Abstract
Toxoplasmosis, which affects more than a billion people worldwide, is a common parasitic infection caused by the obligate intracellular parasite, Toxoplasma gondii. Current treatment strategies have several limitations, including unwanted side effects and poor efficacy. Therefore, newer therapies are needed for toxoplasmosis. Drug repurposing and screening of a vast array of natural and/or synthetic compounds is a viable option for antiparasitic drug discovery. In this study, we screened 62 compounds comprising natural products (NPs) and FDA-approved (FDA) drugs, to identify the hit compounds that suppress the growth of T. gondii. To determine the parasite inhibitory potential of the compounds, host mammalian cells were infected with a transgenic T. gondii strain, and the viability of the parasite was evaluated by luminescence. Of the 62 compounds, tubericidin, sulfuretin, peruvoside, resveratrol, narasin and diacetoxyscirpenol of the natural product isolates, as well as bortezonib, 10-Hydroxycamtothecin, mebendazole, niflumic acid, clindamycin HCl, mecamylamine, chloroquine, mitomycin C, fenbendazole, daunorubicin, atropine, and cerivastatin of FDA molecules were identified as “hits” with ≥ 40 percent anti-parasite action. Additionally, mitomycin C, radicicol, naringenin, gitoxigenin, menadione, botulin, genistin, homobutein, and gelsemin HCl of the natural product isolates, as well as lomofungin, cyclocytidine, prazosin HCl, cerivastatin, camptothecin, flufenamic acid, atropine, daunorubicin, and fenbendazole of the FDA compounds exhibited cytotoxic activity, reducing the host viability by ≥ 30 percent. Our findings not only support the prospects of drug repurposing, but also indicate that screening a vast array of molecules may provide viable sources of alternative therapies for parasitic infection.
Keywords: Antiparasite, Drug discovery, Library of chemical compounds, Medicinal biochemistry, Parasitic infection
Introduction
Toxoplasmosis, which is caused by the obligate intracellular parasite Toxoplasma gondii [1-3], is a common parasitic infection affecting more than a billion people worldwide. The parasite exists in three forms, tachyzoites, bradyzoites, and sporozoites, found in definitive and non-definitive hosts. However, only two forms, tachyzoites and bradyzoites of the parasite exist in human and non-definitive hosts. In healthy individuals, a T. gondii infection can occur without symptoms, but in immune-compromised or pregnant individuals, T. gondii infection often results in serious illness or death [4,5]. For example, T. gondii infection in HIV-AIDS patients is associated with encephalitis, and in pregnant women, numerous cases of miscarriages and fetal deformities have been linked with the Toxoplasma infection [4,5]. Infection of a mammalian host usually comes about by contact with the parasite, either by the consumption of the bradyzoite form in undercooked meat or from the sporozoite form in felid feces, which can contaminate drinking water or occur elsewhere in the environment, such as on unwashed vege or in cat litter.
Currently, antimalarials, such as pyrimethamine, and/or antibiotics, such as clindamycin HCl, are among the therapies of choice for active toxoplasmosis [6]. However, poor tolerance and limited efficacy are major challenges of these therapeutic options. Additionally, the available treatments are not effective against latent infections. Thus, toxoplasmosis remains a growing health burden globally, necessitating the identification of newer therapies. Recent investigations have shown that both drug repurposing and screening a vast array of molecules for antiparasite potential are viable approaches to drug discovery [7-14]. Screening a vast array of chemical compounds, including the repurposing of already approved drugs, may allow the discovery of lead antiparasitic infection candidates in early drug development. For example, testing well-characterized drugs and other types of compounds, which are currently approved to treat other conditions, for potential off-label indications could not only allow the identification of lead targets but also boost efforts toward developing lead candidates. Indeed, emerging reports have identified several potent, new antiparasitic agents by screening well-characterized drugs or compounds that inhibit specific pathways or processes [6,9,10]. In this study, we screened both natural product isolates and FDA-approved compounds, to identify those that possess suppressive action against the in vitro growth of T. gondii.
Materials and Methods
Natural Product Isolates and FDA-approved Drugs
The natural products and FDA-approved compounds in stock solutions of DMSO were provided without charge from the Kanazawa University Cancer Institute, Japan. Pyrimethamine (Sigma Aldrich, St. Louis, MO, USA) was included in all the experiments as the reference drug.
Parasite
In this study, the transgenic T. gondii RH strain 2F (ATCC® 50839), which expresses β-galactosidase, was used, unless indicated otherwise. To sustain the parasite, T. gondii was repeatedly passaged in cultures of human foreskin fibroblast (HFF; ATCC®) monolayers in a medium composed of Dulbecco’s modified Eagle medium (DMEM; Nissui, Tokyo, Japan), 1% L-glutamine (200 mM Gibco, Fisher, UK), fetal calf serum (10% v/v; Gibco, Fisher, UK), and 1% penicillin and streptomycin (100 U/mL Biowhittaker, UK). To obtain a purified suspension of T. gondii, the infected host cells were lysed, filtered, and washed several times with fresh culture medium, as described elsewhere [8].
Assay to Determine Parasite Growth Inhibition
An assay to determine whether the compounds affect parasite growth was performed as described elsewhere [8]. The multiplicity of infection (MOI) was 1:5 (parasite/host cell ratio). In brief, the test compounds were reconstituted in culture medium at five concentrations (between 0 – 1.5 µg/mL), and incubated with newly purified parasites in monolayers of HFF cells grown in 96-well optical bottom plates (Nunc; Fisher Scientific, Pittsburgh, USA). The cells treated with medium only served as the negative drug control, while the medium-only well was used for background signal correction. To validate the assay, pyrimethamine was included as a positive drug control. After a 72-h incubation at 37oC in a 5% CO2 atmosphere, the parasite viability was determined by luminescence using a Beta-Glo assay kit (Promega, Madison, USA). Three independent biological experiments were performed, and each of which had three tests.
Assay to Determine Host Cell Viability
The host cell viability assay was performed as previously reported elsewhere [8]. Cultures of HFF cells were grown to confluence in medium as described above in an atmosphere of 5% CO2 and 37oC. The confluent cells were sub-cultured and seeded in 96-well plates (Nunc; Fisher Scientific, Pittsburgh, USA) at a density of 1 × 105 cells per well, followed by 72-h incubation at 37oC and 5% CO2 atmosphere. Subsequently, the test compounds were added at five concentrations (between 0 – 3 µg/mL) to the culture medium. The negative drug control contained only the culture medium, whereas the medium-only well was used for background noise correction. To validate the assay, a positive control drug, staurosporine (1 µM final concentration) was used [15]. After a 72-h incubation under the tissue culture conditions indicated above, the viability of cells was determined by colorimetric measurement (CellTitre-Aqueous One kit, Promega, Madison, USA) at 490 nm on a microplate reader (MTP 500; Corona Electric, Hitachinaka, Japan). Three independent biological experiments were performed, each of which had three tests. Meanwhile, selectivity index (SI) for the compounds was estimated so at identify promising anti-Toxoplasma compounds with low or no toxicity to host cells. The SI is the ratio of the IC50 concentration in host cells (HFF) to that in parasite (T. gondii). The IC50 is the concentration of the compound that either reduces host cell viability or inhibited Toxoplasma growth by 50 percent.
