Abstract
Lipid droplets are cytosolic fat storage organelles present in most eukaryotic cells. Long regarded merely as inert fat reservoirs, they are now emerging as major regulators of cellular metabolism. They act as hubs that coordinate the pathways of lipid uptake, distribution, storage, and use in the cell. Recent studies have revealed that they are also essential components of the cellular stress response. One of the hallmark characteristics of lipid droplets is their capacity to buffer excess lipids and to finely tune their subsequent release based on specific cellular requirements. This simple feature of lipid droplet biology, buffering and delayed release of lipids, forms the basis for their pleiotropic roles in the cellular stress response. In stressed cells, lipid droplets maintain energy and redox homeostasis and protect against lipotoxicity by sequestering toxic lipids into their neutral lipid core. Their mobility and dynamic interactions with mitochondria enable an efficient delivery of fatty acids for optimal energy production. Lipid droplets are also involved in the maintenance of membrane and organelle homeostasis by regulating membrane composition, preventing lipid peroxidation and removing damaged proteins and lipids. Finally, they also engage in a symbiotic relationship with autophagy and act as reservoirs of bioactive lipids that regulate inflammation and immunity. Thus, lipid droplets are central managers of lipid metabolism that function as safeguards against various types of cellular stress.
Keywords: lipid droplets, fatty acids, nutrient stress, oxidative stress, autophagy, lipophagy, lipotoxicity, mitochondria, β-oxidation, lipid mediators, eicosanoids
Introduction
Cells rely on lipids to provide an efficient means of fuel storage and energy production. Lipids are also the major building blocks of cellular membranes and are precursors for hundreds of signaling molecules. Most eukaryotic cells have the capacity to store lipids in the form of lipid droplets. Being only recently recognized as dynamic, independent organelles rather than inert fat depots, lipid droplets are now emerging as central regulators of lipid uptake, metabolism, trafficking, and signaling in the cell. However, their roles extend well beyond lipid metabolism and an increasing number of studies suggest that they play vital roles in the cellular stress response [1-4].
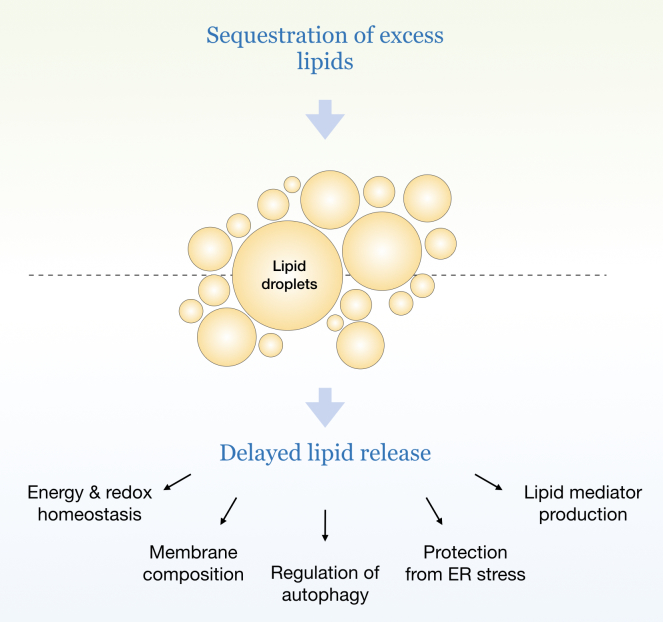
Lipid droplets are dynamically synthesized and broken down in response to cellular needs and environmental signals [5]. Their biogenesis is induced in cells exposed to excess amounts of lipids, to nutrient and oxidative stress, and to various other conditions characterized by energetic and redox imbalances [2]. Intriguingly, lipid droplet biogenesis also occurs in response to complete nutrient deprivation, suggesting that the expense of their synthesis must be worthwhile for the cell and is in fact essential for its response to stress. Indeed, emerging studies reveal that lipid droplets perform numerous tasks crucial for the protection of cellular integrity and function during stress: they sequester potentially toxic lipids and proteins, maintain energy and redox balance, preserve membrane and organelle homeostasis, modulate autophagy, and provide lipids acting as signaling mediators [1,2,4]. Many of these functions are engaged simultaneously and in cooperation with other organelles and we are only beginning to understand their interplay. One of the major properties of lipid droplets is their ability to buffer excess lipids, thereby providing immediate protection from lipotoxicity, and to release them later, gradually and according to cellular needs (Figure 1). This property is particularly important for cells exposed to rapidly changing conditions of stress and it is, for example, exploited by cancer cells during alternating periods of feeding/starvation or hypoxia/reoxygenation [6-10]. This simple aspect of lipid droplet biology – buffering and delayed release of lipids – is indispensable for most of their pleiotropic roles in the cellular stress response.
Figure 1.
Lipid droplet buffering and delayed release of lipids. Various conditions of cellular stress are characterized by lipid overload, arising from an excess of exogenous (e.g. increased uptake of lipids from the circulation) or endogenous lipids (e.g. starvation-induced autophagic breakdown of membranous organelles). Lipid droplets have the capacity to sequester excess lipids, thereby preventing their lipotoxicity, and to release them gradually based on cellular needs to preserve energy, redox, membrane, and organelle homeostasis through various mechanisms. This essential property of lipid droplets – buffering and delayed release of lipids – is particularly important for cells exposed to rapidly changing conditions of nutrient and oxidative stress.
In this article, we discuss emerging functions of lipid droplets in the management of cellular stress. We focus on how lipid droplet turnover and associated mechanisms are engaged during nutrient stress to maintain cellular homeostasis.
Lipid Droplet Biogenesis and Breakdown in Stressed Cells
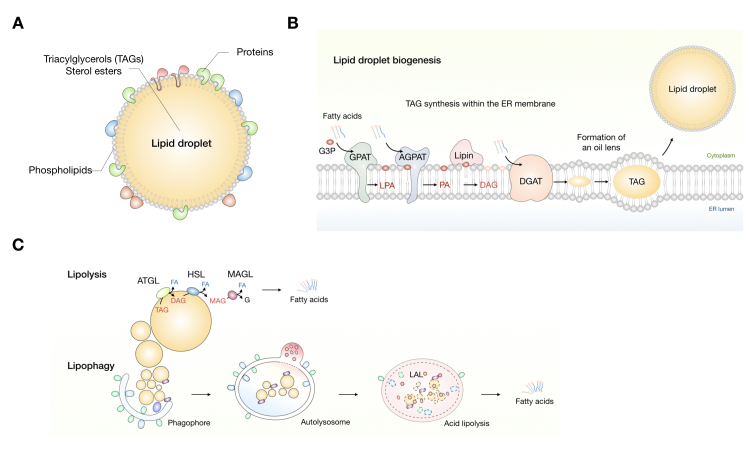
Lipid droplets are unique among organelles with their central hydrophobic core of neutral lipids surrounded by a single layer of phospholipids and proteins (Figure 2A). They are derived from the endoplasmic reticulum (ER†), whereby triacylglycerols (TAGs) are synthesized between the two leaflets of the ER membrane by sequential addition of fatty acids (FAs) to a glycerol backbone (Figure 2B) [11]. Diacylglycerol acyltransferases (DGATs) 1 and 2 catalyze the last and committed step in the TAG biosynthesis pathway. When sufficient neutral lipids accumulate, nascent lipid droplets bud from the ER membrane and are released into the cytosol [4,12,13]. Lipid droplets are broken down by two major mechanisms: lipolysis and lipophagy (Figure 2C) [14-16]. Lipolysis enables a highly regulated release of FAs from TAGs by the sequential action of adipose triglyceride lipase (ATGL), hormone sensitive lipase (HSL) and monoacylglycerol lipase (MAGL) [17-19]. Lipophagy is a recently discovered selective form of autophagy, whereby parts or whole lipid droplets are engulfed within autophagosomal membranes and fused with lysosomes for degradation by hydrolytic enzymes. At the organismal level, lipid droplet breakdown in adipocytes is hormonally regulated and provides FAs for mitochondrial energy production in non-adipose tissues during fasting and exercise [17,18,20]. However, lipid droplets in non-adipose tissues also undergo cycles of biogenesis and breakdown in response to nutrient and other cues from the environment. Their composition, number, size and distribution within cells is dynamically changing depending on the physiological state of the cell [21]. We are only beginning to understand how lipid droplet structure and dynamics define the multitude of their functions.
Figure 2.
Lipid droplet structure, biogenesis, and breakdown. (A) Lipid droplets are composed of a central hydrophobic core of neutral lipids, mostly composed of triacylglycerols (TAGs) and sterol esters, surrounded by a monolayer of phospholipid molecules, wherein numerous proteins are embedded. (B) TAG synthesis occurs in the endoplasmic reticulum membrane by sequential addition of fatty acids (FAs) (in their activated acyl-CoA form) to a glycerol-3-phosphate (G3P) backbone, yielding lysophosphatidic acid (LPA), phosphatidic acid (PA), and diacylglycerol (DAG). These reactions are catalyzed by several acyltransferase enzymes, including glycerol-3-phosphate acyltransferases (GPATs), acylglycerol-3-phosphate acyltransferases (AGPATs), and phosphatidic acid phosphatases (lipins). The last and committed step in the TAG synthesis cascade is catalyzed by the diacyglycerol acyltransferase enzymes (DGATs). (C) Lipid droplet breakdown occurs through lipolysis or lipophagy. TAG lipolysis is carried out by the sequential action of adipose triglyceride lipase (ATGL), hormone sensitive lipase (HSL), and monoacylglycerol lipase (MAGL). Lipophagy is a selective form of autophagy wherein lipid droplets are engulfed within autophagosomal membranes and delivered to lysosomes for degradation by acid lipolysis. Lysosomal TAG lipolysis is catalyzed by lysosomal acid lipase (LAL).
Enhanced lipid droplet accumulation has been observed in various types of cells exposed to complete or partial nutrient restriction [22-26]. Lipid droplet biogenesis in starved cells is essential for buffering autophagy-derived lipids, for protection of mitochondria from lipotoxic damage and for storing lipids for future consumption [25-28]. Lipid droplets also finely regulate the distribution and consumption of lipids during stress in order to maintain energy and redox homeostasis. As discussed in more detail below, starvation-induced dispersion of lipid droplets, their extensive contacts with the network of fused mitochondria and ATGL-mediated lipolysis likely provide the optimal means of FA transfer from lipid droplets to mitochondria [25,27]. In various cells and tissues, including adipocytes, hepatocytes and muscle cells, the delivery of FAs from lipid droplets to mitochondria and its coordination with mitochondrial and oxidative gene expression depends on ATGL activity [18,20,25,29]. In cells exposed to oxidative stress, lipid droplets accumulate in order to protect membranes from peroxidation reactions, maintain membrane saturation and organelle homeostasis, and enable a long-term supply of lipids for energy production and cell survival [6,7,30,31]. By stimulating FA oxidation and increasing NADPH levels in stressed cells, which is required for the maintenance of the glutathione-dependent antioxidant system, lipid droplet-derived FAs simultaneously provide energy and protect from oxidative stress [6,32,33]. As discussed in more detail below, it is therefore not surprising that dysregulated lipolysis may result in lipotoxicity and cell dysfunction. Thus, lipid droplets are dynamic organelles that are essential for the cellular response to metabolic stress.
