Abstract
Introduction
The development and implementation of national strategic plans is a critical component towards successfully addressing antimicrobial resistance (AMR). This study aimed to review the scope and analytical depth of situation analyses conducted to address AMR in human health to inform the development and implementation of national strategic plans.
Methods
A systematic search of the literature was conducted to identify all studies since 2000, that have employed a situation analysis to address AMR. The included studies are analysed against frameworks for strategic analysis, primarily the PESTELI (Political, Economic, Sociological, Technological, Ecological, Legislative, Industry) framework, to understand the depth, scope and utility of current published approaches.
Results
10 studies were included in the final review ranging from single country (6) to regional-level multicountry studies (4). 8 studies carried out documentary review, and 3 of these also included stakeholder interviews. 2 studies were based on expert opinion with no data collection. No study employed the PESTELI framework. Most studies (9) included analysis of the political domain and 1 study included 6 domains of the framework. Technological and industry analyses is a notable gap. Facilitators and inhibitors within the political and legislative domains were the most frequently reported. No facilitators were reported in the economic or industry domains but featured inhibiting factors including: lack of ring-fenced funding for surveillance, perverse financial incentives, cost-shifting to patients; joint-stock drug company ownership complicating regulations.
Conclusion
The PESTELI framework provides further opportunities to combat AMR using a systematic, strategic management approach, rather than a retrospective view. Future analysis of existing quantitative data with interviews of key strategic and operational stakeholders is needed to provide critical insights about where implementation efforts should be focussed, and also how to build contingency at the strategic level for agile responses to macro-level environmental influences.
Keywords: health policy; review; public health; infections, diseases, disorders, injuries
Key questions.
What is already known?
National action plans for addressing antimicrobial resistance (AMR) and country-level situation analyses are under way in individual countries and as part of learning networks (Global Antibiotic Resistance Partnership project) but employ different frameworks and approaches which may have local relevance but with temporal validity for policy design and limited transferability to other contexts.
What are the new findings?
Situation analyses for AMR in human heath have not yet employed a strategic management framework which is critical for building contingency at the strategic level for agile responses to macro-level environmental influences.
Technological and industry analyses is a notable gap.
What do the new findings imply?
Our analysis using a systematic, strategic management approach, rather than a retrospective view shows where important facilitators and inhibitors can be identified and leveraged.
Introduction
Antimicrobial resistance (AMR) as a serious public health threat is well recognised requiring coordination across governments, country borders, and health and non-health sectors to maintain effectiveness of antimicrobials.1–3 While AMR occurs naturally, there are multiple modifiable drivers which accelerate the emergence and spread of resistant pathogens.4 5 Wider political, economic, social and epidemiological contextual factors are relevant when thinking about drivers to resistance as well as solutions.6 7 These wider influences can facilitate or impede progress because they have direct and indirect effects on individuals (public, patient or healthcare professionals), organisations (healthcare and other sectors) and society as a whole.
Strategies and tools to support national-level interventions include the development and implementation of national action plans (NAPs) for AMR, based on best available evidence.8 The evidence base for informing these management strategies requires multidisciplinary approaches including risk assessment,9 10 meta-analysis11 and cost-effectiveness analysis.12
At the global level, in 2011, the WHO initiated a situation analysis of country progress in addressing AMR against four objectives: (1) Improve awareness and understanding of AMR through effective communication, education and training. (2) Strengthen the knowledge and evidence base through surveillance and research. (3) Reduce the incidence of infection through effective sanitation, hygiene and infection prevention measures. (4) Optimise the use of antimicrobial medicines in human and animal health.13 Currently only 19 have a NAP with funding sources identified, being implemented and with a defined monitoring and evaluation process in place. A further 43 have plans and processes in place but without funding commitments. Fifty-three countries are in the process of developing plans and 12 have still not initiated this process. With over 60% of countries worldwide at this developmental and planning stage, it is critical that rapid dissemination of learning from those countries that have progressed is shared. The systematic identification of wider influences which could facilitate or threaten progress is needed. Additionally, with use of strategic management approaches, key stakeholders may then work proactively to formulate contingent strategies particularly where wider influences carry high levels of uncertainty.