Molecular and In Silico Studies: ADME (Absorption, Distribution, Metabolism, Excretion), Bioactivity, and Toxicity
The modeling for physicochemical, pharmacokinetic, and bioactivity properties of some selected compounds were performed using the Molinspiration Cheminformatics server (http://www.molinspiration.com). Compounds were randomly selected from among those with SI> 1. The SMILE formats of the compounds (with their corresponding identities) were obtained from https://pubchem.ncbi.nlm.nih.gov/. Features, including partition coefficient, total polar surface area, molecular weight, molecular volume, number of hydrogen bond acceptors/donors, mean number of rotatable bonds, and degree of violation of Lipinski’s rule, were obtained following the reported standard procedure. The bioactivity score (based on Lipinski’s “Rule of five”), which evaluates the ion channel modulation, GPCR ligand, kinase inhibition, nuclear receptor interaction, protease inhibition, and enzyme inhibition activities, was also determined [16].
The in silico toxicity was determined using ProTox-II [17,18], a web-based computational modeling tool comprising about 33 models that can predict various toxicity endpoints, which include hepatotoxicity, cytotoxicity, carcinogenicity, mutagenicity, immunotoxicity, adverse outcomes pathways, toxicity targets, and acute toxicity.
Toxicity Potential and Classes
For the in silico toxicity evaluation, the toxic doses were given as LD50 values in mg/kg body weight. The model takes LD50 as the median lethal dose, indicating the dose at which 50 % of the test subjects die upon exposure to the test compound. The toxicity classes were defined according to the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). Within the group of substances that showed acute oral toxicity, the GHS classification, class 1 is designated as fatal if swallowed at LD50≤ 5; class 2: fatal if swallowed at 5 < LD50≤ 50; class 3: toxic if swallowed at 50 < LD50≤ 300; class 4 harmful if swallowed at 300 < LD50≤ 2000; class 5: may be harmful if swallowed at 2000 < LD50≤ 5000, and class 6: non-toxic at LD50> 5000 [18,19].
Statistical Analysis
A one-way ANOVA was used to analyze the data utilizing GraphPad Software (San Diego, CA, USA). The results are presented as the mean of three replicates ± standard error of mean (SEM). The Dunnet post-hoc test was used to compare the groups, and the mean values were considered significant at p < .05. A non-linear regression analysis (curve fit) was used to estimate the concentration that caused inhibition of T. gondii growth or cellular toxicity by 50% (IC50).
Results
In this study, we screened a series of chemical entities comprising both natural product isolates (36) and FDA-approved drugs (26), to identify those with suppressive action or blocking capacity against the in vitro growth of T. gondii. Of the 62 compounds evaluated for dose-response antiparasite activity, the results showed that 18 compounds representing, 16.17% and 46.15%, respectively, of the natural product isolates and FDA-approved compounds inhibited (p < .05) parasite growth by ≥ 40 percent (Figures 1 and 2). Meanwhile, determination of host cell viability in the presence of the same compounds showed that a total of 19 compounds representing, 27.77% and 34.62%, respectively, of the natural product isolates and FDA-approved drugs were cytotoxic (p < .05), causing a reduction in host cell viability by ≥ 30 percent (Figures 3 and 4). Meanwhile, ≥ 65 percent of the total compounds from both the natural product isolates and the FDA-approved drugs, showed neither antiparasite activity nor any detectable host cytotoxicity. Furthermore, some of the natural compounds, including tubericidin, peruvoside, narasin, and diacetoxyscirpenol, that strongly blocked the in vitro growth of T. gondii at sub-microgram doses, lacked antiparasite specificity (p < .05), as these compounds were also were cytotoxic to the host cells at the same doses (Tables 1 and 2). Similarly, some of the FDA-approved drugs including 10-hydroxycamptothecin and bortezomib showed excellent anti-T. gondii potential (p < .05), but poor selectivity toward the parasite versus the host cells (Tables 3 and 4). Together, the findings suggest general cellular toxicity of these compounds. To validate our assay, pyrimethamine was included as a reference drug and, as expected, this drug blocked parasite growth.
Figure 1.
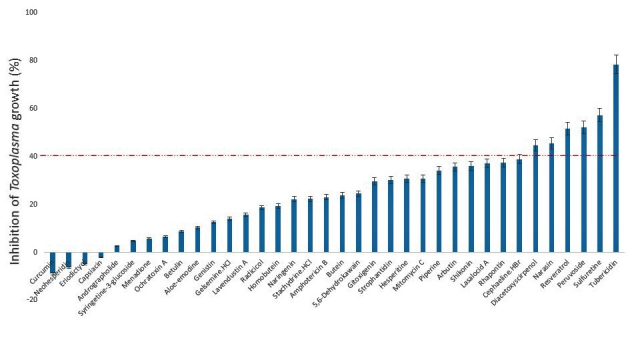
Growth inhibition of Toxoplasma gondii RH-2F strain by natural product isolates at a concentration of 1 μg/mL. DMSO (0.5%) control was calculated as 0% inhibition, and 200 μg/mL pyrimethamine positive drug control was calculated as 100% growth inhibition. Data are presented as mean values of three replicates ± standard errors of the mean.
Figure 2.
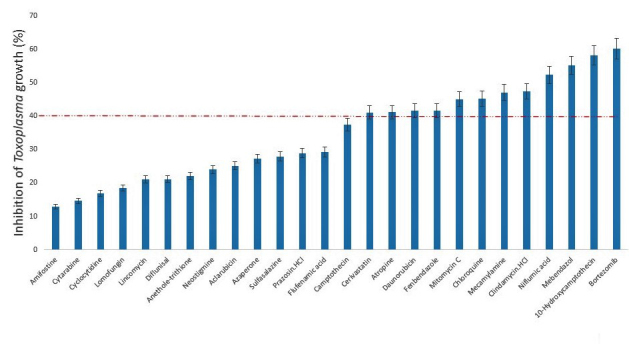
Growth inhibition of Toxoplasma gondii RH-2F strain by FDA-approved compounds at a concentration of 1.5 μg/mL. DMSO (0.5%) control was calculated as 0% inhibition, and 200 μg/mL pyrimethamine positive drug control was calculated as 100% growth inhibition. Data are presented as mean values of three replicates ± standard errors of the mean.
Figure 3.