Lipid Droplets Respond to Nutrient and Energy Imbalances in Stressed Cells
During evolution, cells have developed mechanisms of metabolite sensing in order to cope with the unreliable supply of sugars, amino acids and lipids from the environment [34]. The metabolite-sensing machinery facilitates the coordination of mechanisms that govern nutrient preference, uptake, distribution, recycling, and storage in order to maintain cellular homeostasis. AMP-activated protein kinase (AMPK) and mammalian target of rapamycin complex 1 (mTORC1) are highly conserved and fundamental reciprocal regulators of cellular metabolism [34-37]. AMPK is activated by nutrient and oxidative stress, genotoxins and xenobiotics [37]. It senses minute changes in the intracellular AMP:ATP ratio and responds to low energy states by suppressing anabolic processes, including de novo FA, cholesterol, and TAG synthesis [37-39], while stimulating ATP production through the activation of glycolysis, mitochondrial biogenesis, and FA oxidation [32]. Besides responding to levels of adenine nucleotides, recent reports have shown that AMPK activation may also occur at the lysosomal membrane, where an AMPK-activation complex senses glucose levels independently of the cellular energy status [35,40]. The mTORC1 kinase also localizes to lysosomes, where it is activated by nutrient abundance to promote anabolic cell growth pathways. By regulating the sterol regulatory element-binding protein (SREBP) transcription factors it stimulates FA, cholesterol, and glycerolipid synthesis, whereas it inhibits lipid catabolism by repressing the activity of the transcription factor EB (TFEB) [41-43]. mTORC1 is negatively regulated by AMPK, amino acid, or glucose starvation and is a strong suppressor of autophagy [35,40,44]. Autophagy/lipophagy may be directly regulated by AMPK via a “dual safe” mechanism, involving not only mTORC1 inhibition, but also direct phosphorylation of the Unc-51 like autophagy activating kinase 1 (ULK1) [45].
Different types and severities of nutrient restriction may distinctly affect cellular sensing mechanisms thereby resulting in specific cellular responses. Emerging studies have shown that lipid droplet metabolism is affected by the sensing and regulatory networks of AMPK and mTOR. In growing yeast cells, lipid precursors are mainly used for membrane synthesis. Following rapid exponential growth, nutrients such as glucose or amino acids are eventually exhausted, which leads to a metabolic shift towards mitochondrial oxidative metabolism. This is facilitated by the activation of Snf1 (the yeast homologue of AMPK), which senses glucose depletion and derepresses oxidative gene expression [40]. Intriguingly, the switch from fermentation to respiration also includes a rapid burst of lipid droplet biogenesis in order to redirect lipid precursors away from membrane synthesis and preserve them to enable cell survival during the ensuing starvation [3,46,47]. The shift in lipid metabolism is also dependent on the activation of phosphatidate phosphatase 1 (Pah1), which is necessary for TAG synthesis in the stationary phase and is indirectly regulated by TORC1 [48,49]. And how are lipid droplets consumed during starvation? Yeast cells undergoing sudden glucose restriction consume lipid droplets through microautophagy, i.e. microlipophagy, which is triggered by Snf1/AMPK activation, in order to survive during prolonged nutrient stress [47]. Intriguingly, yeast cells exposed to gradual glucose reduction, amino acid starvation, or TORC1 inhibition cannot activate microlipophagy and do not survive in the long-term, likely because they fail to maintain Snf1/AMPK activation [47].
The mTORC1 kinase may regulate lipid droplet turnover during stress through autophagy-dependent and autophagy-independent mechanisms. In Drosophila, hypoxia-induced TORC1 inhibition leads to autophagy-independent lipid droplet growth and lipid storage in the fat body, which is required for tolerance to hypoxia [50]. In mammalian cells, acute and complete nutrient restriction promotes lipid droplet biogenesis [24]. Experiments in starved mouse embryonic fibroblasts (MEFs) have revealed that amino acid depletion, but not glucose or serum limitation, drives lipid droplet biogenesis [26], which depends on mTORC1 inactivation-induced autophagy [26]. Interestingly, AMPK activation succeeded mTORC1 inhibition and was not required for autophagy initiation, indicating that it might instead be important for sustaining autophagic flux and oxidative metabolism during prolonged starvation [26]. Intriguingly, even in conditions of nutrient sufficiency AMPK may contribute to basal autophagy by activating the ULK1 kinase [51]. Given that lipophagy is activated in milder and prolonged conditions of serum restriction and in the presence of glucose and amino acids [9,25,52], it is not surprising that AMPK stimulates lipophagy through mTORC1-independent activation of ULK1 [51,53]. Thus, it seems that the intricate interplay between mTORC1 and AMPK signaling that regulates autophagy/lipophagy and lipid droplet metabolism depends on the type of nutrients being restricted, the severity and length of stress and the physiological context.
Interestingly, lipolysis and lipophagy are also transcriptionally regulated by common pathways downstream of AMPK and mTORC1 in response to changes in nutrient and energy levels [16,34]. AMPK and mTORC1 signal transduction pathways converge at the TFEB, peroxisome proliferator-activated receptor γ co-activator 1α (PGC1α)-PPARα-retinoid receptor X (RXR) and forkhead box protein O (FOXO) transcription factors that activate the transcription of ATGL and lysosomal acid lipase (LAL), thus promoting both neutral and acid lipolysis [16]. By promoting catabolism and elevating NAD+ levels, AMPK also indirectly activates the deacetylase sirtuin 1 (SIRT1) and its targets PGC1α and FOXO. On the contrary, starvation-induced mTORC1 inhibition leads to translocation of TFEB into the nucleus, thereby increasing the expression of genes that are involved in lysosomal biogenesis and function, autophagy and lysosomal exocytosis [54]. AMPK also regulates the expression and activation of PLIN3, which promotes lipid droplet dispersion during starvation, and the autophagic degradation of PLIN2, which regulates the binding of cytosolic lipases to the lipid droplet surface [55-58]. At the tissue/organismal level, the role of AMPK in regulating mammalian lipid droplet breakdown is still not clear, with numerous contradictory reports suggesting that AMPK has a pro- or anti-lipolytic function, or has no effect on lipolysis [16]. This suggests a marked tissue- and cell type-specific function of AMPK-mediated regulation of lipid droplet breakdown.
Lipid Droplets are Cellular Managers of Lipotoxicity
Lipotoxicity is a condition that occurs when cellular mechanisms fail to overcome cell damage caused by lipids [59]. It is often a consequence of imbalances between lipid uptake, storage and utilization. Cellular mechanisms of protection against lipotoxicity act on the principle of eliminating damaged or surplus lipids by transfer into inert storage pools, breakdown through oxidation or expulsion from the cell. Due to their central role in coordinating lipid uptake, storage, trafficking, and oxidation, lipid droplets are particularly well-suited among organelles to prevent, respond to and alleviate lipotoxic insults. A hallmark feature of lipid droplet biology is their ability to take up large amounts of lipids and release them gradually when needed in response to cellular requirements (Figure 1). Importantly, they do not act solely as simple buffers that minimize cellular lipid fluctuations, but also regulate the multitude of pathways that control the fate of stored lipids, thereby affecting all aspects of cellular homeostasis. Lipid droplets protect cells and tissues against lipotoxicity by mitigating the overload of both exogenous and endogenous lipids [2,4]. They reduce the lipotoxicity of “free,” non-esterified lipids by storing them in their less hazardous, esterified forms within the neutral lipid core [9,26,60-62]. FAs, diacylglycerols (DAGs), cholesterol and ceramides are stored within lipid droplets in the form of TAGs, cholesterol esters (CEs) and acylceramides, respectively. Cells require lipid droplets for protection against lipotoxicity in a variety of stressful conditions, including during lipid overload, e.g. exposure to exogenous FAs [9,61,63-65], hypoxia and oxidative stress [9,30,31], high autophagic flux [26,66] and dysfunctional lipolysis [67,68].
Intriguingly, the protective role of lipid droplets extends also to neighboring cells and tissues, and this cell non-autonomous function of lipid droplets is critical for proper maintenance of tissue homeostasis and stress responses [4,65,69,70]. This phenomenon is not limited to adipose tissue, which is specialized for the protection of cells and tissues from lipotoxicity both locally and at the organismal level, but is also important, for example, during neuronal development, whereby glial lipid droplets protect neighboring neuroblasts from lipotoxicity [30,69,71]. A cell-to-cell heterogeneity in the capacity for storing lipids has also been observed within subpopulations of cells, whereby a subset of cells accumulates more lipid droplets to reduce the risk for lipotoxic damage for the whole population, but also to supply stored lipids to neighboring cells when needed [72]. At the organismal level, lipid droplet biogenesis in different tissues improves insulin sensitivity [73], reduces accumulation of DAG and ceramide [74], and buffers postprandial surges of FAs in order to reduce their lipotoxicity [70]. Dysfunctional lipid droplet turnover may interrupt the ability of cells and tissues to fight against lipotoxic damage and can lead to persistent cellular stress, which is a hallmark of many diseases, including obesity, cardiovascular diseases, metabolic syndrome, diabetes, inflammation, and cancer [2,73,75,76]. The manipulation of lipid droplet turnover thus represents an attractive target for reducing the detrimental consequences of lipotoxicity.
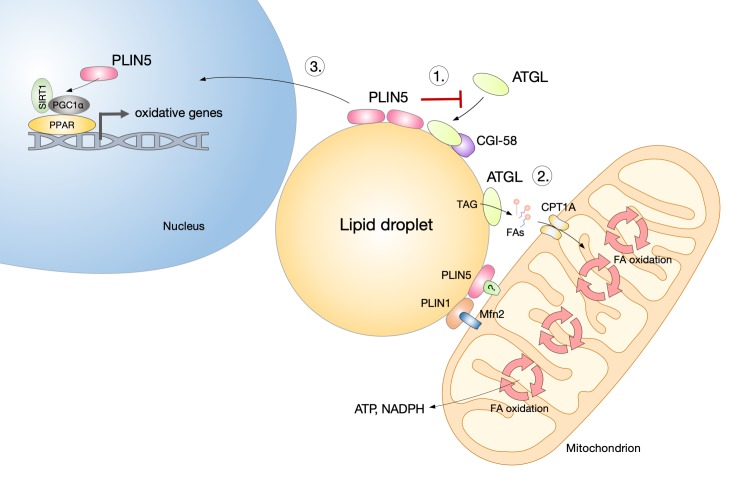
Lipolytic lipid droplet breakdown fuels and regulates mitochondrial oxidative metabolism and is essential for cell survival during nutrient deprivation in various cells [8,9,20,24,25,77]. However, upregulated lipolysis is a hallmark of cellular states and diseases associated with lipid overload. Elevated lipolysis may cause cell damage by increasing the pool of free FAs in the cytosol, altering cell signaling pathways affecting oxidative metabolism, ER homeostasis, and stimulating β-oxidation and reactive oxygen species (ROS) production [78,79]. Under these conditions, inhibition of ATGL-mediated lipolysis protects cells and tissues from lipotoxicity, oxidative stress, insulin resistance, and ER stress [9,67,68,80-83]. The lipid droplet coating protein perilipin 5 (PLIN5) plays a critical role in the regulation of lipolysis and lipotoxicity. It inhibits ATGL and provides a “lipolytic barrier” that suppresses uncontrolled TAG lipolysis, thereby reducing lipotoxic injury and insulin resistance in oxidative tissues (Figure 3) [67,80-82]. It controls the tight coupling between lipolysis and the metabolic demand for FAs, thus preventing ceramide accumulation and reducing oxidative stress, which may be induced by excessive FA oxidation and peroxidation [80,84,85]. The coordination of lipid droplet lipolysis and mitochondrial metabolism is thus critical for the removal of toxic FA species and the prevention of lipotoxicity. On the contrary, ATGL deficiency in macrophages leads to TAG accumulation and apoptotic cell death, suggesting that lipolysis may also play a protective role against lipotoxicity in some cases [86]. Therefore, the precise regulation of ATGL activity is critical for the balance between the protective effects of lipolysis on energy and redox homeostasis, and lipotoxic cell damage.
