Abstract
Simple hepatic cysts are usually asymptomatic but can rarely result in life-threatening complications such as haemoperitoneum secondary to rupture. A 70-year-old woman with known simple hepatic cyst presented with acute chest pain and dyspnoea. The initial diagnosis was pulmonary embolism, and anticoagulation was commenced. However, she subsequently collapsed with haemodynamic instability. CT revealed a large hepatic cyst haematoma with rupture into the peritoneal cavity. The patient underwent emergency laparotomy, haemostasis and partial deroofing of the cyst. Retrospective review of CT scans suggested that the bleed had begun on presentation but was exacerbated by anticoagulation. To our knowledge, this is the first report of haemorrhagic hepatic cyst associated with acute anticoagulation. We discuss several important clinical lessons including cyst rupture as a possible cause of chest pain, the need for careful review of imaging and the choice of anticoagulation in patients with known simple hepatic cyst.
Keywords: gastrointestinal surgery, haematology (drugs and medicines)
Background
Cystic lesions of the liver comprise solitary simple cysts, multiple cysts (such as in polycystic liver disease), neoplastic cysts, parasitic (hydatid) cysts and abscesses.1 According to the American College of Gastroenterology, simple hepatic cysts are thought to arise from ‘congenital exclusions of hyperplastic bile duct rests that lack a communication with biliary ducts.’2 Simple hepatic cysts have an estimated incidence of 5%,3 but the increasing use of cross-sectional imaging means that a growing number of cysts are being detected incidentally. They are uncommon in people below the age of 40 years, with a female to male ratio of approximately 4:1.4 Simple hepatic cysts are usually asymptomatic, but in about 5% of cases, they can cause abdominal pain (the most common symptom), a palpable mass, fever, obstructive jaundice, portal hypertension or cyst rupture.5 6 Compression of adjacent organs may also occur, leading to abdominal distension, early satiety, nausea and vomiting.7 In contrast to parasitic hydatid cysts, in which cyst rupture is a well-recognised complication, simple hepatic cysts very rarely rupture.8
Here we present the first reported case of simple hepatic cyst rupture and haemoperitoneum associated with acute anticoagulation. The hepatic cyst rupture was confounded by an incidental discovery of a small pulmonary embolism. The difficulties encountered in the management of the case are described.
Case presentation
A 70-year-old woman with a known history of simple hepatic cyst and hypertension presented with a 1-day history of pain in the chest and shoulders, with shortness of breath on exertion. She denied recent long-haul travel and had no other risk factors for venous thromboembolism. The hepatic cyst had thus far been managed conservatively by the regional hepatobiliary team due to the lack of secondary effects (figures 1–4). Bedside observations were stable (blood pressure 143/72 mm Hg, heart rate 74 beats/min, respiratory rate 16 breaths/min, oxygen saturation 96% on room air and temperature 36.3°C), and on examination, there was mild tenderness over the epigastrium and the right upper quadrant. Her calves were soft non-tender.
Figure 1.
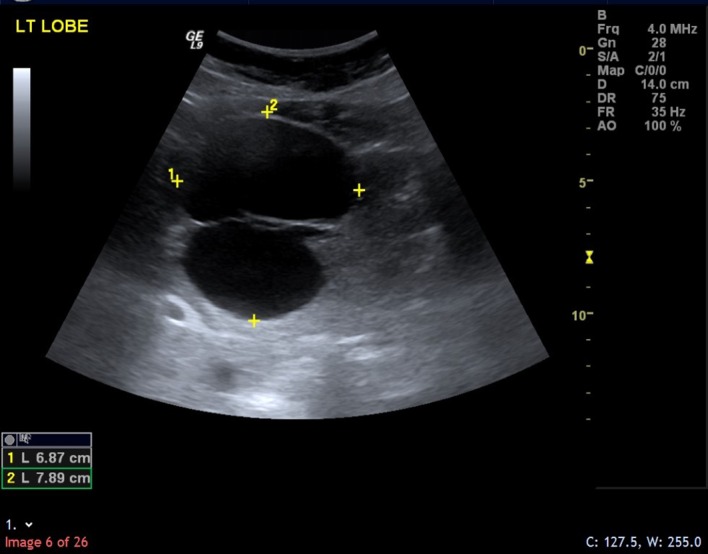
The hepatic cyst was discovered incidentally on ultrasound in April 2013 as a thin-walled bi-locular structure measuring approximately 7.9×6.9 cm.