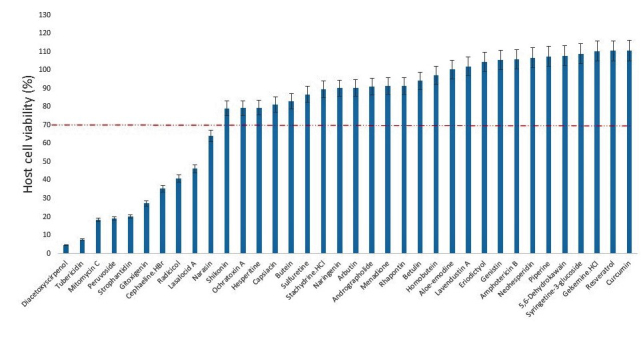
Viability of host cell (human foreskin fibroblast) by natural product isolates at 2 μg/mL. DMSO (0.5%) control was calculated as 100% cell viability. Data are presented as mean values of three replicates ± standard errors of the mean.
Figure 4.
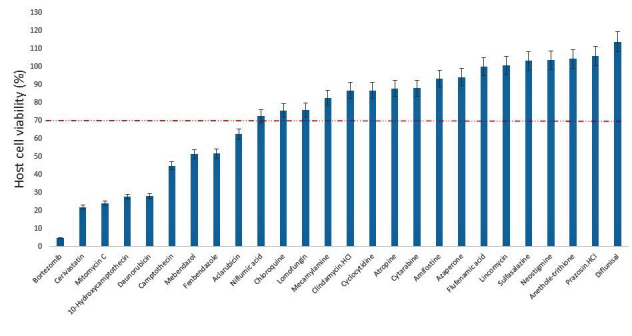
Viability of host cell (human foreskin fibroblast) by FDA-approved compounds at 3 μg/mL. DMSO (0.5%) control was calculated as 100% cell viability. Data are presented as mean values of three replicates ± standard errors of the mean.
Table 1. Inhibition of Toxoplasma gondii growth and host cell viability by natural product isolates.
| Compounds | aInhibition of Toxoplasma gondii (%) | bHost (HFF) cell viability (%) | |
| Tubericidin | 78.45±1.23 | ≥40 % anti-T. gondii activity | 81.17±5.20 |
| Sulfuretine | 57.31±2.58 | ≥40 % anti-T. gondii activity | 110.69±6.46 |
| Peruvoside | 52.09±0.93 | ≥40 % anti-T. gondii activity | 40.96±1.62 |
| Resveratrol | 51.60±1.66 | ≥40 % anti-T. gondii activity | 110.46±5.80 |
| Narasin | 45.56±0.43 | ≥40 % anti-T. gondii activity | 79.09±3.17 |
| Diacetoxyscirpenol | 44.67±2.98 | ≥40 % anti-T. gondii activity | 82.87±1.85 |
| Cephaeline.HBr | 38.92±0.14 | 90.10±9.77 | |
| Rhapontin | 37.46±2.09 | 91.32±4.65 | |
| Lasalocid A | 37.10±2.64 | 105.63±6.02 | |
| Shikonin | 36.05±3.10 | 104.52±4.66 | |
| Arbutin | 35.64±1.22 | 97.26±5.18 | |
| Piperine | 34.16±1.14 | 86.68±3.79 | |
| Mitomycin C | 30.83±1.53 | 4.44±0.57 | |
| Hesperitine | 30.69±1.17 | 90.92±8.80 | |
| Strophantidin | 30.23±1.41 | 108.97±4.43 | |
| Gitoxigenin | 29.57±1.59 | 20.25±0.94 | |
| 5,6-Dehydrokawain | 24.52±2.59 | 107.45±5.59 | |
| Butein | 23.79±0.93 | 89.44±6.59 | |
| Amphotericin B | 23.09±1.21 | 79.21±6.35 | |
| Stachydrine.HCl | 22.39±2.68 | 100.35±5.53 | |
| Naringenin | 22.20±0.73 | 27.28±3.70 | |
| Homobutein | 19.48±1.45 | 64.14±2.07 | |
| Radicicol | 18.68±1.86 | 19.12±1.13 | |
| Lavendustin A | 15.78±0.92 | 91.50±7.90 | |
| Gelsemine.HCl | 14.10±0.42 | 46.17±3.58 | |
| Genistin | 12.55±1.22 | 7.52±0.37 | |
| Aloe-emodine | 10.39±0.18 | 106.75±3.82 | |
| Betulin | 8.87±0.66 | 35.30±2.08 | |
| Ochratoxin A | 6.59±0.84 | 107.83±4.68 | |
| Menadione | 5.76±0.18 | 18.21±1.53 | |
| Syringetine-3-glucoside | 4.78±0.53 | 79.49±12.26 | |
| Andrographolide | 2.60±0.86 | 94.12±6.23 | |
| Capsiacin | -2.14±1.30 | 101.92±6.19 | |
| Eriodictyol | -4.93±1.12 | 110.38±2.89 | |
| Neohesperidin | -6.24±1.71 | 105.79±7.21 | |
| Curcumin | -8.89±0.28 | 90.31±5.66 |
aParasite growth inhibition values from preliminary screening with RH-2F at 1 μg/mL concentration. DMSO (0.5%) control was calculated as 0% inhibition, and 200 μg/mL pyrimethamine control was calculated as 100% growth inhibition. bHost cell viability values from preliminary screening with HFF cells at 2 μg/mL concentration. DMSO control was calculated as 100% cell viability.
Table 2. Estimate of selectivity index for natural product isolates.