Figure 3.
Perilipin 5 regulates lipid droplet and mitochondrial metabolism through metabolic and signaling actions. The lipid droplet coating protein perilipin 5 (PLIN5) plays a critical role in the regulation of lipolysis and mitochondrial metabolism. At least three mechanisms of its action have been implicated in the cellular stress response. 1) In conditions of lipid overload, PLIN5 limits the activity of adipose triglyceride lipase (ATGL) and its co-activator lipid droplet-binding protein CGI-58 and provides a “lipolytic barrier” that suppresses uncontrolled triacylglycerol (TAG) lipolysis, thereby reducing lipotoxic injury and insulin resistance. 2) During nutrient starvation, PLIN5 facilitates fatty acid (FA) flux from lipid droplets to mitochondria and provides a physical link between the organelles. Their close proximity enables a more efficient FA transfer and supports energy and redox homeostasis. Its homologue PLIN1, which is specifically expressed in adipocytes, has been shown to directly interact with the mitochondrial protein mitofusin 2 (Mfn2), a mediator of mitochondrial fusion and mitochondria–lipid droplet interactions. 3) Catecholamine- or fasting-stimulated lipolysis leads to protein kinase A-dependent translocation and enrichment of PLIN5 in the nucleus, whereby it forms transcriptional complexes with sirtuin 1 (SIRT1) and peroxisome proliferator-activated receptor γ co-activator 1α (PGC1α), leading to the transcription of target genes involved in mitochondrial biogenesis and oxidative metabolism.
Lipid Droplets Interact with Other Organelles to Maintain Cellular Homeostasis
Lipid Droplets Interact with Mitochondria to Optimize Lipid Transfer and Energy Production
Energy production during glucose restriction depends on FA oxidation that primarily occurs in mitochondria in mammals, and in peroxisomes in yeast and plants [87]. Recent studies suggest that for an efficient delivery of FAs from lipid droplets to mitochondria a close proximity of both organelles is required, which could minimize the exposure of FAs to the cytosol and prevent lipotoxicity [25,26]. Indeed, lipid droplets form contacts with mitochondria and other organelles including the ER, Golgi, lysosomes, and peroxisomes [4,88,89]. The architecture of lipid droplet–organelle contact sites is mostly unknown, but recent studies suggest that they may include both protein complexes that function as tethers between the organelles and membrane bridges that connect the outer phospholipid monolayers of the opposing membranes [90]. The ability to form membrane bridges at organelle contact sites is unique to lipid droplets, due to the monolayer structure of their phospholipid coat, which may form continuous structures with the outer leaflet of bilayer membranes of other organelles. It may be envisaged that, in contrast to protein tethers, membrane bridges enable direct and efficient transfer of lipids and proteins between the two organelles. Notably, the transport between lipid droplets and mitochondria is probably bidirectional and not limited to FA transfer for the purpose of energy production [90]. For example, FA transfer in the reverse direction may protect mitochondria from lipotoxicity, and mitochondria may also contribute to phospholipid and TAG synthesis for lipid droplet growth [89,90]. Indeed, it has been recently shown in brown adipocytes that a subset of “peridroplet” mitochondria, which display elevated Krebs cycle activity but low FA oxidation capacity, in fact support lipid droplet biogenesis by providing ATP for the synthesis of TAG [91]. Benador et al. [92] have suggested that cells contain two major populations of mitochondria that enable the simultaneous occurrence of lipid synthesis and breakdown: peridroplet mitochondria, that promote lipid droplet biogenesis and protect from lipotoxicity, and non-lipid droplet-bound cytoplasmic mitochondria that specialize in FA oxidation and energy production.
Changes in mitochondrial structure and lipid droplet distribution are necessary for efficient FA trafficking during starvation [25,27]. In nutrient replete conditions, lipid droplets form clusters and are clumped around the nucleus [27]. On the contrary, in starved cells they adopt a dispersed distribution within the cell, which promotes their recruitment to mitochondria. Lipid droplets relocate from the cell center to the periphery by moving on detyrosinated microtubules. Activation of the energy sensor AMPK is essential for lipid droplet mobility along the microtubule network and stimulates their consumption during starvation [27]. Moreover, mitochondrial fusion is necessary for efficient β-oxidation in starved cells, because it ensures efficient FA intake and uniform distribution of FAs within the network of tubulated mitochondria [25]. Mitochondrial fusion in mammals is controlled by mitofusins (Mfn) 1 and 2, GTPase enzymes that control the fusion of the outer mitochondrial membrane [93]. Mfn2 deficiency in murine brown adipose tissue leads to lipid droplet accumulation and mitochondrial dysfunction [94]. In accordance, defects in mitochondrial fusion dynamics reduce β-oxidation and mitochondrial respiration, while promoting lipid droplet accumulation and FA efflux from cells [25]. ATGL activity, but not lipophagy, is essential for supplying mitochondria with lipid droplet-derived FAs in acutely starved MEFs [25], but it is not clear how the transfer of FAs is executed at the molecular level.
Several proteins have been shown to regulate the interaction between lipid droplets and mitochondria, including members of the PLIN family [94,95], however, it is still not clear how they contribute to FA transport from lipid droplets to mitochondria. The lipid droplet-coating protein PLIN5 regulates FA flux from lipid droplets to mitochondria [67] and provides a physical link between the organelles (Figure 3) [91,95]. In accordance, overexpression of PLIN5 has been shown to promote clustering of mitochondria around lipid droplets [95,96]. Interestingly, in adipocytes, Mfn2 binds to PLIN1 to facilitate the interaction between mitochondria and lipid droplets (Figure 3) [94]. Clearly, the protein composition, structure and dynamics of lipid droplet–mitochondria contact sites remain to be elucidated in future studies.
Lipid Droplets Associate with Peroxisomes to Support Lipid Metabolism
Lipid droplets also associate with peroxisomes [88,97,98]. In yeast, lipid droplets form stable interactions with peroxisomes and are enriched with peroxisomal β-oxidation enzymes, suggesting a coupling of lipid droplet lipolysis and peroxisomal FA oxidation [98]. The latter is essential for the breakdown of very-long chain and branched chain FAs [4,87] and for the shortening of polyunsaturated fatty acids (PUFAs), eicosanoids, and epoxy FAs before their final catabolism by mitochondrial oxidation [99]. Furthermore, lipid droplets and peroxisomes interact even in the presence of nutrients, when peroxisomes are not required for growth, indicating that lipid droplets may provide membrane lipids for peroxisome synthesis [98]. In addition, the organelles share a similar ER-mediated pathway for their biogenesis [97]. In mammalian cells, lipid droplets associate with peroxisomes through a tethering complex formed by spastin and ATP binding cassette subfamily D member 1 (ABCD1), which allows direct channeling of FAs from lipid droplets to peroxisomes [100]. Spastin enables lipid droplet–peroxisome interactions by attaching to the lipid droplet phospholipid monolayer and binding to ABCD1 in peroxisomes via its peroxisome-interacting region. Importantly, spastin also recruits two membrane-shaping endosomal sorting complexes required for transport (ESCRT)-III proteins, which facilitate FA trafficking, most likely by inducing morphological changes in the lipid droplet membrane. Moreover, the tethering complex is necessary for the removal of peroxidized lipids from lipid droplets, which suggests a novel role for the lipid droplet–peroxisome relationship in protecting cells against lipotoxicity and oxidative stress. Thus, contact sites between lipid droplets and mitochondria/peroxisomes enable bidirectional communication and effective lipid transfer, which contributes to energy production, maintenance of organelle integrity and prevention of lipotoxicity.
Lipid Droplets Regulate Nuclear Function
Interactions between lipid droplets and the nucleus are an emerging and exciting field of research, currently being addressed mostly from two major perspectives: the involvement of cytosolic lipid droplets in the regulation of nuclear function and the existence of nuclear lipid droplets and their poorly explained origins and function [101]. Cytosolic lipid droplets are often found in close proximity to the nucleus and affect its function via different mechanisms [101]. They are involved in protein exchange with the nucleus, chromatin assembly, and regulation of gene transcription [102-107]. These modulatory functions of lipid droplets are typically associated with the cellular response to stress. For example, the lipid droplet-associated fat-specific protein 27 (Fsp27) sequesters the transcriptional factor nuclear factor of activated T cells 5 (NFAT5) on cytosolic lipid droplets and away from the nucleus, thus directly affecting the execution of an osmoprotective gene expression program that depends on NFAT5 [103]. Furthermore, catecholamine signaling, which is responsible for activation of lipolysis during stress, may stimulate the translocation of the lipid droplet-associated protein PLIN5 to the nucleus, where it physically interacts with PGC-1α/SIRT1 complexes and regulates the transcription of genes that mediate mitochondrial oxidative metabolism (Figure 3) [104]. Thus, besides regulating lipase activity and mitochondria-lipid droplet interactions [67,81,85,91,95], PLIN5 also directly affects the transcription of genes essential for efficient coupling of lipolysis with oxidative metabolism. Intriguingly, bacterial lipid droplets bind directly to DNA using the microorganism lipid droplet small (MLDS) protein, thereby regulating MLDS gene transcription and cell survival under low nitrogen conditions [102]. Finally, lipid droplets are also modulators of chromatin assembly and organization. During early embryonic development of Drosophila, lipid droplets store excess maternal histones to ensure a sufficient supply for rapid nuclear division in the embryo [105]. The sequestration of excess histones also protects embryos from DNA damage [106,107] and, surprisingly, histone-bound LDs also protect the fly against bacterial infections [108]. These findings have begun to reveal many as yet unknown roles of cytosolic lipid droplets in the regulation of nuclear function that are involved in various aspects of the cellular stress response.
Recently, it has been reported that lipid droplets are also present in the nucleus [109-111]. Nuclear lipid droplets have been found in yeast and mammalian cells, and they are particularly abundant in hepatocytes [112,113]. Although it was initially not clear whether these are in fact cytoplasmic lipid droplets trapped in the nucleus [110], it has been confirmed recently that nuclear lipid droplets are formed through distinct mechanisms in the nucleus. These include budding from the inner nuclear membrane (INM) or from the lumen of the type I nucleoplasmic reticulum [112,114]. Studies in yeast have recently shown that nuclear lipid droplets bud from the INM and remain attached to it through seipin-mediated membrane bridges [112]. The latter suggest a dynamic relationship between the two structures, likely enabling bidirectional lipid and protein exchange depending on requirements for membrane synthesis vs storage, protection from lipotoxicity, or regulation of gene expression [4,112]. In hepatocytes, lipid droplets grow within the nucleus and their biogenesis depends on interactions with promyelocytic leukemia nuclear bodies, which are involved in regulation of transcription [113]. A recent study reported that hepatic nuclear lipid droplets derive from a precursor of very low density lipoprotein (VLDL), that arise from the lumen of the type I nucleoplasmic reticulum, and turn into nucleoplasmic lipid droplets by disintegration of the surrounding INM [114].