Country-level situation analyses are under way in individual countries14 and as part of learning networks (Global Antibiotic Resistance Partnership—GARP project) all employing different frameworks and approaches which may have local relevance but with temporal validity for policy design and limited transferability to other contexts.15
In this paper, therefore, we explore the possible contribution to the analysis of the AMR policy context from a very different literature stream, namely from the discipline of strategic management in management studies. Within this very broad field there are many different schools and approaches.16 17 An influential and early school which we access here can be termed the ‘design and planning’ school which seeks to examine the degree of ‘fit’ between an organisation (originally the private firm) and its external environment, using formal methods of rational analysis (rather than more qualitative methods such as visioning or brainstorming). One implication of this design and planning approach is that if the environment is displaying major shifts, then the organisation may need to change too.18 A second is that poor fit may well produce poor performance by the organisation.
But how can the very broad notion of the ‘external environment’ be best conceptualised? The design and planning strategic management tradition has produced two important and influential tools of analysis (originally for the private firm,16 and later for the public agency17), namely SWOT analysis (where Strengths and Weaknesses are internal to the organisation; but Opportunities and Threats come from the external environment), and then the more elaborated PESTEL (and later PESTELI (Political, Economic, Sociological, Technological, Ecological, Legislative, Industry)) framework which draws attention to the following domains: Political factors, Economic influences, Sociological trends, Technological innovations, Ecological factors, Legislative requirements and then Industry analysis.19
By performing a systematic search of the literature, this study aims to identify situation analyses conducted in AMR management in different countries and to assess which domains of the PESTELI are included in the extant literature. By doing so, we assess the depth and scope of previous analyses. We also report on the facilitators in addressing AMR identified in this previous research as well as the inhibitors that have been reported.
Methods
We conducted a literature review to identify situation analyses in addressing AMR.
Study eligibility
Any study published in English since 2000 that has performed a situation analysis to address AMR was considered in this review, in any country(ies) setting(s). The PICO (Population, Intervention, Comparison and Outcomes)20 and SPIDER (sample, phenomenon of interest, design, evaluation, research type)21 inclusion and exclusion criteria were applied at the review stages. To capture those studies that do not mention AMR more generally, but rather specific microbes, 13 clinically relevant bacteria (highlighted by Public Health England)22 were included in the search (eg, Escherichia coli). Studies focussing specifically/solely on drug resistance in tuberculosis, malaria and HIV were excluded. In addition, all of the country-level reports published by the GARP network were included (grey literature).
Search strategy and information sources
The methods used in this review are in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.23 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol (PRISMA-P) checklist was completed as the protocol for literature reviews. The search period was restricted from January 2000 onwards, because there was no policy momentum before this period. The language was limited to English. Studies which did not include AMR in humans were not included. Ovid Medline and Excerpta Medica database (EMBASE), Scopus and EconLit, Healthcare Management Information Consortium (HMIC), PsychInfo, and Institute of Electronical and Electronics Engineers (IEEE) electronic databases were searched. Searches included both controlled vocabulary (predefined subheadings) (eg, Microbials) and text words (eg, gram-negative). The search string used is provided in online supplementary appendix 1.
bmjgh-2019-001730supp001.pdf (19KB, pdf)
Study selection
Each title and abstract was independently reviewed by two researchers. The full text was independently reviewed by two researchers. Any discrepancies (three) were discussed and re-examined by a third researcher until agreement was reached.
Assessment of study quality and risks of bias
Formal quality appraisal of individual studies included was not performed, as this would be beyond the aim of this scoping review.24 The aim here was to identify gaps in the evidence base and to target topic areas for future research.25 26 In addition, use of peer-reviewed publications was used as the proxy for good quality.24 In addition, all of the country-level reports published by the GARP network, though grey literature, were also analysed as this network is recognised and cited by the global community for AMR policy design and evaluation.27
Data extraction and analysis
Data extraction was carried out by three researchers (RA, NJZ and EF) and 25% cross-validation between the researchers using a standardised data extraction table (Microsoft Excel), and then mapped against the PESTELI domains. The following information was extracted: study identifiers (title, author list, year of publication, journal, digital object identifier (DOI)), study characteristics (objectives, country/region setting), methods of data collection. Analysis established the scope of the situation analyses in relation to the PESTELI and if any other framework had been used. Facilitators and inhibitors to addressing AMR were mapped against the PESTELI domains. We anticipated descriptive results given the qualitative nature of the studies and hence presented these in text tables and figures.