| Compounds | IC50Toxoplasma gondii (µg/mL) | IC50 HFF cells (µg/mL) | Selectivity Index (SI) | |
| Rhapontin | 1.22 | 20.05 | 16.38 | SI >1 |
| Homobutein | 4.43 | 68.28 | 15.41 | SI >1 |
| Sulfuretine | 0.68 | 8.20 | 12.10 | SI >1 |
| Arbutin | 17.20 | 84.23 | 4.90 | SI >1 |
| Butein | 2.12 | 7.92 | 3.74 | SI >1 |
| Naringenin | 3.03 | 9.20 | 3.03 | SI >1 |
| Narasin | 0.74 | 2.09 | 2.84 | SI >1 |
| Stachydrine.HCl | 4.40 | 10.41 | 2.36 | SI >1 |
| Shikonin | 3.96 | 5.81 | 1.47 | SI >1 |
| Hesperitine | 13.66 | 12.42 | 0.91 | |
| Lasalocid A | 1.61 | 1.46 | 0.90 | |
| Ochratoxin A | 11.33 | 9.63 | 0.85 | |
| Radicicol | 4.90 | 2.58 | 0.53 | |
| Cephaeline.HBr | 0.95 | 0.45 | 0.47 | |
| Menadione | 44.70 | 17.93 | 0.40 | |
| Peruvoside | 0.37 | 0.11 | 0.30 | |
| Sulfadiazine | 185.20 | 53.35 | 0.29 | |
| Mitomycin C | 1.87 | 0.44 | 0.24 | |
| Gitoxigenin | 2.59 | 0.62 | 0.24 | |
| Pyrimethamine | 205.60 | 42.16 | 0.21 | |
| Strophantidin | 1.30 | 0.19 | 0.15 | |
| Diacetoxyscirpenol | 0.62 | 0.05 | 0.08 | |
| Tubericidin | 0.46 | 0.02 | 0.04 | |
| Capsiacin | ND | 9.69 | ND | |
| Curcumin | ND | ND | ND | |
| Resveratrol | 1.03 | ND | ND | |
| Genistin | 5.46 | ND | ND | |
| Eriodictyol | ND | ND | ND | |
| Andrographolide | 388.10 | ND | ND | |
| Syringetine-3-glucoside | 16.64 | ND | ND | |
| Piperine | 1.71 | ND | ND | |
| Aloe-emodine | 8.14 | ND | ND | |
| Neohesperidin | ND | ND | ND | |
| 5,6-Dehydrokawain | 4.22 | ND | ND | |
| Betulin | 224.40 | ND | ND | |
| Lavendustin A | 37.45 | ND | ND | |
| Gelsemine.HCl | 89.29 | ND | ND |
*ND – Not determined.
Table 3. Inhibition of Toxoplasma gondii growth and host cell viability by FDA-approved chemical compounds.
| Compounds | aInhibition of Toxoplasma gondii (%) | bHost (HFF) cell viability (%) | |
| Bortezomib | 60.13±1.78 | ≥40 % anti-T. gondii activity | 86.64±6.65 |
| 10-Hydroxycamptothecin | 58.16±2.01 | ≥40 % anti-T. gondii activity | 100.55±1.58 |
| Mebendazol | 55.14±0.98 | ≥40 % anti-T. gondii activity | 75.86±3.31 |
| Niflumic acid | 52.31±1.83 | ≥40 % anti-T. gondii activity | 99.96±4.02 |
| Clindamycin HCl | 47.39±1.31 | ≥40 % anti-T. gondii activity | 105.77±2.43 |
| Mecamylamine | 47.06±2.02 | ≥40 % anti-T. gondii activity | 87.76±4.92 |
| Chloroquine | 45.14±2.62 | ≥40 % anti-T. gondii activity | 82.54±4.10 |
| Mitomycin C | 45.06±1.87 | ≥40 % anti-T. gondii activity | 103.37±3.84 |
| Fenbendazole | 41.62±1.70 | ≥40 % anti-T. gondii activity | 21.82±1.24 |
| Daunorubicin | 41.60±1.19 | ≥40 % anti-T. gondii activity | 62.27±0.72 |
| Atropine | 41.10±1.07 | ≥40 % anti-T. gondii activity | 44.73±1.30 |
| Cerivastatin | 41.07±1.44 | ≥40 % anti-T. gondii activity | 24.12±2.47 |
| Camptothecin | 37.38±1.32 | 27.68±2.54 | |
| Flufenamic acid | 29.21±0.16 | 28.01±2.95 | |
| Prazosin.HCl | 28.91±1.82 | 4.70±1.05 | |
| Sulfasalazine | 27.93±1.74 | 104.16±5.22 | |
| Azaperone | 27.24±2.00 | 93.09±3.22 | |
| Aclarubicin | 25.13±0.42 | 94.05±4.80 | |
| Neostigmine | 24.01±1.05 | 75.51±13.61 | |
| Anethole-trithione | 21.98±1.00 | 86.65±4.93 | |
| Diflunisal | 21.13±1.69 | 87.93±8.43 | |
| Lincomycin | 21.04±1.32 | 113.67±8.48 | |
| Lomofungin | 18.43±1.92 | 51.65±6.83 | |
| Cyclocytidine | 16.93±1.05 | 51.24±2.09 | |
| Cytarabine | 14.59±1.18 | 72.36±9.24 | |
| Amifostine | 12.94±1.65 | 102.99±5.23 |
aParasite growth inhibition values from preliminary screening with RH-2F at 1.5 μg/mL concentration. DMSO (0.5%) control was calculated as 0% inhibition, and 200 μg/mL pyrimethamine control was calculated as 100% growth inhibition. bHost cell viability values from preliminary screening with HFF cells at 3 μg/mL concentration. DMSO control was calculated as 100% cell viability.
Table 4. Estimate of selectivity index for FDA-approved chemical compounds.
| Compounds | IC50Toxoplasma gondii (µg/mL) | IC50 HFF cells (µg/mL) | Selectivity Index (SI) | |
| Atropine | 2.20 | 61.35 | 27.85 | SI >1 |
| Niflumic acid | 1.07 | 26.06 | 24.47 | SI >1 |
| Clindamycin HCl | 1.59 | 25.79 | 16.22 | SI >1 |
| Azaperone | 6.40 | 82.00 | 12.82 | SI >1 |
| Chloroquine | 1.97 | 17.54 | 8.90 | SI >1 |
| Mecamylamine | 2.65 | 21.30 | 8.04 | SI >1 |
| Mebendazol | 2.05 | 2.03 | 0.99 | |
| Lomofungin | 31.64 | 18.02 | 0.57 | |
| 10-Hydroxycamptothecin | 1.50 | 0.79 | 0.53 | |
| Fenbendazole | 5.42 | 2.69 | 0.50 | |
| Camptothecin | 4.48 | 1.77 | 0.39 | |
| Mitomycin C | 2.47 | 0.95 | 0.38 | |
| Sulfadiazine | 185.20 | 53.35 | 0.29 | |
| Pyrimethamine | 205.60 | 42.16 | 0.21 | |
| Cerivastatin | 3.29 | 0.60 | 0.18 | |
| Bortezomib | 1.82 | 0.12 | 0.07 | |
| Daunorubicin | 6.39 | 0.35 | 0.05 | |
| Aclarubicin | 110.70 | 3.52 | 0.03 | |
| Lincomycin | 9.39 | ND | ND | |
| Flufenamic acid | 4.32 | ND | ND | |
| Prazosin.HCl | 5.87 | ND | ND | |
| Neostigmine | 17.11 | ND | ND | |
| Anethole-trithione | ND | ND | ND | |
| Amifostine | ND | 59.81 | ND | |
| Cyclocytidine | ND | 27.52 | ND | |
| Cytarabine | ND | 42.01 | ND | |
| Diflunisal | 34.38 | ND | ND | |
| Sulfasalazine | 4.88 | ND | ND |
*ND – Not determined.