During nutrient stress caused by lipid overload, cells are faced with a great demand for additional phosphatidylcholine (PC), the main phospholipid constituent of the lipid droplet monolayer membrane. PC acts as a surfactant that prevents lipid droplet coalescence and its availability is essential for lipid droplet growth and expansion [115]. In Drosophila, local PC synthesis on the lipid droplet surface is mediated by the rate-limiting enzyme in the PC synthesis pathway CTP:phosphocholine cytidylyltransferase alfa (CCTα) [115]. In this case, the coordination of membrane PC synthesis with lipid droplet expansion is mediated by the reversible translocation of CCTα from the nucleus to growing cytosolic lipid droplets [115]. However, in other cell types CCTα is mostly localized to the nucleus, suggesting the presence of other coupling mechanisms [4,116]. Interestingly, new evidence suggests that hepatocyte nuclear lipid droplets are involved in the regulation of phospholipid synthesis [114]. They recruit and activate CCTα to promote PC synthesis, which is additionally modulated by competitive binding of PLIN3 to the nuclear lipid droplet surface. The nuclear regulation of PC synthesis is important for the maintenance of membrane homeostasis in response to ER stress [114]. The important discovery of nuclear lipid droplets elicits new questions about their functions in cell biology. It is worth speculating that they are predominantly involved in the regulation of gene expression, since they are located in the nucleus, but they may also exhibit similar roles to those established for cytosolic lipid droplets.
Lipid Droplets Maintain Membrane and ER Homeostasis
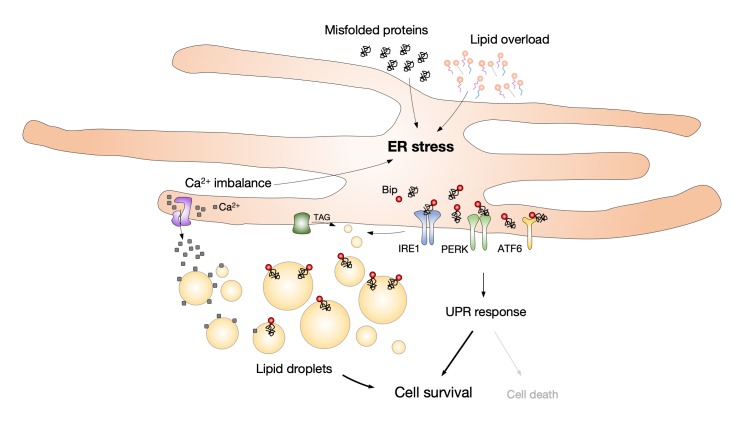
Cell integrity and function depend on the preservation of membrane and ER homeostasis. The ER is composed of a network of membrane cisternae and tubules that extends throughout the cell. It comprises the largest pool of lipids in the cell and forms membrane contact sites with all other organelles [117]. It has a pivotal role in the synthesis, storage, and secretion of proteins and lipids, and is important for calcium dynamics. Various pathophysiological events that interrupt protein folding processes, calcium uptake, or alter ER lipid composition may cause ER stress. Cells cope with ER stress by triggering the unfolded protein response (UPR) in order to restore ER homeostasis. The UPR is an adaptive mechanism whose activation leads to reduced protein translation, increased transcription of genes involved in the ER stress response, and elevated ER-associated protein degradation (ERAD). If any of these mechanisms fail or ER stress is prolonged, the UPR shifts from being an adaptive to a pro-apoptotic mechanism [1,4,118,119]. It is not surprising that lipid droplets, as organelles arising from and intricately associated with the ER, respond to disturbances in ER homeostasis. ER stress promotes lipid droplet formation [120,121] and lipid droplet accumulation has been observed as a general downstream effect of ER stress, including that induced by pharmacological agents, lipid overload, starvation, and impaired lipid biosynthetic pathways (Figure 4) [68,83,120,122,123]. Conversely, dysfunctional lipid droplet turnover also contributes to the induction of ER stress [68,122,124], suggesting a complex and bidirectional relationship between lipid droplets and ER stress. Emerging evidence has revealed that lipid droplets protect against ER stress by removing misfolded proteins, rebalancing ER lipid homeostasis and by regulating other stress response mechanisms, such as autophagy [68,122-127]. It has been shown that lipid droplets act as buffers that not only sequester excess free FAs but also unfolded or misfolded proteins in order to alleviate ER stress (Figure 4) [68,127,128]. The storage of calcium in the ER is affected by ER stress, which leads to the release of calcium in the cytosol and cell death [118,126]. However, it has been suggested that lipid droplets protect from cell death by sequestering calcium and preventing cytosolic calcium overload [129]. Interestingly, ER stress also promotes the formation of nucleoplasmic lipid droplets, which act in a feed-back mechanism that activates the synthesis of PC to alleviate ER stress [114]. Although the mechanisms linking lipid droplets and ER stress are not yet clear, changes in lipid droplet turnover seem to be tightly associated with ER stress. Through their ability to buffer toxic lipids and proteins, and by regulating lipid flux to membranes and organelles, lipid droplets may be essential for protein quality control as well as membrane synthesis, composition, and dynamics. Depending on the particular conditions and cell type, they may act both to prevent the onset of ER stress and to restore ER homeostasis upon UPR activation. Finally, the role of lipid droplets in the regulation of ER stress has been implicated in several pathologies, including insulin resistance, dyslipidemia, hepatic steatosis, heart failure, and inflammation [68,83,130].
Figure 4.
Lipid droplet formation is induced by ER stress to maintain lipid, protein and calcium homeostasis. ER stress may be triggered by various conditions, including the accumulation of misfolded proteins, excess or damaged lipids and calcium imbalance. Cells respond to ER stress by activating the unfolded protein response (UPR) in order to restore homeostasis and enable cell survival, but its prolonged activation may lead to cell death. In mammals, the UPR activation is regulated by three distinct transmembrane proteins: inositol-requiring enzyme 1 (IRE1), PKR (double-stranded-RNA-dependent protein kinase)-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6α (ATF6α). These proteins are activated by dissociating from the ER chaperone imunoglobulin heavy-chain-binding protein BiP/GRP78, which binds to misfolded proteins. ER stress promotes the formation of lipid droplets that bud from the ER membrane. Lipid droplets act as buffers, which sequester misfolded proteins and excess lipids in order to alleviate ER stress. Lipid droplets may also sequester free Ca2+ and protect from cytosolic Ca2+ overload, which has been associated with ER stress. Lipid droplets are thus crucial for the maintenance of lipid, protein and calcium ER homeostasis.
Pharmacological inducers of ER stress, including tunicamycin, brefeldin A, and thapsigargin, promote lipid droplet biogenesis in yeast [120] and mammalian cells [121]. Interestingly, although lipid droplet accumulation correlates with the level of ER stress in yeast cells, it is not essential for their survival [120]. In cardiomyocytes, treatments with exogenous palmitate, which is poorly incorporated into TAGs [61,131,132], upregulates the expression of genes associated with ER stress and causes lipotoxicity, but does not induce lipid droplet biogenesis [68]. When lipid droplet biogenesis was augmented by increasing the expression of peroxisome proliferator-activated receptor γ (PPARγ), palmitate-induced ER stress was mitigated, suggesting that sequestration of palmitate into neutral lipid stores reduces ER stress. However, inhibition of TAG synthesis by DGAT1 knockdown in cultured rat hepatocytes exposed to exogenous FAs did not enhance palmitate lipotoxicity or abolish the ability of oleate to reduce palmitate-induced ER stress [133]. Similarly, rather than promoting the flux of palmitate into TAG, oleate protects myeloid cells from palmitate-induced ER stress by counteracting its incorporation into phospholipids and reducing membrane saturation [134]. In accordance, the protective effect of monounsaturated FAs against ferroptosis, an iron- and lipid peroxidation-dependent form of cell death, was not dependent on lipid droplet formation, but on the displacement of easily oxidizable PUFAs from membrane phospholipids and the concomitant suppression of membrane lipid peroxidation [135]. Thus, the protective effects of monounsaturated FAs against ER stress and lipotoxicity are not always associated with lipid droplet metabolism. Future studies will help elucidate the currently obscure links between lipid droplets, ER stress, FA lipotoxicity, and ferroptosis [136].
In adipocytes, ER stress stimulates lipolysis by activating signaling pathways involved in the regulation of neutral lipases [137] and may contribute to lipotoxicity because of the persistently elevated FA efflux. Conversely, physiological conditions like fasting or intense exercise increase adipose tissue lipolysis to supply other tissues with FAs, but the large amounts of lipolytically released FAs could lead to ER stress and lipotoxicity even in the adipocytes themselves [122]. Therefore, a seemingly futile cycle of lipolysis followed by ATP-consuming FA re-esterification into TAGs occurs simultaneously in adipocytes in order to reduce ER stress. This is indicative of a cell-autonomous protective role of lipid droplet biogenesis against ER stress induced by high levels of lipolysis. In line with this, stimulation of ATGL-mediated lipolysis leads to ER stress even in cells treated with the otherwise beneficial, ER stress-reducing FA, oleate [68]. Similarly, facilitating autophagy-related 14 (ATG14)-mediated lipophagy results in accumulation of free FAs, ER stress, and apoptotic cell death [124]. On the contrary, the absence of ATGL reduces FA saturation levels in the liver and protects mice against tunicamycin-induced hepatic ER stress [83]. These results suggest that the excessive amounts of free FAs released through elevated lipid droplet breakdown are a major cause of ER stress and tissue damage in diseases associated with lipid overload.
ERAD is a mechanism for the elimination of unfolded or misfolded proteins from the ER through ubiquitination and subsequent degradation by the proteasome [138]. Genetic impairment of ERAD-mediated protein degradation or protein glycosylation pathways in yeast leads to lipid droplet accumulation, opening the possibility that lipid droplets are employed by cells to alleviate ER stress associated with protein misfolding [120]. Lipid droplets have been proposed to act as “escape hatches” for removal of damaged ER proteins [128]. Indeed, their role in maintaining protein quality and ER homeostasis has been recently demonstrated [127]. Impairment of yeast PC synthesis led to ER stress, elevated lipid droplet accumulation, and altered the morphology and localization of the ER. The ER was aggregated in the perinuclear area and co-localized with lipid droplets. In addition, chaperone proteins, including heat shock protein 104p, were recruited to the aggregated ER. Importantly, elevated levels of polyubiquitinated proteins were found in lipid droplet isolates, suggesting that unfolded proteins have been transferred from ER aggregates to lipid droplets. Finally, this study suggests that stress-induced lipid droplets are engulfed by and degraded in the vacuole via a process similar to microlipophagy [127]. Thus, lipid droplets likely serve as vehicles for the removal of damaged ER proteins and their delivery to the vacuole independently of the ERAD mechanism. In accordance, other studies have suggested the existence of ERAD-independent vesicle-mediated mechanisms of protein delivery and lysosomal elimination [139,140]. Thus, lipid droplets may regulate the elimination of damaged proteins from the ER by acting as transporters of unfolded or misfolded proteins intended for further elimination.
Lipid Droplets and Autophagy Work in Symbiosis
Autophagy is an evolutionarily conserved process of recycling dispensable cytoplasmic material by lysosomal degradation. It provides building blocks and metabolic substrates for processes essential for cell survival [141-143]. Strong links have been suggested between autophagy and the maintenance of cellular lipid homeostasis under physiological conditions and during stress [14,144]. Both lipid droplet turnover and autophagy are activated during stress and emerging evidence points to a complex, intertwined relationship between these processes [2].
Autophagy may participate in lipid droplet biogenesis in starving cells [25,26,28]. As discussed above, in acutely starved MEFs, autophagy of membranous organelles drives lipid droplet formation by providing a constant supply of FAs, which are then released by ATGL-mediated lipolysis and transferred to mitochondria for energy production [25]. The protective role of autophagy-induced lipid droplet biogenesis has also been observed in other organisms including yeast [145], algae [146], and plants [147]. In white adipose tissue, liver and heart, autophagy-driven lipid droplet biogenesis has been implicated in the regulation of adipocyte differentiation, maintenance of lipid storage, redox homeostasis, and energy metabolism [148-151]. Thus, autophagy contributes to lipid droplet biogenesis under various conditions, and it is possible that lipid droplet biogenesis is a general protective response to high levels of autophagy [26].
