follow‐up period (Chapman 2013; Reifsnider 2018), no other medical conditions that could interfere with breastfeeding (Chapman 2013), agreeing not to participate in another weight loss programme during the trial follow‐up period (Martin 2015), and residing near to the healthcare facility (Rasmussen 2011a; Rasmussen 2011b).
Five of the trials described infant exclusion criteria. Four trials (Carlsen 2013; Rasmussen 2011a; Rasmussen 2011b; Reifsnider 2018), excluded preterm infants and Chapman 2013 only included infants born at 36 or more weeks' gestation. Carlsen 2013 and Chapman 2013 excluded infants who required neonatal admission. Other infant exclusion criteria included one or five minute Apgar scores of less than 6 (Chapman 2013), birthweight ≤ 2.5 kg or ≥ 3.9 kg (Chapman 2013), birthweight less than 2.5 kg (Reifsnider 2018), malformation or congenital disease (Carlsen 2013; Reifsnider 2018), birth injury (Rasmussen 2011a; Rasmussen 2011b), and baby taken into foster care (Rasmussen 2011a; Rasmussen 2011b; Reifsnider 2018).
One trial only included women with a gestational diabetes (GDM) diagnosis (Stuebe 2016). One trial excluded women with medical co‐morbidities that could impact on breastfeeding (Chapman 2013). One trial excluded women with type 1 diabetes but included women with gestational diabetes and pre‐eclampsia (Reifsnider 2018). For the other four trials, one stated that they did not exclude those with co‐morbidities, so their participants included 9% of women with GDM (Martin 2015), and the other three trials made no mention of co‐morbidities such as GDM within their reports (Carlsen 2013; Rasmussen 2011a; Rasmussen 2011b), so we judged them to have included co‐morbidities in line with the general population.
Interventions and comparisons
We found no trials that compared breastfeeding interventions to each other. Of the seven included trials one provided a physical support intervention only versus usual care (Rasmussen 2011b), and the other six provided multiple methods of support versus usual care (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a; Reifsnider 2018; Stuebe 2016).
Physical support versus usual care
The trial providing physical support (Rasmussen 2011b), included three arms, an electric breast pump, a manual breast pump and a control group. We amalgamated the two breast pump arms for the analysis, to make a breast pump versus control comparison. This trial was provided to individual women and was reactive, as women had one scheduled contact in hospital. Once leaving hospital the intervention was woman‐led as women were expected to express for the first two weeks postpartum using the breast pump provided and following the instructions given for continued use of the pump after discharge (Rasmussen 2011b). After the hospital contact the woman continued the intervention, pumping, at home without any further support. We therefore judged this intervention to also be remote support. Although women were recruited in the antenatal period the intervention took place exclusively in the postnatal period (Rasmussen 2011b).
Multiple methods of support versus usual care
Of the six trials providing multiple methods of support, all provided social and educational support. One trial also provided physical support through the use of a breast pump and a breastfeeding sling to promote close mother‐infant contact (Chapman 2013). A further trial provided financial incentives with a gift received by the women in the intervention group at each visit (Reifsnider 2018). Four trials provided face‐to‐face social support as part of their intervention (Chapman 2013; Martin 2015; Reifsnider 2018; Stuebe 2016). Three trials supplemented this by additional remote support through telephone calls (Chapman 2013; Martin 2015) or weekly text messages (Stuebe 2016). The other two trials provided remote social support through telephone support (Carlsen 2013; Rasmussen 2011a). The intervention was provided individually in five trials (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a; Reifsnider 2018) and to a group of women in the final trial (Stuebe 2016). Three trials provided breastfeeding support as part of a bespoke lifestyle intervention (Martin 2015; Reifsnider 2018; Stuebe 2016). One of these trials (Reifsnider 2018), aimed the lifestyle intervention at the infant to prevent infant overweight. The other two trials (Martin 2015; Stuebe 2016), aimed the lifestyle intervention at the mother. Both these trials offered the lifestyle intervention to the control group after completion of trial data collection. Martin 2015 included a diet‐only group, a diet and breastfeeding support group and a control group. We compared the diet and breastfeeding support group with the control group, according to the review inclusion criteria, to keep control groups within the analysis as homogeneous across the trials as possible.
Four trials used a professional to provide the intervention (Carlsen 2013; Martin 2015; Rasmussen 2011a; Stuebe 2016). In all of these cases the professional was a International Board Certified Lactation Consultant. In Chapman 2013, a peer supporter who had breastfed a child for a minimum of six months and had received 30 hours of specialised training provided the intervention. In Reifsnider 2018, a community worker who had 320 hours' training in research, nutrition, breastfeeding support and parenting, as well as two five‐day courses in lactation support provided the intervention.
Timing of the intervention
None of the interventions only occurred in the antenatal period. In five of the trials the intervention took place in both the antenatal and the postnatal periods (Chapman 2013; Martin 2015; Rasmussen 2011a; Reifsnider 2018; Stuebe 2016). In the other trial the intervention took place only in the postnatal period (Carlsen 2013).
Intensity of the intervention
All of the six trials using multiple methods of support were proactive, having scheduled contacts with the woman. The number of scheduled contacts varied from two in Rasmussen 2011a up to 15 in Chapman 2013. Two trials (Martin 2015; Rasmussen 2011a), had a low‐intensity intervention (fewer than four postnatal contacts), one trial (Reifsnider 2018), involved a medium‐intensity intervention (between four and eight postnatal contacts) and we classified the intervention as high‐intensity (nine or more postnatal contacts) in three trials (Carlsen 2013; Chapman 2013; Stuebe 2016). Should they need to do so, women could also access further support outside of the scheduled contacts in four trials (Carlsen 2013; Chapman 2013; Martin 2015; Reifsnider 2018).
Outcomes
Primary outcomes
Five trials reported breastfeeding initiation rates (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a; Rasmussen 2011b). Two trials defined initiation as breastfeeding on day four after delivery (Rasmussen 2011a; Rasmussen 2011b), which differed from the review definition of within 48 hours of delivery, so we did not include these trials within the analysis. All trials collected data on exclusive breastfeeding and any breastfeeding, however the timing of data collection varied. Trials collected data at three and seven days, two and four weeks and then three, four and six months (Carlsen 2013); at two weeks and then monthly until six months (Chapman 2013); at three and six months (Martin 2015); daily until day seven postpartum and then at 30 and 90 days (Rasmussen 2011a; Rasmussen 2011b); at one week and then monthly until 12 months (Reifsnider 2018); and at six weeks followed by four, seven and 10 months (Stuebe 2016). One trial assessed infant feeding using three separate time frames; in the last 24 hours, in the last week and since delivery (Chapman 2013).
Definitions of exclusive breastfeeding varied across the trials. One trial classified infants supplemented with vitamins, mineral supplements and water as exclusively breastfeeding (Carlsen 2013), while two other trials classified infants receiving water, juice, tea or any other liquids as exclusively breastfeeding (Chapman 2013; Martin 2015). Two trials defined the discontinuation of exclusive breastfeeding as the time the baby was offered anything other than breast milk any time after delivery (Rasmussen 2011b), or after discharge from hospital (Rasmussen 2011a). One trial defined stopping exclusive breastfeeding as the time an infant was first given formula milk (Stuebe 2016). One trial defined any breastfeeding, as breastfeeding supplemented with formula milk or solid food (Carlsen 2013). One trial documented breastfeeding according to the WHO definitions, exclusive breastfeeding was not specifically defined, however, any breastfeeding was taken as including low partial breastfeeding or more, but to not include token breastfeeding (Reifsnider 2018).
Secondary outcomes
One trial reported feeding intention using a Feeding Intentions Scale, which was assessed at baseline (Stuebe 2016). Three of the seven trials collected data on maternal postpartum weight outcomes (Martin 2015; Reifsnider 2018; Stuebe 2016). One of these trials collected data on maternal weight, BMI, weight retention and fasting biochemical data at three and six months (Martin 2015), one measured change in fasting glucose and maternal weight from enrolment to 10 months postpartum (Stuebe 2016), and the final one measured maternal BMI at six and 12 months although it was not clear which of these time points was reported.
Only one trial reported infant health outcomes at three and six months including otitis media, hospitalisation, and diarrhoea (Chapman 2013). Three trials measured data on infant weight gain, length, head circumference, skinfold thickness and abdominal circumferences, one at three and six months postpartum (Martin 2015), one at six months postpartum (Carlsen 2013), and the final one at one week, six, 12, 18, 24 and 36 months (Reifsnider 2018); however none of the trials reported the data as infant weight gain from birth. Only one trial reported infant mortality as one infant in the intervention group died shortly after birth (Stuebe 2016).
None of the included trials reported maternal satisfaction with care, maternal satisfaction with feeding method, maternal nipple health or cost‐effectiveness of the intervention.
Declarations of interest in trial reports
All of the publications clearly stated that there were no conflicts of interest.
Sources of trial funding
Funding was provided by the National Institutes of Health in three trials, all of whom stated that the funding body had no role in the trial design or analysis (Chapman 2013; Reifsnider 2018; Stuebe 2016). Chapman 2013 also received funding from the Donaghue Medical Research Foundation. A United States Department of Agriculture (USDA) Hatch grant funded two trials (Rasmussen 2011a; Rasmussen 2011b). A further trial was supported by multiple sources; the Hvidovre Hospital, Copenhagen University, Johannes Fogs Fond, and Dagmar Marshals Fond (Carlsen 2013). Manufacturing companies provided breastfeeding equipment in two trials (Rasmussen 2011b; Stuebe 2016). One trial did not report the source of funding (Martin 2015).
Excluded studies
We excluded fIve trials (see Characteristics of excluded studies). Two trials were ineligible due to population, as they classified women as overweight or obese according to postnatal BMI, not pre‐pregnancy BMI (NCT03640104; Nicklas 2014). Also, the intervention in NCT03640104 was a dietary intervention in women who were breastfeeding, not an intervention to support breastfeeding. We excluded DRKS00012842 due to the intervention being nutritional education around malnutrition, anaemia and vitamin A, and a dietary intervention not breastfeeding support. One trial was ineligible for inclusion as it compared a lifestyle plus breastfeeding support intervention against a lifestyle intervention, rather than against a control group (Lewkowitz 2018). We excluded one trial due to trial design, as it was observational in nature (Chapman 2016).
Risk of bias in included studies
2.
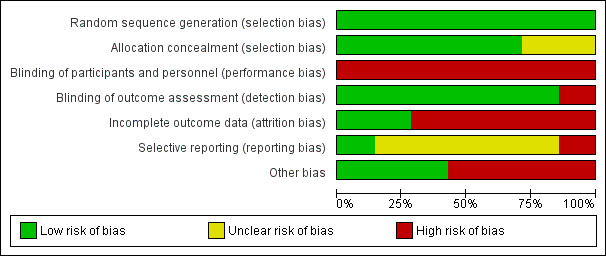
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.
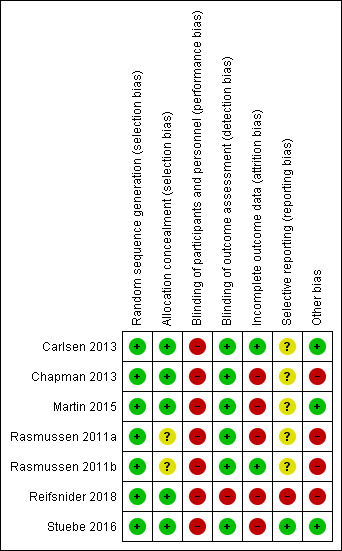
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Random sequence generation
We judged all seven trials to be at low risk of bias. Carlsen 2013; Chapman 2013; Martin 2015; Reifsnider 2018 and Stuebe 2016 all reported using computer‐generated randomisation sequences. The other two trials used a random number table to generate the allocation sequence (Rasmussen 2011a; Rasmussen 2011b).
Allocation concealment
We judged five trials to be at low risk of bias. Carlsen 2013 used an independent web‐based program and Martin 2015 and Reifsnider 2018 reported using sequentially numbered, opaque envelopes. Two trials used independent people to allocate participants, the trial co‐ordinator in Chapman 2013 and the project manager in Stuebe 2016. Newly recruited participants were allocated on a weekly (Chapman 2013), or monthly (Stuebe 2016), basis thus preserving allocation concealment. We judged two trials as unclear risk of bias as no methodological details were given regarding allocation concealment (Rasmussen 2011a; Rasmussen 2011b).
Blinding
Performance bias
Blinding of participants and personnel providing the intervention was not possible due to the nature of the intervention. We therefore judged all seven trials to be at high risk of performance bias, especially given that breastfeeding outcomes were self‐reported in all of the trials.
Detection bias
We judged six trials to be at low risk of detection bias. Trial staff who collected outcome data were blinded to intervention allocation in five of the trials (Carlsen 2013; Martin 2015; Rasmussen 2011a; Rasmussen 2011b; Stuebe 2016). Personnel collecting data in one trial (Chapman 2013), were not completely blinded to the intervention as they asked questions about peer counsellor visits, however care was taken to reduce the risk of bias by asking these questions at the end, after collecting other data. We judged Reifsnider 2018 to be at high risk of detection bias as, although they attempted to blind the assessor, it was noted that unblinding occurred for some participants during the trial.
Incomplete outcome data
Due to the fact that women can be followed up for many months in an intervention aimed at supporting breastfeeding, we specified that trials with 20% missing data or less at six months or trial end if prior to this would be classified as low risk. We judged two trials to be at low risk of attrition bias. Both Carlsen 2013 and Rasmussen 2011b analysed over 80% of recruited women. We judged the other five trials (Chapman 2013; Martin 2015; Rasmussen 2011a; Reifsnider 2018; Stuebe 2016) to be at high risk of bias, as loss to follow‐up exceeded 20% of women in each trial. In Rasmussen 2011a there was 22% loss to follow‐up for all breastfeeding outcomes from initiation to three months postpartum. In Martin 2015, loss to follow‐up at breastfeeding initiation was 19%, whereas for all other breastfeeding outcomes attrition exceeded 20% being 25% at three months and 31% at six months postpartum. In Stuebe 2016 attrition was 22% at six weeks postpartum, and 49% at both four months and 10 months postpartum. Reifsnider 2018 reported breastfeeding outcomes at all time points for only 68% of those originally recruited. In the final trial Chapman 2013, only 25% of the randomised women received the intervention due to post randomisation exclusions and withdrawals. By their final breastfeeding outcome at six months, attrition was 42%. In all five trials where attrition exceeded 20% attrition was even across the intervention and control groups and the reasons for exclusions and attrition were clearly described.