Additionally, a few of the compounds that blocked the in vitro growth of T. gondii, also exhibited multiple-fold specificities (selectivity index (SI) ≥ 3) toward the parasite versus the host cells with EC50 ranging in the sub-microgram concentration. A selectivity index ≥ 1 is considered promising for early drug development against parasitic infection [20]. Some of the natural product isolates with promising antiparasite specificities included sulfuretin, resveratrol, and narasin (Figure 5), while those of the FDA-approved drugs were mecamylamine, atropine, niflumic acid, clindamycin HCl, and chloroquine (Figure 6). Taken together, the findings indicate not only specific antiparasite action by these compounds but also that the antiparasite action of these compounds was not a result of toxicity to the host cells. Given these results, the hit compounds could be considered as early drug development candidates for toxoplasmosis therapy.
Figure 5.
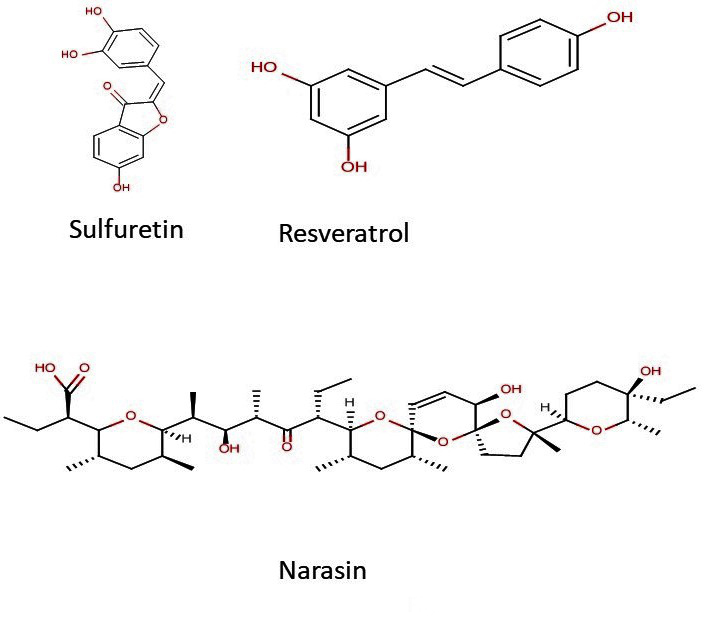
Chemical structures of hit compounds from the natural product isolates.
Figure 6.
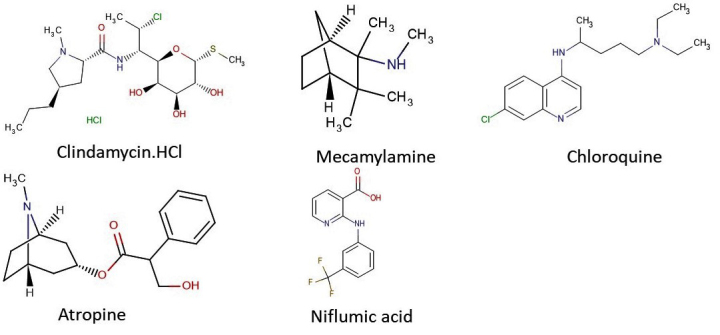
Chemical structures of hit compounds from the FDA-approved drugs.
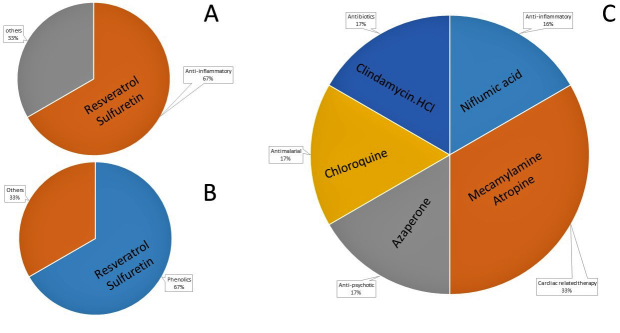
Furthermore, of the identified antiparasitic compounds from both the natural product isolates and the FDA-approved drugs, the majority were effective and/or used against inflammatory related conditions (Figure 7a-c). Similarly, two of the effective natural anti-T. gondii isolates were phenolics. Considered together, the findings reinforce existing investigations that demonstrated that drugs and/or compounds used in inflammatory therapy blocked parasite growth in vitro [6,10,21].
Figure 7.
Distribution of active natural product compounds based on (A) bioactivity, (B) functional groups, and (C) treatment indications for FDA-approved drugs.
Moreover, a few of the natural product isolates in this study showed mild activity against the growth of T. gondii and the same compounds also demonstrated a promising selectivity index (Tables 1 and 2). Among these are butein, homobutein, rhapontin, arbutin, and piperine. While butein and homobutein are flavonoids, rhapontin and arbutin are glycosides and piperine is an alkaloid. Interestingly, our study is the first report showing a mild suppressive action of homobutein, butein, rhapontin, and arbutin against the in vitro growth of T. gondii. However, homobutein has been reported as having antimalarial activity [22,23] and anti-inflammatory, antioxidant, and antitumor potential [24-26]. In separate investigations, the anti-inflammatory, antioxidant, and antibacterial potential of rhapontin and arbutin have each been documented [27-29]. Additionally, piperine has been implicated as having anti-inflammatory properties [30] as well as anti-T. gondii activity [31].
Furthermore, the ADME properties and drug likeness (Lipinski’s rule of five) of the standard anti-Toxoplasma drugs were compared with those of a select few of the FDA-approved drugs and natural product compounds that showed SI>1 (Tables 2, 4, and 5). While only chloroquine and rhapontin showed one violation each of the Lipinski’s rule, mecamylamine had the lowest total polar surface area (12.03 Å2) and molecular volume (186.67 A3) as well as molecular weight (167.30 g/mol). The majority of the compounds had molecular weights and molecular volumes below 500. While all the compounds showed prospects for application as anti-Toxoplama agents, mecamylamine (an FDA-approved drug) and sulfuretin (a natural product) seemed to show greater potential based on their ADME properties.