Figure 2.
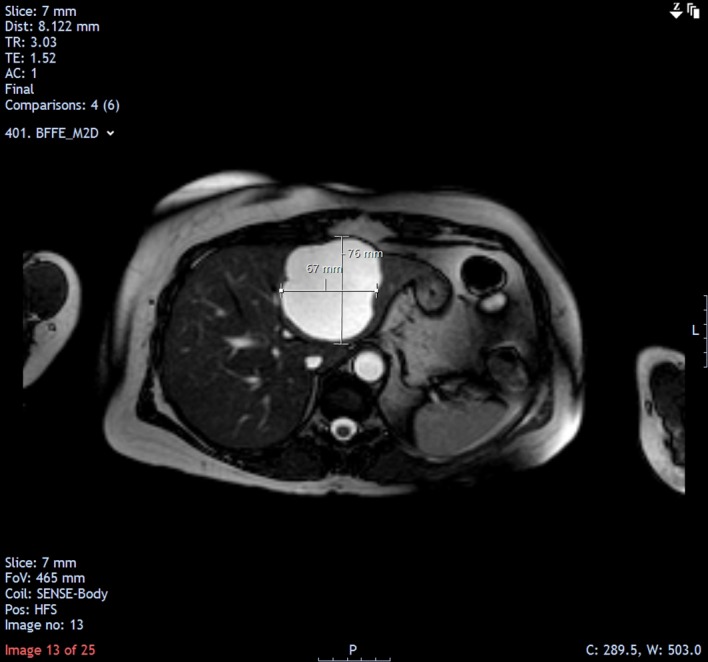
MRI follow-up in November 2013 showed that the size of the cyst was relatively unchanged (7.6×6.7 cm).
Figure 3.
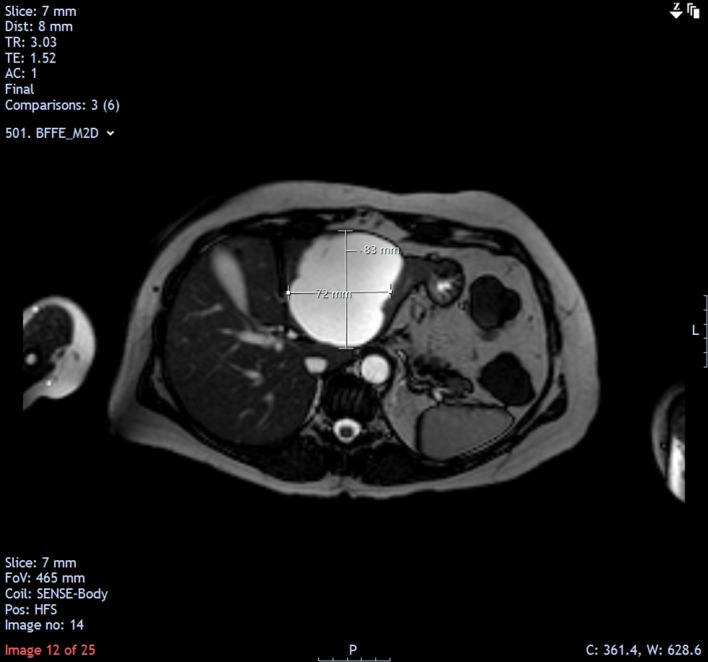
MRI follow-up in May 2014 showed a mild increase in the size of the cyst (8.3×7.2 cm).