Selective reporting
We judged five trials to be at unclear risk of bias as we did not have access to a published protocol or trial registration (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a; Rasmussen 2011b). Under these circumstances it is very difficult to assess the risk of bias due to selective reporting.
A protocol was available for two trials (Reifsnider 2018; Stuebe 2016). Stuebe 2016 did not report all trial outcomes detailed within the protocol but clearly stated that it was focusing on breastfeeding outcomes with other outcomes to be reported in a further article. We therefore judged it to be at low risk of selective reporting. Reifsnider 2018 reported the majority of outcomes as breastfeeding versus not breastfeeding rather than as intervention versus control. We therefore judged it to be at high risk of reporting bias.
Other potential sources of bias
We judged two trials to be at high risk of bias due to lack of fidelity of the intervention. The first (Rasmussen 2011a), did not implement the intervention as planned, with only 24 out of the 40 participants receiving the scheduled antenatal phone call and only 10 out of 20 postpartum intervention phone calls completed. In the other trial (Rasmussen 2011b), three women in the control group requested and received the intervention of a manual breast pump on discharge from the hospital and all women in the control group reported to have used a pump during the early postpartum period. Furthermore within this trial one woman in the electric pump intervention group did not receive any breast pump, two participants who were randomised to receive a manual breast pump actually received an electric pump and one woman refused the hospital‐grade electric pump and used her own battery‐powered one.
Three further trials reported adherence to the intervention and we deemed them to be at low risk for this bias. Carlsen 2013 reported that intervention participants received an average of 6.9 of the intended nine consultations. Stuebe 2016 reported 85% fidelity to the protocol, with 44 out of 50 intervention group participants attending the antenatal breastfeeding group class. Chapman 2013 offered intervention participants proactive peer support and control participants had the option of reactive peer support available. A total of 77% of intervention group participants received one or more antenatal peer counsellor home visits compared to 20% of control participants and at two weeks postpartum 94% of intervention participants compared to 40% of control participants reported having a peer counsellor. This shows that the intervention had a higher amount of support than the control group.
Four trials reported no significant difference in baseline characteristics between the intervention and control groups (Carlsen 2013; Martin 2015; Rasmussen 2011a; Rasmussen 2011b). Two trials (Chapman 2013; Reifsnider 2018), reported differences in mode of delivery; with those receiving the intervention being more likely to have a vaginal delivery in Chapman 2013 and more likely to have a caesarean section in Reifsnider 2018 than those allocated to the control group. Given that delivery occurred after randomisation and after beginning the intervention, we did not consider this to be a baseline imbalance in either trial. We judged Chapman 2013 and Reifsnider 2018 to be at high risk of other bias, however, due to poor reporting. In Chapman 2013 it was noted that numbers reported for infant hospitalisation and percentages did not add up and the sample size at each time point was unclear, and in Reifsnider 2018 the number of infants breastfeeding at two months or longer was reported as 63, 64 and 68 in different parts of the report.
We noted that the sizes of groups differed substantially in Carlsen 2013, with 108 in the intervention group compared to 118 in the control group. As simple 1:1 group allocation was undertaken rather than block randomisation we deemed this to have occurred by chance. Attempts to obtain clarification from the trial authors were not successful.
Within Stuebe 2016, women who scored lower on the intention to breastfeed scale were more likely to drop out of the control group, but not from the intervention group. It was noted that this could have increased the breastfeeding rate in the control group, however as this would potentially bias the results towards the null, we judged it to be of low risk. When considering biases that can affect cluster‐randomised trials, we judged Stuebe 2016 to be low risk of recruitment bias due to clusters being randomised in one‐month blocks after recruitment. We judged it to be at low risk of bias due to baseline imbalances, as although a difference was noted in race, there was no difference in ethnicity between the intervention and control group and groups did not differ in other characteristics such as marital status, occupation, education, income or age. Furthermore, there was no reported loss of clusters, correct analyses were used as intra‐cluster correlations were accounted for within the analyses and the cluster‐randomised trial appeared comparable to the individually randomised trials.
Effects of interventions
Summary of findings for the main comparison. Physical breastfeeding support interventions (electric or manual breast pump) compared to usual care (no pump) for supporting the initiation and continuation of breastfeeding among women who are overweight or obese (comparison 1).
| Physical breastfeeding support interventions (electric or manual breast pump) compared to usual care (no pump) | ||||||
| Patient or population: women with a pre‐pregnancy BMI > 29 kg/m², who were intending to breastfeed, had no history of breast surgery, who were at least 19 years old and had a singleton fetus and were ≤ 35 weeks' gestation at enrolment to the study Setting: hospital setting in rural New York, USA (Rasmussen 2011b) Intervention: physical breastfeeding support intervention: electric or manual breast pump Comparison: usual care (no pump) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with physical breastfeeding support interventions | |||||
| Non‐initiation of breastfeeding | See comments | Outcome not reported by the trial authors | ||||
| Exclusive breastfeeding at 4‐6 weeks | Study population | RR 0.55 (0.20 to 1.51) | 34 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | ||
| 417 per 1000 | 229 per 1000 (83 to 629) | |||||
| Any breastfeeding at 4‐6 weeks | Study population | RR 0.65 (0.41 to 1.03) | 34 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | ||
| 833 per 1000 | 542 per 1000 (342 to 858) | |||||
| Exclusive breastfeeding at 6 months | See comments | Outcome not reported by the trial authors | ||||
| Any breastfeeding at 6 months | See comments | Outcome not reported by the trial authors | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 3 CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aStudy at high risk of bias due to protocol violations reported with control group receiving intervention. Downgraded for limitations in study design (risk of bias; ‐1). bOnly one study with very small sample size, low event rates and wide confidence intervals. Downgraded for very serious concerns around imprecision (‐2). cIt was not possible to blind this type of intervention, so we have not downgraded for lack of blinding.
Summary of findings 2. Multiple methods of breastfeeding support (including social, educational and/or physical support) compared to usual care for supporting the initiation and continuation of breastfeeding among women who are overweight or obese (comparison 2).
| Multiple methods of breastfeeding support (including social, educational and/or physical support) compared to usual care | ||||||
| Patient or population: pregnant or lactating women who were overweight or obese Setting: hospital settings in Denmark (Carlsen 2013), the USA (Chapman 2013; Rasmussen 2011a; Stuebe 2016), and Australia (Martin 2015) Intervention: multiple methods of breastfeeding support including social, educational and/or physical support Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with multiple methods of breastfeeding support | |||||
| Non‐initiation of breastfeeding | Study population | RR 1.03 (0.07 to 16.11) | 380 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | ||
| 5 per 1000 | 5 per 1000 (0 to 84) | |||||
| Exclusive breastfeeding at 4‐6 weeks | Study population | RR 1.21 (0.83 to 1.77) | 445 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d,e | ||
| 412 per 1000 | 498 per 1000 (342 to 729) | |||||
| Any breastfeeding at 4‐6 weeks | Study population | RR 1.04 (0.57 to 1.89) | 103 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d,f,g | ||
| 731 per 1000 | 760 per 1000 (417 to 1000) | |||||
| Exclusive breastfeeding at 6 months | Study population | RR 7.23 (0.38 to 137.08) | 120 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,h | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Any breastfeeding at 6 months | Study population | RR 1.42 (1.08 to 1.87) | 223 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c,i | ||
| 396 per 1000 | 563 per 1000 (428 to 741) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded (‐1) for limitations in study design (risk of bias ‐ most trials high risk for attrition bias). bDowngraded (‐2) for very serious concerns around imprecision (wide confidence interval crossing the line of no effect and few events). cIt was not possible to blind this type of intervention, so we have not downgraded for lack of blinding. dSubstantial heterogeneity. Downgraded for serious concerns around inconsistency (‐1). eDowngraded (‐1) for serious concerns around imprecision (wide confidence intervals crossing the line of no effect). fDowngraded (‐2) for very serious concerns around imprecision (wide confidence intervals crossing the line of no effect, small sample size). gDowngraded (‐2) for limitations in study design (risk of bias ‐ all trials high risk for attrition bias, and protocol violations ‐ control group receiving intervention and interventions not being received on a large scale in one of the two trials reporting this outcome). hDowngraded (‐2) for very serious concerns around imprecision (single study, small sample size, with few events, and wide confidence intervals crossing the line of no effect).
iDowngraded (‐2) for very serious concerns around imprecision (wide confidence intervals, small sample size).
We analysed trials within two comparisons. Comparison 1: physical breastfeeding support interventions versus usual care and Comparison 2: multiple methods of breastfeeding support versus usual care. We did not find any trials that compared educational intervention versus usual care or social support intervention versus usual care, or that compared different types of intervention against each other.
Comparison 1. Physical breastfeeding support (manual or electric breast pump) versus usual care (no pump)
With just one small trial (Rasmussen 2011b), involving 39 women, included under this comparison, meta‐analysis was not possible. This was a trial with two intervention arms (electric breast pump, manual breast pump) and a usual care (no breast pump) control. For the purposes of this review we have combined data from both 'pump' groups.
Our GRADE assessments are presented in Table 1.
Primary outcomes
Non‐initiation of breastfeeding
Non‐initiation of breastfeeding is defined as the baby not being put to the breast or being given any of the mother's breast milk within 48 hours of delivery (NHS England 2014).
Rasmussen 2011b did not report this outcome.
Exclusive breastfeeding at four to six weeks ‐ as defined by the trial authors
We do not know whether physical breastfeeding support (in the form of an electric or manual breast pump) improves or reduces the rate of exclusive breastfeeding at four to six weeks compared to usual care (no pump) because the certainty of this evidence is very low (risk ratio (RR) 0.55, 95% confidence interval (CI) 0.20 to 1.51; 1 trial, 34 women; Analysis 1.1).
1.1. Analysis.
Comparison 1 Physical breastfeeding support interventions versus usual care, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
Any breastfeeding at four to six weeks
We are unclear about the effect of physical breastfeeding support (manual or electric pump) versus usual care (no pump) on the rate of any breastfeeding at four to six weeks due to very low‐certainty evidence (RR 0.65, 95% CI 0.41 to 1.03; 1 trial, 34 women; Analysis 1.2).
1.2. Analysis.
Comparison 1 Physical breastfeeding support interventions versus usual care, Outcome 2 Any breastfeeding at 4‐6 weeks.
Exclusive breastfeeding at six months ‐ as defined by the trial authors
Rasmussen 2011b did not report this outcome.
Any breastfeeding at six months
Rasmussen 2011b did not report this outcome.
Secondary outcomes
Intention to breastfeed
Rasmussen 2011b had intention to breastfeed as one of their trial inclusion criteria so we could not assess this outcome.
Exclusive breastfeeding at one week, two weeks, two, three, four months ‐ as defined by trial authors
We are unclear whether a physical support intervention (electric or manual breast pump) compared to usual care (no pump) improves or reduces the rate of exclusive breastfeeding at one week (RR 0.55, 95% CI 0.30 to 0.99; 1 trial, 34 women; Analysis 1.3) because the certainty of the evidence is very low. Rasmussen 2011b did not measure exclusive breastfeeding at any other time point.
1.3. Analysis.
Comparison 1 Physical breastfeeding support interventions versus usual care, Outcome 3 Exclusive breastfeeding.
Any breastfeeding at two weeks, two, three, four, six, nine and 12 months
There was no evidence of a difference between the intervention and control group in rate of any breastfeeding at three months (RR 0.55, 95% CI 0.28 to 1.08; 1 trial, 34 women; Analysis 1.4). Rasmussen 2011b did not measure rate of any breastfeeding at any other time postpartum.
1.4. Analysis.
Comparison 1 Physical breastfeeding support interventions versus usual care, Outcome 4 Any breastfeeding.
Duration of exclusive breastfeeding ‐ as defined by the trial authors
Rasmussen 2011b reported continuous data for the duration of exclusive breastfeeding but presented these data as medians and interquartile range (IQR), which prohibits further analysis (see Analysis 1.5).
1.5. Analysis.
Comparison 1 Physical breastfeeding support interventions versus usual care, Outcome 5 Duration (median weeks) of exclusive breastfeeding.
| Duration (median weeks) of exclusive breastfeeding | ||||
|---|---|---|---|---|
| Study | Physical intervention [median (IQR)] | Total | Usual care [median (IQR)] | Total |
| Rasmussen 2011b | Manual pump 2.3 (0.4 ‐ 4.4) weeks Electric pump 0.7 (0.1 ‐ 2.7) weeks |
9 13 |
4.4 (1.1 ‐ 9.4) weeks | 12 |
Duration of any breastfeeding
Rasmussen 2011b reported continuous data for the duration of any breastfeeding but presented these data as medians and IQR, which prohibits further analysis (see Analysis 1.6).
1.6. Analysis.
Comparison 1 Physical breastfeeding support interventions versus usual care, Outcome 6 Duration (median weeks) of any breastfeeding.
| Duration (median weeks) of any breastfeeding | ||||
|---|---|---|---|---|
| Study | Physical intervention [median (IQR)] | Total | Usual care [median (IQR)] | Total |
| Rasmussen 2011b | Manual pump 13.4 (2.1 ‐ 36.0) weeks Electric pump 4.0 (2.4 ‐ 8.4) weeks |
9 13 |
26.6 (9.4 ‐ 44.6) weeks | 12 |
Other secondary outcomes
Rasmussen 2011b did not report any of the following secondary outcomes listed in our methods.
Maternal postpartum weight retention at two, three, four, six, nine and 12 months
Maternal postpartum BMI at two, three, four, six, nine and 12 months
All‐cause infant or neonatal morbidity ‐ as reported by trial authors, for example, neonatal hypoglycaemia, low weight gain, infections
All‐cause infant or neonatal mortality
Infant weight gain at two, three, four, six, nine and 12 months
Maternal satisfaction with care
Maternal satisfaction with feeding method
Maternal nipple health ‐ as defined by trial authors, for example, cracked nipples, sore nipples
Cost‐effectiveness of the intervention
Comparison 2. Multiple methods of breastfeeding support versus usual care
Our GRADE assessments are presented in Table 2.