Patient and public involvement
This manuscript is part of a wider study, which has one public representative. This representative is a member of the International Advisory Board, and has commented on the early findings of this work in October 2018. This manuscript being a review, did not require further assessment from a patient and public involvement (PPI) perspective. The representative will be actively supporting dissemination of the published work through patient and public networks in South Africa, India and the UK.
Results
Included studies
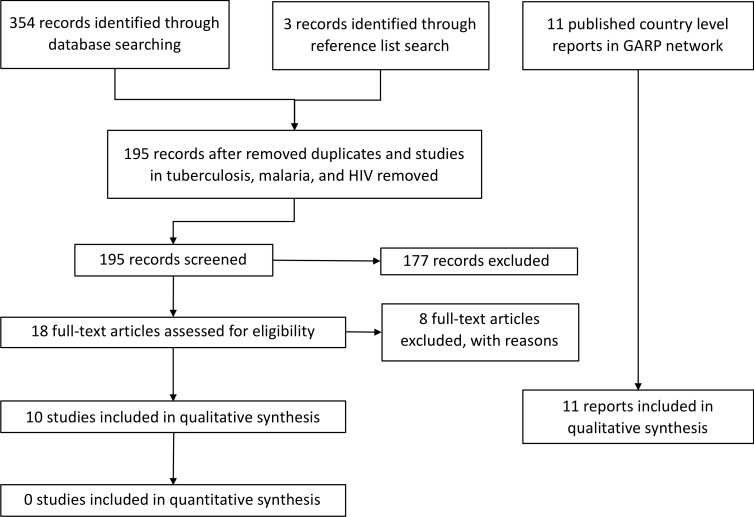
A total of 354 studies was identified from the primary electronic databases with another three from reference list searches. After removal of duplicates and studies in tuberculosis, malaria and HIV, a total of 195 records remained for screening. Eighteen studies were eligible for full-text review and 8 studies were excluded with reasons (2 commentary piece only, 6 organisational-level situation analysis) yielding a total of 10 studies that fit the inclusion criteria. The criteria for study exclusion are provided in online supplementary appendix 2. Figure 1 summarises the flow of literature searching and screening. Additionally, as described above, we included GARP reports published in 11 countries.
Figure 1.
Study flow diagram. GARP, Global Antibiotic Resistance Partnership.
Study characteristics
Of the included studies, six were single-country analysis and four regional-level multicountry studies. In terms of data collection, one study did not collect new data;28 one study used primary data collected through interviews with experts;29 one study carried out documentary review and interviews;30 three studies carried out a documentary review;31–33 two used a review of the literature;34 35 two studies reviewed literature and collected primary data through interviews with stakeholders.36 37
No study employed the PESTELI framework. Most studies (nine) included analysis of the political domain and one study included six domains of the framework. Technological and industry analyses is a notable gap. Only one study employed an established framework (SWOT).28 Table 1 presents the domains included in each study.
Table 1.
Domains of PESTELI (Political, Economic, Sociological, Technological, Ecological, Legislative, Industry) framework covered by the included studies
| Study (author/year) |
Settings | Data collection | PESTELI framework | |||||||
| P | E | S | T | E | L | I | Other frameworks | |||
| 36 | Nepal | Literature review; grey literature; 60 interviews | ✓ | ✓ | ✓ | ✓ | ||||
| 34 | Tanzania | Literature review; grey literature | ✓ | ✓ | SWOT analysis | |||||
| 35 | WHO African countries | Literature review | ✓ | ✓ | ||||||
| 31 | India | Documentary review; case studies | ✓ | ✓ | ✓ | ✓ | ||||
| 37 | Vietnam | Literature review; stakeholder and expert opinion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 28 | Germany | Concept piece—no data | ✓ | ✓ | ✓ | ✓ | ||||
| 30 | Ghana | Documentary review; four interviews | ✓ | ✓ | ✓ | ✓ | ||||
| 32 | South-East Asia | Documentary review | ✓ | ✓ | ||||||
| 33 | South-East Asia | Documentary review | ✓ | ✓ | ||||||
| 29 | South-East Asia | Concept piece—stakeholder and expert opinion | ✓ | ✓ | ||||||
SWOT, Strengths, Weaknesses, Opportunities and Threats.