Figure 4.
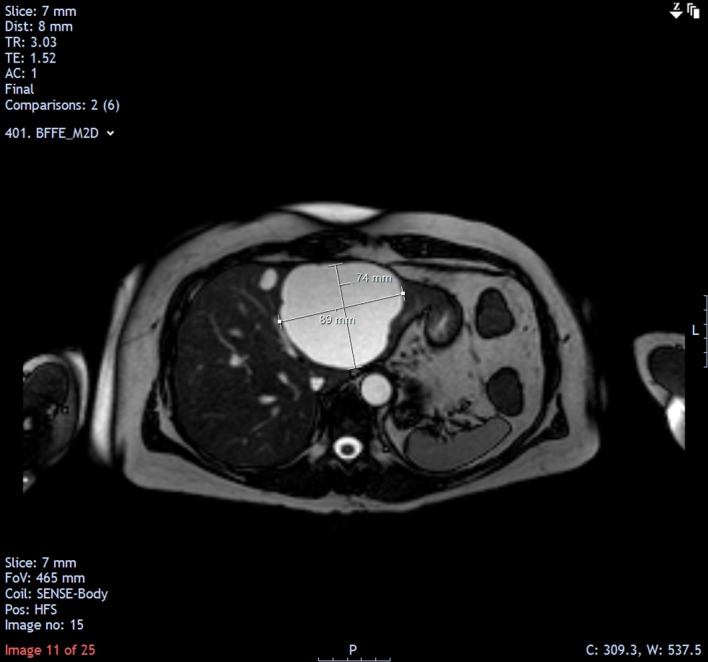
MRI follow-up in April 2015 showed a further mild increase in the size of the cyst (8.9×7.4 cm).
Differential diagnosis
Acute coronary syndrome must be excluded in any presentation of acute chest pain, given the patient’s history of hypertension.
Pulmonary embolism was considered due to the presentation of chest pain and dyspnoea, although the patient did not have any risk factors for venous thromboembolism.
Hepatic cyst complications were also among the differentials due to the known history.
Investigations
An ECG demonstrated sinus rhythm with no acute changes. Blood tests showed troponin I<40 ng/L (ie, not elevated), white cell count 14.1×109/L, C reactive protein 12.9 mg/L and a D-dimer of 582 ng/mL. Due to the elevated D-dimer, a CT pulmonary angiogram was performed, which suggested a faint filling defect in the left upper lobe pulmonary artery. The previously known cyst in the left lobe of the liver was also detected as a 6×8 cm hypodense lesion (figure 5).
Figure 5.
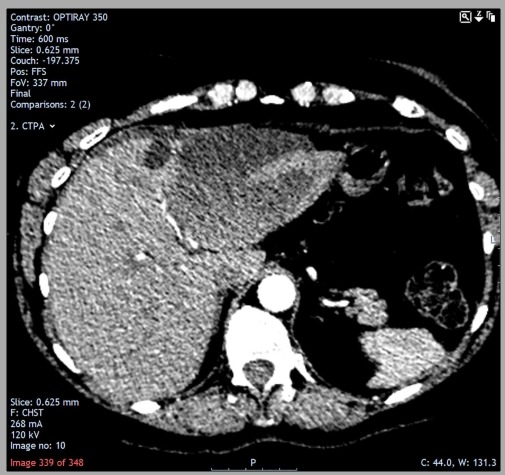
October 2018 (this admission): appearance of the hepatic cyst during the initial CT (pulmonary angiogram). The large cyst in the left lobe was demonstrated as a 6×8 cm hypodense lesion. Within the lesion, there was a subtle hyperdensity, suggesting the evolution of bleed. Adjacent to the large cyst was a smaller cyst that had been virtually unchanged compared with previous scans. Minimal perihepatic fluid was also present.