Six trials (involving 792 women) used multiple methods of support including education and social support through telephone or face‐to‐face contact. One of these trials (Chapman 2013), also provided physical support through providing a breast pump and a baby sling, and one trial (Reifsnider 2018), provided a small gift to the women at each trial visit. Four trials used a professional to provide support (Carlsen 2013; Martin 2015; Rasmussen 2011a; Stuebe 2016); Chapman 2013 used a peer supporter; and Reifsnider 2018 used a community worker. One trial (Stuebe 2016), provided group support, with the other five trials supporting women individually (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a; Reifsnider 2018). For more information about the interventions used by the trials under this comparison, please refer to Included studies and Characteristics of included studies. One trial (Reifsnider 2018; 174 women), did not report on any of our main outcomes of interest.
Primary outcomes
Non‐initiation of breastfeeding
Non‐initiation of breastfeeding is defined as the baby not being put to the breast or being given any of the mother's breast milk within 48 hours of delivery (NHS England 2014).
We do not know whether multiple methods of breastfeeding support (including social, educational or physical support) improves or reduces the incidence of non‐initiation of breastfeeding compared to usual care because the certainty of this evidence is very low (average RR 1.03, 95% CI 0.07 to 16.11; 3 trials, 380 women; Analysis 2.1).
2.1. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 1 Non‐initiation of breastfeeding.
Exclusive breastfeeding at four to six weeks ‐ as defined by the trial authors
We are unclear whether multiple methods of breastfeeding support (including social, educational or physical support) compared to usual care improves or reduces the rate of exclusive breastfeeding at four to six weeks (RR 1.21, 95% CI 0.83 to 1.77; 4 trials, 445 women; Analysis 2.2) because the certainty of the evidence is very low. We observed substantial heterogeneity in this analysis (heterogeneity: Tau² = 0.09, I² = 59%, Chi² for heterogeneity P = 0.06).
2.2. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 2 Exclusive breastfeeding at 4‐6 weeks.
Any breastfeeding at four to six weeks
We found very low‐certainty evidence for the rate of any breastfeeding at four to six weeks, which means we are unclear about whether the interventions improve or reduce this outcome (RR 1.04, 95% CI 0.57 to 1.89; 2 trials, 103 women; Analysis 2.3). We observed substantial heterogeneity in this analysis (heterogeneity: Tau² = 0.16, I² = 86%, Chi² for heterogeneity P = 0.008).
2.3. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 3 Any breastfeeding at 4‐6 weeks.
Exclusive breastfeeding at six months (as defined by the trial authors)
Very low‐certainty evidence from one small trial (120 women) means that we are uncertain about the effects of the intervention on the rate of exclusive breastfeeding at six months (RR 7.23, 95% CI 0.38 to 137.08; 1 trial, 120 women; Analysis 2.4).
2.4. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 4 Exclusive breastfeeding at 6 months.
Any breastfeeding at six months
Two trials (223 women) reported data on the rate of any breastfeeding at six months. The evidence was very low certainty, which means that we are unclear about the effect of the intervention on this outcome (RR 1.42, 95% CI 1.08 to 1.87; 2 trials, 223 women; I² 0%; Analysis 2.5).
2.5. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 5 Any breastfeeding at 6 months.
Secondary outcomes
Intention to breastfeed
Four out of the five trials using multiple methods of breastfeeding support (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a), had 'intention to breastfeed' as one of their trial inclusion criteria. One trial (Stuebe 2016), assessed breastfeeding intention at baseline using the Infant Feeding Intentions (IFI) Scale. IFI scores were similar in the breastfeeding support and the control group (mean ± standard deviation (SD) 11.4 ± 4.2 versus 11.3 ± 4.8).
Exclusive breastfeeding
Very low‐certainty evidence means that we are unclear about the effects of the intervention on the rate of exclusive breastfeeding at the following time points:
at one week (average RR 1.03, 95% CI 0.60 to 1.76; 2 trials, 244 women); we observed substantial heterogeneity (Tau² = 0.13, I² = 87%, Chi² for heterogeneity P = 0.006));
at two weeks (average RR 1.30, 95% CI 1.09 to 1.54; 2 trials, 361 women; I² = 0%);
at two months (RR 0.93, 95% CI 0.53 to 1.64; 1 trial, 133 women);
at three months (average RR 1.42, 95% CI 1.12 to 1.81; 3 trials, 344 women; I² = 0%); and
at four months (RR 1.52, 95% CI 0.51 to 4.53; 1 trial, 119 women).
Data are presented in Analysis 2.6.
2.6. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 6 Exclusive breastfeeding.
Any breastfeeding
Very low‐certainty evidence means that we are unclear about the effects of the intervention on the rate of any breastfeeding at the following time points:
at two weeks (RR 1.12, 95% CI 1.00 to 1.26; 1 trial, 154 women);
at two months (RR 1.17, 95% CI 0.94 to 1.44; 2 trials, 252 women; I² 0%);
at three months (RR 0.87, 95% CI 0.29 to 2.61; 2 trials, 57 women); we observed substantial heterogeneity (Tau² = 0.54, I² = 85%, Chi² for heterogeneity P = 0.010);
at four months (RR 1.35, 95% CI 1.05 to 1.72; 1 trial, 207 women).
Data are presented in Analysis 2.7.
2.7. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 7 Any breastfeeding.
No data were available for rates of any breastfeeding at nine months or 12 months.
Duration of exclusive breastfeeding
Two trials (Carlsen 2013; Rasmussen 2011a), presented continuous data for duration of exclusive breastfeeding. However, they reported these data as medians and IQR because data were not normally distributed. We were unable to include these data in meta‐analysis and data are presented in (Analysis 2.8) for information.
2.8. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 8 Duration (median weeks) of exclusive breastfeeding.
| Duration (median weeks) of exclusive breastfeeding | ||||
|---|---|---|---|---|
| Study | Support [median (IQR)] | Total | Usual care [median (IQR)] | Total |
| Carlsen 2013 | 17.1 (2.0 ‐ 20.3) weeks | 105 | 5.9 (0.4 ‐ 19.0) weeks | 102 |
| Rasmussen 2011a | 3.4 (0.7 ‐ 8.4) weeks | 19 | 8.1 (2.1 ‐ 13.1) weeks | 18 |
Duration of any breastfeeding
Three trials presented continuous data for duration of any breastfeeding but meta‐analysis was not possible. Two trials (Carlsen 2013; Rasmussen 2011a), presented continuous data for duration of any breastfeeding. However, they reported these data as medians and IQR because data were not normally distributed. We report the duration (median week) of any breastfeeding for information only (Analysis 2.10). One very small trial (Martin 2015), provided outcome data as mean and standard deviations (mean difference (MD) 2.30 weeks, 95% CI −7.79 to 12.39; 1 trial, 16 women; very low‐certainty evidence) and these data are presented in Analysis 2.9.
2.10. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 10 Duration (median weeks) of any breastfeeding.
| Duration (median weeks) of any breastfeeding | ||||
|---|---|---|---|---|
| Study | Support [median (IQR)] | Total | Usual care [median (IQR)] | Total |
| Carlsen 2013 | 26.3 (13.1 ‐ 26.4) weeks | 105 | 15.4 (2.3 ‐ 26.4) weeks | 102 |
| Rasmussen 2011a | 8.6 (3.9 ‐ 13.0) weeks | 19 | 12.9 (9.1 ‐ 13.5) weeks | 20 |
2.9. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 9 Duration of any breastfeeding (mean number of weeks).
Maternal postpartum weight retention
One very small trial (Martin 2015), reported maternal weight retention at three and six months postpartum compared to pre‐pregnancy weight at three months (MD −3.30, 95% CI −10.22 to 3.62; 1 trial, 18 women) and at six months (MD −0.30, 95% CI −7.56 to 6.96; 1 trial, 16 women). Data are presented in Analysis 2.11.
2.11. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 11 Maternal postpartum weight retention (mean number of kilograms).
Maternal postpartum BMI
One trial (Martin 2015), reported maternal BMI at three months postpartum (MD −0.50, 95% CI −5.12 to 4.12; 1 trial, 18 women) and at six months postpartum (MD 0.90, 95% CI −2.90 to 4.70; 1 trial, 16 women). Data are presented in Analysis 2.12).
2.12. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 12 Maternal postpartum BMI (mean kg/m²).
All‐cause infant or neonatal morbidity
None of the trials reported on neonatal morbidity but one trial (Chapman 2013), reported the risk of hospitalisation at three months (intervention: 6/57 versus control: 16/62) and six months (6/55 versus 15/53). These data are presented in Analysis 2.13 for information.
2.13. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 13 All cause neonatal or infant morbidity.
All‐cause infant or neonatal mortality
One trial reported this outcome (Stuebe 2016). There was one death in the intervention group. Data are presented in Analysis 2.14.
2.14. Analysis.
Comparison 2 Multiple methods of breastfeeding support versus usual care, Outcome 14 All cause neonatal or infant mortality.
Secondary outcomes not reported in any of the included trials
None of the included trials reported data for the following secondary outcomes.
Infant weight gain
Maternal satisfaction with care
Maternal satisfaction with feeding method
Maternal nipple health
Cost effectiveness of the intervention
Subgroup analysis
There was variation between trials using multiple methods of breastfeeding support versus usual care in intervention delivery, the type of usual care provided to women in the control group, the setting in which the intervention was undertaken, background breastfeeding rates, and the presence of co‐morbidities. There was substantial heterogeneity for the primary outcomes of exclusive breastfeeding at four to six weeks and any breastfeeding at four to six weeks. As per our methods, we planned to explore high levels of heterogeneity through subgroup analysis and whilst we present data and analysis tables detailing data stratified by various subgroups, we have insufficient data to perform meaningful subgroup analyses at this time. Where available, we have presented data as subgroups in Analyses 3 through to 12 for information only, and to inform future updates.
BMI category of the women recruited (see Analysis 3.1; Analysis 3.2)
Who provided the support (see Analysis 4.1; Analysis 4.2)
Type of intervention delivery (Analysis 5.1; Analysis 5.2; Analysis 6.1; Analysis 6.2)
Timing of the intervention (Analysis 7.1; Analysis 7.2)
Setting of the intervention (see Analysis 8.1; Analysis 8.2)
Intensity of the intervention (see Analysis 9.1; Analysis 9.2)
Socioeconomic status of the population (see Analysis 10.1; Analysis 10.2)
Gestational age of the infant at birth (see Analysis 11.1; Analysis 11.2)
Presence of co‐morbidities (see Analysis 12.1; Analysis 12.2)
3.1. Analysis.
Comparison 3 Multiple methods of breastfeeding support versus usual care: subgroup analysis by BMI category, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
3.2. Analysis.
Comparison 3 Multiple methods of breastfeeding support versus usual care: subgroup analysis by BMI category, Outcome 2 Any breastfeeding at 4‐6 weeks.
4.1. Analysis.
Comparison 4 Multiple methods of breastfeeding support versus usual care: subgroup analysis by intervention provider, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
4.2. Analysis.
Comparison 4 Multiple methods of breastfeeding support versus usual care: subgroup analysis by intervention provider, Outcome 2 Any breastfeeding at 4‐6 weeks.
5.1. Analysis.
Comparison 5 Multiple methods of breastfeeding support versus usual care: subgroup analysis by type of intervention, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
5.2. Analysis.
Comparison 5 Multiple methods of breastfeeding support versus usual care: subgroup analysis by type of intervention, Outcome 2 Any breastfeeding at 4‐6 weeks.
6.1. Analysis.
Comparison 6 Multiple methods of breastfeeding support versus usual care: subgroup analysis by type of support, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
6.2. Analysis.
Comparison 6 Multiple methods of breastfeeding support versus usual care: subgroup analysis by type of support, Outcome 2 Any breastfeeding at 4‐6 weeks.
7.1. Analysis.
Comparison 7 Multiple methods of breastfeeding support versus usual care: subgroup analysis by timing of intervention, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
7.2. Analysis.
Comparison 7 Multiple methods of breastfeeding support versus usual care: subgroup analysis by timing of intervention, Outcome 2 Any breastfeeding at 4‐6 weeks.
8.1. Analysis.
Comparison 8 Multiple methods of breastfeeding support versus usual care: subgroup analysis by intervention setting, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
8.2. Analysis.
Comparison 8 Multiple methods of breastfeeding support versus usual care: subgroup analysis by intervention setting, Outcome 2 Any breastfeeding at 4‐6 weeks.
9.1. Analysis.
Comparison 9 Multiple methods of breastfeeding support versus usual care: subgroup analysis by intensity of the intervention, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
9.2. Analysis.
Comparison 9 Multiple methods of breastfeeding support versus usual care: subgroup analysis by intensity of the intervention, Outcome 2 Any breastfeeding at 4‐6 weeks.
10.1. Analysis.
Comparison 10 Multiple methods of breastfeeding support versus usual care: subgroup analysis by socioeconomic status, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
10.2. Analysis.
Comparison 10 Multiple methods of breastfeeding support versus usual care: subgroup analysis by socioeconomic status, Outcome 2 Any breastfeeding at 4‐6 weeks.
11.1. Analysis.
Comparison 11 Multiple methods of breastfeeding support versus usual care: subgroup analysis by gestational age, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
11.2. Analysis.
Comparison 11 Multiple methods of breastfeeding support versus usual care: subgroup analysis by gestational age, Outcome 2 Any breastfeeding at 4‐6 weeks.
12.1. Analysis.
Comparison 12 Multiple methods of breastfeeding support versus usual care: subgroup analysis by co‐morbidities, Outcome 1 Exclusive breastfeeding at 4‐6 weeks.
12.2. Analysis.
Comparison 12 Multiple methods of breastfeeding support versus usual care: subgroup analysis by co‐morbidities, Outcome 2 Any breastfeeding at 4‐6 weeks.
Sensitivity analysis
There were insufficient trials to carry out a sensitivity analysis for risk of bias because we classified only one included trial as high quality due to being at low risk of bias for random sequence generation, allocation concealment and attrition bias (Carlsen 2013).