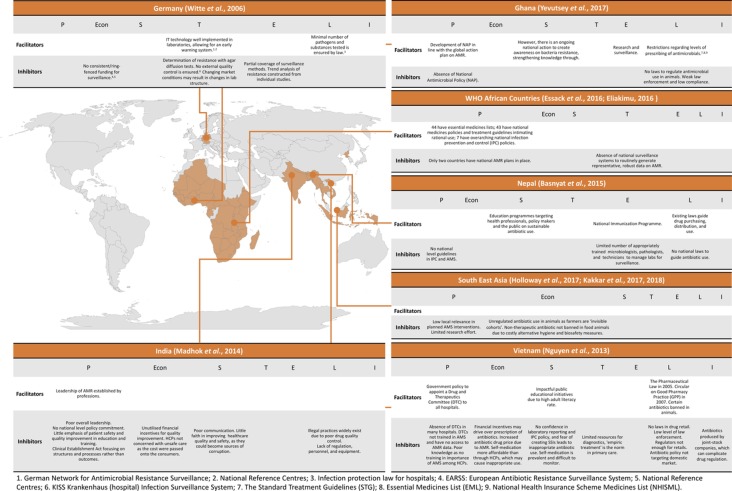
Figure 2 provides details the facilitators and inhibitors against the PESTELI framework and the country setting(s).
Figure 2.
Drivers and inhibitors in addressing antimicrobial resistance (AMR) identified in situation analyses.
Facilitators and inhibitors within the political and legislative domains were the most frequently reported. The political domain is featured in each study expect for one.28 Facilitators and inhibitors within the political and legislative domains were the most frequently reported. No facilitators were reported in the economic or industry domains but featured inhibiting factors.
Economic inhibitors were lack of ring-fenced funding for surveillance,28 unregulated non-therapeutic antibiotics in livestock due to costly alternative hygiene and biosafety measures,29 32 33 perverse financial incentives,37 cost-shifting to patients.31 Industry inhibitors were identified in only one study and reported as complications in regulation arising from joint-stock drug company ownership.37
The sociological domain captured education campaigns for the public and healthcare professionals as facilitators. Low level of confidence and motivation to improve quality among healthcare professionals was cited as an inhibitor31 and similarly low confidence in laboratory results was reported.37 Culture of self-medication and difficulty in monitoring this is reported on the public side.37
Among 15 countries that joined the GARP network, 11 have published a country-level report using situation analysis for AMR. Table 2 presents the main areas of findings included in each GARP country report. The reports have followed a structure to cover key areas such as population demographics, economic and health systems context, burden of diseases in humans and animals, AMR in humans, animals and agriculture, and drug regulation and legislation. There is some variability in terms of which areas are investigated in these situation analyses, but all do examine the burden of diseases and antibiotic use in humans and animals. Estimates of burden of AMR and the influence factors associated with the emergence and spread of AMR are missing in some countries. Except for Pakistan and India, all GARP reports describe the policies for regulating antibiotic drugs. Political context is missing in the analyses of nine countries. The heterogeneity of approaches to analysis, may be explained by the incremental phases of the GARP project. The situation analysis published during the first phase of the GARP network (2008–2011) conducted documentary review to synthesise secondary data from government and healthcare institutes. During the second phase (2012–2014), country-level reports have been produced using information in published literature. During the third phase (2015–current), expert opinion and stakeholder interviews have been included in recent situation analysis.
Table 2.
Areas included in individual GARP country report
| Year | Country | Information sources | Main areas covered in the country-level GARP report | |||||||||
| Demographics | Politic context | Economic context | Health systems setting | Disease burden | Antibiotic use | Drug regulation and supply chain | Other | |||||
| Human | Animal | Human | Animal | |||||||||
| 2010 | Vietnam | Documentary review | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 2011 | India | Literature review | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Interventions to be considered | |||
| 2011 | Kenya | Documentary review | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 2011 | South Africa | Documentary review | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 2014 | Nepal | Literature review | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 2015 | Mozambique | Literature review, expert opinion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 2015 | Tanzania | Documentary review | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 2015 | Uganda | Literature review, interviews | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 2017 | Zimbabwe | Literature review, interviews | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | AMR in agriculture | ||
| 2018 | Bangladesh | Documentary review, literature review | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Surveillance, requirements in establishing antimicrobial stewardship (AMS) (human resources, education, investment) | ||
| 2018 | Pakistan | Literature review, interviews | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Drug accessibility, AMS and interventions | |||
| No report available | Seychelles | |||||||||||
| Laos | ||||||||||||
| Namibia | ||||||||||||
| Nigeria | ||||||||||||
AMR, antimicrobial resistance; GARP, Global Antibiotic Resistance Partnership.