Table 5. ADME properties of the selected compounds/drugs.
| Compound or Drug PubChem ID | MiLogP | TPSA (Å2) | MW (g/mol) | nON | nOHNH | NoV | nRotB | MV (A3) |
| Clindamycin hydrochloride | 2.06 | 102.25 | 424.99 | 7 | 4 | 0 | 7 | 384.72 |
| Mecamylamine | 2.96 | 12.03 | 167.30 | 1 | 1 | 0 | 1 | 186.67 |
| Chloroquine | 5.00 | 28.16 | 319.88 | 3 | 1 | 1 | 8 | 313.12 |
| Azaperone | 2.88 | 36.44 | 327.40 | 4 | 0 | 0 | 6 | 307.55 |
| Atropine | 1.77 | 49.77 | 289.38 | 4 | 1 | 0 | 5 | 279.01 |
| Niflumic acid | 3.42 | 62.22 | 282.22 | 4 | 2 | 0 | 4 | 222.00 |
| Sulfuretin | 1.76 | 90.89 | 270.24 | 5 | 3 | 0 | 1 | 224.05 |
| Homobutein | 2.59 | 86.99 | 286.28 | 5 | 3 | 0 | 4 | 251.45 |
| Arbutin | -0.81 | 119.61 | 272.25 | 7 | 5 | 0 | 3 | 232.20 |
| Rhapontin | 1.02 | 149.07 | 420.41 | 9 | 6 | 1 | 6 | 364.59 |
| Pyrimethamine | 2.84 | 77.83 | 248.72 | 4 | 4 | 0 | 2 | 216.62 |
| Sulfadiazine | -0.04 | 97.98 | 250.28 | 6 | 3 | 0 | 3 | 202.26 |
miLogP - partition coefficient; TPSA - total polar surface area; MW - molecular weight; nON - number of hydrogen bond acceptors; nOHNH - number of hydrogen bond donors; noV - number of violations; nrotb - number of rotatable bonds; MV - molecular volume.
The result of the bioactivity and drug-likeness measures are presented in Table 6. Comparatively, chloroquine exhibited the highest potentials, in terms of its GPCR ligand, ion channel modulation, and kinase inhibition. In the natural products group, arbutin and rhapontin showed parameters comparable with the standard drug, pyrimethamine. All the selected FDA-approved drugs and natural products exhibited potential for further in vivo evaluation. The result of the in silico toxicity evaluation (Table 7) revealed that all the tested drugs and compounds had toxicities in classes 3 and 5. Interestingly, only homobutein and arbutin (natural product compounds) belong to toxicity class 5 (may be harmful if swallowed (2000< LD50≤ 5000) indicating potential relative safety on usage. Clindamycin hydrochloride, chloroquine, azaperone, homobutein, and rhapontin showed mild immunotoxic potential, and sulfuretin and sulfadiazine exhibited potential carcinogenicity. No specific toxicity effect was reported for mecamylamine, atropine, arbutin, or the standard, pyrimethamine in the model used. Like the standard (pyrimethamine), all the natural products showed average potential toxicity to prostaglandin synthase 1 and amine oxidase A.
Table 6. In silico bioactivities for the selected compounds/drugs.
| Compound or Drug Name | PubChem CID | GPCR ligand | ICM | KI | NRL | PI | EI |
| FDA Approved drugs | |||||||
| Clindamycin hydrochloride | 16051951 | 0.33 | -0.11 | -0.54 | -0.27 | 0.47 | 0.23 |
| Mecamylamine | 4032 | -0.70 | 0.03 | -1.47 | -1.18 | -0.80 | -0.66 |
| Chloroquine | 2719 | 0.32 | 0.32 | 0.38 | -0.19 | 0.05 | 0.11 |
| Azaperone | 15443 | 0.26 | 0.09 | 0.15 | -0.17 | -0.02 | 0.12 |
| Atropine | 174174 | 0.44 | 0.26 | -0.09 | -0.06 | 0.13 | 0.21 |
| Niflumic acid | 4488 | 0.04 | 0.04 | 0.29 | -0.03 | -0.11 | 0.11 |
| Natural Products | |||||||
| Sulfuretin | 5281295 | -1.18 | -1.26 | -0.52 | -0.56 | -1.21 | 0.00 |
| Homobutein | 6438092 | -0.10 | -0.19 | -0.24 | 0.02 | -0.31 | 0.06 |
| Arbutin | 440936 | 0.05 | 0.12 | -0.13 | 0.04 | -0.09 | 0.46 |
| Rhapontin | 637213 | 0.10 | 0.02 | 0.01 | 0.14 | -0.02 | 0.30 |
| Standard anti-toxoplasma drugs | |||||||
| Pyrimethamine | 4993 | 0.31 | 0.07 | 0.38 | -0.61 | -0.14 | 0.66 |
| Sulfadiazine | 5215 | -0.29 | -0.39 | -0.14 | -0.92 | -0.47 | -0.01 |
GPCR ligand, ICM - ion channel modulation; KI - kinase inhibition; NRL - nuclear receptor ligand; PI - protease inhibition; EI - enzyme inhibition
Table 7. In silico toxicity model result.
| Compound or Drug Name | Potential Effects | Classification | Probability | Possible toxicity targets | Average Pharmacophore Fit (%) | Predicted LD50 (mg/kg) | Predicted Toxicity Class | ||
| FDA Approved drugs | |||||||||
| Clindamycin hydrochloride | Immuno | TEP | 0.93 | Amine Oxidase A | 70.29 | 1095 | 4 | ||
| Mecamylamine | ND | NA | NA | Dopamine receptor D3 | 0 | 90 | 3 | ||
| Chloroquine | Immuno Mutag |
TEP TEP |
0.99 0.94 |
ND | NA | 311 | 4 | ||
| Azaperone | Immuno | TEP | 0.54 | Histamine Receptor H1 | 56.12 | 63 | 3 | ||
| Atropine | ND | NA | NA | PGH Synthase 1 | 48.05 | 75 | 3 | ||
| Niflumic acid | MMP | TSRP | 1.0 | Amine Oxidase A | 58.28 | 250 | 3 | ||
| Natural Products | |||||||||
| Sulfuretin | Carcino Immuno MMP |
TEP TEP TSRP |
0.71 0.85 0.76 |
PGH Synthase 1 | 50.32 | 500 | 4 | ||
| Homobutein | Immuno MMP |
TEP TSRP |
0.99 0.93 |
Amine Oxidase A | 63.3 | 2100 | 5 | ||
| Arbutin | ND | NA | NA | PGH Synthase 1 | 66.5 | 2420 | 5 | ||
| Rhapontin | Immuno | Toxicity end points | 0.99 | PGH Synthase 1 | 0 | 1380 | 4 | ||
| Standard anti-Toxoplasma drugs | |||||||||
| Pyrimethamine | ND | NA | NA | Amine Oxidase A | 64.07 | 92 | 3 | ||
| Sulfadiazine | Carcino | Toxicity end points | 0.76 | PGH Synthase 1 | 58.4 | 1500 | 4 | ||
Carcino - carcinogenicity; Mutag - mutagenicity; Immuno- immunotoxicity; MMP- mitochondrial membrane potential; TSRP - Tox21-stress response pathways; TEP - toxicity end points; ND - none detected; NA - not applicable; PGH - prostaglandin G/H
Discussion
The screening of a vast array of chemical entities, including drug repurposing, is considered reasonable for identifying new therapeutic targets for the treatment of parasitic infection. For example, drug repurposing, in which well-characterized drugs and other types of chemical entities are tested for potential off-label indications, can not only identify lead targets but can also be useful for drug development for parasitic infections [7].