As we used an estimated ICC to adjust outcomes for the cluster‐randomised trial (Stuebe 2016), we undertook a sensitivity analysis (analyses not listed in the Data and analysis section) to investigate the effect of using differing ICC from other cluster‐randomised breastfeeding interventions. We initially adjusted the sample size for unadjusted outcomes using the ICC of 0.02 reported in Kronborg 2007. We undertook the sensitivity analysis using varying ICC from 0.005 up to 0.07 (Hoddinott 2009; MacArthur 2009). We noted minimal impact on the magnitude of the intervention effect and confidence intervals, and no effect on the direction of the effect for all main analyses in which Stuebe 2016 contributed data (i.e. Analysis 2.2; Analysis 2.3; Analysis 2.14).
Discussion
Summary of main results
This review evaluated evidence on the effect of interventions aimed at supporting the initiation and continuation of breastfeeding in women who are overweight or obese. The review included seven trials published between 2011 and 2018. We found no trials comparing one type of support versus another. We included seven RCTs (including one cluster‐RCT) involving 831 women and the trials were conducted in high‐income countries, USA (5 trials): 5; Denmark (1 trial) and Australia (1 trial), between 2006 and 2015. Sample sizes were generally small, the number of women in each trial ranged from 36 to 226. Three trials only included women who were obese prior to pregnancy and four trials included both women who were overweight and women who were obese.
Physical breastfeeding support (manual or electric breast pump) versus usual care (no breast pump)
We found evidence from one small trial (39 women) looking at a physical support intervention (manual or electric breast pump) versus usual care (no pump) that reported data on two of this review's main outcomes: exclusive breastfeeding at four to six weeks, and any breastfeeding at four to six weeks. Very low‐certainty evidence means we are unclear about the effect of the intervention on these important outcomes. No data were reported for the other main outcomes of interest in this review: non‐initiation of breastfeeding, exclusive or any breastfeeding at six months postpartum. The trial reported on very few of the secondary outcomes of interest in this review. Meta‐analysis was not possible as there was only one trial under this comparison.
Multiple methods of breastfeeding support versus usual care
Six trials (involving 792 women) used multiple methods of support including education and social support through telephone or face‐to‐face contact. One of these trials also provided physical support through providing a breast pump and a baby sling, and one trial provided a small gift to the women at each trial visit. Support in the trials was provided by a professional (four trials) or a peer (two trials). One trial provided group support, with the other five trials supporting women individually. One trial (174 women) did not report on any of our main outcomes of interest.
We are unclear about the effects of the intervention because we identified very low‐certainty evidence for all of the important outcomes in this review: rate of non‐initiation of breastfeeding (3 trials, 380 women); exclusive breastfeeding at four to six weeks (4 trials, 445 women); any breastfeeding at four to six weeks (2 trials, 103 women); rate of exclusive breastfeeding at six months postpartum (1 trial, 120 women); and any breastfeeding at six months postpartum (2 trials, 223 women).
We observed substantial statistical heterogeneity for exclusive breastfeeding at four to six weeks and for any breastfeeding at four to six weeks. We planned to further investigate substantial heterogeneity through subgroup analysis and whilst we do present data stratified for available subgroups, we have insufficient data to carry out any meaningful subgroup analyses.
The included trials under the above comparisons also reported on some of this review's secondary outcomes but very low‐certainty evidence means that we are unclear about the effects of the intervention on those outcomes.
Overall completeness and applicability of evidence
The interventions offered across the trials were very diverse, with physical support only through the provision of a breast pump in one trial (Rasmussen 2011b), and multiple methods of support in the other trials (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a; Reifsnider 2018; Stuebe 2016).
Within the trials providing multiple methods of support, all provided education and social support, one trial (Chapman 2013), also provided physical support through the use of breast pumps and a breastfeeding sling, and another trial provided incentives (Reifsnider 2018). Five trials delivered the intervention in the antenatal period and postnatal period (Chapman 2013; Martin 2015; Rasmussen 2011a; Reifsnider 2018; Stuebe 2016), and the other trial in the postnatal period only (Carlsen 2013). Social support included face‐to‐face support in some trials (Chapman 2013; Martin 2015; Reifsnider 2018; Stuebe 2016), and remote support through telephone or text messages (Carlsen 2013; Chapman 2013; Martin 2015; Rasmussen 2011a; Stuebe 2016). In three trials the breastfeeding support was part of a lifestyle intervention that included dietary as well as breastfeeding components (Martin 2015; Reifsnider 2018; Stuebe 2016). Standard care provided to the control groups also varied between trials, with extensive background support provided in some settings. For example Chapman 2013 examined the impact of specialised breastfeeding peer counsellors as the intervention, but their control group were also offered optional peer support during the antenatal period, while in hospital and postnatally. In total, 87% of women in the control group were noted to have received peer support in hospital and 40% to have had a postnatal peer supporter at two weeks postpartum. It is not surprising therefore that the intervention showed no effect over and above standard care given the extensive support provided to the standard care group. Given the large variations in the format of standard care provided and in the support provided through the intervention, limited conclusions can be drawn from the currently included trials about the effectiveness and types of interventions to support women who are overweight or obese.
The trials had numerous differing end points from three months to 12 months post‐delivery. The period of time between the end of the intervention and the final trial measure also varied. In two trials the intervention carried on until the final breastfeeding outcome was measured at six months (Carlsen 2013; Chapman 2013), while in others the intervention lasted for two weeks with the final breastfeeding measure taken at three months (Rasmussen 2011a; Rasmussen 2011b), or six months (Martin 2015). Caution is therefore required in the interpretation of pooled data.
Many of the included trials were small in size ‐ with three trials recruiting 50 women or fewer. Combined with poor adherence within the intervention and control groups within several trials, the evidence in this review is very limited. The wider applicability of the results is also questioned given that five of the seven currently published trials are from the USA and all included trials were from high‐income countries.
Five of the included trials only recruited women who intended to breastfeed as their aims were to enhance the length of breastfeeding. This limits the applicability of our review findings to assess ways in which breastfeeding initiation could be improved in women who are overweight or obese.
It is important to understand the theoretical background and behavioural‐change techniques that underpin breastfeeding promotional interventions. This can be done by reporting the specific behaviour‐change techniques incorporated in interventions using taxonomies such as Michie 2013. Reporting of the theoretical basis of interventions is important for both the replicability of the trials and to increase the understanding of which specific components of complex interventions may be effective at improving initiation and continuation of breastfeeding in women who are overweight or obese. While the theoretical basis of interventions is not included in this current review it is an important aspect to consider for future updates.
Quality of the evidence
We judged the overall quality of the included trials to be mixed.
Risk of bias
All seven trials were low risk of bias for random sequence generation and five trials for group allocation concealment. There was therefore a relatively low risk of selection bias within the trials. We judged only two trials to be low risk for attrition bias with many trials having incomplete data due to loss of follow‐up, especially for longer‐term breastfeeding outcomes. Overall, we deemed only one included trial to be at low risk of bias in all three areas of random sequence generation, allocation concealment and attrition bias. Due to the nature of the intervention blinding of the participants was not possible in any of the trials, with all trials assessed to be at high risk of performance bias. Rather than being seen as a weakness of the included trials this should be recognised as a limitation of this type of intervention. We considered all but one trial to be at low risk of detection bias, however given the lack of participant blinding and that breastfeeding outcomes were self‐reported in all trials, response bias was likely. Selective reporting was difficult to assess in many of the trials due to the lack of availability of trial protocols.
GRADE assessments
Assessing the body of evidence using the GRADE approach, the overall quality of the evidence for the primary outcomes included in the comparison physical breastfeeding support versus usual care was very low. We downgraded the certainty of evidence for exclusive breastfeeding at four to six weeks, and any breastfeeding at four to six weeks, due to limitations in trial design (risk of bias) and imprecision. We did not downgrade any outcomes for lack of blinding due to the nature of the intervention making blinding not feasible. Non‐initiation of breastfeeding, exclusive breastfeeding at six months, and any breastfeeding at six months, were not reported. See Table 1.
For the comparison multiple methods of support versus usual care, we graded the overall certainty of evidence as very low for all of the outcomes assessed: non‐initiation of breastfeeding; exclusive breastfeeding at four to six weeks; any breastfeeding at four to six weeks; exclusive breastfeeding at six months; any breastfeeding at six months. We did not downgrade any outcomes for lack of blinding due to the nature of the intervention making blinding not feasible. Downgrading decisions were based on limitations in trial design, imprecision, and inconsistency. See Table 2.
Potential biases in the review process
Bias can potentially be introduced at any stage of the review process. To minimise this, two review authors independently assessed eligibility for inclusion, carried out data extraction and assessed risk of bias, with disagreements resolved by discussion with the third review author.
Agreements and disagreements with other studies or reviews
Related Cochrane Reviews have examined education and support interventions to promote breastfeeding in healthy women without any specific health problems. Their findings suggest that education and support interventions by professional and peer supporters may increase the rate of breastfeeding initiation and increase the duration of exclusive and any breastfeeding (Balogun 2016; McFadden 2017). Whilst our review identified outcome data for duration of exclusive and any breastfeeding, the evidence is very low certainty, which means we are unclear about the findings in this review. Furthermore, the target group of women in this review were overweight or obese and therefore may face additional challenges to initiate and continue breastfeeding.
Other research suggests that adherence to Baby Friendly Initiative Principles has been found to have a positive impact on short‐, medium‐ and long‐term outcomes (Pérez‐Escamilla 2016). In Analysis 8.1 and Analysis 8.2, we compared the available data, stratified by intervention setting (BFI‐accredited institution, non‐BFI‐accreditated institution, or unknown BFI accreditation status) but we currently have insufficient data to carry out meaningful subgroup analysis.
The Cochrane Review into breastfeeding interventions to support healthy mothers and healthy, term infants (McFadden 2017) found that face‐to‐face interventions were more effective than telephone‐based support. In Analysis 5.1 and Analysis 5.2, we present the available data for our review, stratified by type of support (face‐to‐face support versus remote support) but we currently have insufficient data to carry out meaningful subgroup analysis.
Authors' conclusions
Implications for practice.
We found limited evidence to inform this review. We identified seven randomised controlled trials (RCTs) involving 831 women, but one trial did not report our main outcomes of interest. We assessed the available evidence as very low certainty, which means that the effectiveness of physical interventions, or multiple methods of support (social, educational or physical) for supporting the initiation or continuation of breastfeeding in women who are overweight or obese remains unclear. We found no RCTs comparing one type of support to another type of support. All of our GRADE assessments resulted in very low‐certainty evidence, with downgrading decisions based on limitations in trial design (e.g. risk of attrition bias), imprecision, inconsistency. The available trials were mostly of variable quality with small numbers of participants, confounded by poor adherence within both the intervention and control groups.
Implications for research.
Well designed, adequately powered research is needed to answer questions about social, educational or physical support, or any combination of these interventions that could potentially help mothers who are overweight or obese to achieve optimal breastfeeding outcomes. Trials are needed that examine interventions designed specifically for women who are overweight or obese and delivered by people with training about how to overcome some of the challenges these women face when establishing and maintaining breastfeeding. Particular attention should be given to the assessment of antenatal interventions aimed at improving breastfeeding initiation in women with a raised body mass index and not just focusing on recruiting women who have an intention to breastfeed. Given that the majority of current trials were undertaken in the USA, a diverse range of countries and settings are required. Future trials need to give consideration to the theoretical basis of the intervention using frameworks such as Michie 2013 to enable replicability by others and to better determine the components of effective interventions.
Acknowledgements
We would like to thank the trial authors (Rasmussen 2011a; Rasmussen 2011b), for responding to our queries regarding their trials. Stuebe 2016 did reply to our enquiry and was hoping to provide further information but no further information was subsequently provided. We had an initial reply from Reifsnider 2018 who provided another person to contact for further information but we did not receive a reply from the additional contact. We attempted to contact the following trial authors but did not receive a reply: Carlsen 2013; Chapman 2013; Martin 2015.