The studies identified are all in low and mid-income countries (LMICs) barring one study.28
Discussion
Our review shows that the PESTELI framework for strategic management has not been used for country-level analysis for addressing AMR. Some of the domains of the framework are included in different studies as reported above (figure 2). Analysis of the technological and industry domains is a notable gap. Facilitators and inhibitors within the political and legislative domains were the most frequently reported. No facilitators were reported in the economic or industry domains but featured inhibiting factors.
Learning from the field of strategic management shows that omitting important wider influences when making strategic decisions for addressing AMR can result in policies unaligned with the local landscape and give rise to unintended adverse consequences.38
By using a consistent and comprehensive framework such as the PESTELI framework, important facilitators and inhibitors can be identified and leveraged. Key stakeholders may then work proactively to formulate contingent strategies particularly where wider influences carry high levels of uncertainty. For example, in countries which perform well in the economic domain, the factors in the political domain can still hinder progress; notably, in Japan, five terms of office, seven general elections since 2000 and an average length of 1.9 years of office (July 1998 and December 2017) may need reliance on strengthening the other domains for sustainable solutions.39
Future analysis of existing quantitative data with interviews of key strategic and operational stakeholders, from each of the domains, is needed to provide critical insights about where implementation efforts should be focussed, and also how to build contingency at the strategic level for agile responses to macro-level environmental influences.
Situation analyses for AMR in human heath have not yet employed a strategic management framework which is critical for building contingency at the strategic level for agile responses to macro-level environmental influences.
Acknowledgments
The lead author thanks Dr Michiyo Iwami and Madeleine Clarkson for early discussions. The authors thank ESRC for support as part of the Antimicrobial Cross Council initiative supported by the seven UK research councils. The authors also thank the Global Challenges Research Fund, for support [ES/P008313/1].
Footnotes
Handling editor: Peter MacGarr Rabinowitz
Collaborators: On behalf of the ASPIRES study co-investigators: Raheelah Ahmad, Gabriel Birgand, Enrique Castro-Sánchez, Esmita Charani, Puneet Dhar, Ewan Balfour Ferlie, Mark Ian Hampton, Alison H Holmes [PI], Andy Leather, Mohamed Reda Lebcir, Marc Mendelson, Jules Ndoli Minega, Franco Sassi, Nick Sevdalis, Sanjeev Singh, Carolyn Clare Tarrant.
Contributors: Inception of the study: RA and EF. RA, NJZ carried out data collection. RA, NJZ, EF, AJML carried out data analysis. NJZ, AJML, EF contributed to the manuscript drafts with input from AH for finalisation. The ASPIRES Study group contributed to validation of the research questions, comment on early findings and high level expert input including identification of relevant grey literature.
Funding: RA, ECS, EC, AH, NJZ were part-funded by the National Institute for Health Research (NIHR) Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in collaboration with Public Health England and Imperial College Healthcare NHS Trust (HPRU-2012-10047). RA is supported by a NIHR fellowship in Knowledge Mobilisation (KMRF2015-007). ECS is NIHR Senior Nurse and Midwife Research Leader, and recognises the support of the NIHR Imperial Patient Safety Translational Research Centre. NS is supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King’s College Hospital NHS Foundation Trust. NS is a member of King’s Improvement Science, which is part of the NIHR CLAHRC South London and comprises a specialist team of improvement scientists and senior researchers based at King’s College London. Its work is funded by King’s Health Partners (Guy’s and St Thomas’ NHS Foundation Trust, King’s College Hospital NHS Foundation Trust, King’s College London and South London and Maudsley NHS Foundation Trust), Guy’s and St Thomas’ Charity, the Maudsley Charity and the Health Foundation. NS is also supported by the NIHR Global Health Research Unit on Health System Strengthening in Sub-Saharan Africa, King’s College London (GHRU 16/136/54). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Competing interests: Nick Sevdalis is the director of the London Safety and Training Solutions Ltd, which offers training in patient safety, implementation solutions and human factors to healthcare organisations.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
Contributor Information
the ASPIRES study co-investigators:
Raheelah Ahmad, Gabriel Birgand, Enrique Castro-Sánchez, Esmita Charani, Puneet Dhar, Ewan Balfour Ferlie, Mark Ian Hampton, Alison H Holmes [PI], Andy Leather, Mohamed Reda Lebcir, Marc Mendelson, Jules Ndoli Minega, Franco Sassi, Nick Sevdalis, Sanjeev Singh, and Carolyn Clare Tarrant
References
- 1. World Health Organization Antimicrobial resistance, 2018. Available: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance [Accessed 7 Apr 2019].