In this study, we screened 62 chemical entities, to identify those that suppress the growth of T. gondii. Altogether, a total of eight compounds from both the natural product isolates and the FDA-approved drugs showed antiparasitic activity at sub-microgram doses. Additionally, some of these same compounds demonstrated promising selectivity with a selectivity index (SI) of a ≥3-fold activity against the parasite versus the host cells. Together, these findings not only underscore the specific antiparasite action shown by the compounds but also indicate that these compounds may be viable prospects in early drug development for toxoplasmosis. In comparison, pyrimethamine, which is one of the current treatment options for toxoplasmosis, exhibited a ≤1-fold activity against the parasite versus the host cell.
Furthermore, most of the “hit” compounds (compounds that blocked parasite growth with promising SIs) among the FDA-approved drugs had not been reported as having anti-T. gondii activity; the exceptions were clindamycin HCl and chloroquine. Respectively, clindamycin HCl and chloroquine are therapeutic options for toxoplasmosis and malaria. Interestingly, the causative agents, T. gondii and Plasmodium spp. for toxoplasmosis and malaria, respectively, are intracellular apicomplexans. This may indicate that most of the FDA-approved drugs that blocked T. gondii growth in the present study have similar clinical indications. For example, two of the hit compounds, mecamylamine and atropine, are used as therapeutic options for cardiac-related conditions, whereas niflumic acid is used to treat inflammatory conditions. Previously, we showed that FDA-approved drugs used as anti-inflammatory therapies potently suppressed the in vitro growth of T. gondii [10]. Taken together, these findings may underscore the prospects of anti-inflammatory agents as a source of alternative anti-T. gondii therapy. However, while the FDA-approved compounds are mostly well-characterized, their mechanisms of action against the growth of T. gondii are yet to be revealed and thus warrant further studies.
Among the hits from the natural product isolates, resveratrol has been previously reported as having anti-T. gondii properties [32], while narasin, a derivative of salinomycin (an ionophoric anticoccidial compound), is a known antiprotozoan agent [33]. Additionally, two of the hit compounds (resveratrol and sulfuretin) from the natural product isolates are phenolics. Interestingly, our study is the first report to show a suppressive action of sulfuretin against the in vitro growth of T. gondii. Nevertheless, some of the hit compounds have been reported as having other bioactive properties. For example, resveratrol has been implicated as having anti-inflammatory properties [34] as well as anti-T. gondii activity [32]. Also, the anti-inflammatory and antiparasitic actions of narasin have been previously documented [35]. It is worth noting that the majority of the hit compounds among the natural product isolates in this study have anti-inflammatory properties. This is consistent with our findings reported elsewhere [10], which revealed not only that the natural compounds active against T. gondii are mostly phenolics but also that the same compounds have anti-inflammatory activity. This same study also revealed that ≥ 75 percent of the FDA-approved drugs with activity against T. gondii are therapeutic options for inflammatory-related conditions. Given these findings, it is plausible to rationalize that the hit compounds identified in the study might have somewhat similar antiparasite mechanisms. Moreover, in the present study, the fact that a majority of the hit compounds possess anti-inflammatory potential may indicate a way to identify new targets that could be further explored for development of more effective therapeutic options for toxoplasmosis. Taken together, the findings in this study not only support our earlier report [10] but also are consistent with those from other studies [6,21] that showed that drugs with anti-inflammatory indications suppressed the in vitro growth of T. gondii. Additionally, the in silico model supported the prospects of the identified anti-Toxoplasma-active compounds from both the FDA-approved and natural products for early drug discovery against toxoplasmosis.
In conclusion, our study identified new chemical compounds from a group of natural product isolates and FDA-approved drugs that suppressed the in vitro growth of T. gondii. The findings are not only new and promising but also further reinforce the concept of screening a vast array of chemical entities, including drug repurposing, as a logical option in early drug development efforts for parasitic infections. Ongoing investigations aim to evaluate these compounds for in vivo efficacy against acute and latent toxoplasmosis in a mouse model.
Acknowledgments
Authors acknowledge (1) the Japan Society for Promotion of Science (JSPS) Fellowship to Dr. OS Adeyemi; (2) the National Centre for Protozoan Diseases (NRCPD) of the Obihiro University of Agriculture and Veterinary Medicine, Japan. (3) Ed and Rhoda Perozzi for Editorial assistance.
Glossary
- NPs
natural products
- MOI
multiplicity of infection
- HFF
Human fibroblast foreskin
Conflicts of Interest.
The authors have no competing interests.
References
- Black MW, Boothroyd JC. Lytic Cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck HP, Blake D, Darde ML, et al. Molecular Approaches to Diversity of Populations of Apicomplexan Parasites. Int J Parasitol. 2009;39:175–89. [DOI] [PubMed] [Google Scholar]
- Hill DE, Chirukandoth S, Dubey JP. Biology and Epidemiology of Toxoplasma gondii in Man and Animals. Anim Health Res Rev. 2005;6:41–61. [DOI] [PubMed] [Google Scholar]
- Kamau ET, Srinivasan AR, Brown MJ, et al. A Focused Small-Molecule Screen Identifies 14 Compounds with Distinct Effects on Toxoplasma gondii. Antimicrob Agents Chemother. 2012;56(11):5581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZD, Liu HH, Ma ZX, et al. Toxoplasma gondii Infection in Immunocompromised Patients: A Systematic Review and Meta-Analysis. Front Microbiol. 2017;8:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar AJ, Drozda AA, Blader IJ. Drug Repurposing Screening Identifies Novel Compounds that Effectively Inhibit Toxoplasma gondii Growth. MSphere. 2016;1(2):e00042–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeyemi OS, Molina MT, Eseola AO, Fonseca-Berzal C, Gómez-Barrio A. New imidazole compounds active against Trypanosoma cruzi. Comb Chem High Throughput Screen. 2017. a;20(1):20–4. [DOI] [PubMed] [Google Scholar]