Autophagy is also involved in lipid droplet breakdown and control of lipid distribution through a lipid droplet-selective form of autophagy called lipophagy [52]. In lipophagy, whole or parts of lipid droplets are engulfed within autophagosomal membranes and delivered to lysosomes for degradation by acid lipolysis (Figure 2C). In yeast, lipid droplet contacts with vacuoles lead to direct invaginations of the vacuolar membrane and lipid droplet breakdown [127]. In mammalian cells, interactions between lipid droplets and lysosomes occur in both prolonged and brief kiss-and-run manners [4] and play an important role in the regulation of lipid droplet breakdown by lipolysis and lipophagy [55]. Lipophagy provides FAs for mitochondrial energy production in hepatocytes [52], participates in the clearance of toxic ceramides in the liver [152], prevents high-fat diet-induced hepatotoxicity and, together with mitophagy, protects against ethanol-induced liver injury [153]. Liver homeostasis may thus depend on constitutive lipophagy. Lipophagy in other tissues is less characterized, but it has been observed in adipocytes [144], macrophages [154], enterocytes [155], hypothalamic neurons [156], and embryos [157]. These studies suggest that lipophagy is involved in food-intake control, lipid distribution and protection against ectopic lipid accumulation. In yeast, microlipophagy, a special form of autophagosome-independent vacuole-mediated lipid droplet breakdown, is involved in the management of ER stress, protein quality control and energy metabolism [47,127,158]. Taken together, lipid droplet mobilization through autophagy occurs in different cell types and has important roles in the protection against cellular stress by reducing lipotoxicity, preserving organelle homeostasis, and maintaining energy metabolism.
Finally, lipid droplets may also promote the autophagic process in several ways, including the provision of lipids for the formation of autophagosomal membranes [159,160], regulation of FA flux and ER homeostasis [123], and through signaling-mediated regulation of autophagy [161]. Interestingly, in hepatocytes, ATGL acts as a signaling node that promotes autophagy/lipophagy through SIRT1-mediated signaling [161]. By orchestrating the extracellular and intracellular lipid fluxes necessary for energy production and membrane synthesis, lipid droplets may be an essential element of the autophagic stress response. In summary, autophagy-mediated lipid droplet metabolism is involved in the maintenance of lipid homeostasis and cell survival under various conditions of stress. On the one hand, autophagy participates in lipid droplet biogenesis or breakdown, while on the other, lipid droplets may provide lipids and signals for the proper execution of autophagy.
Lipid Droplets are Reservoirs of Bioactive Lipids
One of the most underappreciated aspects of lipid droplet biology is their role as cellular storage pools of lipid signaling mediators, i.e. bioactive lipids, and their precursors. Broadly speaking, the lipid composition of lipid droplets depends on the cell type, metabolic state, and environmental conditions. Lipid droplets in adipocytes are predominantly composed of TAGs, those found in Leydig cells of the adrenal cortex and macrophage foam cells mainly contain CEs, whereas retinyl-ester (RE)-rich lipid droplets may be found in hepatic stellate cells (HSCs) [154,162-164]. The fatty acyl group composition of neutral lipids also differs between cells and changes according to cell state. For example, PUFA enrichment in TAGs has been observed in hepatocytes of fasted mice [165], in HSCs during activation [166,167], in glial cells exposed to oxidative stress [30], in visceral adipose tissue of cancer patients [168], cancer cells exposed to exogenous PUFAs [9], and in apoptotic cells [169]. Lipids released from lipid droplets may act directly as signaling molecules, such as FAs, or serve as precursors for the synthesis of other bioactive lipid mediators, including eicosanoids, retinoic acid (RA), endocannabinoids, and ceramides [19,170-173]. Lipid droplet-mediated signaling has been shown to affect mitochondrial function and lipid metabolism [20,164], cell activation [172,173], inflammation [170,171,174-176], and may contribute to tumorigenesis [176-178].
Lipolysis has been associated with the production of inflammatory mediators in several types of immune cells. ATGL-mediated lipolysis has been shown to regulate the generation of pro-inflammatory eicosanoids in mastocytes [171] and mouse neutrophils [170]. In human activated mastocytes, blocking ATGL-mediated lipolysis resulted in lipid droplet accumulation and reduced levels of prostaglandin D2 (PGD2) and leukotriene C4 (LTC4), suggesting that TAG lipolysis is a source of arachidonic acid, a precursor for eicosanoid synthesis [171]. Similarly, pharmacological inhibition of ATGL in murine neutrophils led to a significant decrease in the release of several eicosanoids, including PGD2, LTE4, LTB2, and thromboxane B2. Importantly, exudates from ATGL deficient mice had reduced amounts of eicosanoids, suggesting that the enzyme participates in inflammatory signaling processes in vivo [170]. Additionally, it has recently been suggested that HSL is involved in the generation of lipid mediators in adipocytes [174]. Stimulation of adipocyte lipolysis with a non-selective β-adrenergic agonist resulted in elevated release of cyclooxygenase (COX)-, lipoxygenase (LOX)- and epoxygenase-derived eicosanoids, which was reduced by inhibition of HSL. In addition, elevated lipolysis led to COX-2 upregulation and infiltration of immune cells, suggesting that lipid mediators produced by lipolysis provoke an inflammatory response in adipose tissue [174]. In summary, lipolytic enzymes contribute to the synthesis of pro-inflammatory lipid mediators and are thus potential targets for therapeutic interventions in inflammatory diseases.
Lipid droplet breakdown may also occur through lipophagy and lysosomal degradation. In this case, LAL is involved in neutral lipid catabolism and it may contribute to bioactive lipid synthesis. Macrophages depleted in LAL accumulate neutral lipids within lysosomes, predominantly CEs [175]. These are particularly enriched with arachidonic and linoleic acids, both precursors of pro-inflammatory lipid mediators. Blocking LAL activity in macrophages resulted in a reduced release of several bioactive species derived from COX-, LOX- and cytochrome P450-mediated biosynthetic pathways, suggesting that lipid hydrolysis by LAL is an important source of precursors for bioactive lipid synthesis in macrophages. Further studies are required to establish the connection between LAL-mediated lipid mediator synthesis and lipid droplet breakdown in other immune and non-immune cells.
Lipid droplet-derived lipid mediators are also involved in HSC activation. In HSCs, different pools of lipid droplets have been observed. Quiescent HSCs mainly accumulate RE-rich lipid droplets that are gradually lost during cell activation. On the other hand, activated HSCs contain two structurally distinct pools of lipid droplets: “original,” composed of TAGs and REs, and “new,” transient lipid droplets that are enriched in TAGs containing PUFA acyl chains [163,179]. It has been suggested that the loss of REs from lipid droplets during cell activation promotes the synthesis of bioactive RA, which mitigates HSC activation [173]. Another study has suggested that during HSC activation lipid droplet breakdown and remodeling contribute to the generation of highly potent non-retinoid bioactive lipids, including PUFAs, endocannabinoids, N-acylethanolamides, and ceramides, which affect HSC activation and liver metabolism [172]. Thus, lipid droplets act as dynamic cellular reserves of bioactive lipids with distinct roles in cell metabolism.
Conclusion
Lipid droplets are emerging as major organelles implicated in organellar and membrane lipid homeostasis. Their dynamic turnover is under control of important cellular sensors and effectors of metabolic and oxidative stress, but much remains to be learned in order to understand the mechanisms of regulation of lipid droplet metabolism in stressed cells. It is now clear that lipid droplets interact with virtually all organelles through specific membrane contact sites, thereby engaging in bidirectional communication, which is essential for efficient exchange of lipids and proteins and is critical for various aspects of the cellular stress response. The detailed structures of the contact sites remain to be determined, the mechanisms of nutrient exchange revealed and their physiological significance confirmed in various settings. Likewise, we know very little about the biogenesis and function of nuclear lipid droplets and it will be exciting to witness future discoveries regarding their role in cell biology and diseases. We are only beginning to understand the heterogenous nature of lipid droplets, both in composition and function, within cells and within populations of cells. It will be important to find out how lipid droplets manage the trafficking of different lipid species and how their lipid composition and protein ensemble affects their function. Current evidence suggests that lipid droplet turnover has critical roles in the regulation of membrane lipid composition, lipid trafficking, mitochondrial function, and the protection of other organelles from lipotoxicity and oxidative stress. Their pleiotropic roles in managing lipids and stress will surely expand in the future as new functions and more detailed mechanisms are elucidated. Remarkably, lipid droplets also act as signaling platforms that affect nuclear function and gene expression in the cell, but also signal to neighboring cells through bioactive lipid mediators. Their involvement in the regulation of immune cell function, immunometabolism and inflammatory signaling will be an exciting field of discovery. The clarification of these and many other yet unasked questions in lipid droplet biology in stressed cells may help us uncover their roles in different pathologies, in particular metabolic disorders, cardiovascular diseases, cancer, and neurodegeneration, and may help us design strategies to prevent and fight against these debilitating diseases.
Acknowledgments
We sincerely thank Dr. Nataša Lindič for useful discussions and design of some of the figures and graphical elements. We thank Adrijan Ivanušec for critical reading of the manuscript. This work was supported by the 1000-15-106 Young Researcher grant to E.J., the J7-1818 Research Project to T.P., and the P1-0207 Research Programme grant from the Slovenian Research Agency.
Glossary
- AMPK
AMP-activated protein kinase
- ATGL
adipose triglyceride lipase
- CE
cholesterol ester
- COX
cyclooxygenase
- DGAT
diacylglycerol acyltransferase
- ER
endoplasmic reticulum
- FA
fatty acid
- HSC
hepatic stellate cell
- HSL
hormone sensitive lipase
- LOX
lipoxygenase
- LAL
lysosomal acid lipase
- MAGL
monoacylglycerol lipase
- mTORC1
mammalian target of rapamycin complex 1
- MEF
mouse embryonic fibroblast
- PLIN
perilipin
- PC
phosphatidylcholine
- PPAR
peroxisome-proliferator activated receptor
- PUFA
polyunsaturated fatty acid
- RA
retinoic acid
- RE
retinyl-ester
- TAG
triacylglycerol
- UPR
unfolded protein response
Author Contributions
T.P. conceptualized the study. E.J. and T.P. wrote the manuscript and designed the figures.