- 2. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations, 2014. Available: https://amr-review.org/sites/default/files/AMR Review Paper - Tackling a crisis for the health and wealth of nations_1.pdf [Accessed 21 May 2019].
- 3. McLeod M, Ahmad R, Shebl NA, et al. . A whole-health-economy approach to antimicrobial stewardship: analysis of current models and future direction. PLoS Med 2019;16:e1002774 10.1371/journal.pmed.1002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chereau F, Opatowski L, Tourdjman M, et al. . Risk assessment for antibiotic resistance in South East Asia. BMJ 2017;358 10.1136/bmj.j3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holmes AH, Moore LSP, Sundsfjord A, et al. . Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016;387:176–87. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 6. Dar OA, Hasan R, Schlundt J, et al. . Exploring the evidence base for national and regional policy interventions to combat resistance. The Lancet 2016;387:285–95. 10.1016/S0140-6736(15)00520-6 [DOI] [PubMed] [Google Scholar]
- 7. Årdal C, Outterson K, Hoffman SJ, et al. . International cooperation to improve access to and sustain effectiveness of antimicrobials. The Lancet 2016;387:296–307. 10.1016/S0140-6736(15)00470-5 [DOI] [PubMed] [Google Scholar]
- 8. Rajan D. Situation analysis of the health sector, 2016. Available: https://apps.who.int/iris/bitstream/handle/10665/250221/9789241549745-chapter3-eng.pdf?sequence=19&isAllowed=y
- 9. Acar J, Röstel B. Antimicrobial resistance: an overview. Rev Sci Tech 2001;20:797 10.20506/rst.20.3.1309 [DOI] [PubMed] [Google Scholar]
- 10. Ashbolt NJ, Amézquita A, Backhaus T, et al. . Human health risk assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environ Health Perspect 2013;121:993–1001. 10.1289/ehp.1206316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sazawal S, Hiremath G, Dhingra U, et al. . Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis : 2006. [DOI] [PubMed] [Google Scholar]
- 12. Malfertheiner P, Megraud F, O'Morain C, et al. . Current concepts in the management of Helicobacter pylori infection: the Maastricht III consensus report. Gut 2007;56:772–81. 10.1136/gut.2006.101634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization Monitoring global progress on addressing antimicrobial resistance: analysis report of the second round of results of AMR country self-assessment survey, 2018. Available: https://apps.who.int/iris/bitstream/handle/10665/273128/9789241514422-eng.pdf?ua=1 [Accessed 21 May 2019].
- 14. Federal Ministries of Agriculture Environmental and Health Antimicrobial use and resistance in Nigeria: situation analysis and recommendations, 2017. Available: https://ncdc.gov.ng/themes/common/docs/protocols/56_1510840387.pdf [Accessed 21 May 2019].
- 15. Center for Disease Dynamics Economics & Policy The global antibiotic resistance partnership (GARP), 2019. Available: https://cddep.org/wp-content/uploads/2017/06/garp_factsheet-1.pdf [Accessed 21 May 2019].