As part of the prepublication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewer for her time and comments: Dr Genevieve Becker, Independent researcher and programme consultant, BEST Services, Galway, Ireland.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
ICTRP
breastfeeding AND obese
breastfeeding AND overweight
ClinicalTrials.gov
Advanced search
breastfeeding | Interventional Studies | Obesity
breastfeeding | Interventional Studies | Overweight
Data and analyses
Comparison 1. Physical breastfeeding support interventions versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.51] |
| 2 Any breastfeeding at 4‐6 weeks | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.03] |
| 3 Exclusive breastfeeding | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 At 1 week | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.30, 0.99] |
| 3.2 At 2 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 At 2 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 At 3 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 At 4 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Any breastfeeding | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 At 2 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 At 2 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 At 3 months | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.08] |
| 4.4 At 4 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 At 9 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 At 12 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Duration (median weeks) of exclusive breastfeeding | Other data | No numeric data | ||
| 6 Duration (median weeks) of any breastfeeding | Other data | No numeric data |
Comparison 2. Multiple methods of breastfeeding support versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐initiation of breastfeeding | 3 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.07, 16.11] |
| 2 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 3 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 4 Exclusive breastfeeding at 6 months | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 7.23 [0.38, 137.08] |
| 5 Any breastfeeding at 6 months | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.08, 1.87] |
| 6 Exclusive breastfeeding | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 At 1 week | 2 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.60, 1.76] |
| 6.2 At 2 weeks | 2 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.09, 1.54] |
| 6.3 At 2 months | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.53, 1.64] |
| 6.4 At 3 months | 3 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.12, 1.81] |
| 6.5 At 4 months | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.51, 4.53] |
| 7 Any breastfeeding | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 At 2 weeks | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.00, 1.26] |
| 7.2 At 2 months | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.94, 1.44] |
| 7.3 At 3 months | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.29, 2.61] |
| 7.4 At 4 months | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.05, 1.72] |
| 7.5 At 9 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.6 At 12 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Duration (median weeks) of exclusive breastfeeding | Other data | No numeric data | ||
| 9 Duration of any breastfeeding (mean number of weeks) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐7.79, 12.39] |
| 10 Duration (median weeks) of any breastfeeding | Other data | No numeric data | ||
| 11 Maternal postpartum weight retention (mean number of kilograms) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 At 3 months postpartum | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐3.3 [‐10.22, 3.62] |
| 11.2 At 6 months postpartum | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐7.56, 6.96] |
| 11.3 At 1 year postpartum | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal postpartum BMI (mean kg/m²) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 At 3 months postpartum | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐5.12, 4.12] |
| 12.2 At 6 months postpartum | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐2.90, 4.70] |
| 12.3 At 12 months postpartum | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 All cause neonatal or infant morbidity | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 At 3 months | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.17, 0.97] |
| 13.2 At 6 months | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.16, 0.92] |
| 14 All cause neonatal or infant mortality | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.92] |
Comparison 3. Multiple methods of breastfeeding support versus usual care: subgroup analysis by BMI category.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 1.1 Trials recruiting women who were obese | 2 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.45, 2.20] |
| 1.2 Trials recruiting women who were overweight or obese | 2 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.83, 2.42] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 Trials recruiting women who were obese | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 2.2 Trials recruiting women who were overweight or obese | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [1.04, 1.89] |
Comparison 4. Multiple methods of breastfeeding support versus usual care: subgroup analysis by intervention provider.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 1.1 Professional provider | 3 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.70, 2.12] |
| 1.2 Peer provider | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.68, 1.90] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 Professional provider | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.2 Peer provider | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Multiple methods of breastfeeding support versus usual care: subgroup analysis by type of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 1.1 Face‐to‐face support | 2 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.83, 2.42] |
| 1.2 Remote support | 2 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.45, 2.20] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 Face‐to‐face support | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [1.04, 1.89] |
| 2.2 Remote support | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
Comparison 6. Multiple methods of breastfeeding support versus usual care: subgroup analysis by type of support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 1.1 Individual support | 3 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.68] |
| 1.2 Group support | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.97, 4.06] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 Individual support | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 2.2 Group support | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [1.04, 1.89] |
Comparison 7. Multiple methods of breastfeeding support versus usual care: subgroup analysis by timing of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 1.1 Intervention in the antenatal and postnatal period | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.61, 2.02] |
| 1.2 Intervention in the postnatal period only | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.13, 1.78] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 Intervention in the antenatal and postnatal period | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.2 Intervention in the postnatal period only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Multiple methods of breastfeeding support versus usual care: subgroup analysis by intervention setting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 1.1 Baby‐Friendly Initiative accredited institution | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.68, 1.90] |
| 1.2 Non‐Baby‐Friendly Initiative accredited institution | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.18, 1.81] |
| 1.3 Unknown Baby‐Friendly Initiative accreditation status | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.34, 1.17] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 Baby‐Friendly Initiative accredited institution | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Non‐Baby‐Friendly Initiative accredited institution | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [1.04, 1.89] |
| 2.3 Unknown Baby‐Friendly Initiative accreditation status | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
Comparison 9. Multiple methods of breastfeeding support versus usual care: subgroup analysis by intensity of the intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 1.1 1‐4 scheduled visits | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.34, 1.17] |
| 1.2 5‐8 scheduled visits | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 9 or more scheduled visits | 3 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.16, 1.72] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 1‐4 scheduled visits | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 2.2 5‐8 scheduled visits | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 9 or more scheduled visits | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [1.04, 1.89] |
Comparison 10. Multiple methods of breastfeeding support versus usual care: subgroup analysis by socioeconomic status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.61, 2.02] |
| 1.1 Participants of mixed socioeconomic status | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.35, 3.47] |
| 1.2 More than 75% of participants low socioeconomic status | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.68, 1.90] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 Participants of mixed socioeconomic status | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.2 More than 75% of participants low socioeconomic status | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Multiple methods of breastfeeding support versus usual care: subgroup analysis by gestational age.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 1.1 Term infants only | 3 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.68] |
| 1.2 Preterm and term infants | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.97, 4.06] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 Term infants only | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 2.2 Preterm and term infants | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [1.04, 1.89] |
Comparison 12. Multiple methods of breastfeeding support versus usual care: subgroup analysis by co‐morbidities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding at 4‐6 weeks | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 1.1 Recruiting only participants with co‐morbidities | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.97, 4.06] |
| 1.2 Recruiting participants without medical co‐morbidities | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.68, 1.90] |
| 1.3 Recruiting participant with and without co‐morbidities | 2 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.45, 2.20] |
| 2 Any breastfeeding at 4‐6 weeks | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.89] |
| 2.1 Recruiting only participants with co‐morbidities | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [1.04, 1.89] |
| 2.2 Recruiting participants without medical co‐morbidities | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Recruiting participant with and without co‐morbidities | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
Comparison 13. Multiple methods of breastfeeding support versus usual care: sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐initiation of breastfeeding | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Studies at low risk of bias | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Exclusive breastfeeding at 4‐6 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.13, 1.78] |
| 2.1 Studies at low risk of bias | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.13, 1.78] |
| 3 Any breastfeeding at 6 months | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.03, 1.89] |
| 3.1 Studies at low risk of bias | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.03, 1.89] |
13.1. Analysis.
Comparison 13 Multiple methods of breastfeeding support versus usual care: sensitivity analysis, Outcome 1 Non‐initiation of breastfeeding.
13.2. Analysis.
Comparison 13 Multiple methods of breastfeeding support versus usual care: sensitivity analysis, Outcome 2 Exclusive breastfeeding at 4‐6 weeks.
13.3. Analysis.
Comparison 13 Multiple methods of breastfeeding support versus usual care: sensitivity analysis, Outcome 3 Any breastfeeding at 6 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carlsen 2013.
| Methods | Parallel‐group RCT to determine if telephone‐based support could improve breastfeeding outcomes in obese mothers | |
| Participants | Setting: Denmark, hospital that does not have Baby Friendly Hospital certification Recruitment: December 2010‐June 2012 Inclusion criteria: women‐infant dyads with a healthy singleton infant, born at term (> 258 days of gestation), < 48 h postnatal age. Women intending to breastfeed with no previous history of breast surgery. All women had participated in the Treatment of Obese Pregnant study (TOP‐study) at the hospital, which had inclusion criteria of pre‐pregnancy BMI ≥ 30 kg/m² and an aim to minimise gestational weight gain (i.e. gain max 5 kg). Exclusion criteria: sick infants requiring admission to NICU and infants with congenital diseases or malformations |
|
| Interventions | Breastfeeding support intervention n = 108, usual care n = 118 Telephone‐based breastfeeding support by a International Board Certified Lactation Consultant for 6 months The telephone‐based advisory support service was performed by a single International Board Certified Lactation Consultant. Contacts followed a structured design posing questions of physical and psychological aspects related to breastfeeding and well‐being of the mother and child. Advice was given if the mother was deemed to have insufficient breastfeeding knowledge. Any breastfeeding difficulties were discussed and possible solutions identified. The first contact was approximately 20 min (within the first week), follow‐up contacts were 5‐10 min (x 3 in 1st month, every other week thereafter until 8 weeks and then monthly). The direct contact number of the lactation consultant also given to the women; the lactation consultant was available 7 days/week, so there were extra contacts for specific difficulties. All participants were offered a minimum of 9 consultations in the first 6 months (or until they had ceased all breastfeeding). |
|
| Outcomes | Exclusively breastfeeding and any breastfeeding data were collected by telephone at 3 and 7 days, 2 and 4 weeks, 3, 4 and 6 months Exclusive breastfeeding was defined as breastfeeding only supplemented with vitamins, mineral supplements, or water. Partial breastfeeding was defined as breastfeeding supplemented with formula milk or solid food. Infant weight, length, head circumference and abdominal circumference measured at the level of the umbilicus were taken at birth and 6 months. In addition at 6 months triceps and subscapular skinfold thickness were measured using a Harpendens skinfold calliper. All measures (except weight) were taken in triplicate and a mean obtained. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Mother‐newborn dyads were allocated (1:1) to the intervention by telephone support or control standard care by using a web‐based independent program." |
| Allocation concealment (selection bias) | Low risk | Independent web‐based program used therefore low risk of allocation being known prior to randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants or the lactation consultant to group allocation. This could have led to performance bias and affected outcomes, as breastfeeding outcomes were self‐reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The intervention was blinded to the study staff, which collected data on breastfeeding status and infant growth. The lactation consultant was not involved in measuring infants." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women recruited accounted for in the study flow diagram. < 20% attrition rate for breastfeeding and infant growth outcomes at 6 months |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report without access to the protocol, therefore we cannot be certain whether all pre‐specified outcomes were reported. |
| Other bias | Low risk | Group sizes differed (108 vs 118) however simple randomisation was undertaken, so we judged this to have occurred by chance. |
Chapman 2013.
| Methods | Parallel‐RCT to evaluate if a specialised breastfeeding peer counselling intervention promoted exclusive breastfeeding in low‐income women who were overweight or obese | |
| Participants | Setting: USA, Baby‐Friendly accredited hospital Recruitment: May 2006‐July 2009 Inclusion criteria: women who were overweight or obese (≥ 27.0 kg/m²) according to pre‐pregnancy BMI, women who were on a low income (< 185% of the federal poverty level), were considering breastfeeding, were ≥ 18 years old, ≤ 36 weeks' gestation at enrolment, had a singleton pregnancy and an absence of medical conditions. Women were recruited who were planning to remain in the area for 6/12 months postnatally and who had telephone access Infant inclusion criteria included born at ≥ 36 weeks' gestation, birthweight ≥ 2.5 kg and ≤ 3.9 kg, 1 and 5 min Apgar scores ≥ 6 and not requiring admission to NICU Exclusion criteria: not meeting the above inclusion criteria |
|
| Interventions | Intervention group of additional support n = 103 Usual care n = 103 Usual care: prenatal breastfeeding education was given during routine clinic appointments and written educational materials provided. Standard care included an optional staff peer counsellor who offered telephone support and up to 3 prenatal visits (covering breastfeeding myths, positioning, common problems) as well as daily visits in hospital. Women could have 7 personalised home visits in the first 12 months. Lactation consultants were also available. After discharge participants had access to the hospital ‘warm line’ for breastfeeding questions. Electric pumps were loaned if required. Intervention: women received usual care above (although the optional peer counsellor was not available to intervention group women). In addition to usual care women received 3 x antenatal visits, daily visits in hospital and 11 postnatal visits at home during the first 6 months (x 3 in week 1, x 2 in weeks 2, 3, 4, x 1 in weeks 5 and 6) from a specialised breastfeeding peer supporter. Specialised breastfeeding peer counselling addressed potential obesity‐related breastfeeding barriers (peer counsellors were women from the local community trained to provide support). During the antenatal period the visits assessed previous breastfeeding experience, educated women on breastfeeding logistics, discussed the risks of formula feeding and anticipatory guidance. Women in the intervention group were given a manual pump on discharge and an electric pump if they were returning to school/work during the course of the study. A breastfeeding sling was also provided. |
|
| Outcomes | Basic demographics at recruitment A telephone call at 36 weeks' gestation to determine previous breastfeeding experience and intended breastfeeding duration In‐hospital interview within 24 h to determine infant feeding and peer counsellor contact Medical record review for labour and delivery data Women were followed up by telephone at 2 weeks postnatal and monthly until 6 months. These assessed daily frequency of breastfeeding, expressed breast milk feeding, formula feeding, other solids and liquids. Infant feeding was assessed as in the last 24 h, last week and since delivery. Other outcomes collected included infant health outcomes (e.g. otitis media, hospitalisation, diarrhoea) and peer counsellor contact. Breastfeeding self‐efficacy was collected at recruitment, on the day of delivery and 2 weeks postnatal |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each week, the study coordinator used SPSS software to randomly assign 50% of newly recruited participants to the intervention group, thus preserving allocation concealment". Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Each week, the study coordinator used SPSS software to randomly assign 50% of newly recruited participants to the intervention group, thus preserving allocation concealment." Allocation concealment preserved using an independent allocator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding intervention not possible due to the nature of the study. This could have led to performance bias and affected outcomes as breastfeeding outcomes were self reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were collected by a person separate to the trial, however they were not completely blinded, as they asked about peer counsellor visits. To reduce bias they asked this question after collecting all other data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 108/206 women had outcomes collected at 6 months. This was 52% of those recruited, therefore attrition was > 20% |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report without access to the protocol, therefore we cannot be certain whether all pre‐specified outcomes were reported. |
| Other bias | High risk | Incomplete reporting. Numbers in flow chart at each time point used as 'n' to calculate exclusive breastfeeding rate. Numbers and percentages not corresponding for infant morbidity. Intervention and control groups also differed in baseline characteristics with the intervention group being significantly younger and significantly more likely to have had a vaginal delivery. |
Martin 2015.
| Methods | 3‐armed pilot RCT to examine the feasibility of recruiting and maintaining a cohort of pregnant overweight women and obese women with the view of reducing postpartum weight retention and improving breastfeeding outcomes | |
| Participants | Setting: Teriary Obstetric Unit in New South Wales, Australia Recruitment: October 2010‐September 2011 Inclusion criteria: women with a pre‐pregnancy BMI between 23‐35 kg/m², women intending to breastfeed, ≥ 18 years old, singleton pregnancy, English‐speaking, < 26 weeks' gestation at the initial screening and agreeing not to participate in another weight loss programme in the postnatal period for the duration of the study. Exclusion criteria: not meeting the inclusion criteria above |
|
| Interventions | Dietary advice in the antenatal period and breastfeeding support n = 12 Dietary advice in the antenatal period n = 12 Dietary advice at 3 months postpartum n = 12 The dietary intervention was provided by an Accredited Practicing Dietician in the antenatal period (or for women on the 'waiting list' at 3 months postpartum). Women were directed to implement the “Total Eating Management System” in the postnatal period. Breastfeeding support was provided by an International Board Certified lactation consultant from 35/40. Breastfeeding support only provided advice on lactation issues. In the antenatal period 2 x 30 min face‐to‐face education sessions were provided to discuss the fundamentals of breastfeeding, previous experience of breastfeeding, infant feeding expectations, goals and to build up rapport. In the postnatal period a home visit was provided up to 2 weeks postnatal to ensure breastfeeding was established. At this meeting participants could also discuss concerns. Follow‐up phone calls were provided as required to address concerns raised by the participant. |
|
| Outcomes | Demographics and medical history were obtained. Infant feeding data were collected at 3 and 6 months (obtained by the dietician). This included an infant feeding recall questionnaire (initiation, duration and exclusivity) and current feeding practices to record the infants' breast milk and formula milk intake in the previous 24 h as well as the following: vitamins/minerals/medicine, water/flavoured water, fruit juice, tea, canned/powdered/fresh milk, solid or semi‐solid foods, oral rehydration salts and other foods/fluids Multiple blood bio‐markers were also collected at 35 weeks' gestation, 3 and 6 months postpartum |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation (groups of three) using a computerised generated random number sequence was used to randomise women who were also stratified by pre‐pregnancy weight status categories of overweight (BMI 25–29.99 kg/m²) and obesity (BMI 30–35 kg/m²)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Numbered cards allocating women to an intervention group or the control group were placed in opaque, sequentially numbered envelopes. The person responsible for participant allocation (LMW) did not have direct contact with participants, therefore allocation concealment was maintained." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and lactation consultants was not possible due to the nature of the study. This could have led to performance bias and affected outcomes as breastfeeding outcomes were self‐reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Breastfeeding outcomes were collected by the study dietician who was blinded to allocation to diet or diet and breastfeeding support. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25/36 women enrolled in the study were still in the study at 6 months postpartum. This was 69% of those enrolled so attrition was > 20%. |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report without access to the protocol, therefore we cannot be certain whether all pre‐specified outcomes were reported. Breastfeeding outcomes only reported in brief. |
| Other bias | Low risk | None noted |
Rasmussen 2011a.