References
- Welte MA, Gould AP. Lipid droplet functions beyond energy storage. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. October;1862(10 10 Pt B):1260–72. 10.1016/j.bbalip.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petan T, Jarc E, Jusović M. Lipid Droplets in Cancer: Guardians of Fat in a Stressful World. Molecules. 2018. August;23(8):1941. 10.3390/molecules23081941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Reese ML, Goodman JM. The assembly of lipid droplets and their roles in challenged cells. EMBO J. 2018. June;37(12):e98947. 10.15252/embj.201898947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019. March;20(3):137–55. 10.1038/s41580-018-0085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81(1):687–714. 10.1146/annurev-biochem-061009-102430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, et al. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014. October;9(1):349–65. 10.1016/j.celrep.2014.08.056 [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Nambiar DK, Ramteke A, Kumar R, Dhar D, Agarwal C, et al. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget. 2015. September;6(26):22836–56. 10.18632/oncotarget.4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucer A, Brglez V, Payré C, Pungerčar J, Lambeau G, Petan T. Group X secreted phospholipase A(2) induces lipid droplet formation and prolongs breast cancer cell survival. Mol Cancer. 2013. September;12(1):111. 10.1186/1476-4598-12-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarc E, Kump A, Malavašič P, Eichmann TO, Zimmermann R, Petan T. Lipid droplets induced by secreted phospholipase A2 and unsaturated fatty acids protect breast cancer cells from nutrient and lipotoxic stress. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. March;1863(3):247–65. 10.1016/j.bbalip.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Wang YY, Attané C, Milhas D, Dirat B, Dauvillier S, Guerard A, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017. February;2(4):e87489. 10.1172/jci.insight.87489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfling F, Haas JT, Walther TC, Farese RV., Jr Lipid droplet biogenesis. Curr Opin Cell Biol. 2014. August;29:39–45. 10.1016/j.ceb.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Chung J, Farese RV., Jr Lipid Droplet Biogenesis. Annu Rev Cell Dev Biol. 2017. October;33(4):491–510. 10.1146/annurev-cellbio-100616-060608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam AR, Farese RV, Jr, Walther TC. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol. 2013. December;14(12):775–86. 10.1038/nrm3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CW. Lipid droplets, lipophagy, and beyond. Biochim Biophys Acta. 2016. August;1861(8 8 Pt B):793–805. 10.1016/j.bbalip.2015.12.010 [DOI] [PubMed] [Google Scholar]
- Schulze RJ, Sathyanarayan A, Mashek DG. Breaking fat: the regulation and mechanisms of lipophagy. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. October;1862(10 10 Pt B):1178–87. 10.1016/j.bbalip.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R, Madeo F, Kratky D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol. 2017. November;18(11):671–84. 10.1038/nrm.2017.76 [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004. November;306(5700):1383–6. 10.1126/science.1100747 [DOI] [PubMed] [Google Scholar]
- Schoiswohl G, Schweiger M, Schreiber R, Gorkiewicz G, Preiss-Landl K, Taschler U, et al. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J Lipid Res. 2010. March;51(3):490–9. 10.1194/jlr.M001073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner GF, Zimmermann R, Schicho R, Taschler U. Monoglyceride lipase as a drug target: at the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther. 2017. July;175:35–46. 10.1016/j.pharmthera.2017.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Büttner S, Schmidt A, van de Weijer T, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med. 2011. August;17(9):1076–85. 10.1038/nm.2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam AR, Beller M. The why, when and how of lipid droplet diversity. J Cell Sci. 2017. January;130(2):315–24. 10.1242/jcs.192021 [DOI] [PubMed] [Google Scholar]
- Du L, Hickey RW, Bayir H, Watkins SC, Tyurin VA, Guo F, et al. Starving neurons show sex difference in autophagy. J Biol Chem. 2009. January;284(4):2383–96. 10.1074/jbc.M804396200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubern A, Barceló-Torns M, Casas J, Barneda D, Masgrau R, Picatoste F, et al. Lipid droplet biogenesis induced by stress involves triacylglycerol synthesis that depends on group VIA phospholipase A2. J Biol Chem. 2009. February;284(9):5697–708. 10.1074/jbc.M806173200 [DOI] [PubMed] [Google Scholar]
- Cabodevilla AG, Sánchez-Caballero L, Nintou E, Boiadjieva VG, Picatoste F, Gubern A, et al. Cell survival during complete nutrient deprivation depends on lipid droplet-fueled β-oxidation of fatty acids. J Biol Chem. 2013. September;288(39):27777–88. 10.1074/jbc.M113.466656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold AS, Cohen S, Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell. 2015. March;32(6):678–92. 10.1016/j.devcel.2015.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, et al. DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev Cell. 2017. July;42(1):9–21.e5. 10.1016/j.devcel.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms A, Bosch M, Reddy BJ, Schieber NL, Fajardo A, Rupérez C, et al. AMPK activation promotes lipid droplet dispersion on detyrosinated microtubules to increase mitochondrial fatty acid oxidation. Nat Commun. 2015. May;6(1):7176. 10.1038/ncomms8176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa-Mansergas X, Fadó R, Atari M, Mir JF, Muley H, Serra D, et al. CPT1C promotes human mesenchymal stem cells survival under glucose deprivation through the modulation of autophagy. Sci Rep. 2018. May;8(1):6997. 10.1038/s41598-018-25485-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011. January;53(1):116–26. 10.1002/hep.24006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AP, Koster G, Guillermier C, Hirst EM, MacRae JI, Lechene CP, et al. Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell. 2015. October;163(2):340–53. 10.1016/j.cell.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman D, Tumanov S, Qiu B, Michalopoulou E, Spata M, Azzam A, et al. Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell Rep. 2018. September;24(10):2596–2605.e5. 10.1016/j.celrep.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012. May;485(7400):661–5. 10.1038/nature11066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013. April;13(4):227–32. 10.1038/nrc3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015. January;517(7534):302–10. 10.1038/nature14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D, Shaw RJ. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell. 2017. June;66(6):789–800. 10.1016/j.molcel.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J, Ortells MC, Tejedor S, Buxadé M, López-Rodríguez C. Transcriptional regulation of the stress response by mTOR. Sci Signal. 2014. July;7(332):re2. 10.1126/scisignal.2005326 [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012. March;13(4):251–62. 10.1038/nrm3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta. 2009. June;1791(6):501–6. 10.1016/j.bbalip.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999. March;338(Pt 3):783–91. 10.1042/bj3380783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Hardie DG. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018. February;27(2):299–313. 10.1016/j.cmet.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010. July;39(2):171–83. 10.1016/j.molcel.2010.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JL, Zhang Y, Bae SH, Farooqi MS, Liang G, Hammer RE, et al. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci USA. 2012. October;109(40):16184–9. 10.1073/pnas.1213343109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011. July;14(1):21–32. 10.1016/j.cmet.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen AM, Zoncu R. Emerging Roles for the Lysosome in Lipid Metabolism. Trends Cell Biol. 2017. November;27(11):833–50. 10.1016/j.tcb.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMPK and autophagy get connected. EMBO J. 2011. February;30(4):634–5. 10.1038/emboj.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri H, Rogers S, Ugrankar R, Liu YL, Feathers JR, Henne WM. Lipid droplet biogenesis is spatially coordinated at ER-vacuole contacts under nutritional stress. EMBO Rep. 2018. January;19(1):57–72. 10.15252/embr.201744815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo AY, Lau PW, Feliciano D, Sengupta P, Gros MA, Cinquin B, et al. AMPK and vacuole-associated Atg14p orchestrate μ-lipophagy for energy production and long-term survival under glucose starvation. eLife. 2017. April;6:e21690. 10.7554/eLife.21690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AD, Sembongi H, Su WM, Abreu S, Reggiori F, Carman GM, et al. Lipid partitioning at the nuclear envelope controls membrane biogenesis. Mol Biol Cell. 2015. October;26(20):3641–57. 10.1091/mbc.E15-03-0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubots E, Cottier S, Péli-Gulli MP, Jaquenoud M, Bontron S, Schneiter R, et al. TORC1 regulates Pah1 phosphatidate phosphatase activity via the Nem1/Spo7 protein phosphatase complex. PLoS One. 2014. August;9(8):e104194. 10.1371/journal.pone.0104194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Barretto EC, Grewal SS. TORC1 modulation in adipose tissue is required for organismal adaptation to hypoxia in Drosophila. Nat Commun. 2019. April;10(1):1878. 10.1038/s41467-019-09643-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011. February;13(2):132–41. 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009. April;458(7242):1131–5. 10.1038/nature07976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yang P, Zhao L, Chen Y, Zhang X, Zeng S, et al. CD36 plays a negative role in the regulation of lipophagy in hepatocytes through an AMPK-dependent pathway. J Lipid Res. 2019. April;60(4):844–55. 10.1194/jlr.M090969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci. 2016. July;129(13):2475–81. 10.1242/jcs.146365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015. June;17(6):759–70. 10.1038/ncb3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM. AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy. 2016;12(2):432–8. 10.1080/15548627.2015.1124226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinert M, Parker BL, Chaudhuri R, Fazakerley DJ, Serup A, Thomas KC, et al. mTORC2 and AMPK differentially regulate muscle triglyceride content via Perilipin 3. Mol Metab. 2016. June;5(8):646–55. 10.1016/j.molmet.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Xu M, Liu Y, Zhuang L, Ying K, Liu F, et al. Phosphorylation of PLIN3 by AMPK promotes dispersion of lipid droplets during starvation. Protein Cell. 2019. May;10(5):382–7. 10.1007/s13238-018-0593-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003. June;14(3):281–7. 10.1097/00041433-200306000-00008 [DOI] [PubMed] [Google Scholar]
- Senkal CE, Salama MF, Snider AJ, Allopenna JJ, Rana NA, Koller A, et al. Ceramide Is Metabolized to Acylceramide and Stored in Lipid Droplets. Cell Metab. 2017. March;25(3):686–97. 10.1016/j.cmet.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003. March;100(6):3077–82. 10.1073/pnas.0630588100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M, Kersten S, Hesselink MK, Schrauwen P. Re-evaluating lipotoxic triggers in skeletal muscle: relating intramyocellular lipid metabolism to insulin sensitivity. Prog Lipid Res. 2012. January;51(1):36–49. 10.1016/j.plipres.2011.11.003 [DOI] [PubMed] [Google Scholar]
- Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003. August;278(34):31861–70. 10.1074/jbc.M300190200 [DOI] [PubMed] [Google Scholar]
- Plötz T, Hartmann M, Lenzen S, Elsner M. The role of lipid droplet formation in the protection of unsaturated fatty acids against palmitic acid induced lipotoxicity to rat insulin-producing cells. Nutr Metab (Lond). 2016. February;13(1):16. 10.1186/s12986-016-0076-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 2010. March;120(3):756–67. 10.1172/JCI36066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecker T, Braun RJ, Westermann B. Lipid Droplets Guard Mitochondria during Autophagy. Dev Cell. 2017. July;42(1):1–2. 10.1016/j.devcel.2017.06.018 [DOI] [PubMed] [Google Scholar]
- Wang C, Zhao Y, Gao X, Li L, Yuan Y, Liu F, et al. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology. 2015. March;61(3):870–82. 10.1002/hep.27409 [DOI] [PubMed] [Google Scholar]