Treatment
The patient was initially treated as a case of unprovoked pulmonary embolism. After discussion with the patient, she was commenced on anticoagulation with rivaroxaban. The next morning, however, she became haemodynamically unstable and collapsed. Haemoglobin level dropped from 121 to 77 g/L overnight, prompting suspicion of a severe internal haemorrhage. The major haemorrhage protocol was activated, and an urgent repeat CT of the abdomen and pelvis was performed, which found a large well-defined subacute collection in the left hepatic lobe measuring 12.5×7.5×10 cm, with changing linear areas of hyperdensity within the collection. There was also free fluid around the liver and spleen and in the pelvis (figure 6), consistent with cyst bleed and rupture.
Figure 6.
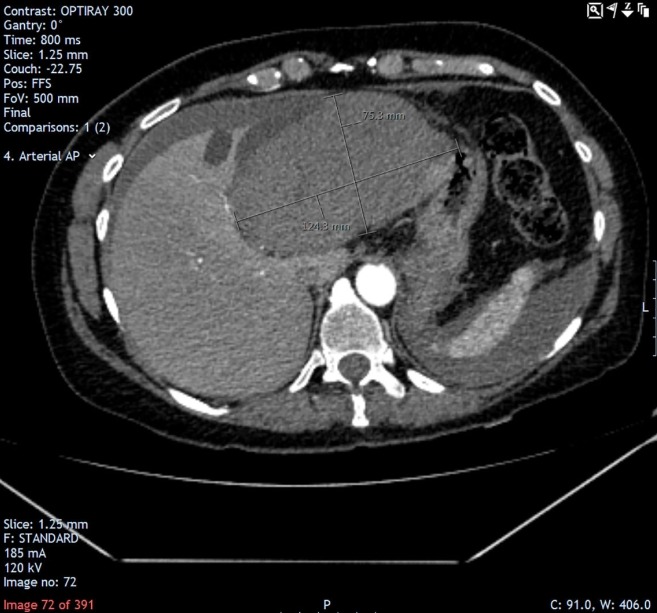
In the subsequent CT (abdomen and pelvis), a large well-defined subacute collection is demonstrated in the left hepatic lobe measuring 12.5×7.5×10 cm, with progression of free fluid around the liver and spleen.
The patient underwent emergency laparotomy with open deroofing of the hepatic cyst. About 2 L of blood was drained from the abdominal cavity. Good haemostasis was achieved, and the abdomen was closed by primary mass closure.
Outcome and follow-up
Postoperative recovery was uneventful, and the patient was closely monitored for 3 days in the intensive care unit. Both preoperative CT scans were reviewed with other radiologists, and interestingly (but with benefit of hindsight), it appeared that the hepatic cyst had begun to bleed even during the first scan, as suggested by a slight hyperdensity within the cyst (figure 5). Moreover, there was minimal perihepatic fluid visible, which progressed significantly in the subsequent CT. The overall impression was that the intracystic bleed with attendant rupture was more likely to be the cause of the patient’s symptoms, rather than the pulmonary embolism, which was deemed very insignificant. The bleed was thought to be exacerbated by the acute anticoagulation, which was therefore discontinued for the rest of her inpatient stay.
The patient was discharged on day 9 postoperatively, with no signs and symptoms of venous thromboembolism. Histological analysis of biopsied tissue confirmed that the lesion was benign. On subsequent follow-up appointments, she remained stable with no further symptoms. Repeat ultrasound scan 9 months later showed no evidence of recurrence of the hepatic cyst (figure 7).
Figure 7.