| Methods | Parallel‐RCT to determine if women who were overweight or obese who received additional breastfeeding support via telephone would breastfeed for longer than those receiving usual care | |
| Participants | Setting: a hospital in a rural part of New York, USA, which has a history of promoting and supporting breastfeeding. Recruitment: infants born May 2006‐Februrary 2007 Inclusion criteria: women with a pre‐pregnancy BMI > 29 kg/m², who were intending to breastfeed, had no history of breast surgery, who were at least 19 years old and had a singleton fetus and were ≤ 35 weeks' gestation at enrolment to the study. Women were recruited if they resided near to the recruiting healthcare centre. Exclusion criteria: after enrolment women were excluded if they gave birth outside of the study data collection period, if their infant was not born at term, if the infant was never put to the breast, if the infant was injured during delivery, if the infant was placed into foster care or cared for elsewhere or if the mother was no longer in telephone contact during the postnatal period. |
|
| Interventions | Breastfeeding support intervention n = 25 Usual care n = 25 In both cases 5 women excluded after enrolment leaving 20 women in each group. Women receiving the intervention received usual care (rooming in with the infant post‐delivery, observed using the Mother‐Baby Assessment tool during at least 1 breastfeed in each 8‐h shift and a peripartum call from 1 of 3 lactation consultants asking them if they had any questions) and targeted breastfeeding support via telephone from lactation consultants. This included a more detailed peripartum call from a lactation consultant, which asked questions on knowledge, expectations and perceptions, answered questions the women had and reviewed practical points about breastfeeding pre‐delivery. After delivery nurses encouraged women to get up and move post‐delivery, nurses asked visitors to leave if they had been there > 2 h or were too numerous for privacy with breastfeeding or for bonding. An additional call from a lactation consultant at 24 h and 72 h after discharge was provided, which followed a script to standardise the assistance given, but also asked questions and addressed issues as necessary. The lactation consultant was able to order a visit if deemed necessary during the call. |
|
| Outcomes | Data were collected via telephone. This included a questionnaire pre‐delivery, collecting demographics, the woman’s goal for the duration of breastfeeding, prior experience with breastfeeding and pumping, and participation in the Special Supplemental Nutrition Program for Women, Infant and Children or the Prenatal Care Assistance Program for low‐income women. Women were contacted daily over the 1st 7 days asking questions to determine timing of lactogenesis and feeding methods. At 30 and 90 days postpartum data around infant feeding and breastfeeding support were collected. Successful breastfeeding initiation was defined as breastfeeding on day 4 after delivery. Duration of any breastfeeding was defined as the difference between the infant's date of birth and the last date they were offered the breast. Duration of exclusive breastfeeding was the date after discharge from hospital when the infant was given anything other than breast milk. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised at enrolment. Additional information from trial author stated that block randomisation was used (blocks of 10). Blocks were created using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned in report. Correspondence with trial author stated that people recruiting were blinded to group allocation, but that they could not remember where group allocation was kept. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and lactation consultants was not possible due to the nature of the study. This could have led to performance bias and affected outcomes as breastfeeding outcomes were self‐reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants collecting data did not know the women's group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 78% of those enrolled in the trial were included in the final analysis at 3 months postpartum, therefore attrition was > 20% therefore at high risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report without access to the protocol, therefore we cannot be certain whether all pre‐specified outcomes were reported. |
| Other bias | High risk | Intervention fidelity: only 11/20 women in each group received the prespecified intervention calls as the intervention was not implemented as planned. Only 24 out of the 40 participants received the scheduled antenatal phone call and only 10 out of 20 postpartum intervention phone calls were completed. |
Rasmussen 2011b.
| Methods | 3‐armed RCT to determine if women who were overweight or obese who received an electric or a manual pump would breastfeed for longer than those receiving usual care | |
| Participants | Setting: a hospital in a rural part of New York, USA, which has a history of promoting and supporting breastfeeding Recruitment: infants born March 2007‐December 2007 Inclusion criteria: women with a pre‐pregnancy BMI > 29 kg/m², who were intending to breastfeed, had no history of breast surgery, who were at least 19 years old and had a singleton fetus and were ≤ 35 weeks' gestation at enrolment to the study. Women were recruited if they resided near to the recruiting healthcare centre. Exclusion criteria: after enrolment women were excluded if they gave birth outside of the study data collection period, if their infant was not born at term, if the infant was never put to the breast, if the infant was injured during delivery, if the infant was placed into foster care or cared for elsewhere or if the mother was no longer in telephone contact during the postnatal period |
|
| Interventions | Electric pump n = 13 Manual pump n = 12 Usual care (no pump) n = 14 Electric breast pump (Symphony pump, Medela) or manual pump (Harmony pump, Medela, Baar, Switzerland) to use for 10‐14 days. Women were provided with the pump to take home, assistance with the pump in hospital and printed instructions. Women were instructed to pump after 5 nursing sessions for 10 min on each breast every day until their milk came in or until the infant was 5 days old. Women with the manual pump were allowed to keep it, electric pumps were picked up on day 14 postpartum. |
|
| Outcomes | Women were asked to complete a log of how often they expressed. Other data were collected via telephone. This included a questionnaire pre‐delivery, collecting demographics, the woman’s goal for the duration of breastfeeding, prior experience with breastfeeding and pumping, and participation in the Special Supplemental Nutrition Program for Women, Infant and Children or the Prenatal Care Assistance Program for low‐income women. Women were contacted daily over the 1st 7 days asking questions to determine timing of lactogenesis and feeding methods. At 30 and 90 days postpartum data around infant feeding and breastfeeding support were collected. Successful breastfeeding initiation was defined as breastfeeding on day 4 after delivery. Duration of any breastfeeding was defined as the difference between the infant's date of birth and the last date they were offered the breast. Duration of exclusive breastfeeding was the difference between the infant's date of birth and the first date the infant was offered something other than breast milk. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised at enrolment. Additional information from trial author stated that block randomisation was used (blocks of 10). Blocks were created using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned in report. Correspondence with trial author stated that people recruiting were blinded to group allocation, but that they could not remember where group allocation was kept. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and lactation consultants was not possible due to the nature of the study. This could have led to performance bias and affected outcomes as breastfeeding measures were self‐reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants collecting data did not know the women's group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34/39 women included in the final analysis at 3 months postpartum which was 87% of those enrolled so attrition was < 20%. |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report without access to the protocol, therefore we cannot be certain whether all pre‐specified outcomes were reported. |
| Other bias | High risk | Intervention fidelity: all control participants pumped, 1 participant in the electric pump group received no pump, with another declining the pump offered and 2 participants in the manual pump group received an electric pump |
Reifsnider 2018.
| Methods | Prospective randomised trial 1:1 randomisation | |
| Participants | Setting: USA, Southwest metropolitan area Recruitment: March 2013‐October 2014 Pregnant obese Latina women were recruited at Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), where the majority of participants were Hispanic, earned < 200% of the federal poverty index, and did not complete high school. Inclusion criteria: participants were healthy Latina women in the third trimester, aged 18‐40 years, with a pre‐pregnancy BMI of ≥ 25 kg/m2, who could receive visitors at home, had a telephone, and did not intend to move away. Exclusion criteria: excluded were mothers with a high‐risk pregnancy, e.g. type 1 diabetes, postpartum complications, hospitalisation, and/or separation from the infant. Infants who were born prior to 38 weeks, low birthweight (< 2500 g) or with endocrine, chromosomal/genetic abnormality, not discharged with mother |
|
| Interventions | Intervention n = 91 (n = 61 within the analysis) Control n = 83 (n = 58 within the analysis) Intervention: women in the intervention group received home visits by trained Spanish fluent community health workers ("promotoras") who provided counselling on infant growth, breastfeeding, nutrition, child development, sleep, physical activity, and safety. Health workers received six months of formal training (320 h over 40 days on research procedures, child development, breastfeeding support, nutrition, parenting, safety, and sleep hygiene) and gained a community health certificate. The 3 research promotoras were Spanish/English‐fluent women experienced in providing health education to the local population. Prenatal and postnatal visits ‐ once before delivery at 36 weeks' gestation and at ages 3 days and 2 weeks, and at 2, 4, 6, 9, and 12, 18 and 24 months (10 visits in total). During visits, the promotoras discussed infants’ growth, health, development, sleep, and play/exercise activities. Promotoras documented and plotted nude weight and length (Doran scale, model DS4100, or calculated as the mean of 3 weights of mother plus nude infant minus mother), weight‐for‐length percentile, 13 breast/formula and solid feedings (number and quantity), and social/developmental issues. Mothers were encouraged to breastfeed and/or to avoid giving excess formula or adding sugar or solids to the bottle. A lactation consultant visited mothers who reported problems with breastfeeding. At each visit, mothers in the intervention group received a small gift such as a children’s book. Control/comparison intervention: promotoras did not visit the control women. Outcomes were measured by the research assistant. The women had access to the standard WIC breastfeeding peer counsellor programme, which included 2 antenatal classes on breastfeeding, 1 session with a breastfeeding peer counsellor, another visit at 7 days and every 3 months after that. |
|
| Outcomes | At the 1‐week postpartum home visit, and at infant ages 1, 6, and 12 months, the research assistant documented the mothers’ postpartum heights and weights (Seca scales model 869‐ 1321004 and 19‐17‐05‐224), the infants’ supine lengths and weights, breastfeeding frequency, duration, and intensity, quantity of formula consumed, pacifier use by the infant, and socio‐demographic data. The research assistant also called the parent monthly until age 12 months (or until the mother stopped breastfeeding) to document breastfeeding status using the WHO definitions: 1 = exclusive, 2 = almost exclusive, 3 = high partial, 4 = medium partial, 5 = low partial, 6 = token, and 7 = no breastfeeding (breastfeeding was classified as 1‐5, not if 6 or 7). Data were used from the Edinburgh Postnatal Depression Scale, the Infant Feeding Observation, the Brief Acculturation Rating Scale for Mexican Americans, the United States Department of Agriculture 16‐item Food Security Questionnaire and the Everyday Stressors Index |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computerized random number generator" was used. Randomization occurred after recruitment and informed consent from the mother. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used for allocation concealment, with envelopes opened for each woman after providing consent to participate in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and lactation consultants was not possible due to the nature of the study. This could have led to performance bias and affected outcomes as breastfeeding outcomes were self‐reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The research assistant "was initially blinded to randomization status, but unblinding occurred for some subjects during the study". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 174 women were randomised. 119 woman‐infant pairs had data collected at 12 months postpartum (no further figures given for 6 months postpartum). The results are only reported for the 119 participants that were still in the trial at 12 months postpartum. This is 68% of the original sample. Therefore it is classified as high risk of bias as attrition is > 20%. |
| Selective reporting (reporting bias) | High risk | Very poor reporting of outcomes for control versus intervention, many outcomes were reported as breastfeeding at 2 months postpartum versus not. Not all outcomes from the protocol were reported |
| Other bias | High risk | Inaccuracies in reporting. Number of women breastfeeding at ≥ 2 months reported as 63, 64 and 68 in different parts of the report Group sizes differed (91 versus 83) however simple randomisation was undertaken, so we judged this to have occurred by chance. |
Stuebe 2016.