- Bosma M, Dapito DH, Drosatos-Tampakaki Z, Huiping-Son N, Huang LS, Kersten S, et al. Sequestration of fatty acids in triglycerides prevents endoplasmic reticulum stress in an in vitro model of cardiomyocyte lipotoxicity. Biochim Biophys Acta. 2014. December;1841(12):1648–55. 10.1016/j.bbalip.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015. January;160(1-2):177–90. 10.1016/j.cell.2014.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A, Lee MY, Sessa WC. Lipid Droplet Biogenesis and Function in the Endothelium. Circ Res. 2017. April;120(8):1289–97. 10.1161/CIRCRESAHA.116.310498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, MacKenzie KR, Putluri N, Maletić-Savatić M, Bellen HJ. The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab. 2017. November;26(5):719–737.e6. 10.1016/j.cmet.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms A, Bosch M, Ariotti N, Reddy BJ, Fajardo A, Fernández-Vidal A, et al. Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr Biol. 2013. August;23(15):1489–96. 10.1016/j.cub.2013.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Stone SJ, Zhou P, Buhman KK, Farese RV., Jr Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 2002. November;51(11):3189–95. 10.2337/diabetes.51.11.3189 [DOI] [PubMed] [Google Scholar]
- Liu L, Trent CM, Fang X, Son NH, Jiang H, Blaner WS, et al. Cardiomyocyte-specific loss of diacylglycerol acyltransferase 1 (DGAT1) reproduces the abnormalities in lipids found in severe heart failure. J Biol Chem. 2014. October;289(43):29881–91. 10.1074/jbc.M114.601864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikci Ertunc M, Hotamisligil GS. Lipid signaling and lipotoxicity in metabolic inflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res. 2016;•••:1–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, et al. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011. June;121(6):2102–10. 10.1172/JCI46069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KT, Mashek MT, Davidson NO, Mashek DG. Hepatic ATGL mediates PPAR-α signaling and fatty acid channeling through an L-FABP independent mechanism. J Lipid Res. 2014. May;55(5):808–15. 10.1194/jlr.M039867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G, Lass A. Genetically modified mouse models to study hepatic neutral lipid mobilization. Biochim Biophys Acta Mol Basis Dis. 2019. May;1865(5):879–94. 10.1016/j.bbadis.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T, Kuramoto K. Heart lipid droplets and lipid droplet-binding proteins: Biochemistry, physiology, and pathology. Exp Cell Res. 2016. January;340(2):198–204. 10.1016/j.yexcr.2015.10.031 [DOI] [PubMed] [Google Scholar]
- Zheng P, Xie Z, Yuan Y, Sui W, Wang C, Gao X, et al. Plin5 alleviates myocardial ischaemia/reperfusion injury by reducing oxidative stress through inhibiting the lipolysis of lipid droplets. Sci Rep. 2017. February;7(1):42574. 10.1038/srep42574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak NM, Schweiger M, Jaeger D, Kolb D, Kumari M, Schreiber R, et al. Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier. J Lipid Res. 2013. April;54(4):1092–102. 10.1194/jlr.M034710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bell M, Sreenivasan U, Hu H, Liu J, Dalen K, et al. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem. 2011. May;286(18):15707–15. 10.1074/jbc.M110.207779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CD, Claudel T, Kumari P, Haemmerle G, Pollheimer MJ, Stojakovic T, et al. Absence of adipose triglyceride lipase protects from hepatic endoplasmic reticulum stress in mice. Hepatology. 2012. July;56(1):270–80. 10.1002/hep.25601 [DOI] [PubMed] [Google Scholar]
- Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, et al. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem. 2012. July;287(28):23852–63. 10.1074/jbc.M111.328708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens C, Bourlier V, Mairal A, Louche K, Badin PM, Mouisel E, et al. Perilipin 5 fine-tunes lipid oxidation to metabolic demand and protects against lipotoxicity in skeletal muscle. Sci Rep. 2016. December;6(1):38310. 10.1038/srep38310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflaki E, Radović B, Chandak PG, Kolb D, Eisenberg T, Ring J, et al. Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages. J Biol Chem. 2011. March;286(9):7418–28. 10.1074/jbc.M110.175703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi IJ, Semenkovich CF. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab. 2014. March;19(3):380–92. 10.1016/j.cmet.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, et al. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature. 2017. June;546(7656):162–7. 10.1038/nature22369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Valm AM, Lippincott-Schwartz J. Interacting organelles. Curr Opin Cell Biol. 2018. August;53:84–91. 10.1016/j.ceb.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner M, Bohnert M. A different kind of love - lipid droplet contact sites. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. October;1862(10 10 Pt B):1188–96. 10.1016/j.bbalip.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Benador IY, Veliova M, Mahdaviani K, Petcherski A, Wikstrom JD, Assali EA, et al. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 2018. April;27(4):869–885.e6. 10.1016/j.cmet.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benador IY, Veliova M, Liesa M, Shirihai OS. Mitochondria Bound to Lipid Droplets: Where Mitochondrial Dynamics Regulate Lipid Storage and Utilization. Cell Metab. 2019. April;29(4):827–35. 10.1016/j.cmet.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Scorrano L. The INs and OUTs of mitofusins. J Cell Biol. 2018. February;217(2):439–40. 10.1083/jcb.201801042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutant M, Kulkarni SS, Joffraud M, Ratajczak J, Valera-Alberni M, Combe R, et al. Mfn2 is critical for brown adipose tissue thermogenic function. EMBO J. 2017. June;36(11):1543–58. 10.15252/embj.201694914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sreenivasan U, Hu H, Saladino A, Polster BM, Lund LM, et al. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res. 2011. December;52(12):2159–68. 10.1194/jlr.M017939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AD, Savage DB, Siniossoglou S. Lipid droplet-organelle interactions: emerging roles in lipid metabolism. Curr Opin Cell Biol. 2015. August;35:91–7. 10.1016/j.ceb.2015.04.017 [DOI] [PubMed] [Google Scholar]
- Joshi AS, Nebenfuehr B, Choudhary V, Satpute-Krishnan P, Levine TP, Golden A, et al. Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Nat Commun. 2018. July;9(1):2940. 10.1038/s41467-018-05277-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns D, Januszewski T, Chen Y, Hill J, Markin VS, Zhao Y, et al. An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol. 2006. June;173(5):719–31. 10.1083/jcb.200511125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven PP. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J Lipid Res. 2010. October;51(10):2863–95. 10.1194/jlr.R005959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CL, Weigel AV, Ioannou MS, Pasolli HA, Xu CS, Peale DR, et al. Spastin tethers lipid droplets to peroxisomes and directs fatty acid trafficking through ESCRT-III. J Cell Biol. 2019. August;218(8):2583–99. 10.1083/jcb.201902061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte MA. Expanding roles for lipid droplets. Curr Biol. 2015. June;25(11):R470–81. 10.1016/j.cub.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yang L, Ding Y, Wang Y, Lan L, Ma Q, et al. Bacterial lipid droplets bind to DNA via an intermediary protein that enhances survival under stress. Nat Commun. 2017. July;8(1):15979. 10.1038/ncomms15979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Shen WJ, Patel S, Greenberg AS, Azhar S, Kraemer FB. Fat-specific protein 27 modulates nuclear factor of activated T cells 5 and the cellular response to stress. J Lipid Res. 2013. March;54(3):734–43. 10.1194/jlr.M033365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo-Montejano VI, Saxena G, Kusminski CM, Yang C, McAfee JL, Hahner L, et al. Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1α/SIRT1-dependent transcriptional regulation of mitochondrial function. Nat Commun. 2016. August;7(1):12723. 10.1038/ncomms12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Thiel K, Thul PJ, Beller M, Kühnlein RP, Welte MA. Lipid droplets control the maternal histone supply of Drosophila embryos. Curr Biol. 2012. November;22(22):2104–13. 10.1016/j.cub.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Johnson MR, Ke Z, Chen L, Welte MA. Drosophila lipid droplets buffer the H2Av supply to protect early embryonic development. Curr Biol. 2014. July;24(13):1485–91. 10.1016/j.cub.2014.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MR, Stephenson RA, Ghaemmaghami S, Welte MA. Developmentally regulated H2Av buffering via dynamic sequestration to lipid droplets in Drosophila embryos. eLife. 2018. July;7:e36021. 10.7554/eLife.36021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Cermelli S, Li Z, Kassan A, Bosch M, Sigua R, et al. A novel role for lipid droplets in the organismal antibacterial response. eLife. 2012. November;1:e00003. 10.7554/eLife.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layerenza JP, González P, García de Bravo MM, Polo MP, Sisti MS, Ves-Losada A. Nuclear lipid droplets: a novel nuclear domain. Biochim Biophys Acta. 2013. February;1831(2):327–40. 10.1016/j.bbalip.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Uzbekov R, Roingeard P. Nuclear lipid droplets identified by electron microscopy of serial sections. BMC Res Notes. 2013. September;6(1):386. 10.1186/1756-0500-6-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang Y, Liang Y, Li J, Liu Y, Zhang J, et al. Specific accumulation of lipid droplets in hepatocyte nuclei of PFOA-exposed BALB/c mice. Sci Rep. 2013;3(1):2174. 10.1038/srep02174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanauska A, Köhler A. The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets. Cell. 2018. July;174(3):700–715.e18. 10.1016/j.cell.2018.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsaki Y, Kawai T, Yoshikawa Y, Cheng J, Jokitalo E, Fujimoto T. PML isoform II plays a critical role in nuclear lipid droplet formation. J Cell Biol. 2016. January;212(1):29–38. 10.1083/jcb.201507122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sołtysik K, Ohsaki Y, Tatematsu T, Cheng J, Fujimoto T. Author Correction: nuclear lipid droplets derive from a lipoprotein precursor and regulate phosphatidylcholine synthesis. Nat Commun. 2019. March;10(1):1230. 10.1038/s41467-019-09294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahmer N, Guo Y, Wilfling F, Hilger M, Lingrell S, Heger K, et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011. October;14(4):504–15. 10.1016/j.cmet.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsaki Y, Suzuki M, Fujimoto T. Open questions in lipid droplet biology. Chem Biol. 2014. January;21(1):86–96. 10.1016/j.chembiol.2013.08.009 [DOI] [PubMed] [Google Scholar]
- Wu H, Carvalho P, Voeltz GK. Here, there, and everywhere: the importance of ER membrane contact sites. Science. 2018. August;361(6401):eaan5835. 10.1126/science.aan5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapala I, Marza E, Ferreira T. Is fat so bad? Modulation of endoplasmic reticulum stress by lipid droplet formation. Biol Cell. 2011. June;103(6):271–85. 10.1042/BC20100144 [DOI] [PubMed] [Google Scholar]
- Han J, Kaufman RJ. The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res. 2016. August;57(8):1329–38. 10.1194/jlr.R067595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei W, Wang H, Fu X, Bielby C, Yang H. Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Biochem J. 2009. October;424(1):61–7. 10.1042/BJ20090785 [DOI] [PubMed] [Google Scholar]
- Lee JS, Mendez R, Heng HH, Yang ZQ, Zhang K. Pharmacological ER stress promotes hepatic lipogenesis and lipid droplet formation. Am J Transl Res. 2012;4(1):102–13. [PMC free article] [PubMed] [Google Scholar]