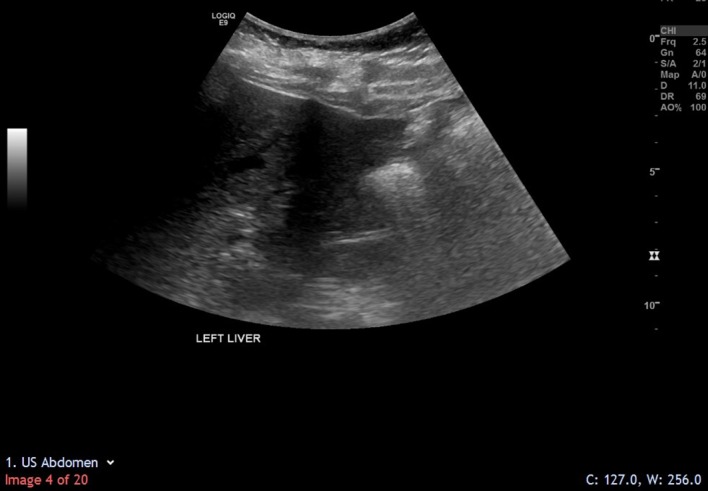
Repeat ultrasound scan in July 2019 showed that the hepatic cyst had not recurred.
Discussion
This case describes a rare event of a simple hepatic cyst rupture that had been aggravated by acute administration of an antithrombotic agent, giving rise to haemoperitoneum and haemodynamic instability. To our knowledge, this is the first report of a life-threatening haemorrhagic hepatic cyst linked to acute anticoagulation. It is unique in its presentation in the form of acute chest and shoulder pain, which can be explained by the phenomenon of referred pain, and the incidental finding of an insignificant pulmonary embolism, which turned out to be a red herring.
The aetiology and pathophysiology of simple hepatic cyst rupture remain unclear due to its rarity. Gaviser et al hypothesised that intracystic haemorrhage begins when high intracystic pressure causes necrosis of the cyst epithelium and injury to fragile blood vessels in the cyst wall.6 In our case, the confirmed intracystic bleed was aggravated by increased bleeding tendency due to anticoagulation, leading to a vicious cycle that raised the tension within Glisson’s capsule sufficiently to cause rupture. Necrosis was absent in histological analysis of the cyst wall, suggesting that this is not a necessary step prior to cyst rupture. Direct trauma has been cited as another possible contributing factor,6 but this was denied in the history.
A systematic review by Imaoka et al identified 18 relevant publications (29 cases) on non-parasitic hepatic cyst ruptures between 1959 and 2015.8 We further identified four publications,9–12 making a total of 34 cases (Imaoka included). Eleven of these resulted in haemoperitoneum, but only two were associated with long-term anticoagulation.10 13 In one case, Simon et al described a 63-year-old man who presented with an acute abdomen on a background of life-long acenocoumarol due to recurrent deep venous thromboses.10 The patient developed bilateral pulmonary embolism when his anticoagulation regime was reversed to treat his haemorrhagic hepatic cyst. He was eventually successfully treated by recommencement of anticoagulation with strict monitoring of international normalised ratio and partial thromboplastin time. In the other case, a 76-year-old man presented with haemoperitoneum from a haemorrhagic hepatic cyst on a background of long-term acenocoumarol use due to a haemodialysis shunt in his arm.13 Exploratory laparotomy was performed, and the bleed was stopped by placing the omentum over the ruptured hepatic cyst. Unfortunately, the patient suffered multiple postoperative complications and eventually died.