- 16. Mintzberg H. Managing. Berrett-Koehler publishers, 2009. https://books.google.co.uk/books?hl=zh-CN&lr=&id=ztZc6XKSBWMC&oi=fnd&pg=PR9&dq=Mintzberg+2009+&ots=Hp_Zi0imit&sig=W0OwaksJQlLnFAc49TV2KTzkQvQ&redir_esc=y#v=onepage&q=Mintzberg 2009&f=false [Google Scholar]
- 17. Ferlie E, Ongaro E. Strategic management in public services organizations. Routledge, 2015. [Google Scholar]
- 18. Greenhalgh T, Papoutsi C. Studying complexity in health services research: desperately seeking an overdue paradigm shift. BMC Med 2018;16:95 10.1186/s12916-018-1089-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cameron M, Cranfield S, Iles V, et al. . Managing change in the NHS: making informed decisions on change: key points for healthcare managers. Change 2001. [Google Scholar]
- 20. Richardson WS, Wilson MC, Nishikawa J, et al. . The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995;123:A12–13. [PubMed] [Google Scholar]
- 21. Cooke A, Smith D, Booth A. Beyond PICO: the spider tool for qualitative evidence synthesis. Qual Health Res 2012;22:1435–43. 10.1177/1049732312452938 [DOI] [PubMed] [Google Scholar]
- 22. Public Health England English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2018; 2018: 1–143.
- 23. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pham MT, Rajić A, Greig JD, et al. . A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods 2014;5:371–85. 10.1002/jrsm.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kastner M, Tricco AC, Soobiah C, et al. . What is the most appropriate knowledge synthesis method to conduct a review? protocol for a scoping review. BMC Med Res Methodol 2012;12:114 10.1186/1471-2288-12-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peters MDJ, Godfrey CM, Khalil H, et al. . Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141–6. 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 27. Gelband H, Laxminarayan R. Tackling antimicrobial resistance at global and local scales. Trends Microbiol 2015;23:524–6. 10.1016/j.tim.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 28. Witte W. Resistance monitoring of human pathogenic bacteria in Germany, SWOT analysis and examples. Int J Med Microbiol 2006;296 Suppl 41. [DOI] [PubMed] [Google Scholar]
- 29. Kakkar M, Chatterjee P, Chauhan AS, et al. . Antimicrobial resistance in South East Asia: time to ask the right questions. Glob Health Action 2018;11:1483637 10.1080/16549716.2018.1483637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yevutsey SK, Buabeng KO, Aikins M, et al. . Situational analysis of antibiotic use and resistance in Ghana: policy and regulation. BMC Public Health 2017;17:896 10.1186/s12889-017-4910-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madhok R, Vaid S, Carson-Stevens A, et al. . Promoting patient safety in India: situational analysis and the way forward. Natl Med J India 2014;27:217–23. [PubMed] [Google Scholar]
- 32. Holloway KA, Batmanabane G, Puri M, et al. . Antibiotic use in South East Asia and policies to promote appropriate use: reports from country situational analyses. BMJ 2017. 10.1136/bmj.j2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kakkar M, Sharma A, Vong S. Developing a situation analysis tool to assess containment of antimicrobial resistance in South East Asia. BMJ 2017. 10.1136/bmj.j3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eliakimu E. Antimicrobial stewardship in Tanzania: a consideration of strengths, weaknesses, opportunities and challenges for maintenance and further development of efforts. Int J Heal Gov 2016;21:150–64. [Google Scholar]
- 35. Essack SY, Desta AT, Abotsi RE, et al. . Antimicrobial resistance in the WHO African region: current status and roadmap for action. J Public Heal 2017;39:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Basnyat B, Pokharel P, Dixit S, et al. . Antibiotic use, its resistance in Nepal and recommendations for action: a situation analysis. J Nepal Health Res Counc 2015;13:102-11. [PubMed] [Google Scholar]
- 37. Nguyen KV, Thi Do NT, Chandna A, et al. . Antibiotic use and resistance in emerging economies: a situation analysis for Viet Nam. BMC Public Health 2013;13:1158 10.1186/1471-2458-13-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haenssgen MJ, Xayavong T, Charoenboon N, et al. . The consequences of AMR education and awareness raising: outputs, outcomes, and behavioural impacts of an antibiotic-related educational activity in Lao PDR. Antibiotics 2018;7 10.3390/antibiotics7040095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mizuno S, Iwami M, Kunisawa S, et al. . Comparison of national strategies to reduce meticillin-resistant Staphylococcus aureus infections in Japan and England. J Hosp Infect 2018;100:280–98. 10.1016/j.jhin.2018.06.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2019-001730supp001.pdf (19KB, pdf)