- Chitraju C, Mejhert N, Haas JT, Diaz-Ramirez LG, Grueter CA, Imbriglio JE, et al. Triglyceride Synthesis by DGAT1 Protects Adipocytes from Lipid-Induced ER Stress during Lipolysis. Cell Metab. 2017. August;26(2):407–418.e3. 10.1016/j.cmet.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez AP, Tatsuta T, Ghillebert R, Drescher I, Graef M. Lipid droplet-mediated ER homeostasis regulates autophagy and cell survival during starvation. J Cell Biol. 2016. March;212(6):621–31. 10.1083/jcb.201508102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Schlaepfer IR, Bergman BC, Panda PK, Praharaj PP, Naik PP, et al. ATG14 facilitated lipophagy in cancer cells induce ER stress mediated mitoptosis through a ROS dependent pathway. Free Radic Biol Med. 2017. March;104:199–213. 10.1016/j.freeradbiomed.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Chen E, Tsai TH, Li L, Saha P, Chan L, Chang BH. PLIN2 is a Key Regulator of the Unfolded Protein Response and Endoplasmic Reticulum Stress Resolution in Pancreatic β Cells. Sci Rep. 2017. January;7(1):40855. 10.1038/srep40855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskás LG, Fehér LZ, Vizler C, Ayaydin F, Rásó E, Molnár E, et al. Polyunsaturated fatty acids synergize with lipid droplet binding thalidomide analogs to induce oxidative stress in cancer cells. Lipids Health Dis. 2010. June;9(1):56. 10.1186/1476-511X-9-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vevea JD, Garcia EJ, Chan RB, Zhou B, Schultz M, Di Paolo G, et al. Role for Lipid Droplet Biogenesis and Microlipophagy in Adaptation to Lipid Imbalance in Yeast. Dev Cell. 2015. December;35(5):584–99. 10.1016/j.devcel.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh HL. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 2007. July;448(7152):435–8. 10.1038/nature06004 [DOI] [PubMed] [Google Scholar]
- Barba I, Chavarria L, Ruiz-Meana M, Mirabet M, Agulló E, Garcia-Dorado D. Effect of intracellular lipid droplets on cytosolic Ca2+ and cell death during ischaemia-reperfusion injury in cardiomyocytes. J Physiol. 2009. March;587(Pt 6):1331–41. 10.1113/jphysiol.2008.163311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greineisen WE, Maaetoft-Udsen K, Speck M, Balajadia J, Shimoda LM, Sung C, et al. Chronic Insulin Exposure Induces ER Stress and Lipid Body Accumulation in Mast Cells at the Expense of Their Secretory Degranulation Response. PLoS One. 2015. August;10(8):e0130198. 10.1371/journal.pone.0130198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001. August;50(8):1771–7. 10.2337/diabetes.50.8.1771 [DOI] [PubMed] [Google Scholar]
- Thörn K, Bergsten P. Fatty acid-induced oxidation and triglyceride formation is higher in insulin-producing MIN6 cells exposed to oleate compared to palmitate. J Cell Biochem. 2010. October;111(2):497–507. 10.1002/jcb.22734 [DOI] [PubMed] [Google Scholar]
- Leamy AK, Hasenour CM, Egnatchik RA, Trenary IA, Yao CH, Patti GJ, et al. Knockdown of triglyceride synthesis does not enhance palmitate lipotoxicity or prevent oleate-mediated rescue in rat hepatocytes. Biochim Biophys Acta. 2016. September;1861(9 9 Pt A):1005–14. 10.1016/j.bbalip.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robblee MM, Kim CC, Porter Abate J, Valdearcos M, Sandlund KL, Shenoy MK, et al. Saturated Fatty Acids Engage an IRE1α-Dependent Pathway to Activate the NLRP3 Inflammasome in Myeloid Cells. Cell Rep. 2016. March;14(11):2611–23. 10.1016/j.celrep.2016.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A, et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem Biol. 2019. March;26(3):420–432.e9. 10.1016/j.chembiol.2018.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Stockwell BR. Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol. 2018. May;16(5):e2006203. 10.1371/journal.pbio.2006203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Liu S, Zou L, Xu C, Geng B, Xu G. Lipolysis response to endoplasmic reticulum stress in adipose cells. J Biol Chem. 2012. February;287(9):6240–9. 10.1074/jbc.M111.299115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J, Huang EY, Olzmann JA. Endoplasmic Reticulum-Associated Degradation and Lipid Homeostasis. Annu Rev Nutr. 2016. July;36(14):511–42. 10.1146/annurev-nutr-071715-051030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Yamamoto A, Kitamura A, Lamandé SR, Yoshimori T, Bateman JF, et al. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell. 2009. June;20(11):2744–54. 10.1091/mbc.e08-11-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute-Krishnan P, Ajinkya M, Bhat S, Itakura E, Hegde RS, Lippincott-Schwartz J. ER stress-induced clearance of misfolded GPI-anchored proteins via the secretory pathway. Cell. 2014. July;158(3):522–33. 10.1016/j.cell.2014.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010. October;40(2):280–93. 10.1016/j.molcel.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010. May;221(1):3–12. 10.1002/path.2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014. December;159(6):1263–76. 10.1016/j.cell.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaishy B, Abel ED. Lipids, lysosomes, and autophagy. J Lipid Res. 2016. September;57(9):1619–35. 10.1194/jlr.R067520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira JB, Masuda CA, Maya-Monteiro CM, Matos GS, Montero-Lomelí M, Bozaquel-Morais BL. TORC1 inhibition induces lipid droplet replenishment in yeast. Mol Cell Biol. 2015. February;35(4):737–46. 10.1128/MCB.01314-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso I, Pérez-Pérez ME, Martínez-Force E, Kim HS, He Y, Umen JG, et al. Autophagic flux is required for the synthesis of triacylglycerols and ribosomal protein turnover in Chlamydomonas. J Exp Bot. 2018. March;69(6):1355–67. 10.1093/jxb/erx372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minina EA, Moschou PN, Vetukuri RR, Sanchez-Vera V, Cardoso C, Liu Q, et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. J Exp Bot. 2018. March;69(6):1415–32. 10.1093/jxb/ery010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, et al. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009. November;119(11):3329–39. 10.1172/JCI39228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yoshimura K, Furuya N, Koike M, Ueno T, Komatsu M, et al. The MAP1-LC3 conjugation system is involved in lipid droplet formation. Biochem Biophys Res Commun. 2009. May;382(2):419–23. 10.1016/j.bbrc.2009.03.039 [DOI] [PubMed] [Google Scholar]
- Shibata M, Yoshimura K, Tamura H, Ueno T, Nishimura T, Inoue T, et al. LC3, a microtubule-associated protein1A/B light chain3, is involved in cytoplasmic lipid droplet formation. Biochem Biophys Res Commun. 2010. March;393(2):274–9. 10.1016/j.bbrc.2010.01.121 [DOI] [PubMed] [Google Scholar]
- Li Y, Chao X, Yang L, Lu Q, Li T, Ding WX, et al. Impaired fasting-induced adaptive lipid droplet biogenesis in liver-specific Atg5-deficient mouse liver is mediated by persistent nuclear factor-like 2 activation. Am J Pathol. 2018. August;188(8):1833–46. 10.1016/j.ajpath.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki A, Gupta SD, Majumder S, Kono M, Tuymetova G, Harmon JM, et al. Autophagy regulates sphingolipid levels in the liver. J Lipid Res. 2014. December;55(12):2521–31. 10.1194/jlr.M051862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010. November;139(5):1740–52. 10.1053/j.gastro.2010.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011. June;13(6):655–67. 10.1016/j.cmet.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaldoun SA, Emond-Boisjoly MA, Chateau D, Carrière V, Lacasa M, Rousset M, et al. Autophagosomes contribute to intracellular lipid distribution in enterocytes. Mol Biol Cell. 2014. January;25(1):118–32. 10.1091/mbc.e13-06-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011. August;14(2):173–83. 10.1016/j.cmet.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi T, Takayama K, Ishii S, Yamamoto A, Hara T, Minami N, et al. Forced lipophagy reveals that lipid droplets are required for early embryonic development in mouse. Development. 2018. February;145(4):dev161893. 10.1242/dev.161893 [DOI] [PubMed] [Google Scholar]
- Garcia EJ, Vevea JD, Pon LA. Lipid droplet autophagy during energy mobilization, lipid homeostasis and protein quality control. Front Biosci. 2018;23:1552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont N, Chauhan S, Arko-Mensah J, Castillo EF, Masedunskas A, Weigert R, et al. Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis. Curr Biol. 2014. March;24(6):609–20. 10.1016/j.cub.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilka T, Welter E, Borovsky N, Amar N, Mari M, Reggiori F, et al. Lipid droplets and their component triglycerides and steryl esters regulate autophagosome biogenesis. EMBO J. 2015. August;34(16):2117–31. 10.15252/embj.201490315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayan A, Mashek MT, Mashek DG. ATGL Promotes Autophagy/Lipophagy via SIRT1 to Control Hepatic Lipid Droplet Catabolism. Cell Rep. 2017. April;19(1):1–9. 10.1016/j.celrep.2017.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer FB, Khor VK, Shen WJ, Azhar S. Cholesterol ester droplets and steroidogenesis. Mol Cell Endocrinol. 2013. May;371(1-2):15–9. 10.1016/j.mce.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar MR, Vaandrager AB, Helms JB. Some Lipid Droplets Are More Equal Than Others: Different Metabolic Lipid Droplet Pools in Hepatic Stellate Cells. Lipid Insights. 2017. December;10:1178635317747281. 10.1177/1178635317747281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, et al. FAT SIGNALS—lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012. March;15(3):279–91. 10.1016/j.cmet.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitraju C, Trötzmüller M, Hartler J, Wolinski H, Thallinger GG, Lass A, et al. Lipidomic analysis of lipid droplets from murine hepatocytes reveals distinct signatures for nutritional stress. J Lipid Res. 2012. October;53(10):2141–52. 10.1194/jlr.M028902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohetahuntila M, Spee B, Kruitwagen HS, Wubbolts R, Brouwers JF, van de Lest CH, et al. Role of long-chain acyl-CoA synthetase 4 in formation of polyunsaturated lipid species in hepatic stellate cells. Biochim Biophys Acta. 2015. February;1851(2):220–30. 10.1016/j.bbalip.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Tuohetahuntila M, Molenaar MR, Spee B, Brouwers JF, Houweling M, Vaandrager AB, et al. ATGL and DGAT1 are involved in the turnover of newly synthesized triacylglycerols in hepatic stellate cells. J Lipid Res. 2016. July;57(7):1162–74. 10.1194/jlr.M066415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lizardo DY, Atilla-Gokcumen GE. Specific Triacylglycerols Accumulate via Increased Lipogenesis During 5-FU-Induced Apoptosis. ACS Chem Biol. 2016. September;11(9):2583–7. 10.1021/acschembio.6b00410 [DOI] [PubMed] [Google Scholar]
- Liesenfeld DB, Grapov D, Fahrmann JF, Salou M, Scherer D, Toth R, et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare study. Am J Clin Nutr. 2015. August;102(2):433–43. 10.3945/ajcn.114.103804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlager S, Goeritzer M, Jandl K, Frei R, Vujic N, Kolb D, et al. Adipose triglyceride lipase acts on neutrophil lipid droplets to regulate substrate availability for lipid mediator synthesis. J Leukoc Biol. 2015. November;98(5):837–50. 10.1189/jlb.3A0515-206R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichlberger A, Schlager S, Maaninka K, Schneider WJ, Kovanen PT. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. J Lipid Res. 2014. December;55(12):2471–8. 10.1194/jlr.M048553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmarakov IO, Jiang H, Liu J, Fernandez EJ, Blaner WS. Hepatic stellate cell activation: A source for bioactive lipids. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. May;1864(5):629–42. 10.1016/j.bbalip.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobowski-Gerard M, Zummo FP, Staels B, Lefebvre P, Eeckhoute J. Retinoids Issued from Hepatic Stellate Cell Lipid Droplet Loss as Potential Signaling Molecules Orchestrating a Multicellular Liver Injury Response. Cells. 2018. September;7(9):137. 10.3390/cells7090137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartung A, Zhao J, Chen S, Mottillo E, VanHecke GC, Ahn YH, et al. Characterization of eicosanoids produced by adipocyte lipolysis: implication of cyclooxygenase-2 in adipose inflammation. J Biol Chem. 2016. July;291(31):16001–10. 10.1074/jbc.M116.725937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlager S, Vujic N, Korbelius M, Duta-Mare M, Dorow J, Leopold C, et al. Lysosomal lipid hydrolysis provides substrates for lipid mediator synthesis in murine macrophages. Oncotarget. 2017. June;8(25):40037–51. 10.18632/oncotarget.16673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umamaheswaran S, Dasari SK, Yang P, Lutgendorf SK, Sood AK. Stress, inflammation, and eicosanoids: an emerging perspective. Cancer Metastasis Rev. 2018. September;37(2-3):203–11. 10.1007/s10555-018-9741-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010; 10(3):181-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza PT Viola JPB. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids. 2010; 82(4-6): 243-50 [DOI] [PubMed] [Google Scholar]
- Tuohetahuntila M Molenaar MR Spee B Brouwers JF, et al. Lysosome-mediated degradation of a distinct pool of lipid droplets during hepatic stellate cell activation. J Biol Chem. 2017; 292(30): 12436-48 [DOI] [PMC free article] [PubMed] [Google Scholar]