- Adeyemi OS, Murata Y, Sugi T, Kato K. Inorganic Nanoparticles Caused Death of Toxoplasma gondii through Alteration of Redox Status and Mitochondrial Membrane Potential. Int J Nanomedicine. 2017. b;12:1647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeyemi OS, Murata Y, Sugi T, Han Y, Kato K. Modulation of Host HIF-1α Activity and the Tryptophan Pathway Contributes to the Anti-Toxoplasma gondii Potential of Nanoparticles. Biochem Biophys Rep. 2017. c;11:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeyemi OS, Sugi T, Han Y, Kato K. Screening of Chemical Compound Libraries Identified New Anti-Toxoplasma gondii Agents. Parasitol Res. 2018. a;117(2):355–63. [DOI] [PubMed] [Google Scholar]
- Adeyemi OS, Murata Y, Sugi T, Han Y, Kato K. Exploring Amino Acid-Capped Nanoparticles for Selective Anti-Parasitic Action and Improved Host Biocompatibility. J Biomed Nanotechnol. 2018. b;14(5):847–67. [DOI] [PubMed] [Google Scholar]
- Adeyemi OS, Molefe NI, Awakan OJ, Nwonuma CO, Alejolowo O, Olaolu T, et al. Metal Nanoparticles Show Potential to Restrict Trypanosoma Growth. Artif Cells Nanomed Biotechnol. 2018. c;46 S3:S86–94. [DOI] [PubMed] [Google Scholar]
- Adeyemi OS, Otohinoyi DA, Awakan OJ, Adeyanju AA. Cellular Apoptosis of HFF Cells by Inorganic Nanoparticles Not Susceptible to Modulation by Toxoplasma gondii Infection In Vitro. Toxicol In Vitro. 2019;54:280–5. [DOI] [PubMed] [Google Scholar]
- Fonseca-Berzal C, Ruiz FA, Escario JA, Kouznetsov VV, Gomez-Barrio A. In vitro Phenotypic Screening of 7-Chloro-4-Amino(Oxy) Quinoline Derivatives as Putative Anti-Trypanosoma Cruzi Agents. Bioorg Med Chem Lett. 2014;24(4):1209–13. [DOI] [PubMed] [Google Scholar]
- Šimenc J, Lipnik-Štangelj M. Staurosporine induces apoptosis and necroptosis in cultured rat astrocytes. Drug Chem Toxicol. 2012;35(4):399–405. [DOI] [PubMed] [Google Scholar]
- Mishra SS, Kumar N, Singh HP, Ranjan S, Sharma CS. In silico Pharmacokinetic, Bioactivity and Toxicity Study of some Selected Anti-Asthmatic Agents. Int J Pharm Sci Drug Res. 2018;10(4):278–82. [Google Scholar]
- Banerjee P, Eckert OA, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46 W1:W257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drwal MN, Banerjee P, Dunkel M, Wettig MR, Preissner R. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014;(Web Server issue):W53-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globally Harmonized System of Classification and Labelling Of Chemicals GHS www.osha.gov. Archived from the original on 2007-07-02. [Retrieved May 2, 2019].
- Molefe NI, Yamasaki S, Macalanda AM, et al. Oral Administration of Azithromycin Ameliorates Trypanosomosis in Trypanosoma Congolense-Infected Mice. Parasitol Res. 2017;116(9):2407–15. [DOI] [PubMed] [Google Scholar]
- Murata Y, Sugi T, Weiss LM, Kato K. Identification of Compounds That Suppress Toxoplasma gondii Tachyzoites and Bradyzoites. PLoS One. 2017;12(6):e0178203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar V, Bhattacharjee G, Rajakumar S, Srivastava K, Puri SK. Synthesis of New Chalcone Derivatives Containing Acridinyl Moiety with Potential Antimalarial Activity. Eur J Med Chem. 2010;45(2):745–51. [DOI] [PubMed] [Google Scholar]
- Yenesew A, Induli M, Derese S, Midiwo JO, Heydenreich M, Peter MG, et al. Anti-Plasmodial Flavonoids from the Stem Bark of Erythrina Abyssinica. Phytochemistry. 2004;65(22):3029–32. [DOI] [PubMed] [Google Scholar]
- Sung J, Lee J. Anti-Inflammatory Activity of Butein and Luteolin Through Suppression of NF κB Activation and Induction of Heme Oxygenase-1. J Med Food. 2015;18(5):557–64. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Kuo SC, Chan SC, Ko FN, Teng CM. Antioxidant Properties of Butein Isolated from Dalbergia Odorifera. Biochimica Et Biophysica Acta (BBA)-. Lipids and Lipid Metabolism. 1998;1392(2-3):291–9. [DOI] [PubMed] [Google Scholar]
- Sivakumar PM, Prabhakar PK, Doble M. Synthesis, Antioxidant Evaluation, and Quantitative Structure–Activity Relationship Studies of Chalcones. Med Chem Res. 2011;20(4):482–92. [Google Scholar]
- Busetto GM, Giovannone R, Ferro M, Tricarico S, Del Giudice F, Matei DV, et al. Chronic Bacterial Prostatitis: Efficacy of Short-Lasting Antibiotic Therapy with Prulifloxacin (Unidrox®) In Association with Saw Palmetto Extract, Lactobacillus Sporogens and Arbutin (Lactorepens®). BMC Urol. 2014;14(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara R, Seto A, Kofujita H, Shiba Y, Oishi Y, Shibasaki Y. Enhanced Antimicrobial Activities of Polymerized Arbutin and its Derivatives Prepared by Oxidative Polymerization of Arbutin. React Funct Polym. 2019 [Google Scholar]
- Zhao W, Wang S, Qin T, Wang W. Arbutin Attenuates Hydrogen Peroxide-Induced Oxidative Injury Through Regulation of Microrna-29a in Retinal Ganglion Cells. Biomed Pharmacother. 2019;112:108729. [DOI] [PubMed] [Google Scholar]
- Bang JS, Oh DH, Choi HM, et al. Anti-Inflammatory and Antiarthritic Effects of Piperine in Human Interleukin 1beta-Stimulated Fibroblast-Like Synoviocytes and in Rat Arthritis Models. Arthritis Res Ther. 2009;11(2):R49 10.1186/ar2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zanbagi NA. In vivo Effect of some Home Spices Extracts on the Toxoplasma gondii Tachyzoites. J Family Community Med. 2009;16(2):59–65. [PMC free article] [PubMed] [Google Scholar]
- Bottari NB, Baldissera MD, Tonin AA, Rech VC, Alves CB, D’Avila F, et al. Synergistic Effects of Resveratrol (Free and Inclusion Complex) And Sulfamethoxazole-Trimetropim Treatment on Pathology, Oxidant/Antioxidant Status and Behavior of Mice Infected with Toxoplasma gondii. Microb Pathog. 2016;95:166–74. 10.1016/j.micpath.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Gerhold RW, Fuller AL, Lollis L, Parr C, McDougald LR. The Efficacy of Anticoccidial Products against Eimeria Spp. In Northern Bobwhites. Avian Dis. 2011;55(1):59–64. [DOI] [PubMed] [Google Scholar]
- de Sá Coutinho D, Pacheco MT, Frozza RL, Bernardi A. Anti-Inflammatory Effects of Resveratrol: mechanistic Insights. Int J Mol Sci. 2018;19(6):1812 10.3390/ijms19061812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevin Ii DA, Meujo DA, Hamann MT. Polyether Ionophores: Broad-Spectrum and Promising Biologically Active Molecules for the Control of Drug-Resistant Bacteria and Parasites. Expert Opin Drug Discov. 2009;4(2):109–46. 10.1517/17460440802661443 [DOI] [PMC free article] [PubMed] [Google Scholar]