| Methods | Cluster‐RCT to determine if a breastfeeding support intervention integrated within a lifestyle intervention would increase duration of any and exclusive breastfeeding among women with GDM. | |
| Participants | Setting: 2 sites in Raleigh, North Carolina, USA. 1 site primarily served privately insured women and the other site served primarily publicly insured women. Neither site was Baby Friendly Hospital Initiative Certified. Recruitment: June 2012‐September 2014 Inclusion criteria: women who were 21 + 0 to 36 + 6 weeks' gestation; diagnosed GDM with ≥ 2 x 100 g oral glucose tolerance tests values exceeding established thresholds: 95 mg/dL (fasting), 180 mg/dL (1 h), 155 mg/dL (2 h) or 140 mg/dL (3 h). Age ≥ 18 and ≤ 45 years old; pre‐pregnancy BMI ≥ 25 kg/m²; ability to read and write English. Exclusion criteria: overt diabetes, indexed as a baseline A1c ≥ 6.5 mg/dL Randomisation: women were cluster‐randomised to the lifestyle intervention or to a waiting list control group. Cluster‐randomisation was used to ensure sufficient women for the group intervention within each time period. Women inducted within a 1‐month period were randomised by a computerised randomisation sequence that was stratified by study site. 8 clusters were included. |
|
| Interventions | Intervention n = 50 Usual care n = 50 Intervention: was a social cognitive theory‐based intervention where breastfeeding support was integrated within a lifestyle intervention. NEST intervention (Nutrition, Exercise and coping Skills Training) including prenatal education about breastfeeding, in group education sessions, phone support, 13 weeks of group nutrition and exercise sessions beginning at 6 weeks postpartum, including weekly group sessions and a home exercise programme. Weekly texts reinforcing intervention themes, i.e. GDM management and breastfeeding tips, were sent from enrolment until the end of the exercise programme (breastfeeding tips were stopped earlier if the woman stopped breastfeeding). Anticipatory messages sent regarding engorgement, growth spurts, returning to work and normal infant sleep patterns and importance of breastfeeding for maternal and infant health. During the exercise programme women returned for monthly group sessions for 3 months. The interventionalist had undertaken an online breastfeeding programme and had group‐lifestyle intervention training. The education class was undertaken by an International Board Certified Lactation Consultant. Breastfeeding education session included benefits of breastfeeding for mother and infant, importance of early skin‐to‐skin contact and early initiation of breastfeeding, feeding based on infant cues and positioning. Women received a breastfeeding pillow No contact during hospitalisation was provided, however following birth the interventionists contacted each woman weekly through text to enquire how breastfeeding was progressing. Questions were answered according to a protocol adapted from an effective breastfeeding intervention. Contacts to text or call for breastfeeding assistance were also provided and if women were struggling the Lactation Conslutant called them to provide additional support. Usual care: these women were on the waiting list, being offered the nutrition, exercise and coping skills components of the intervention at 10 months postpartum once study follow‐up was complete. Women receiving usual care were offered either breastfeeding peer counsellors for home visits or phone calls or an inpatient consultation with an International Board Certified Lactation Consultants depending on which unit they delivered in. |
|
| Outcomes | Primary outcome: change in fasting glucose and maternal weight from enrolment to 10 months postpartum Secondary outcomes: breastfeeding duration and intensity. Duration of exclusive and any breastfeeding were assessed at 6 weeks, 4, 7, and 10 months postpartum. Stopping exclusive breastfeeding was defined as the time when an infant was first given formula (including supplementation during the hospital stay). Breastfeeding intention was measured at baseline using Infant Feeding Intentions scale (higher scores indicating stronger intention) Breastfeeding intensity: using dietary recall questionnaire, categorised as proportion of feeds that were breast milk < 20% (low) 20%‐80% (medium) and > 80% high Reasons for introducing formula or stopping breastfeeding were also assessed. |
|
| Notes | As no ICC was given in this article, the sample size for unadjusted outcomes was adjusted using the ICC of 0.02 reported in Kronborg 2007. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomisation. All women inducted to the study over a 1‐month period at a given site were randomised to either experimental or control group. Quote: “A computerized randomisation program was used to generate the randomisation sequence for the groups of women. The approach to randomisation was stratified by study sites." |
| Allocation concealment (selection bias) | Low risk | Project manager assigned inducted groups in 1‐month blocks, therefore group assignment to the cluster was after induction |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not possible due to the nature of the intervention. This could have led to performance bias and affected outcomes as breastfeeding outcomes were self‐reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “research assistants who were blinded to study group assignment collected all outcome data using a standardized manual.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 51% follow‐up by 4 months postpartum – therefore attrition > 20% at 6 months postpartum so at high risk of bias |
| Selective reporting (reporting bias) | Low risk | Primary outcomes were reported in the protocol as decrease in fasting blood glucose and weight from baseline to 10 months postpartum. This article mentions that other outcomes will be reported in another paper as this paper exclusively reports on the breastfeeding outcomes. No evidence of selective reporting due to significance/not |
| Other bias | Low risk | 44/50 women in the intervention group attended the intervention group breastfeeding class, which is greater than 85%. No significant difference at baseline except for race, the intervention group having a higher percentage of women of white ethnicity and the control group a higher percentage of women of Black/African American ethnicity. Therefore low risk of bias for baseline imbalance. Analyses accounted for within‐cluster correlations. |
BMI: body mass index; GDM: gestational diabetes mellitus; ICC: intra‐cluster correlation; NICU: neonatal intensive care unit; RCT: randomised controlled trial; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chapman 2016 | Observational study, not controlled trial |
| DRKS00012842 | Nutritional intervention around malnutrition, anaemia and vitamin A, no breastfeeding support described as being provided. Therefore not eligible for inclusion as not aimed at supporting mothers who are overweight or obese to breastfeed. |
| Lewkowitz 2018 | Intervention was parent education along with enhanced lifestyle and breastfeeding support. This was compared against a standard parent education intervention. "The randomized control trial did not include a control group receiving routine prenatal care." Neither was the study comparing 2 different types of breastfeeding intervention. Therefore not eligible for inclusion. |
| NCT03640104 | Dietary intervention in women who are breastfeeding. Intervention does not include breastfeeding support. BMI measured at recruitment (postpartum) not at booking. Therefore not eligible for inclusion. |
| Nicklas 2014 | Pre‐pregnancy BMI 18‐40 kg/m², BMI measured at time of recruitment (postnatal) ≥ 24 kg/m² (or ≥ 22 kg/m² for Asian women). Therefore not eligible for inclusion. |
BMI: body mass index
Characteristics of ongoing studies [ordered by study ID]
NCT01668316.
| Trial name or title | Get active and eat right: moms at work (GEM) |
| Methods | Parallel‐RCT |
| Participants | 78 women Setting: USA Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention A 12‐week programme will be provided with biweekly meetings with a registered dietician in the workplace.
Control group: women will be asked not to change their dietary or physical activity habits. |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | July 2012 |
| Contact information | Elyse Shearer and Cheryl Lovelady, University of North Carolina at Greensboro |
| Notes |
NCT02260518.
| Trial name or title | Promoting health in pregnancy and postpartum (HIPP) among overweight/obese women |
| Methods | Parallel RCT. |
| Participants | 400 participants Setting: USA Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention The intervention will focus on women gaining the recommended amount of weight, increasing physical activity to 150 min/week and meeting health eating guidelines. This will be during pregnancy through 1 face‐to‐face counselling session, 10 podcasts and weekly phone calls until delivery. 1 group session on breastfeeding also provided. A private Facebook group will be available for participants to participate in during the prenatal and postnatal periods. Postpartum, participants will be provided with 1 face‐to‐face counselling session, 16 behavioural podcasts, brief weekly check‐in telephone calls for 6 weeks, and up to 8 telephone counselling calls through 6 months after delivery. Control Women will received standard care with usual nutritional advice from physicians, nurses, nutritionists and counsellors. They will receive monthly mailings and podcasts related to healthy pregnancy and on fetal development. Postpartum the mailings will focus on infant development and parenting. |
| Outcomes | Primary outcome measures
Secondary outcome measures
Other outcome measures
|
| Starting date | January 2015 |
| Contact information | Sara Wilcox and Jihong Liu University of South Carolina Prevention Research Center |
| Notes |
NCT02520167.
| Trial name or title | Partnership to improve nutrition and adiposity in prenatal clinical care: a pilot and feasibility study |
| Methods | Parallel RCT, with 1:1 allocation to intervention/control |
| Participants | 24 participants Settting: USA Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention Meet a dietary counsellor at every pre‐natal appointment for 15 min, to receive 11 lessons on diet and lifestyle and 1 further prenatal lesson on breastfeeding. They will also receive access to a private online Facebook page for antenatal education and group support. Control Usual prenatal care, including clinic appointments, ultrasound appointments, recommendations for prenatal multivitamins, a balanced diet and remaining physically active. Early glucose screening at booking and further glucose screening at 24‐28 weeks. Referral to a registered dietician if test positive for pre‐existing or GDM |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | October 2015 |
| Contact information | Katherine A Sauder, University of Colorado, Denver |
| Notes |
NCT02534051.
| Trial name or title | A clinical care pathway for obese pregnant women: a pilot cluster‐RCT |
| Methods | RCT, parallel, with 1:1 allocation of clinics to provide the intervention care pathway or the standard care pathway for obese pregnant women |
| Participants | 142 participants planned (189 recruited) Setting: Canada Inclusion criteria Clinics
Maternal
Exclusion criteria:
|
| Interventions | Intervention: care according to a care pathway with care specific to obese pregnant women, including fetal screening, maternal screening for diabetes, counselling about weight gain, counselling about birth risks and a discussion about breastfeeding Control group: usual perinatal care |
| Outcomes | Primary outcome measures
Secondary outcome measures
Exploratory clinical outcomes:
Provider outcomes:
|
| Starting date | October 2015 |
| Contact information | Sarah D McDonald, McMaster University |
| Notes |
NCT02756169.
| Trial name or title | Maternal obesity and breastfeeding performance |
| Methods | RCT parallel with 1:1 allocation to intervention/control |
| Participants | 200 women Setting: Mexico Inclusion criteria:
Exclusion criteria:
Exit criteria:
|
| Interventions | Intervention
Control: routine care in the prenatal and postnatal periods |
| Outcomes |
|
| Starting date | July 2016 |
| Contact information | Sonia L Hernández Cordero, Universidad Iberoamericana, A.C. |
| Notes |
BMI: body mass index; GDM: gestational diabetes mellitus; HDL: high‐density lipoprotein; NICU: neonatal intensive care unit; LDL: low‐density lipoprotein; ORS: oral rehydration salts; RCT: randomised controlled trial; USDA: United States Department of Agriculture; WHO: World Health Organization
Differences between protocol and review
There are some differences between the published protocol for this review (Soltani 2016), and this full review, which we outline below.
Within the objectives section, we clarified that the main objective was to assess the effectiveness of interventions to support the initiation or continuation of breastfeeding in women who are overweight or obese. We removed the sentence, "We will also examine the effectiveness of different types of interventions based on the intervention delivery format (individual or group and face‐to‐face or mobile technology); style (proactive or reactive); intensity; provider (peer or professional workers); setting (community or hospital, Baby Friendly Initiative accredited; background breastfeeding initiation rate); timing (antenatal, postnatal or both); and co‐morbidities (without complications or with gestational diabetes mellitus or pre‐existing diabetes, caesarean section, preterm birth)" as this is included within the subgroup analyses.
Types of intervention ‐ we added a sentence to clarify that breast milk could be given to the infant either by putting the baby to the breast or by expressing breast milk to give to the infant.
Originally, we had included two comparisons 1. Breastfeeding support versus usual care; 2. One form of breastfeeding support versus another form of breastfeeding support. After editorial advice, we carried out analysis separately on different types of interventions. This led to the consideration of five comparisons.
Social support only versus usual care
Educational support only versus usual care
Pysical support only versus usual care
Multiple methods of support versus usual care
One or multiple forms of breastfeeding support versus another form of breastfeeding support
We made minor changes to primary and secondary outcomes to include timings of assessment of exclusive/any breastfeeding to be in line with other reviews in this area, to include definitions of outcomes and to change 'breastfeeding initiation' to 'non‐initiation of breastfeeding' to make the analysis more meaningful. Primary and secondary outcomes are now:
Primary outcomes
Non‐initiation of breastfeeding ‐ where initiation is defined as the baby being put to the breast or being given any of the mother's breast milk within 48 hours of delivery (NHS England 2014)
Exclusive breastfeeding at four to six weeks ‐ as defined by trial authors
Any breastfeeding at four to six weeks
Exclusive breastfeeding at six months ‐ as defined by trial authors
Any breastfeeding at six months
Secondary outcomes
Breastfeeding intention
Excusive breastfeeding at two weeks, two, three, four months ‐ as defined by trial authors
Any breastfeeding at two weeks, two, three, four, nine, 12 months
Duration of exclusive breastfeeding ‐ as defined by trial authors
Duration of any breastfeeding
Maternal postpartum weight retention at two, three, four, six, nine and 12 months.
Maternal postpartum BMI at two, three, four, six, nine and 12 months.
All‐cause infant or neonatal morbidity ‐ as reported by trial authors, for example, neonatal hypoglycaemia, low weight gain, infections
All‐cause infant or neonatal mortality
Infant weight gain at two, three, four, six, nine and 12 months.
Maternal satisfaction with care
Maternal satisfaction with feeding method
Maternal nipple health ‐ ‐ as defined by trial authors, for example, cracked nipples, sore nipples
Cost‐effectiveness of the intervention
After statistical advice, we reduced the number of subgroups analysed. Subgroup analyses removed were:
Location of the intervention (hospital versus community), as Baby Friendly Accreditation of the institution where the intervention was carried out was similar in concept
Whether the intervention was proactive (scheduled contact) versus reactive (contact requested by the woman), as a similar concept is covered by scheduled number of postnatal contacts
We also added one subgroup:
Gestational age at birth of infant (term infants only versus preterm and term infants)
We changed the subgroup analysis for socioeconomic status of the population from (high and medium versus low) to (mixed versus low (> 75% of participants from low‐income backgrounds)).
Finally, within the sensitivity analysis, we changed the attrition rate to 20% to be consistent with the assessment of risk section in the proposal.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data) ‐ time point added of six months or trial end if sooner to coincide with the primary outcome time point.
Contributions of authors
Hora Soltani and Frankie Fair conceived the review question, planned and developed the review protocol and design.
Frankie Fair assessed trial eligibility and undertook data extraction, 'Risk of bias' assessment, quality assessment using the GRADE approach, analysis and synthesis of the results and interpreting the findings, and drafted text for the Background, Method, Results, Discussion and Conclusions section of the review.
Gemma Ford contributed to assessing trial eligibility, data extraction, 'Risk of bias' assessment, double‐checking data entry into Review Manager 5, assessing quality using the GRADE approach and writing the lay summary and the outcomes part of the description of trial characteristics section.
Hora Soltani is the contact person and guarantor for the review and contributed to trial eligibility, data extraction, 'Risk of bias' assessment, assessing quality using the GRADE approach, synthesis of and interpreting the findings and drafting the Abstract.
All review authors commented on drafts of the review and approved the final version.