Our case was similar to Simon’s but in reverse: the pulmonary embolism was discovered first, leading to initiation of anticoagulation, which then had to be stopped due to the diagnosis of massive haemorrhage from the ruptured simple hepatic cyst. As in Simon’s case, there was a temporary dilemma between treating the pulmonary embolism with anticoagulation and the risk of exacerbating haemorrhage from the ruptured hepatic cyst. Given the rarity of hepatic cysts and their complications, it may be reasonable to treat the pulmonary embolism, but when the suspicion of hepatic cyst rupture arises, the vigilant clinician should delay anticoagulation until the possibility of internal bleeding has been ruled out by meticulously reviewing the radiological images with a consultant radiologist. Depending on the size of the pulmonary embolism and severity of haemorrhagic cyst rupture, if the risk-benefit ratio favours anticoagulation, intravenous unfractionated heparin ought to be chosen by virtue of its reversibility, in keeping with National Institute of Clinical Excellence guidelines.14
The American College of Gastroenterology provides guidance on the management of simple hepatic cysts.2 Asymptomatic cysts should be managed expectantly, whereas symptomatic cysts merit intervention. Simple aspiration is ineffective as the persistence of secretory epithelium leads to cyst recurrence15; concomitant introduction of sclerosing agents aimed at destroying the secretory epithelium (ie, aspiration sclerotherapy) is therefore recommended as the preferred first-line treatment.16 A systematic review by Wijnands et al demonstrated sustained cyst volume reduction ranging between 76% and 100% up to 54 months after aspiration sclerotherapy, with symptom reduction in 72%–100% of patients.17 Ethanol is the most common sclerosing agent,17 but recent advances have seen the use of non-ethanol alternatives such as ethanolamine oleate18 and polidocanol19 with good efficacy. The applicability of aspiration sclerotherapy to patients with haemorrhagic hepatic cysts remains unclear.6
Indications for a primary surgical approach include difficulty in ruling out cystadenoma or malignancy, biliary communication, failure of aspiration sclerotherapy and cyst recurrence.20 Options include laparoscopic or open deroofing/fenestration, cyst enucleation and hepatic resection.6 There have been no trials comparing deroofing/fenestration techniques with aspiration sclerotherapy, but data suggest the former is similarly or more effective in reducing symptoms although with significantly higher morbidity and mortality.16 Hepatic resection is typically reserved for cases where histology suggests cystadenoma or malignancy.6 Resolved haemorrhagic hepatic cysts that proved benign should be followed up with regular ultrasound scans.6
Learning points.
Haemorrhagic rupture is a rare but life-threatening complication of simple hepatic cysts, which may present as acute chest pain.
In patients with suspected hepatic cyst rupture, extreme caution should be taken when considering anticoagulation for pulmonary embolism. Radiological imaging should be meticulously reviewed to guide management.
If the risk-benefit ratio favours anticoagulation, this should be limited to judicious use of intravenous unfractionated heparin with close monitoring of haemodynamic status, international normalised ratio and partial thromboplastin time.
Asymptomatic simple hepatic cysts can be managed expectantly with serial imaging to monitor for progression, while symptomatic cysts merit intervention.
Footnotes
Contributors: AW and JG performed the surgery and contributed to the conception of the work and critical revision of the manuscript. KST and RH drafted the article. All authors gave final approval of the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Knott L, Bonsall A. Liver Cysts and Abscesses [Internet]. 2014. 2014. https://patient.info/doctor/liver-cysts-and-abscesses (14 Jan 2019).