Declarations of interest
Frankie J Fair: none known
Gemma Ford: none known
Hora Soltani: none known
New
References
References to studies included in this review
Carlsen 2013 {published data only}
- Carlsen EM, Kyhnaeb A, Renault KM, Cortes D, Michaelsen KF, Pryds O. Telephone‐based support prolongs breastfeeding duration in obese women: a randomized trial. American Journal of Clinical Nutrition 2013;98(5):1226‐32. [DOI] [PubMed] [Google Scholar]
Chapman 2013 {published data only}
- Chapman DJ, Bermudez‐Millan A, Wetzel K, Damio G, Kyer N, Young S, et al. Breastfeeding education and support trial for obese women. FASEB 2008;22:1080.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DJ, Morel K, Bermudez‐Millan A, Young S, Damio G, Kyer N, et al. Breastfeeding education and support trial for obese women: effects of a specialized peer counseling intervention on breastfeeding and health outcomes. Journal of Human Lactation 2011;27(1):75‐6. [Google Scholar]
- Chapman DJ, Morel K, Bermudez‐Millan A, Young S, Damio G, Perez‐Escamilla R. Breastfeeding education and support trial for overweight and obese women: a randomized trial. Pediatrics 2013;131(1):e162‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DJ, Perez‐Escamilla R. Acculturative type is associated with breastfeeding duration among low‐income Latinas. Maternal and Child Nutrition 2013;9(2):188‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel K, Chapman DJ, Kyer N, Bermudez‐Millan A, Young S, Perez‐Escamilla R. Peer counselors improve breastfeeding technique among low‐income, obese women. FASEB Journal 2010;24(Suppl):[Abstract no. 91.7]. [Google Scholar]
Martin 2015 {published data only}
- Martin J. Weight Retention in the Postpartum Period [thesis]. New South Wales, Australia: University of Newcastle, 2012. [Google Scholar]
- Martin J, MacDonald‐Wicks L, Hure A, Smith R, Collins CE. Reducing postpartum weight retention and improving breastfeeding outcomes in overweight women: a pilot randomised controlled trial. Nutrients 2015;7(3):1464‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rasmussen 2011a {published data only}
- Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers. Journal of Human Lactation 2010;26(4):430. [DOI] [PubMed] [Google Scholar]
- Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers: the Bassett Improving Breastfeeding Study. Breastfeeding Medicine 2011;6:69‐75. [DOI] [PubMed] [Google Scholar]
Rasmussen 2011b {published data only}
- Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers. Journal of Human Lactation 2010;26(4):430. [DOI] [PubMed] [Google Scholar]
- Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers: the Bassett Improving Breastfeeding Study. Breastfeeding Medicine 2011;6:69‐75. [DOI] [PubMed] [Google Scholar]
Reifsnider 2018 {published data only}
- NCT01905072. Preventing childhood obesity through early guidance. clinicaltrials.gov/ct2/show/study/NCT01905072 (first received 23 July 2013).
- Reifsnider E, McCormick DP, Cullen KW, Szalacha L, Moramarco MW, Diaz A, et al. A randomized controlled trial to prevent childhood obesity through early childhood feeding and parenting guidance: rationale and design of study. BMC Public Health 2013;13:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifsnider E, McCormick DP, Cullen KW, Todd M, Moramarco MW, Gallagher MR, et al. A randomized controlled trial to prevent infant overweight in a high‐risk population. Academic Pediatrics 2018;18(3):324‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stuebe 2016 {published data only}
- Berry DC, Neal M, Hall EG, Schwartz TA, Verbiest S, Bonuck K, et al. Rationale, design, and methodology for the optimizing outcomes in women with gestational diabetes mellitus and their infants study. BMC Pregnancy and Childbirth 2013;13:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Bonuck K, Adatorwovor R, Schwartz TA, Berry DC. A cluster randomised trial of tailored breastfeeding support for women with gestational diabetes. Breastfeeding Medicine 2016;11(10):504‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Chapman 2016 {published data only}
- Chapman DJ, Doughty K, Mullin EM, Perez‐Escamilla R. Reliability of lactation assessment tools applied to overweight and obese women. 17th ISRHML Conference: from human milk molecules to population health: research advances; 2014 October 23‐27; Kiawah Island, South Caroline, USA. 2014.
- Chapman DJ, Doughty K, Mullin EM, Perez‐Escamilla R. Reliability of lactation assessment tools applied to overweight and obese women. Journal of Human Lactation 2016;32(2):269‐76. [DOI] [PubMed] [Google Scholar]
DRKS00012842 {published data only}
- DRKS00012842. Nutritional status of mother‐infant pairs in Bukavu, DR Congo. Study part I: impact of breastfeeding practices, dietary intake and food habits of lactating women on the nutritional status of infants aged 0‐6 months. Study part II: impact of specific nutrition interventions on breast milk quality and nutritional status of under‐ and overweight lactating mothers. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00012842 (first received 27 November 2017).
Lewkowitz 2018 {published data only}
- Cahill AG, Haire‐Joshu D, Cade WT, Stein RI, Woolfolk CL, Moley K, et al. Weight control program and gestational weight gain in disadvantaged women with overweight or obesity: a randomized clinical trial. Obesity 2018;26(3):485‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowitz AK, Lopez JD, Stein RI, Rhoades JS, Schulz RC, Woolfolk CL, et al. Effect of a home‐based lifestyle intervention on breastfeeding initiation among socioeconomically disadvantaged African American women with overweight or obesity. Breastfeeding Medicine 2018;13(6):418‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowitz AK, Rhoades JS, Lopez JD, Schulz R, Woolfolk CL, Macones GA, et al. 891: Does a home‐based lifestyle intervention improve breastfeeding rates in socioeconomically disadvantaged (SED) obese women?. American Journal of Obstetrics & Gynecology 2018;218(1 Suppl):S530. [Google Scholar]
NCT03640104 {published data only}
- NCT03640104. Effect of an individualized dietary intervention on the body composition and vitamin A status of breastfeeding women. clinicaltrials.gov/ct2/show/NCT03640104 (first received 21 August 2018).
Nicklas 2014 {published data only}
- Nicklas JM, Zera CA, England LJ, Rosner BA, Horton E, Levkoff SE, et al. A web‐based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstetrics and Gynecology 2014;124(3):563‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01668316 {published data only}
- NCT01668316. Get active and eat right: moms at work (GEM). clinicaltrials.gov/ct2/show/NCT01668316 (first received 20 August 2012).
NCT02260518 {published data only}
- NCT02260518. Promoting health in pregnancy and postpartum (HIPP). clinicaltrials.gov/ct2/show/NCT02260518 (first received 9 October 2014).
NCT02520167 {published data only}
- NCT02520167. Partnership to improve nutrition and adiposity in prenatal clinical care: a pilot and feasibility study. clinicaltrials.gov/ct2/show/study/NCT02520167 (first received 11 August 2015).
NCT02534051 {published data only}
- NCT02534051. A clinical care pathway for obese pregnant women: a pilot cluster RCT. clinicaltrials.gov/ct2/show/NCT02534051 (first received 27 August 2015).
NCT02756169 {published data only}
- NCT02756169. Maternal obesity and breastfeeding performance. clinicaltrials.gov/ct2/show/NCT02756169 (first received 29 April 2016).
Additional references
Amir 2007
- Amir LH, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy and Childbirth 2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Babendure 2015
- Babendure JB, Reifsnider E, Mendias E, Moramarco MW, Davila YR. Reduced breastfeeding rates among obese mothers: a review of contributing factors, clinical considerations and future directions. International Breastfeeding Journal 2015;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2007
- Baker JL, Michaelsen KM, Sørensen TI, Rasmussen KM. High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. American Journal of Clinical Nutrition 2007;86(2):404‐11. [DOI] [PubMed] [Google Scholar]
Balogun 2016
- Balogun OO, O’Sullivan EJ, McFadden A, Ota E, Gavine A, Garner CD, et al. Interventions for promoting the initiation of breastfeeding. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD001688.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlsen 2010
- Carlsen SM, Jacobsen G, Vanky E. Mid‐pregnancy androgen levels are negatively associated with breastfeeding. Acta Obstetrica et Gynecologica Scandinavica 2010;89(1):87‐94. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf ofthe Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Eidelman 2012
- Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, Szucs K, et al. Breastfeeding and the use of human milk. American Academy of Pediatrics policy statement. Pediatrics 2012;129(3):e827‐e841. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 5 July 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
Guelinckx 2012
- Guelinckx I, Devlieger R, Bogaerts A, Pauwels S, Vansant G. The effect of pre‐pregnancy BMI on intention, initiation and duration of breast‐feeding. Public Health Nutrition 2012;15(5):840‐8. [DOI] [PubMed] [Google Scholar]
Haroon 2013
- Haroon S, Das JK, Salam RA, Imdad A, Bhutta ZA. Breastfeeding promotion interventions and breastfeeding practices: a systematic review. BMC Public Health 2013;13(Suppl 3):S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauff 2014
- Hauff LE, Leonard SA, Rasmussen KM. Associations of maternal obesity and psychosocial factors with breastfeeding intention, initiation, and duration. American Journal of Clinical Nutrition 2014;99(3):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesch Anstey 2011
- Hesch Anstey E, Jevitt C. Maternal obesity and breastfeeding. A review of the evidence and implications for practice. Clinical Lactation 2011;2(3):11‐6. [Google Scholar]
Heslehurst 2010
- Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619323 births, 1989‐2007. International Journal of Obesity 2010;34(3):420‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hilson 2004
- Hilson JA, Rasmussen KM, Kjolhede CL. High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. Journal of Human Lactation 2004;20(1):18‐29. [DOI] [PubMed] [Google Scholar]
Hoddinott 2009
- Hoddinott P, Britten J, Prescott GJ, Tappin D, Ludbrook A, Godden DJ. Effectiveness of policy to provide breastfeeding groups (BIG) for pregnant and breastfeeding mothers in primary care: cluster randomised controlled trial. BMJ 2009;338:a3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hossain 2007
- Hossain P, Kawar B, Nahas M. Obesity and diabetes in the developing world – a growing challenge. New England Journal of Medicine 2007;356(3):213‐5. [DOI] [PubMed] [Google Scholar]
Krause 2011
- Krause KM, Lovelady CA, Østbye T. Predictors of breastfeeding in overweight and obese women: data from Active Mothers Postpartum (AMP). Maternal Child Health Journal 2011;15(3):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kronborg 2007
- Kronborg H, Vaeth M, Olsen J, Iversen L, Harder I. Effect of early postnatal breastfeeding support: a cluster randomized community based trial. Acta Paediatrica 2007;96(7):1064‐70. [DOI] [PubMed] [Google Scholar]
Kronborg 2012
- Kronborg H, Vaeth M, Rasmussen KM. Obesity and early cessation of breastfeeding in Denmark. European Journal of Public Health 2012;23(2):316‐22. [DOI] [PubMed] [Google Scholar]
Leonard 2011
- Leonard SA, Labiner‐Wolfe J, Geraghty SR, Rasmussen KM. Associations between high prepregnancy body mass index, breast‐milk expression, and breast‐milk production and feeding. American Journal of Clinical Nutrition 2011;93(3):556‐63. [DOI] [PubMed] [Google Scholar]
Lepe 2011
- Lepe M, Bacardí Gascón M, Castañeda‐González LM, Pérez Morales ME, Jiménez Cruz A. Effect of maternal obesity on lactation: systematic review. Nutrición Hospitalaria 2011;26(6):1266‐9. [DOI] [PubMed] [Google Scholar]
Lessen 2015
- Lessen R, Kavanagh K. Position of the Academy of Nutrition and Dietetics: promoting and supporting breastfeeding. Journal of the Academy of Nutrition and Dietetics 2015;115(3):444–9. [DOI] [PubMed] [Google Scholar]
Li 2003
- Li R, Jewell S, Grummer‐Strawn L. Maternal obesity and breast‐feeding practices. American Journal of Clinical Nutrition 2003;77(4):931‐6. [DOI] [PubMed] [Google Scholar]
MacArthur 2009
- MacArthur C, Jolly K, Ingram L, Freemantle N, Dennis CL, Hamburger R, et al. Antenatal peer support workers and initiation of breast feeding: cluster randomised controlled trial. BMJ 2009;338:b131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchi 2015
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obesity Reviews 2015;16(8):621‐38. [DOI] [PubMed] [Google Scholar]
McFadden 2017
- McFadden A, Gavine A, Renfrew MJ, Wade A, Buchanan P, Taylor JL, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD001141.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2013
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The Behavior Change Technique Taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine 2013;46(1):81‐95. [DOI] [PubMed] [Google Scholar]
Mok 2008
- Mok E, Multon C, Piguel L, Barroso E, Goua V, Christin P, et al. Decreased full breastfeeding, altered practices, perceptions, and infant weight change of prepregnant obese women: a need for extra support. Pediatrics 2008;121(5):e1319‐24. [DOI] [PubMed] [Google Scholar]
Neville 2014
- Nevill CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The relationship between breastfeeding and postpartum weight change—a systematic review and critical evaluation. International Journal of Obesity 2014;38(4):577‐90. [DOI] [PubMed] [Google Scholar]
NHS England 2014
- NHS England. <12 Week Maternal Assessment, Breastfeeding Initiation, 6 & 8 Weeks breastfeeding UNIFY Collections: Guidance. Leeds: NHS England, 2014. [Google Scholar]
Pérez‐Escamilla 2016
- Pérez‐Escamilla R, Martinez JL, Segura‐Pérez S. Impact of the Baby‐friendly Hospital Initiative on breastfeeding and child health outcomes: a systematic review. Maternal & Child Nutrition 2016;12(3):402‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rasmussen 2004
- Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 2004;113(5):e465‐e71. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rollins 2016
- Rollins N, Bhandari N, Hajeebhoy N, Horton S, Lutter C, Martines J, et al. Why invest, and what it will take to improve breastfeeding practices?. Lancet 2016;387:491–504. [DOI] [PubMed] [Google Scholar]
Salone 2013
- Salone LR, Vann WF Jr, Dee DL. Breastfeeding. An overview of oral and general health benefits. Journal of the American Dental Association 2013;144(2):143‐51. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Soltani 2009
- Soltani H, Arden M. Factors associated with breastfeeding up to 6 months postpartum in mothers with diabetes. Journal of Obstetric Gynecologic & Neonatal Nursing 2009;38(5):586‐94. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Stevens 2012
- Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, et al. National, regional, and global trends in adult overweight and obesity prevalences. Population Health Metrics 2012;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2013
- Thompson LA, Zhang S, Black E, Das R, Ryngaert M, Sullivan S, et al. The association of maternal pre‐pregnancy body mass index with breastfeeding initiation. Maternal Child Health Journal 2013;17(10):1842–51. [DOI] [PubMed] [Google Scholar]
Victora 2016
- Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387(10017):475‐90. [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO technical report series 894. Geneva: World Health Organization, 2000. [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. The optimal duration of exclusive breastfeeding: report of an expert consultation. Geneva: World Health Organization, 2001. [Google Scholar]
WHO 2004
- WHO expert consultation. Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363(9403):157‐63. [DOI] [PubMed] [Google Scholar]
Wojcicki 2011
- Wojcicki JM. Maternal prepregnancy body mass index and initiation and duration of breastfeeding: a review of the literature. Journal of Women's Health 2011;20(3):341‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Soltani 2016
- Soltani H, Fair FJ. Interventions for supporting the initiation and continuation of breastfeeding among women who are overweight or obese. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012099] [DOI] [PMC free article] [PubMed] [Google Scholar]