- 2. Marrero JA, Ahn J, Rajender Reddy K. American College of Gastroenterology. ACG Clinical Guideline: The Diagnosis and Management of Focal Liver Lesions. Am J Gastroenterol 2014;109:1328–47. [DOI] [PubMed] [Google Scholar]
- 3. Caremani M, Vincenti A, Benci A, et al. Ecographic epidemiology of non-parasitic hepatic cysts. J Clin Ultrasound 1993;21:115–8. 10.1002/jcu.1870210207 [DOI] [PubMed] [Google Scholar]
- 4. Reid-Lombardo KM, Khan S, Sclabas G. Hepatic cysts and liver abscess. Surg Clin North Am 2010;90:679–97. 10.1016/j.suc.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 5. Inoue K, Iguchi T, Ito S, et al. Rerupture of nonparasitic liver cyst treated with cyst fenestration: a case report. Surg Case Rep 2015;1:71 10.1186/s40792-015-0075-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fong ZV, Wolf AM, Doria C, et al. Hemorrhagic hepatic cyst: report of a case and review of the literature with emphasis on clinical approach and management. J Gastrointest Surg 2012;16:1782–9. 10.1007/s11605-012-1922-6 [DOI] [PubMed] [Google Scholar]
- 7. van Gulick JJ, Gevers TJ, van Keimpema L, et al. Hepatic and renal manifestations in autosomal dominant polycystic kidney disease: a dichotomy of two ends of a spectrum. Neth J Med 2011;69:367–71. [PubMed] [Google Scholar]
- 8. Imaoka Y, Ohira M, Kobayashi T, et al. Elective laparoscopic deroofing to treat the spontaneous rupture of a large simple liver cyst: a case report. Surg Case Rep 2016;2:148 10.1186/s40792-016-0275-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimada S, Hara Y, Wada N, et al. Spontaneously ruptured hepatic cyst treated with laparoscopic deroofing and cystobiliary communication closure: A case report. Asian J Endosc Surg 2016;9:208–10. 10.1111/ases.12284 [DOI] [PubMed] [Google Scholar]
- 10. Simon T, Bakker IS, Penninga L, et al. Haemorrhagic rupture of hepatic simple cysts. BMJ Case Rep 2015;2015:bcr2014208676 10.1136/bcr-2014-208676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee C-C, Lin H-P, Ho W-C, W-c H, et al. Spontaneous Rupture of a Simple Hepatic Cyst Complicated With Intracystic Hemorrhage. J Med Ultrasound 2010;18:39–42. 10.1016/S0929-6441(10)60006-9 [DOI] [Google Scholar]
- 12. Poggi G, Gatti C, Delmonte A, et al. Spontaneous rupture of non-parasitic hepatic cyst. Int J Clin Pract 2005;60:99–103. 10.1111/j.1368-5031.2005.00655.x [DOI] [PubMed] [Google Scholar]
- 13. Carels RA, van Bommel EF. Ruptured giant liver cyst: a rare cause of acute abdomen in a haemodialysis patient with autosomal dominant polycystic kidney disease. Neth J Med 2002;60:363–5. [PubMed] [Google Scholar]
- 14. Venous thromboembolic diseases. diagnosis, management and thrombophilia testing [Internet]. 2015;4:12 https://www.nice.org.uk/guidance/cg144/chapter/Recommendations. [Google Scholar]
- 15. Saini S, Mueller PR, Ferrucci JT, et al. Percutaneous aspiration of hepatic cysts does not provide definitive therapy. AJR Am J Roentgenol 1983;141:559–60. 10.2214/ajr.141.3.559 [DOI] [PubMed] [Google Scholar]
- 16. Lantinga MA, Gevers TJ, Drenth JP. Evaluation of hepatic cystic lesions. World J Gastroenterol 2013;19:3543–54. 10.3748/wjg.v19.i23.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wijnands TF, Görtjes AP, Gevers TJ, et al. Efficacy and Safety of Aspiration Sclerotherapy of Simple Hepatic Cysts: A Systematic Review. AJR Am J Roentgenol 2017;208:201–7. 10.2214/AJR.16.16130 [DOI] [PubMed] [Google Scholar]
- 18. Nakaoka R, Das K, Kudo M, et al. Percutaneous aspiration and ethanolamine oleate sclerotherapy for sustained resolution of symptomatic polycystic liver disease: an initial experience. AJR Am J Roentgenol 2009;193:1540–5. 10.2214/AJR.08.1681 [DOI] [PubMed] [Google Scholar]
- 19. Eso Y, Furuta A, Takai A, et al. Ultrasound-guided microfoam sclerotherapy with polidocanol for symptomatic giant hepatic cyst: Initial experience. Hepatol Res 2018;48:1055–63. 10.1111/hepr.13202 [DOI] [PubMed] [Google Scholar]
- 20. Moorthy K, Mihssin N, Houghton PW. The management of simple hepatic cysts: sclerotherapy or laparoscopic fenestration. Ann R Coll Surg Engl 2001;83:409–14. [PMC free article] [PubMed] [Google Scholar]


